-
磷回收是未来城镇污水处理厂提升经济和环境双重效益的重要途径。目前,从污水中进行磷回收的方法主要有磷酸铵镁结晶法(MAP)、羟基磷酸钙结晶法(HAP)、铝盐和铁盐沉淀以及以离子交换法为代表的新方法[1]。其中,HAP结晶法具有易分离、生成物溶度积小、对进水氮磷比要求低等优点,因而对城镇污水的磷回收有更强的适用性,尤其适用于从污泥浓缩池上清液(磷酸盐浓度12.98~160 mg·L−1)、强化生物除磷系统的厌氧上清液(磷酸盐浓度17.5~28.2 mg·L−1)等富磷溶液中回收磷[2-3]。以往研究大量集中在HAP结晶过程中操作参数的影响,例如结晶体系的pH、钙磷摩尔比(n(Ca)/n(P))、晶种投加量、曝气强度等[4-6],而对城镇污水中不容忽视的重金属离子的影响则鲜有探讨。
城镇污水中的重金属杂质广泛来源于地表径流、工业废水和生活污水,包括Zn、Al、Cd、Cr、Cu、Fe、Mn、Ni、Pb等,其浓度在几微克每升到几千微克每升的范围内波动[7]。Cu、Cd、Zn是污水中常见的生态风险较大的重金属。杨妍妍等[8]研究发现,北京的8家污水厂在2011—2017年的Cu、Cd、Zn含量均值(每千克脱水污泥)分别高达115、1.21和677 mg,属于重污染级别。这些重金属离子易被活性污泥富集,又会于厌氧释磷过程中再次被释放,难以避免地随富磷上清液进入结晶工艺[9]。众所周知,重金属对生物体具有持久危害性、毒性与累积放大性,即使是微量的重金属进入结晶产品也可能对环境造成二次污染。因此,重金属对HAP结晶过程和产品的影响是关乎生产效益与产品安全的现实问题。
根据现有研究结果[10-11],在磷酸铵镁和透钙磷石结晶体系中,重金属离子不能稳定存在于液相,极有可能参与晶核形成,进而会影响产品的纯度。但在HAP结晶体系中,围绕重金属展开的研究仍然较少。因此,本研究着重研究了在适宜的操作参数下,单独和联合投加Cu2+、Cd2+、Zn2+对HAP结晶法回收磷的影响特征,同时考察了重金属离子自身的浓度变化情况,并结合Visual MINTEQ模型验证相关规律。
全文HTML
-
实验仪器:紫外分光光度计(UV 9100 B)、pH测定仪(YSI pH 100,美国)、等离子体发射光谱仪(Optima 8000,美国)、场发射扫描电子显微镜(Quanta 2000 FEI,美国)等。
实验试剂:KH2PO4、CaCl2、Cd(NO3)2·4H2O、Cu(NO3)2·3H2O、ZnCl2、NaOH均为分析纯,所有溶液均使用去离子水配制。
-
采用批量实验研究3种重金属离子在单独投加和联合投加2种形式下对磷去除效果的影响,重金属离子投加量分6个梯度(0、5、10、15、20、25 mg·L−1)。所有反应于500 mL锥形瓶中进行,设定初始pH=9.0、TP=20 mg·L−1、n(Ca)/n(P)=2.0、水温20 ℃、反应时间10 min,以恒温磁力搅拌器维持结晶体系内部流态化。分别在反应前和反应10 min时利用0.22 µm的针孔过滤器取10 mL溶液检测剩余磷浓度和重金属含量。结晶沉淀经抽滤、烘干处理后进行电镜扫描。
-
采用抑制模型[12](式(1))分析重金属离子在单独投加时对结晶体系磷去除率的影响。
式中:Y为重金属离子对除磷的抑制率;E0为空白组的磷去除率;E为投加重金属离子时的磷去除率。重金属离子初始浓度与磷去除率的关系采用修正的Monod方程(式(2))进行拟合。
式中:Ci为重金属离子的初始浓度,mg·L−1;Ki对应重金属离子的抑制常数,mg·L−1。
-
利用Visual MINTEQ模型进行结晶体系的化学平衡计算,以验证和解释重金属对HAP结晶的影响。构建模型所需的反应条件和物料浓度与批量实验保持一致;所有未设定输入数据均采用缺省值;同时考虑“允许”和“不允许”过饱和物质析出的情形,以关注饱和指数(Is)和析出产物的变化。Is用来描述HAP反应体系的饱和状态[13],可根据式(3)进行计算。
式中:Is为某化合物的饱和指数;
$ {K}_{\rm{iap}} $ 、$ {K}_{\rm{sp}} $ 分别指某化合物离子活度积和溶度积常数。Is>0时,溶液过饱和;Is =0时,沉淀溶解平衡;Is<0时,溶液不饱和。
1.1. 实验仪器及药品
1.2. 实验方法
1.3. 抑制模型
1.4. Visual MINTEQ模型的建立
-
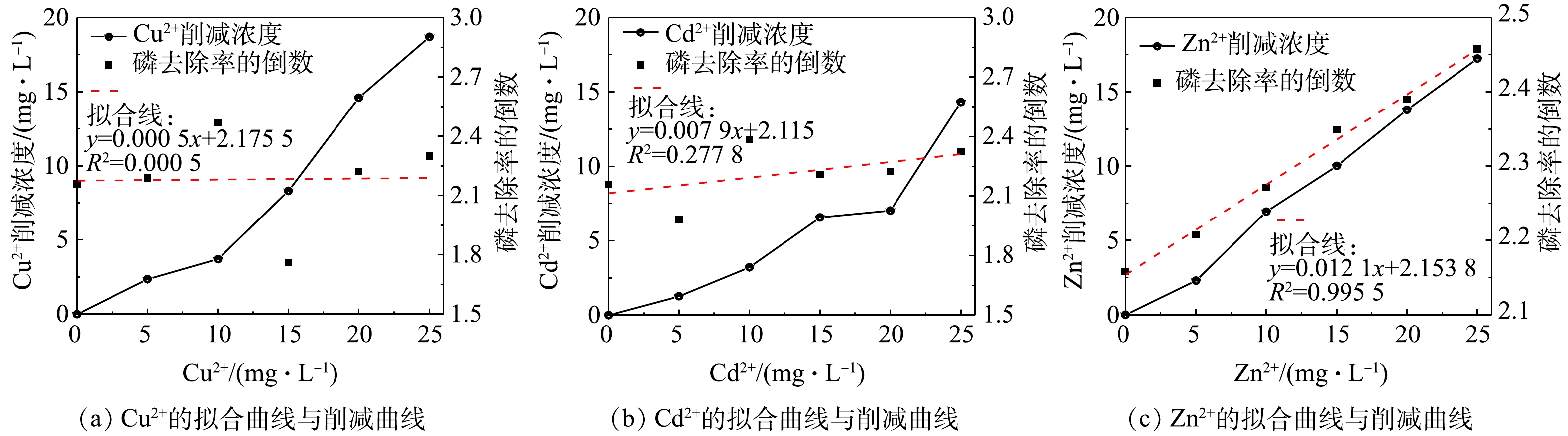
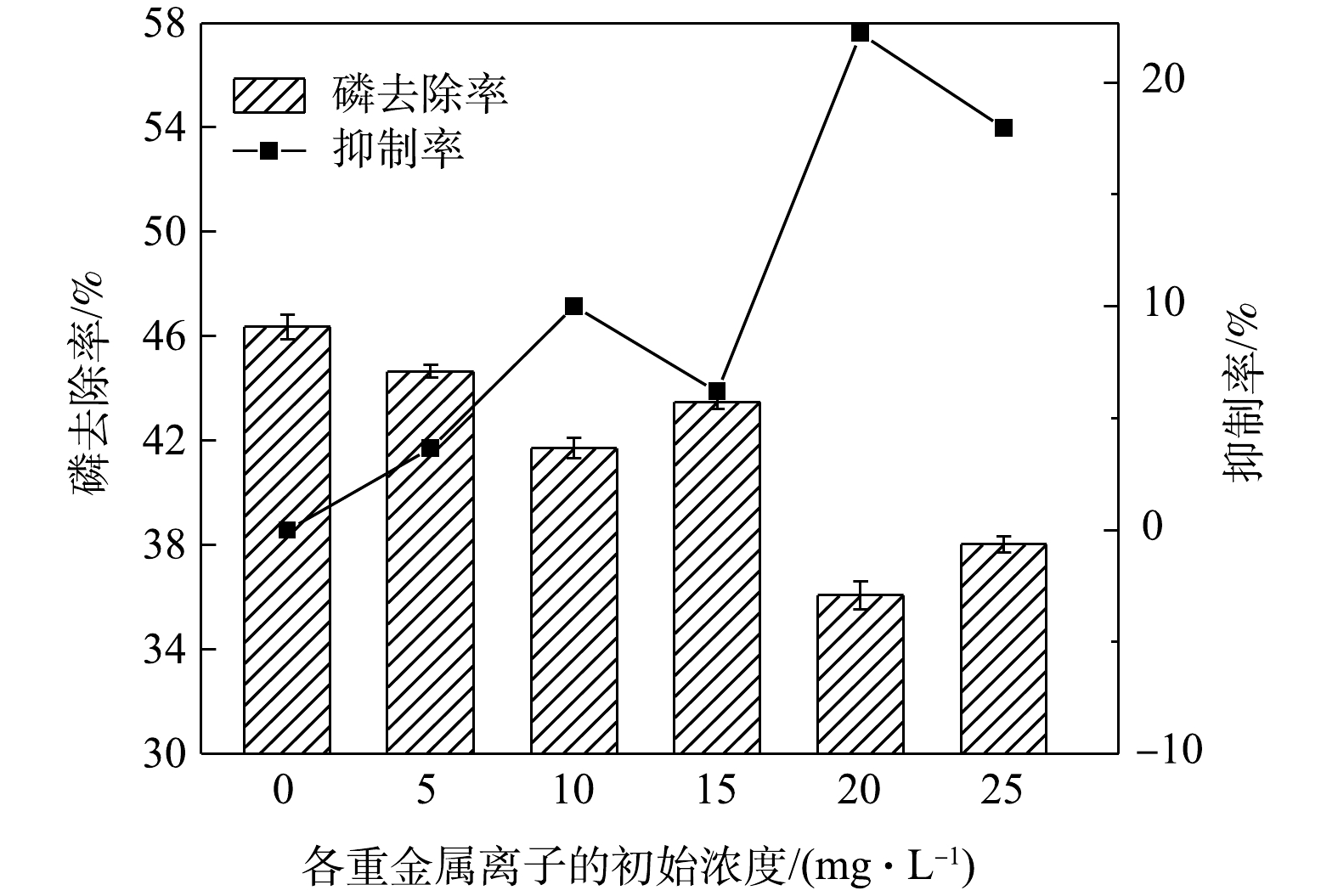
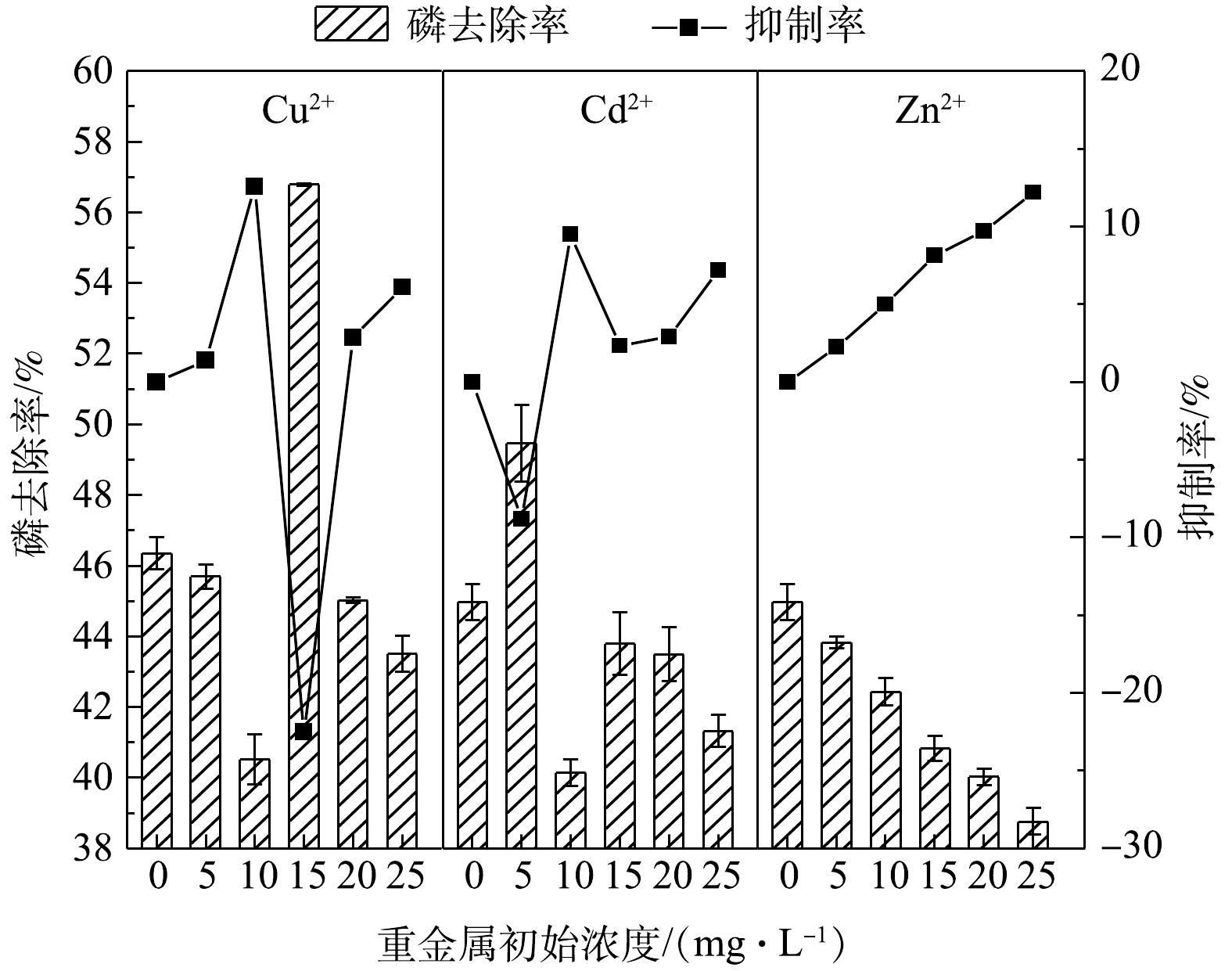
1)单一重金属离子的影响。由图1可知,向HAP结晶体系单独投加Cu2+/Cd2+/Zn2+时,随着重金属投加量的增加,3组反应的磷去除率均发生显著改变。为了进一步分析该影响的特征,利用式(2)计算每种重金属对除磷的抑制率,结果如图1所示。由图1可知,随着重金属浓度的增加,Cu2+和Cd2+对除磷的抑制率呈现波动变化,而Zn2+对除磷的抑制率稳步上升。这说明Zn2+对HAP结晶法除磷的抑制作用比Cu2+和Cd2+的抑制作用更稳定,这一结果与MURYANTO等[14]在研究Zn2+、Cu2+对磷酸铵镁结晶生长的影响时得出的结论相似。此外,Cu2+和Cd2+对除磷的影响以抑制作用为主,仅当Cu2+和Cd2+的浓度分别为15 mg·L−1和10 mg·L−1时,抑制率为负值,呈现反向促进作用。导致这种现象的主要原因是:当Cu2+和Cd2+的浓度分别达到15 mg·L−1和10 mg·L−1附近时,Cu2+、Cd2+开始与
${\rm{PO}}_4^{3 - }$ 结合生成沉淀,反而促进了液相中磷的去除;而随着Cu2+和Cd2+的浓度进一步加大,Cu2+、Cd2+共沉淀消耗大量OH−,导致HAP结晶量大大下降,随之而来的除磷量的下降无法由铜或镉的磷酸盐沉淀完全弥补。DAI等[15]在研究Fe3+、Cu2+对HAP结晶的影响时也发现有类似的现象。3种重金属离子的抑制模型拟合结果如图2(a)~图2(c)所示,Zn2+的投加浓度与磷去除率的倒数(E−1)存在显著的线性关系 (R2=0.995 5),符合修正的Monod方程。根据式(3)求得 Zn2+的抑制常数为178.0 mg·L−1,即Zn2+对磷去除率的半最大抑制浓度。Cu2+和Cd2+的抑制模型拟合效果不佳(R2远远小于0.9)。此外,图2(a)~图2(c)也表明3种重金属离子的浓度在反应过程中会不断削减,削减量随初始浓度的增加而增加。其中,仅Zn2+的削减浓度与E−1存在较显著的正相关关系,R2为0.996 5(P<0.05),Cu2+和Cd2+不存在类似规律。
2)重金属离子的联合影响。由图3可见,联合投加Cu2+、Cd2+、Zn2+时,随着重金属浓度的增加,结晶体系中磷的去除率呈现不同程度的下降。当3种重金属的浓度均达到20 mg·L−1时,他们对磷去除率的联合抑制率达到最大,为22.19%,远远超过Cu2+、Cd2+、Zn2+各自在单独投加时的最大抑制率12.57%、9.49%和12.20%,同时也超过单独投加Cu2+、Cd2+、Zn2+各20 mg·L−1时的抑制率总和(15.51%)。由此可见,相比于单独投加,联合投加Cu2+、Cd2+、Zn2+对结晶体系磷去除率的抑制作用更强。
-
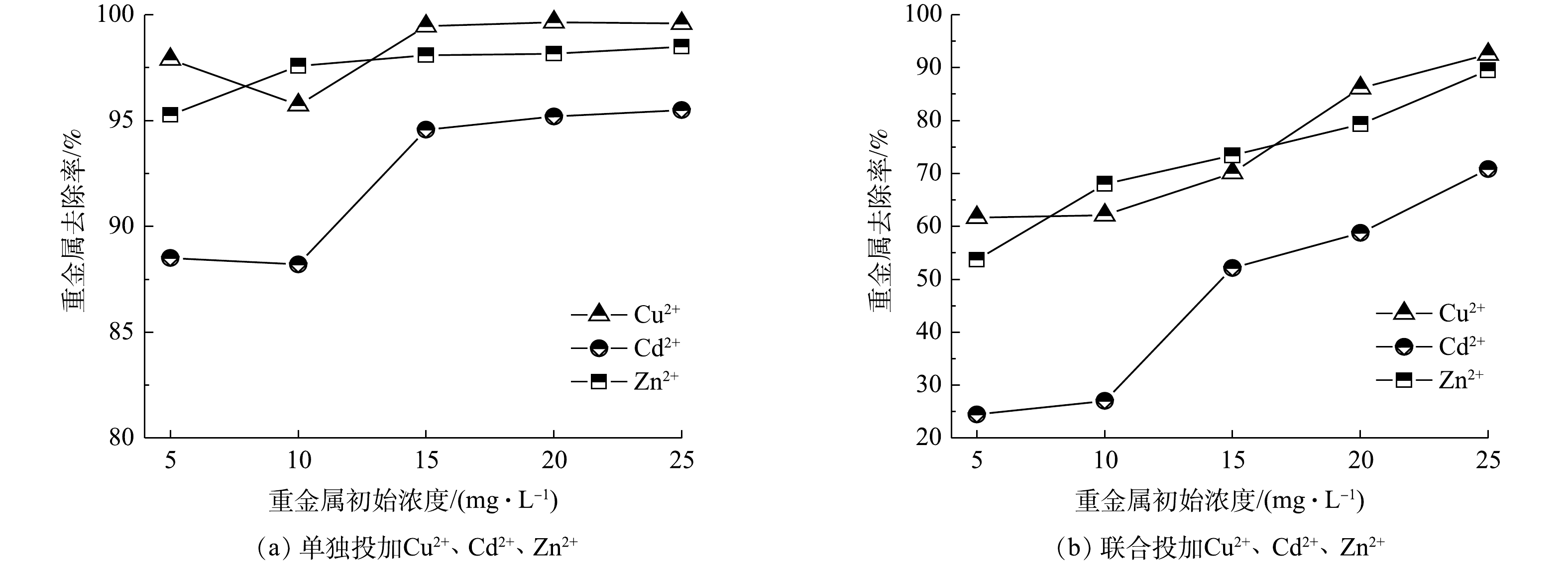
本研究结果表明,HAP结晶体系可以协同去除磷和重金属。如图4(a)所示,在单独投加重金属离子的形式下,随着重金属初始浓度的增加,Cu2+、Cd2+、Zn2+的去除率均呈上升趋势,Cu2+的去除率最大,Zn2+次之,Cd2+最小。对比图4(a)和图4(b)可以发现,3种重金属在联合投加时的去除率均比单独投加时对应的去除率要低。这说明当Cu2+、Cd2+、Zn2+共存于结晶体系时,单个重金属的去除会受到另两者的拮抗。产生以上现象的主要原因是:Cu2+、Cd2+、Zn2+会在碱性条件下竞争OH−,发生如式(4)~式(6)所示的化学反应。
由于Ksp[Cu(OH)2]<Ksp[Zn(OH)2]<Ksp[Cd(OH)2],故Cu2+比 Zn2+、Cd2+更易形成共沉淀,使得液相中Cu2+的去除率最高,Cd2+的去除率最低。此外,重金属离子会取代部分Ca2+[16],或与HAP的表面基团络合,从而发生单分子层化学吸附[17],因此,3种重金属离子也可能竞争结晶体系中HAP前驱物表面的可吸附点位,从而强化相互拮抗作用。
-
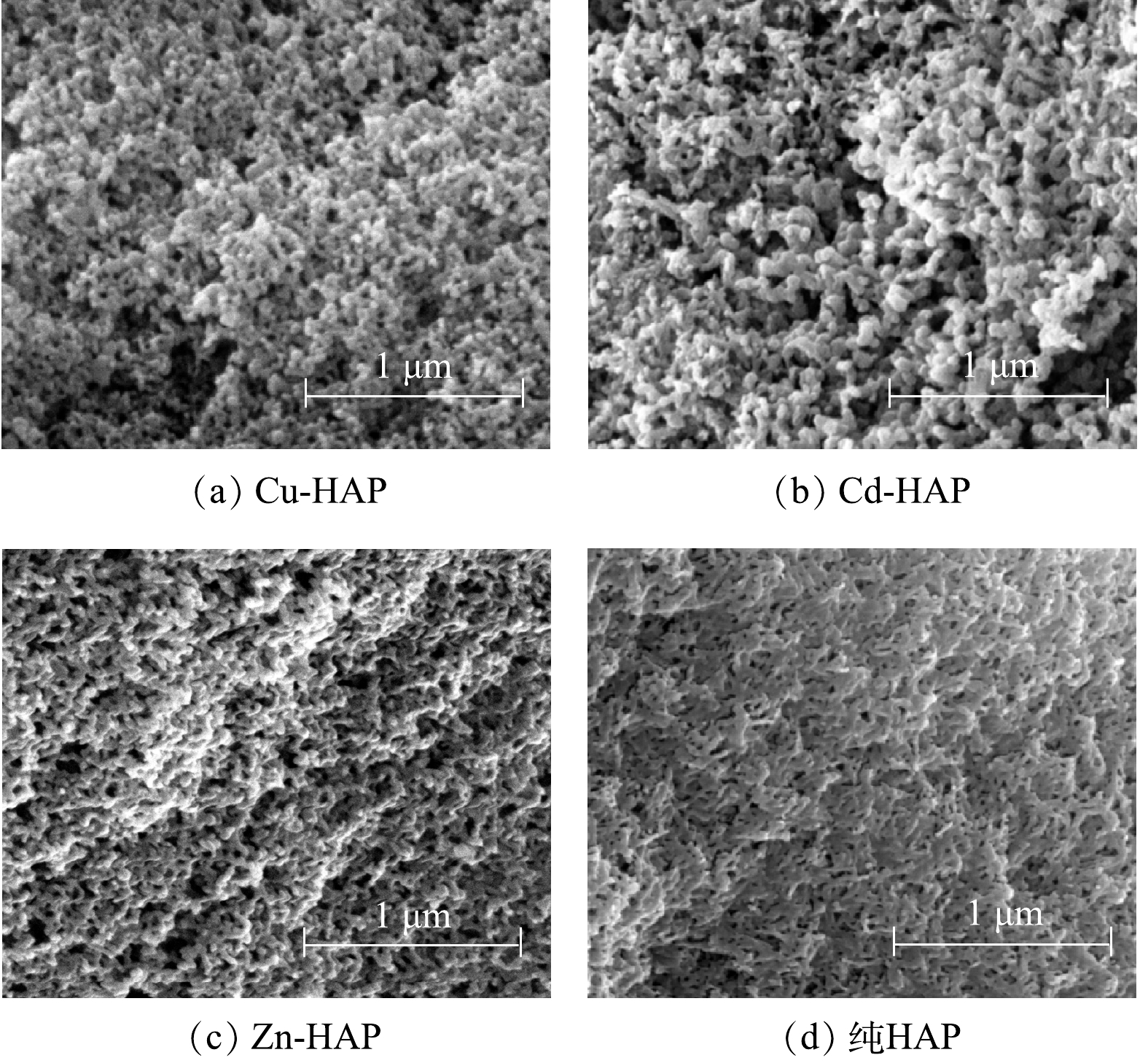
图5为Cu-HAP、Cd-HAP、Zn-HAP和纯HAP晶体的SEM图像。Cu-HAP、Cd-HAP、Zn-HAP分别表示Cu2+、Cd2+、Zn2+的初始浓度为25 mg·L−1时的HAP结晶体系产物。由图5可知,Cu-HAP、Cd-HAP、Zn-HAP和纯HAP晶体均具有疏松多孔的表面特征,但Cu-HAP、Cd-HAP、Zn-HAP的晶体表面比纯HAP晶体更为松散,这一改变可能是金属离子进入晶格和/或这些离子吸附在晶体表面所引起的应力造成的。同时,Cu-HAP、Cd-HAP和Zn-HAP三者的表面形貌无显著差异,这说明3种重金属对晶体表面形貌的影响程度相当。
-
1)模拟单一投加Cu2+/Cd2+/Zn2+。结晶体系中主要的过饱和物质及其Is值如表1、表2、表3所示。可以看出,3种重金属在HAP结晶体系中均可能与OH−和
${\rm{PO}}_4^{3 - }$ 生成多种过饱和物质。随着重金属初始浓度的增加,HAP的Is值减小,结晶速率降低,表明HAP的Kiap值下降,构成化合物的离子有效浓度降低;而含铜/镉/锌化合物的Is值增大,结晶动力增强,说明含铜/镉/锌化合物的Kiap值上升,构成化合物的离子有效浓度升高。这证实了3种重金属离子进入结晶体系后会通过争夺HAP的构晶离子(OH−和${\rm{PO}}_4^{3 - }$ )的方式来抑制HAP结晶。2)模拟联合投加Cu2+、Cd2+、Zn2+各25 mg·L−1。由表4可知,可能形成的沉淀种类复杂:除HAP、Ca3(PO4)2、CaHPO4外,Cu2+主要形成Cu(OH)2及相关的盐、Cu3(PO4)2和CuO,其中的CuO极易由亚稳态的Cu(OH)2脱水转化而来[18];Cd2+主要形成Cd3(PO4)2;Zn2+主要形成Zn(OH)2及相关的盐、Zn3(PO4)2·4H2O和ZnO,其中的ZnO在碱性环境下可由Zn(OH)2转化而来[19]。比较不同化合物的Is值可以看出,与OH−结合的重金属化合物中,Cu(OH)2的Is值最大,Zn(OH)2的Is值次之,Cd(OH)2的Is值接近于0,说明OH−更易与Cu2+结合,其次是Zn2+,与Cd2+几乎不会结合产生沉淀;与
${\rm{PO}}_4^{3 - }$ 结合的重金属化合物中,Zn3(PO4)2·4H2O的Is值最大,Cd3(PO4)2次之,Cu3(PO4)2最小,说明${\rm{PO}}_4^{3 - }$ 更易与Zn2+结合,其次是Cd2+和Cu2+。因此,Cd2+主要通过结合${\rm{PO}}_4^{3 - }$ 来抑制HAP的结晶,而Cu2+和Zn2+通过与${\rm{PO}}_4^{3 - }$ 或OH−结合来抑制HAP的结晶。由表5可知,模拟联合投加时,
${\rm{PO}}_4^{3 - }$ -P的去除率高达89.361%,而固相中的n(Ca)/n(P)为1.08,低于纯HAP的n(Ca)/n(P)(1.67)。这说明${\rm{PO}}_4^{3 - }$ -P并未完全以HAP的形式进入固相,重金属的引入降低了结晶体系的产物纯度。模拟反应体系中Cu2+、Cd2+、Zn2+的沉淀率分别为93.300%、0和79.123%,这表明液相中重金属去除率的排序为Cu2+>Zn2+>Cd2+,这与3.3节的实验结果是一致的。在本研究中,Visual MINTEQ模拟计算仅考虑化学反应,关于重金属与HAP前驱物的吸附特性有待进一步研究。
2.1. 重金属离子对磷去除率的影响
2.2. 重金属离子的浓度削减规律
2.3. 重金属对结晶产物表面形貌的影响
2.4. Visual MINTEQ模拟分析
-
1) 3种重金属(Cu2+、Cd2+、Zn2+)对HAP结晶法除磷均会产生抑制,其中Zn2+的抑制作用最稳定且符合抑制模型,抑制常数为178.0 mg·L−1,Zn2+的削减浓度与E−1正相关。联合投加3种重金属会增强对磷去除率的抑制。
2) HAP结晶法可协同去除重金属,去除率的顺序为Cu2+>Zn2+>Cd2+。重金属的初始浓度越大,去除率越大。联合投加时,Cu2+、Cd2+、Zn2+三者相互拮抗,去除率均有所降低,降幅排序为Cd2+>Zn2+>Cu2+。
3) Cu2+、Cd2+、Zn2+的引入使得结晶产物变得更为松散,但3种重金属对晶体表面形貌的影响程度相当。
4) Visual MINTEQ的模拟计算结果证实,Cu2+、Cd2+、Zn2+主要通过争夺HAP的构晶离子来抑制磷去除率,并通过共沉淀降低产物纯度。HAP的构晶离子中,OH−易与Cu2+结合,
${\rm{PO}}_4^{3 - }$ 易与Zn2+结合。3种重金属进入结晶产物的含量大小为Cu2+>Zn2+>Cd2+。



 下载:
下载: