-
近年来,抗生素等药品和个人护理品(pharmaceutical and personal care products, PPCPs)在地表水和地下水环境样品中不断被检出,这说明其在水生生态系统中存在越来越广泛的分布[1-4],对水生生物和人类健康构成潜在威胁[5-6]。作为PPCP的典型代表之一,磺胺甲恶唑(sulfamethoxazole, SMX)是一种广泛应用的人、兽两用的磺胺类抗生素,由于其消费量巨大且不易降解,导致其在环境水体中广泛赋存[7],从而存在诱发细菌产生抗生素耐药基因的风险[8-9]。因此,开发SMX的有效降解方法很有必要。
高级氧化工艺(advanced oxidation processes, AOPs)是一种通过催化产生自由基等强活性氧物质(reactive oxygen species,ROS)来处理有机污染物的有效方法[10-13]。硫酸根自由基(SO4·−)由于具有较高的氧化还原电位(2.5~3.1 eV)、较长的半衰期(30~40 μs)和对芳烃环污染物具有较强的选择性氧化能力,近年来基于硫酸根自由基的高级氧化(SO4·−-based AOPs, SR-AOPs)技术在降解毒性有机污染物的研究中备受关注[14-15]。过一硫酸盐(peroxymonosulfate, PMS),可通过光化学、声化学和热活化、过渡金属(如钴、铁、银和铜)、碳质材料、电化学活化、碱性条件和氧化剂(如臭氧、过氧化氢、和过氧化钙)等多种途径破坏(氢化)过氧化键(O-O)活化生成SO4•−[15-16]。其中过渡金属钴(Co)活化PMS是目前产生SO4·−的最为高效的一种方法。有研究表明,Co(Ⅱ)/PMS体系在中性pH条件下的催化性能优于传统的芬顿(Fenton)反应,且试剂用量更少[17]。然而,直接使用Co(Ⅱ)作为均相催化剂通常存在可循环性低、Co(Ⅱ)污泥排放存在潜在风险等缺点[11]。为提高催化活性、稳定性和循环使用性能,通常采用其他载体负载Co来制备多相催化剂。常见的载体有碳材料、粘土、沸石和其他金属氧化物等。
近年来,基于静电纺丝工艺制备的碳纤维(carbon nanofibers, CNFs)材料因其制备方法简单、材料比表面积大、电子传输性能好引起了众多研究者的关注[18-19]。CNFs具有较高的长径比,有利于电子传输和反应物扩散,以此为载体可表现出优异的催化活性。目前,钴掺杂碳纳米纤维作为SR-AOPs的高效多相催化剂已有报道[18, 20]。LIN等[21]和ZHANG等[22]发现,负载Co与其他金属元素(Fe和Ag)的双金属纳米颗粒碳纳米纤维,在低剂量下表现出比单金属催化剂更好的性能,且能减少金属离子的浸出。然而,Co/Fe体系通常表现出高磁性和团聚,因此,不可避免地降低了催化活性[23-24];Co/Ag体系应用成本较高,不利于催化剂的广泛使用。Ti也是一种过渡金属,其氧化物具有良好的催化性,在水溶液中可产生氧自由基和羟基自由基等活性基团,当与Co复合时,有望进一步改善Co基催化剂的相关性能。
因此,本研究采用静电纺丝技术,以聚丙烯腈(PAN)为前驱体,适用一步法制备了Co/TiO2@CNFs复合纳米纤维薄膜,通过调整Co和TiO2的复合比例,优化了复合纤维膜的最佳合成工艺;以磺胺甲恶唑(SMX)作为目标污染物,考察了Co/TiO2@CNFs活化PMS降解SMX的效能,且探讨了可能的降解途径及机理。
-
磺胺甲恶唑(SMX)、聚丙烯腈(PAN, FW为150 000 Da)、N, N-二甲基甲酰胺(DMF)和过硫酸氢钾复合盐(Oxone, KHSO5·0.5KHSO4·0.5K2SO4)、对苯醌(p-BQ)、糠醇(FFA)、L-组氨酸(L-histidine)均购自Sigma-Aldrich公司(St. Louis, MO, USA);六水硝酸钴(Co(NO3)2·6H2O)、二氧化钛(TiO2)、氢氧化钠(NaOH)、硫酸(H2SO4)、叔丁醇(TBA)和甲醇(methanol)均购自中国医药化学试剂有限公司(上海);所用材料均为分析纯。高效液相色谱(HPLC)级乙腈(acetonitrile)、甲醇(methanol)和甲酸(methanoic acid)由J.T. Baker (Phillipssburg, NJ, USA)公司购得。使用Millipore Milli-Q Direct 8/16净水系统(中国长沙)制备去离子水(<18.3 MΩ·cm−1)。
-
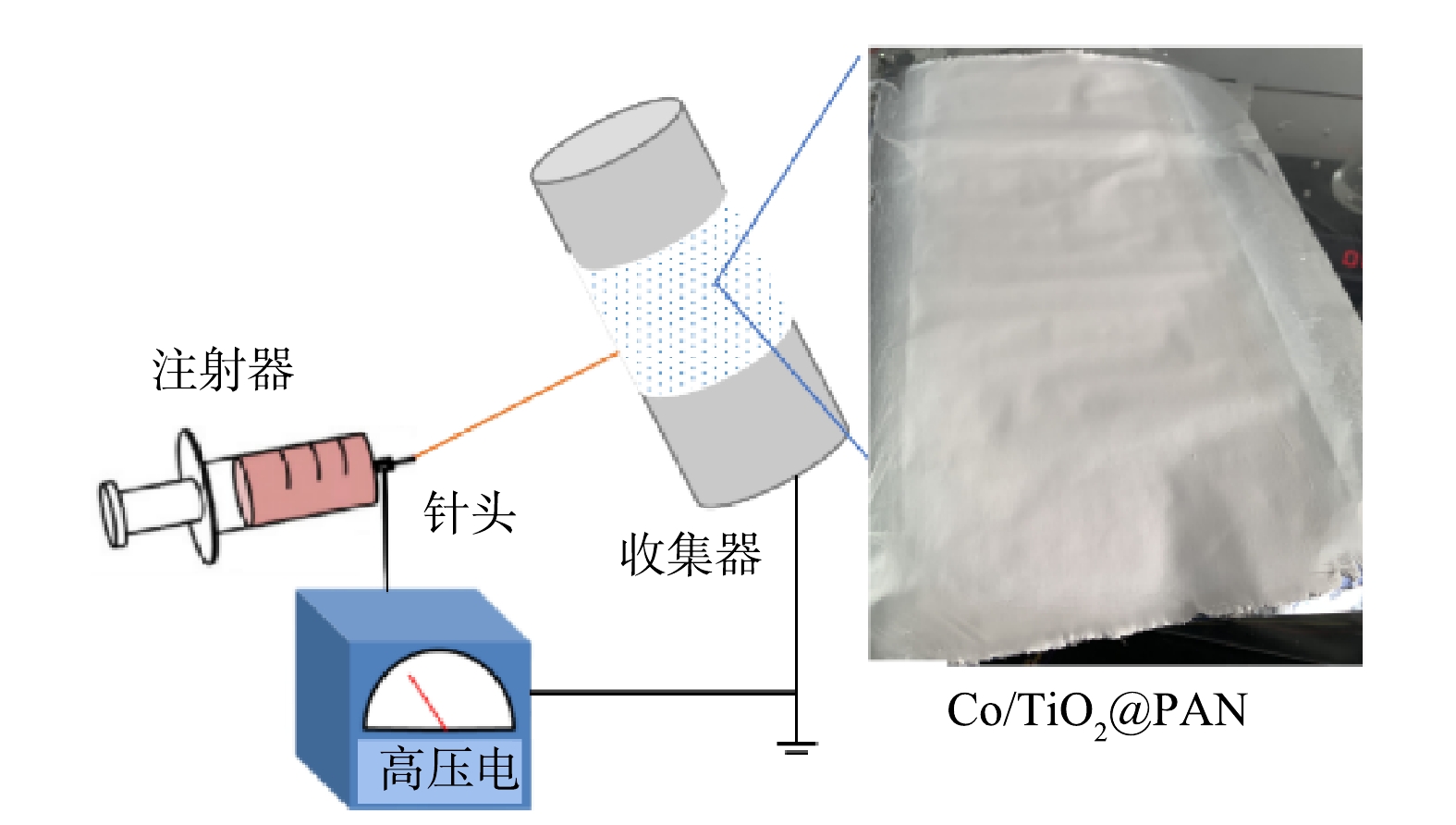
将2.60 g PAN聚合物粉末溶解在25 g DMF溶液中,室温下磁力搅拌至溶液清澈透明。加入0.032 g TiO2,超声分散4 h后添加0.55 g Co(NO3)2·6H2O,充分混合后配得纺丝前驱液。如图1所示,将前驱液转移至注射器,配置1.2 mm×38 mm规格不锈钢针头,在0.4 mL·h−1推进速率、16 KV高压电、6 cm间距条件下进行静电纺丝,同步制备Co/TiO2@PAN纳米纤维膜。对上述所制备的膜进行热处理:在240 ℃空气中煅烧1 h后再在N2保护下600 ℃煅烧3 h。纳米纤维被标记为X Co/TiO2@CNFs,其中X代表钛的质量千分比,本研究采用的催化剂1 Co/TiO2@CNFs简称为Co/TiO2@CNFs。
-
催化剂活化PMS降解SMX实验在100 mL玻璃烧杯中以批实验进行。各因素实验条件为:催化剂质量浓度(0、0.05、0.10和0.20 g·L−1),PMS浓度(0、0.25、0.5、1.0和2.0 mmol·L−1),水温(15、20、25、30和40 ℃),pH(3、5、7、9、11和未调节值5.8)进行性能测试。采用水浴进行温度控制,用0.1 mol·L−1 H2SO4和NaOH溶液调节溶液pH。催化降解实验:将5 mg催化剂投加到50 mL SMX溶液(40 μmol·L−1)中,搅拌3 min至均匀分散,后投加适量的PMS至所需浓度。水样定时采样,经0.22 μm滤膜过滤后再进行甲醇淬灭反应,最终鉴定SMX及降解产物。淬灭实验:先在SMX溶液中分别加入甲醇(MeOH)、叔丁醇(TBA)、对苯醌(p-BQ)、糠醇(FFA)或L-组氨酸(L-histidine)等淬灭剂后采用相同的催化降解步骤。循环测试实验:将使用过的催化剂,经膜过滤后用去离子水和甲醇各洗涤3次,60 ℃烘干待用。
-
采用扫描电子显微镜(SEM, Hitachi s-4800, Japan)和透射电子显微镜(TEM, Feitecnai G2 F30, USA)表征材料的形貌和微观结构;采用能量色散X射线光谱仪(NS7, Thermo Fisher Scientific, USA)与TEM联用表征材料的元素组成;采用X射线衍射仪(D8 Advance, Germany)进行材料的晶格分析;采用傅里叶红外光谱仪(Nexus-470, Thermo Nicolet, USA)表征材料表面官能团化学键结构;采用规格/型号为*inVia Qontor/ inVia Qontor仪器进行拉曼分析;采用热重分析仪(TA Instruments, USA)进行材料热重分析;采用Autosorb-iQ自动气体吸附分析仪(Quanta Chrome Instruments, USA)测量材料的比表面积和孔径分布;采用纳米粒度电位仪(Zetasizer Nano ZS, Malven Instruments, UK)进行Zeta电位分析;采用X射线光电子能谱仪(ESCALAB 250Xi, Thermo Fisher Scientific)表征材料的化学组成和化学状态。
采用高效液相色谱仪(HPLC, Agilent 1260, USA),配置XDB-C18柱(150 mm × 4.6 mm, i.d., 5 μm particle,Waters, USA)和VWD检测器分析SMX浓度。检测条件设置为:流动相采用甲醇和水体积比为55∶45;流速采用0.8 mL·min−1;柱温控制为35 ℃;波长为275 nm。使用电感耦合等离子体质谱仪(ICP-MS, Agilent 7700, USA)测定溶液中金属离子浓度。采用高效液相色谱-电喷雾电离串联质谱(HPLC-ESI-MS/MS)结合TSQ Quantum Access MAX设备(Thermo, USA),配备反相C18分析柱(100 mm × 2.1 mm, 5 μm, Thermo, USA) 对SMX降解中间产物进行鉴定,样品在分析前用MCX (Anpel, China)固相萃取柱提取。采用从50到400 m·z−1全扫描质量方法下的正模式电喷雾电离(ESI +)分析产物中间体,柱温度30 ℃,流量0.15 mL·min−1,流动相是A(乙腈)和B(0.1%甲酸水)。用电子顺磁共振波谱仪(Bruker ESP300E, Germany)进行活性物种鉴别。
活化PMS降解SMX参数采用假一级动力学方程(式(1))进行分析。采用阿伦尼乌斯(Arrhenius)方程(式(2))研究kapp与不同温度之间的关系。
式中:Ct为t时刻污染物浓度,μmol·L−1;C0为污染物初始浓度,μmol·L−1;kapp为表观动力学常数,min−1;t为时间,min。
式中:A为指前因子,g·(mg·min)−1;Ea为SMX降解的活化能,kJ·mol−1;R为通用气体常数,8.314 J·(mol·K)−1;T为溶液的温度,K。
-
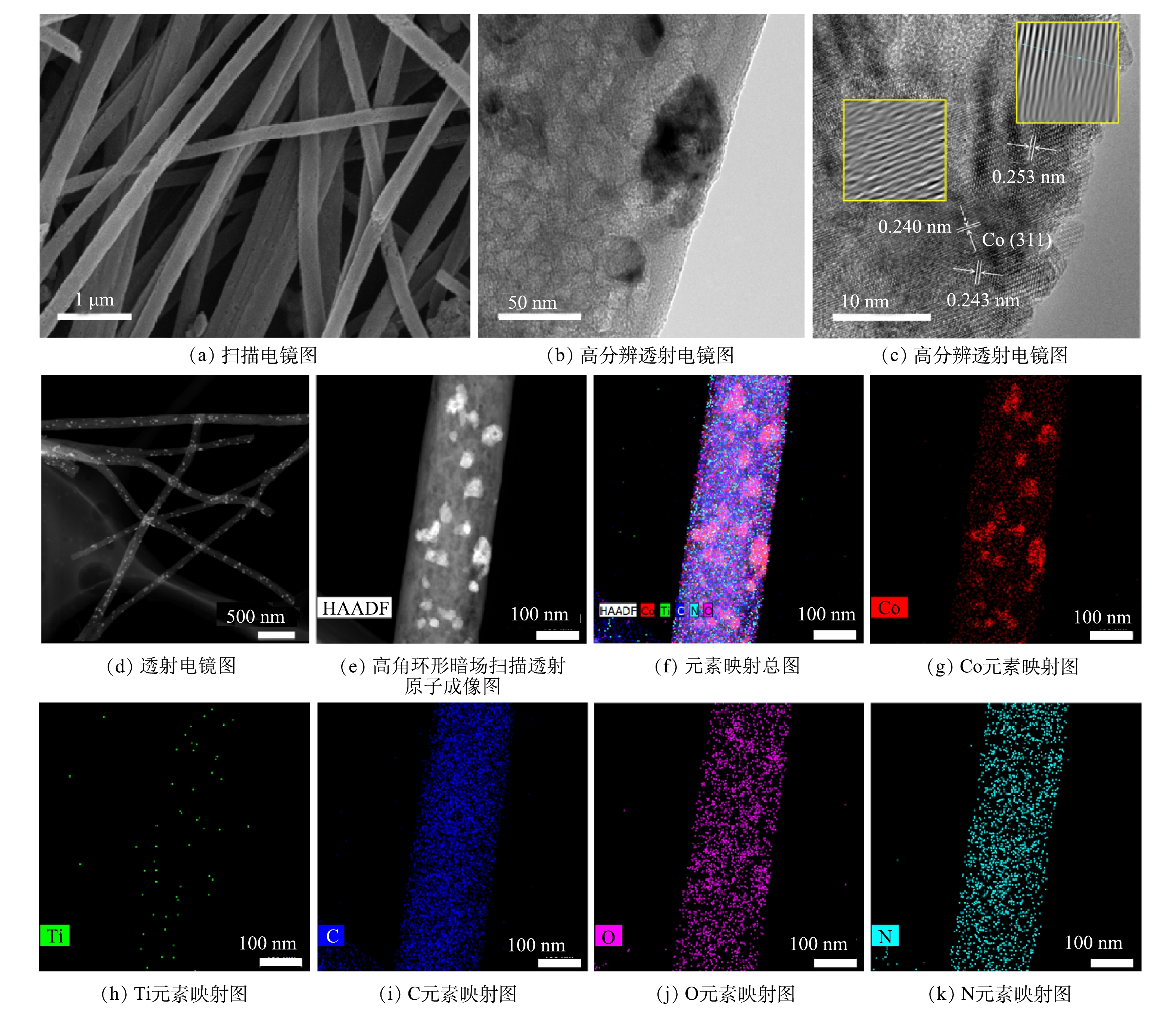
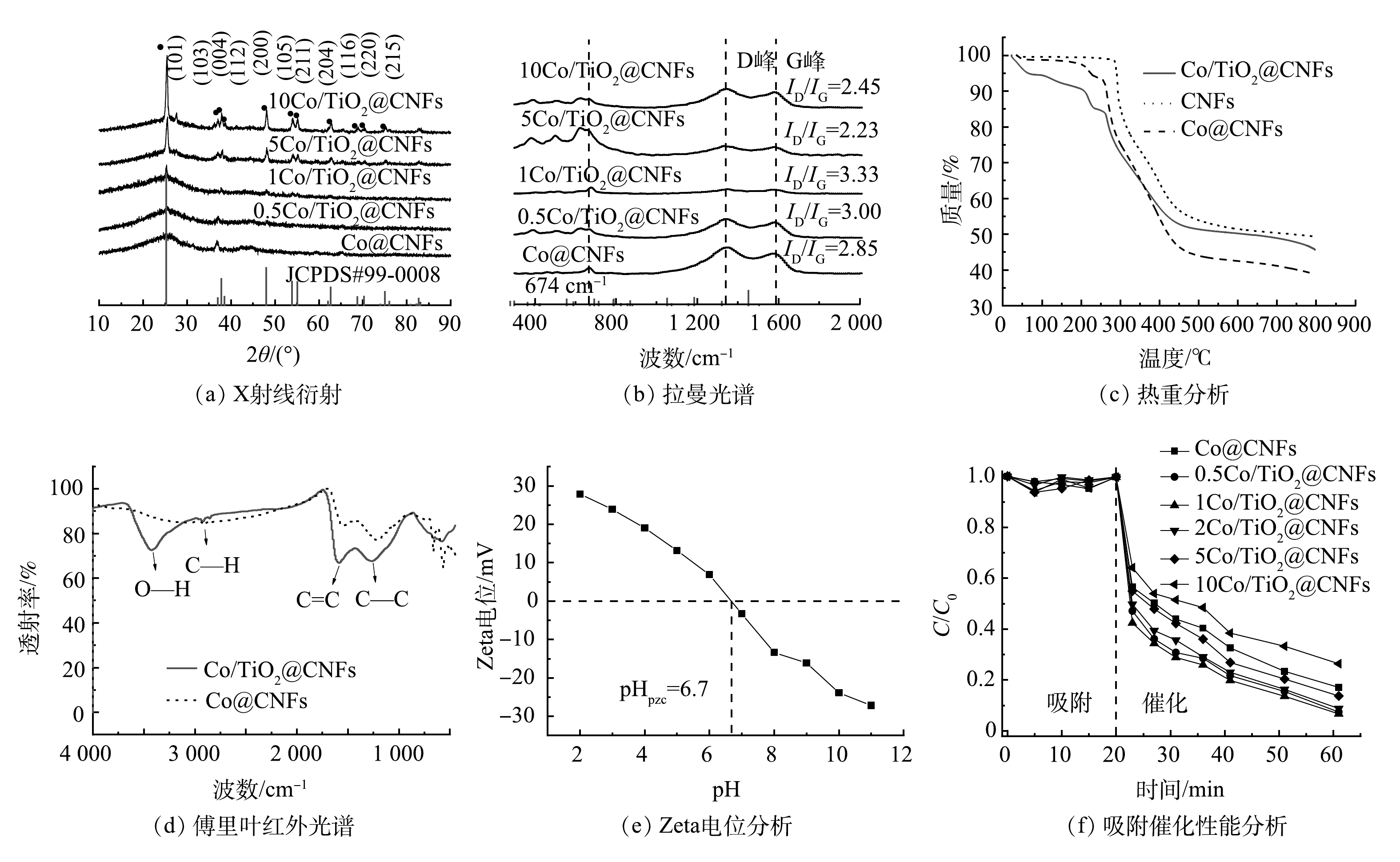
通过SEM、HRTEM、TEM、HAADF-STEM和元素映射对Co/TiO2@CNFs的形貌进行了表征(图2)。由图2(a)可以发现,所制备的材料是平均直径为200~300 nm、相互交织形成开放网状结构的多孔纤维。图2(b)显示,纤维表面负载了颗粒状固体。由图2(c)可见,在材料表面存在晶格条纹间距为0.240~253 nm的钴氧化物(Co3O4),但分布不均匀。在图2(d)中可以看到纤维表面有很多亮点分布。进一步采用高角环形暗场扫描透射原子成像(HAADF-STEM)可发现:这些亮点以亮斑呈现(图2(e))。元素映射分析结果表明(图2(f)~(k)),这些亮斑与Co的分布完美对应, 而O的分布在对应Co的位置也明显明亮。综上所述,由于Ti、N及C在纤维表面分布均匀,因此,不难推测,除了一部分与Ti结合的O(TiO2)及与N结合的O(Co(NO3)2)在表面均匀分布外,还有一部分很可能在局部与Co形成了氧化物。图3(a)是不同成分含量的Co/TiO2@CNFs的XRD测试结果,随着TiO2含量的增加,在(101)、(103)、(004)、(112)、(200)、(105)、(211)、(204)、(116)、(220)、(215)(JCPDS#99-0008)处的TiO2特征衍射峰逐渐增加,这进一步证实了TiO2成功复合在CNFs上。XRD未检测到显著的Co特征峰,可能是材料中Co的结晶度较低,或其特征峰较弱而被TiO2特征峰掩盖。Co@CNFs和X Co/TiO2@CNFs的拉曼光谱如图3(b)所示,在674 cm−1附近的拉曼波段代表晶体Co3O4的A1g模态[25],证实该晶体成功复合到CNFs上。
-
根据拉曼光谱(图3(b)),5种碳材料在1 350 cm−1和1 575 cm−1附近都有显著的特征峰,即D峰和G峰。并且,Co@CNFs、0.5Co/TiO2@CNFs、1Co/TiO2@CNFs、5Co/TiO2@CNFs和10Co/TiO2@CNFs的ID/IG值分别为2.85、3.00、3.33、2.23和2.45,以1Co/TiO2@CNFs的ID/IG值最高,5Co/TiO2@CNFs最低。ID/IG越大,表明晶体缺陷越多,越有利于催化氧化。
相关材料的热重分析(TGA)结果如图3(c)所示。与CNFs相比,Co/TiO2@CNFs和Co/CNFs在100~300 ℃内表现出持续失重。当温度为300~400 ℃时,催化剂的失重更加明显,这可能是由该温度范围内挥发性成分的脱除、脱氢和环化所致[18]。由于Co和TiO2的添加会引起CNFs的缺陷增加,从而导致该温度范围内的脱氢、环化等化学反应增加,因此,表现出比CNFs更明显的失重。在450~800 ℃内,Co/TiO2@CNFs热稳性比Co@CNFs更好,说明掺杂TiO2可提高Co@CNFs的稳定性。
对比Co@CNFs和Co/TiO2@CNFs的FTIR表征结果(图3(d))可发现新增O—H在3 425 cm−1处及C—H在2 919 cm−1处的伸缩振动特征吸收峰;而苯环C=C在1 588 cm−1处以及C—C在1 266 cm−1处的伸缩振动特征吸收峰得到强化。说明添加TiO2后,其碳骨架形成了石墨平面网状层稳定结构,表面含有O—H官能团。图3(e)为Co/TiO2@CNFs 在不同pH下的Zeta电位变化。可见Co/TiO2@CNFs的pHPZC为6.7。因此,当pH低于6.7时,催化剂的表面电荷为正电荷,反之为负电荷。
Co@CNFs和XCo/TiO2@CNFs的N2吸附/脱附等温线分析结果如表1所示。其中1Co/TiO2@CNFs的比表面积为97.15 m2·g−1,总孔容为0.092 cm3·g−1,t-图法微孔为0.018 cm3·g−1,介孔体积是0.074 cm3·g−1,BJH孔径确定为3.93 nm。上述结果表明,1Co/TiO2@CNFs在5种催化剂中具有最大的比表面积和总孔容。
通过以上表征分析可知,1Co/TiO2@CNFs相比其他催化剂缺陷度更多、比表面积更大、总孔容更大,理论上应具有更好的催化性能。
-
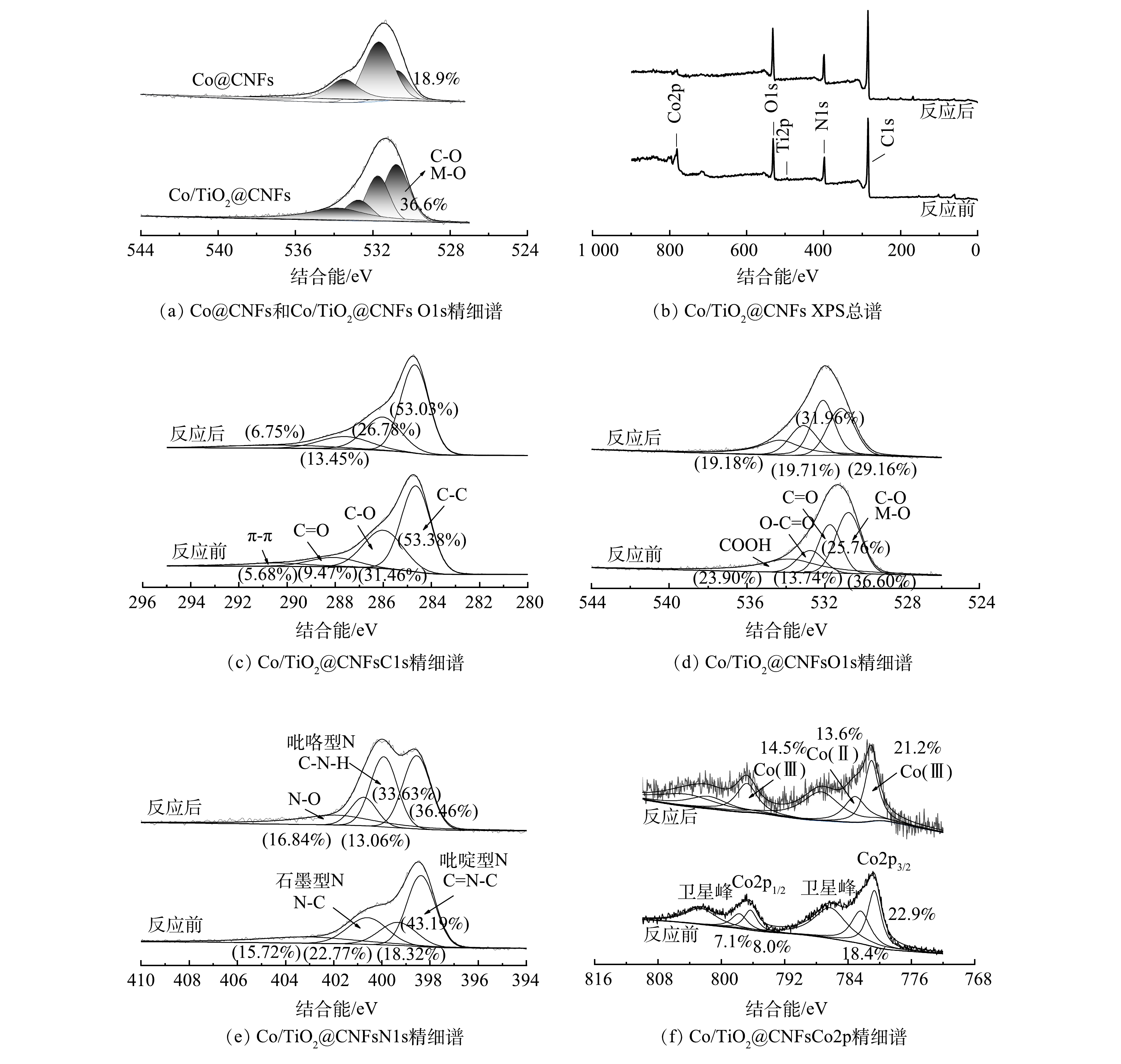
图3(f)为XCo/TiO2@CNFs对SMX的吸附和催化降解效果。前20 min未添加PMS,SMX未出现明显下降,说明该类催化剂对水中的SMX吸附作用几乎可以忽略。20 min后向溶液中加入PMS,SMX浓度迅速下降,60 min时Co@CNFs、0.5Co/TiO2@CNFs、1Co/TiO2@CNFs、2Co/TiO2@CNFs、5Co/TiO2@CNFs、10Co/TiO2@CNFs对SMX的降解率分别为82.9%、92.6%、93.3%、91.2%、86.3%、73.6%。上述结果表明,当催化剂中TiO2添加量从0.5增至5时,催化剂对SMX的降解率分别提升了11.7%、12.5%、10.0%、4.1%。当TiO2添加量为1时,即1Co/TiO2@CNFs活化PMS降解SMX的效果最好。根据之前的表征结果推测,可能原因如下:1) 1Co/TiO2@CNFs具有最大的比表面积,而催化剂的比表面积越大,活性位点越多;2) 1 Co/TiO2@CNFs中碳晶体缺陷度最大,有利于电子转移可促进PMS活化;3) FTIR光谱表明Co/TiO2@CNFs的表面有大量OH基团,可促进催化剂表面Co-OH配合物的形成,从而促进了PMS的多相活化[26-27]。从XPS O1s精细谱对比分析(图4(a))中可以看出,Co/TiO2@CNFs的C-O/Co-O多于Co@CNFs。结合图4(c)~(d),反应后C-O/Co-O有所降低,这可以看出表面Co-OH配合物在降解反应中发挥了重要作用。当TiO2添加量为10时,其催化性能反而略低于Co@CNFs,表明过多的TiO2会导致复合催化剂性能降低。这可能是由于添加过多TiO2后,存在团聚或包覆等问题导致Co3O4作用降低。因此,后续实验催化剂均选用1Co/TiO2@CNFs。
-
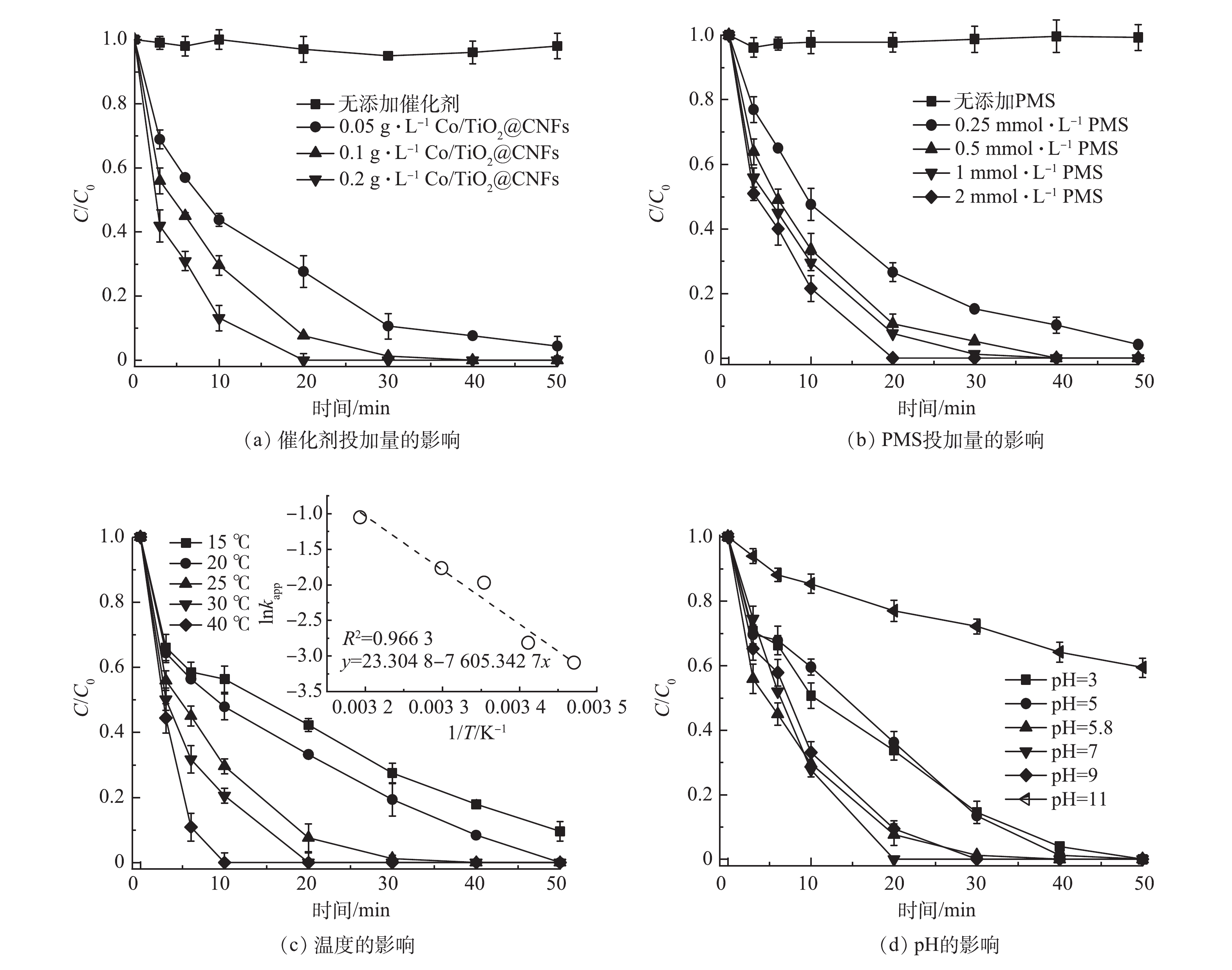
催化剂投加量对SMX降解的影响如图5(a)所示。在PMS浓度为1 mmol·L−1, 反应温度为 25 ℃条件下,当仅添加PMS时,SMX在50 min内几乎无法降解,说明PMS难以自分解去除SMX。当Co/TiO2@CNFs投加量从0.05 g·L−1增至0.2 g·L−1,对应的由式(1)求得的降解动力学常数kapp从0.065 8 min−1提升至0.206 4 min−1。这是因为催化剂越多,可提供的活化PMS位点越多,从而可提升SMX的降解率。
图5(b)反映了PMS剂量对SMX降解的影响。可见,反应动力学和SMX的降解程度受到PMS浓度变化的影响。未添加PMS时,SMX的浓度无明显下降,表明催化剂无吸附效果,这与上述结论一致。当PMS剂量由0.25 mmol·L−1增加到2 mmol·L−1,反应动力学常数 kapp由0.061 8 min−1增长到0.157 6 min−1。这可能是由于PMS剂量越多,PMS与催化剂表面的碰撞概率增大并产生更多活性物种,从而提升了降解效率。
图5(c)反映了温度对SMX降解效果的影响。当温度从15 ℃增至40 ℃时,对应kapp从0.045 4 min−1升至0.349 1 min−1。此结果表明,温度升高可加速SMX的催化降解速率。这是因为温度升高增加了溶液中分子的碰撞概率,促进了PMS的活化,增加活性物质的产生。采用阿伦尼乌斯(Arrhenius)方程(式(2))研究了kapp值与温度之间的关系。由图5(c)中的插图可见,数据点的线性回归拟合较好,计算可得Ea为63.23 kJ·mol−1。
图5(d)反映了pH对SMX降解的影响。SMX溶液的自然pH为5.8,当pH降至3和升高至11时,SMX的降解kapp分别从0.139 6 min−1降为0.071 8 min−1和0.011 0 min−1。可见,SMX降解的最佳pH为5.8~9,而酸性和碱性过强均不利于SMX的降解。其原因归为以下4点。1)在强酸条件下,·OH和SO4·−可被H+灭活(式(3)~式(4))[28-29],从而抑制了PMS的活化和SMX的降解。酸性条件也可抑制CoOH+络合物的形成,从而降低了SO4·−的生成[8]。2)PMS在酸性或中性条件下主要存在形式是HSO5−,活化生成的主要物质是SO4·−。而强碱性条件下主要存在形式是SO52−,其催化活性较低[11]。3) SMX在不同的pH下分别呈现质子化、非质子化和去质子化形式(阳离子、中性和阴离子形式),pKa1为1.6,pKa2为5.7[1, 30]。当pH为3.0时,SMX以非质子化SMX为主;当pH增加到7.0时,SMX主要存在形式为去质子化SMX。Zeta电位结果表明,Co/TiO2@CNFs的pHpzc为6.7(图3(e))。因此,在弱酸性pH下,带负电的去质子化SMX与带正电的催化剂表面的亲和力更强,催化反应效率能得到提升[18];而当碱性pH时,催化剂表面带负电,不利于PMS活化反应的发生。4)过于碱性环境会导致催化剂表面形成覆盖表面活性位点的CoOH+络合物,从而增加SMX与催化剂之间的静电斥力。
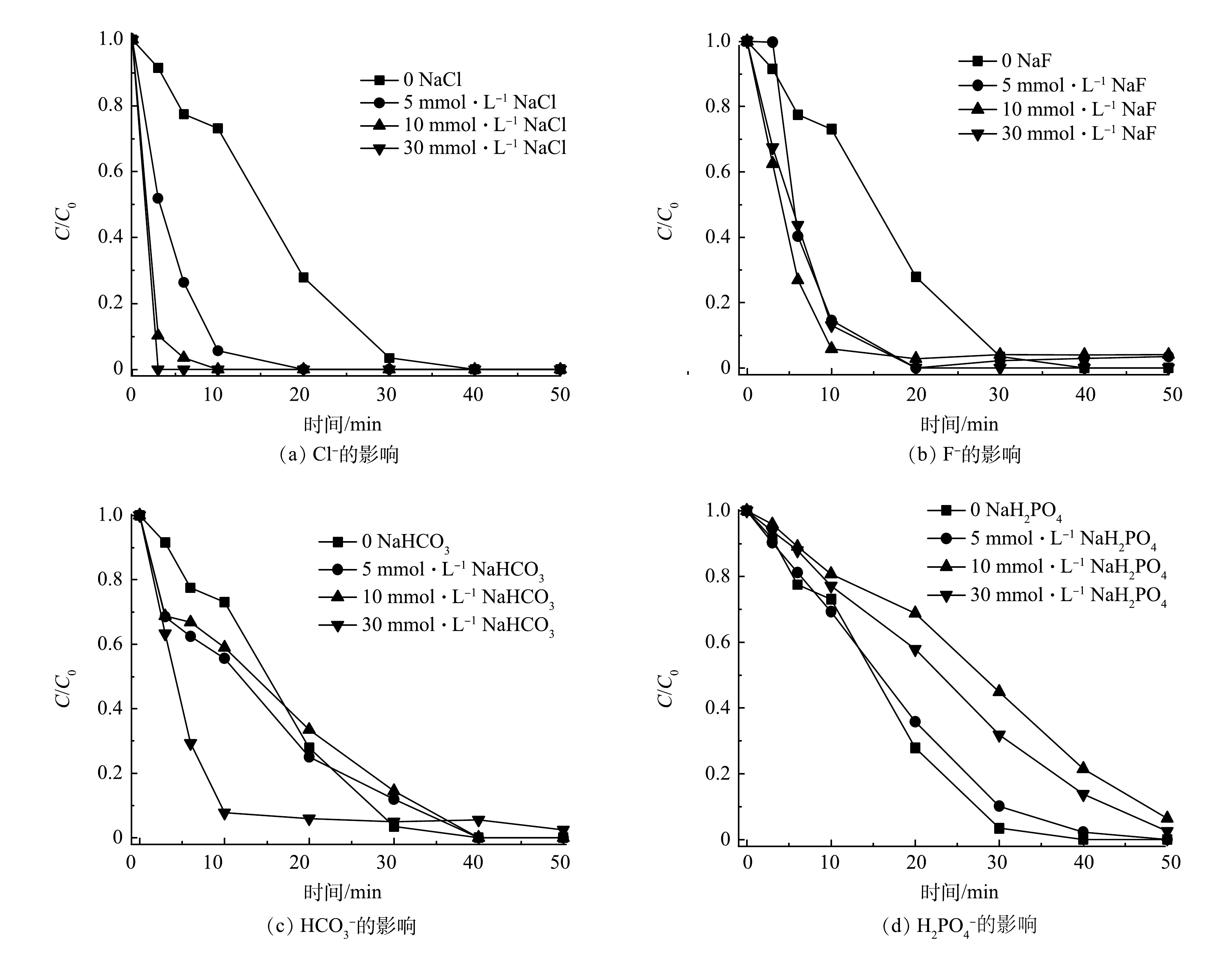
图6反映了共存的无机离子Cl−、F−、HCO3−和H2PO4−对SMX降解的影响。如图6(a)所示,当Cl−为0~30 mmol·L−1时,SMX在3 min内即可完全降解。这可能是Cl−导致SO4·−和·OH产生2.4 V高氧化还原电势的氯自由基Cl•[31-32],生成的Cl·还可与Cl−反应生成Cl2·−(式(5)~式(8))[32-33]。此外,PMS与Cl−反应可生成氧化性氯离子,如HOCl和Cl2 (式(9)~式(10)),Cl·与水反应可生成·OH降解SMX(式(11))[28]。图6(b)表明,F−的添加与Cl−对SMX降解具有相似的促进影响,这可能是因为两者具有相似的卤素元素化学特征。
由图6(c)可知,当HCO3−浓度增加到30 mmol·L−1时,SMX降解反应速率明显加快。这可能是HCO3−的缓冲能力抑制了H+对SO4·−和·OH的捕获[11]。由图6(d)可见,当NaH2PO4为0~30 mmol·L−1时,SMX的降解率有所下降。这可能是H2PO4−和SO4·−或·OH反应形成H2PO4·(式(12)~式(13)),但在50 min时的降解率仍然超过90%。此外,H2PO4−由于与固体表面的强亲和力,易在催化剂表面形成络合物[8,34],这可能导致Co/TiO2@CNFs上的活性位点降低。
-
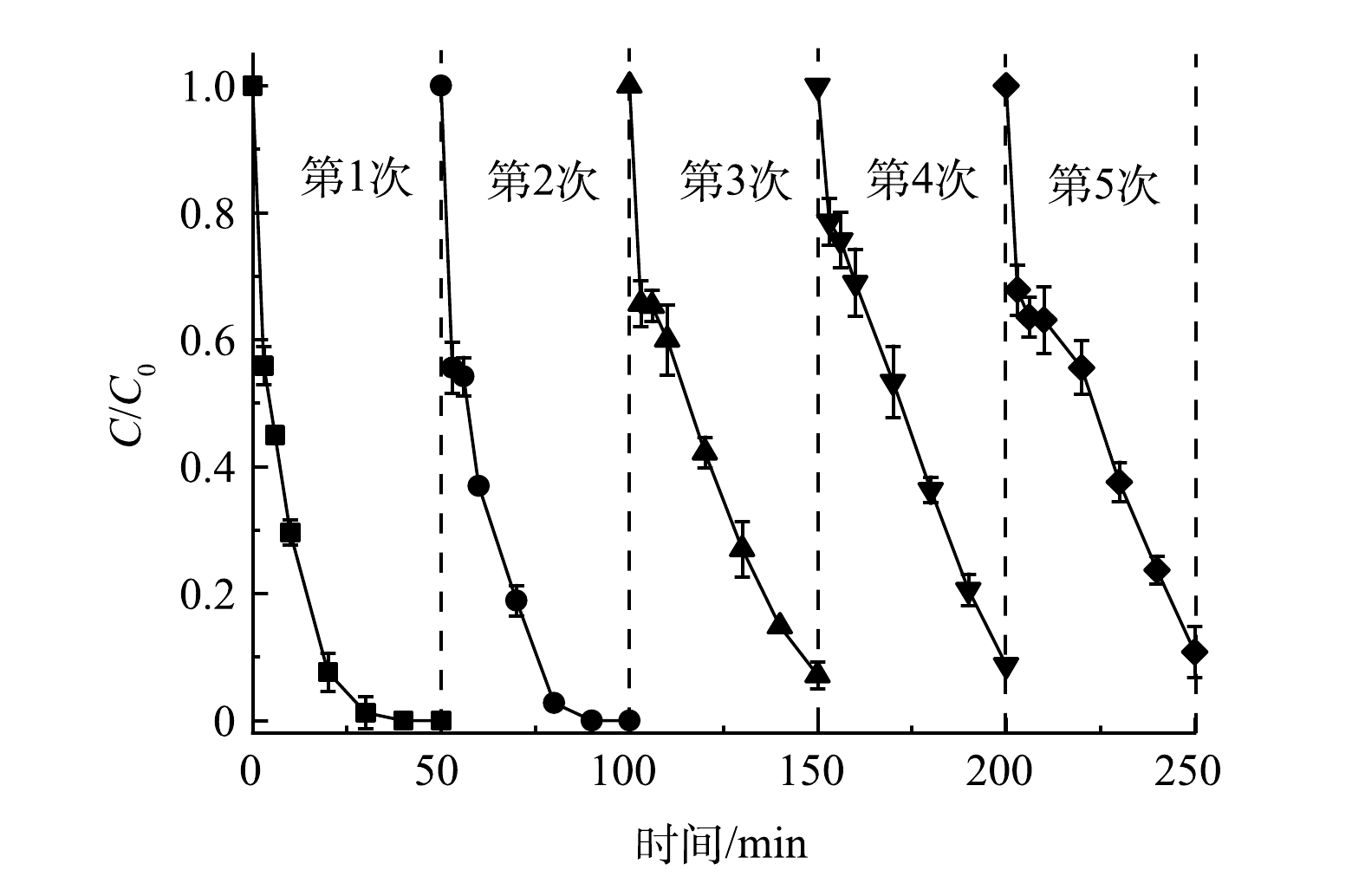
通过循环实验研究了Co/TiO2@CNFs的可重复利用性。如图7所示,在5次循环降解实验中,Co/TiO2@CNFs对SMX的降解率分别为≥99%、99%、93%、91%、89%,对应降解动力学常数kapp分别为0.139 6、0.108 2、0.049 0、0.042 2、0.039 4 min−1。虽然SMX的去除率随着Co/TiO2@CNFs使用频率的增加而有所下降,但经5次循环后的SMX去除率仍保持在较高水平。这可能归因于以下2点。1) 材料表面部分活性位点被降解中间体屏蔽。如图4(e)所示,催化剂表面吡咯型N及C-N-H含量在反应后有所上升,表明催化剂表面活性位点可能存在降解中间体屏蔽。2)材料表面的部分Co金属泄漏。经ICP-MS测试可知,Co@CNFs和Co/TiO2@CNFs在催化反应后溶液中Co泄漏量分别为0.40 mg·L−1和0.36 mg·L−1,均低于1.0 mg·L−1,满足《城镇污水处理厂污染物排放标准》(GB 18918-2002)及《地表水环境质量标准》(GB 3838-2002)的规定要求。该泄漏量也与BAO等[18]制备的5Co/CNF和8Co/CNF在反应120 min后Co泄漏量(0.31 mg·L−1和0.52 mg·L−1)和WANG等[35]制备的Co/Co9S8@N-S-OC在反应250 min后Co泄漏量(0.52 mg·L−1)相当,其泄漏量相对不高,说明稳定性较好,且Co/TiO2@CNFs相比Co@CNFs性能更优。
-
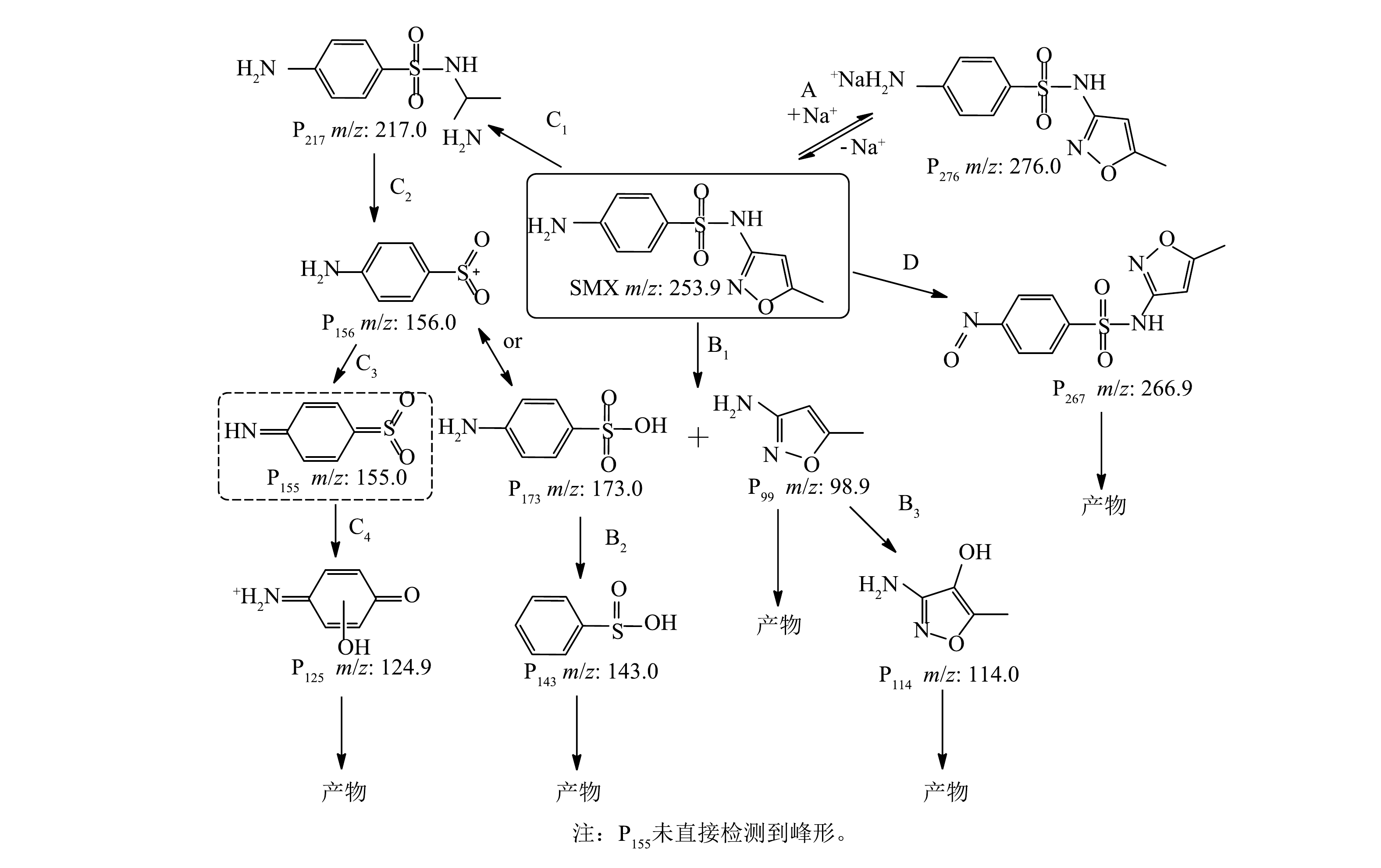
通过液相和质谱联用鉴定出可能的中间产物共10种。根据质荷比(m/z)推测其化学分子式和结构式,进一步分析SMX在Co/TiO2@CNFs活化PMS体系中降解的可能转化途径。中间产物用P和质荷比组合的方式来表述。如图8所示,在路径A中,PMS中的Na+可以与SMX结合形成P276;在路径B中,通过ROS使S—N键断裂而产生P173和P99,即4-氨基-苯磺酸盐和3-氨基-5-甲基-异恶唑[11,36]。此外,自由基可将S—N断裂也可能产生P156和P99[8]。P173通过羟基化作用和氨基的丢失可被进一步氧化生成P143[36]。在路径C中,SMX中异恶唑环氧化开环后形成P217,S—N键进一步发生断裂,产生P156或P173,P156可进一步氧化生成P125。路径D是SMX苯环上的氨基被自由基氧化生成P267和亚硝基衍生物[37],P267可以进一步氧化得到其他小分子产物。
-
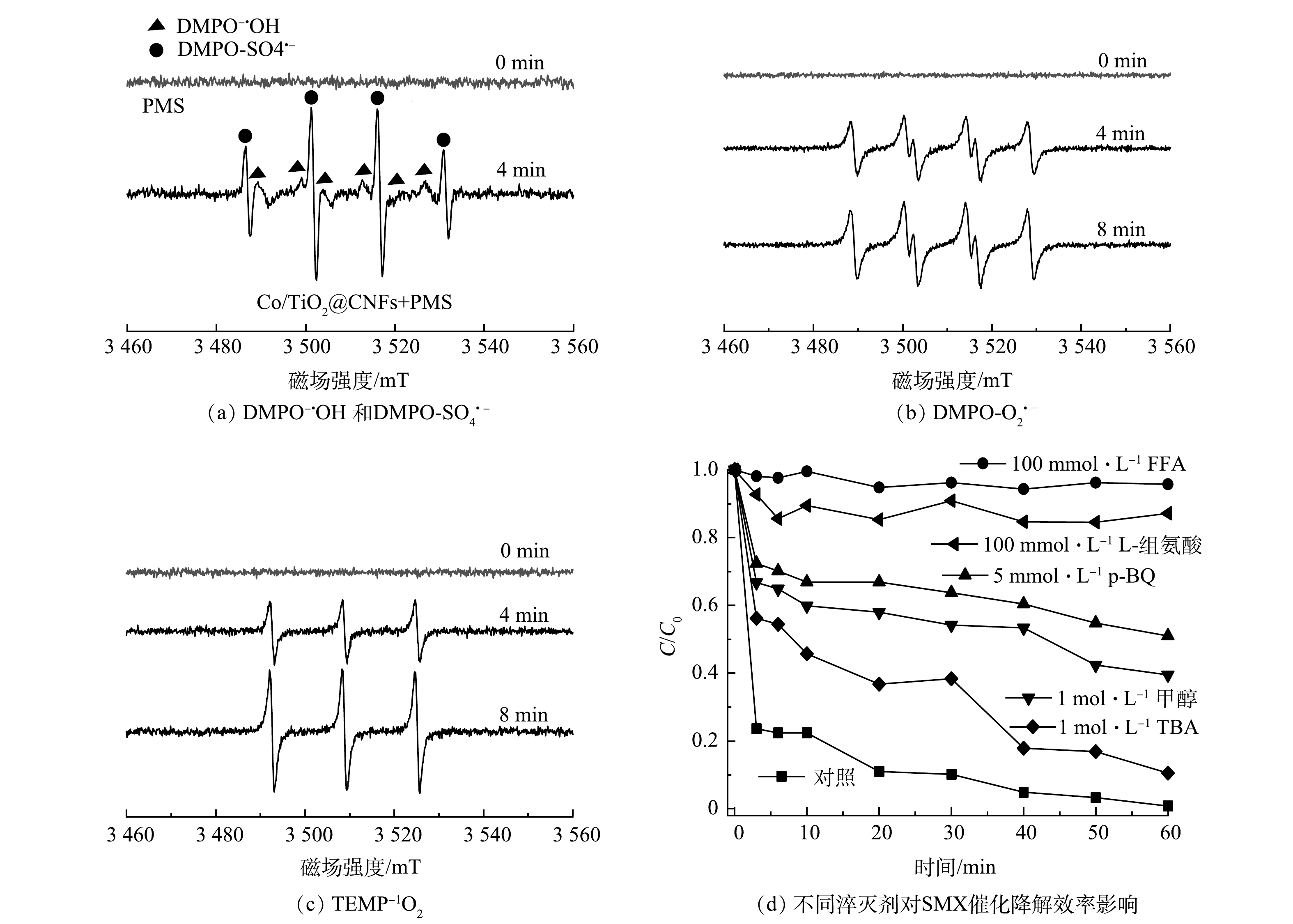
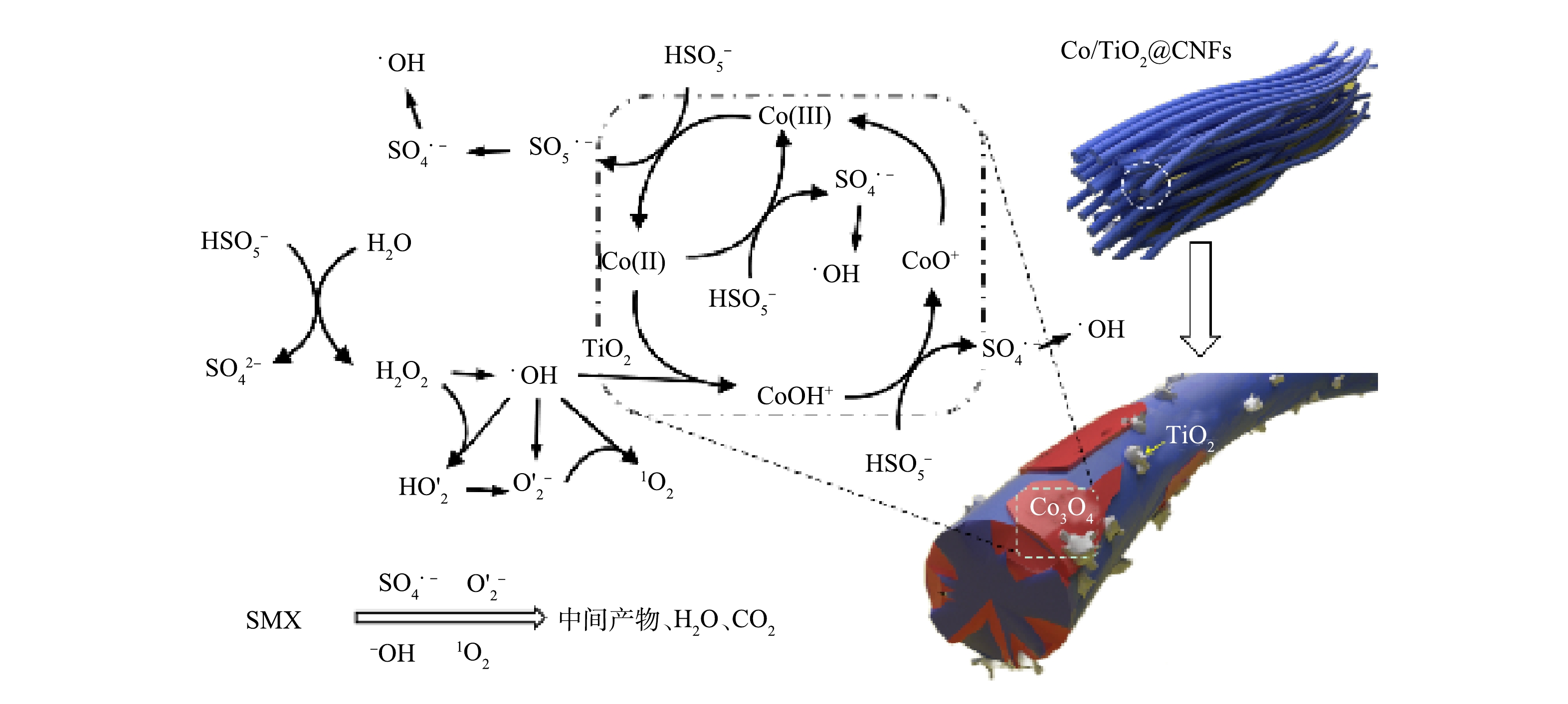
通过ESR分析和淬灭实验对Co/TiO2@CNFs活化PMS降解SMX反应中的主要活性物种进行了鉴定。利用5,5 -二甲基-1-吡咯烷N-氧化物(DMPO)和2,2,6,6-四甲基哌啶(TEMP)作为自旋捕获剂,分别得到了SO4·−、·OH、O2·−和1O2的ESR分析特征峰,如图9(a)~(c)所示。为进一步确定哪种活性氧物种是SMX降解的主要活性物种,采用TBA、甲醇、p-BQ、L-组氨酸和FFA分别作为·OH、SO4·−、O2·−和1O2的淬灭剂,结果显示,60 min时SMX降解率分别为89.5%、60.5%、49.0%、12.9%、4.3%,而未添加淬灭剂的对照组降解率为99.2%。上述结果说明,1 mol·L−1 TBA的添加对活化PMS降解SMX效率的影响较小,而1 mol·L−1甲醇的添加进一步抑制SMX的降解效率,表明实验过程中产生了少量·OH和SO4·−。5 mmol·L−1 p-BQ的添加进一步影响了PMS活化降解SMX,表明溶液中存在一定的O2·−。100 mmol·L−1 L-组氨酸和100 mmol·L−1FFA的添加几乎完全抑制了SMX降解,表明实验过程中产生了大量1O2。综上所述,4种活性物种均有产生,其中1O2在Co/TiO2@CNFs活化PMS过程起到了主导作用。此外,溶液中可能存在的机理反应如式(14)~式(25)[34, 38-41]所示。
为了更深入地解释反应机理,采用XPS分析了Co/TiO2@CNFs在反应前后的元素组成和化学价态。如图4(f)所示,在796.5 eV和780.8 eV的特征峰分别对应于Co2p1/2和Co2p3/2。其中,2个振荡峰分别位于802.7 eV和786.4 eV,Co2p3/2 (780.7 eV)和Co2p1/2 (796.4 eV)的峰属于Co(Ⅲ),Co2p3/2 (782.5 eV)和Co2p1/2 (797.8 eV)的峰属于Co(Ⅱ)。催化反应后Co(Ⅲ)的相对比例略有增加,而Co(Ⅱ)的相对比例下降,说明催化剂在Co/TiO2@CNFs表面上形成了Co(Ⅲ)[17]。这一结果表明在降解过程中发生了氧化还原反应。如式(26)~式(28)所示[22],由于Co(Ⅱ)直接导致SO4·−自由基的生成,因此,催化剂表面的Co(Ⅱ)可以显著促进界面催化反应。有研究表明,催化剂表面生成Co-OH络合物是活化PMS最有效的物种,是反应限速步骤[17]。根据前面的FTIR分析结果,添加TiO2后复合催化剂表面有大量OH基团,可以促进催化剂表面Co-OH络合物的形成,并可强化SMX的降解效率。这说明TiO2强化的Co(Ⅱ)-Co(Ⅲ)-Co(Ⅱ)氧化还原循环参与了催化降解反应。
综上所述,提出了SMX在Co/TiO2@CNFs活化PMS体系中降解的反应机理(图10)。静电纺丝纤维材料表面的Co(Ⅱ)-Co(Ⅲ)-Co(Ⅱ)氧化还原循环活化PMS产生SO4·−和·OH物质,并进一步转化成O2·−和1O2,4种活性物种共同促进SMX的降解。而在Co(Ⅱ)-Co(Ⅲ)-Co(Ⅱ)氧化还原循环当中,TiO2促进了催化剂表面Co-OH的形成,从而提升了催化反应效率。
-
1)通过静电纺丝工艺和热处理技术直接制备得到Co/TiO2@CNFs复合碳纳米纤维催化剂,该材料具有多孔网状结构、较好的稳定性和重复利用性,并能与PMS耦合实现水中SMX的高效降解。在材料投加量为0.1 g·L−1,PMS投加量为1 mmol·L−1条件下,50 min后SMX降解率达99.9%,复合催化剂的最佳pH为5.8~9。
2) TiO2的添加相比于仅仅负载Co的纳米纤维,其催化能力和材料稳定性能均有提高,其中添加比例为0.1%时,Co/TiO2@CNFs具有最大的比表面积、缺陷度,且TiO2添加后可促进催化剂表面Co-OH的形成,因此其催化降解SMX的效率最高。
3)该复合催化剂对SMX进行了有效降解,并根据10种检测到的中间产物推断出其降解过程的4条反应路径。Co/TiO2@CNFs对SMX的高效降解主要通过高效生成1O2和TiO2促进催化剂表面Co-OH的形成来共同实现。
载钴钛碳纳米纤维活化过一硫酸盐降解磺胺甲恶唑的效能及机理分析
Degradation efficiency and mechanism of sulfamethoxazole by activation of peroxymonosulfate via cobalt titanium-loaded carbon nanofibers
-
摘要: 本研究采用静电纺丝和热处理技术制备了一种网状负载钴钛双金属纳米颗粒碳纳米纤维催化剂(Co/TiO2@CNFs),并通过SEM、TEM、HRTEM、HAADF-STEM、元素映射、XRD、氮气吸脱附、TGA、拉曼光谱、FTIR、XPS、Zeta电位等手段对催化剂进行了表征和分析,通过批式降解实验优化了二氧化钛(TiO2)最佳负载量,并研究了催化剂投加量、PMS投加量、温度、pH、共存离子(Cl−、F−、HCO3−和H2PO4−)等因素对磺胺甲恶唑(SMX)降解性能的影响。结果表明,TiO2负载可提高催化剂的催化性能,TiO2与所制备催化剂的最佳质量比为0.1%;在催化剂投加量为0.1 g·L−1,PMS投加量为1 mmol·L−1时,Co/TiO2@CNFs在50 min内降解SMX≥99%;在pH为5.8~9内反应最佳;根据阿伦尼乌斯方程计算出反应体系的活化能为63.23 kJ·mol−1;催化剂在4种共存离子存在下表现出较好的催化性能;经5次循环后,Co/TiO2@CNFs催化降解性能仍保持较好。ESR分析和淬灭实验结果表明,4种活性物种(·OH、SO4·−、O2·−和1O2)参与到SMX的降解过程中,其中,1O2在Co/TiO2@CNFs活化PMS过程中起主导作用。Abstract: The catalytic activation of peroxymonosulfate (PMS) to generate free radicals has received a lot of attention in the environmental catalytic treatment of refractory pollutants. Electrostatic spinning and heat treatment procedures were used to manufacture a porous cobalt-titanium bimetallic nanoparticles supported carbon nanofibers catalyst (Co/TiO2@CNFs) in this research. SEM, TEM, HRTEM, HAADF-STEM, elemental mapping, XRD, nitrogen adsorption/desorption isotherms, TGA, Raman spectroscopy, FTIR, XPS, Zeta potential, and other methods were used to characterize the physical and chemical properties of catalysts. The optimal titanium dioxide (TiO2) loading capacity was explored by batch degradation experiment. The effects of catalyst dosage, PMS dosage, temperature, pH and coexisting ions (Cl−、F−、HCO3− and H2PO4−) on the degradation efficiency of sulfamethoxazole (SMX) were investigated. The results show that TiO2 supports could improve the catalytic performance of the catalyst, and the optimal mass ratio of TiO2 to catalyst was 0.1 %. When catalyst dosage was 0.1 g·L−1 and PMS dosage was 1 mmol·L−1, Co/TiO2@CNFs could degrade SMX≥99% within 50 minutes. The optimum pH value was 5.8-9. According to Arrhenius equation, the activation energy of the reaction system was 63.23 kJ·mol −1. The catalyst had a good catalytic performance under the condition of four coexisting ions. After 5 cycles, Co/TiO2@CNFs still maintained a good catalytic degradation performance. Four active species (·OH、SO4·−、O2·− and 1O2) were involved in the degradation of SMX by ESR analysis and quenching experiments, of which 1O2 played a dominant role in the Co/TiO2@CNFs activated PMS system. Finally, the possible pathway and reaction mechanism of SMX degradation in bimetallic co-catalytic system are proposed. The findings of this research provide a novel perspective on the application of advanced oxidation processes (AOPs) in environmental remediation.
-
Key words:
- peroxymonosulfate /
- electrospinning /
- carbon nanofibers /
- sulfamethoxazole /
- reaction mechanism.
-

-
表 1 Co@CNFs和X Co/TiO2@CNFs的N2吸脱附测试结果
Table 1. Results of N2 adsorption/desorption analysis of the Co@CNFs and X Co/TiO2@CNFs
材料 比表面积/
(m2·g−1)总孔容/
(cm3·g−1)t-图法微孔/
(cm3·g−1)介孔/
(cm3·g−1)孔径/
nmCo@CNFs 64.31 0.073 0.008 0.065 3.94 0.5Co/TiO2@CNFs 7.60 0.017 0.002 0.015 3.94 1Co/TiO2@CNFs 97.15 0.092 0.018 0.074 3.93 5Co/TiO2@CNFs 13.84 0.041 0.002 0.039 1.87 10Co/TiO2@CNFs 27.90 0.085 0.004 0.080 4.31 -
[1] HOU L, ZHANG H, WANG L, et al. Removal of sulfamethoxazole from aqueous solution by sono-ozonation in the presence of a magnetic catalyst[J]. Separation and Purification Technology, 2013, 117: 46-52. doi: 10.1016/j.seppur.2013.05.014 [2] YU X, SUI Q, LYU S, et al. Municipal solid waste landfills: An underestimated source of pharmaceutical and personal care products in the water environment[J]. Environment Science Technology, 2020, 54(16): 9757-9768. doi: 10.1021/acs.est.0c00565 [3] FEKADU S, ALEMAYEHU E, DEWIL R, et al. Pharmaceuticals in freshwater aquatic environments: A comparison of the African and European challenge[J]. Science of the Total Environment, 2019, 654: 324-337. doi: 10.1016/j.scitotenv.2018.11.072 [4] HIRSCH R, TERNES T, HABERER K, et al. Occurrence of antibiotics in the aquatic environment[J]. Science of the Total Environment, 1999, 225(1-2): 109-118. doi: 10.1016/S0048-9697(98)00337-4 [5] LI Y, SALLACH J B, ZHANG W, et al. Insight into the distribution of pharmaceuticals in soil-water-plant systems[J]. Water Research, 2019, 152: 38-46. doi: 10.1016/j.watres.2018.12.039 [6] SHANABLEH A, BHATTACHARJEE S, ALANI S, et al. Assessment of sulfamethoxazole removal by nanoscale zerovalent iron[J]. Science of the Total Environment, 2021, 761: 143307. doi: 10.1016/j.scitotenv.2020.143307 [7] TROVO A G, NOGUEIRA R F, AGUERA A, et al. Degradation of sulfamethoxazole in water by solar photo-Fenton. Chemical and toxicological evaluation[J]. Water Research, 2009, 43(16): 3922-3931. doi: 10.1016/j.watres.2009.04.006 [8] LIU F, ZHOU H, PAN Z, et al. Degradation of sulfamethoxazole by cobalt-nickel powder composite catalyst coupled with peroxymonosulfate: Performance, degradation pathways and mechanistic consideration[J]. Journal of Hazardous Materials, 2020, 400: 123322. doi: 10.1016/j.jhazmat.2020.123322 [9] WALTER M V, VENNES J W. Occurrence of multiple-antibiotic-resistant enteric bacteria in domestic sewage and oxidation lagoons[J]. Applied Environmental Microbiology, 1985, 50(4): 930-933. doi: 10.1128/aem.50.4.930-933.1985 [10] 许晟硕, 钱征, 王龄侦, 等. 氮掺杂碳催化剂活化过一硫酸盐的活性位点分析及其对双酚A的降解机制[J]. 环境工程学报, 2022,16(2): 452-461. [11] ZENG H, DENG L, ZHANG H, et al. Development of oxygen vacancies enriched CoAl hydroxide@hydroxysulfide hollow flowers for peroxymonosulfate activation: A highly efficient singlet oxygen-dominated oxidation process for sulfamethoxazole degradation[J]. Journal of Hazardous Materials, 2020, 400: 123297. doi: 10.1016/j.jhazmat.2020.123297 [12] ANDREOZZI R, CAPRIO V, INSOLA A, et al. Advanced oxidation processes (AOP) for water purification and recovery[J]. Catalysis Today, 1999, 53(1): 51-59. doi: 10.1016/S0920-5861(99)00102-9 [13] ZHANG H, LI G, DENG L, et al. Heterogeneous activation of hydrogen peroxide by cysteine intercalated layered double hydroxide for degradation of organic pollutants: Performance and mechanism[J]. Journal of Colloid and Interface Science, 2019, 543: 183-191. doi: 10.1016/j.jcis.2019.02.059 [14] ZENG H, ZHANG W, DENG L, et al. Degradation of dyes by peroxymonosulfate activated by ternary CoFeNi-layered double hydroxide: Catalytic performance, mechanism and kinetic modeling[J]. Journal of Colloid and Interface Science, 2018, 515: 92-100. doi: 10.1016/j.jcis.2018.01.016 [15] DUAN X, YANG S, WACŁAWEK S, et al. Limitations and prospects of sulfate-radical based advanced oxidation processes[J]. Journal of Environmental Chemical Engineering, 2020, 8(4). [16] CAI T, LIU Y, WANG L, et al. Activation of persulfate by photoexcited dye for antibiotic degradation: Radical and nonradical reactions[J]. Chemical Engineering Journal, 2019: 375. [17] HU P, LONG M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications[J]. Applied Catalysis B:Environmental, 2016, 181: 103-117. doi: 10.1016/j.apcatb.2015.07.024 [18] BAO Y, TIAN M, LUA S K, et al. Spatial confinement of cobalt crystals in carbon nanofibers with oxygen vacancies as a high-efficiency catalyst for organics degradation[J]. Chemosphere, 2020, 245: 125407. doi: 10.1016/j.chemosphere.2019.125407 [19] INAGAKI M, YANG Y, KANG F. Carbon nanofibers prepared via electrospinning[J]. Advanced Materials, 2012, 24(19): 2547-2566. doi: 10.1002/adma.201104940 [20] ZHU Z, JI C, ZHONG L, et al. Magnetic Fe-Co crystal doped hierarchical porous carbon fibers for removal of organic pollutants[J]. Journal of Materials Chemistry A, 2017, 5(34): 18071-18080. doi: 10.1039/C7TA03990E [21] LIN K A, YANG M T, LIN J T, et al. Cobalt ferrite nanoparticles supported on electrospun carbon fiber as a magnetic heterogeneous catalyst for activating peroxymonosulfate[J]. Chemosphere, 2018, 208: 502-511. doi: 10.1016/j.chemosphere.2018.05.127 [22] ZHANG Y, ZHANG B T, TENG Y, et al. Carbon nanofibers supported Co/Ag bimetallic nanoparticles for heterogeneous activation of peroxymonosulfate and efficient oxidation of amoxicillin[J]. Journal of Hazardous Materials, 2020, 400: 123290. doi: 10.1016/j.jhazmat.2020.123290 [23] YANG Z, LI Y, ZHANG X, et al. Sludge activated carbon-based CoFe2O4-SAC nanocomposites used as heterogeneous catalysts for degrading antibiotic norfloxacin through activating peroxymonosulfate[J]. Chemical Engineering Journal, 2020, 384: 123319. doi: 10.1016/j.cej.2019.123319 [24] CAI C, ZHANG H, ZHONG X, et al. Electrochemical enhanced heterogeneous activation of peroxydisulfate by Fe–Co/SBA-15 catalyst for the degradation of Orange II in water[J]. Water Research, 2014, 66: 473-485. doi: 10.1016/j.watres.2014.08.039 [25] CHLEBDA D K, JĘDRZEJCZYK R J, JODŁOWSKI P J, et al. Surface structure of cobalt, palladium, and mixed oxide-based catalysts and their activity in methane combustion studied by means of micro-Raman spectroscopy[J]. Journal of Raman Spectroscopy, 2017, 48(12): 1871-1880. doi: 10.1002/jrs.5261 [26] SUN H, LIANG H, ZHOU G, et al. Supported cobalt catalysts by one-pot aqueous combustion synthesis for catalytic phenol degradation[J]. Journal of Colloid and Interface Science, 2013, 394: 394-400. doi: 10.1016/j.jcis.2012.11.017 [27] YANG Q, CHOI H, DIONYSIOU D D. Nanocrystalline cobalt oxide immobilized on titanium dioxide nanoparticles for the heterogeneous activation of peroxymonosulfate[J]. Applied Catalysis B:Environmental, 2007, 74(1/2): 170-178. doi: 10.1016/j.apcatb.2007.02.001 [28] WANG J, WANG S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants[J]. Chemical Engineering Journal, 2018, 334: 1502-1517. doi: 10.1016/j.cej.2017.11.059 [29] HUANG Y H, HUANG Y F, HUANG C I, et al. Efficient decolorization of azo dye Reactive Black B involving aromatic fragment degradation in buffered Co2+/PMS oxidative processes with a ppb level dosage of Co2+-catalyst[J]. Journal of Hazardous Materials, 2009, 170(2-3): 1110-1118. doi: 10.1016/j.jhazmat.2009.05.091 [30] QI C, LIU X, LIN C, et al. Degradation of sulfamethoxazole by microwave-activated persulfate: Kinetics, mechanism and acute toxicity[J]. Chemical Engineering Journal, 2014, 249: 6-14. doi: 10.1016/j.cej.2014.03.086 [31] ZHANG Q, CHEN J, DAI C, et al. Degradation of carbamazepine and toxicity evaluation using the UV/persulfate process in aqueous solution[J]. Journal of Chemical Technology & Biotechnology, 2015, 90(4): 701-708. [32] AO X, LIU W. Degradation of sulfamethoxazole by medium pressure UV and oxidants: Peroxymonosulfate, persulfate, and hydrogen peroxide[J]. Chemical Engineering Journal, 2017, 313: 629-637. doi: 10.1016/j.cej.2016.12.089 [33] BUXTON G V, BYDDER M, SALMON G A, et al. The reactivity of chlorine atoms in aqueous solution. Part III. The reactions of Cl• with solutes[J]. Physical Chemistry Chemical Physics, 2000, 2(2): 237-245. doi: 10.1039/a907133d [34] YAN J, LI J, PENG J, et al. Efficient degradation of sulfamethoxazole by the CuO@Al2O3 (EPC) coupled PMS system: Optimization, degradation pathways and toxicity evaluation[J]. Chemical Engineering Journal, 2019, 359: 1097-1110. doi: 10.1016/j.cej.2018.11.074 [35] WANG S, LIU H, WANG J. Nitrogen, sulfur and oxygen co-doped carbon-armored Co/Co9S8 rods (Co/Co9S8@N-S-O-C) as efficient activator of peroxymonosulfate for sulfamethoxazole degradation[J]. Journal of Hazardous Materials, 2020, 387: 121669. doi: 10.1016/j.jhazmat.2019.121669 [36] LAI L, YAN J, LI J, et al. Co/Al2O3-EPM as peroxymonosulfate activator for sulfamethoxazole removal: Performance, biotoxicity, degradation pathways and mechanism[J]. Chemical Engineering Journal, 2018, 343: 676-688. doi: 10.1016/j.cej.2018.01.035 [37] AO X, LIU W, SUN W, et al. Mechanisms and toxicity evaluation of the degradation of sulfamethoxazole by MPUV/PMS process[J]. Chemosphere, 2018, 212: 365-375. doi: 10.1016/j.chemosphere.2018.08.031 [38] YANG S, WANG P, YANG X, et al. Degradation efficiencies of azo dye Acid Orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide[J]. Journal of Hazardous Materials, 2010, 179(1-3): 552-558. doi: 10.1016/j.jhazmat.2010.03.039 [39] JI Y, XIE W, FAN Y, et al. Degradation of trimethoprim by thermo-activated persulfate oxidation: Reaction kinetics and transformation mechanisms[J]. Chemical Engineering Journal, 2016, 286: 16-24. doi: 10.1016/j.cej.2015.10.050 [40] WANG C, KIM J, KIM M, et al. Nanoarchitectured metal-organic framework-derived hollow carbon nanofiber filters for advanced oxidation processes[J]. Journal of Materials Chemistry A, 2019, 7(22): 13743-13750. doi: 10.1039/C9TA03128F [41] LIN K Y A, LIN T Y, LU Y C, et al. Electrospun nanofiber of cobalt titanate perovskite as an enhanced heterogeneous catalyst for activating peroxymonosulfate in water[J]. Chemical Engineering Science, 2017, 168: 372-379. doi: 10.1016/j.ces.2017.05.013 -



 下载:
下载: