-
我国资源禀赋决定了煤炭在一次能源供给中的主导地位。燃煤排放的二氧化硫 (SO2) 和氮氧化物 (NOx) 等烟气污染物导致环境问题频发[1–3]。目前,国内燃煤电站已全面实施大气污染物超低排放,工业烟气治理重点已转向非电行业。我国中小型锅炉消耗了全国约25%的煤,却排放了高达燃煤总排放量60%的大气污染物[4]。因此,治理中小型燃煤锅炉和工业炉窑烟气污染物迫在眉睫。
常见烟气脱硫技术有石灰石-石膏法[5-6]、钠碱法[7]、氨法[8]、镁法[9-10]、有机胺法[11-12]、海水法[13]等,但这些方法在脱除大气污染物的同时,也带来了液、固的二次污染[14]。典型地,运用最为广泛的湿法石灰石–石膏法脱硫工艺年产含水石膏约9.5×107 t,石膏堆存占地20000 km2,废弃硫资源量1.3~1.66×107 t,而同期我国硫磺进口量却高达1.078×107 t,形成了硫资源废弃与进口的巨大反差;其烟气处理后排放的脱硫废水所含溶解盐对环境亦造成影响。中国工程院将“工业废水脱盐与水回用关键技术”列为2035环境工程科技关键、共性与颠覆性技术指数前十项之一[15]。因此,通过高效脱除技术并耦合区域产业链,将污染物转化为资源加以利用,同步实现生态与经济效益是环境治理追求的目标。
我国磷矿资源储量丰富,位居世界第二,开采量位居世界第一,主要集中在中西部地区。2019年,全国磷矿石产量高达9.33×107 t,其中约85%的磷矿用于湿法磷酸生产[16-17]。如果将磷化工生产的原料磷矿石用作SO2吸收剂,既能将锅炉烟气中污染物SO2脱除,又能将SO2转化为硫酸分解磷矿石。该过程不仅节约了磷矿浮选过程中需要添加的硫酸用量,产生一定的经济效益,而且脱硫后的废弃矿浆直接输送至磷矿浮选阶段用于湿法磷酸生产,无废水和固体废弃物产生,亦是一条绿色、经济、低成本的脱硫技术[18-19]。磷矿石对SO2的吸收容量 (以每克磷矿粉吸收的SO2质量计) 为3.8×10–2 g·g−1,是同体积水吸收容量的264倍、NaOH饱和溶液的42倍[20]。利用磷矿浆脱除湿法磷酸中过量的SO3,可有效降低硫酸消耗 [21]。武春锦等[22]通过中试研究发现液相F–含量随温度、pH上升而下降;F– 仅在液相中,不会逸出到气相;液相中Mg2+含量随温度的升高而增大,但随浆液pH升高而下降。通过正交实验得出,影响脱硫率的因素排序为:循环量>气量>磷矿浆pH;磷矿浆脱硫最佳工艺条件为气量3.5×103 m3·h−1 (气速 1.94 m·s−1) ,循环量为33 m3·h−1,矿浆pH为6.5。吕武华[23]以动力波设备为反应器,研究了矿浆pH、气量、矿浆循环量等因素对脱硫率的影响;当动力波入口的SO2质量浓度为2.8×103 mg·m−3时,仅用单层喷头,脱硫率即可达到78%以上。此外,吴琼[24]研究了磷矿浆脱硫除镁的影响因素,通过正交实验得出镁溶出率的最佳工艺条件为:SO2质量浓度为1×103 mg·m−3、浆液质量分数为30%、温度为50 ℃、pH等于7。
目前,一些企业已开展了磷矿浆脱硫的应用,瓮福化工公司通过高镁磷精矿处理硫酸尾气,但由于磷矿浆脱硫反应机制不清楚及反应器选型等问题,脱硫效率仅为70%~80%,效果不甚理想[25-26]。在本团队前期研究工作中已探明了磷矿浆高效脱硫的反应原理[18]、矿浆中丰富的金属离子催化促进脱硫机制[27]、含磷复合矿浆同时脱硫脱硝过程中硫氮相互作用促进SO2和NOx脱除机制[28],并与中化云龙有限公司合作建成的75 t·h−1燃煤锅炉烟气脱硫装置,其脱硫率高达100%,回收的SO2作为下一工段的原料使用,并产生一定的经济效益 [18, 23]。然而,磷矿浆脱硫过程中各组分变化情况及脱硫率下降原因尚不清楚。基于此,本研究对磷矿浆脱硫过程开展分析,探究磷矿中的关键组分碳酸镁钙和氟磷酸钙对脱硫的影响,旨在揭示脱硫过程中脱硫活性组分及脱硫率下降的原因,以期为磷矿浆技术的发展和应用提供参考。
-
磷矿石由云南省昆明市中化云龙有限公司提供,粒径小于48 μm。磷矿石主要由碳酸镁钙 (CaMg(CO3)2,占9.2%) 、氟磷酸钙 (Ca5(PO4)3F,占79.37%) 和二氧化硅 (SiO2,占4.74%) 组成[18, 23]。无水氯化钙 (AR) 由天津市风船化学试剂科技有限公司生产。氟磷酸钙 (Ca5(PO4)3F) 是通过磷酸钙 (AR,天津市科密欧化学试剂有限公司) 和氟化钙 (AR,山东西亚化学股份有限公司) 合成[29]。SO2标准气体 (质量分数3%的SO2+质量分数97%的N2) , O2标准气体 (纯度≥ 99.50%) 和N2标准气体 (纯度≥ 99.99%) 从昆明广瑞达特种气体有限责任公司购买。
-
磷矿浆脱硫实验装置 (图1) 由配气系统,吸收系统和检测系统3个部分组成。SO2、O2和N2气体分别由压缩气瓶提供,通过气瓶减压阀控制后进入质量流量控制器进行计量,然后在缓冲罐中混合获得实验条件下的模拟烟气。为检测反应前后模拟烟气的浓度,在管道中设置了2个阀门来切换烟气流向。模拟烟气通过阀门进入鼓泡反应器与配制的磷矿浆反应,通过磁力搅拌反应器控制反应所需的温度和搅拌速度。经磷矿浆吸收后的烟气在无水氯化钙干燥除去水分后,进入烟气分析仪,在线检测SO2质量浓度。在实验过程中,模拟烟气的总流量设置为500 mL·min−1,其中SO2质量浓度为3.3×103 mg·m−3,氧气体积分数为10%。
-
烟气分析仪 (Ecom–J2KN型,德国rbr有限公司) 用于检测模拟烟气中氧气和SO2含量;质量流量计 (CS200型,北京七星华创电子股份有限公司) 用于控制气体的流量;离子色谱仪 (IC,883型,瑞士万通中国有限公司) 用来分析脱硫过程中脱硫液液相中的阴离子;X射线衍射仪 (XRD,Rigaku D/max–1200型,日本) 用于分析脱硫过程中磷矿的组分;pH计 (DHS–3C型,上海仪电科学仪器股份有限公司) 用来检测吸收液中的pH;恒温磁力搅拌器 (RET型,德国IKA) 用于加热和搅拌磷矿浆吸收剂。
-
SO2脱除率 (η) 计算公式见式 (1) 。
式中: C0代表从混合罐出来的模拟烟气中SO2的质量浓度,mg·m−3;C1代表经磷矿浆吸收后,从反应器排出的烟气中SO2的质量浓度,mg·m−3。
-
1) 半连续脱硫性能。在磷矿浆脱硫过程中,pH对脱硫率有很大影响[18]。由于磷矿浆初始pH呈碱性,为排除磷矿浆pH对SO2吸收的影响,以考察磷矿石脱硫性能,对脱硫过程中的脱硫液每隔3 h进行固液分离,将分离获得的脱硫液固体和去离子水配制成新的吸收剂,再次进行脱硫,其结果如图2所示。在脱硫反应持续3 h后,用去离子水替换脱硫液液体,pH增至6.18,但在3 h内快速下降至3.89,在此期间脱硫率保持在100%。当反应6 h后,使用去离子水再次替换脱硫液液体,脱硫率在反应7 h后开始下降,反应至9 h时,脱硫率下降至89%,pH下降至2.29。在此之后,继续使用去离子水替换脱硫液液体,仅能维持瞬时的高脱硫率,pH也基本维持在4以下。这表明磷矿浆脱硫过程中,反复使用去离子水洗涤磷矿,不能维持磷矿浆持续高效脱硫。磷矿石在脱硫过程中本身的变化是导致脱硫率下降的主要原因。
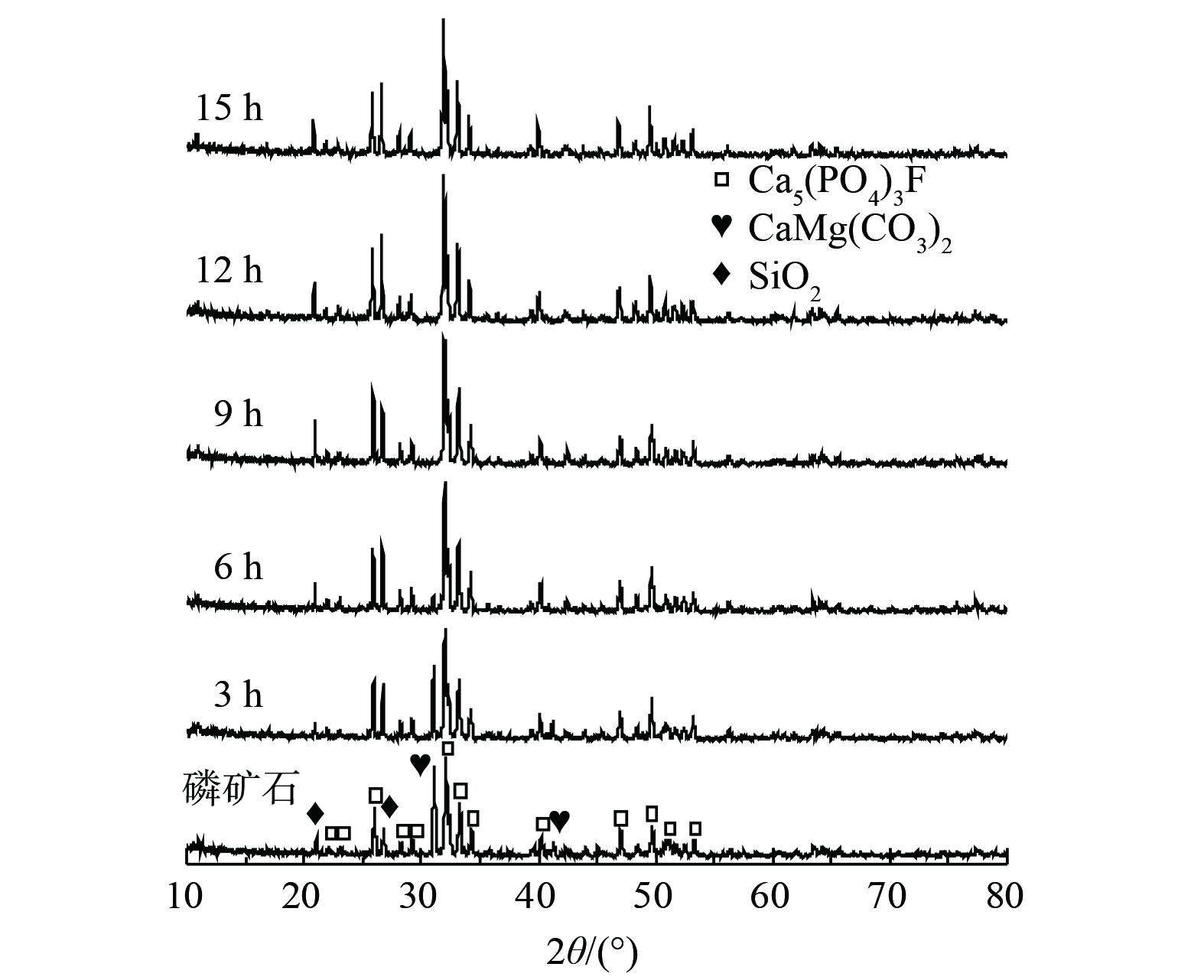
2) 脱硫液固相物质分析。为找出磷矿浆在脱硫过程中脱硫率下降的原因,对不同反应时间下的脱硫液过滤获得的固体物进行XRD表征分析。如图3所示,磷矿石的主要组成成分是CaMg(CO3)2、Ca5(PO4)3F和SiO2 [18, 24]。在脱硫反应过程中,CaMg(CO3)2逐步消失,直至反应至9 h时,CaMg(CO3)2的XRD衍射峰完全消失,Ca5(PO4)3F和SiO2没有明显变化。再加上在反应9 h后,脱硫率迅速下降 (见图2) 。因此,可推断CaMg(CO3)2是磷矿石脱硫的关键活性物质。
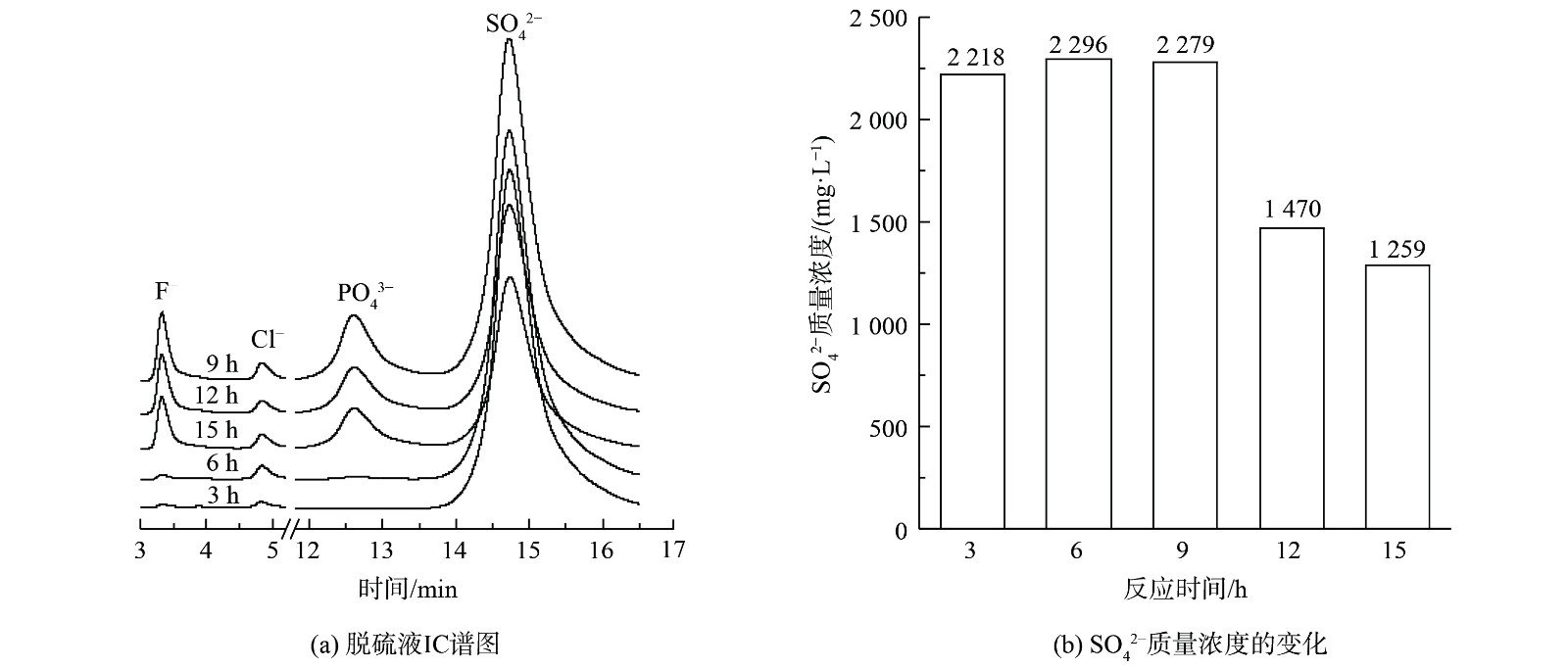
3) 脱硫液液相分析。为进一步确认磷矿浆在脱硫过程中脱硫率下降的原因,对不同反应时间下的脱硫液过滤获得的溶液进行IC分析,结果如图4所示。图4 (a) 表明,在反应前6 h,脱硫液中检测到的F–和PO43–是微量的;在反应进行9 h、12 h和15 h时,脱硫液中检测到大量F–和PO43–。这表明在脱硫反应前6 h,吸收SO2转化形成的H2SO4主要与CaMg(CO3)2反应。当反应至9 h后,CaMg(CO3)2被消耗完,而H2SO4与Ca5(PO4)3F反应生成PO43–和F–。图4 (b) 表明,在反应9 h、12 h和15 h后,脱硫液液相中SO42–质量浓度均低于反应6 h时浆液中的SO42–质量浓度。这表明SO42–不是导致脱硫率下降的原因,CaMg(CO3)2展现出良好的脱硫性能,且优先于Ca5(PO4)3F与硫酸发生反应。
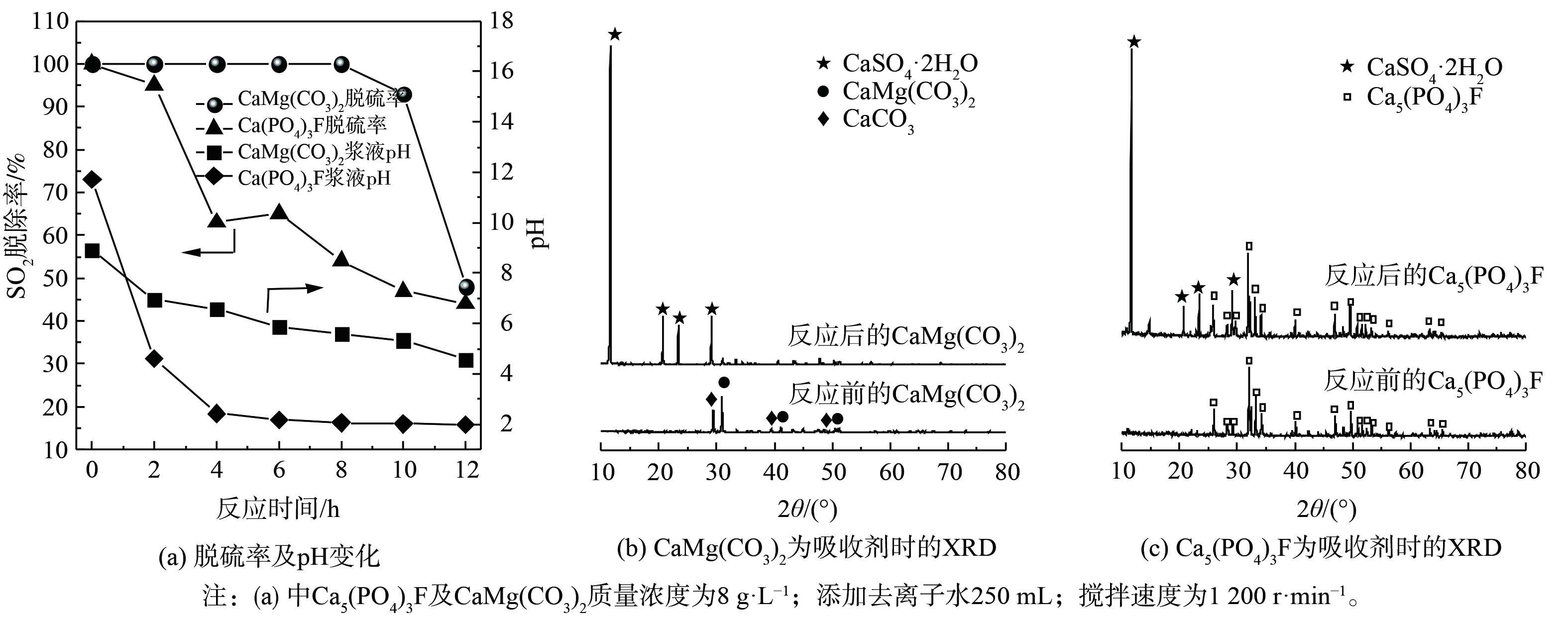
4) 碳酸镁钙和氟磷酸钙脱硫性能分析。为探究磷矿中CaMg(CO3)2和Ca5(PO4)3F这2种组分的脱硫性能,分别用CaMg(CO3)2和Ca5(PO4)3F化学试剂开展脱硫实验,结果见图5。图5 (a) 表明,使用相同质量的CaMg(CO3)2和Ca5(PO4)3F作为吸收剂进行脱硫实验,CaMg(CO3)2吸收剂可维持100%脱硫率的时间长达8 h。CaMg(CO3)2浆液的pH从初始8.93缓慢降至5.58,始终维持在一个较高的pH。直至反应12 h后,脱硫率降至48%,其pH为4.56。然而,Ca5(PO4)3F吸收剂脱硫时,脱硫率在4 h内从100%降至64%,Ca5(PO4)3F浆液pH从11.72迅速降至2.43,随后缓慢下降。高pH有助于SO2吸收脱除,这是CaMg(CO3)2脱硫性能优于Ca5(PO4)3F的关键原因[2]。图5 (b) 和 (c) 表明,对比反应前后的CaMg(CO3)2和Ca5(PO4)3F物质组成,在反应后吸收剂中,均能观察到CaSO4·2H2O衍射峰,表明SO2被转化为CaSO4·2H2O。此外,反应12 h后吸收剂的固体,在CaMg(CO3)2吸收剂的固体中仅检测到CaSO4·2H2O,而在Ca5(PO4)3F吸收剂的固体中仍有大量Ca5(PO4)3F存在。这也进一步表明CaMg(CO3)2具有更好脱硫活性,而Ca5(PO4)3F的脱硫效果较差。
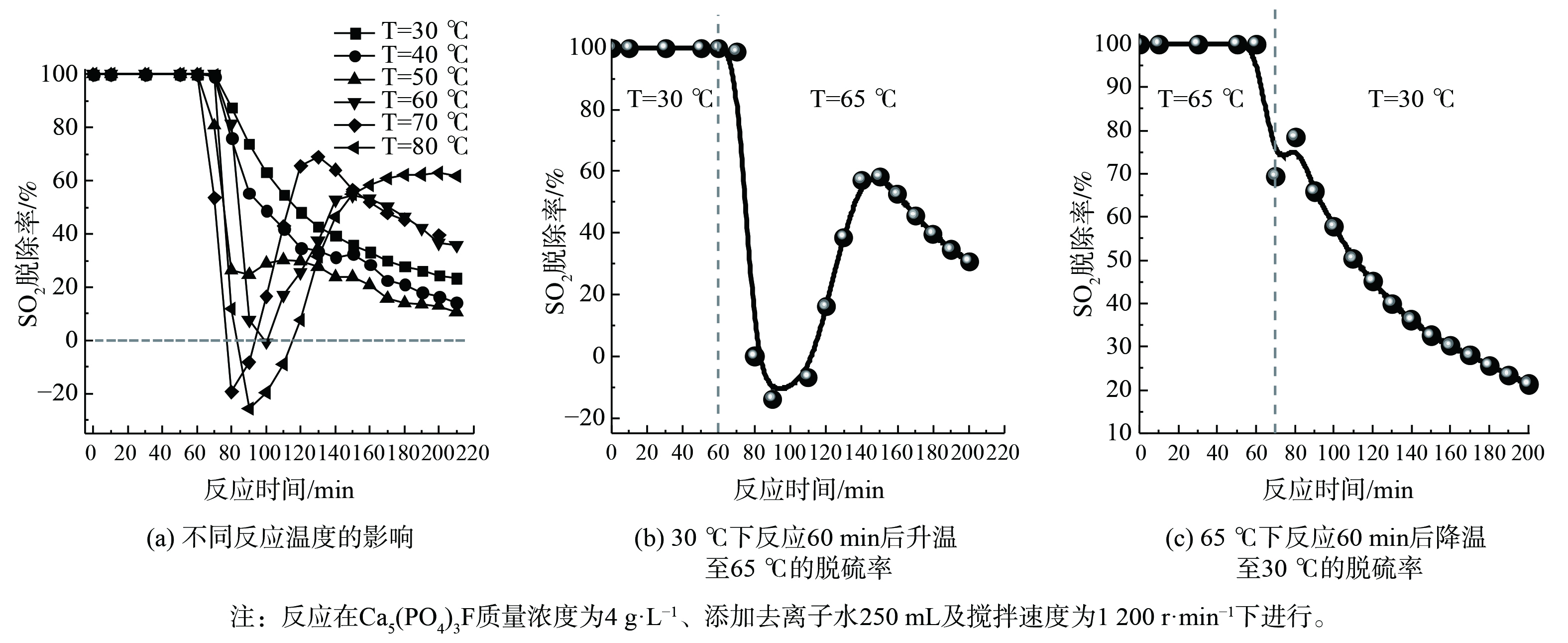
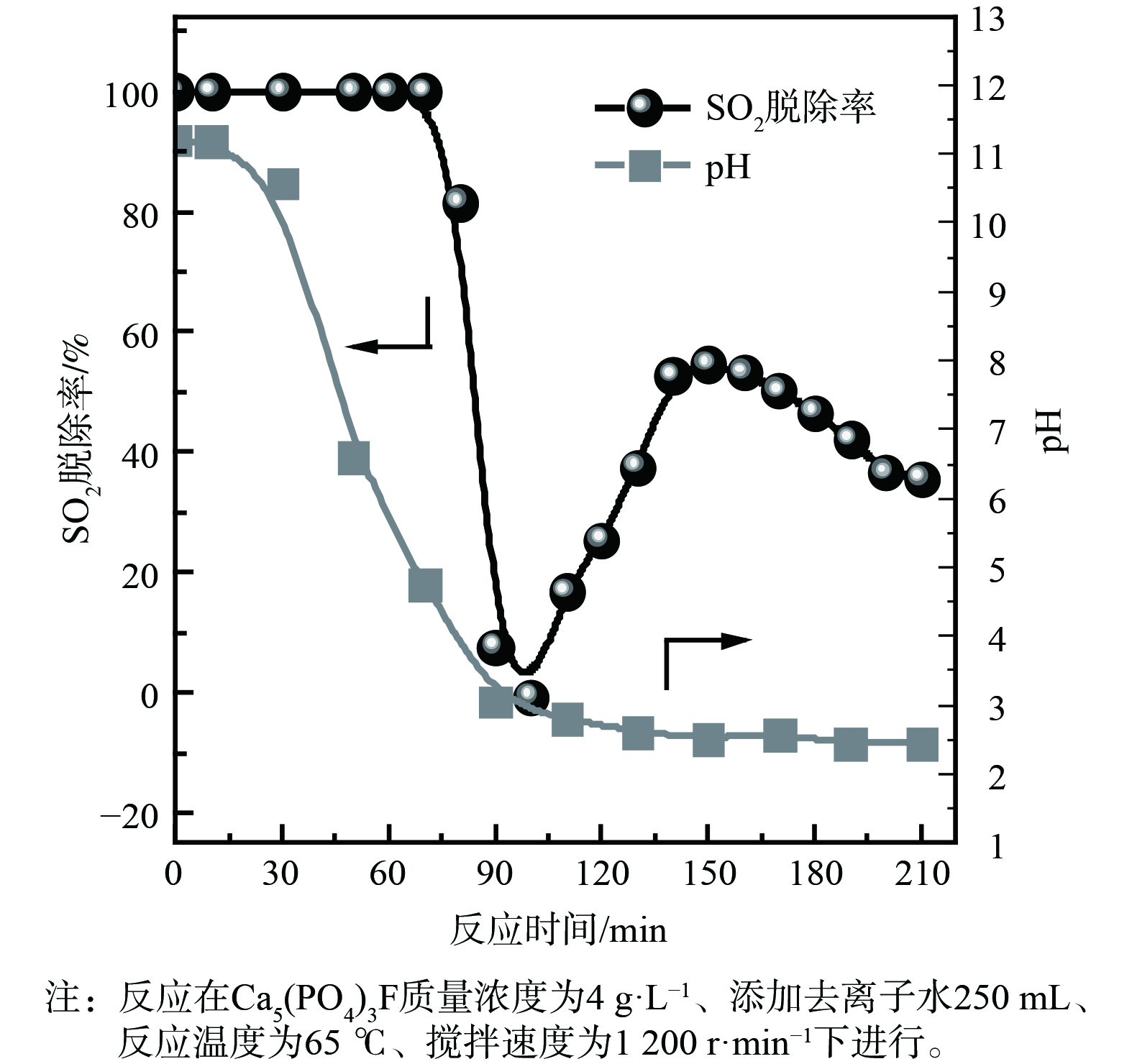
5) Ca5(PO4)3F脱硫性能。Ca5(PO4)3F在磷矿石中的含量高达79%,决定了对SO2的吸收容量。为避免磷矿分解过程中溶出金属离子对脱硫的影响,调查Ca5(PO4)3F脱硫快速失活的原因发现,使用Ca5(PO4)3F和去离子水配制成吸收剂开展脱硫实验。如图6 (a) 所示,Ca5(PO4)3F作为吸收剂脱硫时,脱硫率在反应60 min后快速下降。当反应温度低于55 ℃时,脱硫率呈现逐步下降趋势;当反应温度高于55 ℃时,脱硫率先迅速下降后又逐步回升。当反应温度高于65 ℃、反应时间为70~120 min时,脱硫率为负值,部分SO2被释放出。这表明温度对Ca5(PO4)3F吸收剂吸收SO2影响很大。为进一步确认温度与SO2脱除关系,在同一过程中进行了不同温度下的脱硫实验,结果如图6 (b) 和 (c) 所示。脱硫实验的反应温度首先设置为30 ℃,反应持续60 min,暂停通入模拟SO2烟气,将吸收剂温度升至65 ℃,再通入模拟SO2烟气。从图6 (b) 可知,当反应时间超过60 min后,SO2脱除率呈现先迅速下降后逐步回升的过程。类似地,脱硫实验的反应温度首先设置在65 ℃、反应持续70 min,之后暂停通入模拟SO2烟气,将吸收剂温度降至30 ℃,再通入模拟SO2烟气。如图6 (c) 所示,SO2脱除率呈现逐步下降趋势。这表明Ca5(PO4)3F吸收剂脱硫失活与温度有关,当反应时间为70~120 min时,温度越高,脱硫率下降越快。
当温度为65 ℃时,对Ca5(PO4)3F吸收剂脱硫过程浆液的pH进行在线检测,结果如图7所示。脱硫反应时间为70~130 min时,浆液pH从4.75降至2.61,在此期间脱硫率下降最为显著。当pH降至2.51以下时,脱硫率呈现逐步缓慢下降趋势。这表明pH对Ca5(PO4)3F吸收剂脱硫有显著影响。
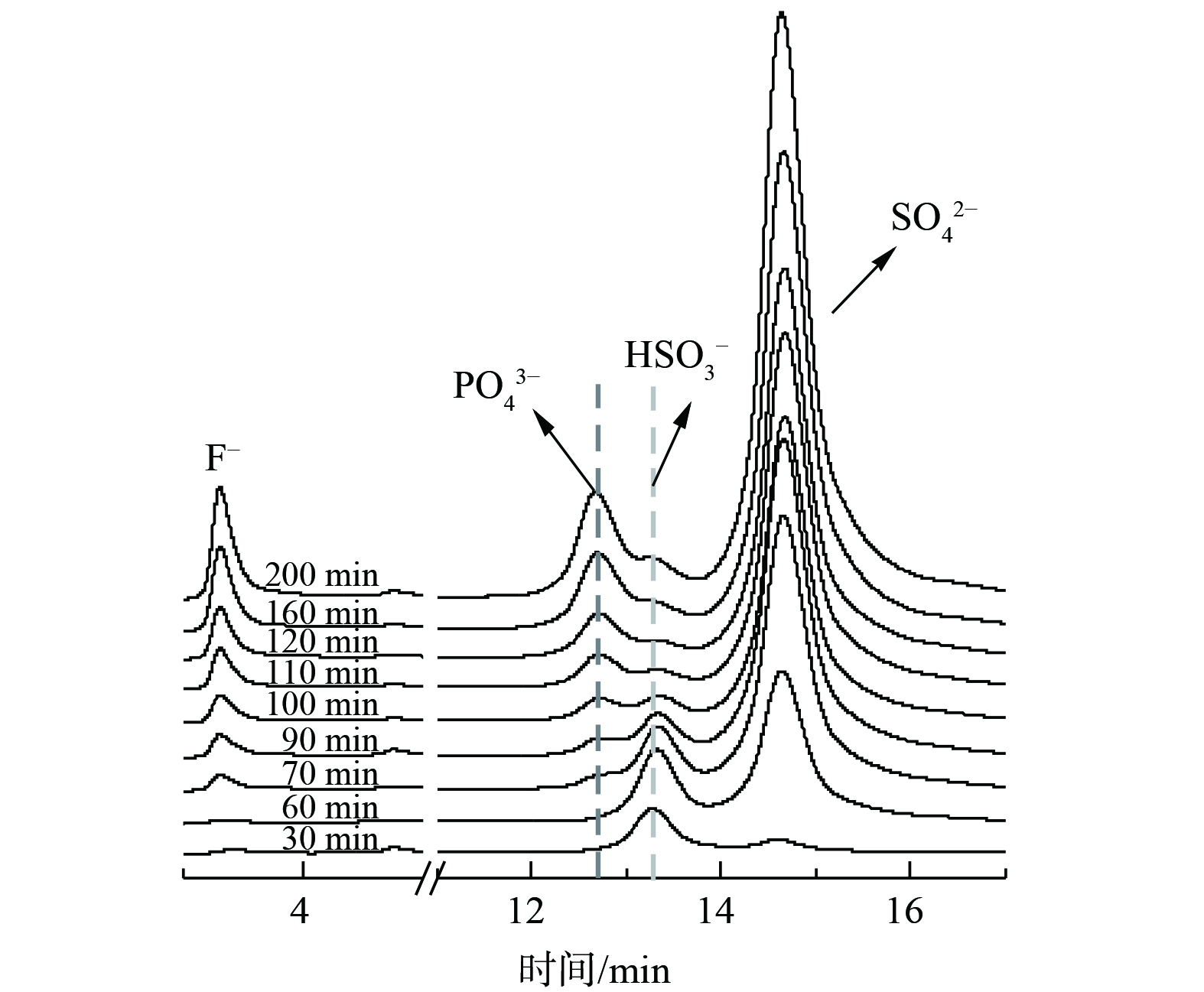
为探究在pH变化较小的情况下,脱硫效率变化较大的原因,对65 ℃条件下氟磷酸钙吸收剂脱硫过程中脱硫液液相进行在线IC分析。结果如图8所示,脱硫液中同时存在PO43–、HSO3–和SO42– [18, 23]。与脱硫反应70 min前脱硫液中的HSO3–浓度相比,在反应70 min后,随着H+和PO43–浓度的增加,脱硫液中HSO3–的浓度逐步降低。这表明H+和PO43–会抑制HSO3–转化为SO42–,并导致SO2溶于水形成的H2SO3发生解离、释放出SO2,从而降低脱硫率。
为确定PO43–对SO2吸收率的影响,在磷矿浆脱硫过程中添加不同含量的KH2PO4开展实验,实验结果如图9所示。图9 (a) 表明,随着H2PO4–浓度从0.004 mol·L−1增至0.024 mol·L−1,SO2的脱除率明显下降,且随着H2PO4–浓度的增加,抑制作用越明显。在脱硫反应时间为0~60 min时,随着反应时间的增加,H2PO4–对SO2脱除抑制作用逐步增加;在反应60 min后,其抑制作用逐步减弱。图9 (b) 表明,当H2PO4–浓度为0.024 mol·L−1时,脱硫过程中浆液pH略高于H2PO4–浓度为0时浆液的pH。添加H2PO4–后,脱硫率下降导致浆液pH升高。这表明Ca5(PO4)3F溶解产生的PO43–对SO2的脱除有抑制作用。
-
在磷矿浆脱硫过程中,SO2先从气相溶于矿浆中通过电离平衡产生H2SO3、HSO3–和SO32– (式 (2)~式 (5) ) ,在酸性条件下主要以HSO3–形式存在[30]。这些S(Ⅳ)迅速被磷矿中溶解出来的铁、锰等金属离子催化氧化为S(Ⅵ) (式 (6)~式 (7) ) [28, 31-33]。S(Ⅵ)先与CaMg(CO3)2溶解产生的Ca2+反应生成CaSO4·2H2O,直至CaMg(CO3)2消耗殆尽,随后Ca5(PO4)3F开始溶解,其产生的Ca2+与S(Ⅵ)反应 (式 (8)~式 (14) ) [30, 32]。然而,Ca5(PO4)3F分解过程中生成的H+和PO43–会抑制SO2的吸收,导致脱硫率逐渐降低,且随着浓度增加,脱硫率下降越显著。当吸收剂温度高于55 ℃且pH为4.75~2.61时,PO43–对脱硫率的抑制作用最明显。PO43–使式 (4) 逆向发生,H2SO3电离释放出SO2,使得降低S(Ⅳ)向S(Ⅵ)转化的效率,从而降低脱硫率。脱硫反应后的固体物仍以Ca5(PO4)3F为主要成分,并伴有一定量的CaSO4·2H2O。
-
1) 在磷矿浆脱硫过程中,脱硫活性组分是CaMg(CO3)2和Ca5(PO4)3F。SO2溶于水形成的亚硫酸被O2氧化转化为硫酸,其先与CaMg(CO3)2反应至其消耗殆尽,随后才与Ca5(PO4)3F反应。CaMg(CO3)2的脱硫活性远高于Ca5(PO4)3F。
2) Ca5(PO4)3F分解过程中产生的H+和PO43–会抑制SO2脱除,液相pH为4.75~2.61时,抑制作用最明显;当液相温度高于55 ℃、反应时间在70~120 min时,脱硫率甚至为负值,会释放出SO2。H+和PO43–浓度增加,会使S(Ⅳ)电离释放SO2,从而降低了S(Ⅳ)向S(Ⅵ)转化的效率,最终降低脱硫率。
3) 磷矿中的碳酸盐是维持其高脱硫性能的关键。碳酸盐含量越高,脱硫容量越大,高脱硫率维持时间越长。因此,可通过磷矿中碳酸盐含量评估磷矿的脱硫性能。
磷矿关键组分对湿法烟气脱硫技术的影响
The effect of primary components of phosphate rock on wet flue gas desulfurization
-
摘要: 磷矿浆湿法烟气脱硫技术将污染物SO2吸收转化为硫酸原位分解磷矿,失活后的磷矿浆用于生产磷酸,节约了湿法磷酸生产中硫酸用量,无废水废液排放,是一种绿色、经济的技术。通过对磷矿浆及其主要组分开展脱硫实验,确立了磷矿中脱硫的关键活性组分及其对脱硫效果的影响。XRD、IC等实验及表征结果表明,SO2吸收后转化形成的硫酸会优先与磷矿中的活性组分碳酸镁钙反应,待其消耗殆尽再与氟磷酸钙反应。与氟磷酸钙相比,碳酸镁钙呈现出优越的脱硫活性。氟磷酸钙分解过程中产生的H+和PO43–会抑制S(Ⅳ)转化为S(Ⅵ)的速率,导致脱硫率下降。PO43–抑制SO2脱除与温度、pH均有关,当温度高于55 ℃、且pH为4.75~2.61时,其抑制作用最明显。本研究可为磷矿浆湿法烟气脱硫技术的优化提供参考。Abstract: Wet flue gas desulfurization with phosphate rock slurry is a green and economic technology. The pollutant SO2 is absorbed and converted into sulfuric acid to decompose phosphate rock in situ, and then the inactivated phosphate rock slurry is directly transported to the flotation section for producing phosphoric acid, which effectively saves the amount of sulfuric acid. In addition, there is no waste water and liquid waste discharged in the whole process. Herein, the key active components for desulfurization in phosphate rock and their influence on desulfurization effect were established, through the desulfurization experiment with phosphate rock and its main components.The XRD, IC and other experiments and characterization results indicated that CaMg(CO3)2 preferentially reacted with sulfuric acid formed by SO2 conversion. When CaMg(CO3)2 was exhausted, Ca5(PO4)3F began to react with sulfuric acid. Significantly, compared with Ca5(PO4)3F, the CaMg(CO3)2 showed superior desulfurization activity. The H+ and PO43–, produced from the decomposition of Ca5(PO4)3F, would inhibit the conversion of S(Ⅳ) towards S(Ⅵ), which was the key reason for the decrease of desulfurization efficiency. The inhibition effect of PO43– on SO2 removal was related to temperature and pH value. It was most significant when the temperature was higher than 55 ℃ and the pH value was between 4.75 and 2.61. Our research can provide a reference for the optimization of wet flue gas desulfurization technology for phosphate slurry.
-

-
-
[1] 汤常金, 孙敬方, 董林. 超低温(< 150 ℃)SCR脱硝技术研究进展[J]. 化工学报, 2020, 71(11): 4873-4884. [2] NIE Y X, DAI J F, HOU Y D, et al. An efficient and environmentally friendly process for the reduction of SO2 by using waste phosphate mine tailings as adsorbent[J]. Journal of Hazardous Materials, 2020, 388: 121748. doi: 10.1016/j.jhazmat.2019.121748 [3] LI S, YANG J Q, WANG C, et al. Removal of NOx from flue gas using yellow phosphorus and phosphate slurry as adsorbent[J]. Energy Fuels, 2018, 32: 5279-5288. doi: 10.1021/acs.energyfuels.7b03964 [4] 田恬, 程茜, 赵雪, 等. 2019年脱硫脱硝行业发展评述及展望[J]. 中国环保产业, 2020, 26(2): 23-25. doi: 10.3969/j.issn.1006-5377.2020.02.007 [5] LANCIA A, MUSMARRA D, PRISCIANDARO M, et al. Catalytic oxidation of calcium bisulfite in the wet limestone–gypsum flue gas desulfurization process[J]. Chemical Engineering Science, 1999, 54(15): 3019-3026. [6] LANCIA A, MUSMARRA D. Calcium bisulfite oxidation rate in the wet limestone gypsum flue gas desulfurization process[J]. Environmental Science and Technology, 1999, 33(11): 1931-1935. doi: 10.1021/es9805425 [7] 刘敦禹, TERRY W, ROHAN S. 富氧燃烧烟气冷凝塔钠碱法脱硫过程SO2和CO2共吸收建模与实验研究[J]. 化工学报, 2018, 69(9): 4019-4029. [8] GAO X, DING H L, DU Z, et al. Gas–liquid absorption reaction between (NH4)2SO3 solution and SO2 for ammonia–based wet flue gas desulfurization[J]. Applied Energy, 2010, 87: 2647-2651. doi: 10.1016/j.apenergy.2010.03.023 [9] VALLE R, NIUBO M, FORMOSA J, et al. Synergistic effect of the parameters affecting wet flue gas desulfurization using magnesium oxides by–products[J]. Chemical Engineering Journal, 2015, 262: 268-277. doi: 10.1016/j.cej.2014.09.085 [10] YAN L Y, LU X F, WANG Q H, et al. Recovery of SO2 and MgO from by–products of MgO wet flue gas desulfurization[J]. Environmental Engineering Science, 2014, 31(11): 621-630. doi: 10.1089/ees.2014.0004 [11] HONG Y H, KIM H, KIM Y J, et al. Nitrile–functionalized tertiary amines as highly efficient and reversible SO2 absorbents[J]. Journal of Hazardous Materials, 2014, 264: 136-143. doi: 10.1016/j.jhazmat.2013.11.026 [12] TAILOR R, ABBOUD M, SAVARJ A. Supported polytertiary amines: highly efficient and selective SO2 adsorbents[J]. Environmental Science & Technology, 2014, 48: 2025-2034. [13] DARAKE S, HATAMIPOUR M S, RAHIMI A, et al. SO2 removal by seawater in a spray tower: experimental study and mathematical modeling[J]. Chemical Engineering Research and Design, 2016, 109: 180-189. doi: 10.1016/j.cherd.2015.11.027 [14] 武春锦, 吕武华, 梅毅, 等. 湿法烟气脱硫技术及运行经济性分析[J]. 化工进展, 2015, 34(12): 4368-4374. doi: 10.16085/j.issn.1000-6613.2015.12.039 [15] 但智钢, 史菲菲, 王志增, 等. 中国环境工程科技2035技术预见研究[J]. 中国工程科学, 2017, 19(1): 80-86. [16] 中华人民共和国自然资源部. 中国矿产资源报告[M]. 北京: 地质出版社, 2020. [17] 吴发富, 王建雄, 刘江涛, 等. 磷矿的分布、特征与开发现状[J]. 中国地质, 2021, 48(1): 82-101. doi: 10.12029/gc20210106 [18] NIE Y X, LI S, WU C J, et al. Efficient removal of SO2 from flue gas with phosphate rock slurry and investigation of reaction mechanism[J]. Industrial & Engineering Chemistry Research, 2018, 57: 15138-15146. [19] 杨加强, 梅毅, 王驰, 等. 湿法烟气脱硝技术现状及发展[J]. 化工进展, 2017, 36(2): 695-704. doi: 10.16085/j.issn.1000-6613.2017.02.041 [20] 贾丽娟, 张冬冬, 殷在飞, 等. 磷矿浆脱硫新技术及工业应用[J]. 磷肥与复肥, 2016, 31(3): 39-41. doi: 10.3969/j.issn.1007-6220.2016.03.015 [21] 马克猛, 杨林台. 用磷矿浆脱除湿法磷酸中过量SO3的研究及其应用[J]. 磷肥与复肥, 2012, 27(1): 23-25. doi: 10.3969/j.issn.1007-6220.2012.01.007 [22] 武春锦. 磷矿浆脱除燃煤锅炉烟气中SO2的研究[D]. 昆明: 昆明理工大学, 2015. [23] 吕武华. 动力波洗涤器用于磷矿浆脱除尾气二氧化硫的研究[D]. 昆明: 昆明理工大学, 2016. [24] 吴琼. 磷矿浆脱硫与磷矿脱镁协同机理研究[D]. 昆明: 昆明理工大学, 2018. [25] 李红林, 蒋世国. 磷精矿脱硫工艺在硫酸尾气处理中的应用[J]. 磷肥与复肥, 2016, 31(9): 7-10. doi: 10.3969/j.issn.1007-6220.2016.09.004 [26] 李红林, 刘海, 赵建勇. 磷矿浆脱硫技术的开发及工业应用[J]. 硫酸工业, 2017, 4: 39-42. doi: 10.3969/j.issn.1002-1507.2017.04.013 [27] NIE Y X, LI S, DAI J F, et al. Catalytic effect of Mn2+, Fe3+ and Mg2+ ions on desulfurization using phosphate rock slurry as absorbent[J]. Chemical Engineering Journal, 2020, 390: 124568. doi: 10.1016/j.cej.2020.124568 [28] NIE Y X, WANG X J, DAI J F, et al. Mutual promotion effect of SO2 and NOx during yellow phosphorus and phosphate rock slurry adsorption process[J]. AIChE Journal, 2021, 67(8): e17236. [29] FABIAN R, KOTSIS I, ZIMANY P, et al. Preparation and chemical characterization of high purity fluorapatite[J]. Talanta, 1998, 46: 1273-1277. doi: 10.1016/S0039-9140(97)00391-3 [30] LIU S Y, XIAO W D. Modeling and simulation of a bubbling SO2 absorber with granular limestone slurry and an organic acid additive[J]. Chemical Engineering & Technology, 2006, 29(10): 1167-1173. [31] KARATZA D, PRISCIANDRAO M, LANCIA A, et al. Calcium bisulfite oxidation in the flue gas desulfurization process catalyzed by iron and manganese ions[J]. Industrial & Engineering Chemistry Research, 2004, 43: 4876-4882. [32] MA X X, KANEKO T, TASHIMO T. Use of limestone for SO2 removal from flue gas in the semidry FGD process with a powder–particle spouted bed[J]. Chemical Engineering Science, 2000, 55: 4643-4652. doi: 10.1016/S0009-2509(00)00090-7 [33] LANCIA A, MUSMARRA D, PEPE F. Model of oxygen absorption into calcium sulfite solutions[J]. Chemical Engineering Journal, 1997, 66: 123-129. doi: 10.1016/S1385-8947(96)03168-3 -




 下载:
下载: