-
榨菜废水是榨菜生产过程中的副产物,具有高盐度、高氨氮、高磷酸盐、高有机质等特点,每年排入三峡水库的榨菜废水量约为3.5×106 m3,这给三峡水库的水环境保护造成了极大的负担[1]。榨菜废水中的盐度对水生动植物具有毒害作用,进一步破坏了水体生态环境[2],因此,有必要去除榨菜废水中的盐度。传统的脱盐工艺(反渗透法、蒸馏法)不仅能耗高,而且还需要较高的水压[3]。有研究[4]表明,传统的盐水淡化技术每生产1 m3淡水,须消耗2~15 kWh电能。因此,有必要寻求经济高效的新方法。
近年来,微生物脱盐燃料电池(MDC)作为一种新型的环境友好型废水处理技术,受到了广泛的关注[5]。MDC相较于传统的脱盐技术,具有能耗更低、操作条件更简单等优点[6],同时,可以在没有外加电源的情况下,实现同步产电脱盐[7]。有研究[6]表明,高盐水中盐度的去除率和电流密度有很大关系,电流密度越大,盐度的去除率越高[8],考虑到阴极电子受体[9-11]对电池的产电性能有较大的影响,为了提高脱盐效率和产电性能,须选择合适的阴极电子受体。常见的阴极有铁氰化钾阴极、空气阴极、生物阴极等。使用铁氰化钾作为阴极电子受体时可以去除约90%的盐度,最大功率密度可以达到2 W·m−2[9],然而,空气阴极中盐度的去除率只有43%~67%,铁氰化钾阴极通常会产生比氧气更高的阴极电位,这在一定程度上会提高盐度的去除率[10]。生物阴极在长周期运行中会产生膜污染,导致电池性能下降[11]。因此,本实验选择铁氰化钾作为阴极电子受体,并以水阴极作为参考。在以往的MDC研究中,中间盐室一般均采用人工配置的NaCl溶液[12],鲜少采用实际榨菜废水。为了促进燃料电池的实际应用,须研究榨菜废水的产电脱盐性能。
本研究以榨菜废水为中间盐室进水,以铁氰化钾和氧气为阴极电子受体,构建MDC,比较其产电和脱盐性能,采用16S rRNA基因测序法对微生物群落和功能微生物菌群进行研究,为后续榨菜废水的处理提供参考。
全文HTML
-
实验所用榨菜废水来源于重庆市涪陵区榨菜集团污水处理厂,废水类型为初沉池出水,阳极进水为生活污水,在实验过程中添加乙酸钠,将阳极进水COD值调整为1 000 mg·L−1。实验所有的样品均保存在4 ℃的冰箱内。实验所用试剂均为分析纯,实验用水为超纯水,电阻率为18.2 MΩ·cm。榨菜废水和生活污水水质如表1所示。
-
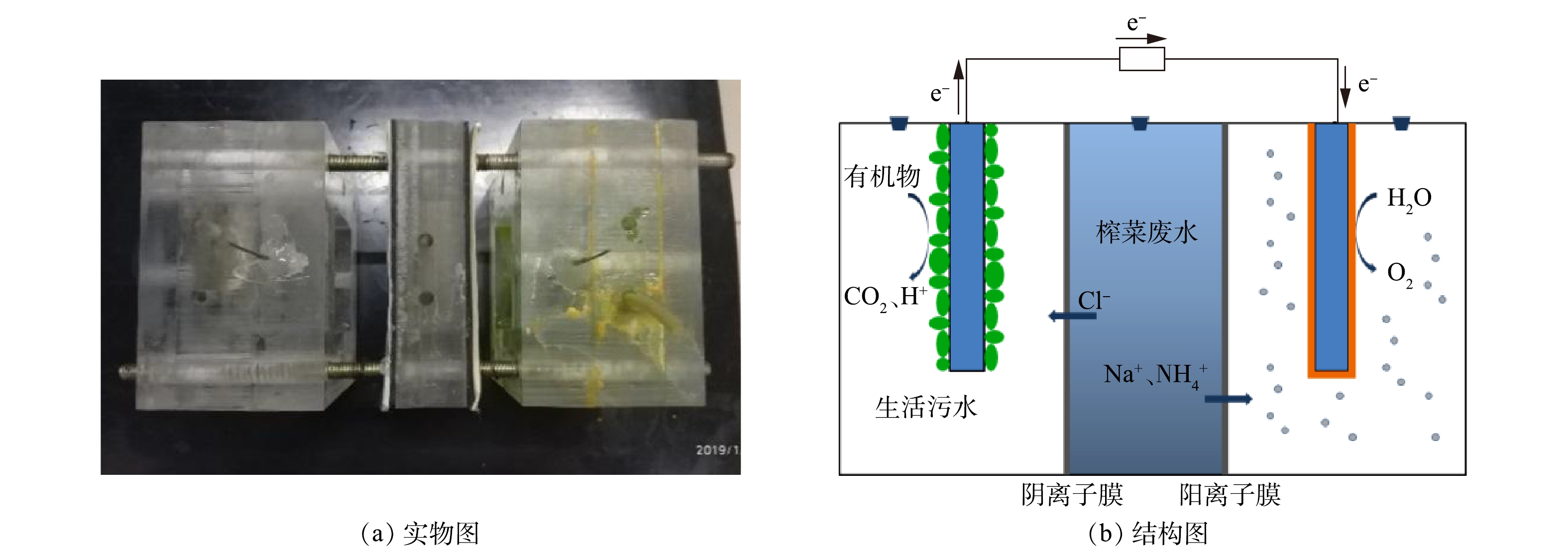
实验装置如图1所示。装置主体由阳极室、脱盐室、阴极室3部分组成,阴、阳极室有效体积均为240 mL(长×宽×高为5 cm×6 cm×8 cm),脱盐室有效体积约为120 mL(长×宽×高为5 cm×3 cm×8 cm),阴、阳离子交换膜的有效面积为40 cm2。其装置实物如图1(a)所示。阳极室与中间盐室用阴离子交换膜隔开,阴极室与中间盐室用阳离子交换膜隔开。两极室均采用碳毡作为电极材料,阳极室采用实验室培养的厌氧污泥进行接种。
在电池的启动阶段,2组电池的阳极室均加入220 mL生活污水以及20 mL厌氧污泥。阴极室中加入220 mL纯水,铁氰化钾组阴极室加入0.05 mol·L−1的铁氰化钾,水极组直接曝入空气,曝气阴量控制在50 mL·min−1。中间盐室直接加入榨菜废水。反应器外阻恒定为500 Ω,在运行过程中保持阳极室的绝对厌氧状态;当电池电压低于100 mV时,则认为完成一个产电周期,此时2组MDC均须更换阳极液,铁氰化钾组须更换阴极液,水阴极组不更换阴极液。MDC装置采用间歇运行模式。如无特殊说明,实验温度均保持在(25±1) ℃。
-
电压采用数据采集卡(DAS,PISO13.Honggeo.Ltd.Taiwan,China),每隔1 min采集1次数据。电流、电流密度、功率、功率密度、极化曲线的测量参考文献中的方法[2];COD和
${\rm{NH}}_4^ + $ 采用便携式分光光度计 (DRB200&DR5000,HACH Co.USA)测定。电导率和TDS(total dissolved solids,TDS)采用电导率仪(FE-30K,Metter-Toledo)测定。实验结束后,将铁氰化钾组阳极、水阴极组阳极和水阴极组阴极生物膜取出,保存于−80 ℃的环境下。DNA通过OMEGA Soil DNA试剂盒进行提取,采用聚合酶链式反应 (PCR) 对提取的DNA进行扩增,引物338F的序列为(ACTCCTACGGGAGGCAGCA),引物806R的序列为(GGACTACHVGGGTWTCTAAT)。高通量测序服务由上海美吉生物平台(上海,中国)提供。
1.1. 实验用水与试剂
1.2. 实验装置与启动方法
1.3. 检测指标及方法
-
如图2所示,经过多周期运行后,铁氰化钾组和水阴极组TDS分别从19.38、19.4 g·L−1降至1.88、2.60 g·L−1,去除率分别为90.30%、86.55%。电导率分别从32.53、33.32 mS·cm−1降至3.40、4.54 mS·cm−1,下降率分别为89.55%、86.37%。盐度的去除率和以往的研究[9]是一致的。从盐度去除效果来看,铁氰化钾组优于水阴极组,原因是铁氰化钾组的电流密度高于水阴极组[8]。
从产电性能来看,铁氰化钾组输出电压均高于600 mV,最大输出电压为625 mV。在本研究中,前期水阴极组最大输出电压只有244 mV,远远低于铁氰化钾组,造成这种差异的原因是曝气水阴极的阴极过电势较大,造成很大的阴极电势损失,使得输出电压变小[13]。由图2可以看出,水阴极组在经历8个周期后,电压出现了明显的增幅,最高达到450 mV。可能的原因是,在非密闭状态下,阴极经过长时间的运行,有微生物不断地附着在碳毡上,逐渐形成了生物膜。生物膜中含有一定的电化学活性菌,可以降低阴极过电势,增加输出电压。
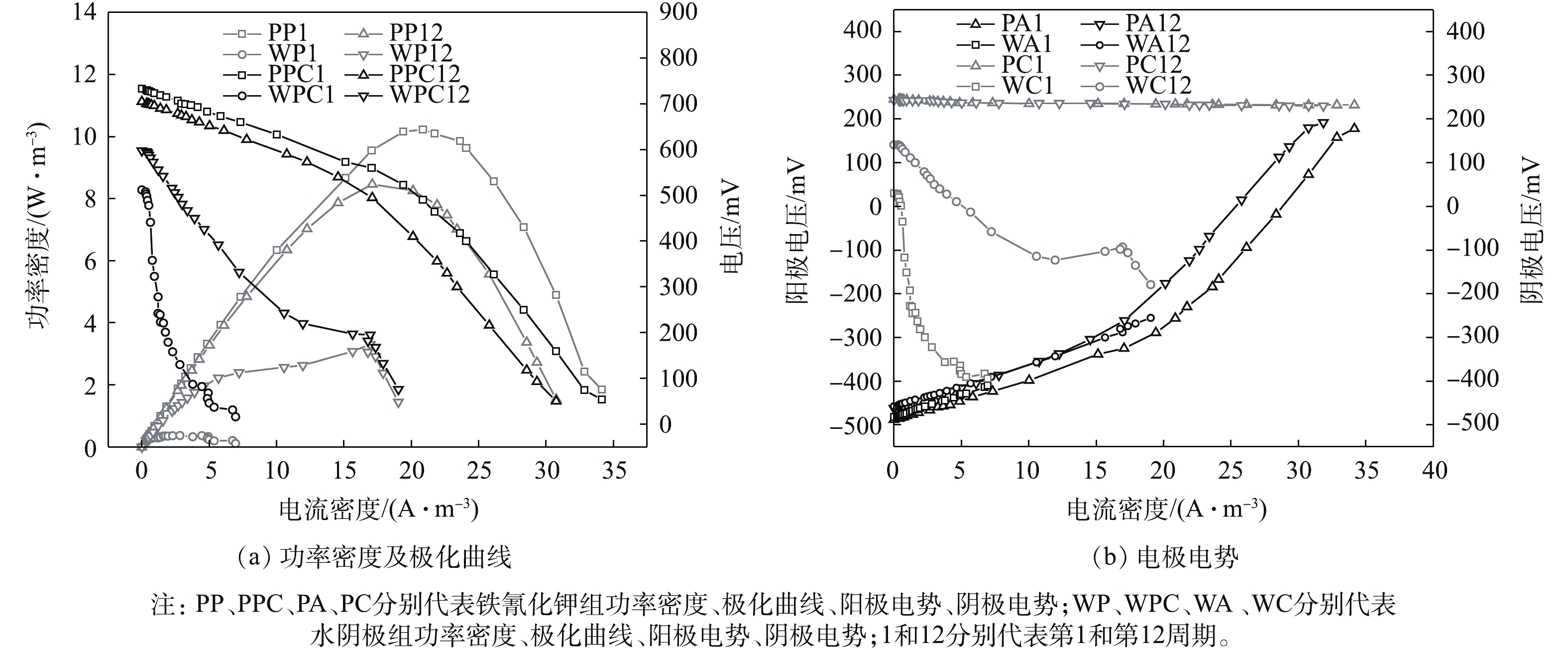
电池稳定运行的第1和第12周期,采用恒电流法测量电池的极化曲线及功率密度曲线。由图3可知,系统的开路电压、最大功率密度和内阻分别是733 mV、10.23 W·m−3和94.87 Ω(铁氰化钾第1周期),705 mV、8.47 W·m−3和105.96 Ω(铁氰化钾第12周期),512 mV、0.36 W·m−3和217.56 Ω(水阴极第1周期),598 mV、3.31 W·m−3和40.02 Ω(水阴极第12周期)。由图3可知,在系统运行的第1个周期,铁氰化钾组的开路电压和功率密度高于水阴极组,原因是阴极的电子受体及浓度不同,使得铁氰化钾组阴极电势远高于水阴极组[14],水阴极组内阻高于铁氰化钾组,原因可能是氧气的扩散阻力很高[15]。经过长周期的运行后,铁氰化钾组产电性能略有下降,然而,水阴极组的情况刚好相反。可能的原因是,随着运行时间的延长,铁氰化钾组阳极产甲烷菌的增殖影响到电池的产电性能[16],而水阴极组的阴极生物膜中富集的产电菌降低了阴极过电势,使得产电性能提高了。由图3还发现,水阴极组和铁氰化钾组阳极电势相差不大,这是因为在实验的全过程中,阳极条件是一致的[14]。铁氰化钾组阴极电势比较稳定,水阴极组阴极电势变化较大,同时,由水阴极组阴极极化曲线可知,阴极传质损失和活化损失是造成电池性能下降的主要原因[17]。
-
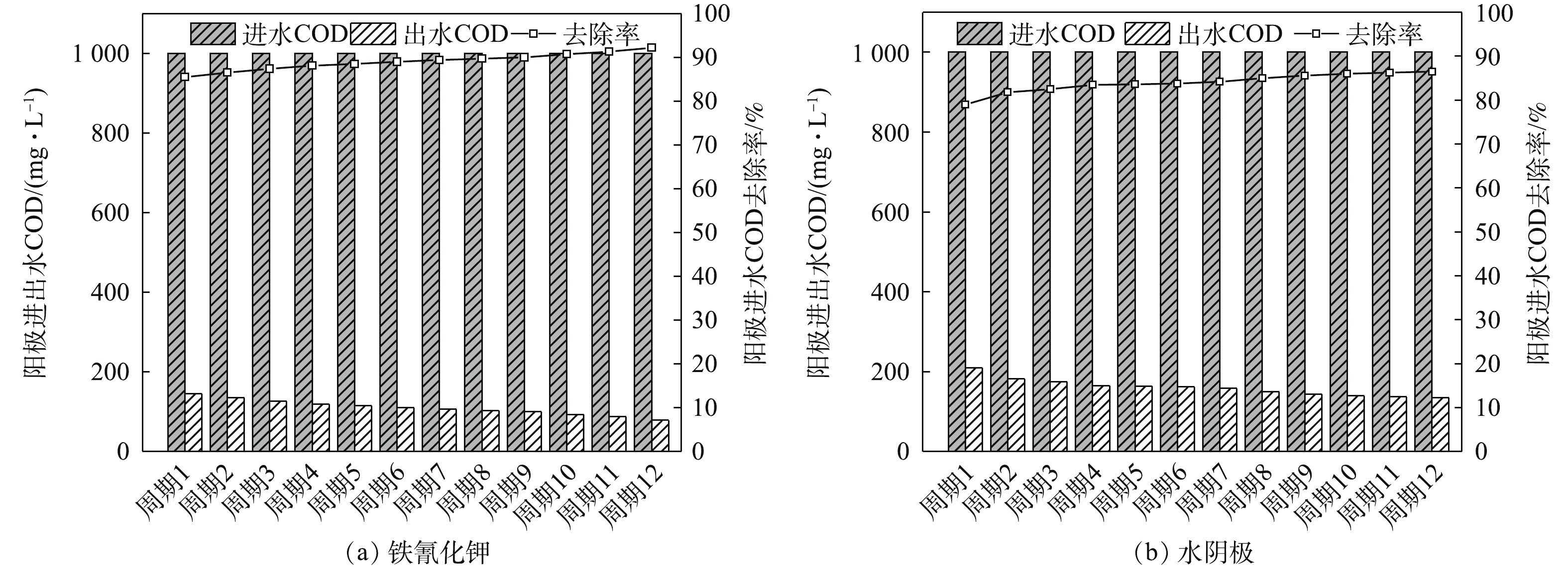
当阳极进水COD值恒定时,在整个脱盐周期中,随着脱盐时间的延长,COD的去除率不断地缓慢提高。最主要的原因可能是装置运行的初期,电极表面微生物富集量较少,COD的去除率较低,随着电极表面产甲烷菌的增殖,COD的去除率也逐步提高[16]。如图4所示,铁氰化钾组COD的去除率最高可达92.2%,实验结果略低于ZHANG等[6]报道的94.2%。水阴极组在系统稳定后,COD的去除率最高也只有86.5%,低于铁氰化钾组。因此,系统的污染物去除率与产电性能有关,产电性能越好,污染物的去除率越高。
由图5可以看出,
${\rm{NH}}_4^ + $ 的迁移主要发生在整个脱盐电池的前期。这是因为中间盐室前期离子的电导率和扩散速率高[18]。在实验结束后,铁氰化钾组${\rm{NH}}_4^ + $ 的去除率为94.41%,水阴极组${\rm{NH}}_4^ + $ 的去除率为97.79%,表明MDC是一种高效、可以同时脱氮除盐的装置。 -
微生物作为MDC系统的核心,对产电和污染物降解具有重要作用,因此,本研究对微生物群落进行了深入研究。微生物多样性指数如表2所示。各组MDC微生物测序的样本文库覆盖率指数均大于0.99,说明测序结果可以很好地反映样本的真实情况。铁氰化钾组、水阴极组阳极和阴极的OUT指数分别为1 201、988和769,Chao指数分别为1 246、1 203和976,说明铁氰化钾组阳极生物膜微生物丰富度高于水阴极组阳极,水阴极组阳极生物膜的微生物丰富度高于水阴极组阴极。铁氰化钾组、水阴极组阳极和阴极的Shannon指数分别为4.52、4.66和4.31,Simpson指数分别0.042、0.024和0.072,表明水阴极组阳极膜微生物多样性较高。
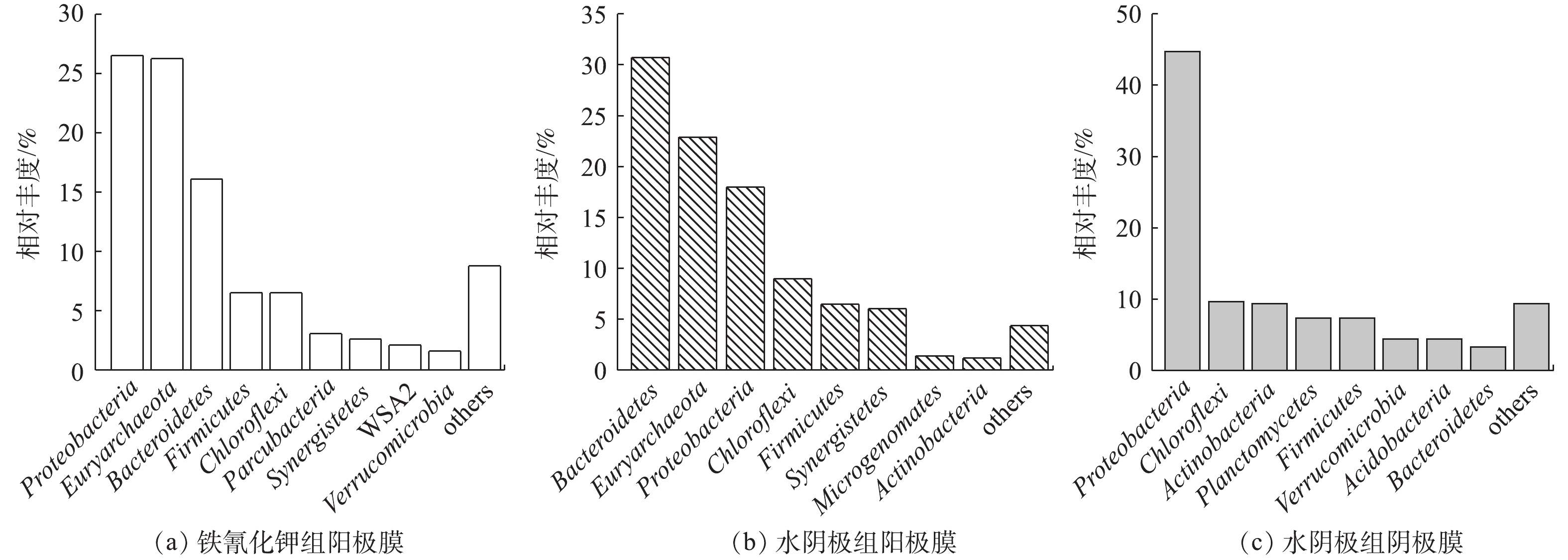
由图6可以看出,在门水平上,铁氰化钾组阳极生物膜的优势菌群为Proteobacteria、Euryarchaeota、Bacteroidetes、Firmicutes、Chloroflexi、Parcubacteria、Synergistetes、WSA2、Verrucomicrobia,相对丰度分别为26.5%、26.2%、16.1%、6.51%、6.51%、3.06%、2.61%、2.09%、1.58%。水阴极组阳极膜优势菌群为Bacteroidetes、Euryarchaeota、Proteobacteria、Chloroflexi、Firmicutes、Synergistetes、Microgenomates、Actinobacteria,相对丰度分别为30.72%、22.90%、17.98%、8.98%、6.47%、6.03%、1.40%、1.17%。水阴极组的阴极膜优势菌群为Proteobacteria、Chloroflexi、Actinobacteria、Planctomycetes、Firmicutes、Verrucomicrobia、Acidobacteria、Bacteroidetes,相对丰度分别为44.73%、9.70%、9.36%、7.36%、7.32%、4.45%、4.41%、3.32%。铁氰化钾和水阴极组的阳极膜菌群中Proteobacteria、Bacteroidetes、Chloroflexi、Firmicutes,作为最常见的电化学活性微生物,对产电有很大的贡献[4,6],广泛存在于阴、阳极生物膜中。Euryarchaeota作为广古菌门,包括产甲烷菌、嗜热菌、噬盐菌等适应极端环境的菌群[19],在阳极膜中的丰度很高。由图6还可以看出,2组阳极生物膜的菌群种类类似,相对丰度不同,可能的原因是,阴极电子受体不同,使得输出电压不同,导致阳极生物膜富集的菌群丰度有所差异[13]。在水阴极组阴极生物膜中发现了Planctomycetes,Planctomycetes几乎包含了所有已知的厌氧氨氧化菌[6],可能对阴极脱氮具有促进作用。
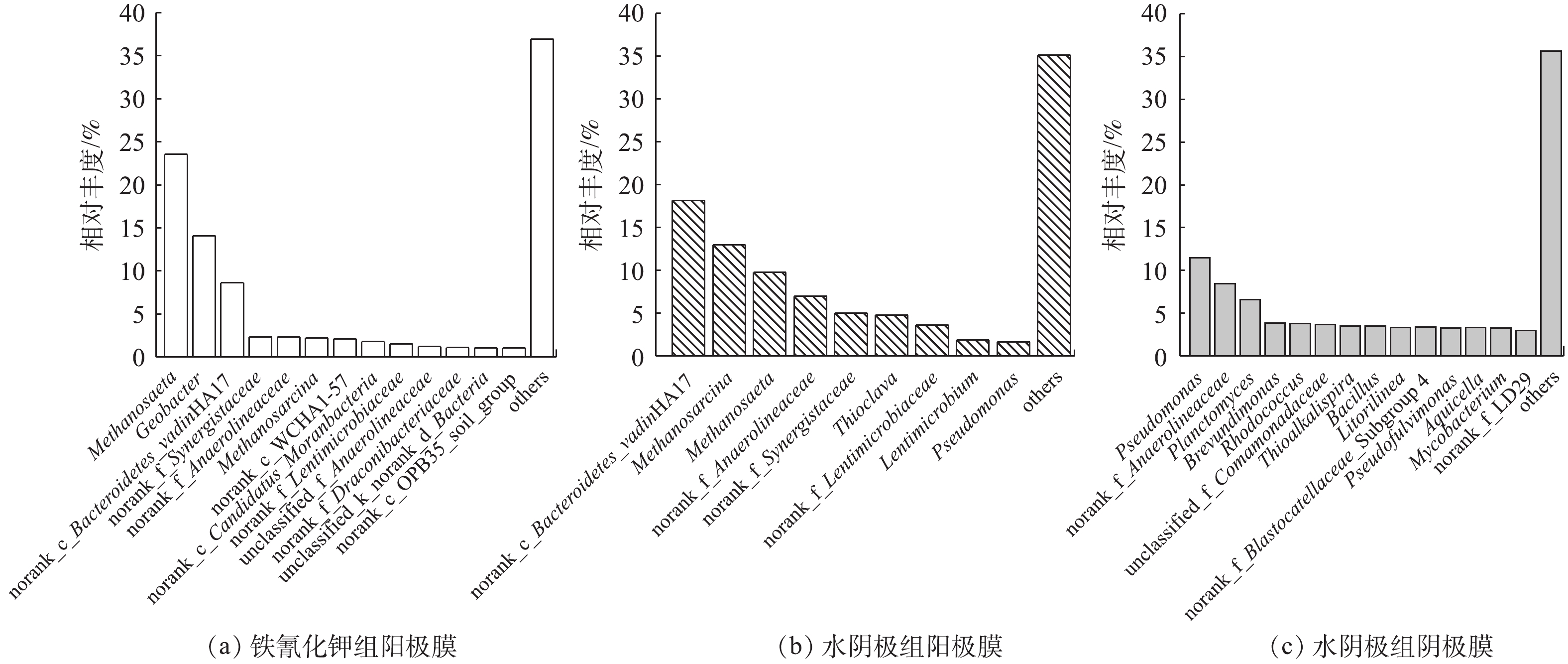
在燃料电池中,产电和污染物去除都和功能微生物菌群密切相关,所以有必要从属水平上来分析微生物的功能。如图7所示:铁氰化钾组阳极生物膜优势菌属为Methanosaeta(23.55%)、Geobacter(14.09%)、norank_c_Bacteroidetes_vadinHA17(8.64%)、norank_f_Synergistaceae(2.33%)、norank_f_Anaerolineaceae(2.31%)、Methanosarcina(2.23%);水阴极组阳极膜优势菌属为norank_c_Bacteroidetes_vadinHA17(18.17%)、Methanosarcina(13.00%)、Methanosaeta(9.79%)、norank_f_Anaerolineaceae(6.96%)、norank_f_Synergistaceae(5.05%);水阴极组阴极膜优势菌属为Pseudomonas(11.49%)、norank_f_Anaerolineaceae(8.46%)、Planctomyces(6.60%)、Brevundimonas(3.86%)、Rhodococcus(3.78%)、Comamonadaceae(3.70%)、Thioalkalispira(3.52%)。由图7可以看出,铁氰化钾和水阴极组阳极膜主要菌属种类相差不大,丰度不同,主要的功能菌群包括水解发酵菌、产甲烷菌。norank_c_Bacteroidetes_vadinHA17、norank_f_Anaerolineaceae和norank_f_Synergistaceae均属于水解发酵菌属,它们的主要作用是将复杂有机物转化为简单有机物,有助于后续产电菌、产甲烷菌发挥作用。Anaerolineaceae是一种专性厌氧菌,可以降解酚类和烃类难降解有机物[20]。vadinHA17的主要作用是降解复杂有机物[21]。Synergistacea是协同菌科的唯一菌属,可以协同产甲烷菌将有机质降解为乙酸、CO2[22]。Geobacter菌属是一种主要的产电菌,具有降解有机物和传递电子给电子受体的功能[23]。Methanosaeta和Methanosarcina均属于产甲烷菌。Methanosaetah和Methanosarcina作为常见的产甲烷菌属,也是唯二可以直接利用乙酸来产甲烷的菌属[24]。水阴极组阴极膜中Pseudomonas和Comamonadaceae不仅是产电菌属,还可以促进水体中氮元素的去除[6]。Brevundimonas是阴极室中一种常见产电菌,通过产生氧化还原介质来提高电流密度[25]。Planctomyces是一种常见的厌氧氨氧化菌属,可以铵盐为电子供体,以硝酸盐或者亚硝酸盐作为电子受体,将水体中的氨氮转化为氮气去除[6]。Thioalkalispira是一种嗜盐碱或者耐盐碱微生物[26]。
在MDC系统中出现的产电菌和厌氧氨氧化菌为高效除盐除氮提供了坚实的基础。阳极生物膜中存在的产甲烷菌属对产电和除盐有不利作用,因此,寻找合适的抑制产甲烷菌产生的方法是未来的研究重点。
2.1. 铁氰化钾和水阴极MDC产电性能及脱盐效果的比较
2.2. 铁氰化钾和水阴极MDC污染物去除效果的比较
2.3. 铁氰化钾和水阴极MDC微生物群落分析
-
1)微生物脱盐燃料电池可以在不消耗能源的情况下淡化高盐榨菜废水。在一个脱盐周期中,铁氰化钾组和水阴极组MDC的电导率及TDS值均下降了85%以上,铁氰化钾组TDS去除率为90.30%,略高于水阴极组。
2)不同阴极的微生物脱盐燃料电池产电性能具有差异性。在整个脱盐周期内,铁氰化钾组产电性能优于水阴极组。随着运行时间的延长,铁氰化钾组产电性能略有下降,而水阴极组产电性能显著增强。
3)微生物群落分析结果表明:运行后期,水阴极组阴极中出现了产电菌,极大地降低了阴极过电势,提高了系统的产电性能;铁氰化钾组阳极和水阴极组阳极中产甲烷菌占比很高,然而,产甲烷菌对系统产电脱盐有不利作用,因此,有必要寻找合适的抑制产甲烷菌产生的方法。



 下载:
下载: