-
化工、医药等行业产生的难降解有机物会排入污水厂。然而,当前以生物处理为核心的传统污水厂无法有效降解这些难降解有机物,如何采用新工艺或者改良当前的污水工艺以应对新型污染物的挑战,已经成为了环境工作者的研究热点之一[1]。高级氧化法可产生具有强氧化能力的羟基自由基(·OH)、硫酸根自由基(SO4·−)等活性氧物种,使难降解有机物氧化成低毒或无毒的小分子物质[2-3],亦可改善其可生化性,故被认为是处理这类污染物的有效方法之一。
各类高级氧化法中,芬顿反应(式(1))因其条件温和、操作简便和价格低廉等优势而被广泛研究[4]。然而,在中性/碱性条件下,Fe2+会以氢氧化物的形式沉淀,失去活性,故该反应体系需要控制pH在3.0~4.0,以保证有高浓度的溶解态亚铁离子(Fe2+)持续驱动自由基的生成[5-6]。调节pH大幅提高了芬顿工艺的运行费用,阻碍了其在含有缓冲溶液的工业废水中的推广使用[7-8]。络合剂能使高浓度Fe2+在中性/碱性条件下以金属络合物(Fe2+-EDTA)形式存在,Fe2+-EDTA在中性/碱性条件下依然具有激活H2O2(式(2))并氧化降解核酸链的能力[9]。在中性条件下,添加NTA(氨三乙酸),TA(对苯二甲酸)等络合剂的芬顿体系可快速降解新诺明(pH=7.0)[10]。然而,络合剂与亚铁离子配比至少为1∶1,这表明将向待处置污水中引入大量的有机物,且络合剂与芬顿反应处置后的Fe3+形成的Fe3+-EDTA提高了体系内去除Fe3+的难度。CHEN等[11]向芬顿体系中投加还原剂(羟胺、NH2OH、HA)以降低铁离子用量,式(1)中失活的Fe3+可被羟胺重新转化成具有活性的Fe2+(式(3)~式(4)),故该强化体系能以痕量铁(2 mg∙L−1)持续驱动芬顿反应的进行与·OH的持续生成[11-12];相似的方法也用于持续驱动Fe2+/过一硫酸根体系和Fe2+/过二硫酸根体系[13-14]。
在后续研究中发现,体系内的羟胺并不会生成对水体健康有害的NO3−和NO2−,而是转化成N2O(式(5)),因此,其不会有二次污染。考虑到羟胺的强还原性,羟胺加入到Fe2+-EDTA/H2O2体系内或许可以实现Fe2+-EDTA的再生,最终实现中性/碱性条件下活性氧自由基(·OH)的持续生成。
基于以上研究结果,本研究拟通过在芬顿体系中投加羟胺与EDTA,以伊文思蓝为·OH的指示剂,考察强化后的芬顿体系持续产生·OH的能力。伊文思蓝可与H2O2、过硫酸盐等过氧化物共存,且其与·OH可快速反应,因此,本研究中伊文思蓝的降解率可反映芬顿体系中·OH的生成情况。本文分析了溶液pH、铁离子浓度、H2O2浓度、EDTA浓度和伊文思蓝浓度对降解过程的影响,优化了体系对伊文思蓝降解的最佳条件,并通过电子自旋共振(ESR)技术和苯基甲基亚砜(PMSO)推测了体系中伊文思蓝的主要降解路径,从而确定该方法的稳定性,以期为芬顿反应在特定应用场景中的应用提供参考。
-
实验用30%过氧化氢(H2O2)购自上海沃凯生物技术有限公司、硫酸亚铁(FeSO4)、盐酸羟胺(HONH2HCl, HA)、乙二胺四乙酸二钠(C10H14N2Na2O8, EDTA)、99.7%乙醇(C2H5OH)、叔丁醇(C4H10O)、甲醇(CH3OH)、乙腈(CH3CN)、伊文思蓝(C34H24N6Na4O14S4)、氢氧化钠(NaOH)、硫酸(H2SO4)等均购自上海麦克林生化科技,叔丁醇、甲醇、乙腈为色谱级纯,伊文思蓝为生物技术级纯,其余均为分析纯。
-
紫外可见分光光度计(UV-3000,上海元析仪器有限公司)、pH计(FE28,梅特勒-托利多仪器(上海)有限公司)、磁力搅拌器(MS-H-Pro+,大龙兴创实验仪器(北京)股份公司)、超纯水机(Milli-Q,厦门锐思捷水纯化技术有限公司)、电热鼓风干燥箱(GFL-45,天津市莱玻特仪器设备有限公司)、高效液相色谱仪(HPLC-20AD,Shimadzu)、顺磁共振波谱仪(EMXnano,BRUKER)。
-
所有实验均于150 mL锥形瓶中进行,室温条件下将反应装置置于磁力搅拌器以350 r∙min−1的速度搅拌,先加入Fe2+、羟胺、EDTA、伊文思蓝药品后使用稀硫酸与氢氧化钠调节pH,再加入H2O2开始计时并定时取样,每次取样品2 mL加入5 mL离心管内,并加入1.5 mL 1 mol∙L−1乙醇淬灭、加入1 mL 10 mmol∙L−1 H2KPO4-HK2PO4 磷酸缓冲液调节样品pH为7,加盖后待测;未及时测定的样品则密封后置于4 ℃冰柜中冷藏保存;实验过程中始终监测系统pH并及时进行调节,保证系统pH始终处于±0.2范围内波动。
-
以超纯水为参比,在波长为611 nm的条件下测定伊文思蓝原始样品及经芬顿/羟胺/EDTA系统处理后样品吸光值,根据朗伯-比尔定律计算样品去除率。使用高效液相色谱(HPLC)检测PMSO(苯基甲基亚砜)与PMSO2(苯基甲基砜),将样品过0.22 μm滤膜后装入1.5 mL玻璃小瓶,按顺序加入HPLC进样板,以1‰乙酸为水相、色谱级纯乙腈为有机相进行测定,设定流速1.0 mL∙min−1,水相80%,有机相20%,停留时间15 min,PMSO测定波长230 nm、PMSO2测定波长254 nm的方法进行测定,测定结果以吸收峰的面积相关去除率进行分析[15]。羟基自由基(·OH)的检测使用电子自旋共振法进行定性分析,以二甲基吡咯氮氧化物(DMPO)为捕获剂,取样后以10∶1加入捕获剂,混匀后置于顺磁共振波谱仪进行测定,若图像中存在比例约为1∶2∶2∶1的4个吸收峰,则表示体系中有·OH存在。
-
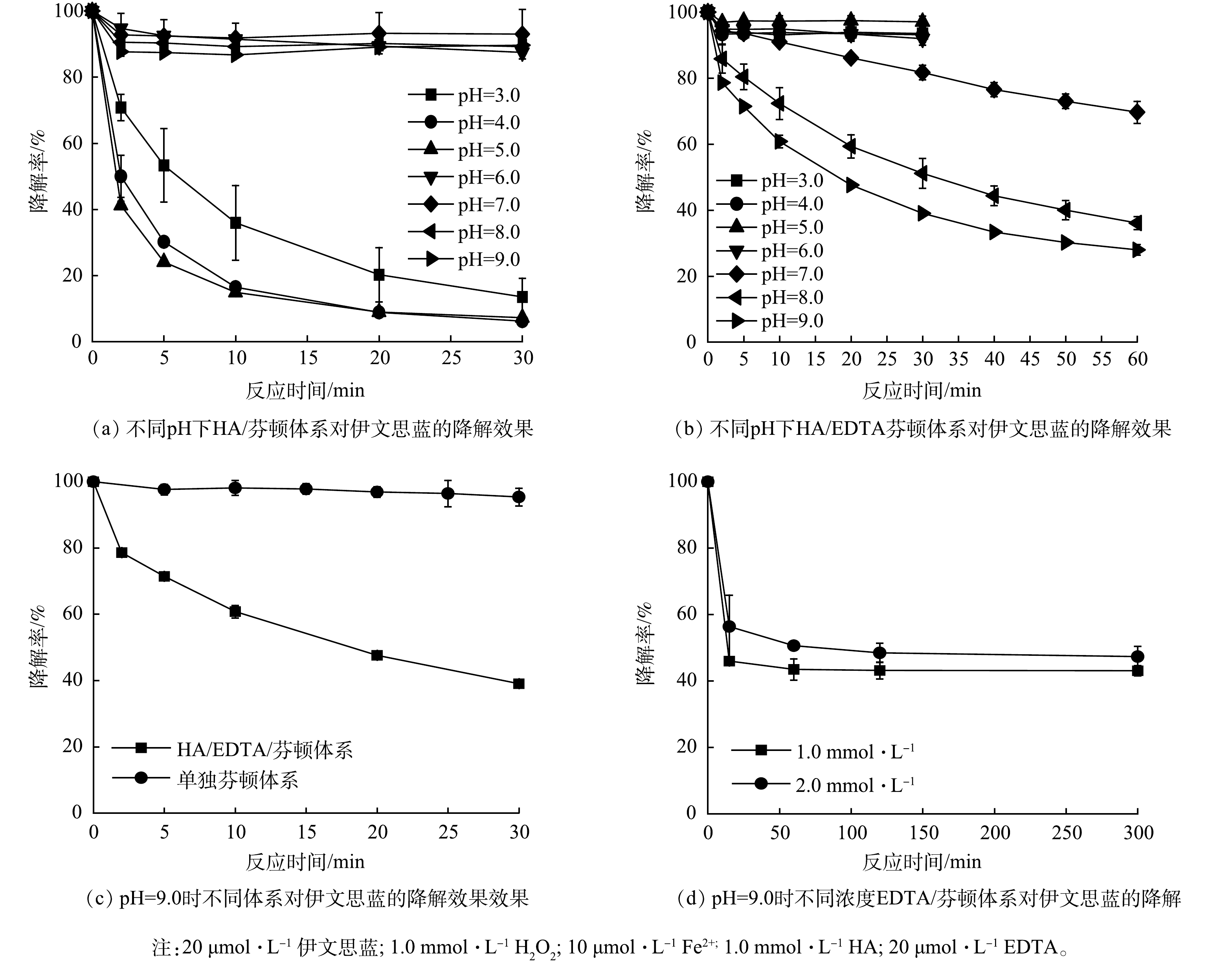
如图1(a)所示,当pH为3.0时,随着时间推移,HA/芬顿体系中伊文思蓝的降解率不断下降,30 min时达到86.5%;随着pH降至4.0或5.0,其降解率分别提升至93.8%和92.8%。CHEN等[11]的研究表明,这主要是因为羟胺的投加可以促进Fe3+向Fe2+转化,溶解态Fe2+的不断再生保证了芬顿体系中活性氧物质的持续生成。但是,随着pH的进一步提高(6.0~9.0),体系对伊文思蓝的降解效果骤然下降,仅维持在约10%。这与传统芬顿的适用pH类似,在中性或者碱性条件下,Fe3+与Fe2+主要以氢氧化物形式存在,故无法有效地被羟胺还原或激发H2O2。
图1(b)为在不同pH条件下HA/EDTA/芬顿体系对伊文思蓝的降解效果。酸性条件下的HA/EDTA/芬顿体系对伊文思蓝几乎没有降解。Fe3+-EDTA/Fe2+-EDTA氧化还原对的电位会因铁离子被络合而偏离Fe3+/Fe2+的氧化还原电位[16]。羟胺的还原性受pH影响,其在酸性条件下还原性较弱,碱性条件下还原性增强。随着pH提高,羟胺可缓慢将Fe3+-EDTA转化成Fe2+-EDTA,进一步用于H2O2的活化(反应2)。在羟胺较强的还原性保证了溶液内溶解氧维持在较低水平的前提下,较高浓度H2O2的竞争使Fe2+-EDTA会更倾向于与H2O2而不是氧气发生反应。在pH为9.0时,体系反应60 min可实现伊文思蓝72.1%的降解效率。值得一提的是,体系内铁离子浓度仅10 μmol∙L−1,而伊文思蓝的降解浓度(14.4 μmol∙L−1)高于铁离子浓度。因此,EDTA与羟胺的共同作用可有效避免碱性条件下铁离子易沉淀的问题,在提高羟胺还原能力的同时,实现Fe3+-EDTA/Fe2+-EDTA循环的持续驱动,而不仅仅是初始的亚铁离子起到了活化作用。
如图1(c)所示,当pH为9.0时,在传统芬顿体系中伊文思蓝基本没有降解(30 min内约5%降解)。而在相同条件下,HA/EDTA/芬顿体系对伊文思蓝的降解率在30 min时达到61.0%。如图1(d)所示,在pH为9.0不添加HA的情况下,Fe2+-EDTA投加量为原体系的100倍与200倍时(分别为1.0 mmol∙L−1和2.0 mmol∙L−1),降解率仅约50%。这主要是因为过量的EDTA与目标污染物竞争·OH导致降解率的下降。因此,HA/EDTA/芬顿体系不仅可降低铁离子及EDTA的用量,还可强化自由基定向氧化伊文思蓝。另外,由于体系在pH=9.0处的优异表现,本研究选取了9.0为后续反应的最佳pH。
-
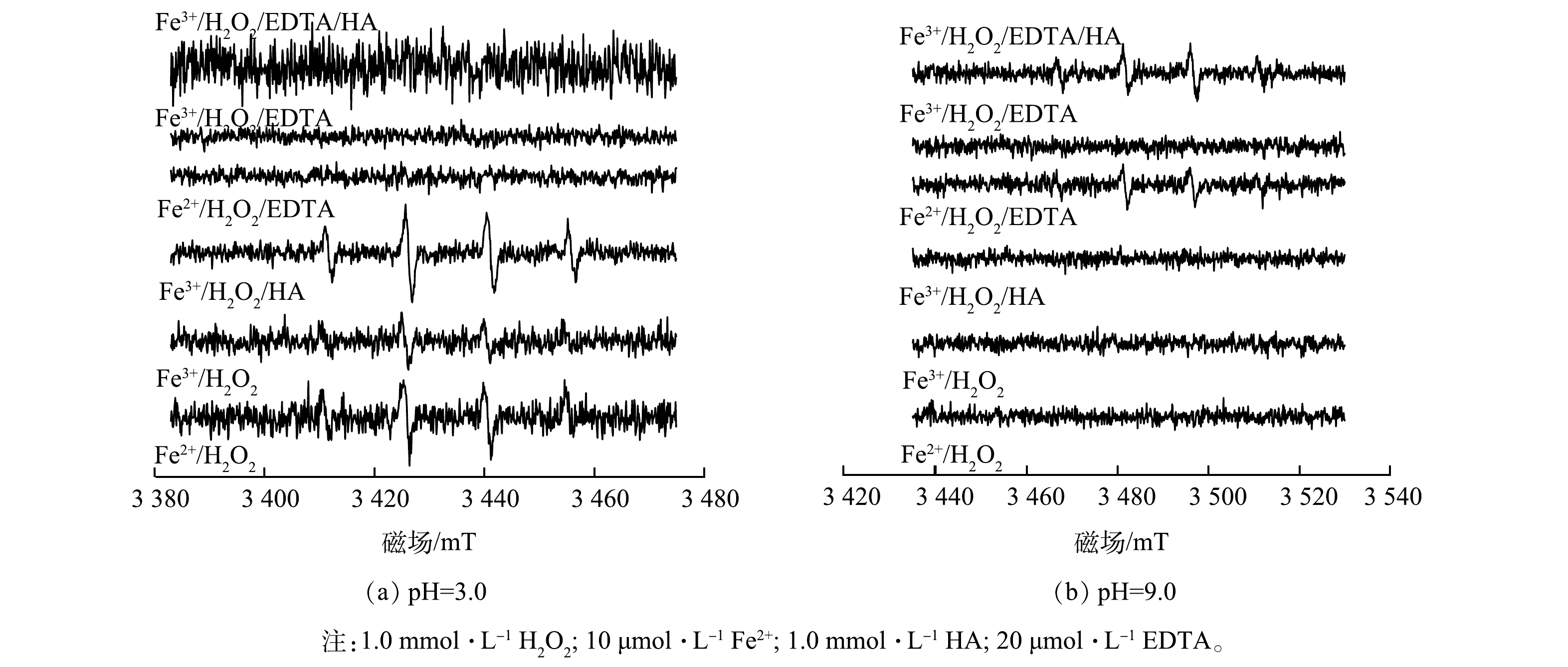
为明确体系中存在的活性物质种类,以DMPO为捕获剂,利用顺磁共振波谱仪检测各体系中的自由基,以便定性判断系统中是否有·OH或其他活性物质存在。如图2所示,当出现比例约为1∶2∶2∶1的吸收峰波形时,代表体系中有DMPO-·OH的存在,即该体系有·OH生成[17-18]。由图2(a)可见,当反应条件为酸性(pH=3.0)时,可检测出·OH的系统有Fe2+/H2O2系统,即酸性条件下的传统芬顿体系;由于酸性条件下有利于Fe3+向Fe2+的转化,故Fe3+/H2O2系统能在一定程度上进行,即图中出现不明显的特征吸收峰;Fe3+/H2O2/HA系统,即HA的存在更有利于铁循环的优化,使反应得以持续进行;其余组别则未出现明显特征吸收峰。
由图2(b)可见,当反应条件为碱性(pH=9.0)时,Fe2+/H2O2系统没有任何波形出现,这与“芬顿反应无法在碱性条件下运行”的传统认知相符;随着EDTA的加入(Fe2+/H2O2/EDTA),明显的DMPO-·OH峰出现,这说明Fe2+-EDTA可有效活化H2O2;相比之下,Fe3+/H2O2/EDTA体系中则没有检测到自由基信号,这说明Fe3+-EDTA体系缺乏活性;而在羟胺和EDTA共存的体系中(Fe3+/H2O2/HA/EDTA),谱图中出现明显的1:2:2:1的·OH的特征峰。综上所述,EDTA的引入可让Fe2+在碱性条件下保持活性,而羟胺的引入则可强化Fe3+-EDTA/Fe2+-EDTA循环,从而实现Fe2+-EDTA源源不断地再生,从而活化H2O2并产生·OH。
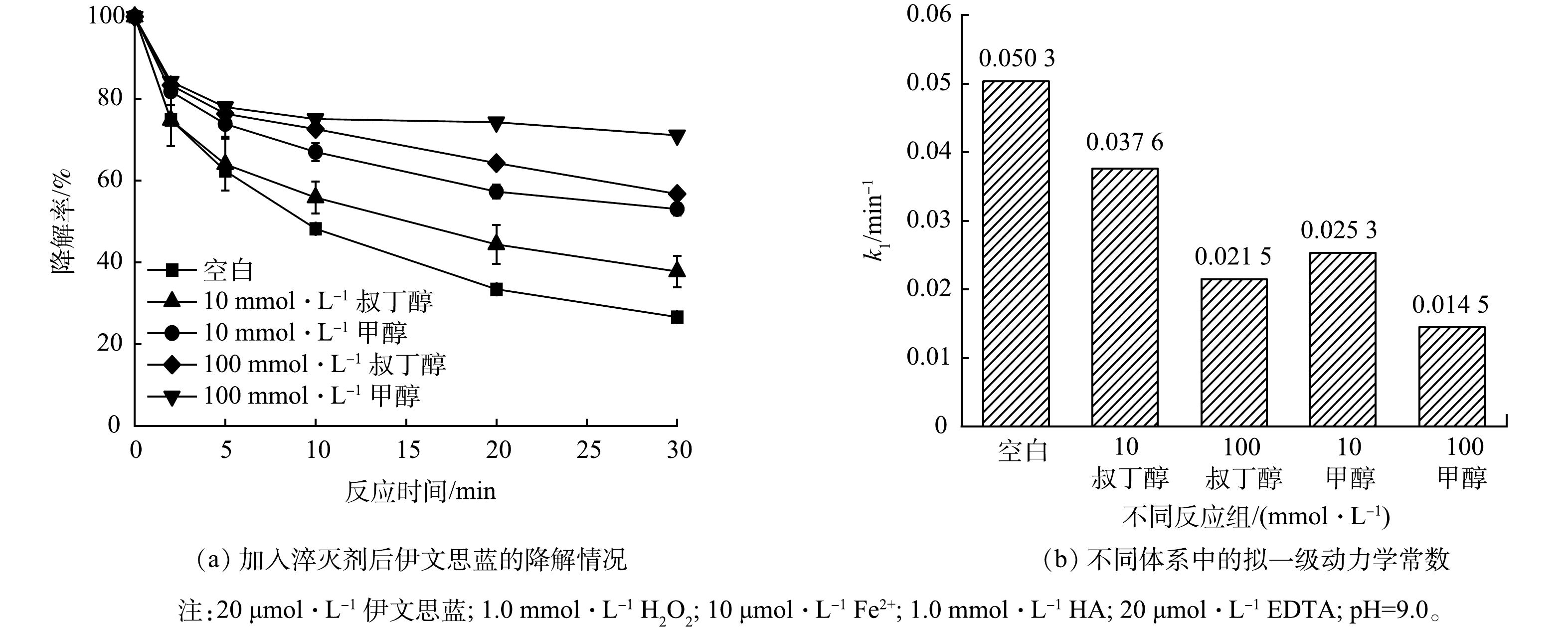
为进一步验证·OH在降解过程中的作用,采用淬灭实验来反推降解体系中可能存在的活性氧物种。·OH与叔丁醇和甲醇的反应动力学常数均较大(3.8×108 L∙mol−1∙s−1与3.0×108 L∙mol−1∙s−1),被认为是一种理想的·OH淬灭剂。如图3(a)所示,HA/EDTA/芬顿体系对伊文思蓝的降解率为72.1%,当在体系内分别投加10 mmol∙L−1的叔丁醇和10 mmol∙L−1甲醇后,伊文思蓝的降解率降至62.3%和47.1%;随着叔丁醇与甲醇浓度分别提高到100 mmol∙L−1,降解率进一步降低至43.3%和28.9%。为了更直观地表达·OH在伊文思蓝降解中的作用,本研究对体系进一步做了一级动力学拟合,叔丁醇的加入使得反应动力学常数(k1)由0.050 3 min−1降至0.037 6 min−1(10 mmol∙L−1)和0.021 5 min−1(100 mmol∙L−1);甲醇的加入则使得伊文思蓝的降解动力学常数变为0.025 3 min−1(10 mmol∙L−1)和0.014 5 min−1(100 mmol∙L−1)。结合ESR的测试结果,叔丁醇和甲醇对伊文思蓝的降解抑制作用表明,在HA/EDTA/芬顿体系降解伊文思蓝的过程中,·OH起到主要作用。
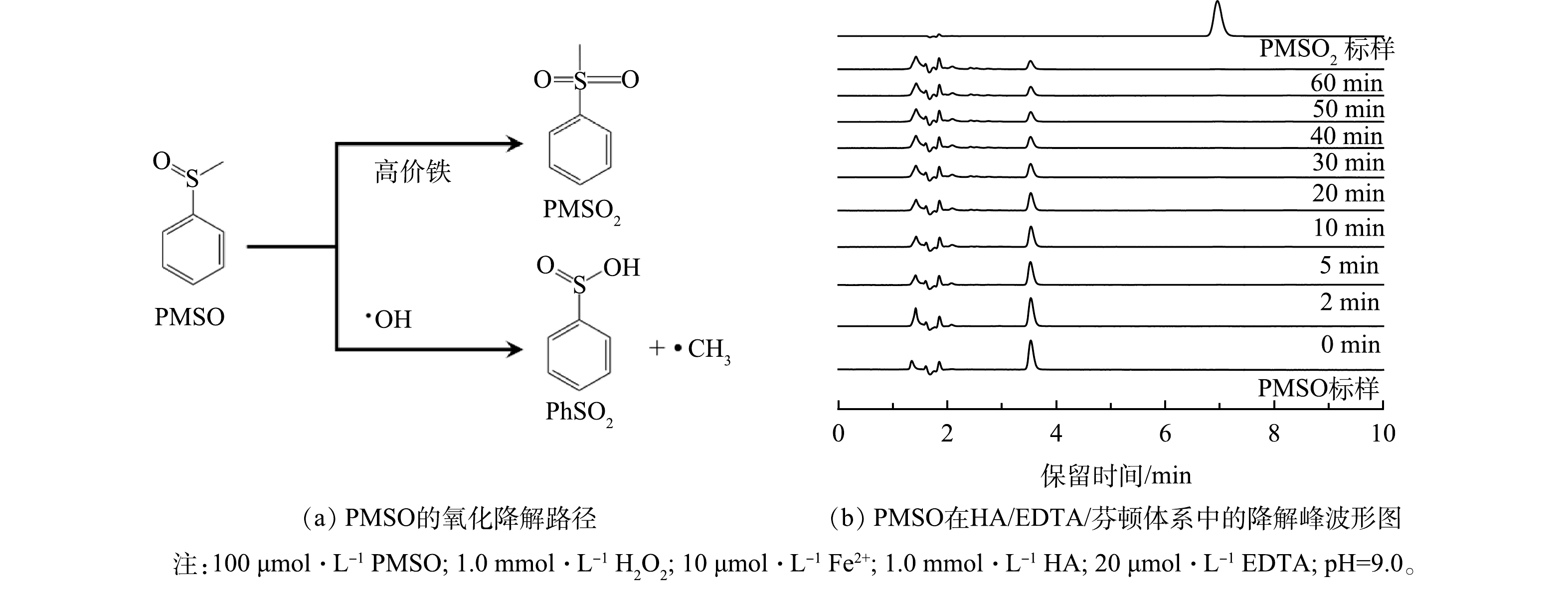
近年来,在芬顿/类芬顿反应研究中,高价铁(FeIV)被认为是芬顿/类芬顿体系中除了·OH以外的另一类核心活性氧物质(式(6))[19]。尤其是在碱性条件下,高价铁被认为是主要活性物质[20]。为确认在新体系中高价铁的作用,采用PMSO为HA/EDTA/芬顿体系的探针物质,PMSO在高价铁作用下会转化成PMSO2,而在·OH的作用下则会转化成苯亚磺酸(PhSO2)[21]。因此,通过鉴定PMSO2是否存在,即可确认高价铁在该过程中的作用。
PMSO标样和PMSO2标样浓度均为100 μmol∙L−1。在高效液相色谱图中,PMSO的出峰位置约在3.6 min,而PMSO2的出峰位置则处于7.0 min。如图4(b)所示,在HA/EDTA/芬顿体系中,随着时间的推移,PMSO的峰强逐渐降低,而代表PMSO2的峰形则始终保持在较低的强度上。这表明PMSO2并不是PMSO在新体系中的主要产物,由此推断即使是在偏碱性的条件下,新体系的活性物质依然以·OH为主。
-
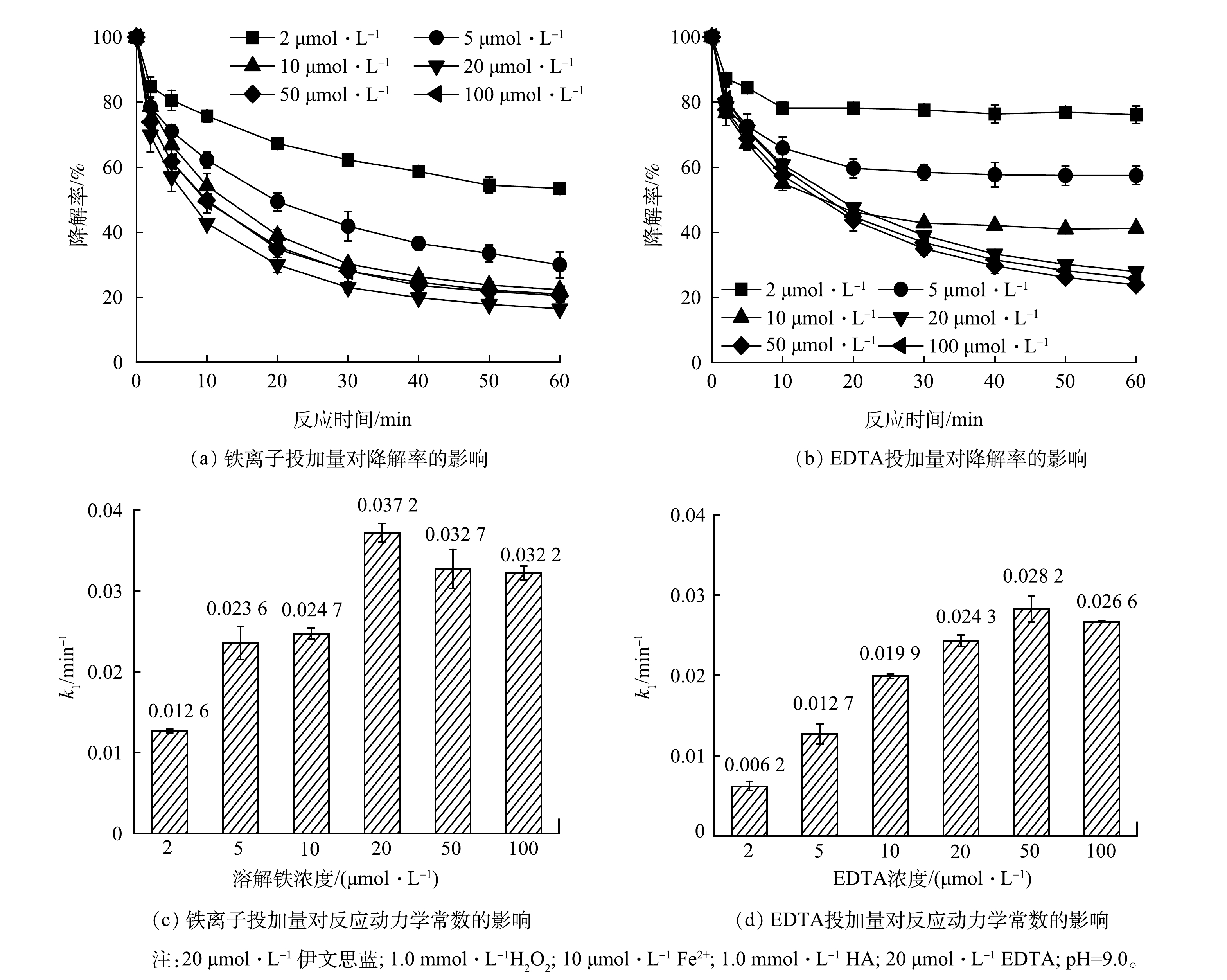
作为Fe3+-EDTA/Fe2+-EDTA循环对的成分,铁离子与EDTA缺一不可,因此本工作考察了铁离子与EDTA对伊文思蓝降解的影响。如图5(a)~(b)所示,铁投加量和EDTA投加量对伊文思蓝降解有明显的正向促进作用。为更好地比较不同投加量药剂的影响,比较了不同铁投加量和EDTA投加量条件下伊文思蓝降解的拟一级动力学常数。如图5(c)所示,在低浓度条件下(Fe2+ <20 μmol∙L−1),铁离子浓度的增加提高了伊文思蓝降解的一级动力学常数;但是当铁离子浓度高于EDTA(20 μmol∙L−1)浓度时,一级动力学常数不再提高。这主要是因为铁离子只有和EDTA络合时才可以发挥作用,因此,当Fe2+<20 μmol∙L−1时,Fe3+-EDTA/Fe2+-EDTA循环才可以驱动H2O2生成·OH;当Fe2+浓度更高时,额外的铁离子则将以氢氧化物形式存在,无法进一步促进体系运行。类似的,当EDTA投加量低于10 μmol∙L−1时,EDTA对伊文思蓝降解有明显的线性提高,随着EDTA投加量提高至10 μmol∙L−1以上,体系降解速度则难以进一步提升。综合经济、效率等因素考虑,可认为铁离子和EDTA的最佳投加摩尔比为1:1。值得一提的是,10 μmol∙L−1的铁离子质量浓度仅为0.56 mg∙L−1,满足污水的排放标准,这表明处理后的污水可直接排放,而无需担心铁离子的超标问题。
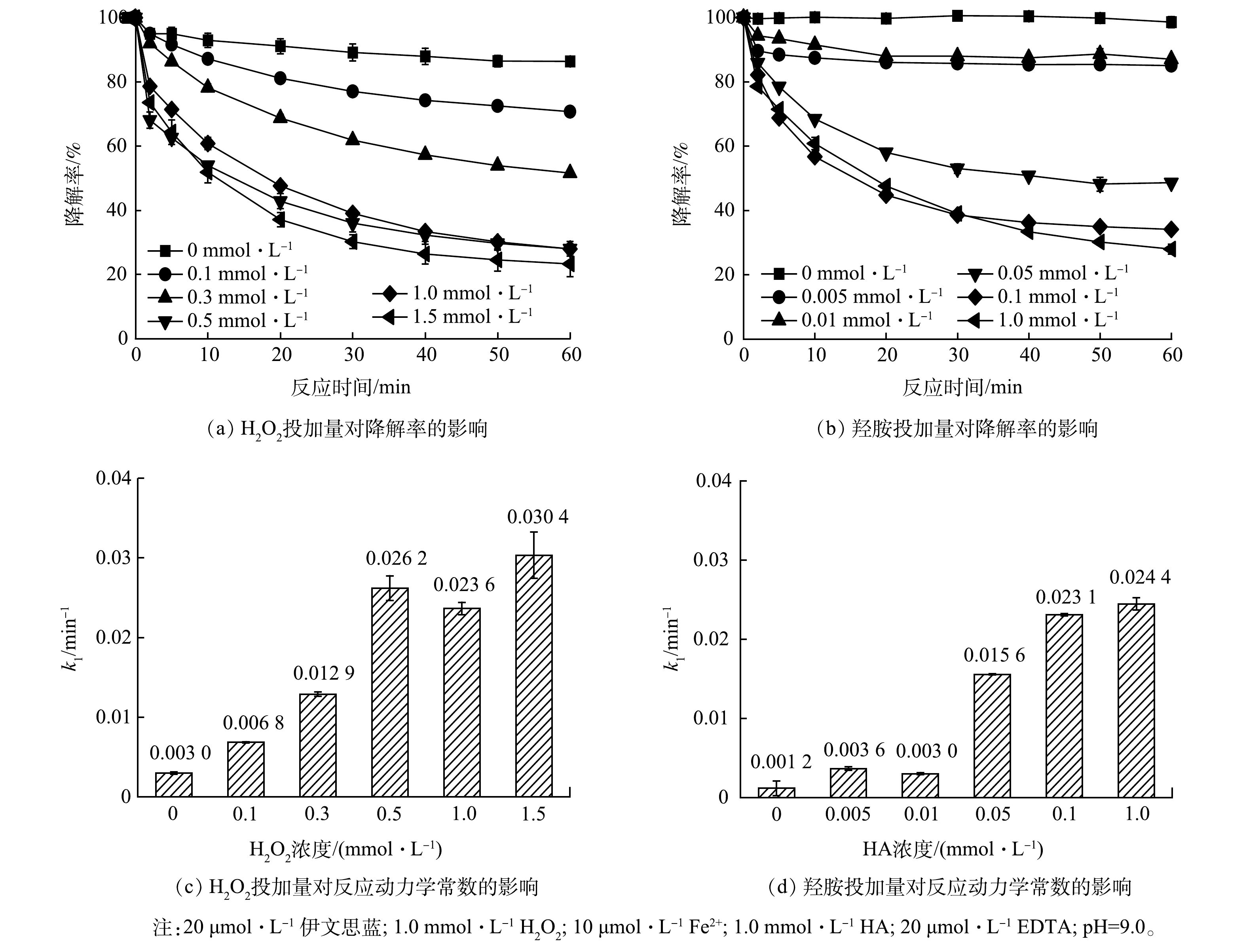
作为体系的电子供体(HA)和·OH前驱体(H2O2),高投加量同样对体系有明显的提升作用(图6(a)~(b))。然而,对H2O2而言,当浓度达到0.5 mmol∙L−1以上时,降解速率的提升的速度明显减缓,60 min的处理效果相似。因此,在一定范围内,提高芬顿/羟胺/EDTA系统中H2O2浓度有助于反应的快速发生,提高处理效率,而当底物浓度过量时,即使继续提升其浓度,但受到其他因素的限制,体系对污染物的处理效率也无法继续提升。使用反应动力学进行拟合,随H2O2浓度的提升,系统的动力学常数k1也随之提升(图6(c)),观察发现H2O2浓度为0~0.5 mmol∙L−1时,与动力学常数之间近似线性关系,对其进行拟合所得R2 = 0.965 4,拟合效果较好;而0.5 mmol∙L−1后这种线性关系便不再显著。当H2O2浓度超过0.5 mmol∙L−1时,其浓度增加对于反应体系效率提升较低,反而可能由于温度等因素的影响导致体系效率产生一定波动,故确定系统中最适H2O2浓度为0.5 mmol∙L−1。
羟胺在体系中能够起到还原Fe3+、促进铁循环的作用。当不加入羟胺时,该体系很快便会停止,故伊文思蓝降解率很低。在加入羟胺后,随加入量的提升,体系的降解效果会逐渐增强。使用反应动力学进行拟合,随羟胺浓度的提升,系统的一级动力学常数k1也随之提升(图6(d)),观察发现0~0.1 mmol∙L−1内羟胺浓度与动力学常数之间近似线性关系,对其进行拟合所得R2=0.973 6,拟合效果较好;而0.1 mmol∙L−1后这种线性关系便不再显著,故认为系统中最适羟胺浓度为0.1 mmol∙L−1。
-
1) EDTA的投加可使芬顿反应在碱性条件下(pH=7~9)运行,而羟胺可以使Fe3+-EDTA持续转化成具有活性的Fe2+-EDTA,从而实现碱性条件下·OH的连续生成与有机污染物的持续降解。当pH为9.0、羟胺浓度为1.0 mmol∙L−1、H2O2浓度为1.0 mmol∙L−1、铁离子浓度为10 μmol∙L−1、EDTA浓度为20 μmol∙L−1时,伊文思蓝在60 min内的降解率可达到72.1%。
2)在HA/EDTA/芬顿体系中主要的活性物种为·OH;PMSO的降解路径证明在碱性体系中高价铁(FeIV)几乎不起任何作用。
3)对于HA/EDTA/芬顿体系降解伊文思蓝(20 μmol∙L−1),铁离子和EDTA的最佳投料比为1:1,H2O2的最优投加量为0.5 mmol∙L−1,HA的最优投加量为0.1 mmol∙L−1。
4)羟胺的引入可缓解EDTA/芬顿体系中铁离子与络合剂的消耗性使用问题,在降低EDTA与铁离子的投加量的同时,避免了体系中络合剂的二次污染问题与铁离子的后续去除问题。
羟胺/EDTA协同强化芬顿反应的机理与动力学
Enhanced Fenton process by a synergistic effect of EDTA and hydroxylamine: Mechanism and kinetics
-
摘要: 经典芬顿反应(Fe2+/H2O2)在实际水处理中存在pH响应范围窄、亚铁盐投加量大和铁泥产量高等技术瓶颈问题。改用乙二胺四乙酸(EDTA)络合的亚铁离子(Fe2+-EDTA)做引发剂后,pH响应范围明显扩大,然而也引入了有机二次污染,同时提高了溶解铁的去除难度。在Fe2+-EDTA/H2O2体系中投加还原性的羟胺(HA),可将失活的Fe3+-EDTA转化成具有活性的Fe2+-EDTA,从而实现低铁投加量/低EDTA投加量条件下有机污染物的消除。结果表明:以伊文思蓝为模型污染物,HA/EDTA/芬顿体系的pH范围可扩大至7.0~9.0;铁离子和EDTA的最佳投料比为1∶1,H2O2的最佳投加量为0.5 mmol∙L−1,HA的最佳投加量为0.1 mmol∙L−1; HA/EDTA/芬顿体系中主要的活性物质为羟基自由基(·OH);苯基甲基亚砜(PMSO)的降解路径分析则证明了新体系中高价铁(FeIV)几乎不起任何作用。以上结果表明,将羟胺引入EDTA/芬顿体系后可同步降低铁离子与络合剂的使用量,故此体系改变了络合剂强化的芬顿反应中药剂投加量过大与后续铁离子不好处置的问题,可为进一步拓展芬顿反应的应用范围提供参考。
-
关键词:
- 芬顿反应 /
- 羟胺 /
- EDTA /
- pH范围 /
- Fe3+-EDTA/Fe2+-EDTA循环
Abstract: Traditional Fenton processes (Fe2+/H2O2) have long suffered from the impractically narrow pH range (around pH 3.0), the addition of ferrous salt and production of a large mass of iron precipitates after neutralization. The employment of ethylene diamine tetraacetic acid (EDTA) for chelating Fe2+ as an initiator (Fe2+-EDTA) of Fenton process can extend the pH range, but it also causes secondary contamination and increases the difficulty on the subsequent removal of dissolved iron. In this study, hydroxylamine was employed for accelerating regeneration of Fe2+-EDTA from Fe3+-EDTA, resulting in continuous degradation of organic contaminants with low dosage of iron salt and EDTA. Using Evans Blue as radical probe, HA/EDTA/Fenton system could output oxidation ability at the pH range of 7.0-9.0; the optimal ratio of iron ions and EDTA was 1:1; taking the cost and efficiency into consideration, the optimal dosages of H2O2 and HA were 0.5 mmol∙L−1 and 0.1 mmol∙L−1, respectively. Quenching experiments, electron spin resonance analysis revealed hydroxyl radical (·OH) as main reactive oxygen species, while degradation analysis of Phenyl methyl sulfoxide (PMSO) excluded the role of iron-oxo (FeIV) in the system. The simultaneous addition of EDTA and HA could reduce the dosage of iron salt and chelating agents, further avoiding the subsequent problem such as larger doses of reagents and the downstream treatment of Fe3+-EDTA. The research can provide a reference for the extension of Fenton process application range.-
Key words:
- Fenton process /
- hydroxylamine /
- EDTA /
- pH range /
- Fe3+-EDTA/Fe2+-EDTA cycle
-

-
-
[1] HODGES B C, CATES E L, KIM J H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials[J]. Nature Nanotechnology, 2018, 13(8): 642-650. doi: 10.1038/s41565-018-0216-x [2] LEE J S, GUNTEN U V, KIM J H. Persulfate-based advanced oxidation: Critical assessment of opportunities and roadblocks[J]. Environmental Science & Technology, 2020, 54(6): 3064-3081. [3] 姚梦东, 岳俊杰, 徐雪婧, 等. 球磨硫化零价铁活化过硫酸盐降解水体中有机氯农药[J]. 环境工程学报, 2021, 15(8): 2563-2575. doi: 10.12030/j.cjee.202103052 [4] 吕来, 胡春. 多相芬顿催化水处理技术与原理[J]. 化学进展, 2017, 29(9): 981-999. doi: 10.7536/PC170552 [5] 姜妍, 蒋林时, 苗时雨, 等. 紫外-Fenton法处理高盐有机废水[J]. 环境工程学报, 2016, 10(5): 2349-2354. doi: 10.12030/j.cjee.201412143 [6] 仙光, 张光明, 刘毓璨, 等. Fenton法处理电镀有机废水[J]. 环境工程学报, 2018, 12(4): 1007-1012. doi: 10.12030/j.cjee.201710049 [7] REN Y M, LIN L Q, MA J, et al. Sulfate radicals induced from peroxymonosulfate by magnetic ferrospinel MFe2O4 (M = Co, Cu, Mn, and Zn) as heterogeneous catalysts in the water[J]. Applied Catalysis B: Environmental, 2015, 165: 572-578. doi: 10.1016/j.apcatb.2014.10.051 [8] SUN M, CHU C H, GENG F L, et al. Reinventing Fenton chemistry: Iron oxychloride nanosheet for pH-insensitive H2O2 activation[J]. Environmental Science & Technology Letters, 2018, 5(3): 186-191. [9] WENDY K P, TIMOTHY J M, THOMAS D T. What species is responsible for strand scission in the reaction of [FeIIEDTA]2- and H2O2 with DNA?[J]. Journal of the American Chemical Society, 1995, 117(24): 6428-643. doi: 10.1021/ja00129a002 [10] ANTONELL D L, RENATO F D, SANTIAGO E. Assessment of iron chelates efficiency for photo-Fenton at neutral pH[J]. Water Research, 2014, 61: 232-242. doi: 10.1016/j.watres.2014.05.033 [11] CHEN L W, MA J, LI X C, et al. Strong enhancement on Fenton oxidation by addition of hydroxylamine to accelerate the ferric and ferrous iron cycles[J]. Environmental Science & Technology, 2011, 45(9): 3925-3930. [12] ZHOU H Y, ZHANG H, HE Y L, et al. Critical review of reductant-enhanced peroxide activation processes: Trade-off between accelerated Fe3+/Fe2+ cycle and quenching reactions[J]. Applied Catalysis B:Environmental, 2021, 286: 119900. doi: 10.1016/j.apcatb.2021.119900 [13] ZOU J, MA J, LI X C, et al. Rapid acceleration of ferrous iron/peroxymonosulfate oxidation of organic pollutants by promoting Fe(III)/Fe(II) cycle with hydroxylamine[J]. Environmental Science & Technology[J], 2013, 47(20): 11685-11691. [14] LI Z Y, WANG L, LIU Y L, et al. Unraveling the interaction of hydroxylamine and Fe(III) in Fe(II)/Persulfate system: A kinetic and simulating study[J]. Water Research, 2020, 168: 115093. doi: 10.1016/j.watres.2019.115093 [15] QIAO J L, FENG L Y, DONG H Y, et al. Overlooked role of sulfur-centered radicals during bromate reduction by sulfite[J]. Environmental Science & Technology, 2019, 53(17): 10320-10328. [16] PAN Y, SU H R, ZHU Y T, et al. CaO2 based Fenton-like reaction at neutral pH: Accelerated reduction of ferric species and production of superoxide radicals[J]. Water Research, 2018, 145: 731-740. doi: 10.1016/j.watres.2018.09.020 [17] ZENG H B, ZHANG G, JI Q H, et al. pH-independent production of hydroxyl radical from atomic H*-mediated electrocatalytic H2O2 reduction: A green Fenton process without byproducts[J]. Environmental Science & Technology, 2020, 54(22): 14725-14731. [18] 张娟娟, 刘蕴晗, 乔梦, 等. TiO2纳米管阳极光电催化氧化次磷酸盐同时阴极回收金属铜[J]. 环境工程学报, 2022, 16(4): 1145-1153. doi: 10.12030/j.cjee.202202092 [19] DONG H Y, LI Y, WANG S C, et al. Both Fe(IV) and radicals are active oxidants in the Fe(II)/peroxydisulfate process[J]. Environmental Science & Technology Letters, 2020, 7(3): 219-224. [20] WANG Z, JIANG J, PANG S Y, et al. Is sulfate radical really generated from peroxydisulfate activated by iron(II) for environmental decontamination?[J]. Environmental Science & Technology, 2018, 52(19): 11276-11284. [21] ZENG H B, LAN H C, AN X Q, et al. Insight into electroreductive activation process of peroxydisulfate for eliminating organic pollution: Essential role of atomic hydrogen[J]. Chemical Engineering Journal, 2021, 426: 128355. doi: 10.1016/j.cej.2020.128355 -



 下载:
下载: