-
微纳米曝气作为一种新型曝气技术,具有较高的气液传质效率,可以快速提高水体溶解氧含量[1],调整水体微生物种群结构,增加好氧微生物数量[2-3],改善水环境水质,因而广泛应用于河道黑臭水体治理、城市景观水体治理、湖泊河流生态修复等领域[4-8]。但针对该技术应用于饮用水源地水库及其对沉积物微生物影响的研究相对较少[9-10]。沉积物微生物是湖泊水库生态系统物质循环和能量流动的主要动力,在沉积物-水体的物质循环、能量流动及水华形成过程中发挥着重要作用[10-11]。磷功能菌(包括聚磷菌和解磷菌)作为参与沉积物内源磷代谢循环的主要菌群,与沉积物的各形态磷转化息息相关[12-13]。目前,国内外关于磷功能菌多样性及其群落组成、结构研究多见于强化生物除磷工艺中[14-15]、农田土壤植物促生[16-17]等方面,对于饮用水源地水库沉积物这种特殊生境的研究鲜有报道[13,18-19]。而此方面的研究,特别是结合微纳米曝气工程的实施,开展曝气前后沉积物微生物群落结构动态变化的相关研究,不仅可以为微纳米曝气技术的实施提供参考,而且可以为水库沉积物磷循环的微生物驱动机理研究提供基础数据。
福建省东牙溪水库是三明市区的重要饮用水水源地,肩负着为三明市30万人提供饮水的重任。近些年来,随着三明市的经济发展及城镇化建设的加快,周边环境的改变,使得东牙溪水库面临的一些水体污染问题逐渐显现[20-21]。为了保护饮用水源地,在2017年实施微纳米曝气生态修复工程项目。为探究微纳米曝气工程实施是否对沉积物的微生物及磷代谢功能菌的群落产生影响,本研究采用Illumina高通量测序技术对曝气前后沉积物微生物及磷功能菌的群落组成、结构进行了分析,探究了曝气实施对磷功能菌群落组成和结构的影响,其结果可以补充完善水库沉积物磷功能菌群落组成、结构的相关基础数据,为微纳米曝气技术机理研究以及实际应用提供参考。
-
东牙溪水库位于福建省三明市三元区东南部的中村乡境内,总库容约有2 263×104 m3,年平均径流量约1.36×108 m3;东牙溪水库属山谷型月调节水库,最大水深为63.4 m,主河道全长约30 km,全流域面积188 km2[9,21]。东牙溪流域属亚热带季风气候,年降水量一般在1 600~1 800 mm,相对湿度78%,年平均气温为16.5~18.1 ℃,无霜期为260~290 d[22]。
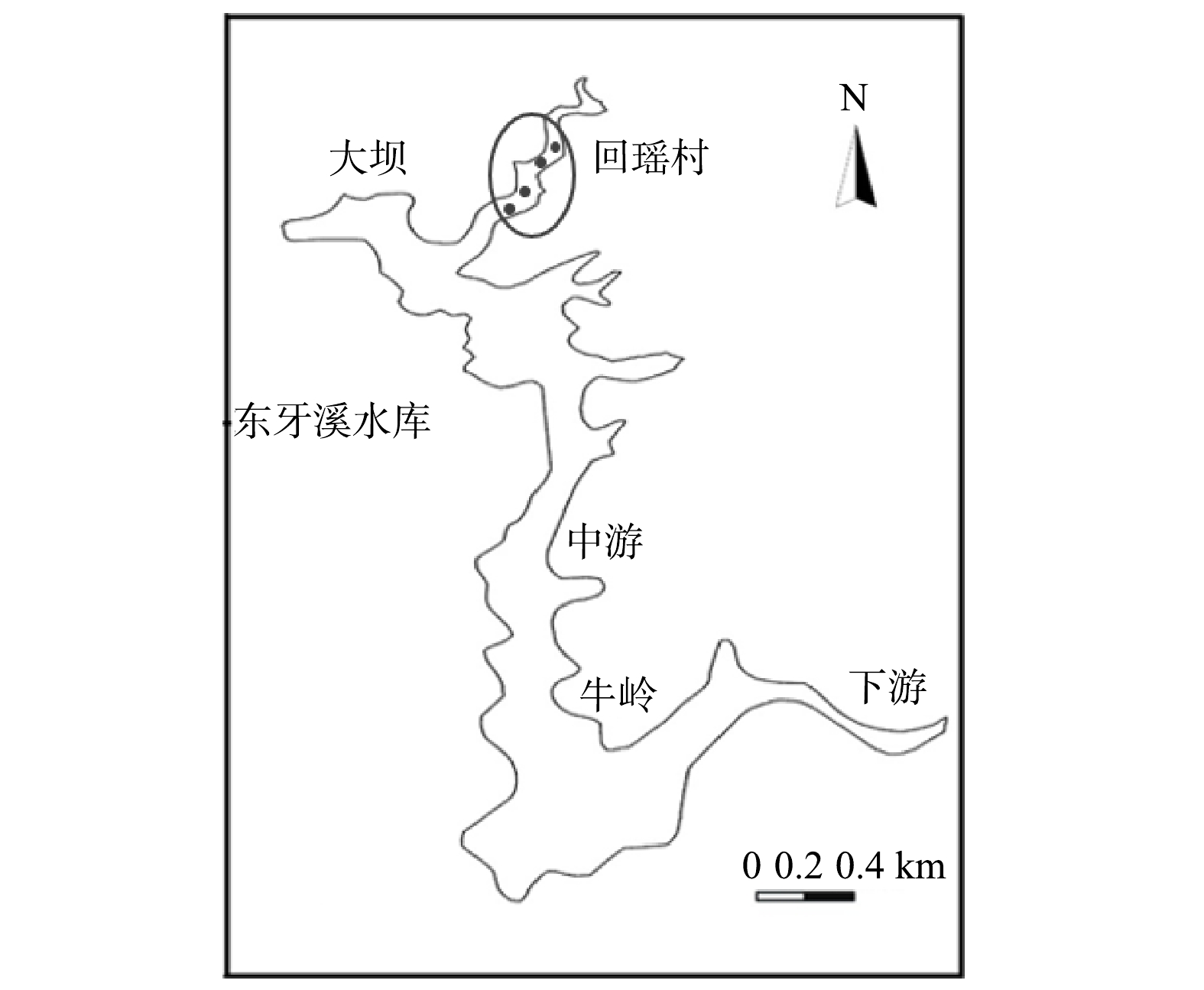
采样区域位于东牙溪水库的回瑶湾库区(26°11′56.87″,117°37′55.79″),位于大坝偏上方的回瑶村旁,见图1。设置4个不同的采样断面进行采样,使用抓斗采泥器分别采集微纳米曝气工程实施前(10月份)和实施后(12月份)的回瑶湾表层沉积物。以上样品均5个重复,混匀后,分为2份,1份供沉积物理化性质分析,1份供微生物分析。其中,微生物分析用的沉积物样品用无菌自封袋封存,4 ℃冷藏保存,并尽快带回实验室−80 ℃冻存,以备后续分子生物学分析使用。同时分别采集该工程实施前后的水样,以监测其水质主要理化指标的变化规律。高通量测序是4个样点取样后提取DNA后混合在一起进行测定,以表示曝气前后微生物的变化情况。对于水体、沉积物理化指标测定值为各样点测定值的均值加标准差。
-
总氮(TN)用碱性过硫酸钾消解分光光度法测定( GB /T 11894—1989 ) ;总磷(TP)用钼酸铵分光光度法测定GB / T 11893—1989) ;溶解氧( DO) 和水温使用德国WTW公司的Oxi 3310IDS 便携式溶解氧分析仪测定;pH使用pH计测定。沉积物各形态磷的测定采用SMT法[23]:将SMT法提取的各形态磷,采用钼-锑抗分光光度法进行测定。沉积物碳氮用碳氮元素分析仪(VarioELⅢ)测定。
-
本研究所用的微纳米曝气装置采用福建省华川技术有限公司专利生产的湖库流域生态基因纳米调控装置[24]。微纳米曝气装置所用的曝气头为福建省华川技术有限公司与澳大利亚公司合作设计生产的陶瓷微纳米气泡扩散曝气头[25],曝气方式为高压切割。
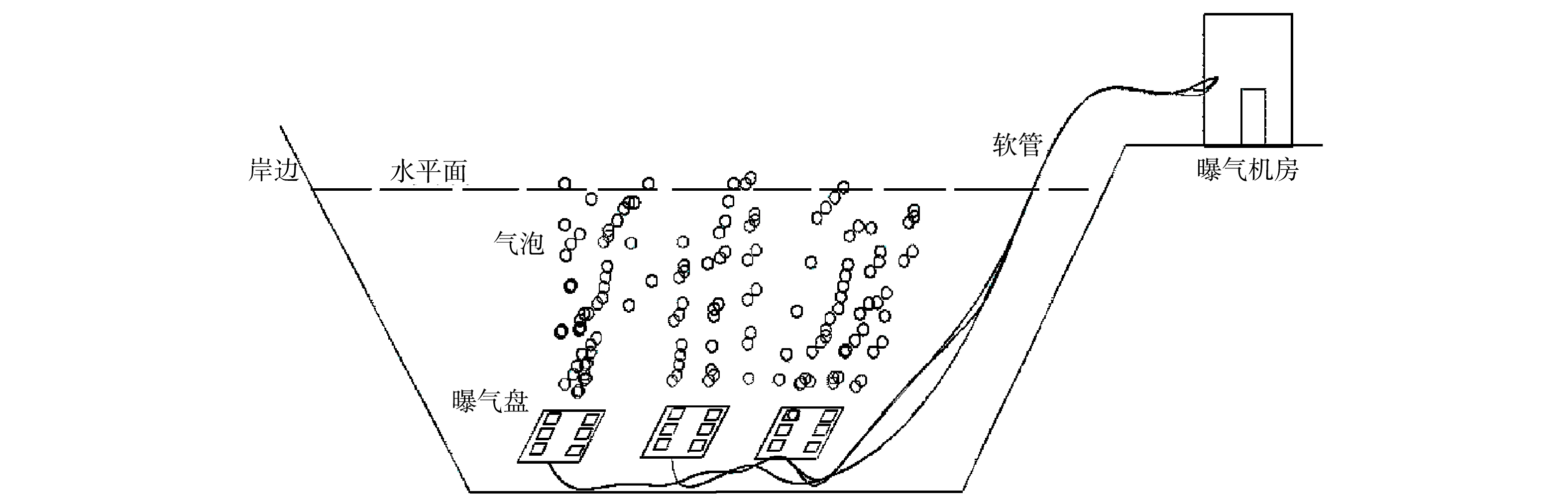
本微纳米曝气工程的微纳米曝气发生装置安置于牛岭库湾岸边的工程项目建设机房内,阵列可控溶氧盘则安置在水库回瑶湾库湾区域的河道底部,回瑶湾安装深度约为15~20 m,由机房内的曝气装置产生的气体通过管道输送到阵列可控溶氧盘上,再由阵列可控溶氧盘上的微纳米气泡扩散曝气头将微纳米气泡扩散至水体中。该微纳米曝气工程的具体示意图见图2。回瑶湾区域微纳米曝气区域面积约为46 666 m2,为24 h连续曝气。
-
样品基因组总DNA 的提取及纯化按照Power Soil DNA isolation kit(MO BIO Laboratories, Inc., USA)说明书进行操作。DNA纯度和浓度通过紫外分光光度计(Nano Drop,USA)进行检测,收集的DNA样品在分析之前存于−20 ℃冰箱备用。提取纯化好的DNA样品经琼脂糖凝胶电泳确认后送上海生工生物工程有限公司进行细菌的高通量测序。扩增区域V3~V4区,采用细菌16S测序引物为Nobar_341F (CCTACGGGNGGCWGCAG)和Nobar_805R(GACTACHVGGGTATCTAATCC)。
-
聚磷菌ppk1基因的扩增采用特异性引物ppk1 F:′5-AAYYTIGAYGARTTYTTYATGGT-3′,ppk1 R:′5-TTIKYITSYTCRTCRAAICKIGC-3′[26]。PCR扩增体系(25 μL):10×PCR缓冲液 2 μL、dNTP 2.5 μL、上游引物(ppk1 F)2 μL、下游引物(ppk1 R)2 μL、Taq DNA聚合酶 0.5 μL、模板DNA 1 μL、灭菌双蒸水15 μL。PCR扩增反应程序:94 ℃预变性2 min,94 ℃加热变性30 s,55 ℃退火45 s,68 ℃延伸2 min,33个循环,最后68 ℃延伸10 min。
有机解磷菌phoX基因的扩增采用特异性phoX F:′5-GGCAAAACGCCNTGGGGNAC-3′,phoX R:′5-GGGTCGACCTCGACNAYVYAGCC-3′ [27]。PCR 反应体系(25 μL):10×PCR缓冲液 2.5 μL、dNTP 2 μL、上游引物(phoX F)0.5 μL、下游引物(phoX R)0.5 μL、灭菌双蒸水16.2 μL、Taq DNA聚合酶 0.3 μL、模板DNA 3 μL。PCR反应程序:94 ℃预变性4 min,94 ℃加热变性45 s,56 ℃退火30 s,72 ℃延伸1 min,30个循环,最后72 ℃延伸8 min。
-
经PCR扩增及琼脂糖凝胶电泳验证存在ppk1基因、phoX基因的DNA样品,外送上海生工生物工程有限公司进行Illumina高通量测序。选取高通量测序得到的代表性OTU序列,采用MEGA4软件包[28]中的Neighbor-Joining法,设自展值为1000次,构建聚磷菌及有机解磷菌的系统发育树。
-
使用QIIME软件对获得的高通量测序数据进行分析,同时对获得的序列进行降噪处理。OTU划分和归并按97%的序列相似度进行。使用Mothur软件分析细菌、聚磷菌及有机解磷菌的多样性指数。利用Canoco 4.5软件中的冗余分析(RDA)分析聚磷菌和有机解磷菌的群落组成与沉积物主要理化因子的关系。使用Excel和SPSS22.0软件对数据进行统计分析。
-
1)水体主要理化指标变化。表1为东牙溪水库回瑶湾微纳米曝气前后水体的主要理化指标。结果表明,水体呈现中性状态,pH值高低不受微纳米曝气的影响。曝气前的水体表层DO含量为4.15 mg·L−1,底层较低,为2.97 mg·L−1;而曝气后,水体表层DO升高到8.17 mg·L−1,沉积物上覆水中的DO也达到7.63 mg·L−1。这表明微纳米气泡能够增加水体中的DO含量,且与对提升氧的传质效率特性相符[1]。10月份水体水温在25.5 ℃左右,而在12月份,其水温则在17.2 ℃左右,表层与底层水温无显著差异,表明回瑶湾区域在冬季并无水温分层现象。回瑶湾断面在曝气前后的总氮含量并无差异,曝气前为0.94 mg·L−1,曝气后为0.92 mg·L−1,表明在回瑶湾区域实施微纳米曝气对水体总氮的削减效果并不显著。曝气前,回瑶湾断面的总磷质量浓度为0.02 mg·L−1,曝气后为0.03 mg·L−1。王骁等[6]在对黑臭河道的研究中发现,水体中的总磷会随着微纳米曝气时间发生变化,呈现先升后降的趋势,在曝气57 d时总磷浓度小幅升高。这与本研究结果相近。总体而言,微纳米曝气在10—12月对水体的氮磷浓度的削减并不显著。氮磷浓度的削减不显著的原因涉及多因素的影响,如外界环境因素、生物因素、曝气工程实施时间长短等。该水库牛岭库湾的微纳米曝气区(工程实施周期1 a左右)在不同季节的总磷平均削减率为14.0%~29.1%;不同季节的总氮平均削减率为 1.1%~11.0%,整体削减率较低。这表明纯粹地对水体进行曝气,并不能有效降低水体中的总氮浓度[9]。此外,冬季温度相对较低,也可能使得水体中的微生物对水体的氮磷营养盐代谢循环的能力变弱[29-31]。
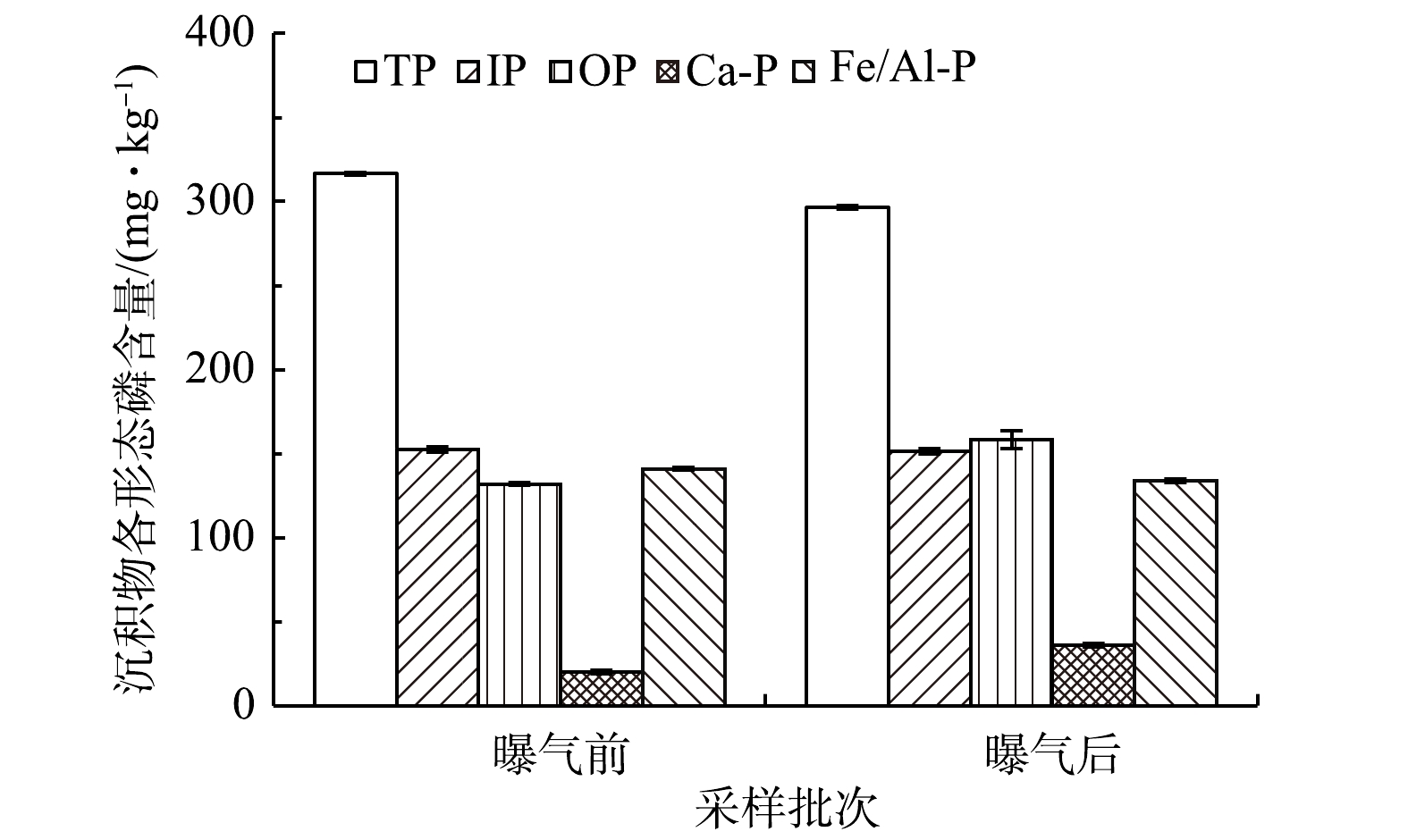
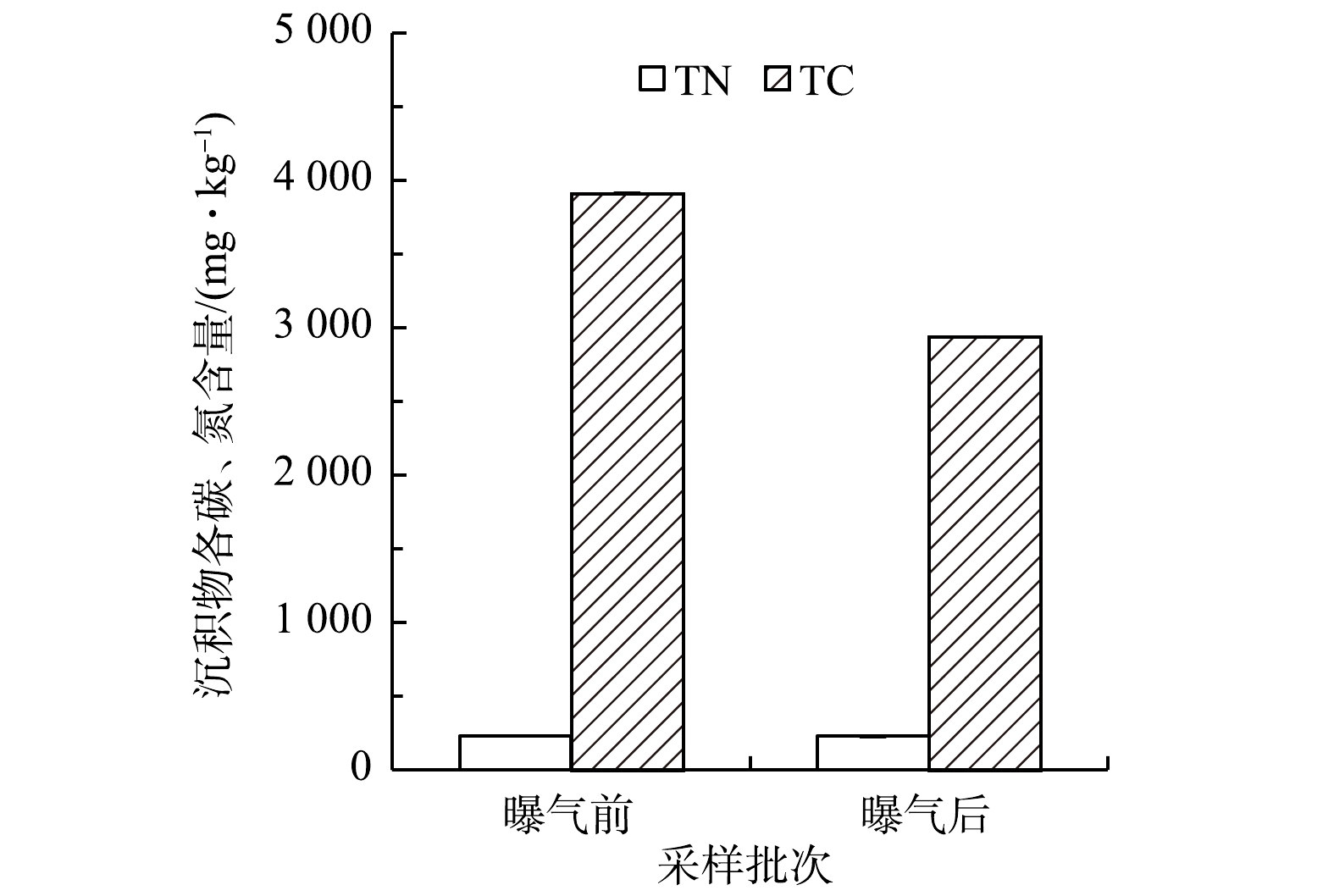
2)沉积物主要理化指标变化。微纳米曝气工程实施前后沉积物各形态磷含量变化情况见图3。回瑶湾曝气2个月后的沉积物总磷含量为296.4 mg·kg−1,略低于曝气前(316.5 mg·kg−1)。这与WU等[2]对城市黑臭河流的研究结果不一致,可能是由于两者水环境中的营养条件不一样,回瑶湾沉积物磷含量较低,污染程度较轻,内源磷释放的风险较低。曝气前后的沉积物各形态磷含量的变化并无明显的差异。沉积物TC和TN含量如图4所示。曝气前的TC含量为3 910.4 mg·kg−1,曝气后下降了971.0 mg·kg−1,而TN含量曝气前后无差别;则曝气前,沉积物中的C/N值达到16.8,曝气后则为12.6,表明微纳米曝气有利于降低了C/N值。有研究[32]表明,在高C/N条件下,虽然氮去除效率较高,但也会消耗更多的可溶性氧,会限制好氧硝化过程。因此,曝气有利于沉积物含碳有机质氧化,减少过量的碳源带来的二次污染[33]。
-
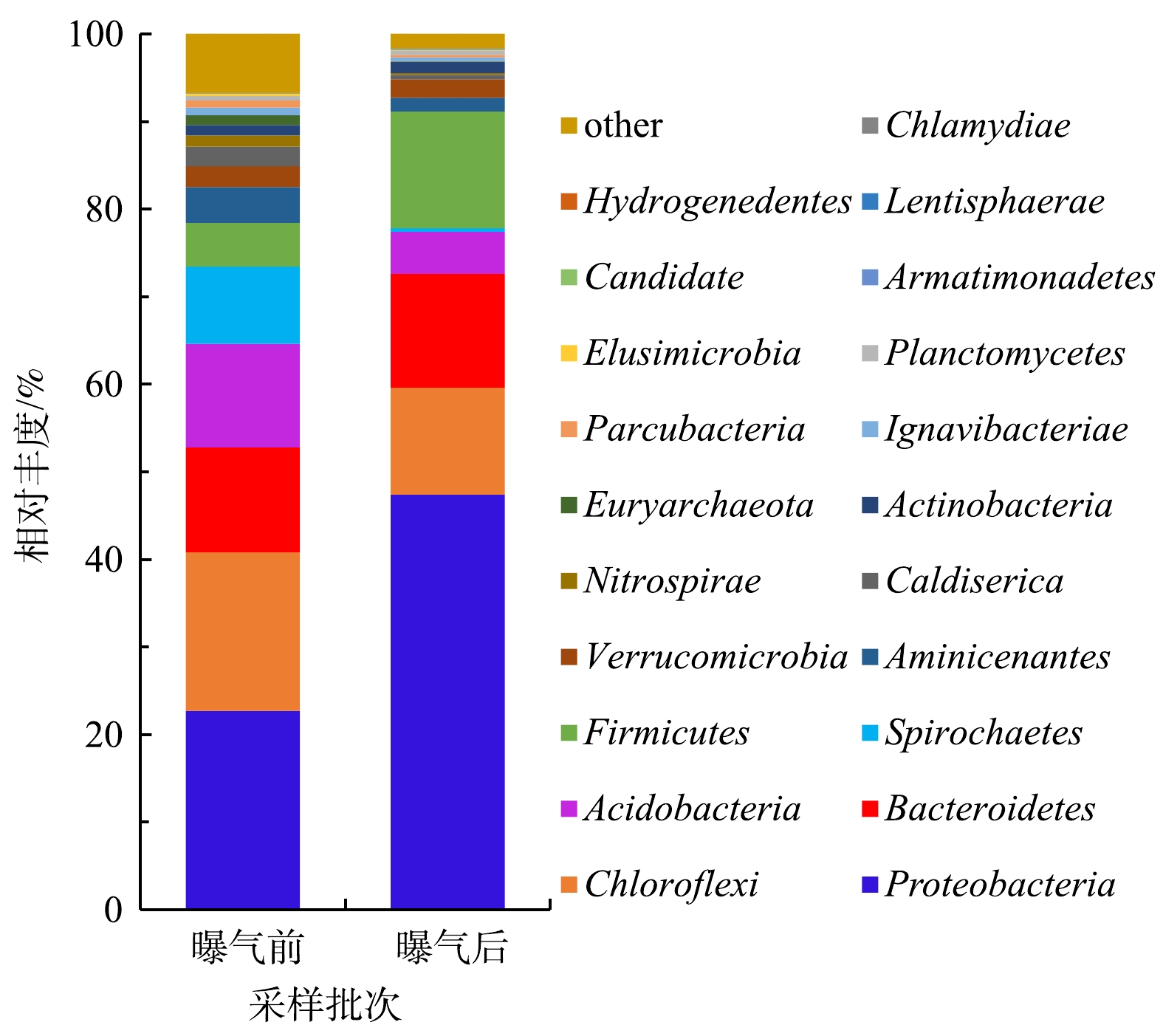
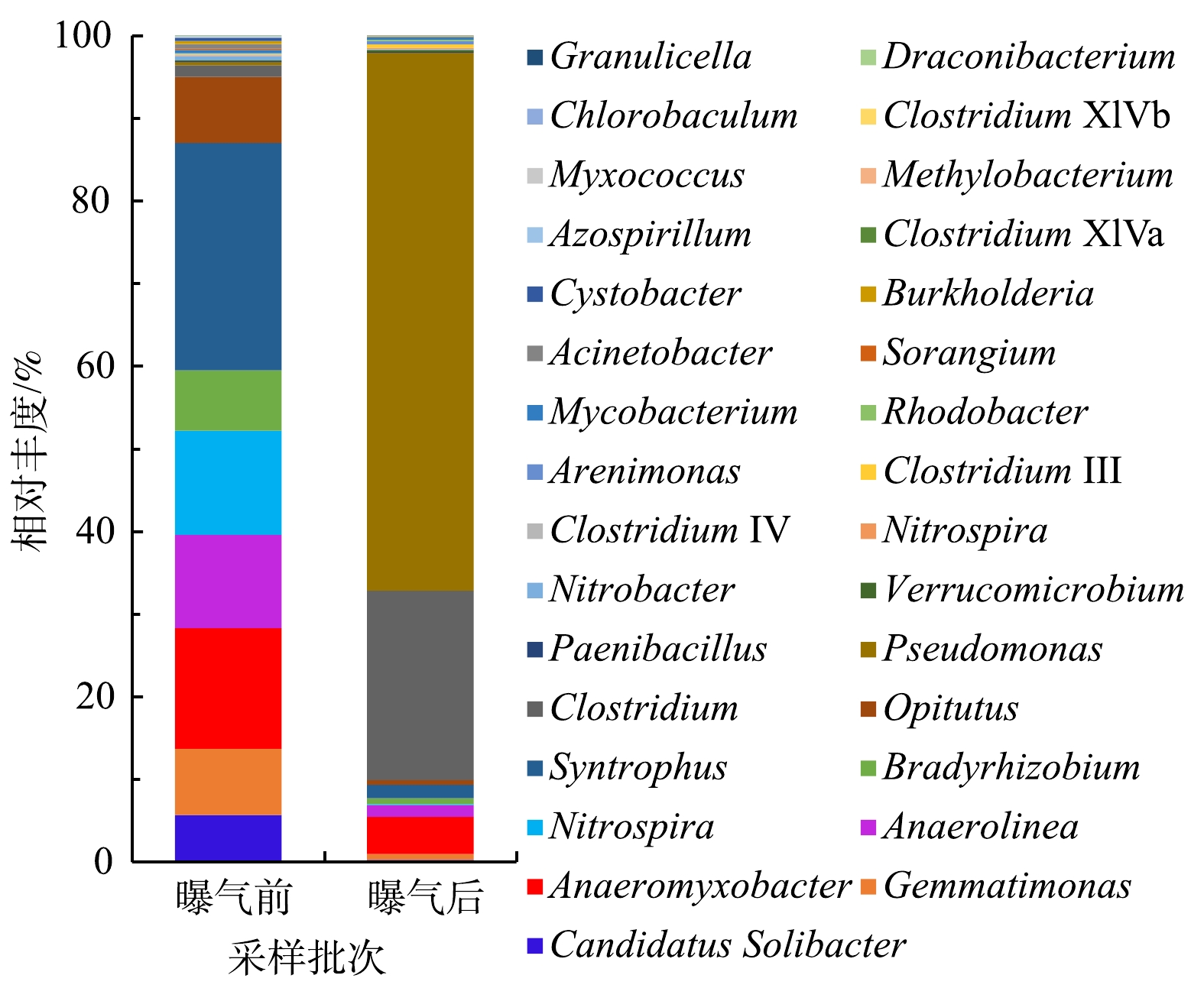
沉积物中的微生物群落结构的变化与水体环境的变化往往是相互影响的[3,34]。东牙溪水库回瑶湾微纳米曝气前后沉积物微生物群落结构变化如图5所示。本研究检测到沉积物中的细菌共有21门、113属。其中所占比例较高的主要门类有变形菌门(Proteobacteria)、绿弯菌门(Chloroflexi)、拟杆菌门(Bacteroidetes)、酸杆菌门(Acidobacteria)、螺旋体门(Spirochaetes)和厚壁菌门(Firmicutes),其分别占微生物总量的22.7%、18.1%、12.0%、11.8%、8.8%和5.0%,而曝气2个月后,其占微生物总量的占比变为47.4%、12.2%、13.0%、4.8%、0.4%和13.3%,曝气前后沉积物微生物群落结构存在着一定的差异。与曝气实施前相比,Proteobacteria的相对丰度上升了24.7%,始终处于优势地位,这与姚丽平[35]的研究结果一致;Firmicutes上升了8.3%,而Chloroflexi下降了5.9%,Acidobacteria下降了7.0%,Spirochaetes下降了8.4%,其他门类则相对丰度的变化率较低。在属的水平上,种类最多的是Proteobacteria,共检出40属细菌,其次是Firmicutes,检出12属细菌,Chloroflexi则检出10属细菌。另外,高通量测序结果表明(表2),曝气实施前的Shannon指数为8.78,实施后为7.29。这表明曝气对沉积物底部的充氧作用会对细菌的多样性造成一定影响,使得沉积物细菌的多样性指数略有下降,但变化不是很明显。SUN等[36]对2条黑臭河流的研究中发现,曝气后河流的微生物群落多样性指数变化不大,这与本研究结果基本一致。
WU等[2]分析了城市河流水体微纳米曝气前后微生物的群落组成变化,发现在属水平上,曝气前优势菌为厌氧的喜营养菌(如Arcobacter sp.、Azonexus sp.和Citrobacter sp.),曝气后为好氧的功能性菌群 (例如,Perlucidibaca sp.、Pseudarcicella sp.、Rhodoluna sp.和Sediminibacterium sp.) 的相对丰度得到提高。王硕等[3]发现,微纳米气泡曝气对于水体微生物群落的改变比底泥微生物的改变更显著,在没有对底床直接冲击的条件下,微纳米气泡的作用范围主要在水体,而底泥内的微生物群落保持稳定。而本研究微纳米曝气工程的阵列可控溶氧盘安置在河道底部,对沉积物的充氧作用较为显著,所以曝气前后沉积物的微生物群落变化相对明显。
-
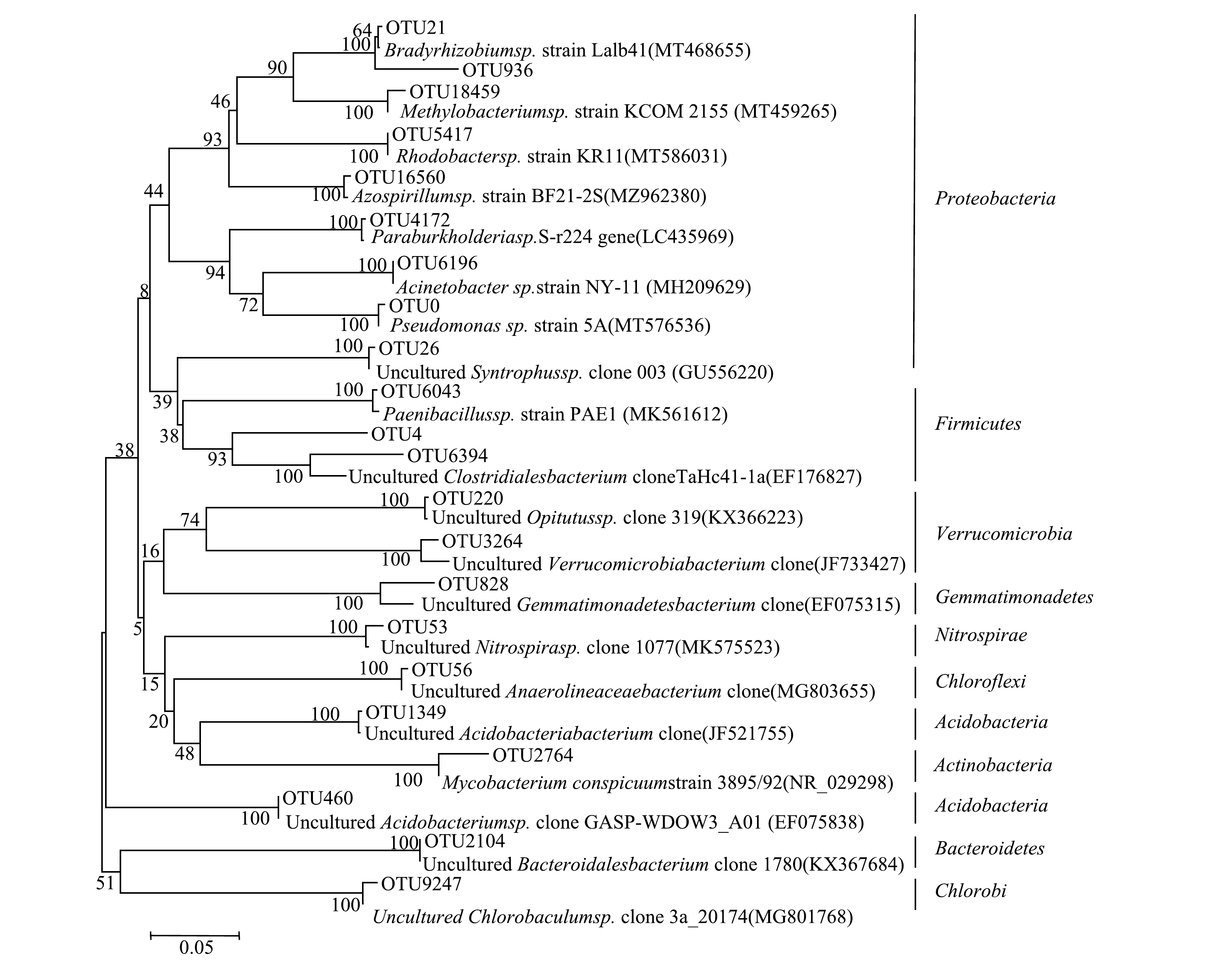
1)东牙溪水库样品聚磷菌的基因测序和系统发育分析。选取沉积物样品代表性OTU序列构建聚磷菌系统发育树图(图6)。由系统发育图可见,东牙溪水库回瑶湾采样点沉积物中所检测到的聚磷菌可以聚类为10个门类,分别为:Proteobacteria、放线菌门(Actinobacteria) 、Firmicutes、绿菌门(Chlorobi)、Chloroflexi、Bacteroidetes、Acidobacteria、硝化螺旋菌门(Nitrospirae)、芽单胞菌门(Gemmatimonadetes) 和疣微菌门(Verrucomicrobia)。其中,Proteobacteria和Firmicutes是聚磷菌的主要门类。曝气前,属于Proteobacteria的优势菌属约占49.4%;曝气后,属于Proteobacteria和Firmicutes的优势菌属分别占65.1%和22.9%。不同类型的聚磷菌具有特定的生态位,或与不同生境中特定的环境因子相关。对太湖的沉积物研究显示[11],污染重和污染轻的位点沉积物中的CandidatusAccumulibacter类聚磷菌均属于I型多聚磷酸盐激酶基因的ⅡD分支。而对海南东寨港红树林淤泥研究[37]发现,所筛的聚磷菌多为不动杆菌属(Acinetobacter),也有芽孢杆菌属(Bacillus)、寡养单胞菌属(Stenotrophomonas)和假单孢菌属(Pseudomonas),并且表现出一定的差异性。佘晨兴等[18]对福建省3座水库库心沉积物样品中聚磷菌研究发现,优势菌属差异较为明显,但均以Proteobacteria、Actinobacteria和Acidobacteria为主,其中Proteobacteria中的厌氧粘细菌属(Anaeromyxobacter)、Acidobacteria中的Solibacter属在3座水库库心沉积物样品中均为优势菌属。上述研究均表明,在门的水平上,Proteobacteria是沉积物聚磷菌的一个重要门类,而在属的水平上,由于生境不同,优势的聚磷菌菌属可能存在差异性。
2)微纳米曝气前后聚磷菌菌群结构对比。由图7可以看出,在曝气前后聚磷菌的群落结构总体变化不大。从门类上看,曝气前共检测到10个门,曝气后检测到9个门,且施工前后检测到的9个门均相同,唯一的不同在于施工后未检测出绿菌门;再从菌属上看,曝气前检测到28个属,曝气后检测到29个属,前后2次的检测结果中有26个属是相同的。曝气后没有出现Proteobacteria中的粘球菌属(Myxococcus)和Chlorobi中的绿棒菌属(Chlorobaculum),表明这2个属对溶氧条件的选择性较强,他们更适合于厌氧环境下生存;而新出现了Proteobacteria中的甲基杆菌属(Methylobacterium)和Firmicutes中的梭状芽胞杆菌XlVa属(Clostridium XlVa)、梭状芽胞杆菌XlVb属(Clostridium XlVb)3个属。此外,微纳米曝气施工前后优势菌属差异较为明显。在施工前的优势菌属是Proteobacteria中的互营菌属(Syntrophus)(27.5%)、Anaeromyxobacter(14.6%)和慢生根瘤菌属(Bradyrhizobium)(7.3%),Nitrospirae中的硝化螺菌属(Nitrospira)(12.6%),Chloroflexi中的厌氧绳菌属(Anaerolinea)(11.3%),Verrucomicrobia中的丰祐菌属(Opitutus)(8.0%),Gemmatimonadetes中的芽孢单菌属(Gemmatimonas)(8.0%),Acidobacteria中的CandidatusSolibacter属(5.7%)。而曝气后聚磷菌的优势菌属是Proteobacteria中的Pseudomonas和Firmicutes中的Clostridium,所占百分比分别是65.1%和22.9%,说明这2个属对溶解氧量有较高的敏感性,被微纳米曝气激活了活性,成为高氧环境下新的聚磷菌优势种。这与WU等[2]的研究结果一致,他们发现经微纳米曝气后,Pseudomonas有所增加。此外,高通量测序结果显示(见表3),曝气实施前的Shannon指数为4.01,实施后为2.71。这表明曝气对沉积物底部的充氧作用会对聚磷菌的多样性造成一定的影响,使得沉积物聚磷菌的多样性指数下降。
-
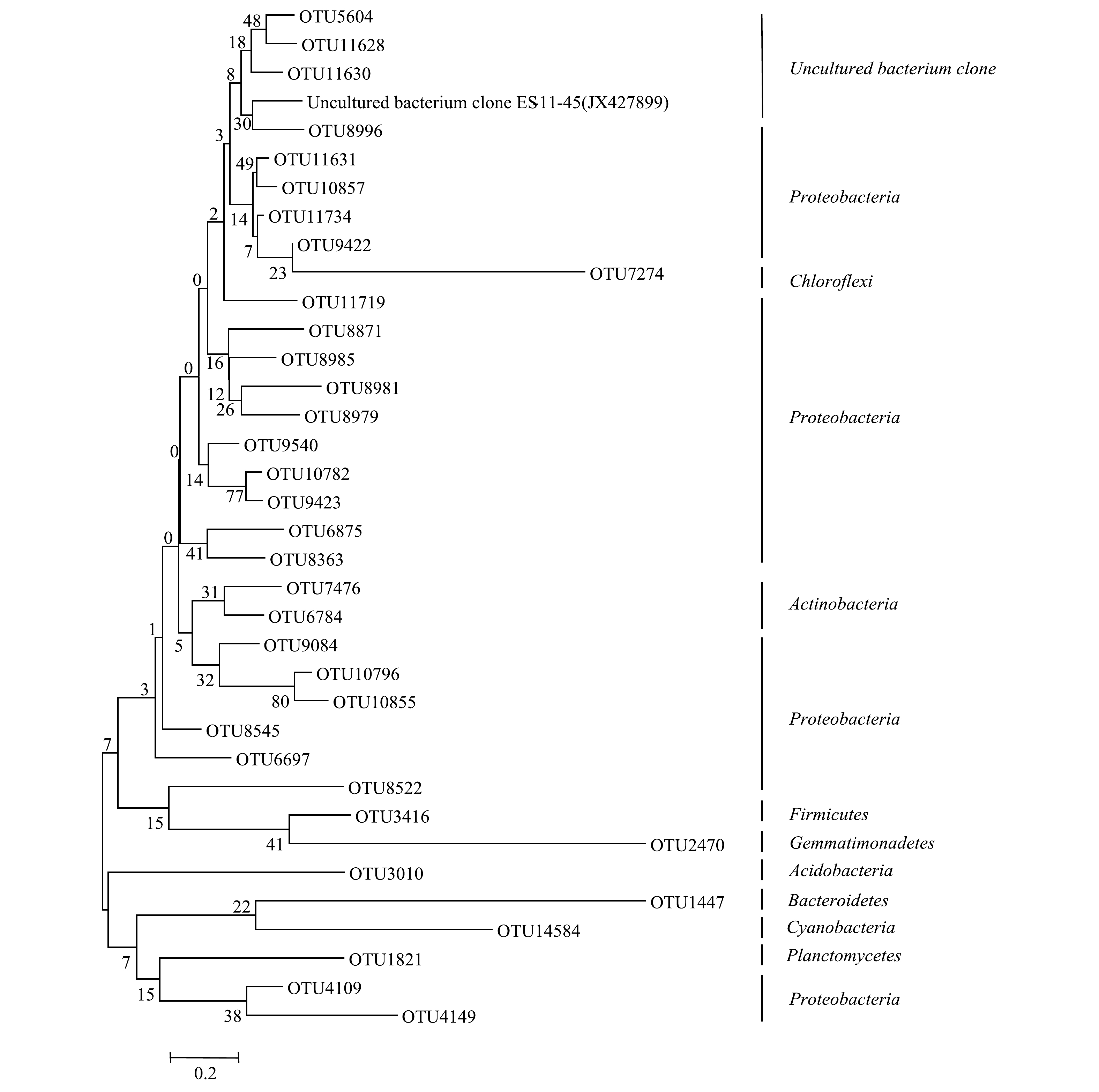
1)东牙溪水库样品有机解磷菌的基因测序和系统发育分析。选取沉积物样品代表性OTU序列构建有机解磷菌系统发育树图(图8)。可见,东牙溪水库回瑶湾采样点沉积物中所检测到的有机解磷菌可以聚类为9个门类,分别为:Proteobacteria、Actinobacteria、Firmicutes、浮霉状菌门(Planctomycetes)、Chloroflexi、Acidobacteria、Bacteroidetes、Gemmatimonadetes和蓝细菌门(Cyanobacteria)。其中,Proteobacteria是有机解磷菌的主要门类。曝气前,属于Proteobacteria的优势菌属约占29.7%;曝气后,属于Proteobacteria的优势菌属占35.6%。当然,对于具体种类,不同生境中检测到的有机解磷菌会存在差异性。李莹杰[38]从7个湖泊沉积物样品中共筛选出35株有机磷细菌,优势菌株属于Proteobacteria的Pseudomonas、类芽孢杆菌属(Paenibacillus)和Bacillus。在海水水域环境,王琛等[39]从九龙江口海水和沉积物中分离纯化得到了10株有机解磷菌,其中有8株菌为Bacillus,2株菌为弧菌属(Vibrio)菌株,均属于Proteobacteria门。对闽江口-平潭海域有机解磷菌的研究[40]表明,表层海水有机解磷菌优势门是Proteobacteria和Cyanobacteria,而沉积物中为Proteobacteria和Bacteroidetes。上述研究结果表明,Proteobacteria是不同生境中有机解磷菌的优势类群,这与本研究结果相符。
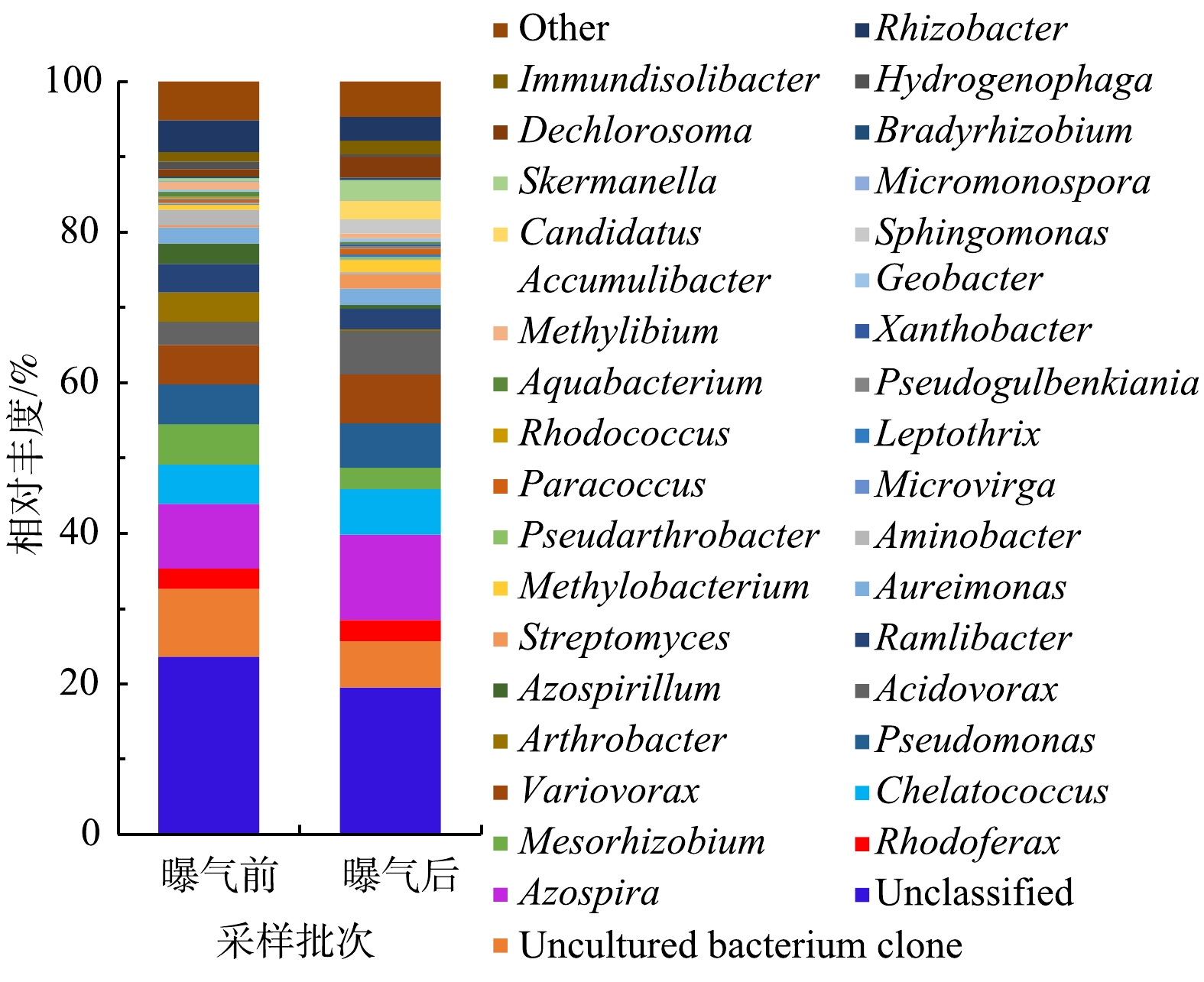
2)微纳米曝气前后有机解磷菌菌群结构对比。从门水平上看,微纳米曝气施工前后均检测到的门有7个,唯一的不同在于施工后多检测出Chloroflexi和Gemmatimonadetes这2个门。从属水平上看,曝气前检测到51个属,曝气后52个属,曝气前后均检测到的有42个属。曝气后有9个属没有出现,分别是Phreatobacter和Marmoricola、Alloactinosynnema、类诺卡氏菌属(Nocardioides)、太阳杆菌属(Heliobacterium)、Paludisphaera、Terriglobus、Petrimonas和念珠藻属(Nostoc);曝气后新出现的有10个属,分别是CandidatusAccumulibacter、Chelativorans、丛毛单胞菌(Comamonas)、Ralstonia、Singulisphaera、Caldilinea、CandidatusKoribacter、Mucilaginibacter、Gemmatirosa和纤发鞘丝蓝细菌属(Leptolyngbya)。微纳米曝气前后有机解磷菌菌属的相对丰度图见图9。由图9可以看出,在门的水平上,施工前后优势菌群变化不大,都为Proteobacteria,但在属的水平上,优势的菌属有所变化。曝气前,检测到沉积物有机解磷菌的优势菌属主要为Proteobacteria中的Azospira(8.6%)、中慢生根瘤菌(Mesorhizobium)(5.4%)、Pseudomonas(5.3%)、Chelatococcus(5.2%)、Variovorax(5.2%)。曝气后,优势菌群为Proteobacteria中的Azospira(11.3%)、Variovorax(6.5%)、Chelatococcus(6.1%)、Pseudomonas(5.9%)、食酸菌属(Acidovorax)(5.8%)。由此可见,在微纳米曝气实施后,有机解磷菌菌群会对沉积物理化性质变化做出响应,通过激活一些好氧微生物,使适应曝气环境下的微生物成为优势物种[3]。此外,由高通量测序结果(表4)可知,曝气前后23个样品的覆盖度均较高(99%),能较好反映样品的多样性情况;Shannon指数显示曝气对有机解磷菌的多样性影响不大;综合ACE和Chao1指数可知,曝气前有机解磷菌的丰富度高于曝气后。
-
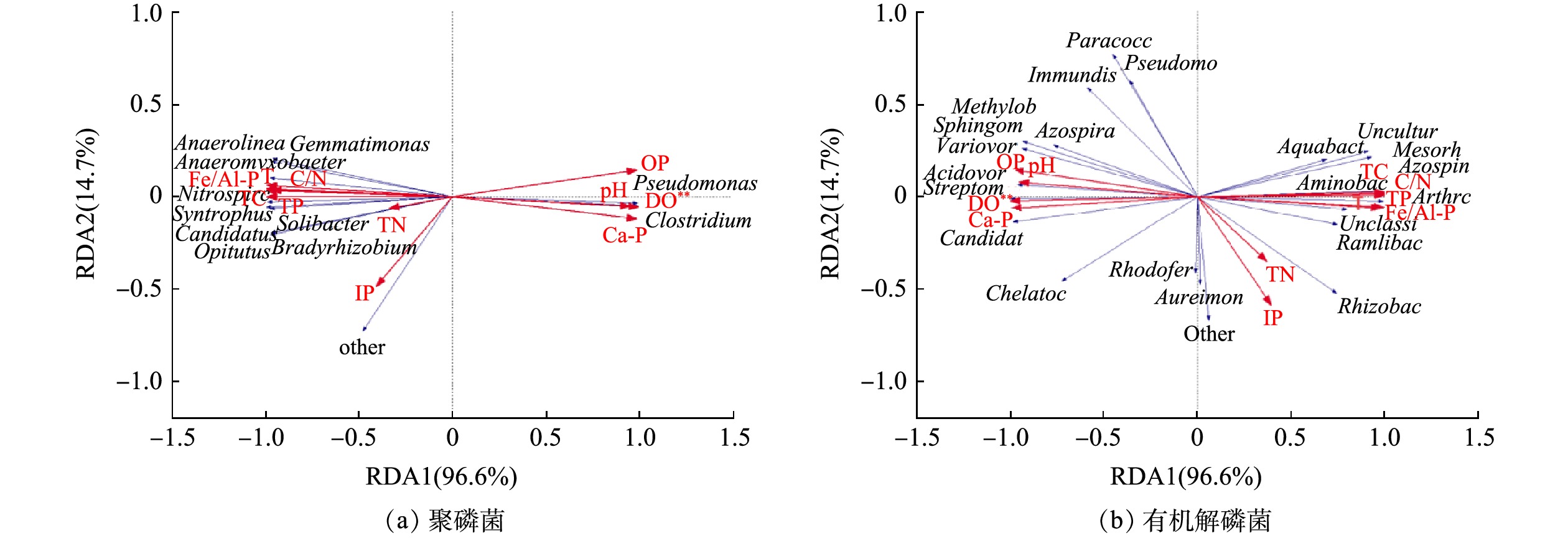
环境因子对沉积物微生物的群落结构有着重要影响。选取相对丰度排名相对较前的主要聚磷菌和有机解磷菌菌属与理化因子进行冗余(RDA)分析,结果见图10。聚磷菌的RDA分析结果表明,RDA1和RDA2共同解释了总体变化的98.3%,表明前两轴的贡献值可以较好地解释各因子对聚磷菌群落组成的影响。曝气前的聚磷菌群落组成主要与Fe/Al-P、T、C/N、TC、TP、TN、IP等环境因子呈正相关,而与DO、OP、Ca-P和pH呈负相关。曝气后的聚磷菌群落组成主要与DO、OP、Ca-P和pH呈正相关,而与Fe/Al-P、T、C/N、TC、TP、TN、IP等环境因子呈负相关。值得注意的是,DO与聚磷菌的群落组成显著相关(P<0.05)。另外,有机解磷菌的RDA分析结果表明,RDA1和RDA2共同解释了总体变化的90.0%,前两轴的贡献值可以较好地解释各因子对有机解磷菌群落组成的影响。曝气前的有机解磷菌群落组成主要与DO、OP、Ca-P和pH等环境因子呈正相关,而与Fe/Al-P、T、C/N、TC、TP、TN、IP呈负相关。曝气后的有机解磷菌群落组成主要与Fe/Al-P、T、C/N、TC、TP、TN、IP呈正相关,而与DO、OP、Ca-P和pH等环境因子呈负相关。其中,DO与有机解磷菌的群落组成显著相关(P<0.01),这证明DO是影响沉积物微生物群落的重要因素之一[41-42]。有研究表明,磷素、pH和TOC是影响沉积物细菌群落结构组成分布的主要环境因子[43-44]。其中,碱性磷酸酶活性影响磷功能菌的群落组成 [31, 45-46]。有研究表明,碱性磷酸酶活性随着溶解氧浓度的降低而增大,可促使水体中有机磷的分解和释放[46],这是OP与曝气后的磷功能菌群落组成呈负相关的原因。王晓蓉等[47]在研究太湖沉积物磷释放过程中发现,在厌氧释放过程中,存在着铁磷、铝磷向钙磷、闭蓄态磷转化的趋势。这释了本文曝气后阻止厌氧细菌释放Ca-P,从而与Ca-P的形成呈负相关。上述结果表明,由于微纳米曝气使得水库沉积物表层的DO发生改变,进而对磷功能菌的群落组成造成影响[48];此外,影响曝气前后磷功能菌群落组成的主要环境因子不同,说明他们在功能多样性上的差异。
当然,不同生境差异性大,影响微生物群落结构的环境因子也各不相同[46,49]。杜萍等[49]对长江口低氧高发海域的研究发现,底层水的有机磷细菌与DO呈显著正相关,表层沉积物中的无机磷细菌与DO呈显著正相关。刘欣[46]发现,长江口表层沉积物中的大部分微生物群落结构与磷浓度、温度、DO浓度具有明显的相关性。因此,对于特定生境,环境因子与磷功能菌之间存在何种关系,后续还需进一步深入研究,以期获得水库沉积物磷代谢微生物机制更为详细的信息。
-
1)微纳米曝气显著增加水体中的溶解氧。秋冬季,微纳米曝气的生态修复效果并不明显,可能与曝气实施时间较短,水体水温较低、微生物新陈代谢作用较弱等因素相关。
2)曝气对沉积物底部的充氧作用在一定程度上会对微生物的群落组成、结构造成影响,表现为曝气后,沉积物中Proteobacteria相对丰度上升了24.7%,Firmicutes上升了8.3%,而Chloroflexi下降了5.9%,Acidobacteria下降了7.0%,Spirochaetes下降了8.4%,其他门类含量则变化较小。
3)微纳米曝气促使适应曝气环境下的微生物成为优势菌属。曝气前检测到沉积物的聚磷菌优势菌属为Syntrophus、Anaeromyxobacter、Nitrospira和Anaerolinea,而曝气后变为Pseudomonas和Candidatus Solibacte;对于有机解磷菌,曝气前后均检测到的优势菌属有Azospira、Variovorax、Chelatococcus和Pseudomonas,区别在于曝气前多了一个优势菌属Mesorhizobium,而曝气后则多了一个优势菌属Acidovorax。
4) RDA分析结果表明,不管是聚磷菌还是有机解磷菌,其群落组成与沉积物表层的DO均显著相关(P<0.05),表明微纳米曝气通过提高DO浓度,对沉积物磷功能菌的群落组成、结构造成一定影响。
微纳米曝气对水库沉积物细菌及磷功能菌群落的影响
Effects of micro-nanometer aeration on bacterial and phosphorus functional bacterial communities in reservoir sediments
-
摘要: 为探究微纳米曝气对水库沉积物细菌及磷功能菌群落的影响,以三明市东牙溪水库为研究对象,通过Illumina高通量测序,分析了曝气前后沉积物细菌、聚磷菌与有机解磷菌的群落组成、结构及其与环境因子的相关性。结果表明,微纳米曝气对底部产生充氧作用,进而对沉积物微生物的群落造成一定影响,微纳米曝气后,变形菌门(Proteobacteria)的相对丰度上升了24.7%,厚壁菌门(Firmicutes)上升了8.3%,而绿弯菌门(Chloroflexi)下降了5.9%,酸杆菌门(Acidobacteria)下降了7.0%。曝气前,沉积物中聚磷菌的优势菌属包括Syntrophus(26.79%)、Anaeromyxobacter(14.6%)、Nitrospira(12.6%)和Anaerolinea(11.3%);而曝气后,聚磷菌优势菌属则为Pseudomonas(65.1%)和Clostridium(22.9%)。有机解磷菌的优势菌属在微纳米曝气前后同样发生了改变:在曝气前,优势菌属为Azospira、Mesorhizobium、Pseudomonas、Chelatococcus、Variovorax,分别占8.6%、5.4%、5.3%、5.2%、和5.2%;在曝气后,优势菌属则为Azospira、Variovorax、Chelatococcus、Pseudomonas、Acidovorax,分别占11.3%、6.5%、6.1%、5.9%和5.8%。冗余分析结果表明,不管是聚磷菌还是有机解磷菌,其群落组成与沉积物表层的溶解氧(DO)均显著相关(P<0.05)。微纳米曝气工程的实施,对水库沉积物微生物的群落组成、结构有一定影响。Abstract: In order to explore the effects of micro-nanometer aeration on bacterial and phosphorus functional bacterial communities in reservoir sediments, Dongyaxi reservoir in Sanming city was taken as the research object in this study. Illumina high-throughput sequencing was used to analyze the community composition and structure of bacteria, phosphorus-accumulating bacteria and organic phosphorus solubilizing bacteria before and after micro-nanometer aeration, as well as the correlation with environmental factors. The results show that micro-nanometer aeration had a certain influence on the microbial community in the sediment through oxygenation. After aeration, the abundance of Proteobacteria and Firmicutes increased by 24.7% and 8.3%, respectively. The abundance of Chloroflexi and Acidobacteria decreased by 5.9% and 7.0%, respectively. Before aeration, the dominant genera of phosphorus-accumulating bacteria in sediments included: Syntrophus (26.79%), Anaeromyxobacter(14.6%), Nitrospira(12.6%) and Anaerolinea (11.3%). After aeration, the dominant species were Pseudomonas (65.1%) and Clostridium (22.9%). The dominant genera of organic phosphorus solubilizing bacteria also changed before and after micro-nanometer aeration. Before micro-nanometer aeration, the dominant genera were Azospira, Mesorhizobium, Pseudomonas, Chelatococcus and Variovorax, accounting for 8.6%, 5.4%, 5.3%, 5.2% and 5.2%, respectively. After micro-nanometer aeration, the dominant genera were Azospira, Variovorax, Chelatococcus, Pseudomonas and Acidovorax, accounting for 11.3%, 6.5%, 6.1%, 5.9% and 5.8%, respectively. The results of redundancy analysis showed that there was a significant correlation between the community composition and the dissolved oxygen (DO) in sediment surface layer (P<0.05), regardless of phosphorus-accumulating bacteria or organic phosphorus solubilizing bacteria. The implementation of micro-nanometer aeration project has a certain influence on the composition and structure of microbial community in reservoir sediment.
-

-
表 1 微纳米曝气前后水体主要理化指标的变化
Table 1. Changes of water quality indexes after micro-nanometer aeration
采样批次 水层 pH DO/(mg·L−1) 水温/℃ 总氮/(mg·L−1) 总磷/(mg·L−1) 曝气前 表层 6.9±0.00 4.2±0.06 25.8±0.06 0.94±0.01 0.02±0.00 底层 6.8±0.10 3.0±0.15 25.4±0.25 曝气后 表层 7.2±0.06 8.2±0.06 17.3±0.06 0.92±0.01 0.03±0.00 底层 7.0±0.06 7.6±0.06 17.2±0.06 表 2 微纳米曝气前后沉积物细菌的多样性特征
Table 2. Diversity features of bacteria in sediments before and after micro-nanometer aeration
采样批次 OUT
数目Shannon指数 Simpson
指数ACE
指数Chao1
指数覆盖度 曝气前 18 632 8.78 6.7e-04 141 877.38 72 435.31 0.91 曝气后 12 377 7.29 0.01 102 868.55 50 368.36 0.99 表 3 微纳米曝气前后沉积物聚磷菌的多样性特征
Table 3. Diversity features of phosphorus-accumulating bacteria in sediments before and after micro-nanometer aeration
采样批次 OUT
数目Shannon指数 Simpson
指数ACE
指数Chao1
指数覆盖度/% 曝气前 672 4.01 0.05 878.26 838.92 99 曝气后 342 2.71 0.15 455.33 455.33 100 表 4 微纳米曝气前后沉积物有机解磷菌的多样性特征
Table 4. Diversity features of organic phosphorus solubilizing bacteria in sediments before and after micro-nanometer aeration
采样批次 OUT
数目Shannon指数 Simpson
指数ACE
指数Chao1
指数覆盖度/% 曝气前 4 973 6.63 6.6e-03 7 883.83 7 283.04 99 曝气后 4 029 6.74 3.9e-03 5 985.53 5 737.62 99 -
[1] UCHIDA T, YAMAZAKI K, GOHARA K. Generation of micro-and nano-bubbles in water by dissociation of gas hydrates[J]. Korean Journal of Chemical Engineering, 2016, 33(5): 1749-1755. doi: 10.1007/s11814-016-0032-7 [2] WU Y, LIN H, YIN W, et al. Water quality and microbial community changes in an urban river after micro-nano bubble technology in situ treatment[J]. Water, 2019, 11(1): 66. doi: 10.3390/w11010066 [3] 王硕, 刘蕴思, 李攀. 微纳米气泡对小微水体中好氧微生物群落的影响[J]. 中国给水排水, 2020, 36(15): 29-34. [4] 陆晖, 胡湛波, 蒋哲, 等. 微纳米曝气技术对城市景观水体修复的影响[J]. 环境工程学报, 2016, 10(4): 1755-1760. doi: 10.12030/j.cjee.20160427 [5] 潘俊, 孙舶洋, 魏炜, 等. 微纳米曝气-生态浮岛联合技术处理氮磷污染水体[J]. 环境工程, 2020, 38(5): 49-53. [6] 王骁, 吉贵祥, 周林军, 等. 微纳米气泡特性及黑臭河道环境修复工程应用[J]. 《环境工程》2019年全国学术年会论文集[C], 2019, 37: 116-120. [7] JING Z, HUANG G Q, LIU C, et al. Synergistic effect of microbubbles and activated carbon on the ozonation treatment of synthetic dyeing wastewater[J]. Separation and Purification Technology, 2018, 201: 10-18. doi: 10.1016/j.seppur.2018.02.003 [8] MOVAHED S, SARMAH A K. Global trends and characteristics of nano- and micro-bubbles research in environmental engineering over the past two decades: A scientometric analysis[J]. Science of the Total Environment, 2021, 785: 147362. doi: 10.1016/j.scitotenv.2021.147362 [9] 黄磊, 张太阳女, 苏玉萍, 等. 微纳米曝气工程对东牙溪水库水质改善效果[J]. 渔业研究, 2019, 41(5): 374-384. [10] 彭宇科, 路俊玲, 陈慧萍, 等. 蓝藻水华形成过程对氮磷转化功能细菌群的影响[J]. 环境科学, 2018, 39(11): 4938-4945. [11] 陈楠. 太湖沉积物微生物群落组成与物质循环及藻华爆发的相关性[D]. 北京: 中国农业大学, 2015. [12] 鲁小曼, 尚琨, 刘欣, 等. 长江口表层沉积物中可培养有机解磷菌多样性特征[J]. 海洋科学进展, 2019, 37(3): 495-507. doi: 10.3969/j.issn.1671-6647.2019.03.013 [13] 佘晨兴, 王静, 苏玉萍, 等. 福建省3座水库库心沉积物聚磷菌的群落特征[J]. 应用生态学报, 2019, 30(7): 2393-2403. [14] PAN Y W, CHENG K Y, KRISHNA K, et al. Improvement of carbon usage for phosphorus recovery in EBPR-r and the shift in microbial community[J]. Journal of Environmental Management, 2018, 218: 569-578. doi: 10.1016/j.jenvman.2018.03.130 [15] 张丽敏, 曾薇, 王安其, 等. 城市污水处理厂Candidatus Accumulibacter的菌群结构及定量分析[J]. 环境科学学报, 2016, 36(4): 1226-1235. [16] 王向英, 武欣, 张杰, 等. 解磷菌在复垦土壤中的定殖及促生效果研究[J]. 东北农业大学学报, 2021, 52(7): 40-47. doi: 10.3969/j.issn.1005-9369.2021.07.005 [17] 宋娟, 徐国芳, 赵邢, 等. 枫香根际解有机磷细菌筛选及其促生效应(英文)[J]. 南京林业大学学报(自然科学版), 2020, 44(3): 95-104. [18] 佘晨兴, 王静, 苏玉萍, 等. 三十六脚湖水库沉积物聚磷菌多样性及群落组成[J]. 应用与环境生物学报, 2018, 24(6): 1254-1262. [19] LIU Y Q, CAO X Y, LI H, et al. Distribution of phosphorus solubilizing bacteria in relation to fractionation and sorption behaviors of phosphorus in sediment of The Three Gorges Reservoir[J]. Environmental Science and Pollution Research, 2017, 24(21): 17679-17687. doi: 10.1007/s11356-017-9339-0 [20] 陈燕明. 东牙溪水库水质富营养化原因分析及防治对策[J]. 海峡科学, 2016, 114(6): 44-46. doi: 10.3969/j.issn.1673-8683.2016.06.014 [21] 林映津, 曾小妹, 陈倩, 等. 东牙溪水库蓝藻水华的应急与长效管控效果[J]. 渔业研究, 2020, 42(5): 429-444. [22] 李精精, 张玉珍. 综合营养状态指数法在东牙溪富营养化评价中的应用[J]. 海峡科学, 2017, 127(7): 3-5. doi: 10.3969/j.issn.1673-8683.2017.07.001 [23] RUBAN V, J F LÓPEZ-SÁNCHEZ, P PARDO, et al. Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments-a synthesis of recent works[J]. Analytical and Bioanalytical Chemistry, 2001, 370(2): 224. [24] 林映津, 陈瑜. 湖库流域生态基因纳米调控装置: CN103739099. A[P]. 2014-04-23. [25] 林映津. 微气泡及微纳米气泡扩散装置: CN102351267. B[P]. 2013-05-08. [26] MEHLIG L, LUDWIG F, ESCHENHAGEN M, et al. Development of new primer systems for the detection of the polyphosphate kinase gene in activated sludge[J]. Water Science and Technology, 2009, 30(2): 213-218. [27] 廖梓鹏. 农田土壤微生物碱性磷酸酶基因的多样性及其对磷素响应[D]. 广州: 华南理工大学, 2017. [28] KOICHIRO T, JOEL D, MASATOSHI N, et al. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0[J]. Molecular Biology and Evolution, 2007, 24(8): 1596-1599. doi: 10.1093/molbev/msm092 [29] 王丽娜, 陈辉煌, 刘乐冕, 等. 亚热带分层水库固氮微生物时空分布格局[J]. 生态学报, 2016, 36(18): 5827-5837. [30] YU Z, ZHOU J, YANG J, et al. Vertical distribution of diazotrophic bacterial community associated with temperature and oxygen gradients in a subtropical reservoir[J]. Hydrobiologia, 2014, 741(1): 69-77. doi: 10.1007/s10750-014-1832-6 [31] 张翠英, 徐德兰, 万蕾, 等. 云龙湖沉积物中氮磷含量和碱性磷酸酶活性季节变化分析[J]. 江西农业大学学报, 2013, 35(1): 209-215. doi: 10.3969/j.issn.1000-2286.2013.01.037 [32] 潘福霞, 来晓双, 王树志, 等. 曝气条件下进水C/N对水平潜流型人工湿地脱氮效果和氮转化功能微生物丰度的影响[J]. 环境工程学报, 2021, 15(4): 1386-1394. doi: 10.12030/j.cjee.202010136 [33] ZHAO Y, LIU B, ZHANG W, et al. Performance of pilot-scale vertical flow constructed wetlands in responding to variation ininfluent C/N ratios of simulated urban sewage[J]. Bioresource Technology, 2010, 101(6): 1693-1700. doi: 10.1016/j.biortech.2009.10.002 [34] ROBERTO A A, GRAY J, LEFF L G. Sediment bacteria in an urban stream: Spatiotemporal patterns in community composition[J]. Water Resource, 2018, 134: 353-369. [35] 姚丽平. 城市黑臭河道底泥微生物群落结构对人工曝气的响应特征及机理研究[D]. 上海: 华东师范大学, 2014. [36] SUN Y, WANG S, NIU J. Microbial community evolution of black and stinking rivers during in situ remediation through micro-nano bubble and submerged resin floating bed technology[J]. Bioresource Technology, 2018, 258: 187-194. doi: 10.1016/j.biortech.2018.03.008 [37] 伍思宇, 周志如, 尤青, 等. 海南东寨港红树林聚磷菌的筛选及其特性[J]. 应用与环境生物学报, 2016, 22(3): 397-401. [38] 李莹杰. 长江中下游湖泊沉积物中磷的GIS分布特征及解磷菌的研究[D]. 武汉: 武汉理工大学, 2016. [39] 王琛, 田欣欣, 曲凌云. 九龙江口解有机磷细菌的解磷特性[J]. 海洋环境科学, 2013, 32(5): 736-740. [40] 佘晨兴, 林洪, 苏玉萍, 等. 闽江口-平潭海域有机解磷菌多样性及群落特征[J]. 应用生态学报, 2021, 32(5): 1863-1872. [41] IZADI P, IZADI P, ELDYASTI A. Understanding microbial shift of enhanced biological phosphorus removal process (EBPR) under different dissolved oxygen (DO) concentrations and hydraulic retention time (HRTs)[J]. Biochemical Engineering Journal, 2020, 166: 107833. [42] 夏超. 溶解氧对水源水库多相界面微生物群落的影响研究[D]. 西安: 西安建筑科技大学, 2020. [43] HOLLISTER E B, ENGLEDOW A S, HAMMETT A J M, et al. Shifts in microbial community structure along anecological gradient of hypersaline soils and sediments[J]. The ISME Journal, 2010, 4(6): 829-838. doi: 10.1038/ismej.2010.3 [44] 李勇. 三岔湖微生物群落结构及其在磷素迁移转化中的作用[D]. 成都: 西南交通大学, 2020. [45] 苏玉萍, 郑达贤, 曾花森, 等. 浅层湖泊沉积物碱性磷酸酶活性垂向特征初探[J]. 福建师范大学学报(自然科学版), 2005, 21(3): 35-38. [46] 刘欣. 长江口低氧区表层沉积物中微生物群落及phoD碱性磷酸酶基因的时空变化[D]. 山东: 齐鲁工业大学, 2019. [47] 王晓蓉, 华兆哲, 徐菱, 等. 环境条件变化对太湖沉积物磷释放的影响[J]. 环境化学, 1996, 15(1): 15-19. [48] SIMON H M, SMITH M W, HERFORT L. Metagenomic insights into particles and their associated microbiota in a coastal margin ecosystem[J]. Frontiers in Microbiology, 2014, 5(466): 1-10. [49] 杜萍, 刘晶晶, 曾江宁, 等. 长江口低氧区异养细菌及氮磷细菌分布[J]. 应用生态学报, 2011, 22(5): 1316-1324. -



 下载:
下载: