-
黄铁矿作为地球上含量最丰富的天然硫化矿物之一,广泛分布于地壳与沉积物之中[1-3]。环境中的黄铁矿容易被氧化溶出铁离子而发生异相Fenton反应[4],且黄铁矿的半导体特性使其在自然光条件能产生光电响应[5]。因此,来源广、成本低的黄铁矿已被尝试用于处理难降解的抗生素废水[6],其中黄铁矿的Fenton法[7]和电化学法[8-9]降解抗生素的效果优良。虽然Fenton及电化学法反应速度快,但Fenton法对pH和H2O2的要求及大量含铁污泥的产生限制了其应用。而电化学法则存在传质效率有限,催化剂电腐蚀严重等问题[10]。
作为一种天然半导体材料,黄铁矿较窄的带隙有利于黄铁矿充分利用自然光,产生·OH等具有强氧化性的活性物种降解污染物[11],从而大幅降低了光催化降解技术中光催化材料普遍要求的紫外光源所产生的处理成本。黄铁矿的半导体特性和Fenton效应使其在等离子体降解技术中也有着很好的发展潜力。等离子体装置放电过程会产生高能电子(e−*),并伴随有光能的释放[12]。同时,等离子体辐射会产生大量的H2O2、O3、·OH等活性氧化物种。单一的等离子体放电过程中,光能不能被充分利用,生成的H2O2和O3不能被高效转化成高氧化性的·OH。当黄铁矿作为催化剂和等离子耦合时,黄铁矿半导体特性可以促进其充分吸收等离子体所释放的光能进行光催化反应。同时,黄铁矿中释放的Fe2+/Fe3+及其pH自调节能力,能够利用等离子体产生的H2O2促成Fenton反应的发生[13]。与天然黄铁矿的Fenton法和电化学方法相比,同时利用黄铁矿的半导体特征和Fenton特性的光催化技术和黄铁矿耦合等离子体技术的相关研究机制探讨还不充分,综合对比分析两者处理抗生素废水的降解效果、机制及产物的研究还比较缺乏。
因此,本研究以广泛存在于地壳中的天然半导体材料黄铁矿为催化剂,以畜牧养殖业中大量使用的难降解兽用抗生素泰乐霉素(tylosin tartrate,TYL)为模型污染物,采用等离子体技术和光催化技术分别对其进行了降解,对比分析了2种技术的降解效果、降解机制、降解产物、适用条件和经济成本。本研究结果以期为难降解的抗生素类污染物的处理提供参考。
-
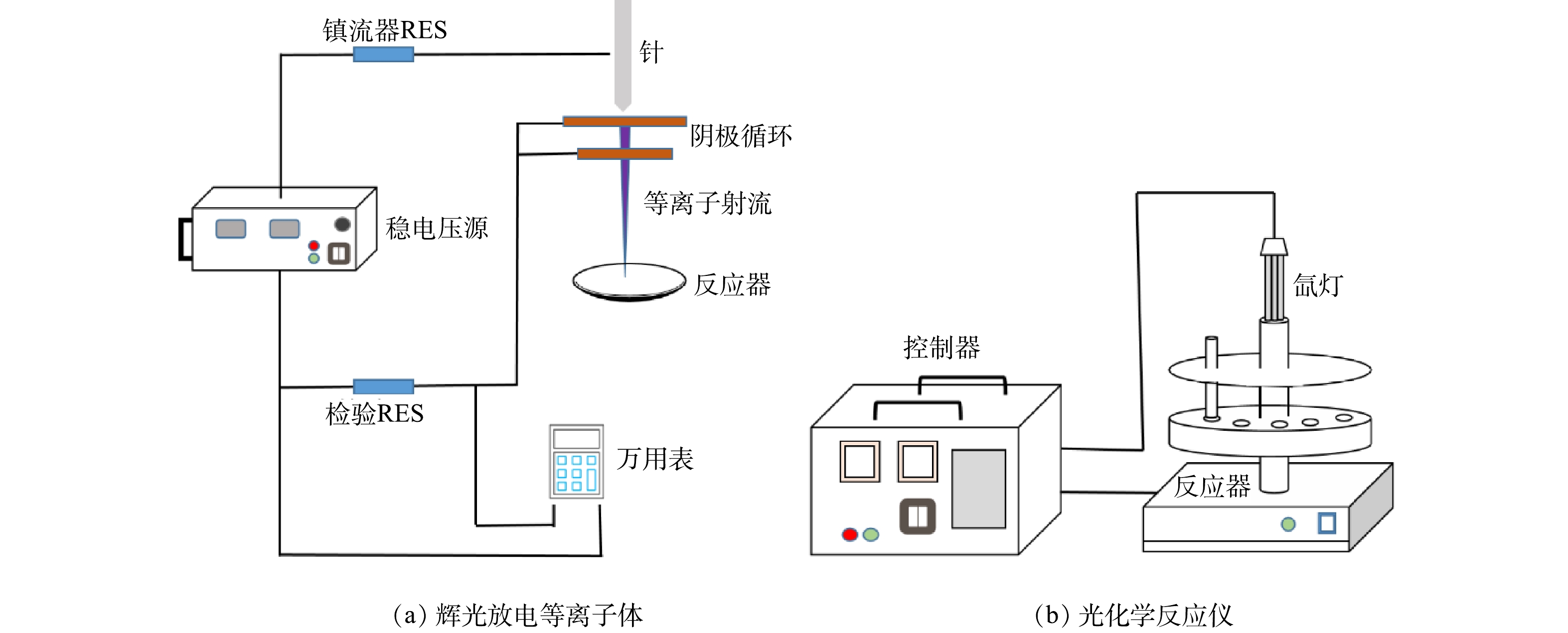
泰乐霉素(tylosin tartrate,纯度大于98%,分子式C46H77NO17.C4H6O6,分子质量为1 066.19,CAS编号为1405-54-5)购自阿拉丁化学试剂公司,在-20 ℃下保存待用。黄铁矿购自中国桂林的万宝矿物有限公司,将其研磨过100目筛,然后用超纯水清洗数次,干燥后储存在密封的棕色玻璃瓶中待用。其他分析纯化学试剂购自上海麦克林生物化学有限公司。实验用辉光放电等离子体(glow discharge plasma, GDP)由溶液池、针和阴极环组成(图1(a))。反应器为石英玻璃材质,内径为90 mm,溶液体积为50 mL。实验电压为9.5 kV,循环电流由直流稳压电源提供,放电电流为0~150 μA(可调)。光降解反应器见图1(b),采用500 W的氙灯模拟自然光源,50 mL石英光反应管为反应容器。
-
pH对黄铁矿的理化性质与TYL的存在形态可产生影响。首先考察了pH对TYL在2个降解体系中降解效果的影响。用盐酸与氢氧化钠将TYL溶液初始pH调整为3、5、7、9和11,分别进行光反应实验与等离子体实验。2种降解体系中TYL的初始质量浓度(10 mg·L−1)与固液比(1.4 g·L−1)相同。溶液反应不同时间后取样,样品过0.22 μm的滤膜待测。根据以上实验结果确定TYL降解实验溶液pH。批量去除实验TYL初始浓度及固液比与上相同。黄铁矿和50 mL TYL溶液加入光反应器后,立即打开光源,在不同反应时间点(0~120 min)取样。在等离子体实验中反应时间为0~30 min。取样后样品根据实验需求测定TYL和总有机碳(TOC)浓度。活性物种捕捉实验以异丙醇、草酸铵、对苯醌和硝酸银分别作为·OH、h+、·O2−和e−的淬灭剂,分别向含有0.07 g黄铁矿的50 mL,10 mg·L−1的TYL溶液中加入250 μL 1 mol·L−1的草酸铵、对苯醌、硝酸银及异丙醇,在不同反应时间取样分析溶液中的TYL浓度。其他实验步骤与上述批处理实验一致。
-
通过高效液相色谱法(HPLC,Shimadzu,LC-2030C 3D plus)检测TYL的浓度,具体检测参数与课题组先前的研究一致[14]。降解后的溶液通过固相萃取-液相色谱-质谱(SPE-LC-MS)检测TYL的降解中间产物。LC-MS分析在四极杆飞行时间质谱仪 (Agilent Technologies)上进行,流动相为去离子水(A相)、含1%甲酸的5 mol·L−1乙酸铵水(B相,pH=4.5)和乙腈(C相),梯度洗脱程序见表1。质谱选择电喷雾离子源,采用正离子扫描模式,雾化气压力40.0 psi,雾化气温度276.8 K;辅助气体流速为6 L·min−1。
-
TYL降解率根据式(1)进行计算,矿化率通过计算反应后减少的TOC占原始TOC含量的百分比来确定。所有实验数据均采用 Excel 2016 和 Origin 8.5 软件处理。
式中:η为TYL的降解率,%;C0和Ct分别为TYL的初始和t时刻的质量浓度 mg·L−1。
-
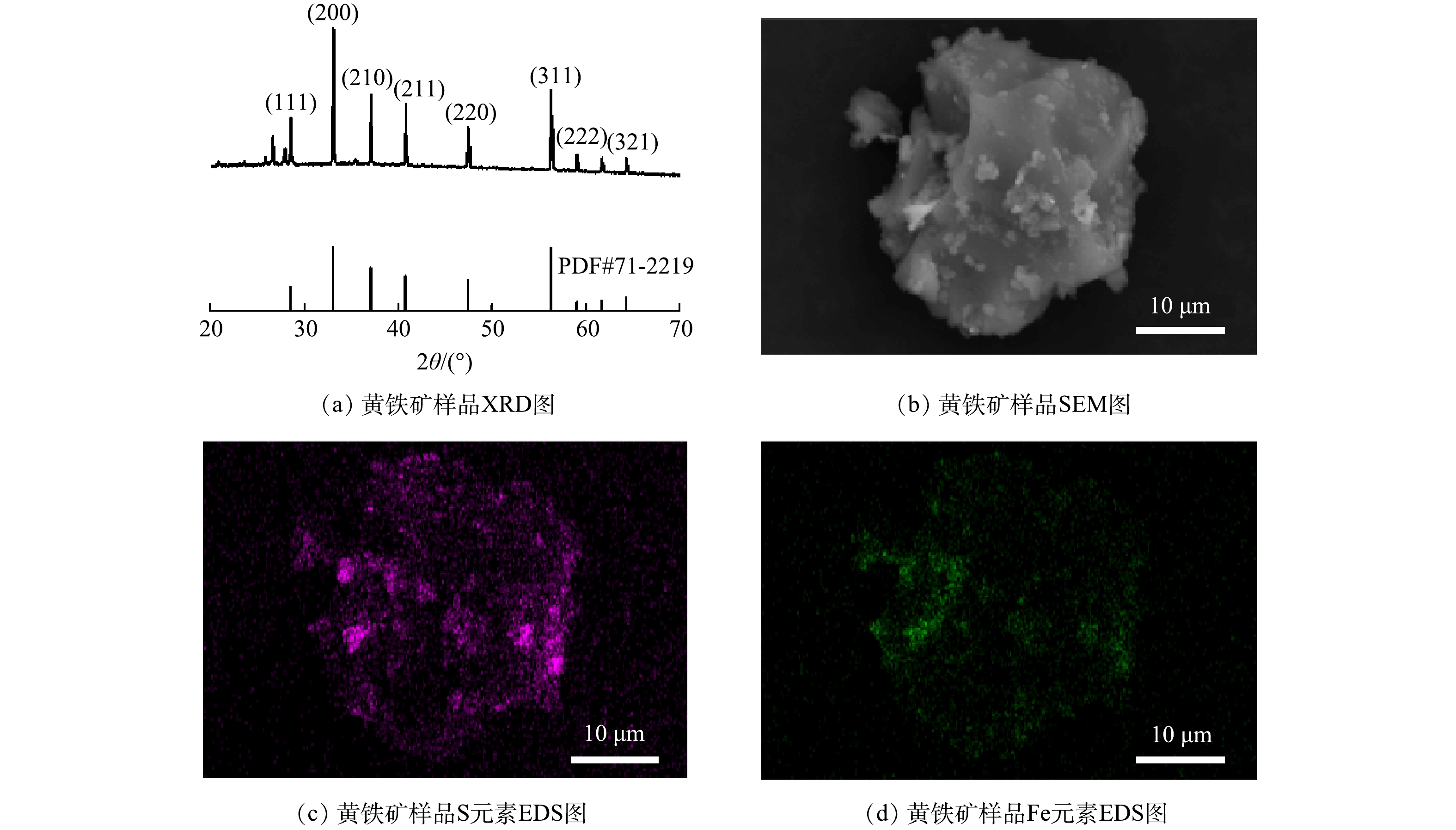
黄铁矿样品的XRD主要衍射峰的2θ为28.5、33.0、37.1、40.8、47.4、56.3、59.0和64.3(图2(a)),对应于的黄铁矿(111)、(200)、(210)、(210)、(211)、(220)、(311)、(222)和(321)晶面,与卡号为71-2219的标准卡匹配。SEM结果显示黄铁矿为不规则的晶体颗粒,表面伴有细小的颗粒状凸起(图2(b)),样品中S、Fe元素均匀分布在样品表面(图2(c)~(d))。XRD、SEM与EDS结果表明实验使用的黄铁矿有着较高的纯度。黄铁矿样品的比表面积为0.3052 m2·g−1,Fe、S元素的质量比分别为46.99%和44.32%。黄铁矿的等电点为2.61,当溶液pH小于2.61时,黄铁矿表面带正电;当pH大于2.61时,黄铁矿表面带负电。
-
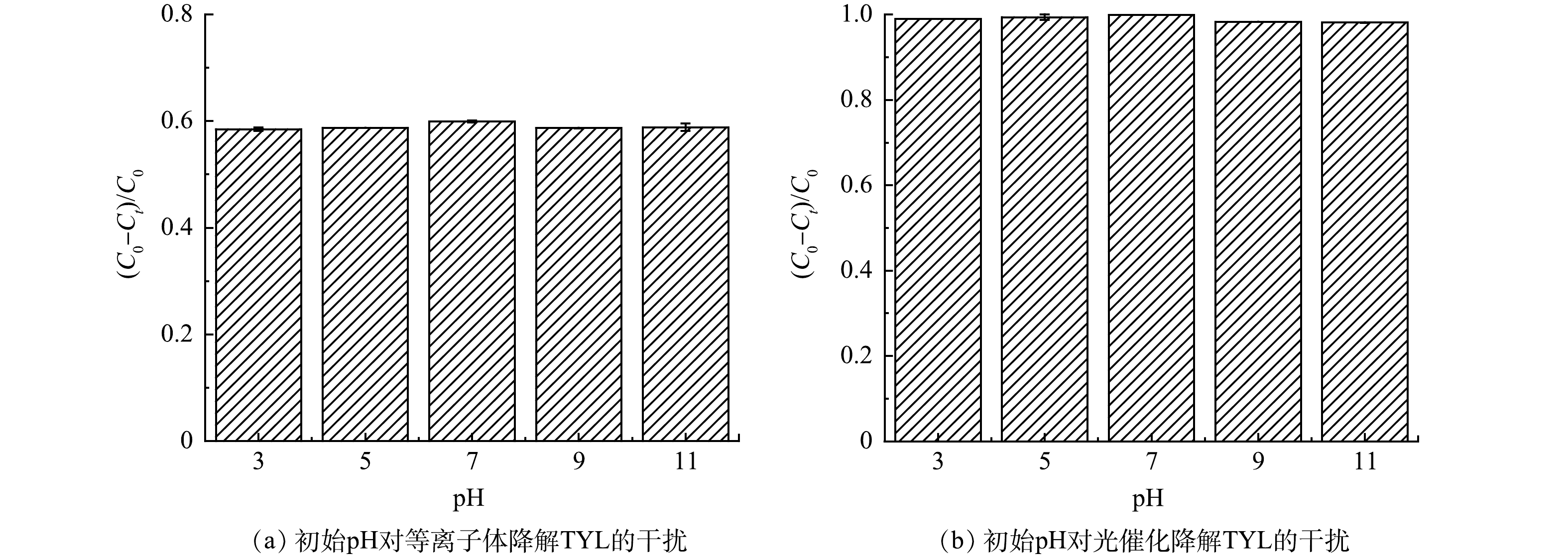
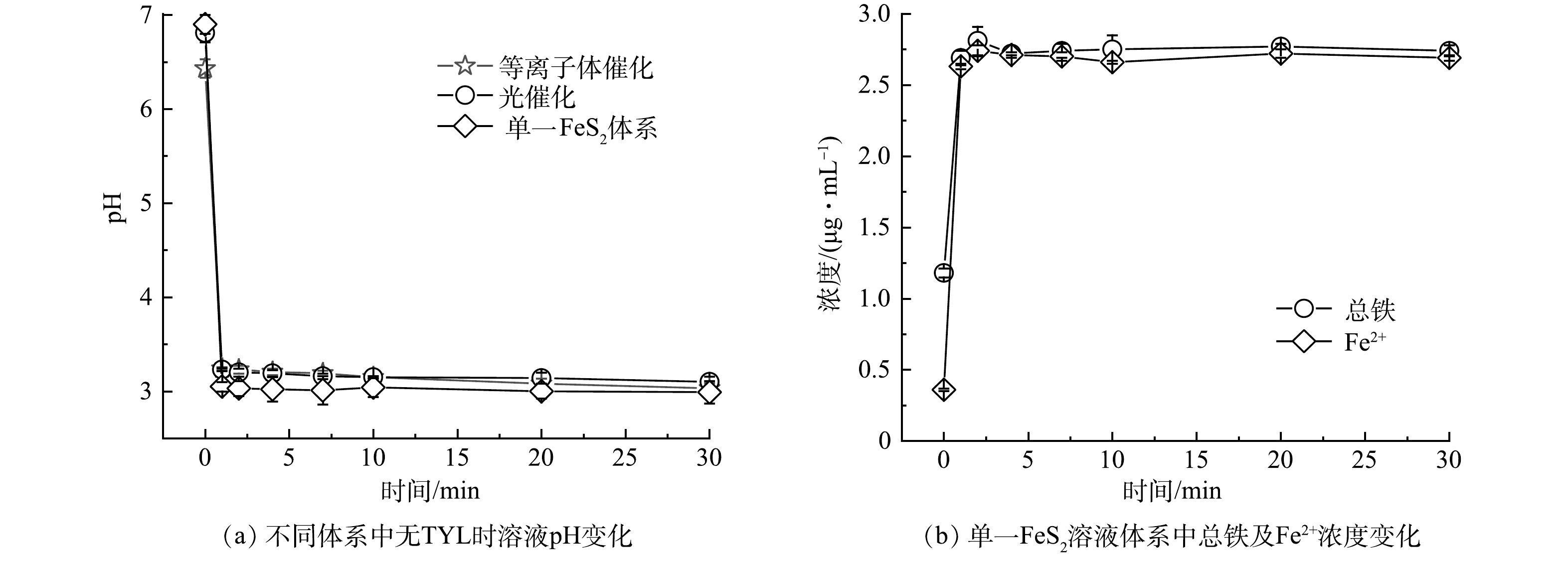
溶液pH会影响黄铁矿表面电荷。TYL作为一种典型的两性污染物,当溶液呈酸性时,TYL一般带正电;而当溶液pH增大后,TYL一般为不带电的分子态。除此以外,pH的改变还会对污染物降解的动力学速率产生影响[15]。pH对黄铁矿参与的等离子体与光催化技术降解TYL效果的影响见图3。在碱性、中性或是酸性条件下,黄铁矿耦合等离子体体系对TYL去除率均在60%(图3(a))。在实验pH范围内,黄铁矿对TYL的光催化降解率始终保持在90%以上(图3(b))。在不含TYL的不同反应体系中,溶液的pH变化情况见图4(a)。结果表明,3种体系中溶液的pH均会在黄铁矿添加后迅速下降,并在pH=3左右达到稳定。这是因为黄铁矿在水溶液中会与水与氧气反应生成大量H+,导致溶液pH迅速下降(图4(a))[13]。为了进一步确定溶液pH的下降主要是由黄铁矿氧化导致的,实验考察了单一的黄铁矿体系中的铁离子的溶出情况。如图4(b)所示,体系中Fe2+含量随时间的增加而迅速上升,而后变化缓慢。这表明黄铁矿在溶液体系中被不断氧化,并导致溶液pH降低
$ (\text{Fe}{\text{S}}_{\text{2}}\text{+3.5}{\text{O}}_{\text{2}}\text{+}{\text{H}}_{\text{2}}\text{O→}{\text{Fe}}^{\text{2+}}\text{+2}{\text{SO}}_{\text{4}}^{\text{2-}}\text{+2}{\text{H}}^{\text{+}} $ )。黄铁矿等电点为2.61,在pH稳定在3时,黄铁矿表面电荷始终为负,初始pH的变化不影响黄铁矿的表面带电情况。同时,最终pH的稳定使TYL(pKb=7.1)在体系中始终带正电。因此,初始溶液pH对2种技术降解TYL几乎没有影响。可见黄铁矿耦合等离子体技术与光催化技术降解TYL的溶液pH适用范围均比较广。 -
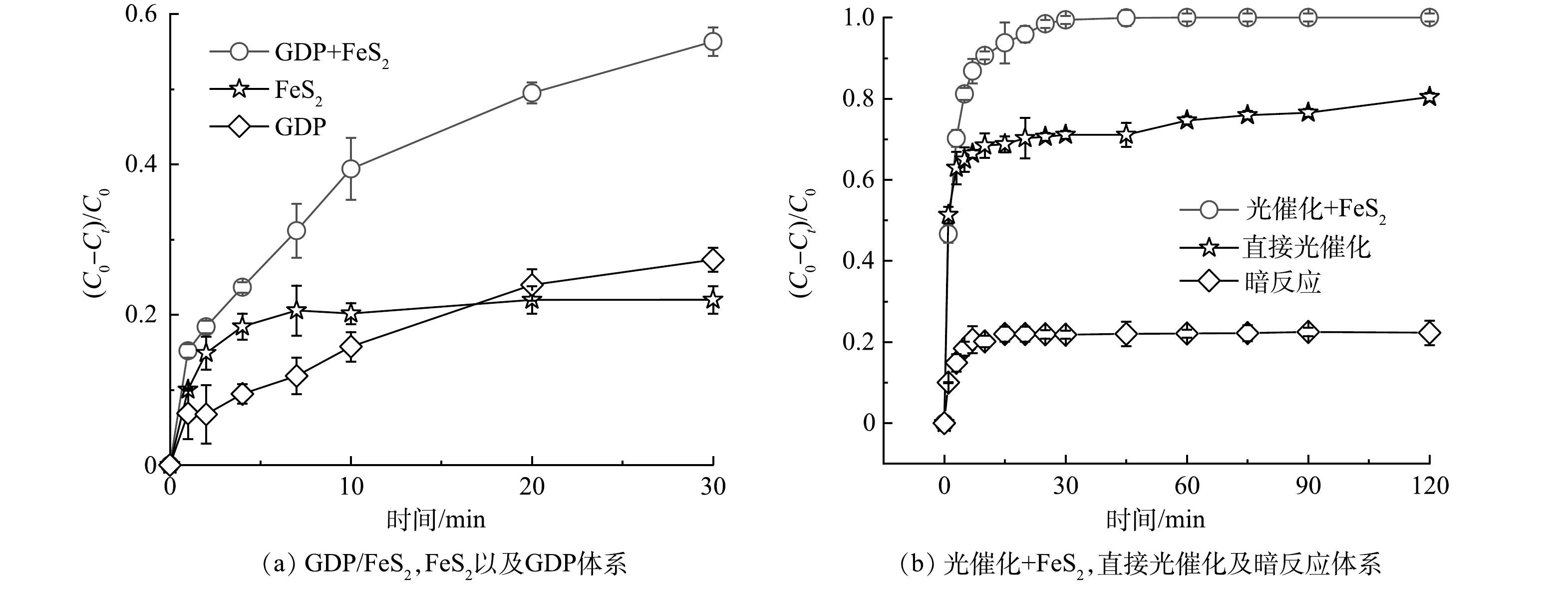
根据溶液pH对TYL降解影响的结果,在不调节溶液初始pH的条件下(pH=5.81),对比考察了黄铁矿在等离子体系和光降解体系中对TYL的降解效果和矿化效果的影响。在黄铁矿耦合等离子(GDP+FeS2)和单一等离子体(GDP)体系中,TYL浓度在前10 min迅速减小,之后去除速率逐渐降低,30 min其去除率达到最大(图5(a))。在FeS2体系中,TYL的去除率在7 min达到21%后不变,是黄铁矿快速吸附TYL的结果。30 min后,不同对照组对TYL的去除效果大小顺序为GDP+FeS2>GDP>FeS2。GDP+FeS2体系对TYL的去除率最高(56.33%),和GDP体系相比,其去效率提升了29.01%。WANG等[16]研究表明,添加催化剂后等离子体体系对四环素的降解率最高可提升30%。结果表明,催化剂的加入可以显著提高等离子体的降解性能。这主要是因为在等离子体反应器中引入催化剂可以产生更多活性物种,利于等离子体诱导污染物的深度氧化反应[17]。而在单一的FeS2体系中TYL的去除量最低,仅为21.98%,说明吸附作用对TYL去除的影响相对较小,这与黄铁矿本身较小的比表面积和黄铁矿用量密切相关。在黄铁矿光催化(光催化+FeS2)和直接光催化体系中(图5(b)),反应的前30 min内,2种体系中的TYL的降解效率都迅速增加,之后反应速率逐渐降低。在暗反应条件下,TYL的去除率在15 min达到最大值(22.30%),这说明部分的TYL被黄铁矿吸附。在反应30 min后,光催化+FeS2体系中的TYL几乎被完全降解。加入光照后体系对TYL去除率的提升表明吸附并不是体系中TYL去除的主要机制。本研究中黄铁矿的光催化技术对抗生素类污染物有着很好的去除效果,这与其他研究结果一致[18]。值得注意的是,TYL直接光照后的去除率也达到71.09%。这意味着在光催化体系中,大部分的TYL可能被水解成其他中间产物,而非完全矿化。尽管从TYL的去除效果看,黄铁矿光催化对TYL的去除效果远高于黄铁矿耦合等离子体体系,但仍需要进一步考察2个体系对TYL的矿化情况。
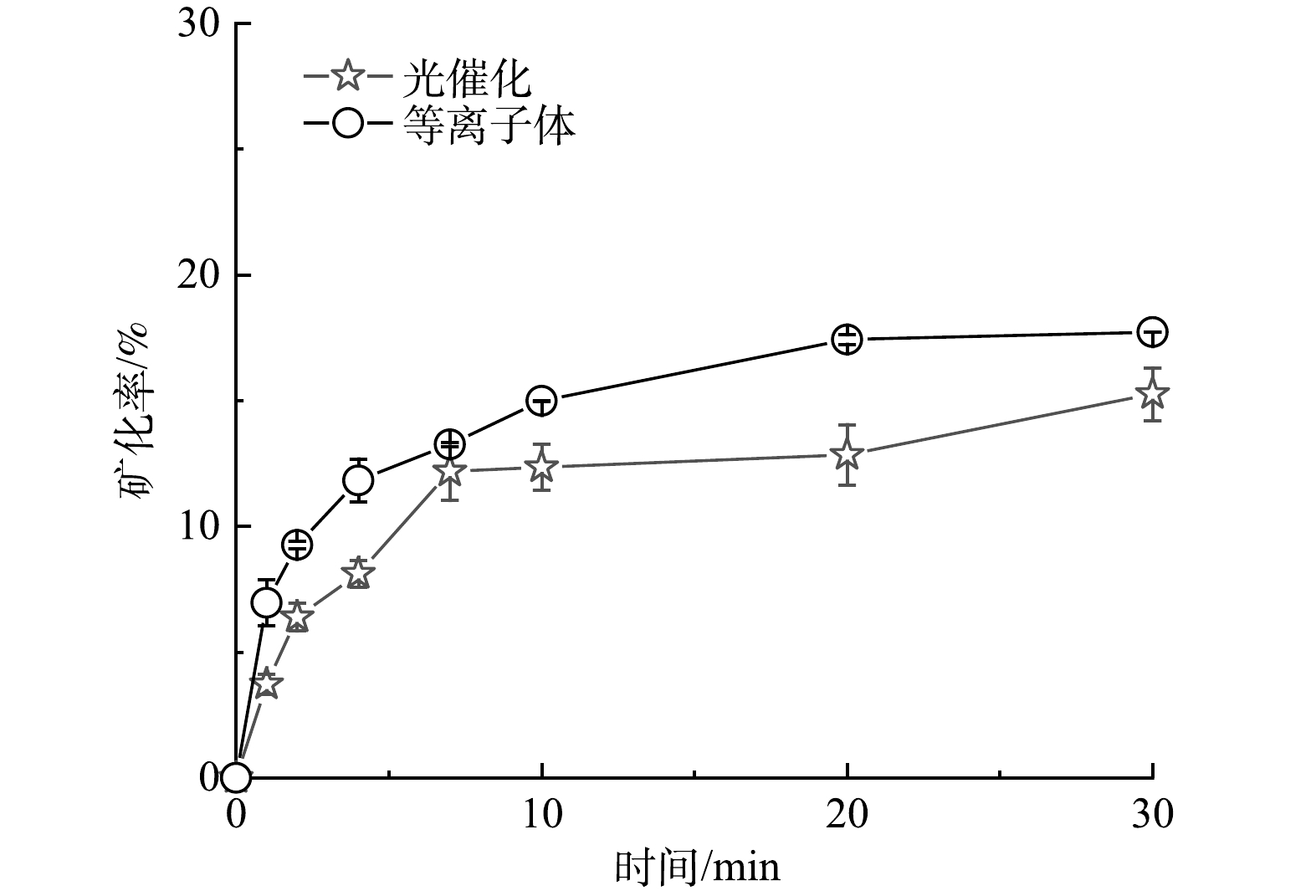
如图6所示,2个反应体系对TYL的矿化率都表现出先快速上升而后逐渐平缓的过程。反应30 min后,黄铁矿耦合等离子体与黄铁矿光催化技术对TYL的矿化率分别为17.72%和15.26%。FERRAG-SIAGH[19]使用电芬顿法降解TYL, 2 h后TYL的矿化率为45%,而在反应的前30 min内TYL的矿化率不足20%。由此可见,TYL是一种难降解的大分子抗生素。从去除效果看,等离子体耦合黄铁矿技术对TYL的去除率(56.33%)远低于黄铁矿光催化的去除率(99.99%)。但从矿化角度来看,该技术对TYL的矿化率要优于黄铁矿光催化技术。需要指出的是,采用黄铁矿耦合等离子和黄铁矿光催化降解TYL的去除效果是在相同固液比(1.4 g·L−1)、相同初始溶液条件(C0=10 mg·L−1)下进行的。辉光放电等离子体系反应器的溶液深度及其位置均可能影响GDP对污染物的降解效果[20]。因此,本研究中黄铁矿耦合GDP技术对TYL降解效果可以通过改进反应器得到提升。
-
TYL在黄铁矿耦合等离子和光催化降解体系中表现出不同的去除和矿化效果与溶液中的活性氧化物种密切相关[21]。在黄铁矿耦合等离子体体系中(图7(a)),添加硝酸银后,TYL的去除率下降了36.42%。硝酸银明显抑制了TYL的去除。这表明电子在耦合等离子体体系中对TYL的降解起重要的作用。在黄铁矿耦合等离子体体系中,高能电子(e−*)首先会与空气中的水和氧气发生反应,产生·OH、·O等活性物质进入溶液,进而促进TYL的降解。同时,e−*还会与溶液表面的水分子发生反应生成H2O+*,而生成的H2O+*又可以进一步转化为·OH等活性物质[22]。在溶液中添加草酸铵、异丙醇以及对苯醌后,TYL的降解率分别下降了34.52%、31.38%和11.90%。这说明h+、·OH以及·O2−在黄铁矿耦合等离子体体系中对TYL降解也不容忽视,其中h+和·OH对TYL降解的影响与电子的影响差异不大。结果表明,除了高能电子本身引起的·OH等活性物质对TYL的降解之外,FeS2对放电过程中光的吸收而产生的光生电子和空穴的分离及FeS2的光芬顿作用也参与了TYL的降解。光催化体系溶液中添加异丙醇、草酸铵和对苯醌抑制剂后,TYL的降解率分别下降了20.56%、19.94%以及20.11%(图7(b))。这说明·OH、h+、·O2−在光催化反应中都发挥了重要作用。当以硝酸银为抑制剂时,淬灭实验对TYL降解的抑制效果略有下降(18.93%)。通过对比可以发现,黄铁矿耦合等离子体体系中的各活性物种的贡献各不相同,其中e−的贡献最高。而在光催化体系中·OH、h+、·O2−对TYL的降解贡献较大。
-
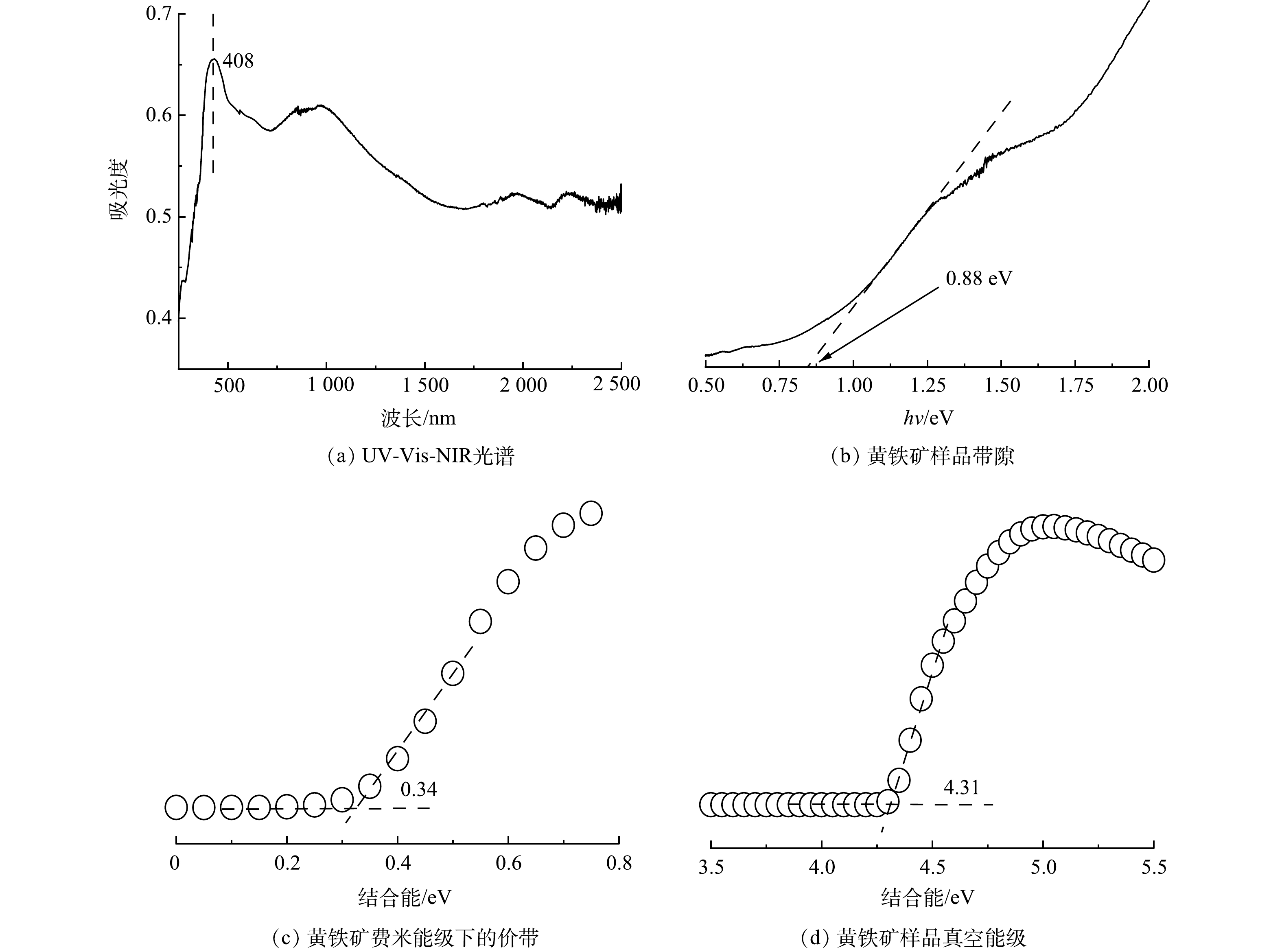
在黄铁矿耦合等离子和黄铁矿光催化技术降解TYL的过程中,黄铁矿都能被光激发产生光生电子。分离的光生电子和空穴可能会与氧气和水反应,产生活性物种进而降解TYL。为推导黄铁矿在光催化体系和等离子体系中活性物种的生成途径,分析了黄铁矿的能带结构。在250~2 500 nm整个紫外可见近红外区域内,黄铁矿样品可以全波段吸光(图8(a)),表明实验用黄铁矿对紫外可见光有较好的吸收能力。图8(b)为黄铁矿Kubelka-Munk函数(hν·F(R))2与光能hv的关系图,根据Tauc原理可以算出样品的禁带宽度Egpyrite为0.88 eV。同步辐射光电子能谱(SR-PES)的测试结果见图8(c)~(d)。由黄铁矿二次电子截止边可以确定黄铁矿样品的真空能级为−4.31 V (vs RHE),进一步可以算出黄铁矿的费米能级EF为−0.19 V。图8(c)显示了黄铁矿样品费米能级下的价带为0.34 V。根据等式EVB=|费米能级下的价带|+EF可得出实验所使用的黄铁矿价带(VB)为0.15 V,进而得到黄铁矿样品的导带(CB)为−0.73 V。
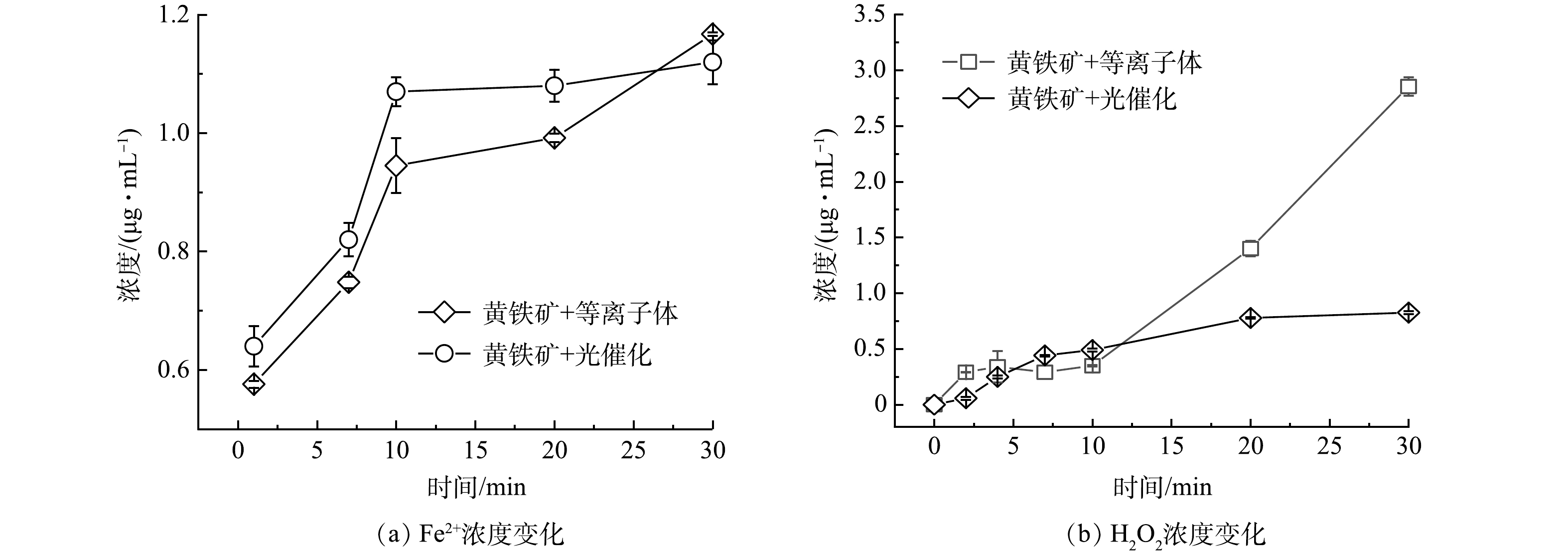
在光催化反应中,黄铁矿会吸收紫外可见光产生光生h+与e−(式(2))。由于黄铁矿CB值为−0.73 V,光电子与黄铁矿表面上的溶解氧之间可以相互作用产生·O2− (O2/O2− = −0.33 eV,pH=7) (式(3))[23]。随后,溶液环境中·O2−会与H+作用,并经过进一步反应生成H2O2(式(4)~(5)),而生成的H2O2又会与黄铁矿所释放出的Fe2+发生反应,促成Fenton反应(式(6)),由图9(a)~(b)可见,黄铁矿光催化反应过程中Fe2+与H2O2浓度均随时间的增加而上升。说明黄铁矿光催化体系中具备Fenton反应发生的条件。另一方面,由于黄铁矿的价带位置较低(VB=0.15 V),空穴不能直接将H2O和OH− 氧化成羟基自由基(H2O/·OH=2.27 eV,OH−/·OH=1.99 eV, pH=7)[24]。黄铁矿光催化降解TYL过程中活性物种的生成路径如式(2)~式(7)所示。在黄铁矿光催化降解TYL体系中,活性物种主要通过光催化与Fenton反应生成。
在黄铁矿耦合等离子体体系中,反应过程中的Fe2+与H2O2浓度也随时间的增加而上升(图9(a)~(b))。同时由于光能的激发,式(2)~式(6)所代表的光催化及Fenton反应在黄铁矿耦合等离子体体系中也会发生。与光催化体系不同的是,在耦合等离子体体系中,等离子体放电产生能量为1~25 eV的e−*[25],e−*迅速与空气中的O2和H2O反应,生成·O以及·OH(式(7)~式(9))。之后,被解离出的·OH又会通过自由基重组形成H2O2(式(10))并释放到溶液当中,从而更有利于为Fenton反应的发生。不仅如此,在生成H2O2的同时,溶液中的O2还会与·O发生反应并生成一定量的O3(式(11))。由于e−和Fe2+的存在,O3并不稳定,而是会转化为·OH进一步促进了TYL的降解(式(12)~式(16))[26-27]。式(2)~式(16)提出了黄铁矿耦合等离子体体系中活性物种产生的可能途径。与黄铁矿光催化体系相比,黄铁矿耦合等离子体体系中e−*的产生为TYL的降解提供更多的活性物种。这可能也是该体系对TYL的矿化率要高于黄铁矿光催化体系的原因之一。黄铁矿耦合等离子体体系通过等式(8)~式(9)产生了更多的e−,这从另一个角度解释了淬灭实验中e−在耦合等离子体体系的降解过程中发挥最重要的作用。
-
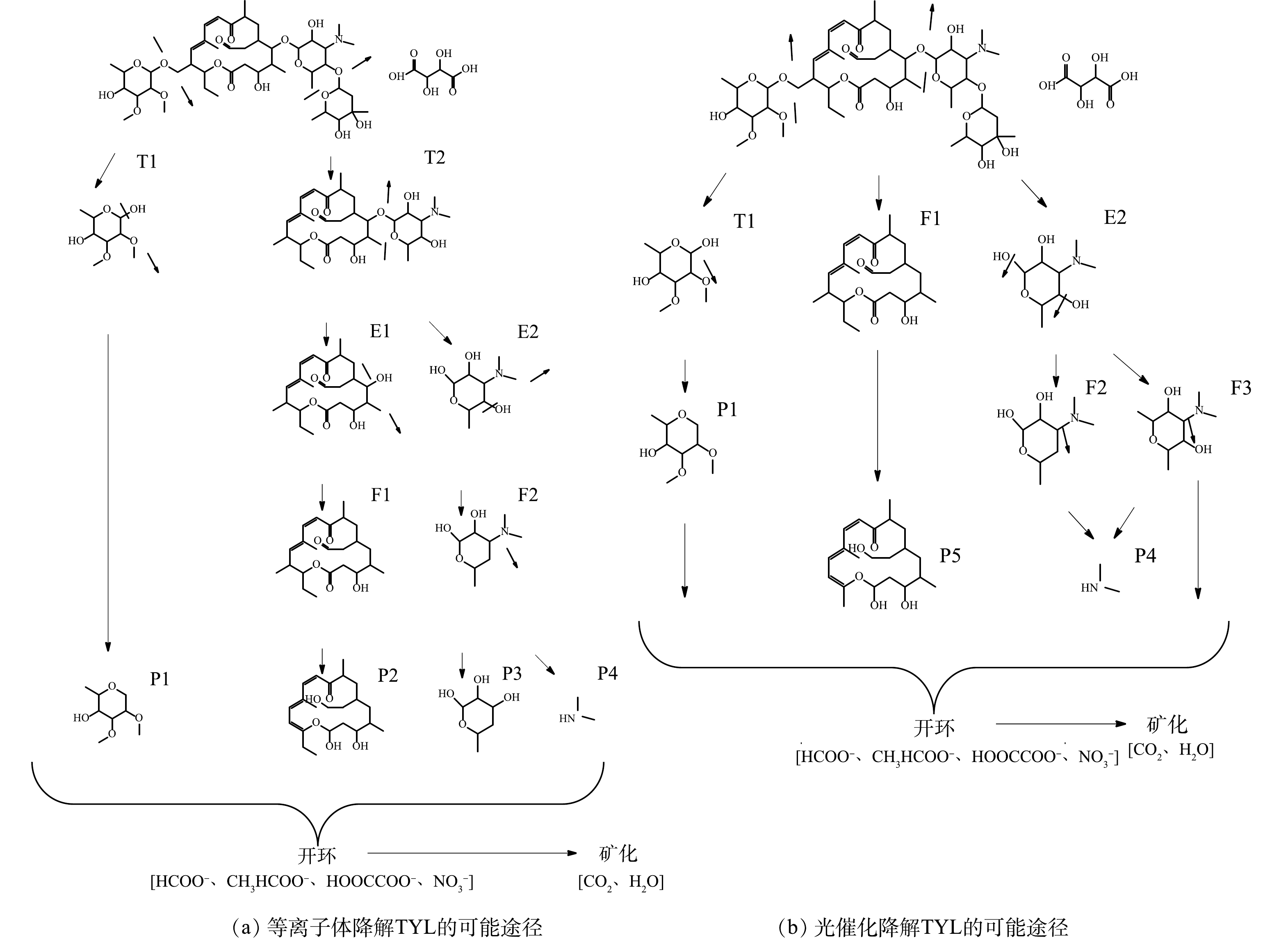
TYL在黄铁矿耦合等离子体和黄铁矿光催化降解体系中的降解路径如图10所示。在黄铁矿耦合等离子体体系中(图10(a)),TYL首先接受等离子体所释放出的能量,使得C—O键断裂,生成水解产物T1和T2。HU[28]等在降解水和土壤中的TYL时也发现,在降解初期TYL会迅速分解为多种极性中间产物,这与本研究的结果相一致。而后,T1和T2可以进一步通过C—O键的断裂分解为P1、E1和E2[29]。由于溶液中·OH和·O2−等高氧化性的活性物种的存在,中间产物E1及E2的羟基被脱去[30],转变为有机化合物F1、F2。之后,F1中的甲基和C=O键被进一步破坏,生成P2。同时,F2中的C-N键被活性物质攻击,分解为产物P3和P4[31]。最后,环状中间体经过开环被氧化成小分子有机酸,并最终被矿化为H2O和CO2。黄铁矿光催化技术对TYL的降解途径如图10(b)所示。与黄铁矿耦合等离子体技术相同,反应开始后TYL首先被迅速水解,生成中间产物T1、F1和E2。之后,F1中的C=C和C=O键断裂,形成产物P5。同时,T1通过C—O断裂生成P1,E2中的羟基受到活性物质的攻击而分解为F2和F3。随后,F2和F3中的C-N键又会被进一步破坏,并生成产物P4。最后,经过开环和氧化作用,部分中间产物会被矿化为无害的H2O和CO2。总体来说,黄铁矿耦合等离子体技术与黄铁矿光催化技术对TYL的降解路径是十分相似的,TYL均是首先被转化为小分子有机酸以及环状化合物,而后再被矿化为H2O和CO2。在降解初期,在黄铁矿光催化体系中检测到了更多的初期水解产物。由前文的研究可知,TYL光解的去除率可以达到70%以上,因此初期更多的水解产物很可能是光照导致的TYL分解和黄铁矿的光催化作用共同造成的。光照促进了TYL的分解,但并不能完全将中间产物矿化,这可能也是光催化体系中TYL的降解率较高但矿化率却较低的原因。
-
以实验的降解效率和降解条件为基础,初步分析对比了2种AOP工艺在实验室条件下降解TYL的经济性。2种技术降解TYL的成本主要包括电能和催化剂药剂费用。在本研究中,2种工艺中黄铁矿的使用量相同,成本均为0.056 元·g−1。但2种工艺对电能的消耗各不相同,其中光催化电费成本为5 kWh·L−1,等离子体技术电费成本为1.045 kWh·L−1。经济性是通过计算在单位电能内对TYL的去除效果。结合2种工艺的电能成本及光催化和等离子体技术对TYL的去除率(99.99%和56.33%),可以计算出单位电能成本下TYL的降解量。结合2种工艺中黄铁矿的用量及各自对TYL的去除效率,可以计算出黄铁矿光催化技术对TYL的处理效率为2 mg·(kWh)−1,低于黄铁矿耦合等离子体对TYL的处理效率(5.39 mg·(kWh)−1)。因此,黄铁矿光催化降解TYL的经济性要低于黄体矿耦合等离子体技术。
-
1)由于黄铁矿对溶液pH的自调节作用,黄铁矿光催化和黄铁矿耦合等离子技术对溶液pH的适应范围均比较广。
2)黄铁矿光催化对TYL的降解率(99%)明显高于黄铁矿耦合等离子技术(56.33%),但前者的矿化效果不佳。
3)在黄铁矿光催化体系中,·OH、h+和·O2−是造成TYL降解的主要原因;而在黄铁矿耦合等离子体体系中,除上述活性物种,e−在TYL降解中发挥的作用更为突出。
4) 2种技术降解TYL的过程十分相似。TYL均先后经过水解、C-O键断裂、去羟基、去甲基、C=O、C-N断裂和开环后最终被矿化为H2O和CO2。
5)在实验室条件下,黄铁矿耦合等离子技术对TYL的处理效能要优于光催化技术。
黄铁矿的光催化及其辉光放电等离子体催化降解水体中泰乐霉素的性能对比
Comparison on the performance of tylosin tartrate degradation by photocatalysis and glow discharge plasma catalysis of pyrite
-
摘要: 黄铁矿的异相Fenton和半导体特性使其具有良好的污染物降解潜力。基于黄铁矿的光催化技术和辉光放电等离子体技术可以同时利用这2种特性。但目前仍缺乏对这2种技术降解抗生素废水的对比研究。因此,考察了黄铁矿参与的2种高级氧化技术(AOP)对泰乐霉素(tylosin tartrate,TYL)的降解效果、机制、产物、适用条件和经济成本。结果表明,2种技术在pH=3~11内对TYL均有稳定的降解性能。黄铁矿光催化技术对TYL的降解效率(99%)要明显高于黄铁矿耦合等离子技术(56.33%),但其矿化效果却低于等离子技术。2种反应体系中的·OH、h+、·O2−以及e−均参与到TYL的降解中。其中,在黄铁矿光催化体系中·OH、h+和·O2−是TYL降解的主要活性物种。活性物种主要通过光催化与Fenton反应生成。而在黄铁矿耦合等离子体体系中,e−在TYL降解中发挥的作用则更为突出。与黄铁矿光催化相比,黄铁矿耦合等离子体中e−*的产生为TYL的降解提供了更多的活性物种。2种催化降解技术对TYL的降解过程相似。TYL经过水解、C-O键断裂、去羟基、去甲基、C=O、C-N断裂、开环后最终被矿化为H2O和CO2。在相同实验条件下,黄铁矿耦合等离子体技术对TYL的处理效能要优于光催化技术。以上研究结果有助于更好地理解黄铁矿参与的高级氧化技术对污染物的降解机理,并为难降解的抗生素类污染物的处理提供参考。Abstract: Pyrite shows an excellent contamination degrading ability due to its heterogeneous Fenton and semiconductor properties, which could be used by pyrite-based photocatalysis and glow discharge plasma technology. However, a comprehensive comparison of these two technologies for the degradation of antibiotic wastewater is still limited. Therefore, two kinds of advanced oxidation techniques (AOPs) based on pyrite were studied to investigate the efficiency, mechanisms, products, application conditions and economic costs of tylosin tartrate (TYL) degradation. The results showed that both techniques exhibited a stable TYL degradation performance within pH 3~11. Pyrite-based photocatalysis had a significantly higher degradation efficiency for TYL (99%) than pyrite-based plasma technology (56.33%), but the mineralization efficiency of the former was lower than that of the latter. The ·OH, h+, ·O2− as well as e− in both reaction systems took part in the degradation of TYL. Yet ·OH, h+ and ·O2− were the main active species in the pyrite photocatalytic system for TYL degradation. Of which, in pyrite-based photocatalysis system, ·OH、h+ and ·O2− were the active species for TYL degradation, they were mainly produced through photocatalytic and Fenton reactions. In the pyrite-based plasma system, e− played a more prominent role in the degradation of TYL. The production of e−* in the pyrite-based plasma system provided more active species for the degradation of TYL than in photocatalysis. The degradation process of TYL was similar in the two catalytic degradation techniques. TYL was mineralized to H2O and CO2 lastly through hydrolysis, breakage of C-O bond, de-hydroxylation, de-methylation, breakage of C=O, C-N and ring opening. Under the same experimental conditions, the pyrite-based plasma technique was more effective than photocatalysis for the degradation of TYL. The results of this study contribute to a better understanding of the processes and mechanisms involved in the degradation of pollutants by advanced oxidation techniques based on pyrite. And it provides a theoretical reference for the treatment of refractory antibiotic contaminants.
-
Key words:
- pyrite /
- tylosin tartrate /
- photocatalytic technology /
- plasma
-

-
表 1 LC-MS梯度洗脱程序
Table 1. LC-MS gradient elution procedure
时间/min A/% B/% C/% 0.0 70 10 20 6.0 0 20 80 8.0 0 5 95 10.0 0 20 80 12.0 0 80 20 18.0 70 10 20 -
[1] ZENG L Y, GONG J Y, DAN J F, et al. Novel visible light enhanced Pyrite-Fenton system toward ultrarapid oxidation of p-nitrophenol: Catalytic activity, characterization and mechanism[J]. Chemosphere, 2019, 228: 232-240. doi: 10.1016/j.chemosphere.2019.04.103 [2] 乔雯雯, 王宇晖, 宋新山. 黄铁矿强化人工湿地反硝化处理含氮废水的研究[J]. 工业水处理, 2021, 41(4): 77-83. [3] 胡俊松, 李睿华, 孙茜茜, 等. 天然黄铁矿对草甘膦的吸附性能[J]. 环境工程学报, 2015, 9(11): 5463-5469. doi: 10.12030/j.cjee.20151152 [4] LIU W, WANG Y Y, AI Z H, et al. Hydrothermal synthesis of FeS2 as a high-efficiency fenton reagent to degrade alachlor via superoxide-mediated Fe(II)/Fe(III) cycle[J]. ACS Applied Materials & Interfaces, 2015, 7(51): 28534-28544. [5] 李海东, 田世洪, 江卫兵, 等. 粤东北桃源铀矿床黄铁矿地球化学特征及其地质意义[J]. 地质论评, 2021, 67(4): 1157-1167. [6] 陈上锋. 黄铁矿在环境净化方面的应用[J]. 中山大学研究生学刊(自然科学医学版), 2014, 35(2): 22-36. [7] 王汉林, 陈天虎, 刘海波. 硫化鲕状赤铁矿非均相Fenton法降解四环素, 中国矿物岩石地球化学学会第17届学术年会, 中国浙江杭州, F, 2019 C].
[8] GOLSHEIKH A M, HUANG N M, LIM H N, et al. One-pot hydrothermal synthesis and characterization of FeS2 (pyrite)/graphene nanocomposite[J]. Chemical Engineering Journal, 2013, 218: 276-284. doi: 10.1016/j.cej.2012.09.082 [9] PUTHUSSERY J, SEEFELD S, BERRY N, et al. Colloidal iron pyrite (FeS2) nanocrystal inks for thin-film photovoltaics[J]. Journal of the American Chemical Society, 2011, 133(4): 716-719. doi: 10.1021/ja1096368 [10] WANG J L, ZHUAN R. Degradation of antibiotics by advanced oxidation processes: An overview[J]. Science of the Total Environment, 2020, 701: 135023. doi: 10.1016/j.scitotenv.2019.135023 [11] SCHOONEN M A A, HARRINGTON A D, LAFFERS R, et al. Role of hydrogen peroxide and hydroxyl radical in pyrite oxidation by molecular oxygen[J]. Geochimica Cosmochimica Acta, 2010, 74(17): 4971-4987. doi: 10.1016/j.gca.2010.05.028 [12] HE D, SUN Y, XIN L, et al. Aqueous tetracycline degradation by non-thermal plasma combined with nano-TiO2[J]. Chemical Engineering Journal, 2014, 258: 18-25. doi: 10.1016/j.cej.2014.07.089 [13] DIAO Z H, LIU J J, HU Y X, et al. Comparative study of Rhodamine B degradation by the systems pyrite/H2O2 and pyrite/persulfate: Reactivity, stability, products and mechanism[J]. Separation Purification Technology, 2017, 184: 374-383. doi: 10.1016/j.seppur.2017.05.016 [14] ZHANG Q, PENG Q, SHU X, et al. Spectroscopic analysis of tylosin adsorption on extracellular DNA reveals its interaction mechanism[J]. Colloids and Surfaces B: 2019, 183: 110431. [15] GUO H, LI Z, LIN S, et al. Multi-catalysis induced by pulsed discharge plasma coupled with graphene-Fe3O4 nanocomposites for efficient removal of ofloxacin in water: Mechanism, degradation pathway and potential toxicity[J]. Chemosphere, 2021, 265: 129089. doi: 10.1016/j.chemosphere.2020.129089 [16] WANG B W, WANG C, YAO S M, et al. Plasma-catalytic degradation of tetracycline hydrochloride over Mn/gamma-Al2O3 catalysts in a dielectric barrier discharge reactor[J]. Plasma Science & Technology, 2019, 21(6): 132-139. [17] JIANG B, ZHENG J T, QIU S, et al. Review on electrical discharge plasma technology for wastewater remediation[J]. Chemical Engineering Journal, 2014, 236: 348-368. doi: 10.1016/j.cej.2013.09.090 [18] 艾翠玲, 周丹丹, 张嵘嵘, 等. β-In2S3的制备及其太阳光下降解土霉素[J]. 环境科学, 2015, 36(8): 2911-2917. [19] FERRAG-SIAGH F, FOURCADE F, SOUTREL I, et al. Electro-Fenton pretreatment for the improvement of tylosin biodegradability[J]. Environmental Science and Pollution Research, 2014, 21(14): 8534-8542. doi: 10.1007/s11356-014-2771-5 [20] CHANG Z S, WANG C, ZHANG G J. Progress in degradation of volatile organic compounds based on low-temperature plasma technology[J]. Plasma Processes and Polymers, 2020, 17(4): 201900131. [21] ZHU F, CHEN J X, MA J F, et al. Degradation of organic pollutants by ZnMn2O4/organic acid system: Identification of active species[J]. Materials Letters, 2021, 293: 129725. doi: 10.1016/j.matlet.2021.129725 [22] SHEN C S, WU S S, CHEN H, et al. Phthalate degradation by glow discharge plasma enhanced with pyrite in aqueous solution[J]. Water Science and Technology, 2016, 74(6): 1365-1375. doi: 10.2166/wst.2016.316 [23] CHEN S, WANG H, SHI M, et al. Deep oxidation of NO by a hybrid system of plasma-N-type semiconductors: High-Energy electron-activated "Pseudo Photocatalysis" behavior[J]. Environmental Science & Technology, 2018, 52(15): 8568-8577. [24] CUI Y, HUANG J, FU X, et al. Metal-free photocatalytic degradation of 4-chlorophenol in water by mesoporous carbon nitride semiconductors[J]. Catalysis Science & Technology, 2012, 2(7): 1396-1402. [25] HARLING A M, GLOVER D J, WHITEHEAD J C, et al. Novel method for enhancing the destruction of environmental pollutants by the combination of multiple plasma discharges[J]. Environmental Science & Technol, 2008, 42(12): 4546-4550. [26] LIU Y, SUN B, WANG L, et al. Characteristics of light emission and radicals formed by contact glow discharge electrolysis of an aqueous solution[J]. Plasma Chemistry and Plasma Processing, 2012, 32(2): 359-368. doi: 10.1007/s11090-011-9347-7 [27] SUN B, KUNITOMO S, IGARASHI C. Characteristics of ultraviolet light and radicals formed by pulsed discharge in water[J]. Journal of Physics D:Applied Physics, 2006, 39(17): 3814-3820. doi: 10.1088/0022-3727/39/17/016 [28] HU D, COATS J R. Aerobic degradation and photolysis of tylosin in water and soil[J]. Environmental Toxicology and Chemistry, 2007, 26(5): 884-889. doi: 10.1897/06-197R.1 [29] VON EYKEN A, BAYEN S. Non-targeted study of the thermal degradation of tylosin in honey, water and water: honey mixtures[J]. Food Additives and Contaminants:Part A, 2020, 37(3): 421-437. doi: 10.1080/19440049.2019.1704442 [30] ZHANG G, HUANG G, YANG C, et al. Efficient photoelectrocatalytic degradation of tylosin on TiO2 nanotube arrays with tunable phosphorus dopants[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104742. doi: 10.1016/j.jece.2020.104742 [31] KOSJEK T, HEATH E. Applications of mass spectrometry to identifying pharmaceutical transformation products in water treatment[J]. Trends in Analytical Chemistry, 2008, 27(10): 807-820. doi: 10.1016/j.trac.2008.08.014 -



 下载:
下载: