-
随着中国城市化进程加快,对铁矿石的需求量也不断增加,但中国的铁矿资源多数依靠进口[1-2]。中国是钢铁生产大国,国内铁矿石品位低、结构复杂、难选,导致高品质铁矿石严重短缺。过去几年 (2008-2022年) ,中国铁矿石进口量从4.4×108 t增加到11.7×108 t,外部依赖性逐年增大[3]。铁矿石的长期供应压力,已经成为中国钢铁工业可持续发展的重大隐患。
据统计,中国铁尾矿总堆存量超过1×1010 t[4],每年新增约6×108 t,利用率低,铁尾矿平均总铁品位达11%以上[1],但过去部分矿山采用“粗放式开采、选富弃贫”的方式,使部分尾矿中总铁品位甚至高达30%左右。由于技术限制,铁尾矿一般在尾矿库堆填,这样既造成了大量的土地浪费,也对周围生态环境造成了极大破坏[5-6]。
磁化焙烧技术是指将物料或矿石在一定的气氛条件下加热进行化学反应,选择性地使弱磁性铁矿物转变为强磁性的磁铁矿或磁赤铁矿,而脉石矿物磁性几乎不变。通过磁化焙烧人为地增大铁氧化物与脉石矿物的磁性差异,提高铁矿石的可选性,是难选铁矿分选的最有效方法。同时,焙烧过程还能去除矿石中的结晶水、硫、砷等有害杂质,使矿石结构疏松,有利于提高后续磨矿效率。LI等[7]采用碳作为还原剂对铁尾矿进行磁化焙烧,在750 °C、60 min焙烧条件下,磁选后得到铁品位58.6%,铁回收率68.4%的磁精矿;DENG等[8]采用生物质作为还原剂,在650 °C、20 min焙烧条件下,得到铁品位62.04%,铁回收率95.29%的磁精矿;YUAN等[9]使用纯CO还原铁锰矿,在600 °C、20 min焙烧条件下,得到铁品位为68.31%,铁回收率96.34%的磁精矿。当前磁化焙烧的还原剂主要由煤炭、石油等化石能源制备,具有成本高,碳排放强度大,环保落地困难等不足。
印染行业是我国传统支柱产业,据2021年中国环境统计年鉴[10],2020年全国印染废水量达2.06×109 t,居工业行业第三,并产生5.15×106 t (80%含水率计) 印染污泥。其含有毒有机物质,例如染色剂、表面活性剂、添加剂、多环芳烃(PAHs)、持久性有机污染物(POPs)、芳香胺(AAs)和重金属等[11-13]。目前印染污泥主要是厂内锅炉掺烧以及厂外集中焚烧,少量填埋或者烧砖等[14-15],处理过程中的污染控制是印染污泥无害化处理的关键环节。
中国“十四五”规划中,鼓励污泥在实现稳定化、无害化处置前提下,稳步推进污泥能量资源化回收利用。污泥热解产生的不凝性气体主要由CO、CO2、CH4和H2组成,具有强还原性,可作为铁尾矿磁化焙烧的还原剂,此外印染废水混凝沉淀过程中投加的聚合硫酸铁会产生大量含铁高电荷聚合阳离子,例如[Fe(H2O)6]3+、[Fe(OH)x](3−x)+、[Fem(OH)x]n(3m−x)n+等[16],这些多核络合离子能与水以任意比例互溶,发挥电性中和、吸附架桥作用,当溶液的pH值升高后,单核羟基络离子就会生成羟基桥联的多核络合物[17]。印染污泥含铁多核络合物在焙烧的过程中发生热分解,生成Fe2O3。通过与铁尾矿共同磁化焙烧,可实现印染污泥与铁尾矿中铁的高效协同回收。
本研究利用印染污泥热解产生的还原性气体将印染污泥和铁尾矿中的铁资源还原成四氧化三铁,再通过磁选得到铁精矿,研究了焙烧温度、焙烧时间和印染污泥掺烧量对铁品位和回收率的影响及作用机制。有望开辟以废治废,低碳排放的大宗固体废物资源化利用新途径,具有非常重要的理论价值和实际应用前景。
-
铁尾矿从广东省韶关市大宝山的尾矿库中取样获得。铁尾矿取样后置于105 °C烘箱中干燥处理24 h,将干燥后的铁尾矿破碎至粒度分布确保40%通过200目(−0.074 mm),密封保存。
印染污泥从广东省佛山市一家印染废水处理厂获得。印染污泥自然风干后,置于105 °C烘箱中干燥处理24 h,将干燥后的印染污泥样品粉碎并过200目筛网(−0.074 mm),密封保存。
本研究中的铁精矿由焙烧产物通过120 mT湿法磁选方式获得。
-
印染污泥(−0.074 mm)与40%过200目铁尾矿(−0.074 mm)磁化焙烧采用高温真空气氛管式炉(SK-G06123K, TJZH, China),印染污泥与铁尾矿按照一定比例混合均匀放入刚玉瓷舟中,将高温真空气氛管式炉通入一定速率的N2 (99.9%纯度, 100 mL·min−1) 以便排净空气。当反应器温度达到预定温度时,关闭N2,将装有混合物料的刚玉瓷舟置于高温真空气氛管式炉中进行快速热解,当达到预定反应时间后,停止加热持续通入一定速率的N2冷却至室温,取出刚玉瓷舟,刚玉瓷舟内的样品即为焙烧产物。
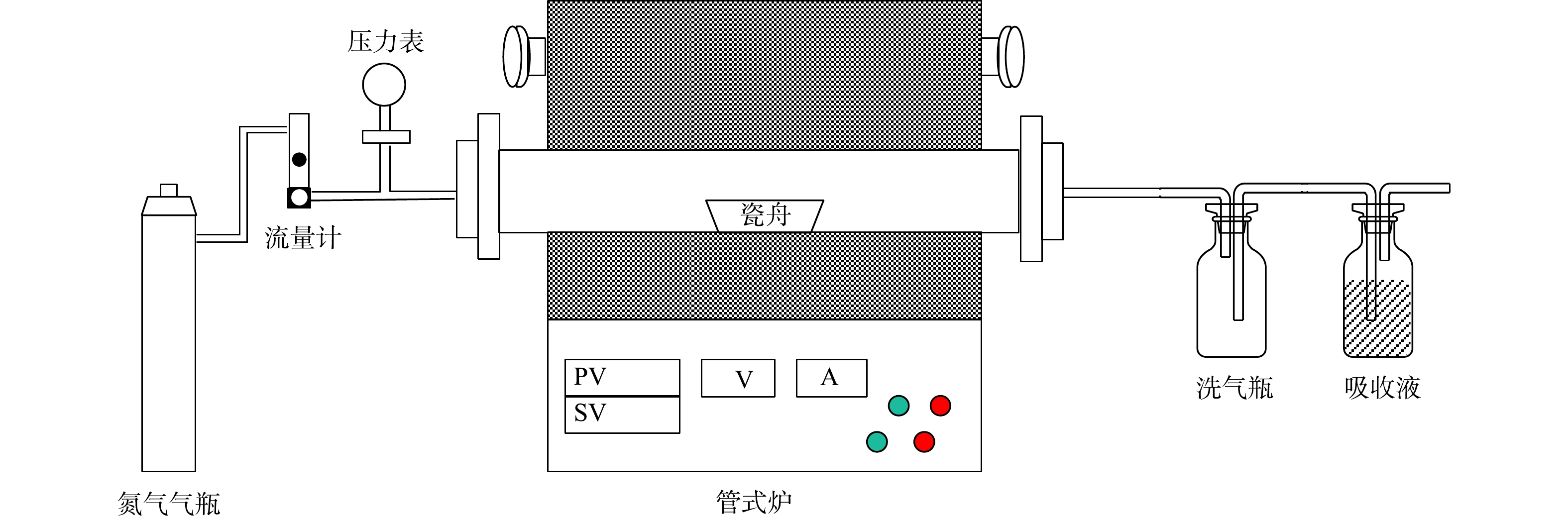
本实验装置如图1所示,包括供气装置、气流控制器、温度控制器等。在最佳磁化焙烧条件探究中,焙烧温度、焙烧时间和印染污泥掺烧比量的范围分别为600~850 °C、10~60 min和2.5%~25%。通过XRD (D8 ADVANCE, Bruker, Germany,40 kV, 30 mA, 10◦ min−1, 10◦~80◦)分析、扫描电子显微镜(Sigma 300, Zeiss, Germany)、XPS分析仪(Thermo Scientific K-Alpha, America, 12 kV, 6 mA)以及振动样品磁强计(Lake Shore 7404, LakeShore, America)对焙烧产品进行研究。用球磨机将焙烧样品破碎至过200目筛网(−0.074 mm),并在磁场强度为120 mT条件下,使用磁选机进行湿法磁选。磁性精矿和非磁性尾矿在分离后都经过过滤、干燥和称重,干燥过程在温度为65 °C的烘箱中进行。对铁品位采用GB/T 6730.65-2009进行分析测定,铁品位、铁回收率的计算如式(1)和(2)所示:
式中:ω为样品的铁品位;c为测定铁品位时重铬酸钾标准滴定溶液的浓度;V1为测定铁品位时滴定试料所消耗的重铬酸钾标准滴定溶液的体积;V0为滴定空白试验溶液消耗重铬酸钾标准滴定溶液体积;m0为试料的质量。
-
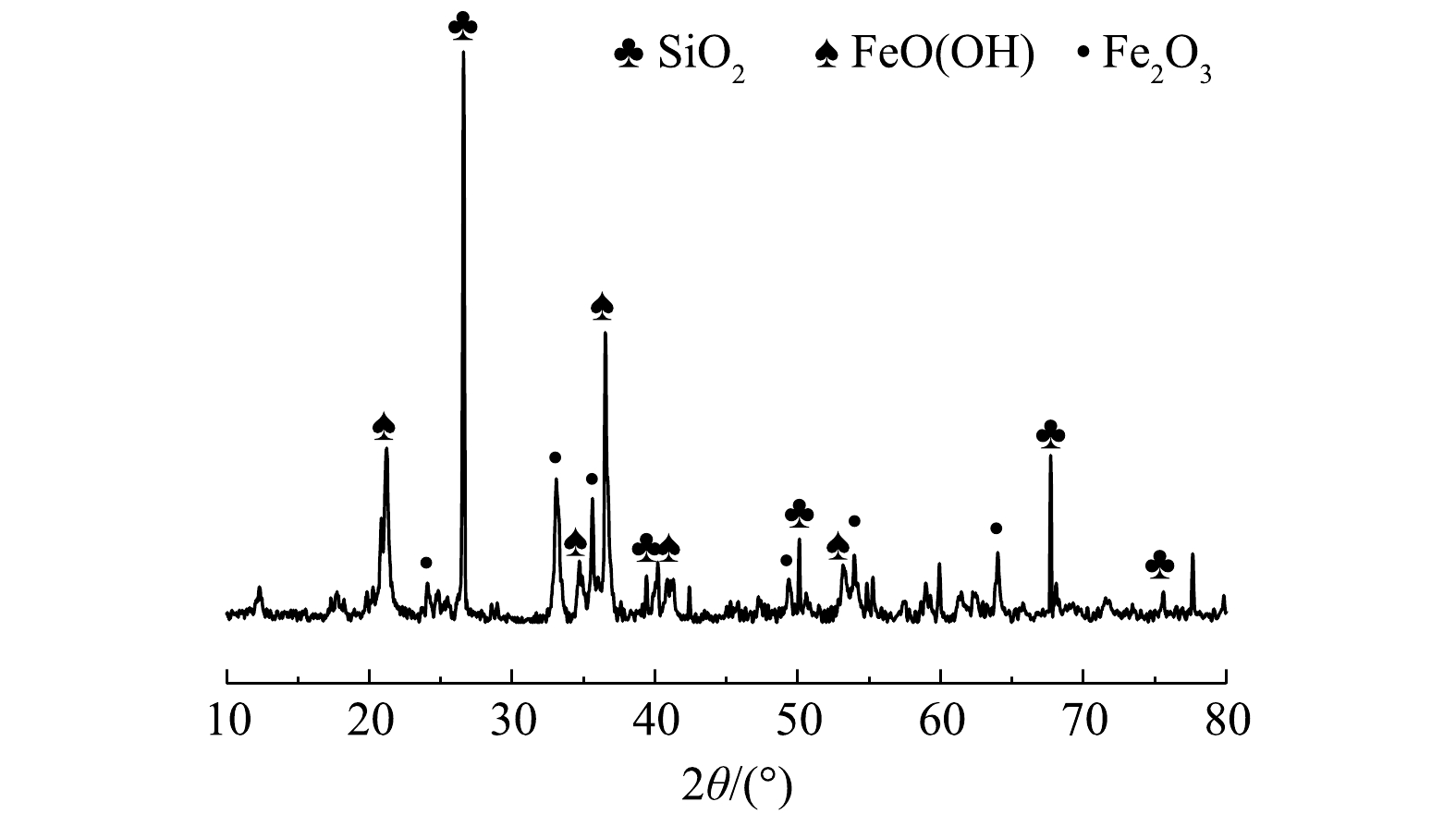
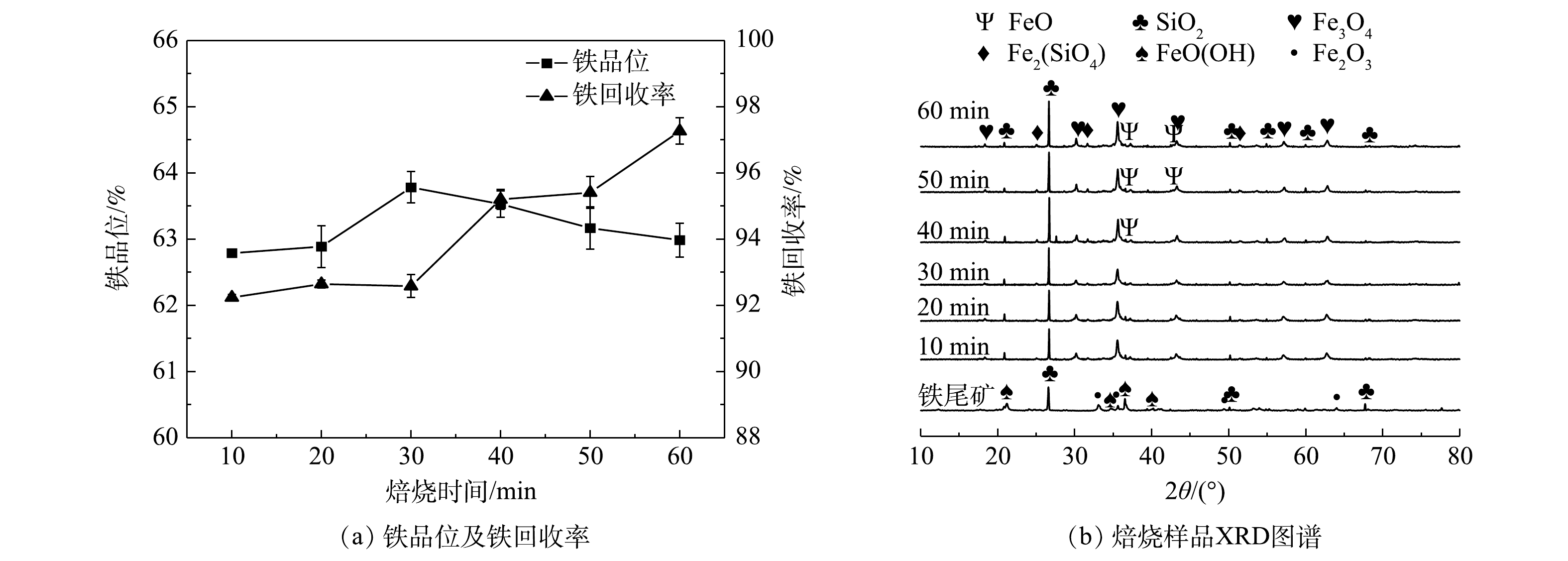
1) 成分分析。铁尾矿样品组成如表1所示。铁尾矿中总铁含量为40.35%,通过XRF-7000 (Shimadzu, Japan) 表征可知,铁尾矿中主要的元素为Fe、Si、Al等 (以氧化物形式表示) 。铁尾矿XRD图谱如图2(a)所示,主要成分为赤铁矿(Fe2O3),针铁矿(FeO(OH)),主要脉石矿物为石英(SiO2)。
印染污泥样品组成依据GB/T 28731-2012进行工业分析和XRF表征,其结果如表1、表2所示。印染污泥中总铁含量为20.04%,其中还含有22.09%的碳,与氢、氮、氧等元素组成有机质部分。
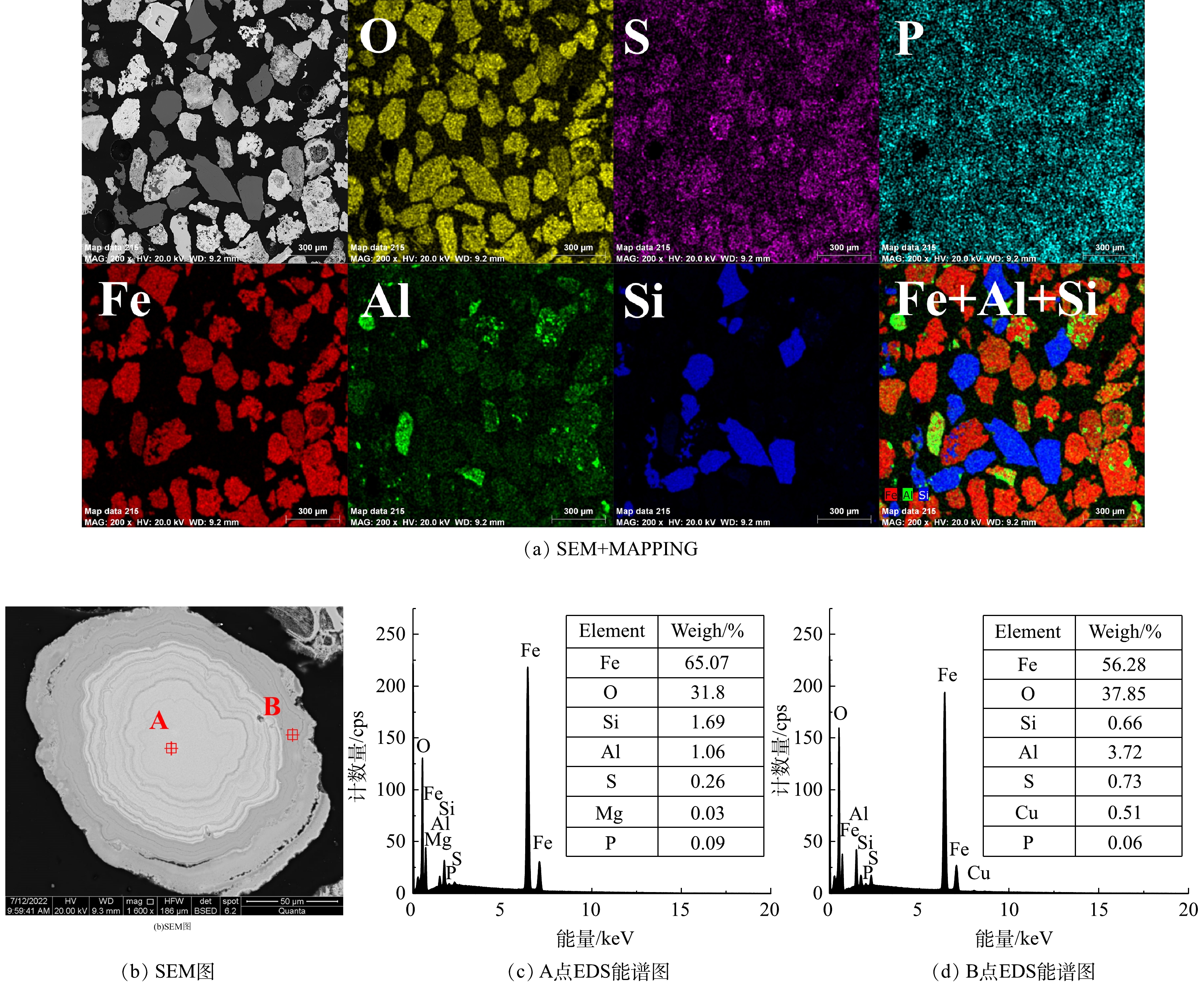
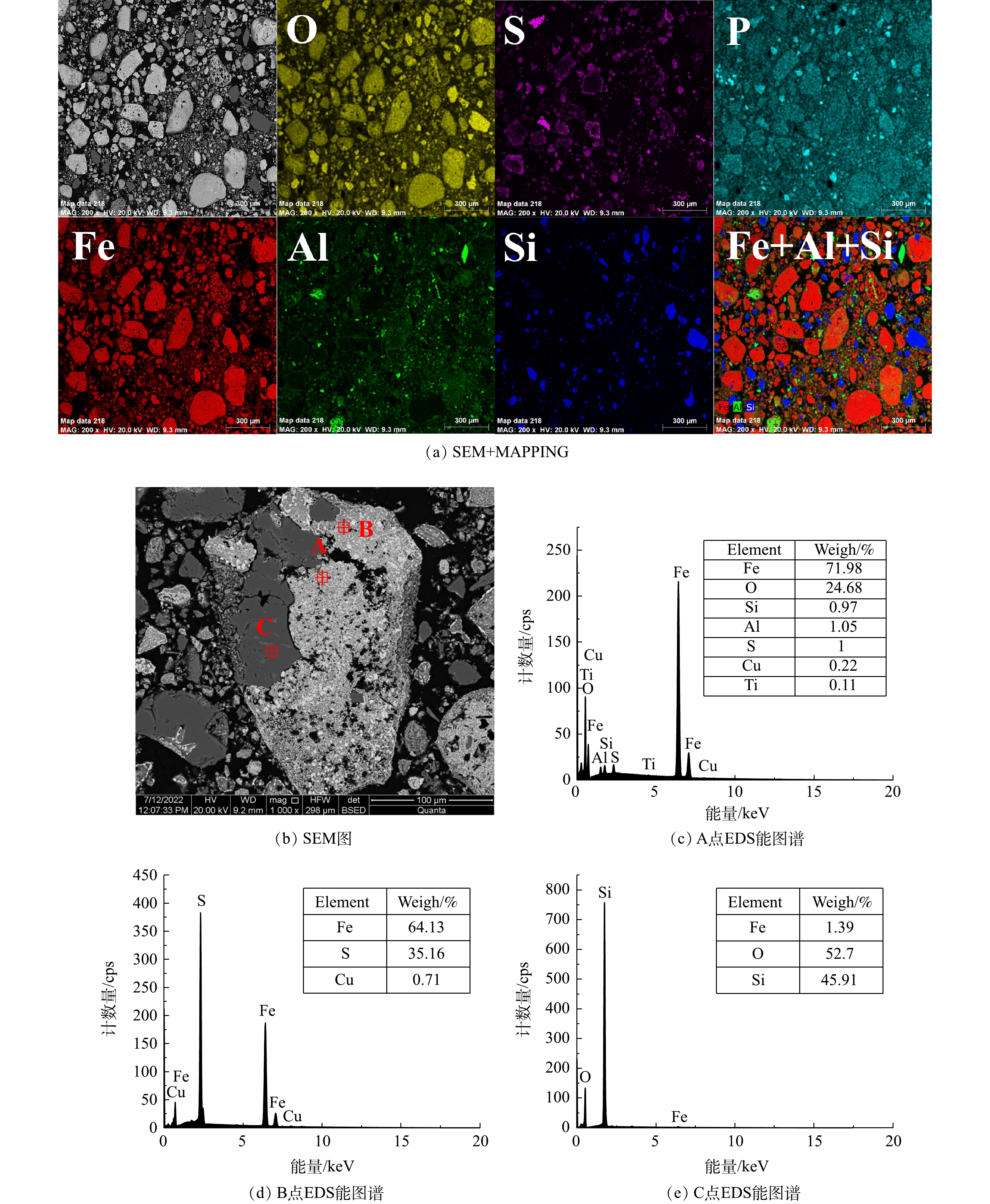
2) 铁尾矿微观形貌分析。铁尾矿原矿SEM表征如图3所示,铁尾矿呈不规则颗粒状,通过切面SEM+MAPPING可以看出,原矿中Fe、Al、Si、O和S分布较为集中均匀,而Fe、Al、Si存在共掺杂现象。铝元素和铁元素相互交错,表明Fe2O3和Al2O3存在相互结合或包裹。Al与Fe较Si与Fe的结合包裹更明显, Si则与氧结合,主要以SiO2形式存在。选取其中一块铁尾矿切面并对其中两个点位进行EDS分析,可以发现铁尾矿中心内部与外部形貌有较大区别。通过对原子质量的分析,点A对应的质量比ωFe∶ωO=2.05,而FeO(OH)理论比ωFe∶ωO=1.75,表明中心区域矿物为针铁矿。同理,点B对应区域可表述为赤铁矿,表明铁尾矿中部分针铁矿是被赤铁矿包裹在内。
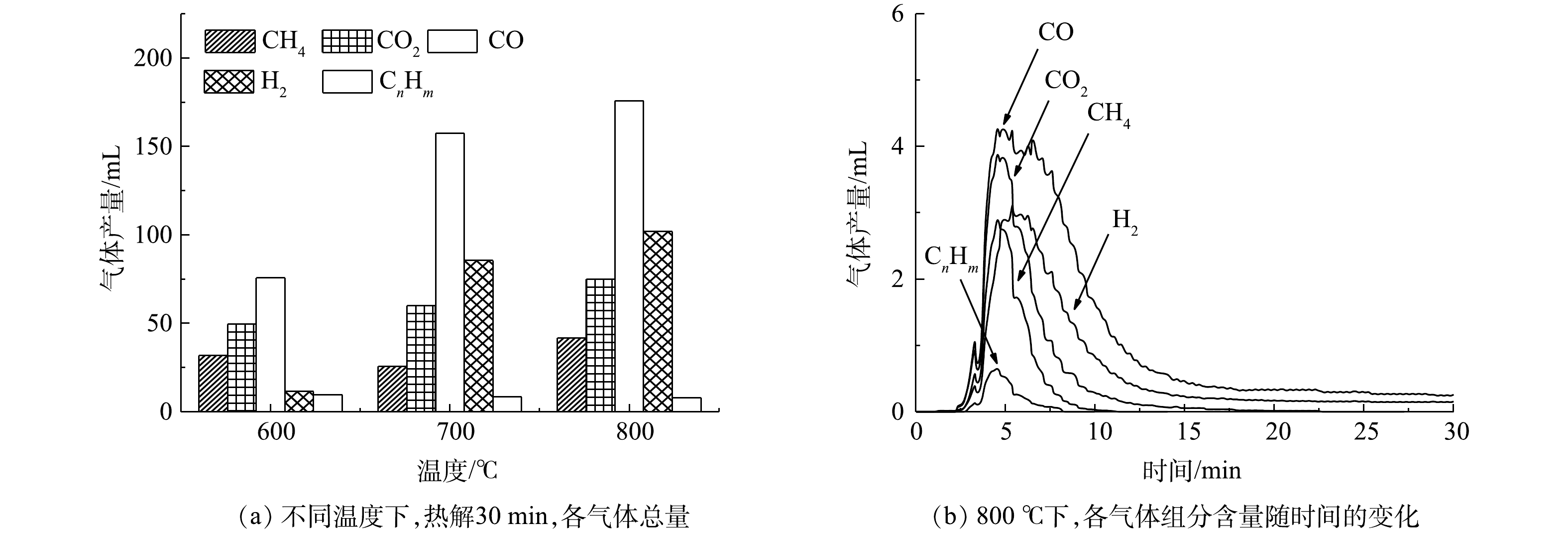
3) 印染污泥热解分析。有机物热分解是印染污泥热解气的主要来源[18],因为在高温情况下,有机物中的大分子物质 (苯酚,醚,甲氧基和含氧杂环结构等氧官能团) 裂解成为更加稳定的小分子物质 (CO、CH4、H2、CO2等) 。利用烟气分析仪(TY-6300P, Wuhan- Tianyu, China)对印染污泥的热解产气进行研究。从图4(a)中可知,随着热解温度升高,还原性气体CO、H2和CH4逐渐增多,为赤铁矿脱氧还原提供足够还原剂。由图4(b)可知,在800 °C下前10 min为印染污泥热解产气,这与DING的研究相一致[19];在10~15 min时,体系中CO2与碳发生Boudouard反应 (式4) 以及CH4与CO2反应 (式8) ,使得体系中CO2、CH4含量减少,但仍有CO与H2被检出。根据印染污泥热解产气的组分及含量变化,该过程可能存在的化学反应见式(3)~式(9)。
-
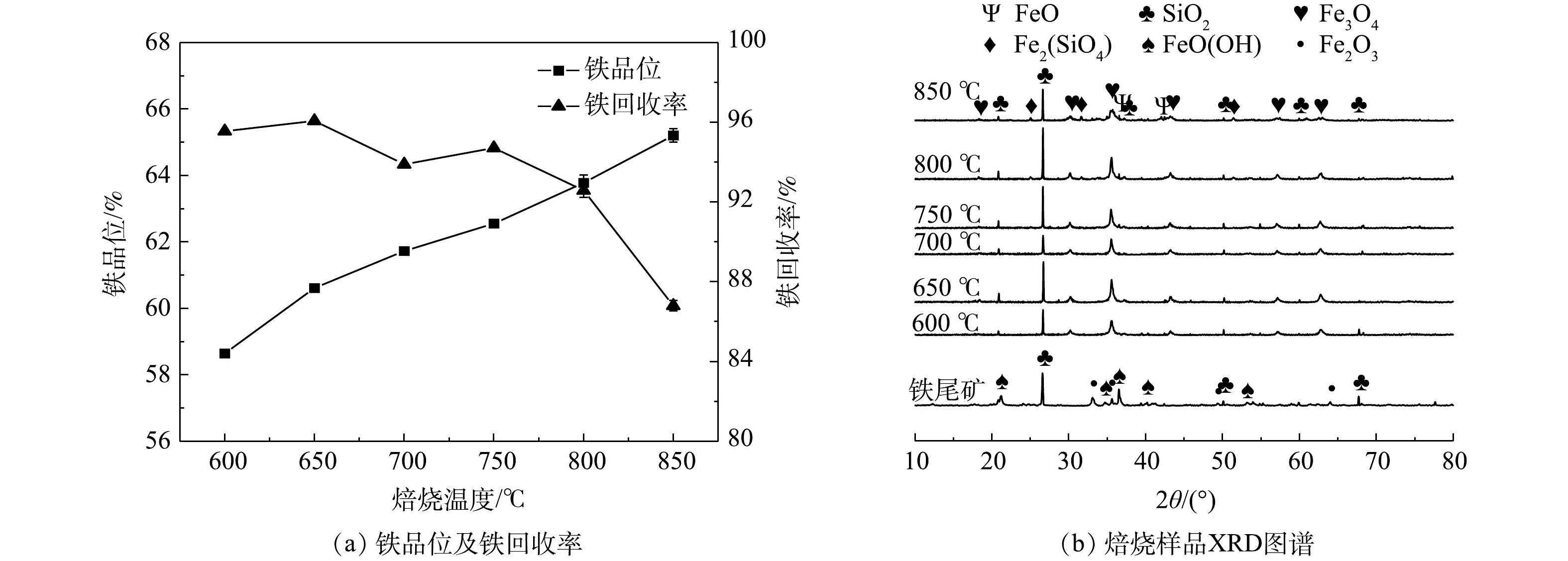
1) 焙烧温度。在焙烧时间30 min,印染污泥掺烧量15%的条件下,探究焙烧温度的影响。从图5(a)中可知,随着焙烧温度从600 °C增加至850 °C,铁品位从58.64%增加至65.20%,而铁回收率呈现下降趋势。当温度超过800 °C时,铁回收率有明显下降,约下降了5.78%。这是因为焙烧温度过高时,一些赤铁矿过度还原为弱磁性的氧化亚铁而难以回收[20]。此外高温还会导致磁性矿物之间磁团聚作用增强,脉石矿物难以与铁精矿分离[21]。由焙烧产物XRD图谱 (图5(b)) 可知,随着焙烧温度增加,Fe3O4峰强度先增加后减少,在850 °C出现了氧化亚铁(FeO)及铁橄榄石(Fe2(SiO4))峰,均无法被磁选回收,这是铁回收率下降的主要原因,因此800 °C为最佳焙烧温度。
2) 焙烧时间。在焙烧温度800 °C,印染污泥掺烧量15%条件下,焙烧时间对铁品位以及铁回收率的影响如图6(a)所示。随着焙烧时间从10 min到60 min,铁品位呈现先上升再下降的趋势,在30 min达到最高铁品位63.78%。焙烧产物进行XRD图谱分析如图6(b)所示。随着焙烧时间增加,在40 min开始出现氧化亚铁(FeO)及铁橄榄石(Fe2(SiO4))峰,铁橄榄石以及一些脉石矿物在磁团聚作用下,被磁铁矿包裹,在磁选过程中进入铁精矿中,导致铁品位下降而铁回收率升高。此外,磁团聚作用容易阻止还原性气体扩散[22],无法对赤铁矿进行有效还原,进而铁品位不断下降。
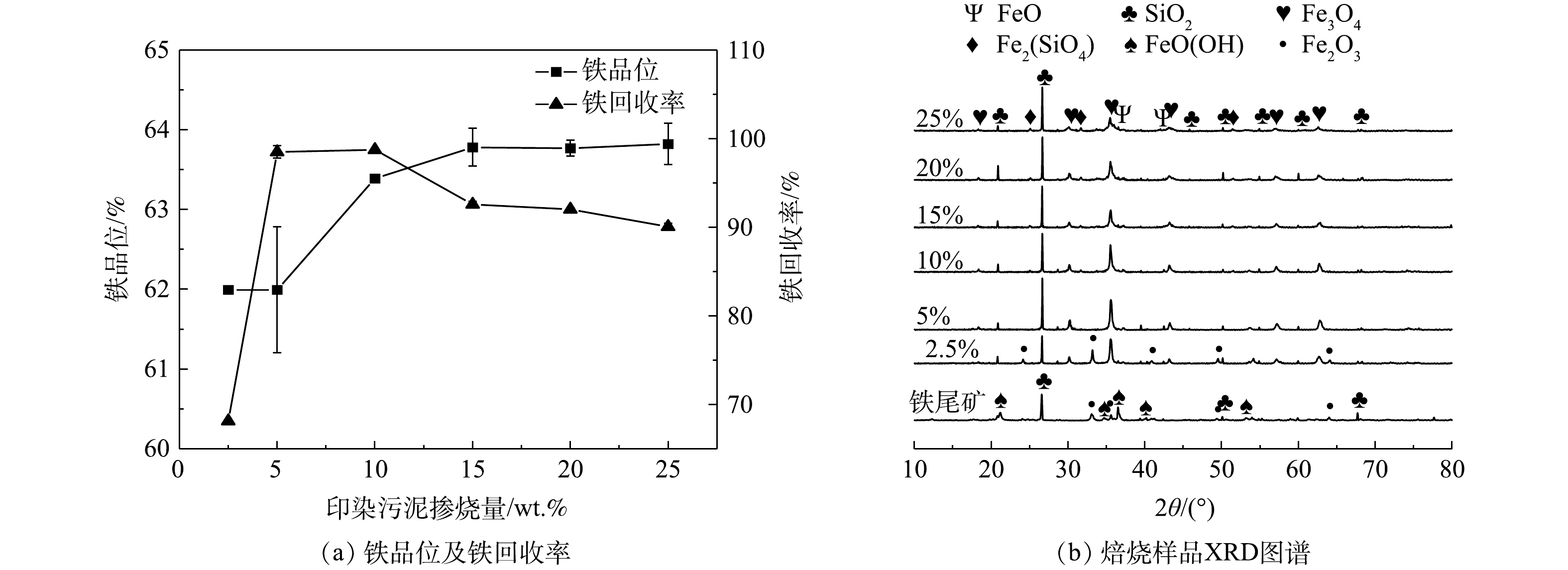
3) 印染污泥掺烧量。在焙烧温度800 °C、焙烧时间30 min下,印染污泥掺烧量对铁品位以及铁回收率的影响如图7(a)所示。随着印染污泥掺烧量从2.5%到25%,铁品位总体呈现上升的趋势,当掺烧量为15%时,铁品位达到63.78%。焙烧产物的XRD图谱分析如图7(b)所示,当印染污泥掺烧量较低时,焙烧样中还有较强的赤铁矿衍射峰,这是因为污泥热解提供的还原气不足以将赤铁矿全部还原。随着印染污泥掺烧量增加,印染污泥产生了较多还原性气体,赤铁矿衍射峰逐渐消失,磁铁矿衍射峰增强。当掺烧量达到25%时,出现了FeO特征峰,表明了赤铁矿发生了过还原现象,使得回收率下降。因此15%为最佳印染污泥掺烧量。
在800 °C、30 min,印染污泥掺烧量15%的最佳焙烧条件下,得到铁品位63.78%,铁回收率92.58%的铁精矿。相较于QIU等[23]采用悬浮磁化焙烧,将生物质热解气作为还原剂还原铁尾矿,得到铁回收率为93.32%、铁品位为61.50%的铁精矿,本研究将40.35%品位铁尾矿转化为63.78%铁品位的铁精矿,不仅简化流程,更节省成本,具有更好的经济性。
-
采用SEM+MAPPING图像和EDS能谱分析了焙烧样微观形貌及赋存形态 (图8) ,相比于原矿,焙烧样中Si、Al被明显解离成小块,Fe、Al和Si掺杂现象明显减少,表明Si、Al与Fe连生体在磁化焙烧过程中发生了解离现象,这些细小颗粒部分是在磁化焙烧过程中和赤铁矿分离的铝硅酸盐,通过磁选可以进行有效分离[8]。选取一块焙烧矿的切面图并对其中3个点位进行EDS分析。通过对原子质量分析可知,点位A对应为磁铁矿,点位B对应为硫铁矿物,点位C对应为石英,铁矿物和脉石之间的微裂纹清晰可见,通过后续破碎研磨以及磁选,能够使得部分石英以及铝硅酸盐与磁铁矿分离。焙烧样中存在少部分硫铁矿物,可能是印染污泥的硫酸根发生了还原反应生成活性硫,进而与铁的化合物反应生成硫铁矿物,这些硫铁矿物附着在磁铁矿物表面,使得部分焙烧矿出现铁矿石表面硫化。
-
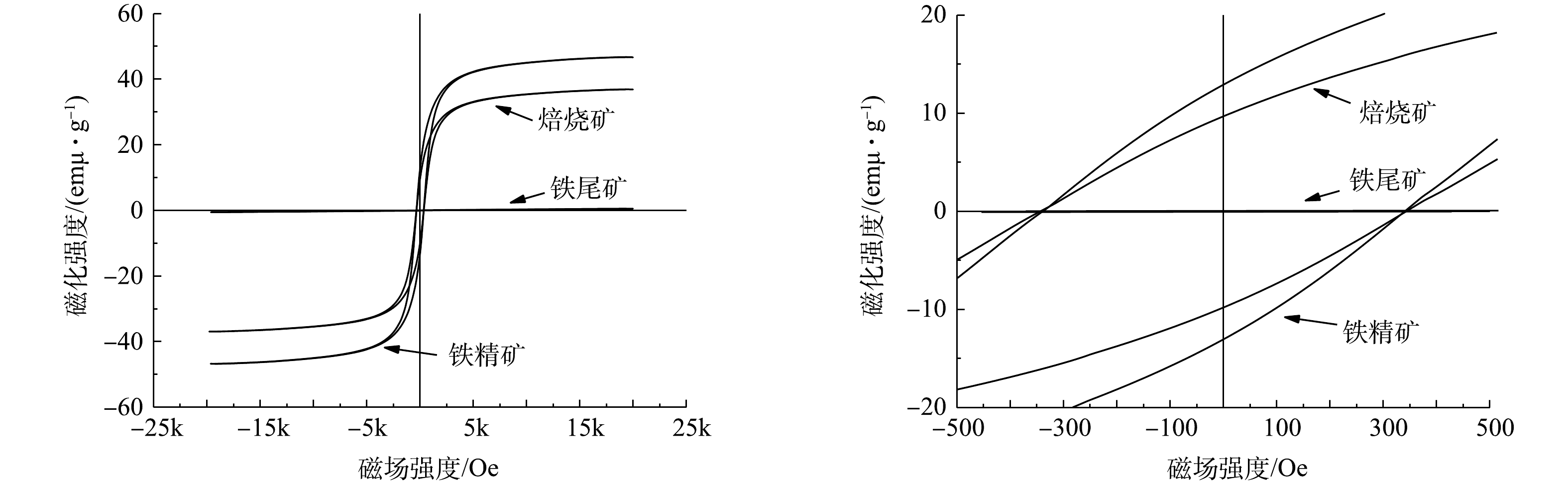
对铁尾矿、焙烧矿、磁选精矿磁性进行VSM分析,VSM磁滞回线结果如图9所示。磁滞回线可以确定材料许多磁性,例如剩磁、饱和磁化强度和矫顽力。铁尾矿饱和磁化强度为0.55 emu·g−1,混合磁化焙烧后焙烧矿饱和磁化强度为36.88 emu·g−1,磁选后磁选精矿饱和磁化强度增加至46.68 emu·g−1,焙烧矿剩磁为9.72 emu·g−1,磁精矿剩磁为12.82 emu·g−1,原矿、焙烧矿和磁选精矿之间饱和磁化强度和剩磁差异表明,印染污泥与铁尾矿混合磁化焙烧可将物料中铁氧化物还原成磁铁矿,并通过磁选实现脉石矿物与磁铁矿物的分离。
-
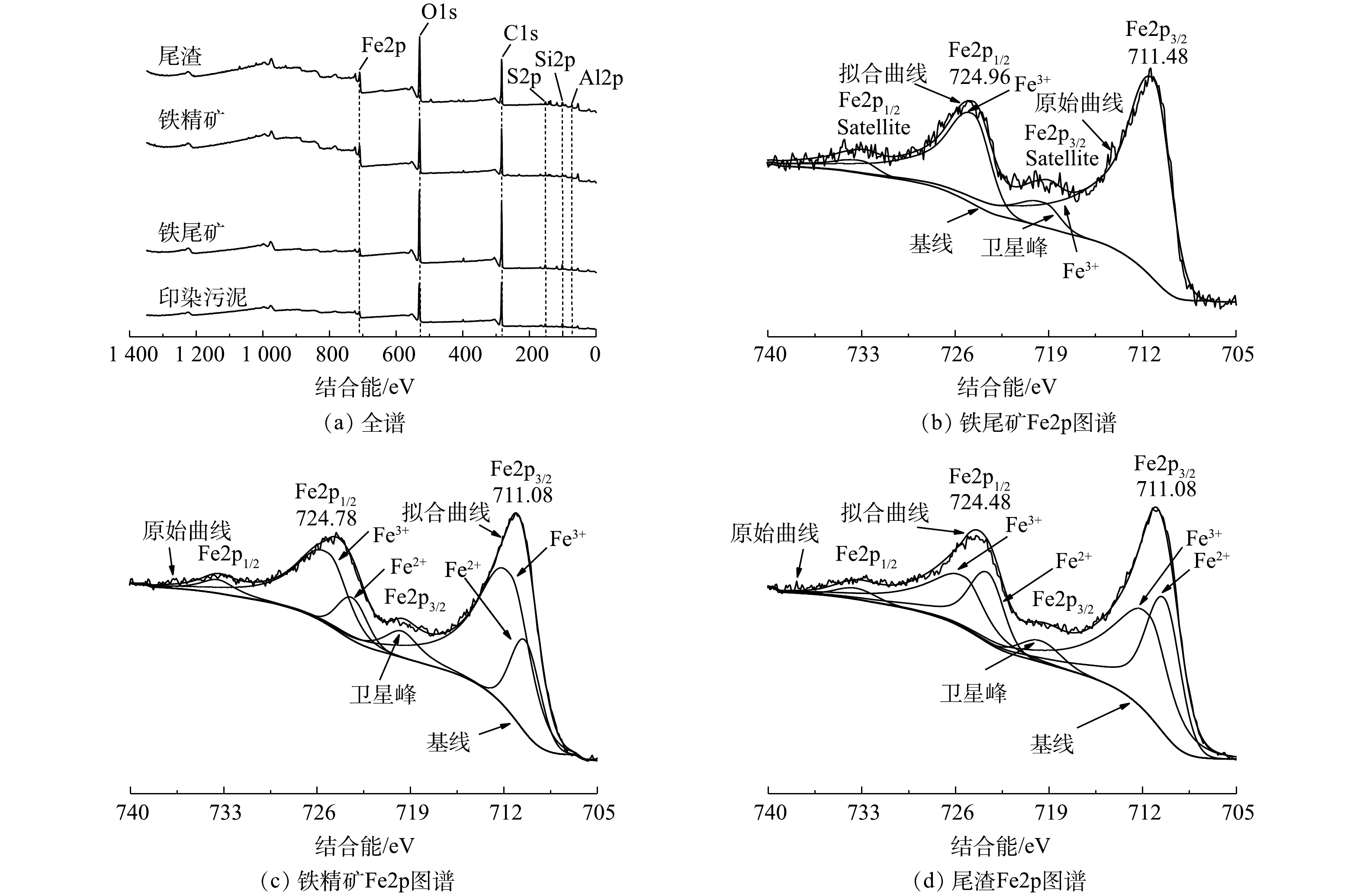
为了进一步证实磁化焙烧过程中铁的物相转变机理,研究了印染污泥、铁尾矿、铁精矿和尾渣XPS图谱,图谱均采用C1s的284.80 eV进行电荷校正。由图10(a)可知,样品中存在Fe、O、Si、Al、S和C元素,这与XRF和EDS结果一致。
由图10(b)可知,铁尾矿Fe2p图谱在711.48 eV和724.96 eV结合能位置具有Fe2p3/2和Fe2p1/2两个峰,两个峰之间结合能差约13.48 eV,分别对应于铁尾矿中赤铁矿(Fe2O3)和针铁矿(FeO(OH))[24]。由图10(c)可知,铁精矿中的Fe2+在Fe2p图谱上出现,表明铁尾矿中部分Fe3+被还原成Fe2+,赤铁矿和针铁矿基本转化为磁铁矿(Fe3O4)[25-26]。值得注意的是,如图10(d)所示,在磁选渣中Fe2+含量(45.6%)较铁精矿中Fe2+含量(29.6%)高,说明部分过还原铁富集在尾渣中无法被磁选。XPS结果进一步证实了在磁化焙烧过程中,铁转化路径为FeO(OH)→Fe2O3→Fe3O4。
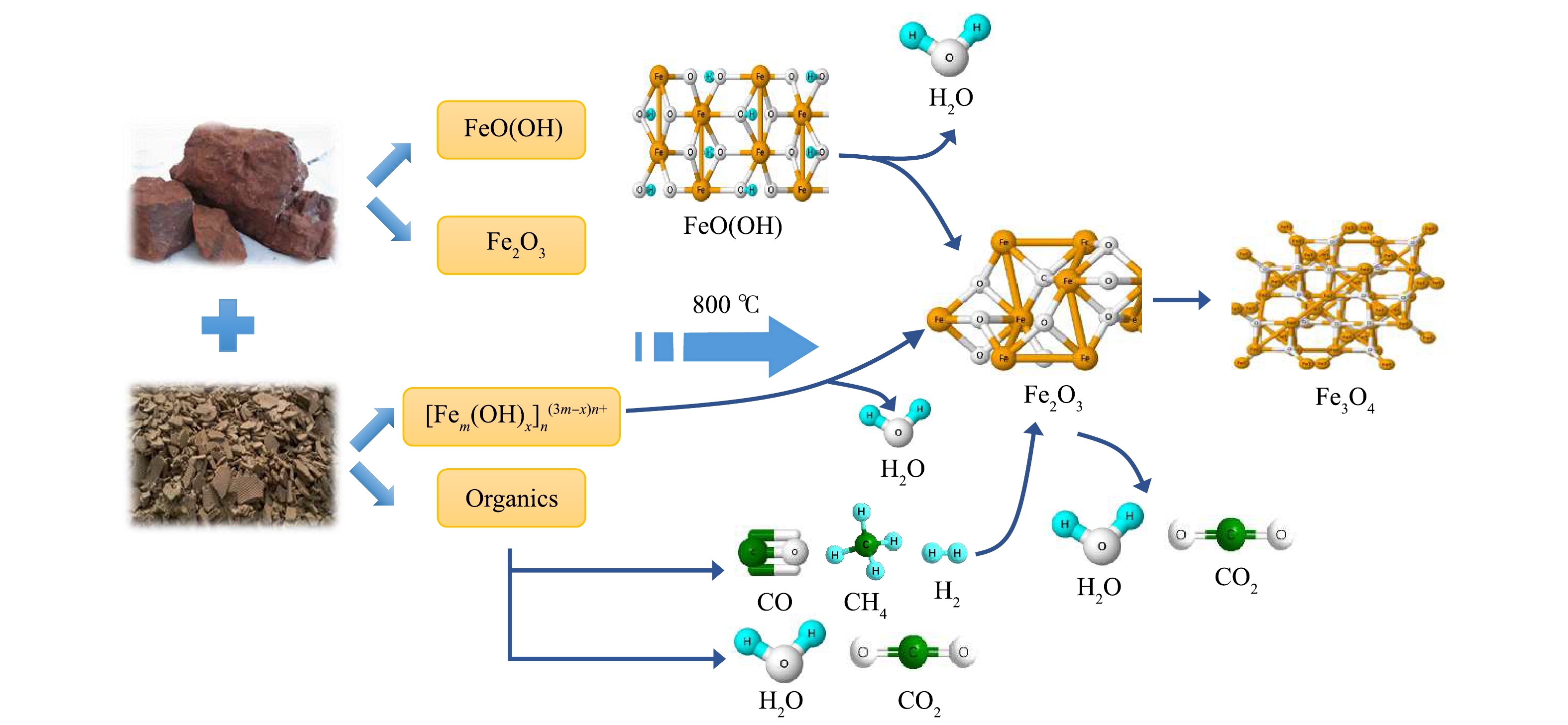
基于XRD、XPS、SEM+MAPPING等表征结果,印染污泥和铁尾矿共磁化焙烧,铁的迁移转化过程为:铁尾矿中针铁矿首先失水,在矿物颗粒表面造成孔道进而转化为赤铁矿 (式(10)) 。此时印染污泥有机组分热解产生的还原性气体通过孔道可以更好地作用于赤铁矿,赤铁矿脱氧转化为具有磁性的Fe3O4 (式(11)~(13)) 。对于印染污泥中含铁物质([Fem(OH)x]n(3m−x)n+),首先[Fem(OH)x]n(3m−x)n+受热脱水生成Fe2O3,经由H2和CO等还原后转化为Fe3O4,铁尾矿及印染污泥中铁的转化路径如图11所示。
-
1) 印染污泥与铁尾矿混合磁化焙烧最佳工艺参数为焙烧温度800 °C、焙烧时间30 min,印染污泥掺烧量15%,对焙烧产物进行120 mT湿法磁选,获得铁品位63.78%,回收率92.58%的铁精矿。
2) 焙烧过程中,铁尾矿中铝与赤铁矿的连生体结合发生了明显解离,提高了铁精矿的铁品位和回收率。
3) 印染污泥和铁尾矿协同磁化焙烧技术,有望开辟以废治废,低碳排放的大宗固体废物资源化利用新途径。
印染污泥与铁尾矿磁化焙烧回收铁资源
Recovery of iron from iron tailings and textile dyeing sludge with co-magnetization roasting
-
摘要: 我国铁尾矿累计堆存量超1×1010 t,主要为难选的赤铁矿、褐铁矿、菱铁矿等,由于脉石矿物组成复杂,重金属等有害杂质含量高,难以直接资源化利用。利用铁尾矿与印染污泥共同磁化焙烧,回收铁尾矿和印染污泥中铁资源,研究了焙烧温度、焙烧时间和印染污泥掺烧量对铁品位和铁回收率的影响及作用机制。最佳焙烧条件为,800 °C、30 min、印染污泥掺烧量15%,对焙烧产物进行120 mT的湿法磁选,得到铁品位63.78%,回收率92.58%的铁精矿。铁尾矿中的针铁矿失水留下孔隙转化为赤铁矿,进而被还原为磁铁矿,其还原路径为FeO(OH)→Fe2O3→Fe3O4。SEM+MAPPING分析结果表明,磁化焙烧后赤铁矿和铝化合物的连生体被破坏,通过磁选可提升铁精矿品位及回收率。本研究可为印染污泥和铁尾矿的协同处理及资源化利用提供参考。Abstract: The accumulated stockpile of iron tailings in China exceeds 1×1010 t, mainly difficult to select hematite, limonite, and rhodochrosite, etc., which are difficult to resource directly due to the complex composition of vein minerals and high content of heavy metals and other harmful impurities. In this study, iron tailings and textile dyeing sludge were co-magnetized and roasted to recover iron resources from iron tailings and textile dyeing sludge, and the effects of roasting temperature, roasting time and the amount of textile dyeing sludge blending on iron grade and iron recovery and the mechanism of action were investigated. The optimum roasting conditions were: 800 °C, 30 min, 15% textile dyeing sludge blending, 120 mT wet magnetic separation of the roasted product, and the iron concentrate with 63.78% Fe grade and 92.58% recovery was obtained. The goethite in the iron tailings lost water leaving pores transformed into hematite, which was then reduced to magnetite with the reduction path of FeO(OH)→Fe2O3→Fe3O4. SEM+MAPPING analysis showed that the congeners of hematite and aluminum compounds were destroyed after magnetization roasting, and the iron concentrate grade and recovery could be enhanced by magnetic separation. This study provides a scientific basis for the synergistic treatment and resource utilization of textile dyeing sludge and iron tailings.
-
Key words:
- magnetization roasting /
- textile dyeing sludge /
- iron tailings
-

-
表 1 样品化学及成分分析
Table 1. Chemical analysis of the sample
% 组分 TFe Fe2O3 SiO2 Al2O3 SO3 CaO ZnO MnO K2O CuO 铁尾矿 40.35 52.04 28.90 14.79 3.00 0.12 0.18 0.10 0.51 0.24 印染污泥 20.04 40.31 7.48 5.52 46.71 1.33 0.97 0.24 0.11 0.05 表 2 印染污泥有机元素分析及工业分析
Table 2. Textile dyeing sludge organic elemental analysis and industrial analysis
% 组分 C H N O S Mad Aad Vad FCad 含量 22.09 4.27 2.61 16.75 11.03 6.72 59.06 30.72 3.50 注:Mad为水分;Aad为灰分;Vad为挥发分;FCad为固定碳。 -
[1] LI Q, DAI T, WANG G, et al. Iron material flow analysis for production, consumption, and trade in China from 2010 to 2015[J]. J Clean Prod, 2018, 172: 1807-1813. doi: 10.1016/j.jclepro.2017.12.006 [2] ZHOU W, LIU X, LYU X, et al. Extraction and separation of copper and iron from copper smelting slag: A review[J]. J Clean Prod, 2022, 368: 133095. doi: 10.1016/j.jclepro.2022.133095 [3] SUN Y, ZHU X, HAN Y, et al. Iron recovery from refractory limonite ore using suspension magnetization roasting: A pilot-scale study[J]. J Clean Prod, 2020, 261: 121221. doi: 10.1016/j.jclepro.2020.121221 [4] LV X, SHEN W, WANG L, et al. A comparative study on the practical utilization of iron tailings as a complete replacement of normal aggregates in dam concrete with different gradation[J]. J Clean Prod, 2019, 211: 704-715. doi: 10.1016/j.jclepro.2018.11.107 [5] LI S, WU J, HUO Y, et al. Profiling multiple heavy metal contamination and bacterial communities surrounding an iron tailing pond in Northwest China[J]. Sci Total Environ, 2021, 752: 141827. doi: 10.1016/j.scitotenv.2020.141827 [6] WANG P, SUN Z, HU Y, et al. Leaching of heavy metals from abandoned mine tailings brought by precipitation and the associated environmental impact[J]. Sci Total Environ, 2019, 695: 133893. doi: 10.1016/j.scitotenv.2019.133893 [7] LI M, PENG B, CHAI L, et al. Recovery of iron from zinc leaching residue by selective reduction roasting with carbon[J]. J Hazard Mater, 2012, 237-238: 323-330. doi: 10.1016/j.jhazmat.2012.08.052 [8] DENG J, NING X-A, SHEN J, et al. Biomass waste as a clean reductant for iron recovery of iron tailings by magnetization roasting[J]. J Environ Manage, 2022, 317: 115435. doi: 10.1016/j.jenvman.2022.115435 [9] YUAN S, ZHOU W, HAN Y, et al. Individual enrichment of manganese and iron from complex refractory ferromanganese ore by suspension magnetization roasting and magnetic separation[J]. Powder Technol, 2020, 373: 689-701. doi: 10.1016/j.powtec.2020.07.005 [10] 中华人民共和国国家统计局生态环境部. 2021中国环境统计年鉴 [M]. 北京: 中国统计出版社, 2021. [11] CHEN X, NING X-A, LAI X, et al. Chlorophenols in textile dyeing sludge: Pollution characteristics and environmental risk control[J]. J Hazard Mater, 2021, 416: 125721. doi: 10.1016/j.jhazmat.2021.125721 [12] NING X-A, LIN M-Q, SHEN L-Z, et al. Levels, composition profiles and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in sludge from ten textile dyeing plants[J]. Environ Res, 2014, 132: 112-118. doi: 10.1016/j.envres.2014.03.041 [13] ZHOU W, CHEN X, WANG Y, et al. Anaerobic co-digestion of textile dyeing sludge: Digestion efficiency and heavy metal stability[J]. Sci Total Environ, 2021, 801: 149722. doi: 10.1016/j.scitotenv.2021.149722 [14] HAO X, CHEN Q, VAN LOOSDRECHT M C M, et al. Sustainable disposal of excess sludge: Incineration without anaerobic digestion[J]. Water Res, 2020, 170: 115298. doi: 10.1016/j.watres.2019.115298 [15] LIU J, HUANG L, ZOU H, et al. Do FeCl3 and FeCl3/CaO conditioners change pyrolysis and incineration performances, emissions, and elemental fates of textile dyeing sludge?[J]. J Hazard Mater, 2021, 413: 125334. doi: 10.1016/j.jhazmat.2021.125334 [16] CHENG W P. Hydrolysis characteristic of polyferric sulfate coagulant and its optimal condition of preparation[J]. Colloids Surf Physicochem Eng Aspects, 2001, 182(1): 57-63. [17] LIU X, WU Y, XU Q, et al. Mechanistic insights into the effect of poly ferric sulfate on anaerobic digestion of waste activated sludge[J]. Water Res, 2021, 189: 116645. doi: 10.1016/j.watres.2020.116645 [18] SONG Y, HU J, LIU J, et al. CO2-assisted co-pyrolysis of textile dyeing sludge and hyperaccumulator biomass: Dynamic and comparative analyses of evolved gases, bio-oils, biochars, and reaction mechanisms[J]. J Hazard Mater, 2020, 400: 123190. doi: 10.1016/j.jhazmat.2020.123190 [19] DING Z, LIU J, CHEN H, et al. Co-pyrolysis performances, synergistic mechanisms, and products of textile dyeing sludge and medical plastic wastes[J]. Sci Total Environ, 2021, 799: 149397. doi: 10.1016/j.scitotenv.2021.149397 [20] YUAN S, WANG X, ZHANG H, et al. Experimental and mechanism research of the effects of alkali on the reduction reaction of hematite during roasting reduction reaction[J]. Adv Powder Technol, 2022, 33(6): 103592. doi: 10.1016/j.apt.2022.103592 [21] YU J, LI Y, LV Y, et al. Recovery of iron from high-iron red mud using suspension magnetization roasting and magnetic separation[J]. Miner Eng, 2022, 178: 107394. doi: 10.1016/j.mineng.2022.107394 [22] ZHANG Y, LI H, YU X. Recovery of iron from cyanide tailings with reduction roasting–water leaching followed by magnetic separation[J]. J Hazard Mater, 2012, 213-214: 167-174. doi: 10.1016/j.jhazmat.2012.01.076 [23] QIU G, NING X, SHEN J, WANG Y, ZHANG D, DENG J. Recovery of iron from iron tailings by suspension magnetization roasting with biomass-derived pyrolytic gas[J]. Waste Manage, 2023, 156: 255-263. doi: 10.1016/j.wasman.2022.11.034 [24] LI Y, ZHANG Q, YUAN S, et al. High-efficiency extraction of iron from early iron tailings via the suspension roasting-magnetic separation[J]. Powder Technol, 2021, 379: 466-477. doi: 10.1016/j.powtec.2020.10.005 [25] SALAMA W, EL AREF M, GAUPP R. Spectroscopic characterization of iron ores formed in different geological environments using FTIR, XPS, Mössbauer spectroscopy and thermoanalyses[J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2015, 136: 1816-1826. doi: 10.1016/j.saa.2014.10.090 [26] OMRAN M, FABRITIUS T, ELMAHDY A M, et al. XPS and FTIR spectroscopic study on microwave treated high phosphorus iron ore[J]. Appl Surf Sci, 2015, 345: 127-140. doi: 10.1016/j.apsusc.2015.03.209 -



 下载:
下载: