-
由于矿山开采、金属冶炼[1]、不合格农药使用等人类生产活动而导致大量含有重金属的废水进入自然环境,已造成严重的土壤和地下水污染[2]。根据2014年原环境保护部与原国土资源部联合发布的全国土壤污染调查公报,我国污水灌溉区超标点位占26.4%,重金属元素镉(Cd)的超标率位列第一。而且,Cd具有不能被分解或降解的特性,对环境中的生物体表现不同程度的毒性,会通过食物链进行传递,严重威胁人类身体健康[3]。
近年来,已有多种物理和化学技术应用于水体中Cd(Ⅱ)的污染修复,其中包括化学沉淀、离子交换、膜分离和吸附[4]。在这些方法中,吸附方法因其能高效固定水体中的重金属且操作简单而被广泛应用。目前,已有多种吸附材料已经被用于水体中Cd(Ⅱ)的污染修复,包括活性炭、沸石、生物炭等[5]。有研究表明,生物炭因其具有高比表面积、高阳离子交换容量(CEC)、丰富的含氧官能团和高矿物质含量,能够将Cd(Ⅱ)吸附在其表面,成为Cd(Ⅱ)污染修复应用中的有效吸附剂[6]。HAN等利用生物炭吸附水体Cd(Ⅱ),其最大吸附量为74.04 mg·g−1[7]。然而,传统生物炭存在对Cd(Ⅱ)的吸附效果不理想,难以分离和回收重金属资源,并且容易引起二次污染等问题[8],因而限制了其在水污染修复中的应用。有研究表明,铁改性生物炭不仅可以增强Cd(Ⅱ)的吸附能力,而且可磁性回收[9]。纳米零价铁(nZVI)因其粒径小、表面积大、活性强,在反应过程中产生的铁氧化物能够高效吸附Cd(Ⅱ)[10],被广泛应用于生物炭改性吸附剂。然而,之前研究中通过化学还原方法制备的nZVI改性生物炭存在易氧化和抗干扰性不足,还原剂成本高等问题,故难以广泛应用。因此,如何研发出既能高效吸附Cd(Ⅱ),同时抗干扰能力强,且可以回收的铁炭复合材料是当前Cd(Ⅱ)污染修复的关键和难点。
综上所述,本研究使用废弃木屑生物质和铁盐为原料,使用碳热厌氧还原方法制备出一种新型多孔生物质铁炭基功能材料,并采用多种固相技术对材料的组成、结构和性质进行了表征和分析;研究了材料对水体Cd(Ⅱ)的吸附效果及其构效关系,考察了pH和干扰离子等对Cd(Ⅱ)吸附的影响;揭示了新型多孔生物质铁炭基功能材料对Cd(Ⅱ)的固定机制。
-
氢氧化钠、无水亚硫酸钠、氯化镉、乙醇、30%过氧化氢等化学试剂均为分析纯(广州化学试剂厂),九水硝酸铁(分析纯)购自上海阿拉丁生化科技有限公司(中国上海)。所有实验均使用超纯水(18 MΩ·cm−1)。
-
将废弃的球状天然毫米木球浸泡在NaOH和Na2SO3的混合溶液中,超声30 min后转移到反应釜中,并在100 ℃中反应10 h。反应后将木球取出,用超纯水冲洗,然后转移到反应釜中并加入H2O2,加热至100 ℃,继续保持6 h,反应后冷冻干燥,所得生物质简称为MC。将MC浸泡在Fe(NO3)3·9H2O溶液中,持续振荡10 h,使得Fe3+充分吸附在MC上。最后置于氮气保护的真空管式炉中,分别在热解温度为400、600、800 ℃条件下保持1 h,升温速率为10 ℃·min−1。制备出新型多孔生物质铁炭基功能材料,分别简称为MCFe-400、MCFe-600、MCFe-800。其中,将MC置于氮气保护的真空管式炉中在热解温度为800 ℃,其他条件与制备无铁负载的生物炭材料(BC)相同。
-
扫描电子显微镜(SU8220,HIT ACHI,日本)用于分析MCFe的形貌。比表面积和孔隙分析仪(Tristar II 3020 M,Micromeritics,美国)用于分析MCFe的比表面积和孔容。此外,使用Smartlab X射线衍射仪在2θ为10~90°(9 kW)内使用Cu靶测量反应前后样品的X射线衍射。X射线光电子能谱(ESCALAB 250Xi,Thermo Fischer,美国)用于对反应前后MCFe表面元素组成和价态进行表征。振动样品磁强计仪(PPMS-9,Quantum Design,美国)用于表征MCFe的磁滞回线。
-
使用不同热解温度制备的MCFe和生物炭(MCFe-400、MCFe-600、MCFe-800、BC)进行动力学实验,以研究材料对水体Cd(Ⅱ)的吸附性能。实验材料的投加量为0.8 g·L−1;Cd(Ⅱ)的初始质量浓度为10 mg·L−1;反应体系为40 mL。所有批次实验均在50 mL离心管中进行(200 r·min−1,(25±1) ℃)。反应一段时间(0、5、15、30、60、90、120、240、360 min)后,取反应混合液过0.22 μm的滤膜,用于Cd(Ⅱ)质量浓度的测定。所有实验均设置3组平行。
为探究MCFe对Cd(Ⅱ)的吸附行为,采用伪二级动力学模型(式(1))对实验数据进行拟合。采用Langmuir(式(2))和Freundlich(式(4))模型对实验结果进行拟合[11]。
式中:qt和qe分别是在平衡和t时刻的吸附量,mg·g−1;k2是吸附速率常数,g·(mg·min)−1。
式中:qe为吸附达到平衡时的吸附量,mg·g−1;Qm为最大吸附量,mg·g−1;Ce为平衡时溶液中污染物的质量浓度,mg·L−1;KL为Langmuir模型中结合位点的附着性相关吸附平衡常数,L·mg−1;KF为Freundlich吸附平衡常数;n为非均质性因子。
本研究考察了pH和共存的阳离子或阴离子对吸附效果的影响。实验的初始条件同上。使用HCl (0.1 mol·L−1)和NaOH (0.1 mol·L−1)将水溶液的初始pH调至4、6、7、9,反应6 h后测定反应体系中Cd(Ⅱ)的质量浓度。此外,磷酸根(PO43-)、硫酸根(SO42-)、碳酸氢根(CO32-)、硝酸根(NO3−)、钙离子(Ca2+)和镁离子(Mg2+)为水体终常见的阴阳离子,研究其对MCFe吸附效果的影响。
研究MCFe-800对实际水体中Cd(Ⅱ)的吸附效果。选用湖泊水、农田灌溉水和河水配制初始质量浓度为10 mg·L−1的Cd(Ⅱ)溶液,用于循环吸附实验,其他实验条件同上。反应1 h后,将MCFe-800从溶液中分离出来,加入准备好的40 mL溶液中,重复4次。在带有恒温水浴夹套的层析柱(长100 mm,直径10 mm)中进行实验。每根柱子填满MCFe-800。将含有1 000 µg·L−1和2 000 µg·L−1 Cd(Ⅱ)的农田灌溉水(pH=7.15±0.2)由蠕动泵(LongerPump,中国)以2 mL·min−1的恒定流速自下而上模式连续送入层析柱,空床接触时间(EBCT)为4 min。在预设时间收集流出溶液样品,并分析Cd(Ⅱ)的质量浓度以确定吸附效率。
-
采用电感耦合等离子体发射光谱仪(ICP-OES)测定Cd(Ⅱ)的质量浓度。将材料分散到不同pH的溶液中,超声至充分分散的悬浊液,使用Zeta电位仪测定材料表面在不同pH条件下的电位。将不同材料负载在工作电极上,然后浸入0.1 mol·L−1 KCl溶液中在CHI-920d电化学工作站上进行腐蚀电流测定。
-
MCFe-(400~800)材料的SEM表征结果如图1所示。可见,MCFe-400材料碳表面较为光滑且出现很多小颗粒。随着热解温度升高到600 ℃和800 ℃,由图1(b)、图1(c)可以观察到,在生物炭表面上承载着较为均匀分散的球形颗粒,且在MCFe-800表面出现褶皱,该褶皱结构可以增大生物炭比表面积[12]。此外,通过XRD分析了MCFe的晶型矿物组成(图1(d))。结果表明,MCFe-400无明显的晶型峰生成,而随着热解温度的升高,MCFe-600和MCFe-800在2θ为44.7°和82.3°出现了明显的特征峰,与零价铁(Fe0)的标准卡片(PDF#06-0696)一致。这表明材料表面生成的球形颗粒为nZVI,即MCFe复合材料成功制备。此外,MCFe-800在26.4°出现石墨的晶型峰,而且,由图1(d)还可以看到Fe3C的特征峰,与其标准卡片(PDF#35-0772)一致[13]。复合材料的氮吸附-脱附等温线如图1(e)和表1所示。可见,MCFe-800的比表面积为209.286 m2·g−1,明显大于MCFe-600和MCFe-400。随着热解温度的升高,材料孔容由0.010 cm3·g−1增加到0.216 cm3·g−1,表明热解温度升高可使材料形成多孔结构,同时也增加了材料的比表面积[14-15]。因而,MCFe-800表现出最大的比表面积和多孔结构。图1(f)显示了MCFe材料的磁性。磁滞回线表明所制备的材料均具有磁性。MCFe-400、MCFe-600和MCFe-800的饱和磁化强度值分别为8.22、27.35和60.38 emu·g−1。这表明热解温度为800 ℃时制备的材料磁性最大,可以更容易实现材料的固液分离[16]。
-
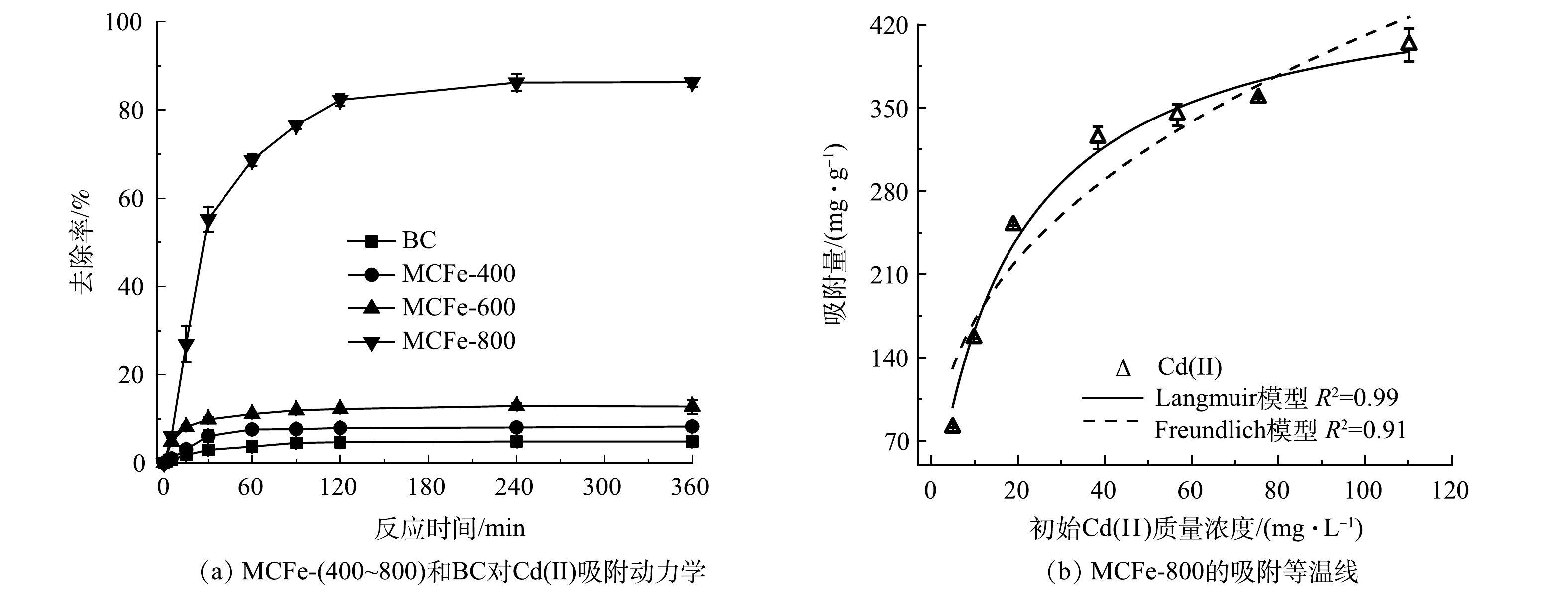
通过动力学实验研究了不同材料对Cd(Ⅱ)的吸附效果。如图2(a)所示,BC、MCFe-400和MCFe-600在反应360 min对Cd(Ⅱ)的去除率分别为4.9%、8.3%和12.8%。这表明BC、MCFe-400和MCFe-600对Cd(Ⅱ)的吸附效果较低。而MCFe-800加入到反应体系中后,Cd(Ⅱ)的质量浓度迅速降低,在120 min后逐渐趋于平衡,反应结束时MCFe-800对Cd(Ⅱ)的去除率为85.5%。反应后,不同材料对Cd(Ⅱ)去除率从高到低依次为MCFe-800>MCFe-600>MCFe-400>BC。这主要是因为MCFe-800具有更大的比表面积,能提供更多的镉吸附位点[17],而且随着热解温度的升高,材料表面生成更多的纳米零价铁颗粒,有利于Cd(Ⅱ)的吸附。同时,MCFe-(400~800)对Cd(Ⅱ)吸附动力学数据能够较好地拟合伪二级动力学模型(R2>0.98)。这表明MCFe-(400~800)对Cd(Ⅱ)的吸附主要以化学吸附为主[18]。动力学实验结果表明,MCFe-800对Cd(Ⅱ)的吸附效果最佳。
MCFe-800对Cd(Ⅱ)的吸附等温线结果如图2(b)所示。采用Langmuir和Freundlich等温吸附模型分别拟合MCFe-800对溶液Cd(Ⅱ)的吸附过程,其等温吸附的拟合结果如表2所示。MCFe-800的Langmuir和Freundlich等温吸附模型的R2分别为0.99和0.91,可见,Langmuir模型更适合描述MCFe-800对Cd(Ⅱ)的等温吸附过程。这表明MCFe-800对Cd(Ⅱ)吸附过程中单层吸附占主导作用[19]。此外,考虑到单独的BC对镉的去除率(4.9%)较低,根据Langmuir模型,MCFe-800对Cd(Ⅱ)的最大吸附量归一化到Fe为463.84 mg·g−1。基于上述结果,选取MCFe-800进行后续实验。
-
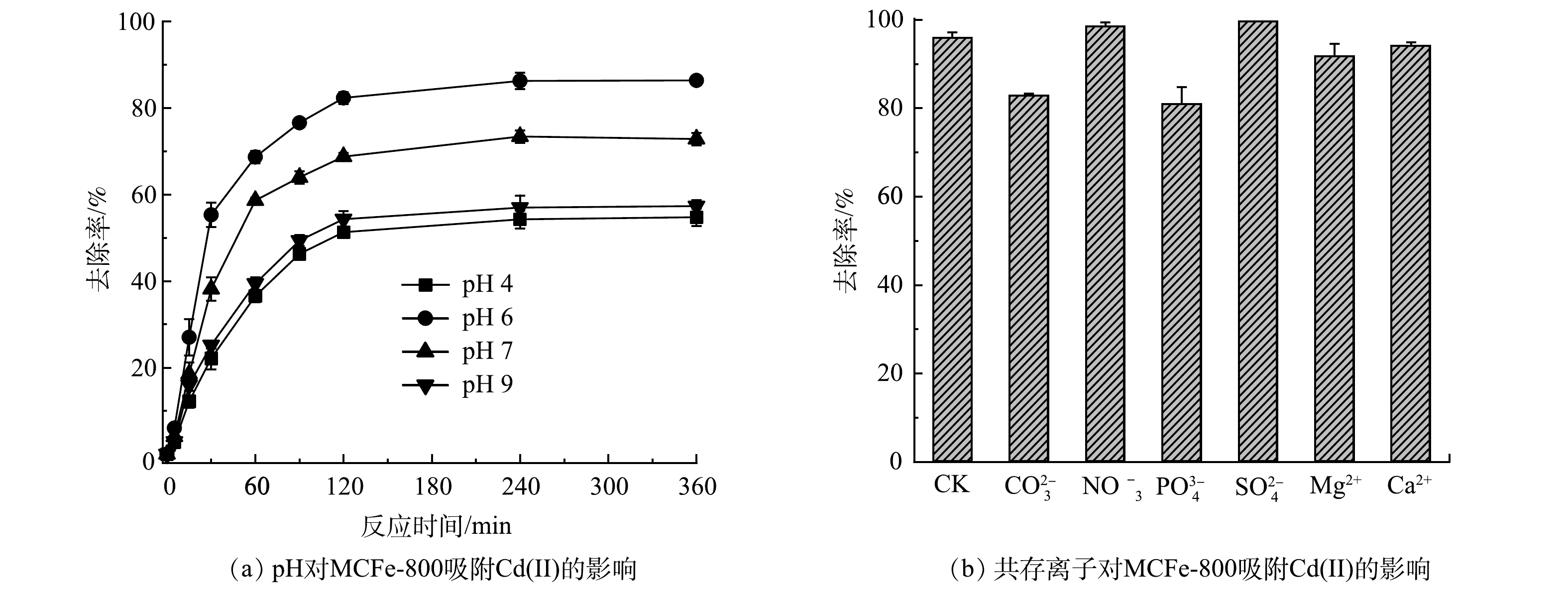
溶液的pH不仅会影响吸附剂表面的物理化学性质,还会影响水溶液中Cd(Ⅱ)的赋存形态[20]。因此,考察了初始pH对MCFe-800吸附Cd(Ⅱ)的影响,结果如图3(a)所示。在不同pH条件下,反应开始时溶液中Cd(Ⅱ)的质量浓度迅速下降,120 min后溶液中Cd(Ⅱ)的质量浓度逐渐趋于平衡。随着初始pH由4增加到6,Cd(Ⅱ)去除率由53.04%升高到85.48%。当pH达到9时,Cd(Ⅱ)的去除率明显下降,表明溶液pH为6时对Cd(Ⅱ)的去除率最大。这主要是因为:在酸性条件下(pH<5),铁氧化物表面主要带正电荷,不利于阳离子镉与材料表面发生静电吸附[21];同时质子也会与Cd(Ⅱ)竞争吸附位点,导致对镉的吸附能力下降。pH的升高会导致反应生成的铁氧化物表面向带负电转化,Cd(Ⅱ)与吸附剂表面之间会发生静电结合,从而导致吸附量的增加[22]。然而,当pH偏碱性时不利于nZVI的腐蚀,反应体系中生成铁氧化物减少,抑制了Cd(Ⅱ)的与铁氧化物的共沉淀和表面络合等吸附反应[23-24],并且当反应体系pH为7~9时,溶液中Cd(Ⅱ)还未开始沉淀[25]。因此,本研究中pH升高可抑制MCFe-800中的nZVI的腐蚀,从而降低MCFe-800对Cd(Ⅱ)的去除率。Cd(Ⅱ)吸附动力学数据符合伪二级动力学模型(R2>0.95),二级动力学常数显示pH为6的反应速率为0.004 g·(mg·min)−1,大于pH为4、7和9的反应速率。因此,偏中性条件下MCFe-800对Cd(Ⅱ)吸附效果最佳。
常见共存阳离子和阴离子对Cd(Ⅱ)吸附的影响如图3(b)所示。结果表明,当CO32-和PO43-存在,MCFe-800对Cd(Ⅱ)去除率分别由95.93%降到83.79%和80.98%,表明CO32-和PO43-抑制了MCFe-800对Cd(Ⅱ)的吸附。有研究表明,CO32-通过与铁氧化物形成内球表面复合物,能够被吸附固定到非晶型铁氧化物表面[26]。此外,CO32-可能与生成的Fe2+反应形成菱铁矿(FeCO3)或Fe2+/Fe3+(氧化物)羟基碳酸盐,从而消耗铁离子并阻塞反应位点[27]。与此同时,PO43-通过与铁氧化物形成内球复合物与Cd(Ⅱ)竞争部分的结合位点[28-29]。因此,CO32-和PO43-会降低MCFe-800对Cd(Ⅱ)的去除率,在实际应用过程中需要考虑水体中CO32-和PO43-的质量浓度。针对共存阳离子,当Ca2+和Mg2+存在时,MCFe-800对Cd(Ⅱ)去除率分别为91.23%和94.18%,MCFe-800对Cd(Ⅱ)吸附能力只有微弱下降。这主要是因为阳离子间存在吸附位点的直接竞争[30],但不明显。综上所述,MCFe-800对水体Cd(Ⅱ)污染修复中具有较强的抗干扰离子能力。
-
为揭示MCFe-800对Cd(Ⅱ)的固定机理,使用XRD、XPS对反应前后的MCFe-800进行了详细的表征,以分析材料的表面组成和化学性质的变化。如图4(a)所示,根据Fe2p结合能的值,Fe峰可以进一步分峰为Fe0、Fe(Ⅱ)和Fe(III)[25]。结合能为710.8 eV和724.2 eV的Fe2p峰属于Fe(Ⅱ),714.2 eV和727 eV处的峰对应Fe(III)[31]。反应前在706.9 eV处有一个小峰为Fe0。然而,该峰在反应后明显减弱,并且Fe(Ⅱ)和Fe(III)与总铁的比值分别从49.06%和39.58%增加到55.39%和43.92%,表明MCFe-800表面的nZVI在反应后被氧化。与此同时,对比图4(b)可见,反应后Fe0的峰(44.64°、82.33°、65.02°)明显减弱。该结果与Fe2p分峰结果一致。而反应前后材料Fe3C的峰无明显变化。有研究表明,Fe3C和石墨在反应过程中具有一定的催化作用,可以促进电子转移,从而提高材料中nZVI的腐蚀,生成铁氧化物,有利于Cd(Ⅱ)的吸附[32-33]。电化学结果进一步的证实,如图4(c)所示,MCFe-800的腐蚀电压为0.023 V,小于MCFe-600和MCFe-400。同时,不同材料的腐蚀电流结果表明 MCFe-800电流最高为1.49×10−7 A,明显大于其他材料。有研究[34]表明,腐蚀电流越高,表明材料失去电子的能力越强,材料中nZVI更容易被氧化。因此,在好氧情况下,Cd(Ⅱ)会与MCFe-800中释放的Fe(Ⅱ)或铁的氢氧化物发生共沉淀和络合反应(式(4)和式(5))[22, 35],从而把Cd固定在MCFe-800表面上。
图4(d)为3种材料的zeta电位图。可见,当pH从3升高到10,MCFe-400、MCFe-600的Zeta电位分别从−2.61 mV和−4.15 mV下降到−27.05 mV和−25.20 mV,其表面电荷始终为负。而MCFe-800由19.95 mV下降到−14.40 mV时,其等电位(pHPZC)为3.74。当溶液pH大于3.74时,其表面总是带负电,这有利于阳离子Cd(Ⅱ)的吸附[36]。根据上述分析,MCFe-800在好氧情况下固定Cd(Ⅱ)的机制主要是通过静电吸附、共沉淀和表面络合反应。
-
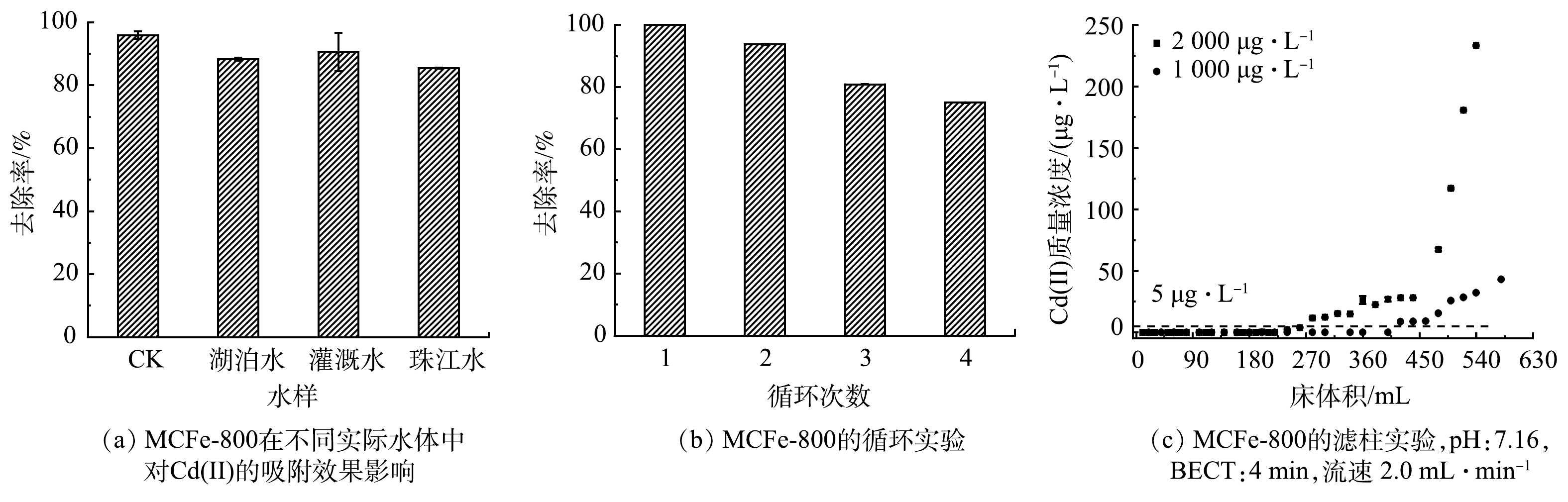
图5(a)描述了MCFe-800对实际水体中Cd(Ⅱ)的吸附及循环利用。可见,MCFe-800在农田灌溉水、湖泊水和河水中的去除率分别为88.3%、90.5%和85.4%,相较于在超纯水反应体系中的95.9%去除率分别降低了7.6%、5.4%和10.5%。这表明MCFe-800在实际水体中依然对Cd(Ⅱ)保持良好的吸附效果,具有较强的抗干扰能力。在连续4次的循环中(图5(b)),MCFe-800对水体Cd(Ⅱ)的去除率在循环吸附过程中逐渐下降。其原因可能是,每次循环后吸附剂表面nZVI的损失导致活性位点的减少,进而导致Cd(Ⅱ)的吸附容量降低[37]。然而,其去除率在第4次循环中仍然保持在75.0%,表明MCFe-800能够实现多次的循环使用。
此外,为了评估MCFe-800在从污染农田灌溉水中吸附Cd(Ⅱ)的工程应用,选取了实际的农田灌溉水进行滤柱实验,其结果如图5(c)所示。Cd(Ⅱ)质量浓度能快速降低到国家饮用水标准5 μg·L−1以下,分别在经过200 min和135 min后出水Cd(Ⅱ)质量浓度才缓慢增加,表明MCFe-800作为层析柱填充材料能够高效吸附农田灌溉水中的Cd(Ⅱ)。Cd(Ⅱ)初始质量浓度为1 000 μg·L−1和2 000 μg·L−1,其有效处理量分别为400 BV和270 BV。结合前面对镉的最大吸附量(463.84 mg·g−1),本研究中MCFe-800材料对镉的吸附容量和效率明显优于以往研究中使用的单一零价铁、生物炭和生物炭负载零价铁[17, 25]。综上所述,MCFe-800在农田灌溉水镉污染修复中具有潜在的应用价值。
-
1)对本研究制备的新型多孔生物质铁炭基功能材料的表征结果表明,随者热解温度升高,MCFe比表面积和孔容增加,在800℃制备的MCFe-800材料表面为分散均匀的纳米零价铁颗粒;磁滞回线表明,MCFe-800的磁性最强,更容易实现固液分离。
2) MCFe-800对水体Cd(Ⅱ)的去除率明显高于其他热解温度,符合伪二级动力学,并且根据Langmuir等温线模型,MCFe对Cd(Ⅱ)的最大吸附量高达463.84 mg·g−1。当pH为6~7时,其对Cd(Ⅱ)的吸附效果最佳,高浓度的CO32-和PO43-会抑制材料对Cd(Ⅱ)的吸附。MCFe800固定Cd(Ⅱ)的主要机制有静电吸附、共沉淀和表面络合。
3) MCFe-800对Cd(Ⅱ)的吸附具有较强的抗干扰能力,还能实现多次循环。滤柱实验结果显示,该材料能够将农田灌溉水中1 000 μg·L−1和2 000 μg·L−1的Cd(Ⅱ)快速降低至饮用水标准,其有效处理量分别为400 BV和270 BV。
新型多孔生物质铁炭基功能材料高效去除镉及其作用机制
Novel biomass-derived porous iron/carbon materials for highly efficient cadmium removal and its mechanisms
-
摘要: 通过高温厌氧碳热还原方法制备出一种新型多孔生物质铁炭基功能材料,并详细分析了材料的结构和性质;考察了制备过程中的热解温度、pH和干扰离子等环境因子对镉吸附性能的影响,并揭示了铁炭基功能材料对镉的固定机制。结果表明,材料比表面积和孔容随热解温度升高而增加,在800 ℃材料中形成了纳米零价铁和碳化铁,制备出了具有磁性的铁炭基功能材料(MCFe-800),有利于材料的磁性回收。动力学实验结果表明,MCFe-800对水体镉的去除率明显高于其他热解温度,最大吸附容量归一化到铁为463.84 mg·g−1。在偏中性条件下更有利于对镉的去除。MCFe-800对镉的固定机制主要为静电吸附、共沉淀和表面络合。此外,经过4次循环实验后,MCFe-800对实际水体中镉的去除率仍为75.0%。滤柱实验结果表明,当镉初始质量浓度为1 000 μg·L−1和2 000 μg·L−1时,有效处理量分别为400 BV和270 BV。因此,新型多孔生物质铁炭基功能材料在水体镉污染修复方面具有很大应用潜力。Abstract: In this study, a type of novel biomass-derived porous iron/carbon material was prepared by the high-temperature carbothermal reduction method under anaerobic conditions, and its structure and properties were analyzed in detail. The effects of environmental factors during preparation including pyrolysis temperature, pH, and co-existing ions on the performance of cadmium (Cd) removal were investigated, and the corresponding Cd fixation mechanism by this iron/carbon material was revealed. The results show that the specific surface area and pore volume of the material increased with the increase of the pyrolysis temperature, and nanoscale zero-valent iron and iron carbide occurred at 800℃, a magnetic iron/carbon-based functional material (MCFe-800) was prepared, which was beneficial to the magnetic recovery. The adsorption kinetic experiment results show that the removal efficiency of Cd by MCFe-800 was significantly higher than that of other pyrolysis temperatures, and the maximum removal capacity normalized to iron was 463.84 mg·g−1. It is more favorable for Cd removal by MCFe-800 under neutral conditions. The removal mechanisms of Cd were mainly electrostatic adsorption, co-precipitation, and surface complexation. In addition, after four cycles, the removal efficiency of Cd by MCFe-800 in real water was still 75.0%. Filter column experiments show that the treatment capacities of MCFe-800 were 400 BV and 270 BV when the initial concentrations of Cd(II) were 1 000 μg·L−1 and 2 000 μg·L−1, respectively. Therefore, the novel biomass-derived porous iron/carbon material has a great application potential for the remediation of Cd pollution in water.
-
Key words:
- iron/carbon materials /
- cadmium /
- water treatment
-

-
表 1 MCFe的结构性能
Table 1. Structural properties of MCFe
吸附材料 比表面积/(m2·g−1) 孔容/(cm3·g−1) 孔径/(nm) MCFe-400 5.38 0.01 14.01 MCFe-600 166.77 0.11 3.63 MCFe-800 209.29 0.22 4.12 表 2 吸附等温模型和动力学参数
Table 2. Adsorption isotherm model and kinetic parameters
材料 Langmuir模型 伪二级动力学拟合 Qm/(mg·g−1) KL/(L·mg−1) R2 k2/(g·(mg·min)−1) R2 MCFe-400 — — — 0.031 0.98 MCFe-600 — — — 0.057 0.99 MCFe-800(pH=4) — — — 0.002 0.95 MCFe-800(pH=6) 463.84 0.05 0.99 0.004 0.99 MCFe-800(pH=7) — — — 0.002 0.95 MCFe-800(pH=9) — — — 0.002 0.95 -
[1] DU Y, HU X F, WU X H, et al. Effects of mining activities on Cd pollution to the paddy soils and rice grain in Hunan province, Central South China[J]. Environmental Monitoring and Assessment, 2013, 185(12): 9843-9856. doi: 10.1007/s10661-013-3296-y [2] TALABI A O, KAYODE T J. Groundwater pollution and remediation[J]. Journal of Water Resource and Protection, 2019, 11(01): 1. doi: 10.4236/jwarp.2019.111001 [3] WANG P, CHEN H, KOPITTKE P M, et al. Cadmium contamination in agricultural soils of China and the impact on food safety[J]. Environmental Pollution, 2019, 249: 1038-1048. doi: 10.1016/j.envpol.2019.03.063 [4] ZHANG S, CUI M, CHEN J, et al. Modification of synthetic zeolite X by thiourea and its adsorption for Cd (II)[J]. Materials Letters, 2019, 236: 233-235. doi: 10.1016/j.matlet.2018.10.100 [5] YAP M W, MUBARAK N M, SAHU J N, et al. Microwave induced synthesis of magnetic biochar from agricultural biomass for removal of lead and cadmium from wastewater[J]. Journal of Industrial and Engineering Chemistry, 2017, 45: 287-295. doi: 10.1016/j.jiec.2016.09.036 [6] ZHANG Y, CAO B, ZHAO L, et al. Biochar-supported reduced graphene oxide composite for adsorption and coadsorption of atrazine and lead ions[J]. Applied Surface Science, 2018, 427: 147-155. doi: 10.1016/j.apsusc.2017.07.237 [7] HAN C, WANG M, REN Y, et al. Characterization of pruned tea branch biochar and the mechanisms underlying its adsorption for cadmium in aqueous solution[J]. RSC Advances, 2021, 11(43): 26832-26843. doi: 10.1039/D1RA04235A [8] ZHANG G, QU J, LIU H, et al. CuFe2O4/activated carbon composite: a novel magnetic adsorbent for the removal of acid orange II and catalytic regeneration[J]. Chemosphere, 2007, 68(6): 1058-1066. doi: 10.1016/j.chemosphere.2007.01.081 [9] KARUNANAYAKE A G, TODD O A, CROWLEY M, et al. Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir[J]. Chemical Engineering Journal, 2018, 331: 480-491. doi: 10.1016/j.cej.2017.08.124 [10] LI S, LI S, WEN N, et al. Highly effective removal of lead and cadmium ions from wastewater by bifunctional magnetic mesoporous silica[J]. Separation and Purification Technology, 2021, 265: 118341. doi: 10.1016/j.seppur.2021.118341 [11] WANG S, ZHAO M, ZHOU M, et al. Biomass facilitated phase transformation of natural hematite at high temperatures and sorption of Cd2+ and Cu2+[J]. Environment International, 2019, 124: 473-481. doi: 10.1016/j.envint.2019.01.004 [12] WANG Q, LAI Z, MU J, et al. Converting industrial waste cork to biochar as Cu (II) adsorbent via slow pyrolysis[J]. Waste Management, 2020, 105: 102-109. doi: 10.1016/j.wasman.2020.01.041 [13] LIANG X, XIAO J, WENG W, et al. Electrochemical reduction of carbon dioxide and iron oxide in molten salts to Fe/Fe3C modified carbon for electrocatalytic oxygen evolution[J]. Angewandte Chemie International Edition, 2021, 60(4): 2120-2124. doi: 10.1002/anie.202013257 [14] Al-WABEL M I, Al-OMRAN A, El-NAGGAR A H, et al. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes[J]. Bioresource Technology, 2013, 131: 374-379. doi: 10.1016/j.biortech.2012.12.165 [15] RAFIQ M K, BACHMANN R T, RAFIQ M T, et al. Influence of pyrolysis temperature on physico-chemical properties of corn stover (Zea mays L. ) biochar and feasibility for carbon capture and energy balance[J]. PloS one, 2016, 11(6): e0156894. doi: 10.1371/journal.pone.0156894 [16] FAN G, JIANG Y, XIN J, et al. Facile synthesis of Fe@ Fe3C/C nanocomposites derived from bulrush for excellent electromagnetic wave-absorbing properties[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(23): 18765-18774. [17] TOMCZYK A, SOKOLOWSKA Z, BOGUTA P. Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects[J]. Reviews in Environmental Science and Biotechnology, 2020, 19(1): 191-215. doi: 10.1007/s11157-020-09523-3 [18] 孙俊芝, 王静霞, 倪茂君, 等. 改性海藻酸钠微球对Pb吸附性能的研究[J]. 环境科学与技术, 2019(7): 6. [19] KHAN Z H, GAO M, QIU W, et al. Mechanisms for cadmium adsorption by magnetic biochar composites in an aqueous solution[J]. Chemosphere, 2020, 246: 125701. doi: 10.1016/j.chemosphere.2019.125701 [20] KAKAVANDI B, JONIDI JAFARI A, REZAEI KALANTARY R, et al. Simultaneous adsorption of lead and aniline onto magnetically recoverable carbon: Optimization, modeling and mechanism[J]. Journal of Chemical Technology & Biotechnology, 2016, 91(12): 3000-3010. [21] AHMADI M, FOLADIVANDA M, JAAFARZADEH N, et al. Synthesis of chitosan zero-valent iron nanoparticles-supported for cadmium removal: characterization, optimization and modeling approach[J]. Journal of Water Supply:Research and Technology—Aqua, 2017, 66(2): 116-130. doi: 10.2166/aqua.2017.027 [22] HUANG P, YE Z, XIE W, et al. Rapid magnetic removal of aqueous heavy metals and their relevant mechanisms using nanoscale zero valent iron (nZVI) particles[J]. Water Research, 2013, 47(12): 4050-4058. doi: 10.1016/j.watres.2013.01.054 [23] KAKAVANDI B, KALANTARY R R, JAFARI A J, et al. Pb (II) adsorption onto a magnetic composite of activated carbon and superparamagnetic Fe3O4 nanoparticles: experimental and modeling study[J]. CLEAN–Soil, Air, Water, 2015, 43(8): 1157-1166. doi: 10.1002/clen.201400568 [24] TANG L, FENG H, TANG J, et al. Treatment of arsenic in acid wastewater and river sediment by Fe@ Fe2O3 nanobunches: The effect of environmental conditions and reaction mechanism[J]. Water research, 2017, 117: 175-186. doi: 10.1016/j.watres.2017.03.059 [25] LIU K, LI F, CUI J, et al. Simultaneous removal of Cd (II) and As (III) by graphene-like biochar-supported zero-valent iron from irrigation waters under aerobic conditions: synergistic effects and mechanisms[J]. Journal of Hazardous Materials, 2020, 395: 122623. doi: 10.1016/j.jhazmat.2020.122623 [26] LV X, HU Y, TANG J, et al. Effects of co-existing ions and natural organic matter on removal of chromium (VI) from aqueous solution by nanoscale zero valent iron (nZVI)-Fe3O4 nanocomposites[J]. Chemical Engineering Journal, 2013, 218: 55-64. doi: 10.1016/j.cej.2012.12.026 [27] MAK M S H, RAO P, LO I M C. Effects of hardness and alkalinity on the removal of arsenic (V) from humic acid-deficient and humic acid-rich groundwater by zero-valent iron[J]. Water research, 2009, 43(17): 4296-4304. doi: 10.1016/j.watres.2009.06.022 [28] TANBOONCHUY V, GRISDANURAK N, LIAO C H. Background species effect on aqueous arsenic removal by nano zero-valent iron using fractional factorial design[J]. Journal of hazardous materials, 2012, 205: 40-46. [29] TYROVOLA K, NIKOLAIDIS N P, VERANIS N, et al. Arsenic removal from geothermal waters with zero-valent iron—effect of temperature, phosphate and nitrate[J]. Water Research, 2006, 40(12): 2375-2386. doi: 10.1016/j.watres.2006.04.006 [30] LIU R, LIU F, HU C, et al. Simultaneous removal of Cd (II) and Sb (V) by Fe–Mn binary oxide: Positive effects of Cd (II) on Sb (V) adsorption[J]. Journal of hazardous materials, 2015, 300: 847-854. doi: 10.1016/j.jhazmat.2015.08.020 [31] GROSVENOR A P, KOBE B A, BIESINGER M C, et al. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds[J]. Surface and Interface Analysis:An International Journal devoted to the development and application of techniques for the analysis of surfaces, interfaces and thin films, 2004, 36(12): 1564-1574. [32] HU Y, JENSEN J O, ZHANG W, et al. Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts[J]. Angewandte Chemie, 2014, 126(14): 3749-3753. doi: 10.1002/ange.201400358 [33] LI J, LAN H, LIU H, et al. Intercalation of nanosized Fe3C in iron/carbon to construct multifunctional interface with reduction, catalysis, corrosion resistance, and immobilization capabilities[J]. ACS applied materials & interfaces, 2019, 11(17): 15709-15717. [34] MISHRA D, FARRELL J. Understanding nitrate reactions with zerovalent iron using tafel analysis and electrochemical impedance spectroscopy[J]. Environmental science & technology, 2005, 39(2): 645-650. [35] ZHU S, ZHAO J, ZHAO N, et al. Goethite modified biochar as a multifunctional amendment for cationic Cd (II), anionic As (III), roxarsone, and phosphorus in soil and water[J]. Journal of Cleaner Production, 2020, 247: 119579. doi: 10.1016/j.jclepro.2019.119579 [36] WAN S, YU C, LI Y, et al. Highly selective and ultrafast removal of cadmium and copper from water by magnetic core-shell microsphere[J]. Chemical Engineering Journal, 2021, 405: 126576. doi: 10.1016/j.cej.2020.126576 [37] LIU C M, DIAO Z H, HUO W Y, et al. Simultaneous removal of Cu2+ and bisphenol A by a novel biochar-supported zero valent iron from aqueous solution: Synthesis, reactivity and mechanism[J]. Environmental pollution, 2018, 239: 698-705. doi: 10.1016/j.envpol.2018.04.084 -




 下载:
下载: