-
天然有机物(natural organic matter,NOM)广泛存在于地表水、水库水等饮用水源中。在饮用水氯消毒处理过程中,氯会和NOM发生反应,生成一系列具有三致效应的卤代消毒副产物。因此,需要在消毒单元前将水体中的NOM去除[1-2]。常规去除NOM的技术主要包括混凝法、化学氧化法、吸附法等,但这些方法在实际应用中均存在一定的问题[3-4]。近些年来,电絮凝(electrocoagulation,EC)作为一种高效去除NOM的技术得到了广泛的关注,其具有操作简单、无需外部投加试剂、去除效率高、产泥量少等优势[5-6]。但在EC水处理过程中,电极钝化问题严重影响了该技术在实际工程中的应用[7]。
电极钝化是指在EC反应过程中,阳极板表面形成的较薄的金属氧化物组成的钝化层。其会影响金属阳极的溶解,从而降低金属离子以及其水解络合物的产量,致使了对NOM较差的处理效果[8]。此外,钝化层导电性较差,增加了EC技术的电能消耗与运行成本[9-10]。
金属阳极在发生溶解的同时还伴随着副反应的发生,形成金属氧化物层(钝化层)[11]。此外,沉积在极板表面的絮体也可能转化为钝化层[10, 12-13]。现有抑制极板钝化的技术包括添加侵蚀离子(氯离子)、采用交流脉冲电流和超声等,但分别存在影响出水水质、增加电源负担、能耗过高等问题[14-16]。因此,通过直接调节EC的运行参数来延缓金属氧化物的形成以及控制电极表面絮体浓度是一种更高效、低耗且绿色的抑制阳极钝化的方法。
本研究以腐殖酸(humic acid, HA)为污染物模拟含天然有机物的地表水,考察了极板表面粗糙度、溶液初始pH、初始HA浓度对阳极板钝化的影响,并通过对EC后溶液残余Al3+浓度及阳极板表面形貌的分析,明确了抑制阳极板钝化的内在机制。本研究可为EC在实际工程应用中抑制阳极钝化提供参考。
-
模拟实验用水:使用分析天平准确称取 1 g HA,溶解于200 mL 0.1 mol·L−1 NaOH溶液中,并将此溶液放置于磁力搅拌器上搅拌 12 h,然后用 1 L超纯水进行定容,静置 12 h后用 0.45 μm微滤膜进行抽滤,抽滤得到的溶液作为HA储备液放置在棕色瓶中并冷藏保存,待使用时进行稀释。同时模拟实验用水中还要加入200 mg·L−1 Na2SO4溶液作为电解质,另加入0.5 mmol·L−1的 NaHCO3溶液模拟自然水体的缓冲能力,采用 0.1 mol·L−1 NaOH 溶液和 0.1 mol·L−1 H2SO4 溶液调节溶液的 pH。实验所用药品均为分析纯。
极板及活化处理:本实验阳极、阴极选取的极板材料均为纯度 99.9% 的高纯度铝,购置的铝阳极材料可能会在运输和保存的过程中被空气氧化产生一层氧化膜,因此在使用前需要经过一定的前处理来保持极板的活性。前处理的过程如下:先用一定目数的SiC砂纸将极板待反应区域均匀打磨至光滑,然后将打磨后的极板放置到无水乙醇中,用超声波清洗机超声10 min以清除极板表面的残余 SiC 颗粒,最后擦干放入密封袋中待用[17]。
-
本研究中EC系统主要由稳压直流电源、反应室、纯铝电极(阳极、阴极)、磁力搅拌器组成,装置图如图1 所示。
-
EC实验:电源采用直流稳压电源,阴阳极板均采用铝电极,EC反应在50 mL烧杯中进行。直流稳压电源采用恒电流模式,实时记录电压的变化,2块平行电极板垂直放入反应室中,极板间距为 1 cm,极板的反应面积恒定为2 cm2(1 cm×2 cm)。为保持极板反应面积一致,用聚酰亚胺绝缘胶带遮盖住极板的非反应部分,电流密度设定为 20 A·m−2,磁力搅拌器转速恒定为 300 r·min−1,每组EC实验反应时间为25 min,每隔 5 min取1 mL的水样,水样经膜(0.45 μm)过滤后分析测试。需要说明的是,本实验探究的是不同操作参数对于铝阳极钝化初期的影响,与长时间的电絮凝钝化相比,主要区别在于钝化初期极板表面没有生成肉眼可见的钝化成分,但在25 min的反应时间内已出现包括电压升高、阻抗增大等明显的钝化现象。MANSOURI [17]等认为,电絮凝所生成钝化层为双层结构,内层为致密的金属氧化层,外层是疏松的多孔层,因此可推测,在电絮凝阳极钝化初期生成的是内部致密钝化层,且致密层影响着阳极钝化后续的宏观发展,这对于钝化初期的研究具有十分重要的意义。
-
用总有机碳分析仪 (TOC-VCPH, SHIMADZU, Japan) 测定HA浓度,采用pH测定仪(720,Thermo Orion,USA)测试溶液pH,采用紫外/可见分光光度计 (U-3010, Hitachi High-Technologes Co, Japan)测定水样的UV254参数,利用表面轮廓仪(Contour Elite I, Bruker, USA)定量极板表面算数平均粗糙度Ra,采用电感耦合等离子-原子发射光谱 (OPTIMA-2000, Perkin Elmer, USA)测定不同初始HA质量浓度下溶液中残余Al3+的量,采用场发射扫描电镜(SEM, JSM7401F, JEDL, Japan)分析反应后电极表面的形貌。关于阳极钝化程度的测定采用两种手段,首先观测恒电流条件下电压的变化,溶液电导率一致时钝化层的出现使得电压增大,电压可通过直流电源(DAHUA DH1766, China)直接记录,其次用电化学交流阻抗量化钝化程度,电化学阻抗谱测试使用电化学工作站(CHI 760E, Chenhua, China),在标准三电极体系下进行,工作电极由电絮凝25 min后的铝阳极制成,铂片电极用作对电极,Ag/AgCl电极作为参比电极。用聚酰亚胺绝缘胶带覆盖工作电极表面,保证测量面积恒定为 1 cm2,所有电化学交流阻抗实验均在 pH为11的30 g·L−1 硫酸钠溶液中进行,电压变化幅度为10 mV,测量频率为10−1~104 Hz,使用ZView软件对实验数据进行拟合。
-
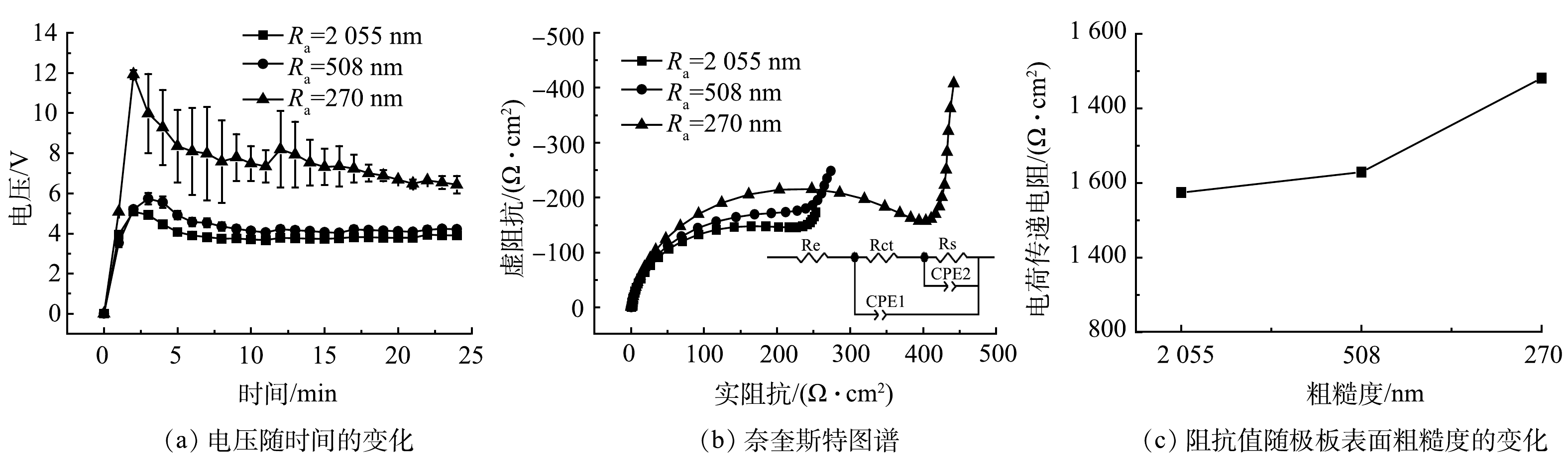
为了探究电极表面Ra对阳极板的钝化的影响,用500、2 000、3 000 目的砂纸打磨极板表面,得到Ra分别为2 055、508、270 nm的铝电极。在初始pH为7, HA质量浓度为5 mg·L−1的条件下,在不同粗糙度的阳极表面的电压变化情况如图2 (a)所示。Ra由2 055 nm 降到 270 nm时,25 min 后EC的电压从(3.9±0.1) V左右增长到(6.4 ± 0.4) V左右。随着极板表面粗糙度的降低,操作电压会出现明显的上升。这说明极板的高粗糙度有助于抑制钝化层形成。对比不同粗糙度条件下阳极板的电化学交流阻抗谱结果如图2(b)和图2(c)所示,利用Zview软件对阻抗谱图进行分析,本研究采用的等效电路见图2(b),其中Rct代表由钝化层导致的电荷传递电阻,其大小可以定量表示极板的钝化程度[18]。当Ra由2 055 nm降至 270 nm 时,对应极板的Rct由1 174.0 Ω·cm2增至1 481.2 Ω·cm2,说明随着极板表面粗糙度的降低,Rct值会随之增大,这与图2 (a)的结果相吻合。
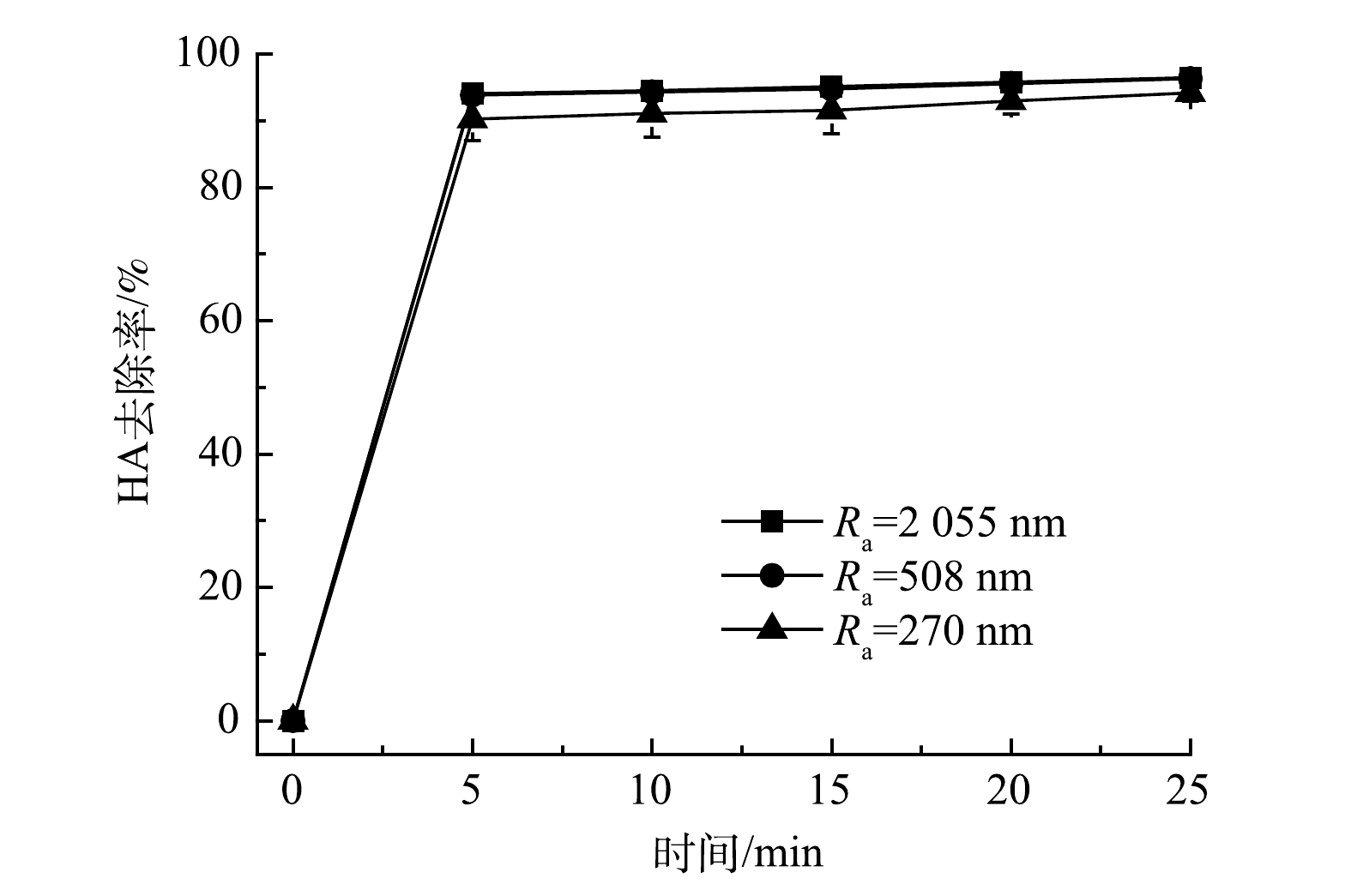
不同粗糙度下HA的去除率变化如图3所示,随着Ra从2 055 nm 降到 270 nm,5 min时,HA的去除率从(94.0±0.5)%降到(90.2±3.2)%。25 min时,HA的去除率从(96.4±0.5)%降到(94.1±1.2)%,表明随着钝化程度的提高,EC效率下降,导致HA的去除率下降。电压和电化学交流阻抗的结论说明在阳极板的表面越粗糙,越有利于抑制钝化层的生成。究其原因,粗糙度高的阳极板微观上来看具有更大的表面积,相同电流条件下其真实电流密度更小,一定范围内,电流密度大小与钝化层厚度呈正相关[19],因此粗糙度高的阳极板钝化程度低。另一方面,这可能是因为粗糙的极板表面空间结构曲折,不利于钝化层的均匀生长,从而遏制了钝化的发生[20]。同时HA的去除率也会随着粗糙度的降低而降低,极板表面钝化程度和HA去除率均与极板表面粗糙度存在一定的相关性。可以推测,极板表面粗糙度可能会影响极板表面的钝化程度进而影响HA的去除率,具体表现为极板粗糙度越高,钝化程度越低,对HA的去除率越高。
-
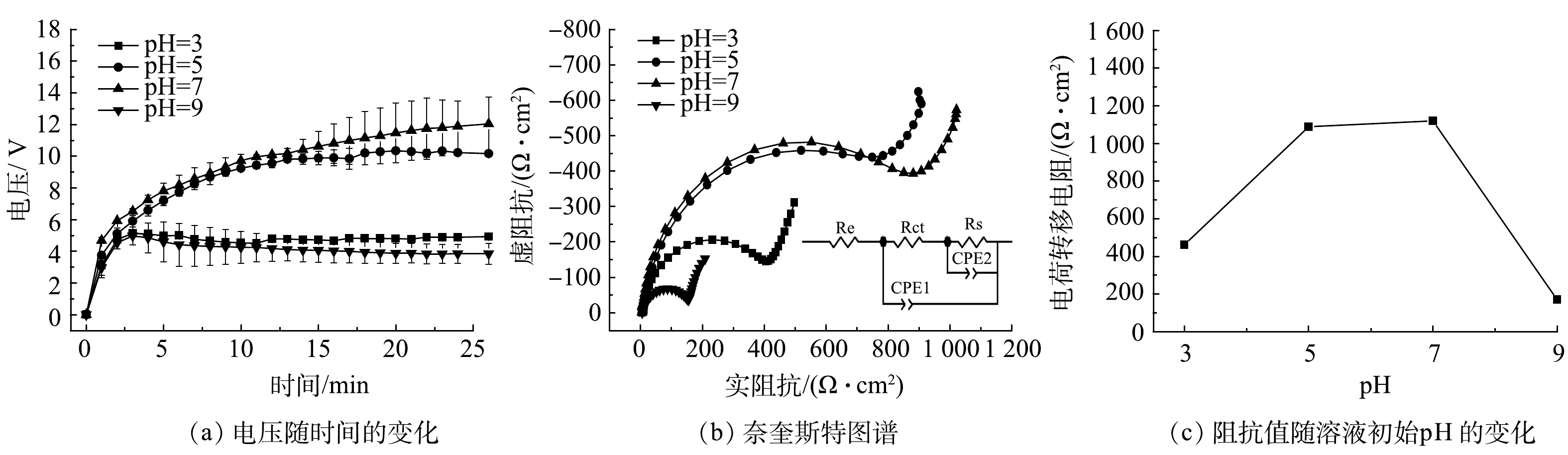
在Ra为508 nm,初始HA质量浓度为5 mg·L−1的条件下,探究了初始pH对阳极板钝化的影响。如图4(a) 所示,当pH从3 提高到 7 时, 25 min 后EC的电压从(4.9±0.1) V左右增长到(12.0±1.7) V左右;当pH由7 提高到 9 时,电压又从(12.0±1.7) V左右降低到(3.9±0.2) V左右。随着pH的增大,操作电压都会出现明显的上升,但当pH上升到7以上时电压又会随着pH增大而下降。
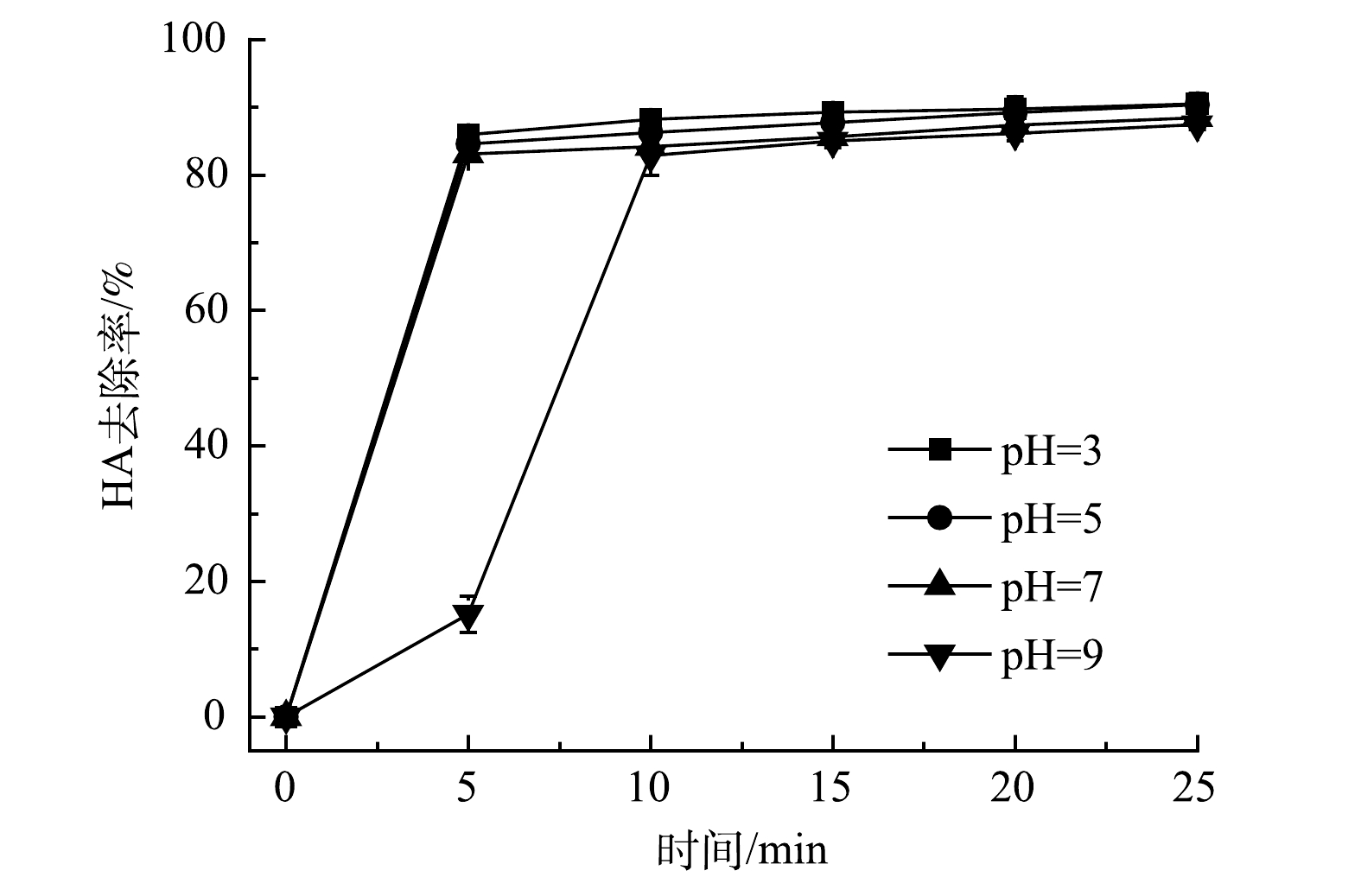
对比不同pH条件下阳极板的电化学交流阻抗,探究了不同pH条件下阳极板的钝化情况。如图4(b)和图4(c)所示,当pH由3 增加到 7 时,阳极板的Rct由461.0 Ω·cm2升高到1 120.2 Ω·cm2;当pH由7 增加到 9 时,阳极板的Rct又从1 120.2 Ω·cm2降低到169.5 Ω·cm2。随着pH的增大,Rct会出现明显的上升,但当pH增加到7以上时Rct又会随着pH增大而下降。如图5所示,当pH由3 升至 9时,EC处理5 min后的HA去除率由(85.9±0.5)%降到(15.2±2.6)%;在反应25 min时,HA的去除率由(90.5±0.4)%降到(87.4±0.7)%。这说明随着溶液初始pH的升高,HA的去除率也会随之降低。
电压-时间曲线和电化学交流阻抗变化的结果表明,在pH为7以下,随着pH增大极板表面生成更厚、电阻更大的钝化层,当溶液呈现碱性时钝化程度又会减弱。出现这种现象可能是因为在不同的pH条件下体系中生成铝的产物不同。当溶液呈酸性时,铝阳极主要发生溶解反应生成Al3+,而在偏中性时则更有利于Al2O3的生成,碱性时阳极主要生成偏铝酸盐 (AlO2−),阳极钝化层的主要成分一般为对应金属氧化物,所以中性条件下阳极钝化更严重[20-21]。HA的去除效果随着pH的升高逐渐下降,这是由于HA中的羧基在酸性条件下发生电离,其更容易和低pH条件下带正电Al3+和Al(OH)2+发生吸附电中和,从而去除率较高,随着pH的升高,这种效应减弱,所以HA去除率也会随之降低[22]。溶液初始pH对HA去除率和阳极板面钝化程度的影响结果并不一致。
-
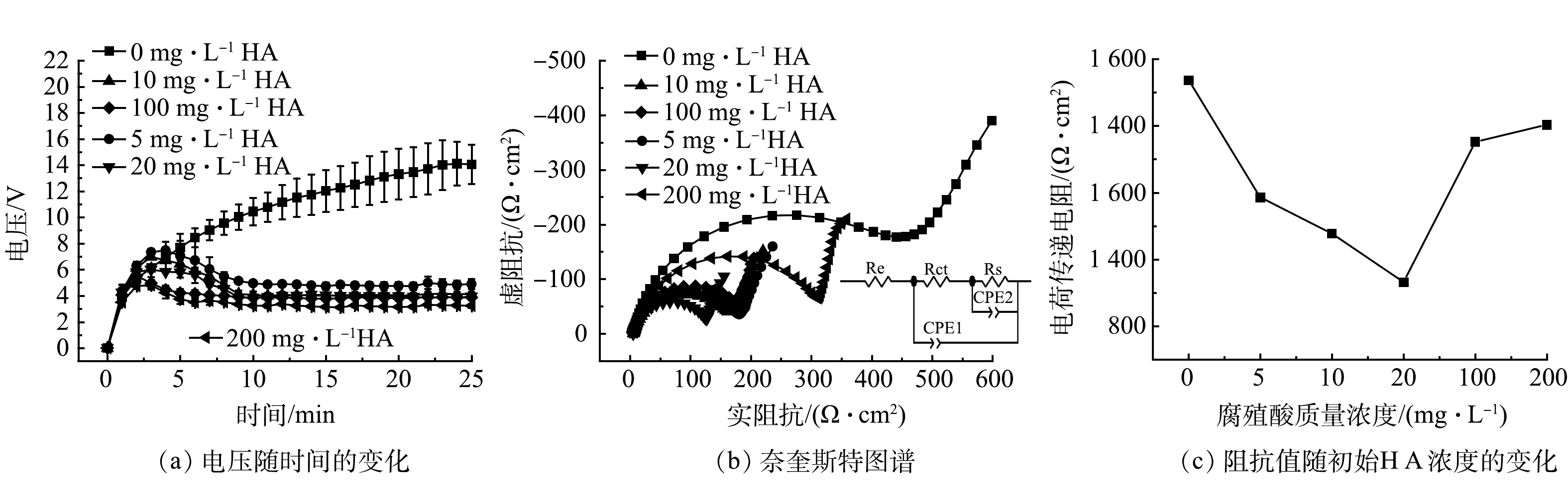
在初始pH为7,Ra为508 nm的条件下,对比在恒电流时不同初始HA浓度下电压随时间变化情况以及不同初始HA浓度阳极板的钝化情况。如图6(a)所示,HA的质量浓度从0 mg·L−1提高到 5 mg·L−1时,EC 25 min 后的电压从(15.2±3.1) V左右降低到(4.9±0.5) V左右,随后随着HA初始质量浓度的增大,操作电压都会出现明显的下降,但这应该是由于增大了HA浓度后电导率增大导致的。当HA质量浓度从5 mg·L−1 提高到 200 mg·L−1 时,电导率从400 mS·cm−1增至520 mS·cm−1。因此,本节中电压的变化难以判断极板的钝化情况,但HA质量浓度从0 mg·L−1提高到 5 mg·L−1时电导率的变化并不大,电压也发生了明显的下降,且添加了HA后,电压均呈现一种先上升,后下降的趋势。对比不同初始HA浓度下阳极板的电化学交流阻抗,探究了不同初始HA浓度条件下阳极板的钝化情况。如图6(b)和图6(c)所示,当HA质量浓度从0 mg·L−1提高到5 mg·L−1 时,对应极板的Rct从1 536.4 Ω·cm2降低到1 186.8 Ω·cm2;当HA浓度从5 mg·L−1 提高到20 mg·L−1 时,对应极板的Rct接着从1 186.8 Ω·cm2降低到932.9 Ω·cm2。在这个范围内,随着初始HA浓度的增大,Rct会出现明显的下降。随着HA质量浓度进一步上升从20 mg·L−1升至200 mg·L−1,对应极板的Rct又会由932.9 Ω·cm2增大到1 403.2 Ω·cm2。
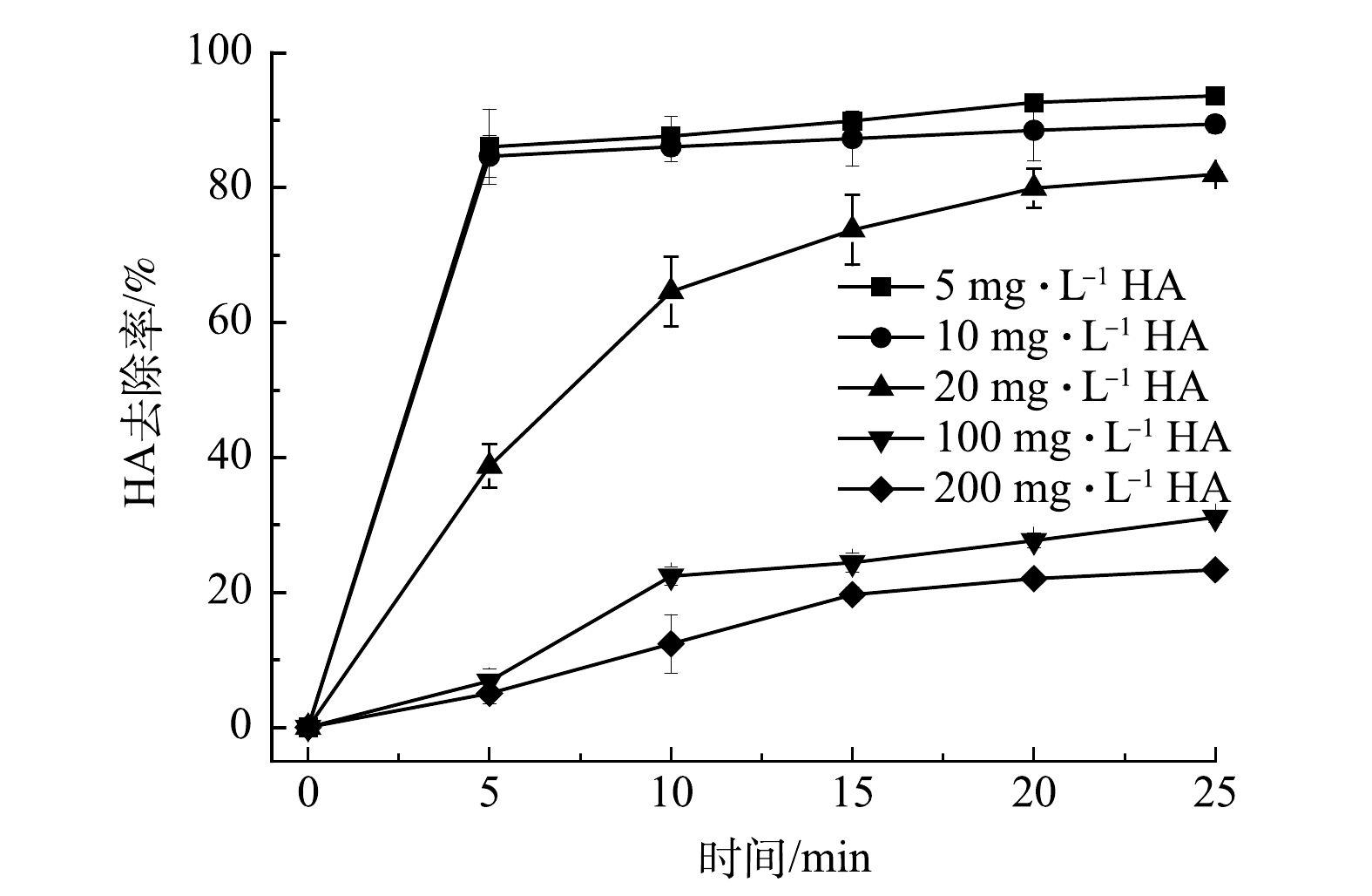
不同初始HA浓度下HA的去除率变化如图7所示。当HA质量浓度从5 mg·L−1 提高到 200 mg·L−1时,反应5 min后HA的去除率由(86.1±5.6)%降到(5.1±1.6)%;反应25 min后HA的去除率由(93.1±0.8)%降到(23.4±0.7)%,随着溶液HA浓度的升高,HA的去除率也会随之降低。由图6(a)和图6(b)可见,随着HA浓度的升高,阳极表面的钝化程度会有所缓解,但HA浓度过高时,钝化又会加重。这可能是因为阳极表面存在一个低pH界面[18],在pH较低时,在一定HA浓度范围内,HA中的羧基与阳极板表面溶解的Al3+发生配位[23],这个过程缓解了Al3+在极板表面的聚集程度,抑制了金属氧化物的生成。当溶液中的HA浓度过大时,一方面会提高阳极板界面的pH,影响HA与Al3+的络合反应[24];另一方面高浓度的HA会影响Al3+向溶液中扩散,严重时会形成凝胶层[25],污染极板表面,因此,加剧局部钝化,降低有效电极面积。
-
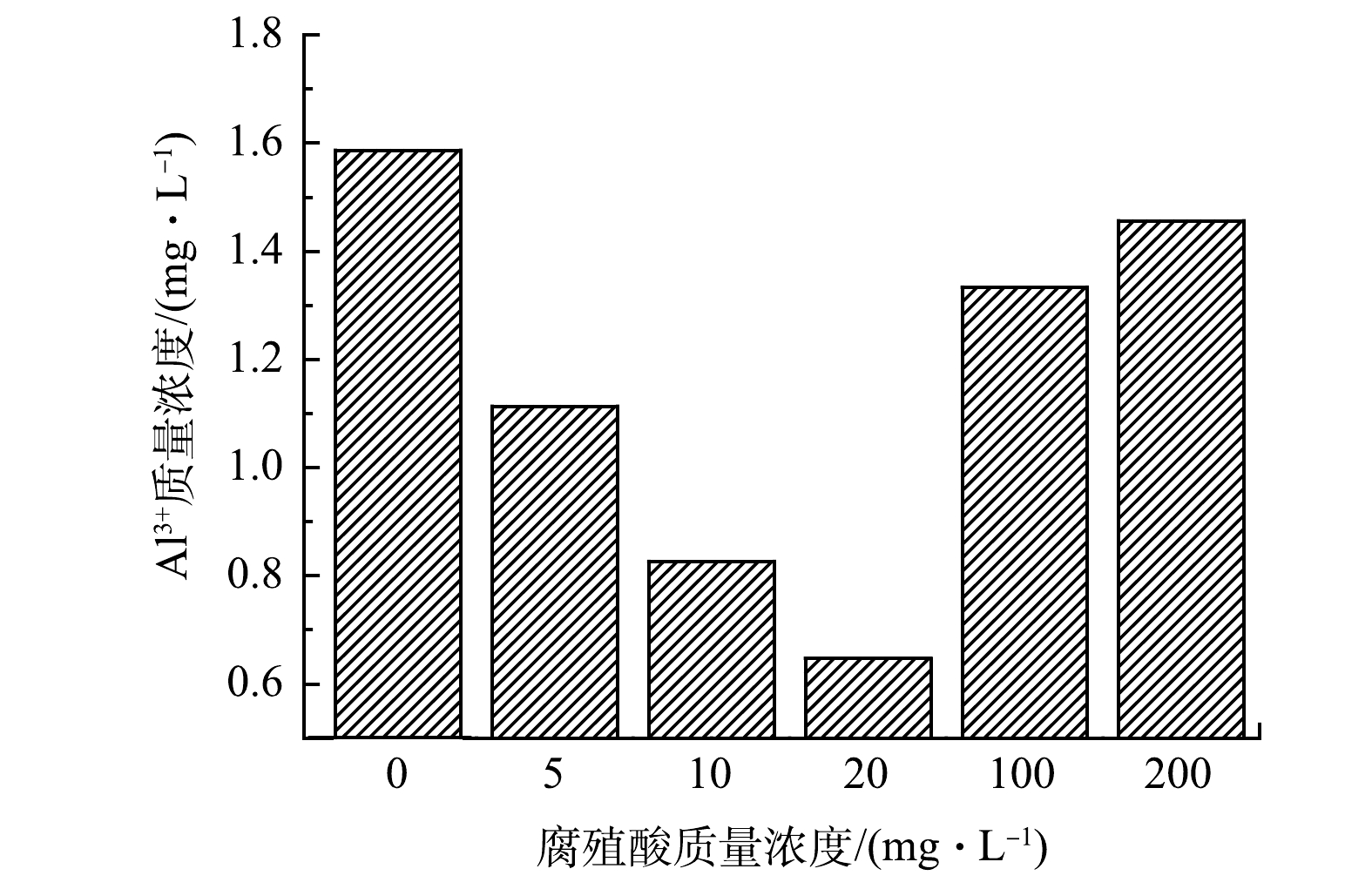
在初始pH为7,Ra为508 nm的条件下,比较了不同初始HA浓度下反应后溶液中的残留Al3+的浓度,结果如图8所示。当HA质量浓度从0 mg·L−1 提高到 5 mg·L−1 时,Al3+的质量浓度由1.59 mg·L−1下降到1.11 mg·L−1;当初始HA质量浓度从5 mg·L−1 提高到20 mg·L−1时,Al3+的质量浓度由1.11 mg·L−1下降到0.65 mg·L−1。可见,相较于未添加HA的电解液来说,加入HA之后溶液中剩余的Al3+量明显下降,但随着初始HA质量浓度由20 mg·L−1进一步升至200 mg·L−1,Al3+的质量浓度进一步回升,由0.65 mg·L−1上升到1.46 mg·L−1。Al3+的浓度的变化验证了上一节的推测,即一定量的HA可以通过减少在极板表面聚集的Al3+浓度,进而缓解钝化层的生成。
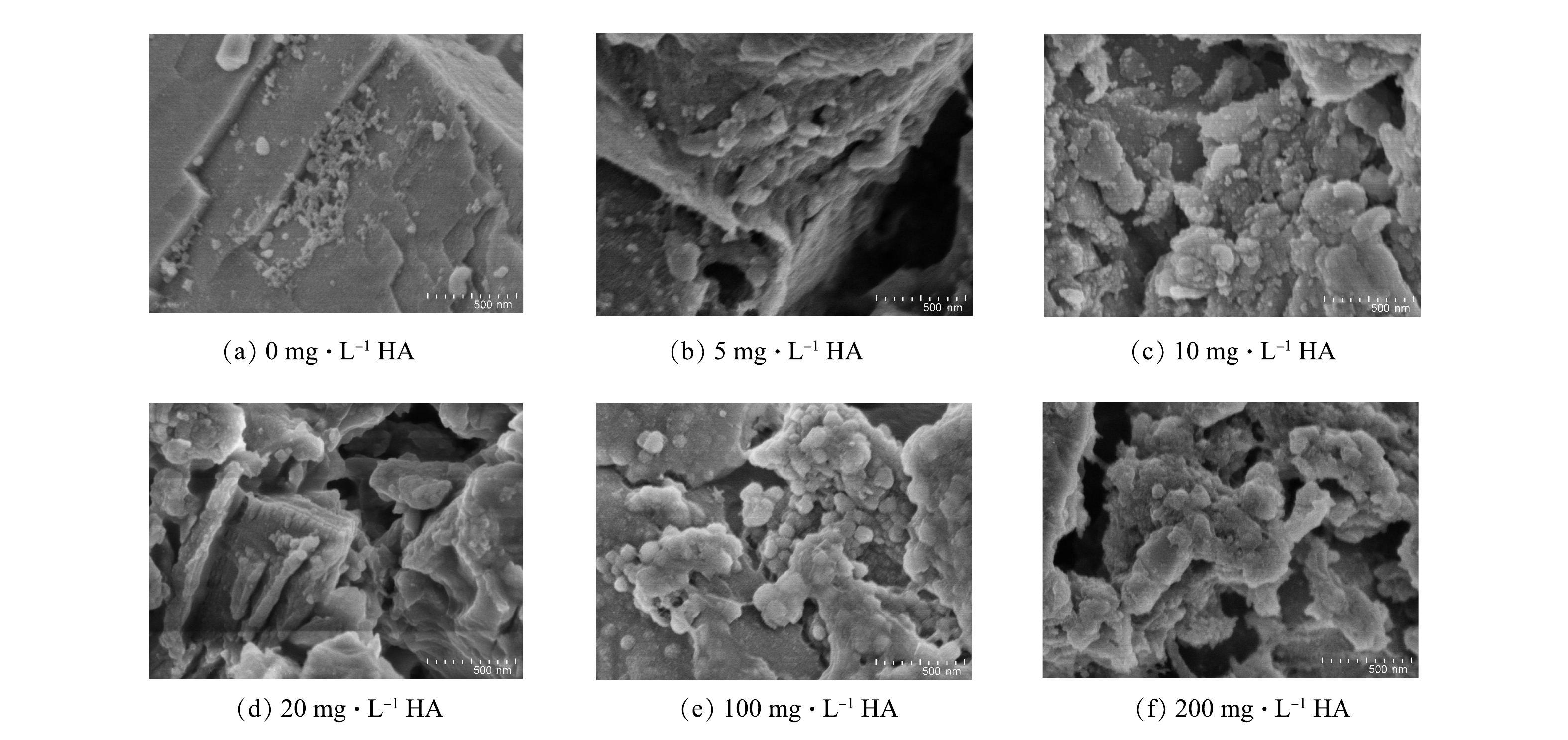
不同初始HA质量浓度下反应后阳极板表面的形貌变化如图9(a)~(f)所示。可见,极板表面越疏松多孔,说明极板溶解的越充分,钝化程度越低。由图9(a)可见,对于未添加HA的溶液来说,EC后极板表面还是大块平整的结构,极板溶解的很不充分,钝化很严重。由图9(b)~(d)可见,极板表面逐渐变的凹凸不平,极板溶解的比较充分,钝化程度逐渐降低。极板表面的细碎颗粒认为是当溶液中加入HA后,吸附了HA的部分絮体黏附在极板上形成的。如图9(e)和图9(f)所示,在初始HA质量浓度很高时,可以观察到HA的絮体分布在极板的表面,形成连续的片状结构,阻碍了阳极板的溶解。
-
1)当阳极表面Ra从2 055 nm降至270 nm时,电絮凝后阳极板的Rct增至26.2%,HA去除率下降了4%。这表明阳极表面粗糙度可以影响极板的钝化程度以及HA的去除率,即极板粗糙度越高,钝化程度越低,对HA的去除率越高。因此,可以在实际应用中通过适当增大极板表面的粗糙程度来抑制钝化。
2)当溶液初始pH从3 提高到 7 时,极板的Rct从461.0 Ω·cm2增大到1 120.2 Ω·cm2,几乎增大了1.4倍;而当pH继续升高到 9 时,极板的Rct相较于pH为3时下降了63.2%。这表明中性溶液条件下有利于金属氧化物的生成,不利于控制钝化。
3)当初始HA质量浓度从0 mg·L−1 提高到20 mg·L−1 时,对应极板的Rct降低了39.3%;HA质量浓度进一步上升至200 mg·L−1时,对应极板的Rct又会增大到1 403.2 Ω·cm2,几乎与初始HA为0 mg·L−1时一致。这是因为一定量的 HA会与阳极板表面的Al3+发生络合反应,从而减缓钝化层的生成。而过量的HA会阻碍Al3+的扩散,加剧阳极钝化。因此,在处理含有HA的水体时,通过调控污染负荷,可以实现在保证去除率的同时最大程度的缓解极板钝化。
电絮凝处理腐殖酸过程中阳极钝化影响因素分析
Influence factors of anode passivation during humic acid electrocoagulation treatment
-
摘要: 针对电絮凝(electrocoagulation,EC)处理腐殖酸(humic acid,HA)过程中电极易发生钝化,造成处理效能下降的问题,主要探究了阳极表面粗糙度、初始pH、初始HA浓度对于铝阳极钝化的影响,随后通过检测溶液中残留Al3+浓度和阳极板表面形貌,进一步分析了HA浓度对于铝阳极钝化发展的影响机制。结果表明:铝阳极钝化程度与阳极板表面算数平均粗糙度(arithmetic mean roughness,Ra)呈负相关,随着Ra从2 055 nm下降到270 nm,阳极板的电荷转移电阻(charge transfer resistor,Rct)从1 174.0 Ω·cm2增大到1 481.2 Ω·cm2;当溶液初始pH从3 提高到 7 时,极板的Rct从461.0 Ω·cm2增大到1 120.2 Ω·cm2,而当pH继续升高到 9 时,极板的Rct下降到169.5 Ω·cm2;铝阳极钝化程度随溶液初始HA浓度呈现先减小后增大的规律。HA的质量浓度从0 mg·L−1 提高到20 mg·L−1 时,对应极板的Rct从1 536.4 Ω·cm2减小到932.9 Ω·cm2,HA的质量浓度进一步上升至200 mg·L−1时,对应极板的Rct又从932.9 Ω·cm2增大到1 403.2 Ω·cm2。本研究结果可为EC在实际应用中抑制阳极钝化提供参考。Abstract: Aiming at the passivation problem of the anode during the electrocoagulation (EC) treatment of humic acid (HA) which can decrease the removal efficiency, the effects of surface roughness, initial pH, and initial HA concentration on the passivation degrees of aluminum anodes were investigated in this study. The passivation mechanism was further analyzed by detecting the concentration of residual Al3+ in the solution and the surface morphologies of the anodes. The results showed that the passivation degree of the aluminum anode was negatively correlated with the arithmetic mean roughness (Ra) of its surface. The charge transfer resistance (Rct) of the anode increased from 1 174.0 Ω·cm2 to 1 481.2 Ω·cm2 when Ra decreased from 2 055 nm to 270 nm. In addition, when the initial pH of the solution increased from 3 to 7, Rct of the anode increased from 461.0 Ω·cm2 to 1 120.2 Ω·cm2. And when the pH continued to rise to 9, Rct decreased to 169.5 Ω·cm2. The passivation degree of the anode first decreased and then increased with the increase of the initial HA concentration. When the HA concentration increased from 0 mg·L−1 to 20 mg·L−1, Rct of the corresponding anode decreased from 1 536.4 Ω·cm2 to 932.9 Ω·cm2. And when the HA concentration further increased to 200 mg·L−1, the corresponding Rct increased from 932.9 Ω·cm2 to 1 403.2 Ω·cm2. This study provides a theoretical and technical support for anode passivation mitigation in practical application of EC.
-
Key words:
- anode passivation /
- electrocoagulation /
- humic acid /
- water treatment
-

-
-
[1] DE OLIVEIRA A G, RIBEIRO J P, NETO E F A, et al. Removal of natural organic matter from aqueous solutions using electrocoagulation pulsed current: Optimization using response surface methodology[J]. Water Science and Technology, 2020, 82(1): 56-66. [2] METSÄMUURONEN S, SILLANPÄÄ M, BHATNAGAR A, et al. Natural organic matter removal from drinking water by membrane technology[J]. Separation & Purification Reviews, 2014, 43(1): 1-61. [3] SÄRKKÄ H, VEPSÄLÄINEN M, SILLANPÄÄ M. Natural organic matter (NOM) removal by electrochemical methods: A review[J]. Journal of Electroanalytical Chemistry, 2015, 755: 100-108. doi: 10.1016/j.jelechem.2015.07.029 [4] INAM M A, KHAN R, PARK D R, et al. Complexation of antimony with natural organic matter: Performance evaluation during coagulation-flocculation process[J]. International Journal of Environmental Research and Public Health, 2019, 16(7): 1092. doi: 10.3390/ijerph16071092 [5] HAKIZIMANA J N, GOURICH B, CHAFI M, et al. Electrocoagulation process in water treatment: A review of electrocoagulation modeling approaches[J]. Desalination, 2017, 404: 1-21. doi: 10.1016/j.desal.2016.10.011 [6] KLIAUGAITĖ D, YASADI K, EUVERINK G, et al. Electrochemical removal and recovery of humic-like substances from wastewater[J]. Separation and Purification Technology, 2013, 108: 37-44. doi: 10.1016/j.seppur.2013.01.055 [7] BIAN Y, GE Z, ALBANO C, et al. Oily bilge water treatment using DC/AC powered electrocoagulation[J]. Environmental Science:Water Research & Technology, 2019, 5(10): 1654-1660. [8] HUANG C, CHEN L, YANG C. Effect of anions on electrochemical coagulation for cadmium removal[J]. Separation and Purification Technology, 2009, 65(2): 137-146. doi: 10.1016/j.seppur.2008.10.029 [9] VAN GENUCHTEN C M, BANDARU S R S, SUROROVA E, et al. Formation of macroscopic surface layers on Fe(0) electrocoagulation electrodes during an extended field trial of arsenic treatment[J]. Chemosphere, 2016, 153: 270-279. doi: 10.1016/j.chemosphere.2016.03.027 [10] YANG Z, XU H, ZENG G, et al. The behavior of dissolution/passivation and the transformation of passive films during electrocoagulation: Influences of initial pH, Cr(VI) concentration, and alternating pulsed current[J]. Electrochimica Acta, 2015, 153: 149-158. doi: 10.1016/j.electacta.2014.11.183 [11] 徐龙乾, 刘树丽, 徐晓军, 等. 电絮凝法在重金属废水处理中钝化机理的研究进展[J]. 现代化工, 2017, 37(12): 33-37. [12] MAHESH S, GARG K K, SRIVASTAVA V C, et al. Continuous electrocoagulation treatment of pulp and paper mill wastewater: Operating cost and sludge study[J]. RSC Advances, 2016, 6(20): 16223-16233. doi: 10.1039/C5RA27486A [13] BRAHMI K, BOUGUERRA W, HAMROUNI B. Use of electrocoagulation with aluminum electrodes to reduce hardness in tunisian phosphate mining process water[J]. Mine Water & the Environment, 2015, 35(3): 1-8. [14] CHELLAM S, SARI M A. Aluminum electrocoagulation as pretreatment during microfiltration of surface water containing NOM: A review of fouling, NOM, DBP, and virus control[J]. Journal of Hazardous Materials, 2016, 304: 490-501. doi: 10.1016/j.jhazmat.2015.10.054 [15] DUBRAWSKI K L, VAN GENUCHTEN C M, DELAIRE C, et al. Production and transformation of mixed-valent nanoparticles generated by Fe(0) electrocoagulation[J]. Environmental Science & Technology, 2015, 49(4): 2171-2179. [16] ASHRAF S N, RAJAPAKSE J, DAWES L A, et al. Electrocoagulation for the purification of highly concentrated brine produced from reverse osmosis desalination of coal seam gas associated water[J]. Journal of Water Process Engineering, 2019, 28: 300-310. doi: 10.1016/j.jwpe.2019.02.003 [17] MANSOURI K, IBRIK K, BENSALAH N, et al. Anodic dissolution of pure aluminum during electrocoagulation process: Influence of supporting electrolyte, initial pH, and current density[J]. Industrial & Engineering Chemistry Research, 2011, 50(23): 13362-13372. [18] CHEN X, CHEN G, YUE P L. Investigation on the electrolysis voltage of electrocoagulation[J]. Chemical Engineering Science, 2002, 57(13): 2449-2455. doi: 10.1016/S0009-2509(02)00147-1 [19] INGELSSON M, YASRI N, ROBERTS E P L. Electrode passivation, faradaic efficiency, and performance enhancement strategies in electrocoagulation: A review[J]. Water Research, 2020, 187: 116433. doi: 10.1016/j.watres.2020.116433 [20] MECHELHOFF M, KELSALL G H, GRAHAM N J D. Electrochemical behaviour of aluminium in electrocoagulation processes[J]. Chemical Engineering Science, 2013, 95: 301-312. doi: 10.1016/j.ces.2013.03.010 [21] AMIN M A, ABD EL REHIM S S, EL-LITHY A S. Corrosion, passivation and breakdown of passivity of Al and Al–Cu alloys in gluconic acid solutions[J]. Electrochimica Acta, 2010, 55(20): 5996-6003. doi: 10.1016/j.electacta.2010.05.055 [22] ULU F, BARISCI S, KOBYA M, et al. Removal of humic substances by electrocoagulation (EC) process and characterization of floc size growth mechanism under optimum conditions[J]. Separation and Purification Technology, 2014, 133: 246-253. doi: 10.1016/j.seppur.2014.07.003 [23] SONG J, JIN X, WANG X C, et al. Preferential binding properties of carboxyl and hydroxyl groups with aluminium salts for humic acid removal[J]. Chemosphere, 2019, 234: 478-487. doi: 10.1016/j.chemosphere.2019.06.107 [24] 宋吉娜. 腐殖酸质子化基团与铝离子在水中的迁变及凝聚行为表征[D]. 西安: 西安建筑科技大学, 2018 [25] YILDIZ Y S, KOPARAL A S, KESKINLER B. Effect of initial pH and supporting electrolyte on the treatment of water containing high concentration of humic substances by electrocoagulation[J]. Chemical Engineering Journal, 2008, 138(1): 63-72. -



 下载:
下载: