-
反硝化除磷菌(DPBs)具有与普通聚磷菌类似的代谢机理。在厌氧条件下DPBs利用胞内聚磷(Poly-P)及糖原(Gly)分解所获得的能量将挥发性脂肪酸(VFA)转移至体内合成PHA,宏观表现为
PO3−4 -P浓度的升高,该阶段为释磷阶段。在缺氧条件下,DPBs利用NO−x -N代替O2作为电子受体,将厌氧合成的PHA分解,产生的能量用于吸收PO3−4 -P并合成Poly-P存储于胞内,同时伴随着糖原的再生,即缺氧吸磷,从而实现了氮磷的同步去除[1]。目前大多数的反硝化除磷研究都是以NO−3 -N作为电子受体,并且效果良好。而NO−2 -N是硝化和反硝化过程的中间产物,DPBs若能以其作为电子受体则能减少碳源消耗及曝气量,并且由于其生长速率相对较慢,因此,也能减少污泥的产量。近期有研究[2-3]表明,当NO−2 -N浓度较低时,DPBs能够以NO−2 -N为电子受体吸磷,并且未受到抑制。而短程硝化与全程硝化相比具有节省曝气量、反应速率快等优点[4],因此,短程反硝化除磷工艺受到更多学者的关注。近年来,多数研究者采用批次实验验证了
NO−2 -N作为反硝化除磷电子受体的可行性,然而,鲜有研究者考察NO−2 -N对长期运行效能的影响。ABR反应器具有微生物相分离以及对底物不同阶段和程度转化的优势,可产生VFA等优质碳源,同时,MBR具有高效的生物截留作用而被日益广泛地使用。本研究采用ABR-MBR工艺,驯化DPBs对NO−2 -N的耐受程度,考察了在长期运行条件下NO−2 -N对反硝化吸磷的抑制程度及耐受限度,以期寻找出最佳的运行负荷,以实现碳氮磷的同步高效去除。
全文HTML
-
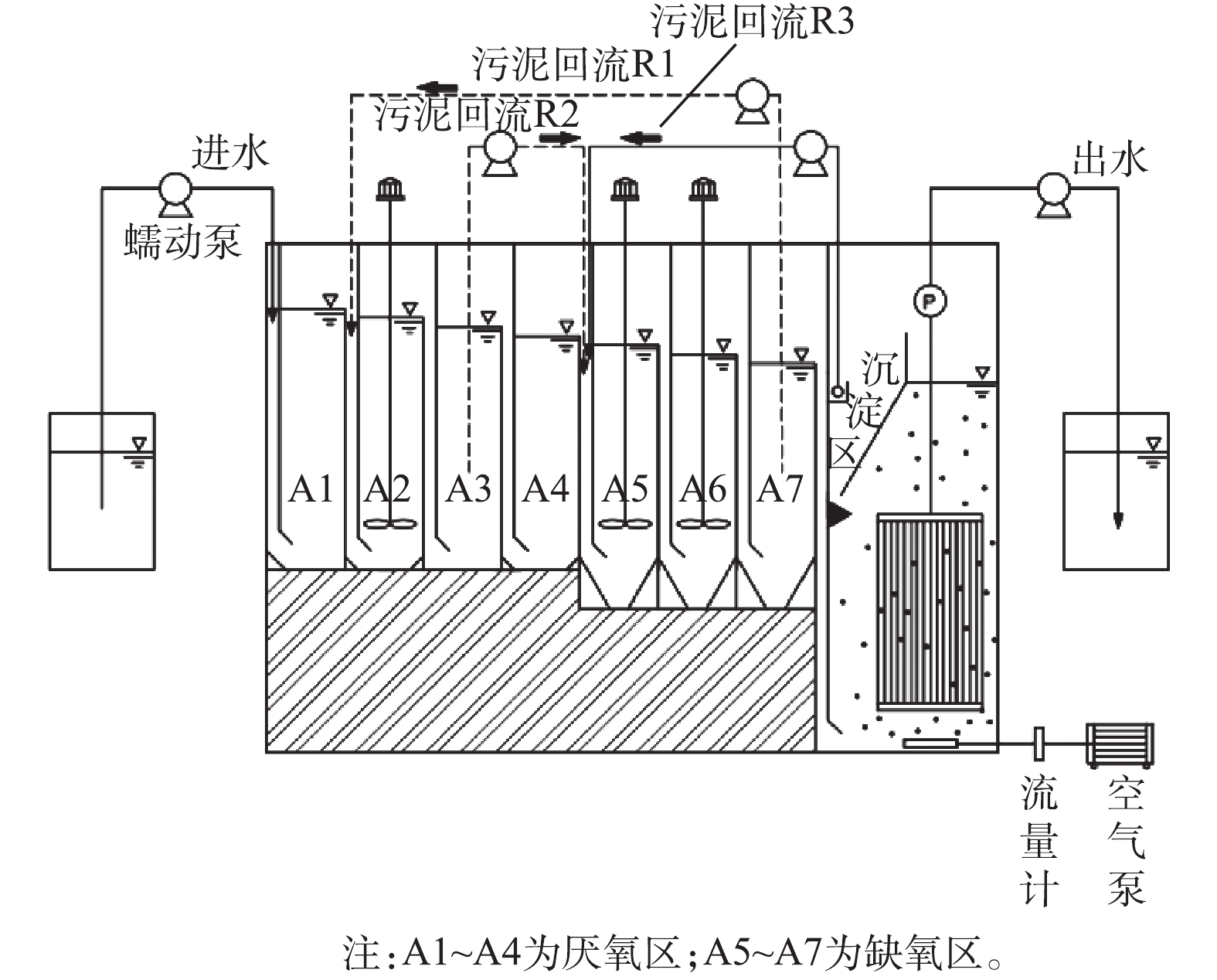
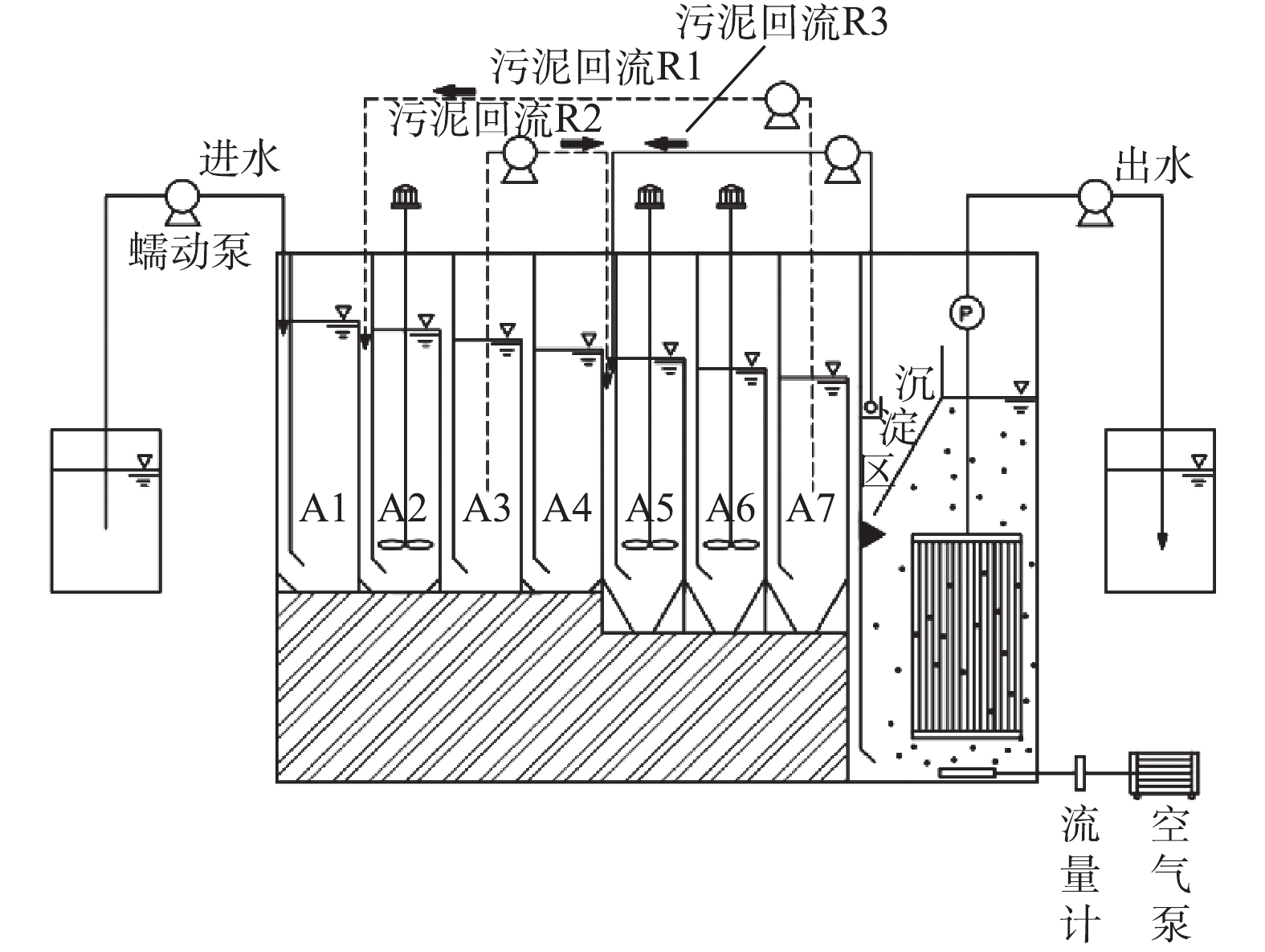
ABR-MBR工艺实验装置及处理流程如图1所示,其由含有7个隔室的ABR反应器及好氧MBR反应器组成。反应器总有效容积为11.4 L,其中ABR有效容积为7.6 L,A1~A4为厌氧区,A5~A7为缺氧区,MBR设有沉淀区进行硝化液回流,有效容积为3.8 L,采用间歇抽吸出水,抽吸周期为10 min (8 min抽吸出水和2 min反冲洗)。ABR-MBR工艺设有3个回流为R1、R2、R3。污泥回流R1(污泥为A7~A2),将富含DPBs的污泥回流至厌氧A2隔室,通过A1隔室的水解酸化作用,旨在为DPBs提供优质碳源;污泥回流R2(污泥为A3~A5),利用A4隔室对剩余碳源进一步降解,消除传统反硝化菌对DPBs电子受体的竞争;硝化液回流R3从MBR沉淀区回流至A5,为DPBs提供电子受体。
表1为实验过程及其中对应的参数。由表1可知,实验分为4个工况,第1工况共运行22 d,其他每个工况均运行14 d。实验前152 d主要包括反硝化除磷的启动和考察HRT对系统的影响,得出最佳条件:ABR反应器HRT为9 h、污泥回流比保持在80%、硝化液回流比稳定在300%、反硝化除磷功能区(A2、A3、A5~A7)污泥龄(SRT)为25 d。控制MBR反应器内溶解氧在0.5~1.0 mg·L−1,水温通过水浴加热维持在(30±2) ℃,污泥龄为15 d。控制系统进水C/N/P值不变,逐步提高进水基质浓度,稳定MBR内短程硝化的运行,以实现ABR-MBR短程反硝化除磷工艺的优化与稳定。
-
COD、
NH+4 -N、NO−2 -N、NO−3 -N、PO3−4 -P、TN等指标采用标准方法[5]测定,水样采用0.45 μm中速滤纸过滤,以去除悬浮物的影响,其中COD采用快速消解法;NH+4 -N采用纳氏试剂光度法测定;NO−2 -N采用N-(1-萘基)-乙二胺光度法测定;NO−3 -N采用紫外分光光度法;PO3−4 -P采用钼锑抗分光光度法;TN采用过硫酸钾氧化-紫外分光光度法;MLSS采用滤纸称重法测定。亚硝酸盐积累率(NAR)按照式(1)计算。游离亚硝酸(FNA)及游离氨(FA)值按照式(2)和式(3)进行计算。
式中:η为亚硝酸盐积累率;
CNO−2-N 和CNO−3-N 为反应器中NO−2 -N及NO−3 -N的质量浓度,mg·L−1。式中:CFNA为游离亚硝酸的浓度,mg·L−1;CFA为游离氨的浓度,mg·L−1;T为该系统的温度,℃;
C+NH4-N 为反应器中NH+4 -N的质量浓度,mg·L−1。
1.1. 实验装置及运行
1.2. 分析方法
-
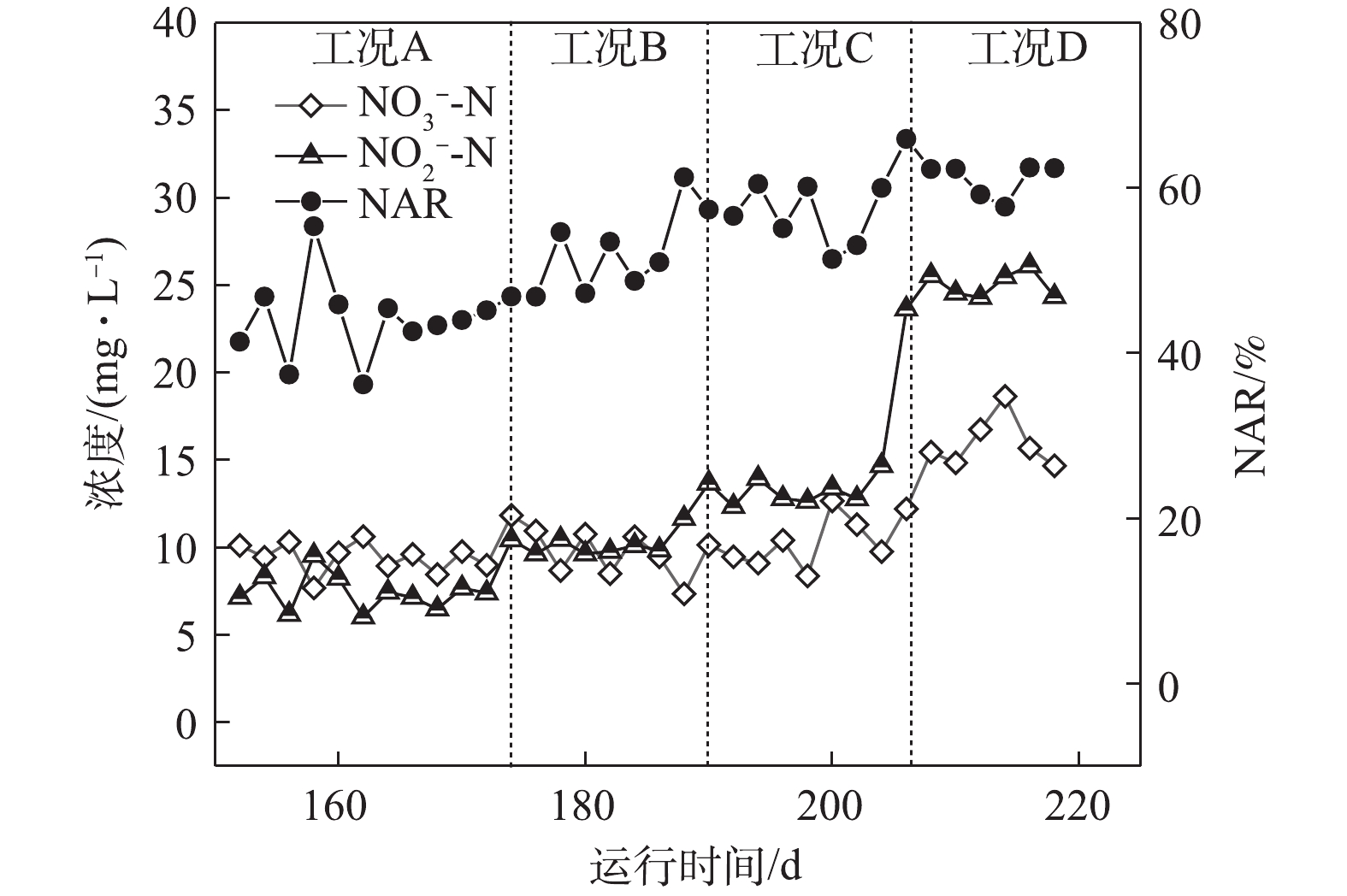
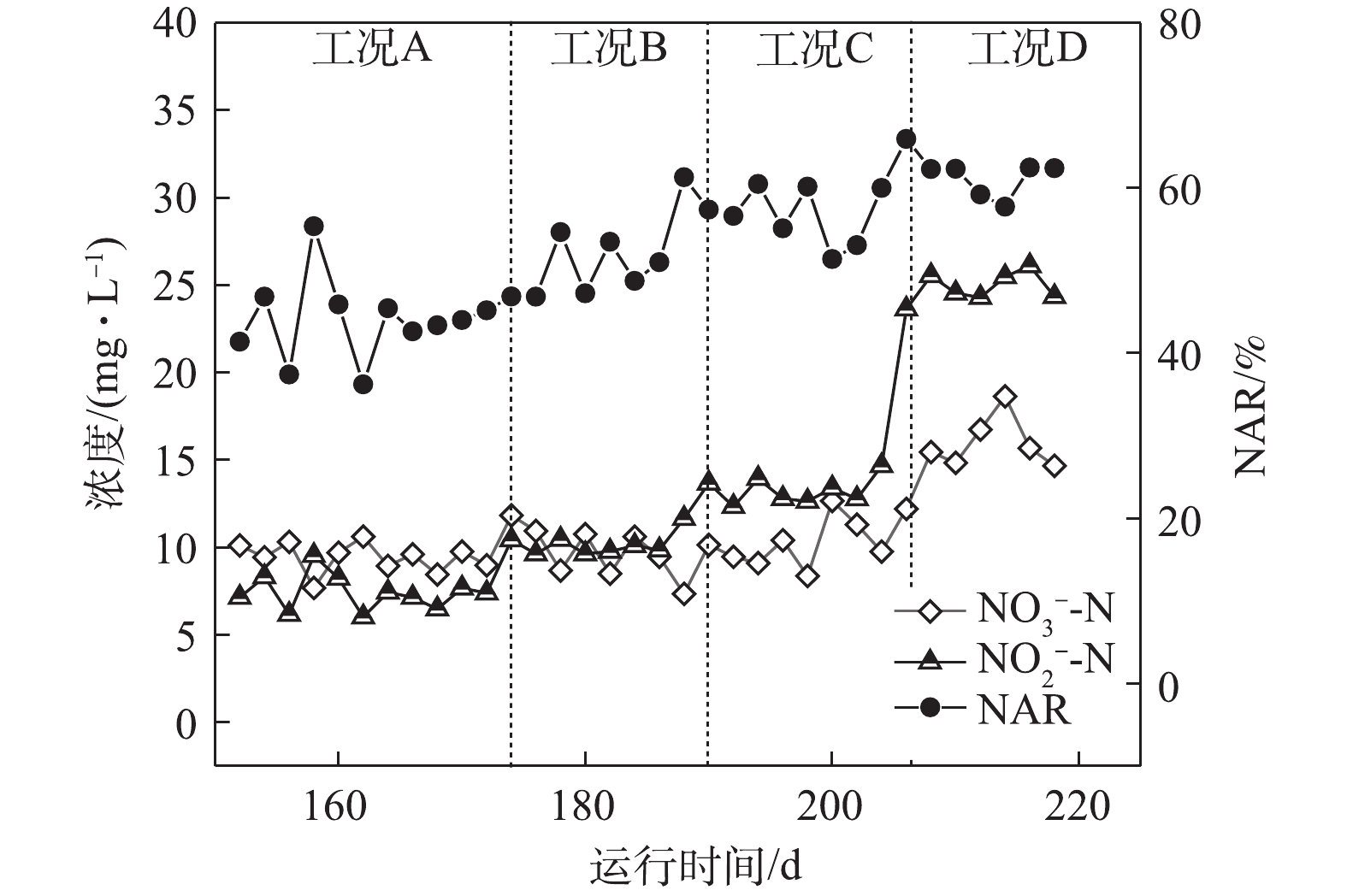
如图2所示,在工况A、B、C和D下,MBR反应器内对应的平均亚硝酸盐积累率分别为43.9%、51.3%、56.8%和61.7%。在工况A下,
NH+4 -N转化率高达98%;随着反应的持续进行,较低的NH+4 -N负荷使得AOBs的基质逐步缺失,同时较低的NH+4 -N浓度也使得FA值较低,因此,MBR反应器对NOBs的抑制效果较差,具有较强活性的NOBs会将NO−2 -N继续氧化为NO−3 -N,从而使得NAR较低,所以,保证充足的氨氮浓度和抑制NOBs的生长是短程硝化稳定运行的关键所在[6]。随着基质浓度的上升,NH+4 -N负荷逐渐升高,在工况C和工况D下,出水NH+4 -N出现少量剩余,这说明AOBs基质浓度充足,且其活性逐渐增强。本研究中,短程硝化稳定运行的因素主要归于以下几点:首先是合适的SRT。MULDER等[7]的研究表明,在14 ℃以上时,AOBs和NOBs的世代周期分别为8~36 h和12~59 h。因此,将污泥龄控制在AOBs与NOBs最小世代周期内,NOBs就会被逐渐淘洗掉,使AOBs成为优势菌群,本研究通过排除泥水混合液将泥龄控制在15 d。其次是适宜的温度。在20 ℃时,AOBs和NOBs的比增长速率μmax分别为0.801 d−1和0.788 d−1;当温度低于20 ℃时,AOBs的μmax小于NOBs,大于20 ℃时则相反[8]。再次是维持较低的DO浓度。有研究[9]表明,AOBs的氧饱和系数为0.2~0.4 mg·L−1,NOBs的氧饱和系数为1.2~1.6 mg·L−1,因此,控制DO浓度是实现短程硝化的限制性因素,本研究将DO浓度基本维持在0.7 mg·L−1左右,由于NOBs的活性长期受到抑制,故使得NAR逐渐升高。最后是控制较高的FA值,从而抑制NOBs的生长[10]。有研究[11]表明,当FA浓度为0.1~1.0 mg·L−1时,NOBs则会受到其抑制作用,FA浓度达到6 mg·L−1时,则NOBs的生长代谢则几乎被完全抑制,而AOBs对FA的受抑制范围则为10~150 mg·L−1。在工况A、B、C和D下,FA的浓度分别为4.48、6.72、8.96和11.19 mg·L−1,在工况D下,
NH+4 -N浓度为150 mg·L−1,NAR稳定在60%以上,通过FA选择性抑制NOBs,可使系统短程硝化高效稳定运行。 -
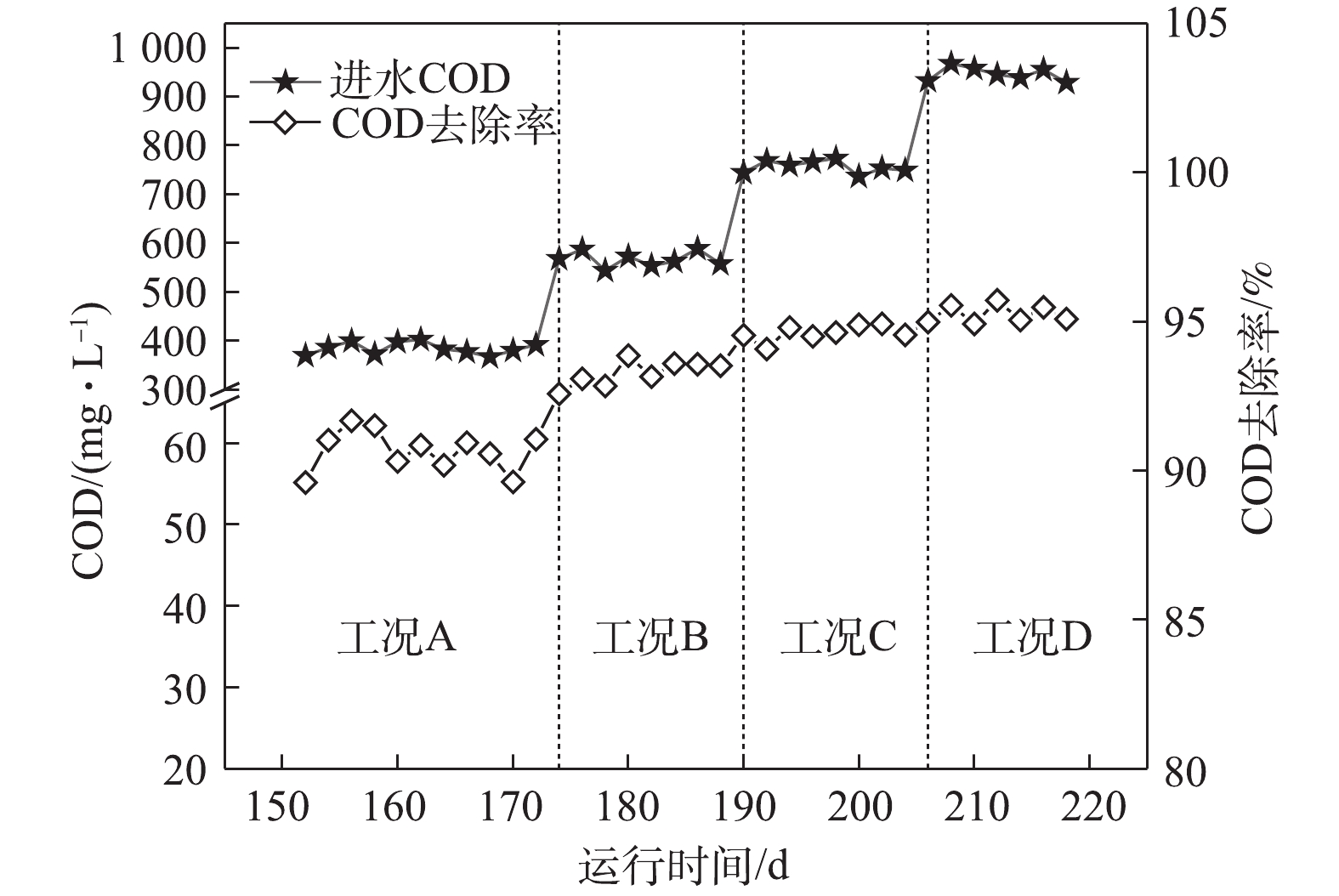
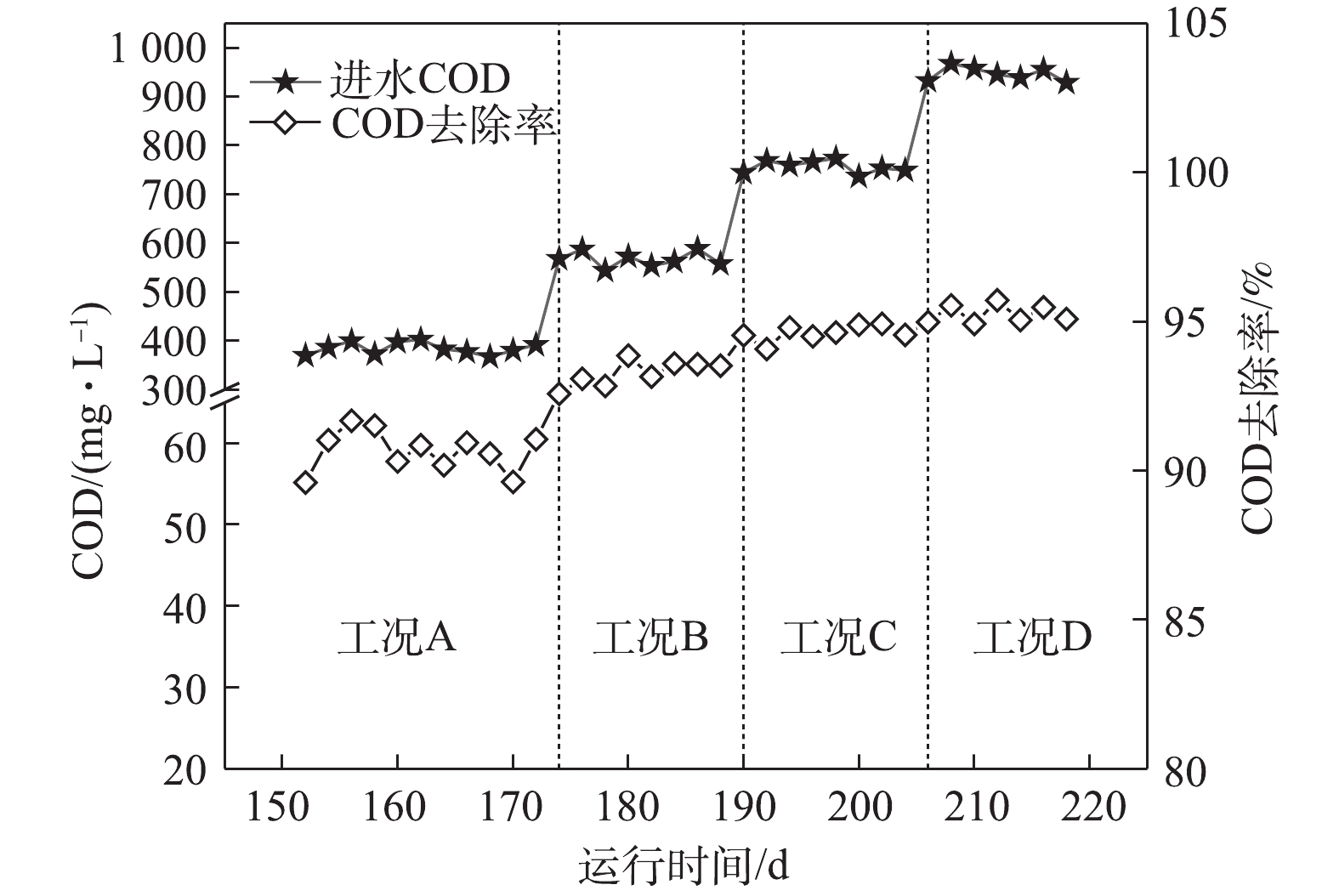
图3为系统对COD的去除情况。由图3可知,在工况A、B、C和D下,系统对COD的去除率分别为90.6 %、93.3 %、94.6 %和95.3%。随着进水COD逐渐上升,其对应的ABR容积负荷(以COD计)分别为1.02、1.53、2.04和2.55 kg·(m3·d)−1。在工况A和工况B时,对COD去除占主导作用的是前3个隔室,主要是通过A1厌氧隔室的水解酸化作用产生VFA,使DPBs利用优质碳源进行厌氧释磷完成对COD的去除。而随着基质浓度的升高,容积负荷(VLR)的升高使得前3个隔室对COD的去除效率下降。在工况C和工况D时,对COD去除作用的功能隔室则逐渐后移,A4厌氧隔室通过产甲烷菌对剩余的COD进一步去除,剩余耗氧有机污染物(以COD计)则通过常规异养反硝化菌在缺氧段利用硝化液中的
NO−x -N被去除,至此大部分耗氧有机污染物(以COD计)已在ABR反应器去除,ABR反应器的出水分别为35.9、38.1、40.8和45.1 mg·L−1。从工况A至工况D,虽然COD呈梯度上升,但前端厌氧及缺氧隔室对NH+4 -N仅有少量同化作用而去除,而经过前端ABR反应器对COD较高的去除率,使得进入MBR反应器的C/N值逐渐降低。张婷等[12]研究表明,当C/N=1时,有利于短程硝化的运行,而过高的C/N值会导致普通异养菌的快速增殖,进而降低硝化反应的速率。本研究中进入MBR反应器的C/N值低于1,因此,C/N不会成为短程硝化的限制性因素。 -
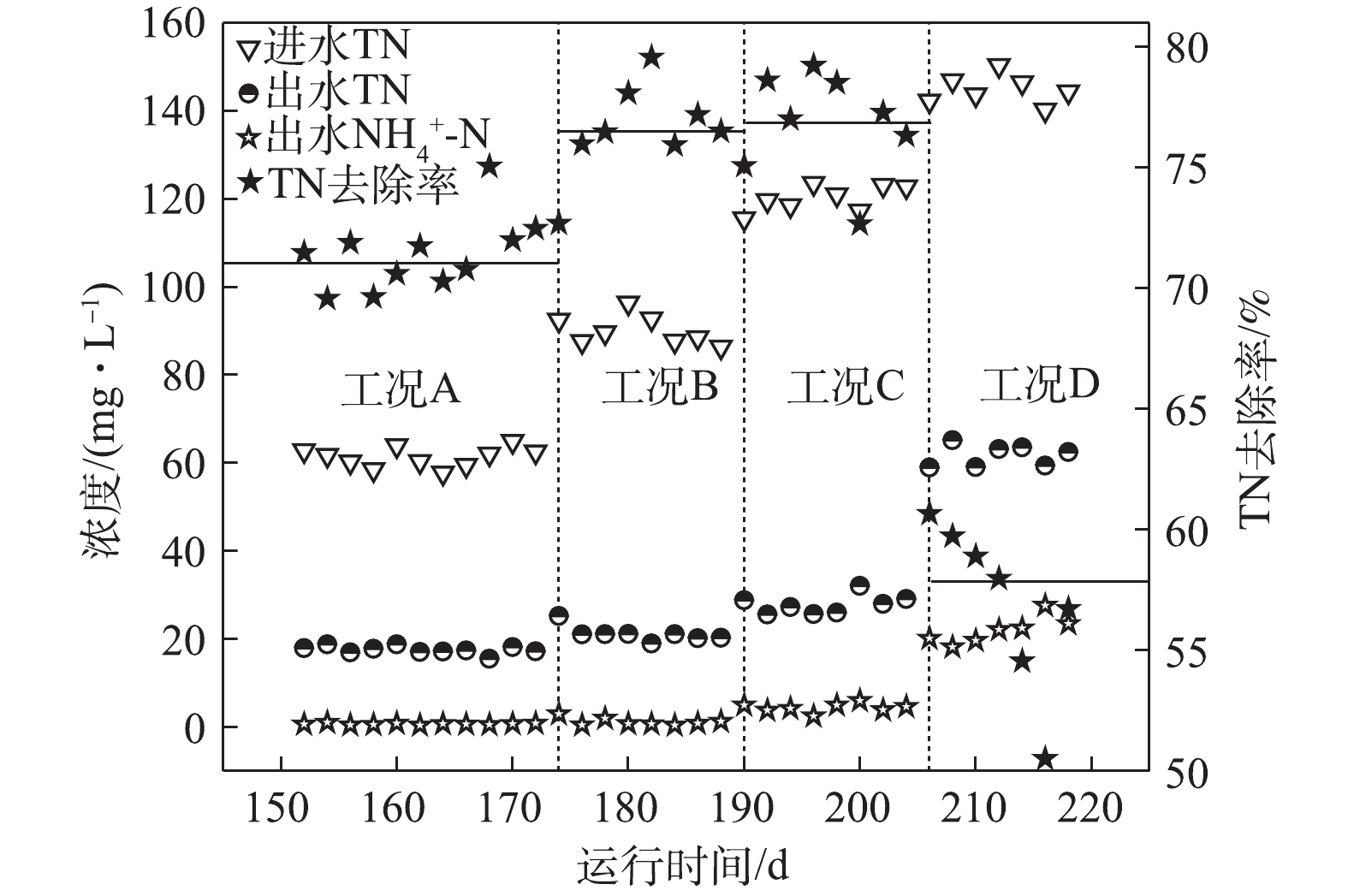
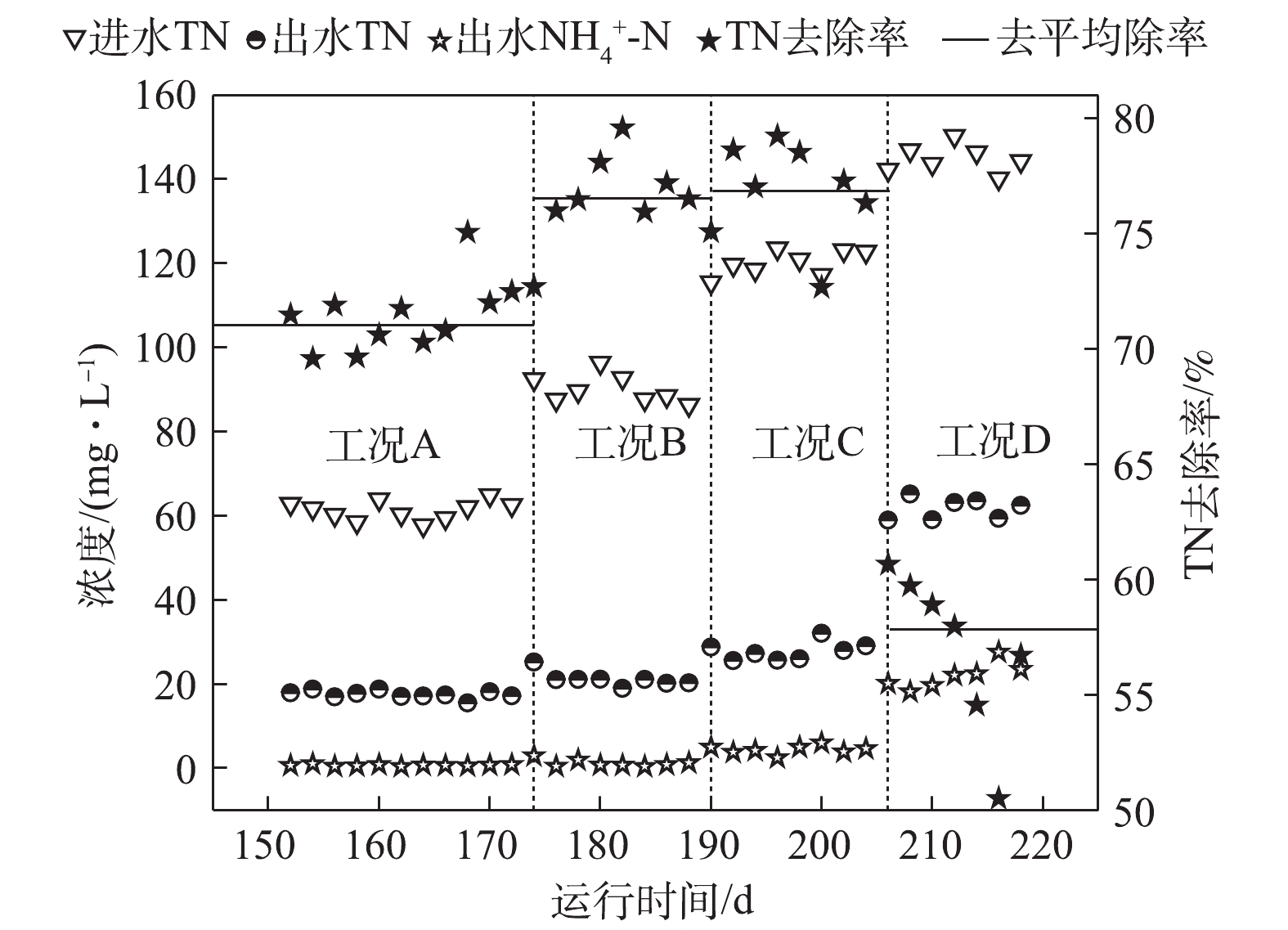
如图4所示,在工况A、B、C和D下,TN平均去除率分别为71.4%、76.6%、76.8%和56.3%。在工况A下,基质浓度较低,大部分耗氧有机污染物(以COD计)在缺氧段之前已经被完全去除,因此,系统脱氮则完全由DPBs主导,此时脱氮效率相对较低。在工况B和工况C条件下,虽然NAR稳定在55%以上,但由于回流硝化液中
NO−2 -N浓度较低,尚未达到抑制缺氧吸磷的阈值,所以系统反硝化除磷脱氮效果依然很好,并且有剩余耗氧有机污染物(以COD计)参与了常规反硝化的作用,使得脱氮效率逐步提高。同时,GAOs可能对脱氮也具有一定的贡献。GAOs与PAOs具有相似的代谢机制,在反硝化除磷过程中,GAOs的增殖始终伴随着PAOs的富集[13]。有研究[14]报道,在缺氧条件下,GAOs可以进行与有氧条件下相同的代谢,GAOs在厌氧环境下形成的PHAs,在缺氧中利用其完成糖原的再生以及脱氮的作用,在脱氮过程中发挥了重要作用。在工况D条件下,由于基质浓度的升高使得MBR反应器内的NAR较高,进而使得回流硝化液中NO−2 -N浓度超过了DPBs的耐受限度,导致反硝化吸磷效果较低,从而降低了脱氮的效能。而较高的基质浓度使得AOBs对氨氮的转化率也下降至84.2%,测定到的出水NH+4 -N平均浓度为22.92 mg·L−1,因此,一定的基质浓度范围有利于系统的脱氮效能。 -
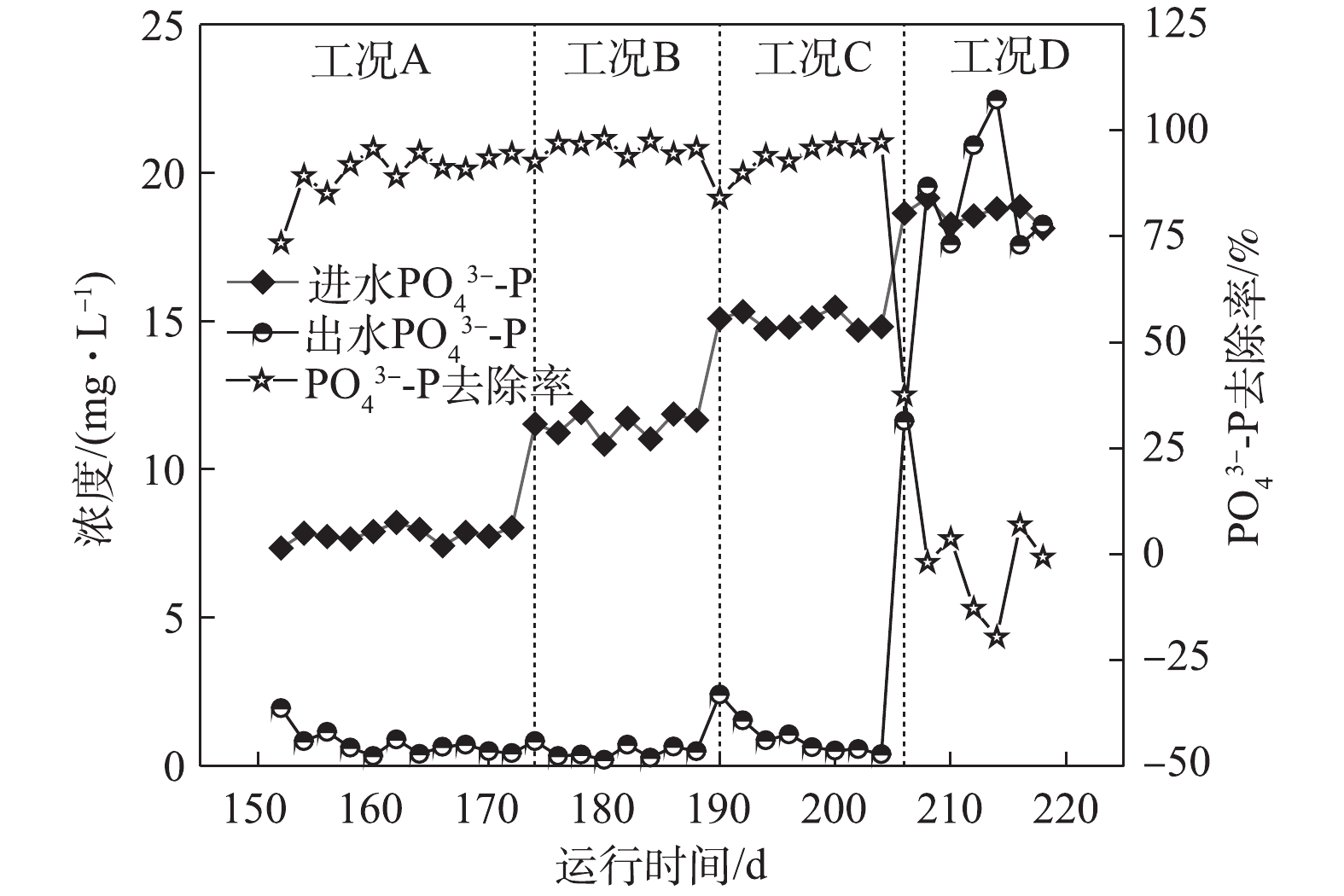
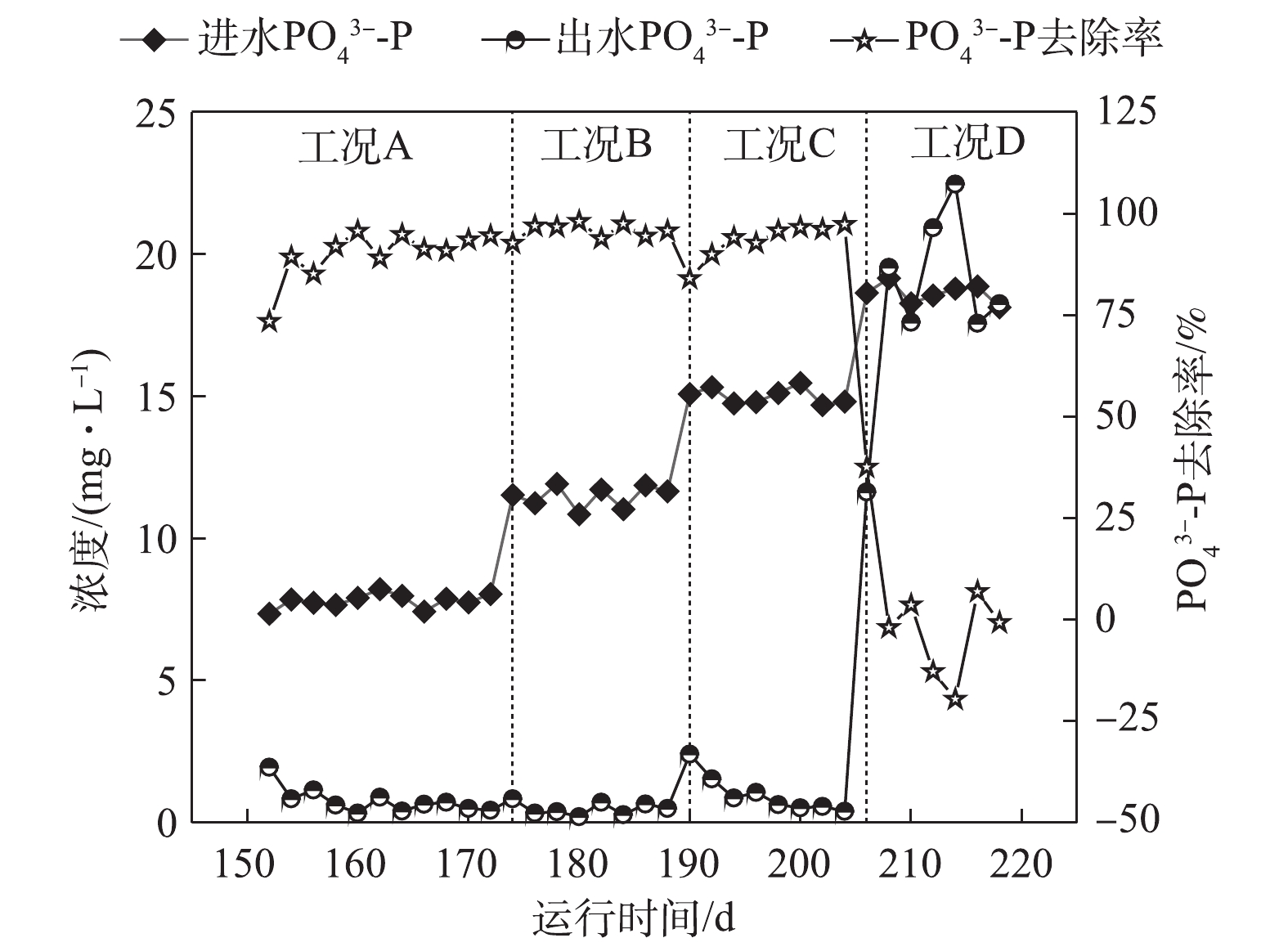
如图5所示,在工况A、B、C和D下,对应的ABR反应器中容积负荷(以
PO3−4 -P计)分别为0.021、0.032、0.043和0.053 kg·(m3·d)−1,系统对磷的平均去除率分别为89.96%、95.74%、93.3%和1.84%。在工况A条件下,MBR反应器NAR平均为43.9%,且系统除磷率达到89.96%。这表明NO−2 -N能够作为电子受体实现除磷,与ZHANG等[15]的研究结论一致,即低浓度的NO−2 -N并未抑制反硝化吸磷作用的进行。RUBIO-RINCÓN等[13]使用16S rRNA基因扩增子测序和多聚磷酸盐激酶基因(ppk1)作为遗传标记,结果表明,“Candidatus Accumulibacter ”分为2个主要的支系(PAO Ⅰ和PAO Ⅱ)。同时,通过宏基因组分析表明,PAO II的宏基因组缺乏呼吸硝酸盐还原酶(nar),但具有利用亚硝酸盐还原为氮气的能力,这表明PAO Ⅱ不能利用硝酸盐进行除磷,而PAO Ⅰ则能够利用O2、NO−2 -N及NO−3 -N。在工况B条件下,虽然ABR进水磷负荷升高至0.032 kg·(m3·d)−1,MBR反应器平均NAR为51.3%,
NO−2 -N平均浓度达到10.60 mg·L−1,但出水PO3−4 -P依旧稳定在0.5 mg·L−1左右,PO3−4 -P的去除率高达95.74%,表明DPBs对NO−2 -N的耐受并未达到极限。在工况C条件下,随着容积负荷的升高,出水NO−2 -N平均浓度为13.28 mg·L−1,回流硝化液中NO−2 -N浓度随之升高,这对DPBs有一定的影响,在第190天时,出水PO3−4 -P增至2.41 mg·L−1。随着ABR反应器内反硝化除磷功能区对DPBs的驯化,出水平均PO3−4 -P稳定至1.0 mg·L−1以下。在工况D下,回流NO−x -N中NO−2 -N/NO−3 -N为0.617,出水NO−2 -N平均浓度高达24.9 mg·L−1,磷去除率瞬间下降,甚至出现了负值,磷吸收阶段受到严重的抑制作用。诸多研究[16-18]表明,亚硝酸质子化产物—游离亚硝酸(FNA)是缺氧吸磷真正的抑制剂,而并非是亚硝酸盐的影响所致。在工况D条件下,FNA为0.001 3 mg·L−1,低于ZHOU等[19]所报道的FNA对缺氧磷吸收完全抑制的浓度。FNA对吸磷的抑制作用主要是通过对微生物新陈代谢的影响得以实现:首先,FNA影响ATP的合成,能够提高质子透过膜的透过性,导致质子推动力的效果变差,从而影响了胞内聚磷的合成[17];其次,FNA抑制反硝化酶的活性以及缺氧过程PHA的氧化,ZENG等[18]的研究表明,在缺氧条件下,当FNA完全抑制吸磷时,PHA首先被用于NO−2 -N的还原从而实现解毒作用,而不是作为吸磷的能量来源被DPBs分解。
2.1. 基质浓度对短程硝化的影响
2.2. 系统对COD的去除特性
2.3. 系统对氮的去除特性
2.4. 系统对磷的去除特性
-
1)控制ABR反应器HRT为9 h、C/N/P值不变、提高进水基质浓度,在此条件下,MBR反应器在工况A、B、C和D下平均亚硝酸盐积累率分别为43.9%、51.3%、56.8%和61.7%;通过SRT、DO浓度、温度及FA的协同作用稳定短程硝化的运行,控制FA选择性抑制NOBs的功能,可使AOBs逐渐成为优势菌群。
2)随着基质浓度的上升,负荷的升高使得前3隔室的COD去除率下降。在工况C和工况D下,对COD去除作用的功能隔室则逐渐后移,剩余耗氧有机污染物(以COD计)则通过常规异养反硝化菌在缺氧段利用硝化液中的
NO−x -N被去除;同时基质浓度的上升也提高了脱氮率,在一定的基质浓度范围内有利于系统的处理效能。3) FNA是缺氧吸磷真正的抑制剂。在FNA为0.001 3 mg·L−1时,磷去除率骤降,缺氧吸磷受到严重的抑制,这主要是因为FNA影响了ATP的合成,并且FNA抑制反硝化酶的活性以及缺氧过程中PHA的氧化。







 下载:
下载: