-
水是生命之源,随着采矿业、金属制造业、造纸业和电镀等工业的快速发展,含有重金属的工业废水大量直接或间接地排放到水环境中,威胁着生态环境的稳定[1]。汞(Hg)、镉(Cd)、铅(Pb)、铬(Cr)等重金属由于不易被生物降解,在食物链中不断传递富集,对肾脏、血液系统以及神经系统产生威胁,最终损害人类的生命健康[2-4] ,重金属水污染处理成为当今世界热点议题之一。
水处理技术包含:化学沉淀法、混凝絮凝法、电化学处理法、吸附法、离子交换法、光催化法和生物处理法等。其中,吸附法具有去除效率高、操作简单和成本低等优点,是有效去除污染物的方式之一[5]。常见的吸附材料有活性炭、碳纳米管、生物炭、膨润土等材料[6-8]。碳纳米管具有许多独特的机械、电子、物理和化学性质,其热稳定性好,热阻率高,灵敏度高、在水中分散性好、化学性能稳定,制备简单[9-10]。在处理重金属废水方面,碳纳米管因其比表面积大,官能团多,具有优异的吸附性能和较高的吸附效率,是很好的候选吸附材料[11-12]。Lu等[13]通过吸附实验比较研究了碳纳米管和活性炭对Zn2+的吸附能力。利用Langmuir模型得到单壁碳纳米管、多壁碳纳米管和活性炭对Zn2+的最大吸附量分别为43.66、32.68、13.04 mg·g−1,表明碳纳米管对Zn2+的去除效果比活性炭显著。碳纳米管在表面引入分子链或者羰基(C=O)、羧基(—COOH)、氨基(—NH2)和羟基(—OH)等活性基团不仅能够提高碳纳米管的溶解性和稳定性,还提高碳纳米管对重金属离子的吸附能力[14]。Yang等[15]比较了原始和氨基改性多壁碳纳米管对Pb2+的吸附能力。改性后多壁碳纳米管对Pb2+的最大吸附量从6.8 mg·g−1提高到了147 mg·g−1。Neto等[16]将磁性氧化铁和多壁碳纳米管掺杂在一起,并与交联壳聚糖复合,制备的改性CLCh/MWCNT/Fe复合材料对Cr6+有较好的吸附能力,1 h内可以达到最大吸附量449.3 mg·g−1。众多的吸附实验表明,碳纳米管对重金属离子有很好的去除效果,是一种很有前景的废水处理材料。然而,吸附实验中通常采用例如扫描电镜图象观察吸附材料的表面形貌和微观结构;Langmuir、Freundlich模型拟合平衡等温线,计算最大吸附容量;准一阶、准二阶动力学模型分析吸附速率;红外光谱(FTIR)和X射线光电子能谱(XPS)探究可能的成键吸附方式[17]。以上传统的吸附实验分析手段对深层次的理解吸附材料与重金属之间的相互作用还存在着局限性。
近年来随着计算机技术的发展与应用,量子力学等理论方法的完善,计算化学发展日趋成熟。利用密度泛函理论(density functional theory, DFT)等方法计算与模拟,不仅能够实现对分子结构的优化,计算键长、键角,得到稳定的分子构型,还能够计算反应的吸附能来探究碳纳米管吸附重金属的稳定性;分子动力学模拟(molecular dynamics, MD)可以用来分析重金属离子在碳纳米管上吸附的动态特征[18]。一般来说,计算化学的理论计算结果能够定性分析吸附实验拟合的吸附量,实现了从微观角度探索反应机理,从原子角度更加直观的表达了碳纳米管吸附重金属离子的过程,为实验化学提供理论支持。因此,本文总结了近年来国内外研究人员利用计算化学研究碳纳米管对重金属的吸附研究进展,为探究两者之间的吸附机理提供理论支持。
-
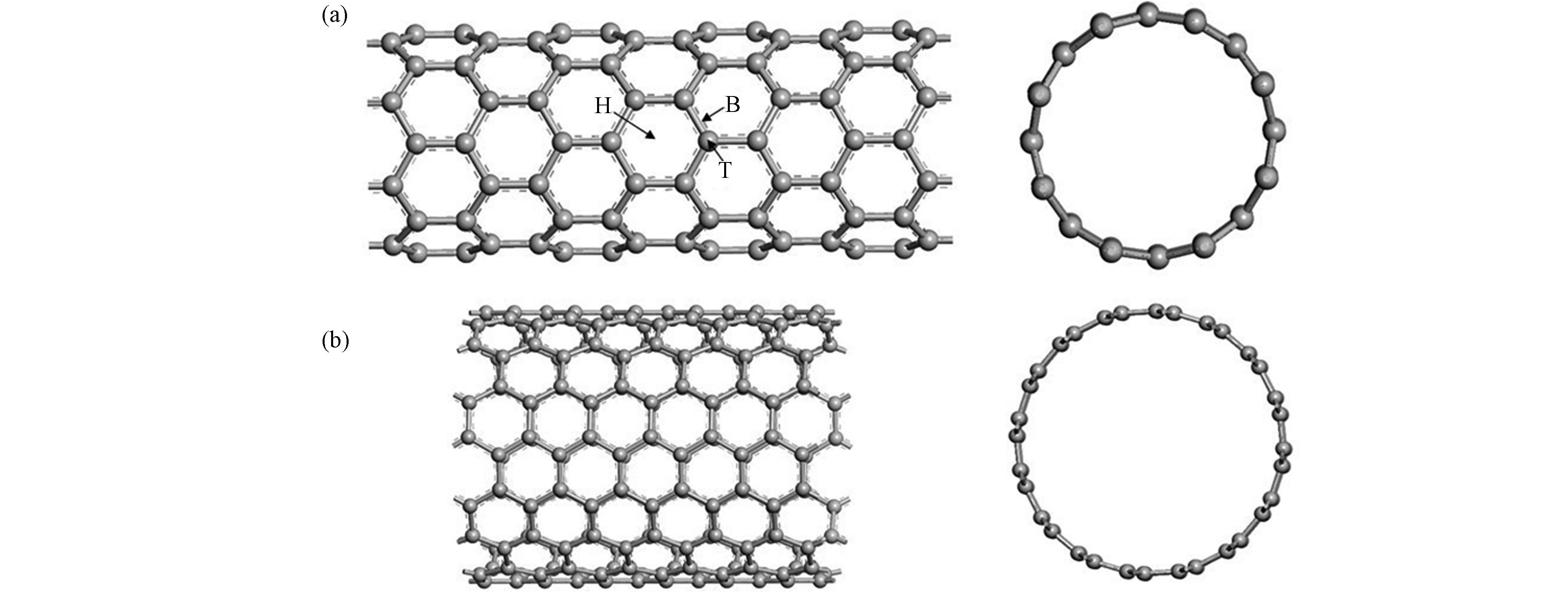
自从1991年lijima发现了碳纳米管(CNTs),近年来随着生产规模的不断扩大,碳纳米管被广泛应用于纳米二极管、晶体管等微电子器件、场发射、催化剂载体、药物载体、废水处理、气体传感器和电化学传感器等领域[19-24]。碳纳米管的基本结构是由sp2杂化碳原子组成的管状结构,其直径为1—3 nm,长度为100—1000 nm。同时,碳纳米管也可以看成石墨烯卷成的无缝管状结构,根据结构可以将其分为由一个石墨烯片的圆柱体组成的单壁碳纳米管(SWCNTs)和由几个同心圆柱形石墨烯片组成的多壁碳纳米管(MWCNTs)[25]。由于碳纳米管可以看作由石墨烯的卷曲而来,因此其具有与石墨烯相同的成键方式,即碳原子以sp2杂化轨道与相邻C原子形成σ共价键。与此同时,每个碳原子互相平行的p轨道的电子形成π电子云。根据石墨烯的卷曲方式将单壁碳纳米管分为扶手椅型,锯齿形和手性螺旋型[26]。其结构示意图见图1。在理论模拟计算中通常选取一段碳纳米管作为计算模型,其长度可长可短。常见的扶手椅形碳纳米管模拟结构有CNT(5,5)、(6,6)、(8,8)、(9,9)、(10,10),半径依次为0.34、0.41、0.545、0.612、0.678 nm,碳纳米管尺寸依次递增。锯齿形碳纳米管常见的模拟结构有CNT(8,0)、(9,0)、(10,0),半径分别为0.315、0.358、0.395 nm[27]。碳纳米管上有六碳环中心的中心位(H)、C—C键中心的桥位(B)和C原子顶端的顶位(T),3个常见的吸附位置,如图1(a)所示。娄昀璟等[28]基于密度泛函理论,采用管径0.6 nm,管长1.2 nm的(8,0)CNT作为碳纳米管模型来研究对氧分子的吸附作用,计算得到最佳吸附构型为氧分子处于碳纳米管六元环中心且平行于碳纳米管表面。
-
密度泛函理论是一种通过密度函数代替波函数研究多电子体系电子结构的方法,在量子化学中有着广泛的应用[29]。研究人员对碳纳米管吸附重金属的体系选择合适的计算模型,经过DFT计算模拟得到吸附的结构、能量及电子性质等相关信息,从而定性分析碳纳米管与重金属之间相互作用机理,为碳纳米管在废水处理中的广泛应用提供理论支持。
成键键长和吸附能大小分析,是目前DFT计算分析碳纳米管材料与金属之间相互作用的常见手段。成键键长能够反映出不同金属在碳纳米管上吸附构型的微观差异,而吸附能的大小能够直观地表示金属与吸附剂之间相互作用的强弱,通常吸附能的表达式为:
Eads-metal是吸附后吸附剂与金属离子的总能量;Eads是吸附前吸附剂的能量;Emetal是金属离子的能量。吸附能的值为负表明金属离子在碳纳米管上的吸附过程是自发放热的,形成的吸附结构是稳定的并且吸附能值越负,两者之间的相互作用越强。表1和表2分别总结了基于密度泛函理论模拟碳纳米管吸附Hg(Ⅱ)、Cu(Ⅱ)、Pb(Ⅱ)和Cr(Ⅲ)等重金属离子后的键长以及吸附能的情况。由表格数据表明碳纳米管与重金属离子之间的键长约在0.19 nm到0.43 nm,水相中的吸附能约在−0.3 eV到−10.2 eV范围内。
碳纳米管对重金属的吸附形式有物理吸附和化学吸附。Zhu等[30]构建了由64个C原子组成的(8,0)CNT,研究了碳纳米管上的中心位(H)、桥位(B)和顶位(T)的3个吸附位置对二价重金属离子(Zn2+、Cu2+、Pb2+和Sn2+)的吸附性能。Zn2+、Cu2+、Pb2+和Sn2+与碳纳米管中C原子的原始距离分别为0.359、0.335、0.381、0.382 nm。从表1中的数据分析可知,对于同一种重金属离子,吸附在桥位上时,金属与碳原子之间键长最短;而对于同一个碳纳米管的吸附位置,在CNT分别吸附4种重金属离子之后,Zn—C的距离基本没有改变,说明Zn2+与碳纳米管之间的相互作用较弱。而Cu、Pb和Sn与C原子之间的成键距离有明显缩短,说明Cu2+、Pb2+和Sn2+与碳纳米管之间存在着较强的相互作用。这种较强的相互作用有利于碳纳米管与重金属离子之间形成化学键以及稳定的吸附结构。除了分析键长以外,研究人员又进一步比较4种金属离子与碳纳米管之间吸附能的强弱。碳纳米管与Sn2+具有最大的吸附能,其在顶位、中心位和桥位吸附Sn2+的吸附能约为−4.1 eV。其次,吸附Cu2+的吸附能约为−3.3 eV,吸附Pb2+的吸附能约为−2.9 eV。而碳纳米管与Zn2+的吸附能最小,在3个吸附位点的吸附能约为−1.0 eV。由于二价重金属离子是缺电子状态,所以当CNT与二价重金属离子之间存在着较强的相互作用时,电子会从CNT转移到重金属离子。从电荷转移的角度来看, Zn2+与CNT的3个吸附位置之间的电子转移量为~0.014 e,几乎可以忽略不计。Cu2+、Pb2+和Sn2+与CNT之间则有着较为丰富的电荷转移量,分别为~0.52 e、1.00 e和0.77 e。因此可以推测碳纳米管与锌离子之间主要是物理吸附,而与铜、铅和锡离子的3种重金属离子之间形成了化学吸附。
-
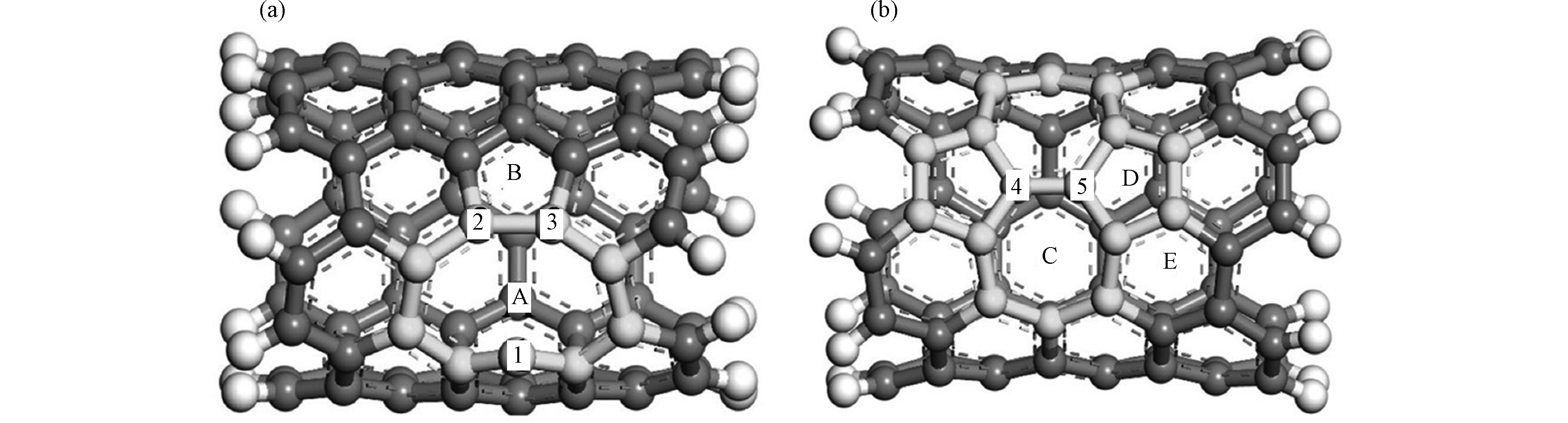
众所周知,对碳纳米管进行改性修饰能够为重金属提供更多有效吸附位点,从而提高材料的吸附性能。材料的表面化学改性有缺陷/空位、表面杂原子掺杂和表面分子功能化等形式。常见的碳纳米管缺陷有单原子空位缺陷(Single—Vacancy Defect,SV又称5—1DB缺陷)和拓扑缺陷(又称Stone—Wales缺陷,SW缺陷)两种。图2为几何优化后的含5—1DB缺陷的碳纳米管(a)和拓扑缺陷的碳纳米管(b)。单原子空位缺陷是在原始碳纳米管上移除1个C原子形成单空位;拓扑缺陷从结构上可以看作原始碳纳米管中1个C—C键旋转90°使得六元环变成七元环和五元环[31]。图2中A是SV缺陷中的空位处,B是SV缺陷形成的五元环;C,D和E是SW缺陷形成的七元环、五元环和六元环。缺陷破环了碳纳米管中的π电子云,导致电子发生转移。比如,SV缺陷的存在形式为1个五元环和1个带有悬挂键的碳原子。由于带有悬挂键的碳原子上有未配对的电子,所以此位置的化学活性较强,可以与其他原子或基团发生相互作用。因此缺陷/空位能够提高碳纳米管对金属的吸附能力。张变霞等[32]基于密度泛函理论,计算并比较了(5,5)CNT、存在5—1DB缺陷和拓扑缺陷的(5,5)CNT对铜原子的吸附。对于不含缺陷的碳纳米管,铜原子在(5,5)CNT的顶位(T)具有最大的吸附能(−1.22 eV),以及最短的Cu—C键长(0.199 nm)。因此吸附单个Cu原子的最佳位置是C原子顶端的顶位。通过比较铜原子在不同缺陷位置的能量大小,铜原子与碳纳米管在5—1DB缺陷的空位处和五元环处的相互作用能分别为−3.26 eV和−0.89 eV,在拓扑缺陷五元环、六元环和七元环处相互作用能分别为−0.91、−0.72、−1.65 eV。缺陷结构不仅增强了对铜原子的吸附并且空位处的相互作用最强。王清云等[33]基于密度泛函理论,构建了含有96个碳原子的(6,6)CNT模型,研究碳纳米管单空位缺陷和SW缺陷对金属原子钛(Ti)的吸附能力,比较碳纳米管不含缺陷以及SV和SW两种缺陷对Ti原子吸附能的相对大小。研究表明,Ti原子吸附在碳纳米管内外的吸附能分别为−2.71 eV和−2.58 eV,而SV缺陷内外的吸附能分别为−9.68 eV和−8.04 eV,并且SW缺陷的最大吸附能为−4.91 eV。以上研究充分说明,缺陷的存在使得铜原子和钛原子与碳纳米管吸附能增加,增强了碳纳米管的吸附能力并且空位缺陷与两种金属原子的相互作用最强,结合最稳定。缺陷对碳纳米管吸附的作用,有时也会受到碳纳米管尺寸的影响。例如,Li等[34]研究了碳纳米管的尺寸与缺陷对铅离子的吸附作用。从(4,0)CNTs到(6,0)CNTs,随着CNTs的孔径增加,Pb离子与CNTs表面的C原子距离从0.2685 nm逐渐缩小到0.2619 nm,吸附能从−1.822 eV增加到−2.856 eV。碳纳米管的孔径变大,碳纳米管与Pb离子能力的相互作用变得更强,吸附结构更加稳定;当CNTs表面引入单空位缺陷后,例如SV—(4,0)CNTs 中Pb—C键距(0.2478 nm)较原始(4,0)CNTs中的Pb—C键距(0.2685 nm)显著缩短,吸附能从−3.808 eV减小到−1.822 eV。说明了单空位缺陷能够显著增强Pb离子与CNTs表面的相互作用。而随着缺陷碳纳米管尺寸的增加,Pb2+与CNTs表面的C原子距离从0.2478 nm逐渐缩小到0.2392 nm。从吸附能的角度来看,SV—(4,0)CNTs、SV—(5,0)CNTs和SV—(6,0)CNTs对Pb2+的吸附能分别为−3.808 eV、−6.538 eV和−5.166 eV。与碳纳米管的能量变化规律不同,缺陷碳纳米管的吸附能随着尺寸的增加呈现出先增大后减小的趋势。在实验上发现采用化学气相沉积法制备碳纳米管的方法可以改善其缺陷结构。这一现象可以从理论计算的角度得以证明。Zhou等[35]研究了碳氢基团(CH、CH2、CH3)对SV缺陷碳纳米管的修复。碳氢基团因带有悬挂键而更容易与SV缺陷上的带有悬挂键的碳原子发生相互作用。在模拟过程中可以看到CH、CH2、CH3能够自发的与缺陷碳纳米管发生吸附作用并且CH和CH2碳氢基团能够提供修复空位所必须的碳原子进而修复缺陷结构。其修复过程可以简单概括为CH和CH2中的C原子首先吸附空位处,与图2(a)中C1连接成键,在升温过程中CH和CH2中的C原子再与C2和C3连接成键,C2—C3键断开,最后碳氢基团中的H原子脱离碳纳米管,缺陷结构修复完成。
表面掺杂中,硼(元素符号B)和氮(元素符号N)原子是常见的两种掺杂原子。掺杂原子的引入改变了碳纳米管自身周围的电荷分布,进而影响了其吸附过程。刘贵立等[36]基于密度泛函理论选用(5,5)CNT来研究 B(N)共掺杂CNT的形成能。优化得到的(5,5)CNT构型中C—C键长约在 0.141—0.142 nm之间, B(N)原子掺杂(5,5)CNT的优化结构B—C、B—N键长分别增大4.2%和2.6%,N—C键长和C—C键长分别减小0.5%和0.99%。虽然B(N)掺杂后(5,5)CNT的键长发生改变,但是其仍然保持着六元环结构。另外,B(N)原子掺杂(5,5)CNT的形成能为−79.384 eV,说明B(N)原子掺杂是释放能量的过程,掺杂后的碳纳米管能够稳定存在。在表面掺杂结构基础上,为了研究表面杂原子掺杂对重金属的吸附作用,Hizhnyide等[37]进行了未掺杂和B/N掺杂的CNT吸附铬酸根(CrO42−)的理论计算。计算结果如表1所示,吸附CrO42−后碳纳米管上的 C、N和B原子均能够与铬酸根的氧原子结合构成C—O、B—O和N—O键。在吸附过程中电荷从CrO42−转移到CNT表面,CNT的电荷转移量约为0.8 e,B/N掺杂CNT的电荷转移量分别约为0.6 e和1.11 e。除此之外,N掺杂CNT与铬酸根的吸附能最大(约为−7.0 eV),B掺杂CNT的吸附能最小(约为−2.0 eV),而未掺杂CNT的吸附能约为−4.7 eV。由于B的电负性比C低,所以B掺杂周围的C原子带负电,进而削弱了CNT与铬酸根的相互作用。N的电负性比C高,所以N掺杂周围的C原子表现出缺电子特性,促进了与铬酸根的相互作用。杨忠华等[38]研究了N掺杂碳纳米管对Fe原子的吸附影响。经过吸附能计算发现未掺杂碳纳米管对Fe原子的吸附能为−1.245 eV,掺杂碳纳米管对Fe原子的吸附能为−3.239 eV。姚洁等[39]基于密度泛函理论建立了管径为0.4 nm的(3,3)B/N掺杂碳纳米管模型,并计算其对第四周期过渡金属(Sc~Cu)的吸附情况。计算结果发现N掺杂碳纳米管相对于未掺杂碳纳米管对第四周期过渡金属原子的吸附能提高了1.5 eV左右,而B掺杂碳纳米管对金属原子的吸附能与未掺杂碳纳米管的吸附能相比,变化不大。上述的例子说明了N掺杂能够提高碳纳米管对过渡金属原子的吸附性能,因此表面原子掺杂是改善碳纳米管对重金属吸附性能的手段之一。
-
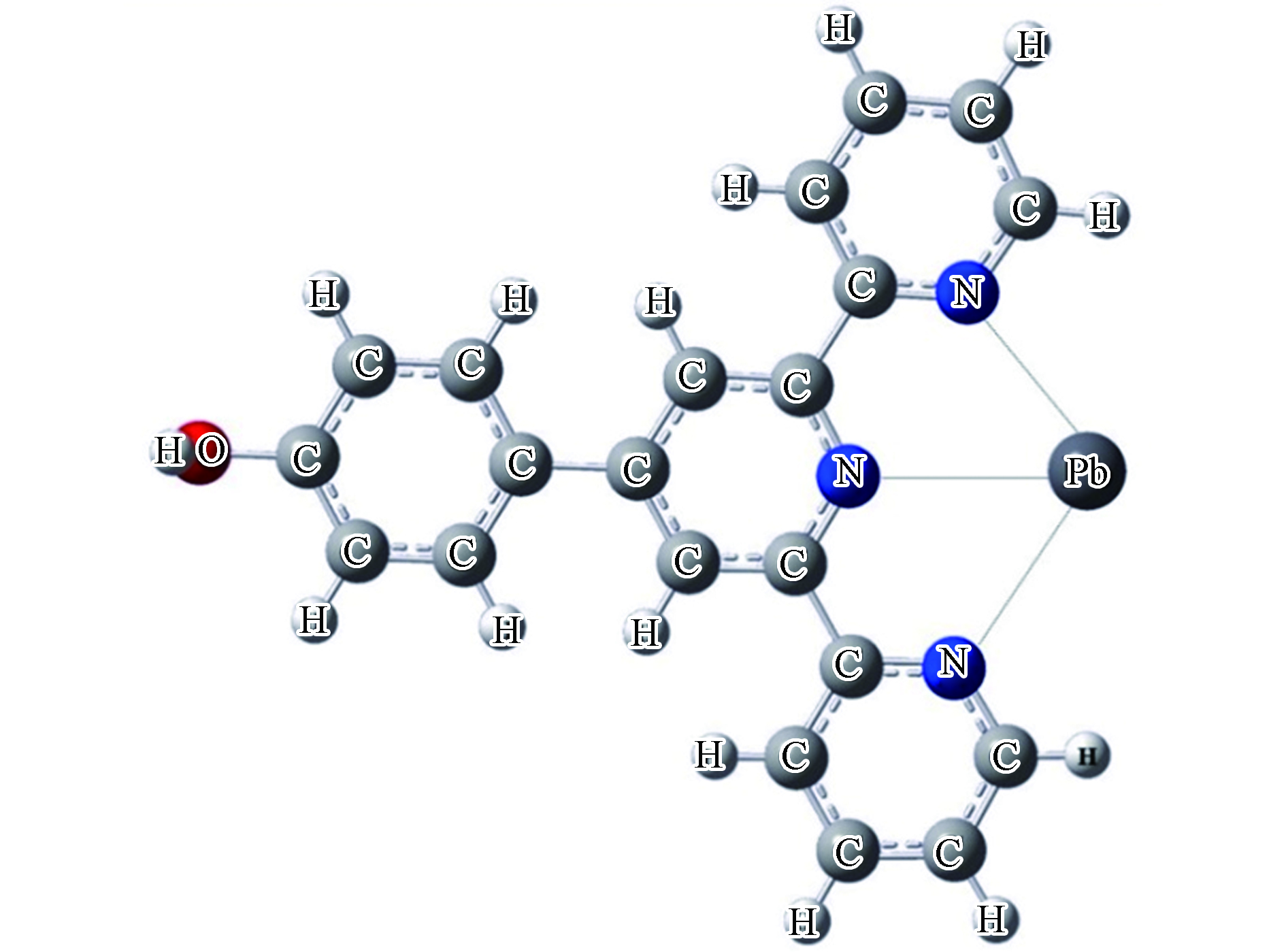
表面分子功能化有共价功能化和非共价功能化两种化学修饰方式,共价功能化是碳纳米管通过共价键与分子或官能团结合,而非共价功能化则是通过离子键的相互作用。首先,在结构上,功能化的碳纳米管会发生结构畸变,Zhao等[40]研究了羧基官能团与碳纳米管共价功能化的电子态,他们发现羧基与碳纳米管之间形成共价键并且官能团羧基沿碳纳米管径向发生了局部畸变,畸变局限在键合位点附近。此外,畸变结构影响了可取代官能团数量。Chełmecka等[41]在扶手椅形和锯齿形两种构型的碳纳米管末端依次添加1—9个羧基基团。研究发现锯齿形碳纳米管的末端可以实现由9个羧基基团完全取代的稳定结构,而扶手椅形碳纳米管由于碳骨架的空间拥挤结构和轮辋变形其末端的1个氢原子不能被取代从而不能实现完全功能化。Wang等[42]研究了羧基基团与SW缺陷锯齿碳纳米管的相互作用。研究发现碳纳米管的电子特性受结构的影响,缺陷由于产生施主能态从而促进其吸附或者氧化羧基基团。其次,官能团在碳纳米管中不同位点引入的吸附效能不同。功能化碳纳米管上的基团可以负载在材料的侧壁或者边缘。Singha Deb课题组首先研究了酰胺基团(AA)的负载位置和Hg2+与HgCl2的配位方式对吸附性能的影响[43]。如表1所示,当AA基团负载在SWCNT的侧壁和边缘时,SWCNT—AA中 C=O和C—N键长变化区别并不明显。从SWCNT—AA分别吸附Hg2+与HgCl2后的键长的变化可以发现:在单配位的吸附模式下,金属Hg2+和分子HgCl2与AA基团中的N原子相互作用,而在双配位的吸附模式下,金属Hg2+和分子HgCl2与AA基团中的N原子和O原子相互作用。当Hg2+和HgCl2以单配位的方式与SWCNT—AA相互作用时,从表1中可以看到AA基团负载在碳纳米管边缘时Hg—N键更短。这说明了侧壁基团与Hg2+和HgCl2以单配位结合时相互作用较弱。并且与吸附Hg2+相比,HgCl2分子与碳纳米管之间的Hg—N键更短一些。当Hg2+以双配位的方式与SWCNT—AA相互作用时,Hg2+与碳纳米管在AA基团负载在边缘时具有更短的 Hg—N键(0.369 nm),而AA基团负载在侧壁时具有更短的 Hg—O键(0.379 nm)。在当HgCl2以双配位的方式与SWCNT—AA相互作用时,可以看到AA基团负载在碳纳米管边缘时Hg—N和Hg—O键更短。说明了边缘基团与HgCl2以双配位结合时具有较强的相互作用。为了进一步比较Hg2+和HgCl2与碳纳米管之间相互作用的强弱,Singha Deb课题组从能量角度分析了Hg2+和HgCl2与SWCNT—AA不同配位方式的吸附能。从表2中可以看到,由于水环境中水分子的影响,气相中计算的吸附能相较于水相中要低。在水相中对于Hg2+与AA 基团单独的单配位或者双配位结合模式来说,侧壁吸附的吸附能(−8.6 eV左右)略高于边缘吸附(−8.2 eV左右),表示Hg2+与SWCNT—AA侧壁的相互作用强于边缘的相互作用并且从所有的四种结合模式来看,侧壁双配位吸附能最低(−8.69 eV),即结合作用最强。对比分析Hg2+和HgCl2与碳纳米管之间吸附能可以发现,汞金属以HgCl2分子形式与碳纳米管的吸附能(~−0.6 eV)普遍低于Hg2+的(~−8.2 eV),这说明汞以二价离子形式存在时与SWCNT—AA的相互作用更强。研究了酰胺基团的影响之后,Singha Deb课题组又在碳纳米管上负载了硫醇(SH)和二硫代氨基甲酸(DTC)配体,分别探究对Hg2+与HgCl2的吸附性能影响[44]。如表1所示,Hg—S和Hg—O的成键形式说明了金属Hg2+与SH和DTC基团中的S原子和O原子相互作用。从表2的吸附能的角度来看,SWCNT—DTC和SWCNT—SH对于相同吸附质的吸附能并没有太大的能量差别,而在Hg2+和HgCl2两者之间存在着较大差别。相较于对HgCl2的吸附,两种改性碳纳米管吸附Hg2+的吸附能更低,这也说明了Hg2+与改性碳纳米管的结合更稳定。通过以上研究发现官能团改性后的碳纳米管中含氮和含硫等官能团是金属离子吸附的有效位点,并且这些官能团在碳纳米管中不同位点引入效果不同。除了毒性较强的汞金属,铅也是废水污染中不能忽视的一种金属。Oyetade等[45]将4'—(4—羟基苯基) —2,2':6',2"—三联吡啶基团(HO—Phttpy)负载到多壁碳纳米管制备了一种改性材料(MWCNT—ttpy),并对比研究该基团与羧基基团改性对Pb2+和Zn2+的作用效果。通过比较表2的吸附能大小可以看到MWCNT—ttpy吸附Pb2+的吸附能最低(−10.21eV),对Pb2+吸附效果最好。通过比较两种材料对同一金属离子的吸附,以Pb2+为例,MWCNT—ttpy与Pb2+的吸附能(−10.21 eV)要比MWCNT—COOH的(−1.51 eV)低得多,Zn2+的吸附能大小比较与Pb2+的相似。这说明两种基团中,HO—Phttpy基团改性比COOH基团改性吸附Pb2+和Zn2+的效果更好。图3为HO—Phttpy基团与Pb2+相互作用的示例图。
由于HO—Phttpy基团中的N原子可以为金属离子提供更多电子,与金属离子有很强的螯合作用,所以HO—Phttpy基团改性后的碳纳米管具有了更多与金属离子配位的螯合位点,从而高效去除溶液中的重金属离子。除了在碳纳米管表面引入羧基基团,羟基改性也是一种有效的改性方式。Hizhnyi等[46]研究了羧基和羟基功能化碳纳米管(CNT—COOH、CNT—COOH—OH和CNT—COO−—OH)对Cr(VI)的吸附。分别考虑水相中Cr(VI)的CrO42−、Cr2O72−和HCrO4−三种存在形式与碳纳米管相互作用的吸附能。从表2中可以看到,碳纳米管吸附CrO42−的吸附能在水相中ΔE值均为正值,而吸附Cr2O72−和HCrO4−的吸附能ΔE值均为负。这种吸附能的差异性使得羧基和羟基功能化碳纳米管对Cr(VI)的吸附具有选择性,能有效吸附Cr2O72−和HCrO4−离子,而不利于吸附水体中的CrO42−。比较同一种改性材料对Cr2O72−和HCrO4−的吸附能,CNT—COOH和CNT—COOH—OH吸附Cr2O72−的能量要比HCrO4−略大一点。一般来说,六价铬的毒性比三价铬的毒性要大得多。Liu等[47]的研究中发现电场能够将Cr(VI)转化为Cr(Ⅲ),从而影响了CrO42−的活性。电场的存在影响了碳纳米管材料对重金属离子的吸附。如表2所示,聚苯胺修饰的碳纳米管(PANI—CNT)对Cr3+的吸附能在施加电场后从−0.07 eV下降到−1.94 eV;同样的施加电场后PANI—CNT 对CrO42−的吸附能从−0.58 eV下降到−1.39 eV。施加电场使得PANI—CNT对金属铬的吸附能下降,表明了电场的存在能够使PANI—CNT更有效的去除铬离子。除此之外,Liu等[48]还将钛酸盐纳米线与碳纳米管复合,探究该复合材料对三价锑离子的氧化和吸附。研究发现施加电场后,吸附能增加了0.38 eV,电子转移量从1.27 e增加到1.32 e。说明施加电场能够增强碳纳米管复合材料对Sb(Ⅲ)的吸附。
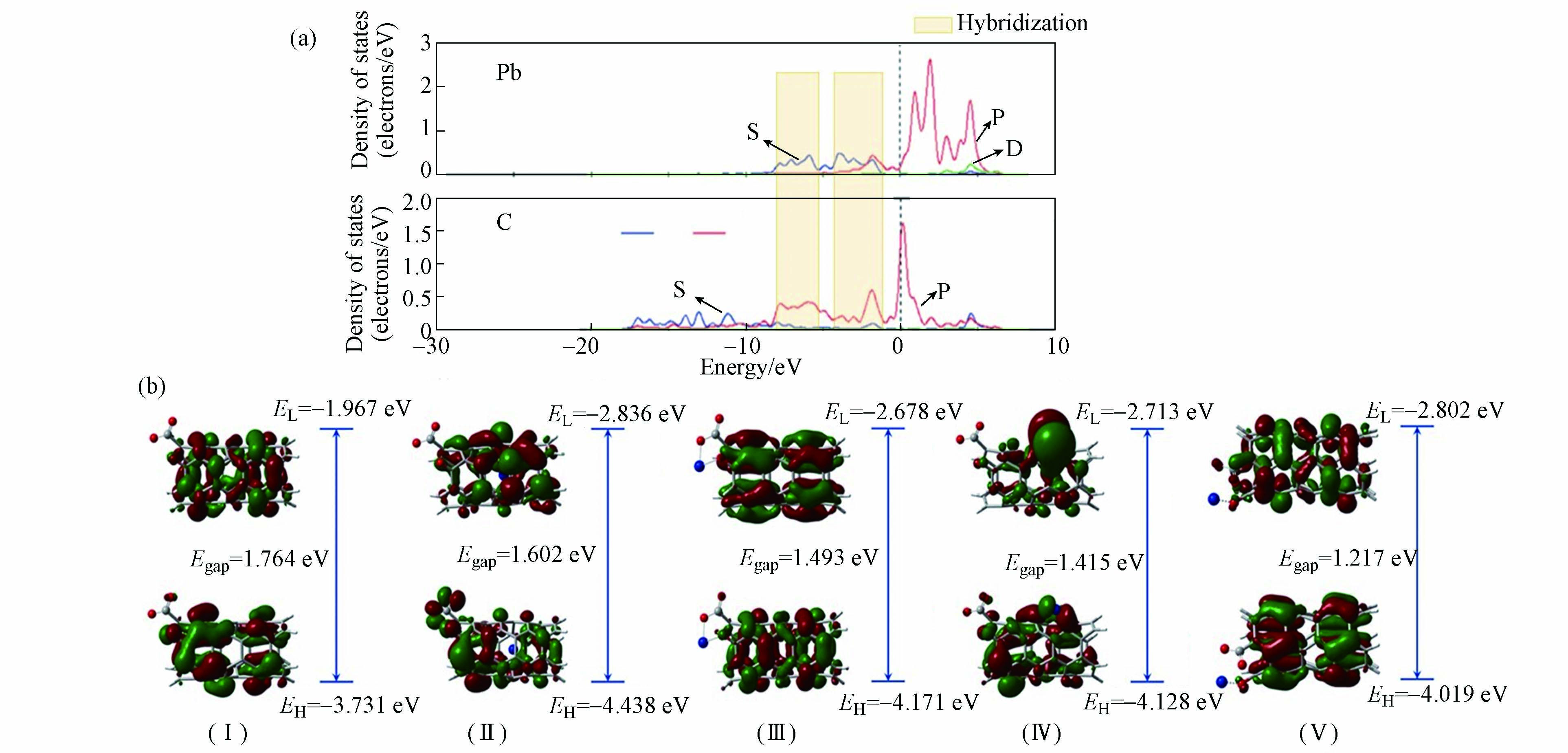
在DFT计算中,态密度(density of state, DOS)表示某一能量范围内的电子分布情况,HOMO(highest occupied molecule orbital)表示最高占据分子轨道,LUMO(lowest unoccupied molecule orbital)表示最低未占分子轨道,他们可以从电子特性角度分析碳纳米管与金属离子之间的相互作用。碳纳米管的尺寸大小影响了其本身的电子特性。Satio等[49] 从(9,0)和(10,0)两种锯齿型碳纳米管的DOS图发现随着直径的增加,碳纳米管的带隙(Band gap)变窄,当碳纳米管无限增大时,碳纳米管带隙可以变为零。Li等[34]用局部态密度图(PDOS)分析SV—(6,0)CNTs对Pb2+的吸附机理。从图4(a)可以看到,能量在−10—0 eV之间时,C的p轨道和Pb的s轨道之间有重叠峰,说明了Pb离子与碳纳米管之间有强烈杂化作用。目前,较多的碳纳米管对重金属的吸附研究着眼于碳纳米管表面,而缺乏对碳纳米管孔径内的研究。因此,Zhang等[50]研究了COO−改性的碳纳米管(O—CNTs2−)表面及其孔径内对Pb2+的吸附机理。从图4(b)中吸附Pb2+之前O—CNTs2−的HOMO和LUMO轨道(Ⅰ)可以看出,吸附Pb2+之前O—CNTs2−中的LUMO轨道集中分布在碳纳米管表面,羧基周围分布着HOMO轨道。从碳纳米管孔径内阳离子—π(Ⅱ)、络合(Ⅲ)、碳纳米管表面阳离子—π(Ⅳ)和离子交换(Ⅴ)的4种Pb2+与O—CNTs2−的作用方式下的HOMO和LUMO图中可以看到,HOMO和LUMO轨道均产生了变化,说明了在吸附Pb2+之后电子发生了明显转移。通过比较O—CNTs2−与Pb2+的4种作用方式下HOMO—LUMO能隙(Egap)的大小可以发现孔径内阳离子—π相互作用的能隙最大(1.602 eV)。这说明碳纳米管孔径内的阳离子—π相互作用在吸附过程中起着重要作用。除此之外,阳离子与离域极化的π电子相互作用近期也得到了广泛关注。这种作用引起的交联效应有助于增强吸附[51]。
运用密度泛函理论能够对重金属离子在碳纳米管上的静态吸附结构、相互作用能以及电子特性等性质进行深入的研究。对于分析探讨碳纳米管与重金属离子的微观相互作用具有重要的作用。然而,目前大部分的DFT计算仅仅从结构和能量角度说明了重金属与碳纳米管较强的相互作用。对于一些弱相互作用,例如溶剂的影响,范德华力等具有一定的局限性。金属离子在水溶液中多数以水合物的存在形式,然而目前DFT的研究对于金属水合物没有进行深入的考虑。此外,由于碳纳米管的孔径结构,其扩散过程对吸附具有至关重要的影响。静态DFT计算无法对扩散的动态过程给予很好的理论解释。今后的DFT理论计算在偏重于对碳纳米管构造缺陷、掺杂或改性的研究外,可以进一步深入以上的研究内容。
-
分子动力学模拟是一种基于经典牛顿力学方程模拟体系中粒子的受力和坐标等随时间动态变化的计算方法。为了进一步观察溶液中的金属离子在CNTs上的动态吸附过程,探究CNTs与重金属离子之间相互作用的动态变化,研究人员利用MD模拟通过分析溶液中金属离子的均方位移(mean square displacement, MSD)及其在CNTs上的径向分布函数(radial distribution function, RDF)和平均力势(potential of mean force, PMF)揭示了金属离子在CNTs上吸附的微观结构,并通过对金属离子与CNTs之间的相互作用能量及其吸附自由能的计算明确了吸附过程的驱动力,为更好的解释实验现象提供理论支持[52-55]。其中,RDF、MSD以及PMF的计算公式和相应的物理意义如表3所示。
-
通过对MD模拟轨迹的分析能够直观地观察到吸附质随着时间的推移在吸附剂中的运动过程。Shang等[56]将多根(3,3)CNT扭转成束,并研究其对金属离子的吸附。图5(a)展示了0 fs、400 fs、3.0 ps和4.0 ps等不同时刻下金属离子在CNT束中的微观吸附结构。从图5可以看出,金属离子首先慢慢接近CNT束表面,再进入到碳纳米管的缝隙中,有些金属离子最终可以进入碳纳米管的孔道内。研究者们进一步探究了碳纳米管的自身特性及其周围的溶液环境对重金属离子吸附的影响。Dezfoli课题组通过MD模拟研究带电碳纳米管的电荷量[57]以及金属离子的种类[58]对吸附过程的影响。对于同一重金属离子,带电碳纳米管的电荷量对其吸附速率的影响效果不同。首先,Dezfoli等[57]研究了带电量分别为−0.005 e、−0.01 e、−0.02 e和−0.03 e的CNT(Ⅰ)、CNT(Ⅱ)、CNT(Ⅲ)和CNT(Ⅳ)这4种碳纳米管对水溶液中Zn2+的吸附性能。结果表明溶液中Zn2+在CNT(Ⅰ)、CNT(Ⅱ)、CNT(Ⅲ)和CNT(Ⅳ)上的吸附分别在7.5 ps、6.5 ps、8.3 ps、8.59 ps后达到平衡。即随着碳纳米管表面负电荷量的增加,Zn2+在CNTs上的吸附平衡时间表现出先减小后增加的趋势。为了进一步探究这一吸附现象的本质,Dezfoli等首先计算了CNTs与锌离子之间的相互作用能量,发现CNTs与锌离子之间的静电相互作用能量约为范德华相互作用能量的600—700倍,说明静电相互作用是锌离子在CNTs上吸附过程的主要驱动力。因此,在静电相互作用的驱动下碳纳米管表面带电量的增加本应促进对Zn2+的吸附速率,但是从吸附结果上来看,带电量为−0.02 e和−0.03 e的碳纳米管反而要比带电量−0.005 e、−0.01 e的碳纳米管达到吸附平衡时间要长。Dezfoli等分析这4种碳纳米管周围水分子的自扩散系数发现:随着碳纳米管的表面电荷从−0.005 e增加到−0.03 e,体系中水的自扩散系数从1.70×10−9 m2·s−1减小到1.32×10−9 m2·s−1,水分子自扩散系数的减小使得CNTs周围水分子的运动能力降低,阻碍了溶液中金属离子向碳纳米管表面的扩散,从而使得金属离子的吸附速率降低。此外,Dezfoli等[58]通过研究水溶液中Zn2+和Cd2+两种金属离子分别在带电量为−0.01 e和−0.02 e的CNT(Ⅱ)和CNT(Ⅲ)上的吸附过程进一步探究不同金属离子在CNTs上吸附的差异。从图5(b)中Zn2+与CNTs之间的最短距离随模拟时间的变化可以发现:随着CNTs表面电荷的增加,Zn2+吸附的平衡时间逐渐增加。这与他们之前的研究结果一致。但是Cd2+的吸附平衡时间却随着CNTs表面电荷的增加反而大大减小。考虑到CNTs周围水分子的影响,Dezfoli等通过分析CNTs周围水分子的密度分布发现:在CNTs外侧,随着距离的增加,溶液中的水分子依次呈现出高密度区域(Region I)、低密度区域(Region Ⅱ)和本体区域(Region Ⅲ)。与Zn2+和水分子形成的稳定Zn2+—(H2O)6配位形式相比,Cd2+周围络合的水分子数能够在4~8之间变化。Cd2+这种丰富的配位类型使其平衡动力学更高,也使其能够更容易穿过CNTs外侧的Region I区域,最终吸附到CNTs上。对于带电的CNTs,外侧高密度区域的水分子大大削弱了CNTs与重金属离子之间的静电相互作用,从而降低了溶液中重金属离子的吸附速率。不仅如此,体系的温度变化也能够使得CNTs周围水分子的密度发生变化,进而影响重金属离子的吸附过程。Ansari等[18]模拟了298 K,303 K和338 K的3个温度下碳纳米管吸附Zn2+的过程来探究温度对CNTs吸附重金属离子的影响。随着温度从298 K增加到338 K,碳纳米管与Zn2+的吸附平衡时间从24 ps减小到5 ps左右,吸附速率逐渐增加。研究人员通过比较不同温度下碳纳米管周围水分子的径向分布函数发现,298 K下水分子在0.24 nm处的RDF峰强最高,而随着温度升高,水分子在0.24 nm处的RDF最强峰的强度逐渐降低。这表示在298 K的温度下碳纳米管周围水分子分布更密集,阻碍了重金属离子向碳纳米管表面的运动。而随着体系温度的增加,碳纳米管周围水分子的分布密度逐渐降低,能够有效地促进碳纳米管对溶液中Zn2+的吸附。研究者们采用分子动力学模拟的方法通过对带电碳纳米管的带电量、体系温度以及金属离子种类的探究发现这些因素能够通过影响溶液中水分子的分布或者通过与水分子之间形成的不同配位形式来影响碳纳米管对金属离子的吸附性能。
-
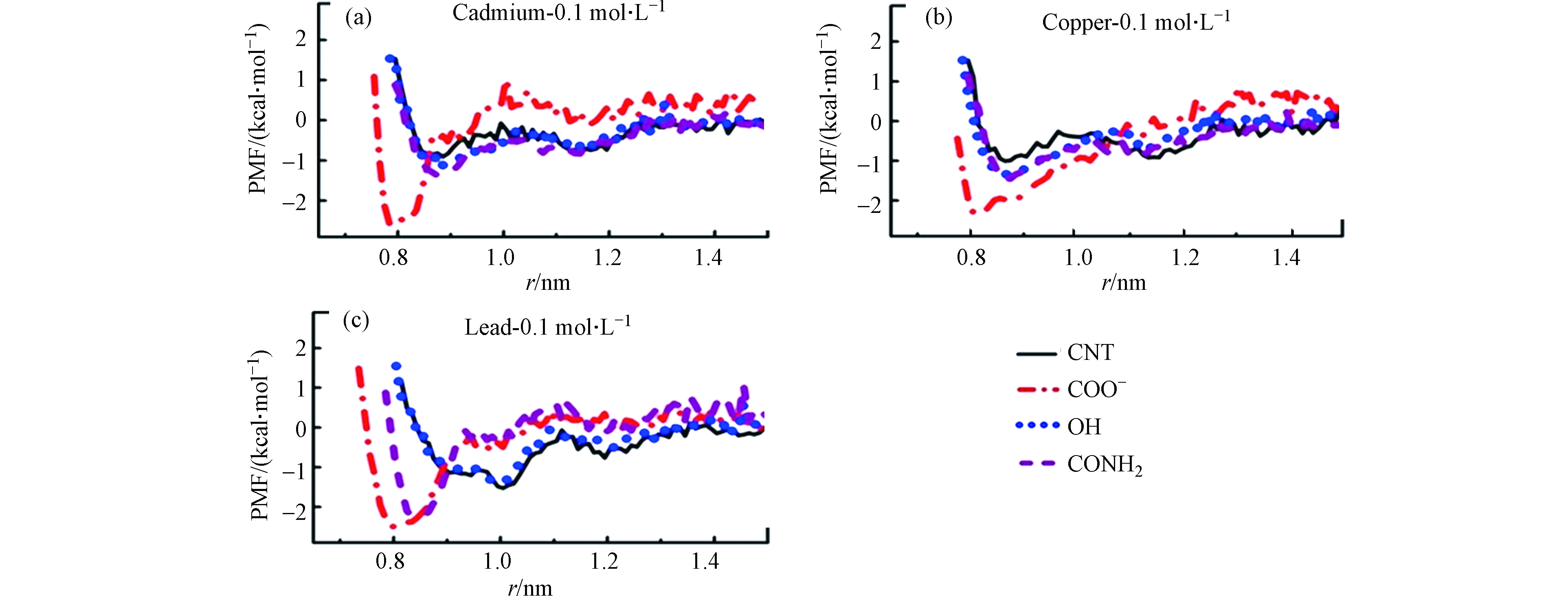
碳纳米管中官能团的引入能够为溶液中金属离子提供更多的吸附位点,从而进一步提高碳纳米管对金属离子的吸附性能。能够引入到CNTs中的改性官能团有:羟基(OH)、羧基(COOH)、酰胺基(CONH2)等官能团,研究者们采用MD模拟的方法对这些官能团改性的CNTs吸附重金属离子的相互作用机理进行了深入的研究。首先,Ansari等[18]研究了羧基和羟基改性碳纳米管对Zn2+的吸附。CNT的体系中Zn2+达到稳定吸附所需的时间约为24 ps,而在羧基改性的碳纳米管(CNT—COOH)和羟基改性的碳纳米管(CNT—OH)体系中Zn2+的吸附平衡时间大大缩减,分别为7 ps和5 ps。相比于未加修饰的碳纳米管,羟基和羧基的引入都能显著提高对Zn2+的吸附速率。进一步通过相互作用能量的分析发现:当体系达到吸附平衡时,CNT—COOH与Zn2+之间总的相互作用能量约为−125 kcal·mol−1,其中静电相互作用能量和范德华相互作用能量分别为−150 kcal·mol−1和25 kcal·mol−1。与CNT—COOH的体系相比,吸附平衡时CNT—OH与Zn2+的总相互作用能更大,约为−600 kcal·mol−1,其中静电相互作用能量和范德华相互作用能量分别为−700 kcal·mol−1和100 kcal·mol−1。这表明静电相互作用在羧基和羟基改性的碳纳米管吸附Zn2+的过程中占主导,而且羟基官能团的引入更有利于提高碳纳米管对Zn2+的吸附。此外,Anitha等[59]还研究了羧基、羟基和酰胺官能团改性的碳纳米管对不同金属离子(如:Cd2+、Cu2+和Pb2+)的吸附性能。负的吸附自由能说明吸附过程是自发的放热过程;吸附自由能越负,表明金属离子吸附在CNTs上的结构越稳定。通过比较不同官能团改性的CNTs体系中重金属离子的吸附自由能,可以得到不同改性官能团对重金属离子的吸附性能差异。首先从图6中可以得到:Cd2+在纯的CNT体系中的吸附自由能约为−0.9 kcal·mol−1,相比之下,Cd2+在官能团改性体系中的吸附自由能均有所提高。其中,在羧基改性的CNT体系中Cd2+的吸附自由能约为其在纯CNT体系中的3倍(−2.5 kcal·mol−1),而在羟基和酰胺基改性的CNT体系中Cd2+的吸附自由能分别约为−1.0 kcal·mol−1和−1.1 kcal·mol−1。其次从图中很明显地发现不同体系中Cu2+的吸附自由能变化与Cd2+的相似,其在羧基改性的CNT体系中具有最大的吸附自由能(−2.0 kcal·mol−1),在羟基和酰胺基改性的CNT体系中具有相似的吸附自由能(~−1.2 kcal·mol−1)而在纯的CNT体系中吸附自由能最小(约为−1.0 kcal·mol−1)。与Cd2+和Cu2+的吸附自由能变化不同,Pb2+吸附在纯的CNT体系和羟基改性的CNT体系中的自由能相似约为−1.5 kcal·mol−1,而在羧基和酰胺基改性的CNT体系中吸附自由能分别为−2.5 kcal·mol−1和−2.0 kcal·mol−1。综上,可以发现官能团的引入能够有效提高CNTs对金属离子的吸附自由能,其中羧基修饰的CNTs在吸附Cd2+、Cu2+和Pb2+等3种金属离子时均具有最大的自由能,这说明羧基基团的引入能够显著增强碳纳米管与这3种金属离子之间的相互作用,从而提高其吸附性能。Sahu等[60]利用分子动力学模拟研究Cd2+分别在—COOH、—CONH(CH2)2NH2和—CONH(CH2)2SH这3种官能团改性的碳纳米管上的吸附行为和相互作用机理。
图7(a—c)分别为模拟轨迹中Cd2+在这3种功能化碳纳米管上吸附的稳定构型。从图7可以看出,金属离子主要吸附在这些官能团的O原子,N原子和S原子附近。这是因为这些原子的最外层上有孤对电子,能够通过配位作用与金属离子形成络合物,从而可以作为溶液中的金属离子有效的吸附位点。此外,O原子的电负性比N原子和S原子的电负性强,使得金属离子更易于吸附在O原子附近。从图7(d)中可以看出,Cd2+在3种功能化碳纳米管的体系中均在0.22 nm处出现较强的尖峰。相比于—CONH(CH2)2NH2和—CONH(CH2)2SH改性的碳纳米管体系,—COOH改性的碳纳米管体系中Cd2+的g(r)曲线在0.22 nm处尖峰的强度更高,这表明Cd2+在O原子吸附位点处的吸附强度比其在N原子和S原子这两个吸附位点的吸附强度更高。除了对碳纳米管直接进行官能团修饰外,CNTs与其他材料的复合也是一种可行的提高吸附性能的方式。Poorsargol等[61]将石墨烯(Graphene)与碳纳米管复合构造了一种复合材料(Graphene—CNT),并研究了羧基和羟基分别修饰的碳纳米管及其复合材料(CNT—COOH、CNT—OH、Graphene—CNT—COOH和Graphene—CNT—OH)对水溶液中Cu2+的吸附性能。从图7(e)中Cu2+在这四种材料中的MSD可以看到Cu2+在碳纳米管中的MSD值约为复合材料中的两倍,例如15000 ps时刻下Cu2+在碳纳米管中的MSD值约为100 nm2而在复合材料中约为50 nm2。复合材料中Cu2+的均方位移显著下降说明复合材料与Cu2+的相互作用更强,降低了Cu2+在溶液中的扩散能力。
分子动力学模拟的轨迹首先直观地展现了溶液中重金属离子在CNTs上动态吸附的微观过程。其次,通过对轨迹中吸附微观结构的分析能够清楚的看到CNTs上可能的吸附位点。然而,当前对于吸附位点的分析MD模拟中选取大多为含氧官能团,其中引入的羟基和羧基等含氧官能团中的氧原子是重金属离子的主要吸附位点,而对于其他含N和含S等基团研究较为缺乏。带电碳纳米管自身的带电特性以及体系的模拟温度能够直接影响体系中CNTs周围的水分子密度,从而影响重金属离子的吸附速率。除了环境温度的影响,pH和压力等变化也是吸附过程中不可忽略的因素,而MD模拟的优势之一就在于可以模拟一些实验中实现较为困难的极端环境(高温、高压),在这方面有待进一步研究。除此之外,目前大多数吸附研究的模拟体系为碳纳米管与单金属离子体系,而实际的水处理环境为多种重金属离子共存,因此探讨多种重金属离子之间竞争吸附也具有着良好的研究前景。
-
碳纳米管在废水处理方面具有良好的应用前景。近年来,计算机技术和量子理论的不断发展为从微观角度探索重金属与碳纳米管材料的作用过程与机理提供了可能。本文从静态密度泛函理论计算和动态分子动力学模拟两个角度出发,综述了近年来碳纳米管吸附重金属的理论计算研究进展。密度泛函理论研究表明,构造缺陷、杂原子掺杂以及表面官能团修饰对碳纳米管以及金属离子的吸附构型、能量以及电子特性具有重要的影响,进而对其吸附性能和机理产生一定作用。而分子动力学模拟能直观的反映重金属离子在碳纳米管中的运动过程以及环境影响。对吸附速率、相互作用能、吸附机理等给予理论解释。
在现有的理论计算和实验研究中,研究者致力于制备吸附性能好的碳纳米管材料。大量的实验以及理论计算结果表示碳纳米管对单金属离子体系表现出优异的吸附效果。然而在实际的废水处理环境中存在着多种污染物,对纳米材料与多种重金属离子之间的竞争吸附以及特异性吸附有待进一步研究。除此之外,重金属离子在溶液中多与水分子配位,以金属水合物的形式存在。因此探讨碳纳米管与金属水合物之间的相互作用也是今后的研究方向之一。碳纳米管是一种多孔材料,其多孔的结构有利于吸附各种污染物。现如今更多的吸附过程聚焦在材料表面以及表面基团的影响。理论计算的优势就在于可以获得实验中难以测得的结构模型键长、能量以及一些动力学参数,Gaussian、VASP、CP2K、Gromacs和Lammps等计算软件的广泛使用对研究污染物在孔径内的扩散运动以及材料的孔径效应提供了可行性。在模拟环境中除了温度对吸附的影响外,溶液的pH、压力以及干扰离子等因素对吸附的影响也是今后的研究方向之一。除此之外,利用机器学习在有限的实验数据中挖掘信息也是目前理论研究的关注热点。由于化学环境的复杂性,虽然密度泛函理论和分子动力学模拟中的真空环境或模拟环境与实际实验中的溶液环境不一致,导致理论计算与实验结果存在一定偏差。但是在保证一定准确度的前提下,理论计算不仅能够节省大量时间,降低实验成本,还能够从微观角度探索相互作用过程与吸附机理,为解释实验现象提供理论支持,为新材料的合成提供设计思路。碳纳米管材料在环境修复中有巨大的发展潜力,但是理论计算的研究仍处于初始阶段,重金属与碳纳米管的作用机理尚不明确,我们期待理论计算在环境领域中能够有更广泛的应用。
碳纳米管吸附环境重金属机制研究——理论计算进展
Mechanism of adsorption of heavy metal ions by carbon nanotubes——progress in theoretical calculation
-
摘要: 重金属污染严重威胁着生态环境的平衡与稳定。碳纳米管因其比表面积较大,官能团丰富以及结构稳定等特性,对重金属离子具有良好的吸附能力,是废水处理中一种具有广泛应用前景的纳米材料。近年来,随着计算机技术和量子理论的不断发展,理论计算发展迅速。理论计算能够从微观角度分析吸附剂与吸附质之间可能存在的相互作用,探究两者之间的作用机理,为解释实验现象提供理论参考。本文从理论计算角度,以静态密度泛函理论计算与动态分子动力学模拟两方面,总结了碳纳米管在吸附重金属方面的研究进展。从碳纳米管的计算模型、优化结构、电子特性和能量等角度进行分析综述,探究碳纳米管与重金属离子之间的相互作用机理,以期能够为碳纳米管等纳米材料在废水处理中的广泛应用提供理论支持。Abstract: Heavy metal pollution is a serious threat to the balance and stability of the ecological environment. Due to their large specific surface area, rich functional groups and stable structure, carbon nanotubes have good adsorption capacity for heavy metal, and become a kind of nano materials with wide application prospects in wastewater treatment. In recent years, with the continuous development of computer technology and quantum theory, theoretical calculation has developed rapidly. We can analyze the possible interaction between adsorbent and adsorbate from the micro perspective, explore the interaction mechanism between them, and provide theoretical reference for explaining experimental phenomena. From the perspective of theoretical calculation, this work summarized the research progress of carbon nanotubes in the adsorption of heavy metal from aspects of density functional theory calculation and molecular dynamics simulation. In order to provide theoretical support for the wide application of carbon nanotubes and other nano materials in the wastewater treatment, we analyzed and summarized the calculational model, optimized configuration, electronic properties and interaction energy of carbon nanotubes, and explored the interaction mechanism between carbon nanotubes and heavy metal.
-
Key words:
- heavy metal /
- carbon nanotube /
- density functional theory /
- molecular dynamics simulation /
- adsorption
-

-
图 2 碳纳米管的5—1DB缺陷图(a)和拓扑缺陷图(b) [31]
Figure 2. 5—1DB defect graph (a) and Stone—Wales defect graph (b) of carbon nanotubes, in which 1, 2, 3, 4 and 5 represent different carbon atoms respectively, A and B are vacancy and a five-membered ring in SV defects ,C, D and E are seven-membered ring , five-membered ring and six-membered ring in SW defect[31]
图 4 (a) SV—(6,0)CNTs吸附Pb2+后C和Pb的PDOS图[34]; (b) O—CNTs2−的HOMO和LUMO图(Ⅰ),Pb2+与 O—CNTs2−孔径内阳离子—π相互作用(Ⅱ)、络合(Ⅲ)、碳纳米管表面阳离子—π相互作用(Ⅳ)和离子交换(Ⅴ)的HOMO和LUMO图[49]
Figure 4. (a) The PDOS of C and Pb in Pb2+—SV—(6, 0) CNTs [34];(b) HOMO and LUMO plots of O—CNTs2− (Ⅰ), O—CNTs2−+Pb2+ Inner—cation—π (Ⅱ), O—CNTs2−+Pb2+ Complexation (Ⅲ), O—CNTs2−+Pb2+ Outer—cation—π (Ⅳ) and O—CNTs2−+Pb2+ Eletrostatic (Ⅴ) with B3LYP/6—31G* level of theory[49]
图 7 Cd2+分别在(a)CNT—COOH、(b)CNT—CONH(CH2)2NH2和(c)CNT—CONH(CH2)2SH上的吸附结构。颜色符号:Cd2+:黄色;O:红色;N:蓝色;S:黄色;C:青色;H:白色,(d)三种官能团改性的CNTs体系中Cd2+的径向分布函数[60] ,(e) Cu2+在不同体系中的均方位移[61]
Figure 7. Adsorption structures of Cd2+on (a) CNT-COOH, (b) CNT-CONH(CH2)2NH2 and (c) CNT-CONH(CH2)2SH (Cd2+: yellow; O:red; N:blue;S:yellow;C:cyan;H:white), (d) radial distribution function of Cd2+ associate with COOH, CONH(CH2)2NH2 and CONH(CH2)2SH, respectively [60] , (e) mean square displacement of Cu2+ on CNT and hybrid graphene–CNT functionalized with OH and COOH [61].
表 1 碳纳米管吸附金属后的键距变化(单位 nm)
Table 1. The change of bond distance of carbon nanotubes after adsorption of metal, in nm.
结构
StructureZn—C Cu—C Pb—C Sn—C — 参考文献
ReferenceCNT—T 0.3590 0.2084 0.2628 0.2492 — [30] CNT—H 0.3589 0.2358 0.2555 0.2406 — CNT—B 0.3585 0.1940 0.2474 0.2389 — 结构
StructureC—O B/N—O Cr—O C—Cr B/N—Cr 参考文献
ReferenceCNT(3,3) 0.1416 — 0.1838 0.2931 — [37] CNT(5,5) 0.1436 — 0.1854 0.2909 — CNT(3,3)—B 0.2534 0.1448 0.1789 0.3554 0.2970 CNT(5,5)—B 0.2561 0.1476 0.1801 0.3488 0.2925 CNT(3,3)—N 0.2335 0.1481 0.1884 0.3581 0.2971 CNT(5,5)—N 0.2441 0.1520 0.1892 0.3313 0.2942 结构
StructureHg—O Hg—N C—N(NH2) C=O Hg—Cl 参考文献
ReferenceSWCNT—AA(侧壁) — — 0.1456 0.1219 — [43] SWCNT—AA(边缘) — — 0.1454 0.1230 — SWCNT—AA(侧壁)—Hg2+—单配位 — 0.3671 0.1443 0.1217 — SWCNT—AA(边缘)—Hg2+—单配位 — 0.3640 0.1441 0.1224 — SWCNT—AA(侧壁)—Hg2+—双配位 0.3792 0.6159 0.1460 0.1210 — SWCNT—AA(边缘)—Hg2+—双配位 0.5832 0.3690 0.1448 0.1224 — SWCNT—AA(侧壁)—HgCl2—单配位 — 0.2681 0.1465 0.1217 0.2360,0.2350 SWCNT—AA(边缘)—HgCl2—单配位 — 0.2640 0.1469 0.1229 0.2356,0.2354 SWCNT—AA(侧壁)—HgCl2—双配位 0.2708 0.2687 0.1468 0.1225 0.2387,0.2363 SWCNT—AA(边缘)—HgCl2—双配位 0.2601 0.2668 0.1467 0.1241 0.2392,0.2379 结构
StructureS—Hg (C=)O—Hg C—Hg — — 参考文献
ReferenceSWCNT—SH—Hg2+ 0.3868 0.5502 0.3214 — — [44] SWCNT—DTC—Hg2+ 0.4095 0.3339 0.5430 — — 0.5825 — — — — SWCNT—SH—HgCl2 0.5262 0.2537 0.4320 — — SWCNT—DTC—HgCl2 0.3684 0.2611 0.4295 — — 0.6170 — — — — 表 2 基于DFT计算改性单壁或多壁碳纳米管在水相和气相吸附金属的吸附能(eV)
Table 2. Based on DFT calculation, the adsorption energy of metal adsorption in water and gas phase of modified single-walled or multi walled carbon nanotubes
吸附剂
Adsorbent吸附质
Adsorbate水相ΔE/eV
Aqueous phase气相ΔE/eV
Gas phase计算方法
Method of calculation计算软件
Software参考文献
ReferenceSWCNT—AA(侧壁)—单配位 Hg2+ −8.63 −16.85 B3LYP, TZVP,SVP Turbomole [43] HgCl2 −0.39 −0.53 SWCNT—AA(侧壁)—双配位 Hg2+ −8.69 −17.14 HgCl2 −0.42 −0.65 SWCNT—AA(边缘)—单配位 Hg2+ −8.25 −16.56 HgCl2 −0.36 −0.45 SWCNT—AA(边缘)—双配位 Hg2+ −8.21 −16.56 HgCl2 −0.33 −0.50 SWCNT—SH Hg2+ −8.63 −17.07 B3LYP, TZVP, Hg2+( Grimme D3) Turbomole [44] HgCl2 −0.65 −0.90 SWCNT—DTC Hg2+ −8.63 −17.47 HgCl2 −0.71 −0.94 MWCNT—ttpy Pb2+ −10.21 — M06—2X, LANL2DZ—ECP(Pb,Zn), 6—31G(d,p)(其他原子),IEFPCM Gaussian09W [45] Zn2+ −6.23 — MWCNT—COOH Pb2+ −1.51 — Zn2+ −0.72 — CNT—COOH HCrO4− −0.46 — B3LYP/6-31G(C,H),cc—pVDZ(Cr,O),BBSE Gaussian09-E01 [46] Cr2O72− −0.53 — CrO42− 3.68 −5.09 CNT—COOH—OH HCrO4− −0.12 — Cr2O72− −0.36 — CrO42− 3.93 −4.15 CNT—COO−—OH HCrO4− −0.77 — Cr2O72− −0.19 — CrO42− 2.29 — PANI—CNT(未加电场) CrO42− — −0.58 PBE(Grimme D3)/ GTH, DZVPMOLOPT—GTH CP2K [47] Cr3+ — −0.07 PANI—CNT(施加电场) CrO42− — −1.39 Cr3+ — −1.94 表 3 RDF、MSD和PMF的公式及物理意义
Table 3. Formulas and physical meanings of RDF, MSD and PMF
符号
Symbol公式
Formula物理意义
Physical meaningRDF dN/4πρr2dr 以体系中某一粒子为中心,距该粒子r处出现另一个粒子的概率 MSD <[r(t+dt)-r(t)]2> 用于描述分子运动轨迹,其中< >表示对所有元素的平均值 PMF −kBT ln(P(r)) 反应了自由能随反应坐标的变化,其中kB为波尔兹曼常数,T为体系温度,P(r)为平均位置概率 -
[1] MUBARAK N M, SAHU J N, ABDULLAH E C, et al. Removal of heavy metals from wastewater using carbon nanotubes [J]. Separation & Purification Reviews, 2014, 43(4): 311-338. [2] FU F L, WANG Q. Removal of heavy metal ions from wastewaters: A review [J]. Journal of Environmental Management, 2011, 92(3): 407-418. doi: 10.1016/j.jenvman.2010.11.011 [3] KRISHNA KUMAR A S, JIANG S J, TSENG W L. Effective adsorption of chromium(VI)/Cr(III) from aqueous solution using ionic liquid functionalized multiwalled carbon nanotubes as a super sorbent [J]. Journal of Materials Chemistry A, 2015, 3(13): 7044-7057. doi: 10.1039/C4TA06948J [4] DIMPE K M, NOMNGONGO P N. A review on the efficacy of the application of myriad carbonaceous materials for the removal of toxic trace elements in the environment [J]. Trends in Environmental Analytical Chemistry, 2017, 16: 24-31. doi: 10.1016/j.teac.2017.10.001 [5] SANCEY B, TRUNFIO G, CHARLES J, et al. Heavy metal removal from industrial effluents by sorption on cross-linked starch: Chemical study and impact on water toxicity [J]. Journal of Environmental Management, 2011, 92(3): 765-772. doi: 10.1016/j.jenvman.2010.10.033 [6] SHARMA G, NAUSHAD M. Adsorptive removal of noxious cadmium ions from aqueous medium using activated carbon/zirconium oxide composite: Isotherm and kinetic modelling [J]. Journal of Molecular Liquids, 2020, 310: 113025. doi: 10.1016/j.molliq.2020.113025 [7] HAN H W, RAFIQ M K, ZHOU T Y, et al. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants [J]. Journal of Hazardous Materials, 2019, 369: 780-796. doi: 10.1016/j.jhazmat.2019.02.003 [8] KAM C S, LEUNG T L, LIU F Z, et al. Lead removal from water – dependence on the form of carbon and surface functionalization [J]. RSC Advances, 2018, 8(33): 18355-18362. doi: 10.1039/C8RA02264J [9] ZENG L, ZHANG Z H, ZHOU C Y, et al. Molecular dynamics simulation and DFT calculations on the oil-water mixture separation by single-walled carbon nanotubes [J]. Applied Surface Science, 2020, 523: 146446. doi: 10.1016/j.apsusc.2020.146446 [10] SHARMA R, BAIK J H, PERERA C J, et al. Anomalously large reactivity of single graphene layers and edges toward electron transfer chemistries [J]. Nano Letters, 2010, 10(2): 398-405. doi: 10.1021/nl902741x [11] LI Y H, WANG S G, WEI J Q, et al. Lead adsorption on carbon nanotubes [J]. Chemical Physics Letters, 2002, 357(3/4): 263-266. [12] IHSANULLAH, ABBAS A, AL-AMER A M, et al. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications [J]. Separation and Purification Technology, 2016, 157: 141-161. doi: 10.1016/j.seppur.2015.11.039 [13] LU C, CHIU H. Adsorption of zinc(II) from water with purified carbon nanotubes [J]. Chemical Engineering Science, 2006, 61(4): 1138-1145. doi: 10.1016/j.ces.2005.08.007 [14] HAMON M A, HUI H, BHOWMIK P, et al. Ester-functionalized soluble single-walled carbon nanotubes [J]. Applied Physics A, 2002, 74(3): 333-338. doi: 10.1007/s003390201281 [15] YANG K L, LOU Z M, FU R Q, et al. Multiwalled carbon nanotubes incorporated with or without amino groups for aqueous Pb(II) removal: Comparison and mechanism study [J]. Journal of Molecular Liquids, 2018, 260: 149-158. doi: 10.1016/j.molliq.2018.03.082 [16] MARQUES NETO J D O, BELLATO C R, SILVA D D C. Iron oxide/carbon nanotubes/chitosan magnetic composite film for chromium species removal [J]. Chemosphere, 2019, 218: 391-401. doi: 10.1016/j.chemosphere.2018.11.080 [17] SOUNTHARARAJAH D P, LOGANATHAN P, KANDASAMY J, et al. Removing heavy metals using permeable pavement system with a titanate nano-fibrous adsorbent column as a post treatment [J]. Chemosphere, 2017, 168: 467-473. doi: 10.1016/j.chemosphere.2016.11.045 [18] ANSARI A, MEHRABIAN M A, HASHEMIPOUR H. Zinc ion adsorption on carbon nanotubes in an aqueous solution [J]. Polish Journal of Chemical Technology, 2012, 14(3): 29-37. doi: 10.2478/v10026-012-0081-6 [19] DEHGHANI M H, MOSTOFI M, ALIMOHAMMADI M, et al. High-performance removal of toxic phenol by single-walled and multi-walled carbon nanotubes: Kinetics, adsorption, mechanism and optimization studies [J]. Journal of Industrial and Engineering Chemistry, 2016, 35: 63-74. doi: 10.1016/j.jiec.2015.12.010 [20] JAWED A, SAXENA V, PANDEY L M. Engineered nanomaterials and their surface functionalization for the removal of heavy metals: A review [J]. Journal of Water Process Engineering, 2020, 33: 101009. doi: 10.1016/j.jwpe.2019.101009 [21] AFKHAMI A, SHIRZADMEHR A, MADRAKIAN T, et al. Improvement in the performance of a Pb2 + selective potentiometric sensor using modified core/shell SiO2/Fe3O4 nano-structure [J]. Journal of Molecular Liquids, 2014, 199: 108-114. doi: 10.1016/j.molliq.2014.08.027 [22] ZHOU X, ZHAO C H, CHEN C T, et al. DFT study on adsorption of formaldehyde on pure, Pd-doped, Si-doped single-walled carbon nanotube [J]. Applied Surface Science, 2020, 525: 146595. doi: 10.1016/j.apsusc.2020.146595 [23] CHEN R J, CHOI H C, BANGSARUNTIP S, et al. An investigation of the mechanisms of electronic sensing of protein adsorption on carbon nanotube devices [J]. Journal of the American Chemical Society, 2004, 126(5): 1563-1568. doi: 10.1021/ja038702m [24] IIJIMA S. Helical microtubules of graphitic carbon [J]. Nature, 1991, 354(6348): 56-58. doi: 10.1038/354056a0 [25] CHEN T, LI A G. Synthesizing carbon nanotubes in space [J]. Astronomy & Astrophysics, 2019, 631: A54. [26] DRESSELHAUS M S, DRESSELHAUS G, SAITO R. Physics of Carbon Nanotubes[J]. Carbon, 1995, 33(7): 883-891. [27] KOZINSKY B, MARZARI N. Static dielectric properties of carbon nanotubes from first principles [J]. Physical Review Letters, 2006, 96(16): 166801. doi: 10.1103/PhysRevLett.96.166801 [28] 娄昀璟, 李雪花, 陈景文. 氧分子在碳纳米颗粒表面吸附的密度泛函理论研究 [J]. 环境化学, 2015, 34(9): 1587-1593. doi: 10.7524/j.issn.0254-6108.2015.09.2015042201 LOU Y J, LI X H, CHEN J W. Oxygen adsorption on carbon nanoparticles: A density functional theory study [J]. Environmental Chemistry, 2015, 34(9): 1587-1593(in Chinese). doi: 10.7524/j.issn.0254-6108.2015.09.2015042201
[29] ZHANG C, WANG W J, DUAN A, et al. Adsorption behavior of engineered carbons and carbon nanomaterials for metal endocrine disruptors: Experiments and theoretical calculation [J]. Chemosphere, 2019, 222: 184-194. doi: 10.1016/j.chemosphere.2019.01.128 [30] ZHU Z, AN L, CHEN T, et al. The adsorption of divalent heavy metal ions on (8, 0) carbon nanotubes: The first-principles study [J]. Modern Physics Letters B, 2020, 34(32): 2050368. doi: 10.1142/S0217984920503686 [31] 代利峰, 安立宝. 含缺陷碳纳米管吸附Al原子的第一性原理研究 [J]. 特种铸造及有色合金, 2017, 37(3): 340-344. doi: 10.15980/j.tzzz.2017.03.029 DAI L F, AN L B. The first principles investigation on the adsorption of Al atoms on carbon nanotubes with defect [J]. Special Casting & Nonferrous Alloys, 2017, 37(3): 340-344(in Chinese). doi: 10.15980/j.tzzz.2017.03.029
[32] 张变霞, 杨春, 冯玉芳, 等. 碳纳米管吸附铜原子的密度泛函理论研究 [J]. 物理学报, 2009, 58(6): 4066-4071. doi: 10.3321/j.issn:1000-3290.2009.06.070 ZHANG B X, YANG C, FENG Y F, et al. A density functional theory study of the absorption behavior of copper on single-walled carbon nanotubes [J]. Acta Physica Sinica, 2009, 58(6): 4066-4071(in Chinese). doi: 10.3321/j.issn:1000-3290.2009.06.070
[33] 王清云, 佟永纯, 闫盆吉, 等. 缺陷碳纳米管限域金属Ti原子的理论研究 [J]. 原子与分子物理学报, 2019, 36(4): 588-593. doi: 10.3969/j.issn.1000-0364.2019.04.010 WANG Q Y, TONG Y C, YAN P J, et al. Theoretical study of Ti interaction with defect carbon nanotubes [J]. Journal of Atomic and Molecular Physics, 2019, 36(4): 588-593(in Chinese). doi: 10.3969/j.issn.1000-0364.2019.04.010
[34] LI W, ZHAO Y, WANG T. Study of Pb ion adsorption on (n, 0) CNTs (n=4, 5, 6) [J]. Nanotechnology Reviews, 2018, 7(6): 469-473. doi: 10.1515/ntrev-2018-0087 [35] ZHOU R L, HE H Y, PAN B C. Enhancing the topological structures of defected carbon nanotubes with adsorbed hydrocarbon radicals at low temperatures [J]. Physical Review B, 2007, 75(11): 113401. doi: 10.1103/PhysRevB.75.113401 [36] 刘贵立, 宋媛媛, 姜艳, 等. 拉压变形对B(N)掺杂碳纳米管Al吸附性能的影响 [J]. 沈阳工业大学学报, 2016, 38(4): 391-396. doi: 10.7688/j.issn.1000-1646.2016.04.06 LIU G L, SONG Y Y, JIANG Y, et al. Effect of tension and compression deformation on adsorption properties between Al and carbon nanotubes with B(N) doping [J]. Journal of Shenyang University of Technology, 2016, 38(4): 391-396(in Chinese). doi: 10.7688/j.issn.1000-1646.2016.04.06
[37] HIZHNYI Y, NEDILKO S, BORYSIUK V, et al. Ab initio computational study of chromate molecular anion adsorption on the surfaces of pristine and B- or N-doped carbon nanotubes and graphene [J]. Nanoscale Research Letters, 2017, 12(1): 71. doi: 10.1186/s11671-017-1846-x [38] 杨忠华, 刘贵立, 曲迎东, 等. N掺杂碳纳米管环吸附Fe原子的第一性原理研究 [J]. 计算物理, 2016, 33(3): 374-378. doi: 10.3969/j.issn.1001-246X.2016.03.014 YANG Z H, LIU G L, QU Y D, et al. First principles study on adsorbing of Fe on N doping carbon nanotube rings [J]. Chinese Journal of Computational Physics, 2016, 33(3): 374-378(in Chinese). doi: 10.3969/j.issn.1001-246X.2016.03.014
[39] 姚洁. 硼/氮掺杂碳纳米管与石墨烯吸附性能的第一性原理研究[D]. 武汉: 华中科技大学, 2013. YAO J. First principles investigation of the adsorption on boron-or nitrogen-doped carbon nanotube and graphene[D]. Wuhan: Huazhong University of Science and Technology, 2013(in Chinese).
[40] ZHAO J J, PARK H, HAN J, et al. Electronic properties of carbon nanotubes with covalent sidewall functionalization [J]. The Journal of Physical Chemistry B, 2004, 108(14): 4227-4230. doi: 10.1021/jp036814u [41] CHEŁMECKA E, PASTERNY K, KUPKA T, et al. DFT studies of COOH tip-functionalized zigzag and armchair single wall carbon nanotubes [J]. Journal of Molecular Modeling, 2012, 18(5): 2241-2246. doi: 10.1007/s00894-011-1242-x [42] WANG C C, ZHOU G, LIU H T, et al. Chemical functionalization of carbon nanotubes by carboxyl groups on stone-wales defects: A density functional theory study [J]. The Journal of Physical Chemistry B, 2006, 110(21): 10266-10271. doi: 10.1021/jp060412f [43] SINGHA DEB A K, DWIVEDI V, DASGUPTA K, et al. Novel amidoamine functionalized multi-walled carbon nanotubes for removal of mercury(II) ions from wastewater: Combined experimental and density functional theoretical approach [J]. Chemical Engineering Journal, 2017, 313: 899-911. doi: 10.1016/j.cej.2016.10.126 [44] SINGHA DEB A K, DHUME N, DASGUPTA K, et al. Sulphur ligand functionalized carbon nanotubes for removal of mercury from waste water - experimental and density functional theoretical study [J]. Separation Science and Technology, 2019, 54(10): 1573-1587. doi: 10.1080/01496395.2018.1529044 [45] OYETADE O A, SKELTON A A, NYAMORI V O, et al. Experimental and DFT studies on the selective adsorption of Pb2+ and Zn2+ from aqueous solution by nitrogen-functionalized multiwalled carbon nanotubes [J]. Separation and Purification Technology, 2017, 188: 174-187. doi: 10.1016/j.seppur.2017.07.022 [46] HIZHNYI Y, NEDILKO S, BORYSIUK V, et al. Removal of oxoanions of MVI(MVI=Cr, Mo, W) metals by carbon nanostructures: Insights into mechanisms from DFT calculations [J]. International Journal of Quantum Chemistry, 2018, 118(20): e25715. doi: 10.1002/qua.25715 [47] LIU Y B, LIU F Q, DING N, et al. Boosting Cr(VI) detoxification and sequestration efficiency with carbon nanotube electrochemical filter functionalized with nanoscale polyaniline: Performance and mechanism [J]. Science of the Total Environment, 2019, 695: 133926. doi: 10.1016/j.scitotenv.2019.133926 [48] LIU Y B, LIU F Q, QI Z L, et al. Simultaneous oxidation and sorption of highly toxic Sb(III) using a dual-functional electroactive filter [J]. Environmental Pollution, 2019, 251: 72-80. doi: 10.1016/j.envpol.2019.04.116 [49] SAITO R, FUJITA M, DRESSELHAUS G, et al. Electronic structure of chiral graphene tubules [J]. Applied Physics Letters, 1992, 60(18): 2204-2206. doi: 10.1063/1.107080 [50] ZHANG J L, LI T, LI X Y, et al. A key role of inner-cation-π interaction in adsorption of Pb(II) on carbon nanotubes: Experimental and DFT studies [J]. Journal of Hazardous Materials, 2021, 412: 125187. doi: 10.1016/j.jhazmat.2021.125187 [51] ZHAO G K, ZHU H W. Cation-π interactions in graphene-containing systems for water treatment and beyond [J]. Advanced Materials (Deerfield Beach, Fla. ), 2020, 32(22): e1905756. doi: 10.1002/adma.201905756 [52] 张志森, 王琦, 陈尔余. 自由能计算方法及其在生物大分子体系中的适用性问题 [J]. 中国科学:化学, 2014, 44(6): 854-863. doi: 10.1360/N032014-00005 ZHANG Z S, WANG Q, CHEN E Y. Free energy calculation and its application in bio-complex system [J]. Scientia Sinica (Chimica), 2014, 44(6): 854-863(in Chinese). doi: 10.1360/N032014-00005
[53] KALINICHEV A G, KIRKPATRICK R J. Molecular dynamics simulation of cationic complexation with natural organic matter [J]. European Journal of Soil Science, 2007, 58(4): 909-917. doi: 10.1111/j.1365-2389.2007.00929.x [54] ALLEN M P. Molecular graphics and the computer simulation of liquid crystals [J]. Molecular Simulation, 1989, 2(4/5/6): 301-306. [55] 赵超锋, 金佳人, 霍英忠, 等. 氧化石墨烯吸附水体中酚类有机污染物的分子动力学模拟 [J]. 无机材料学报, 2020, 35(3): 277-285. doi: 10.15541/jim20190377 ZHAO C F, JIN J R, HUO Y Z, et al. Adsorption of phenolic organic pollutants on graphene oxide: Molecular dynamics study [J]. Journal of Inorganic Materials, 2020, 35(3): 277-285(in Chinese). doi: 10.15541/jim20190377
[56] SHANG J J, YANG Q S, YAN X H, et al. Ionic adsorption and desorption of CNT nanoropes [J]. Nanomaterials, 2016, 6(10): 177. doi: 10.3390/nano6100177 [57] DEZFOLI A R A, MEHRABIAN M A, HASHEMIPOUR H. Study of interaction energies in zinc ion adsorption on charged carbon nano-tubes using molecular dynamics simulation [J]. Journal of Computational and Theoretical Nanoscience, 2013, 10(10): 2411-2417. doi: 10.1166/jctn.2013.3223 [58] ANSARI DEZFOLI A R, MEHRABIAN M A, HASHEMIPOUR H. Comparative study of Zn(II) and Cd(II) ions adsorption on charged carbon nano tubes: Molecular dynamics approach [J]. Adsorption, 2013, 19(6): 1253-1261. doi: 10.1007/s10450-013-9567-7 [59] ANITHA K, NAMSANI S, SINGH J K. Removal of heavy metal ions using a functionalized single-walled carbon nanotube: A molecular dynamics study [J]. The Journal of Physical Chemistry A, 2015, 119(30): 8349-8358. doi: 10.1021/acs.jpca.5b03352 [60] SAHU P, SINGHA DEB A K, ALI S K M, et al. Tailoring of carbon nanotubes for the adsorption of heavy metal ions: Molecular dynamics and experimental investigations [J]. Molecular Systems Design & Engineering, 2018, 3(6): 917-929. [61] POORSARGOL M, RAZMARA Z, AMIRI M M. The role of hydroxyl and carboxyl functional groups in adsorption of copper by carbon nanotube and hybrid graphene-carbon nanotube: Insights from molecular dynamic simulation [J]. Adsorption, 2020, 26(3): 397-405. doi: 10.1007/s10450-020-00214-7 -



 下载:
下载: