-
纳米材料(如碳纳米材料、金属及氧化物纳米材料、纳米聚合物和量子点)因其尺寸小、比表面积大和表面活性高而具有表面效应、小尺寸效应、介电限域和量子效应等,已被广泛应用于医学、生物、交通、能源化工、环境保护、农业、计算机和电子电气等诸多领域。据统计,2017年全球纳米材料市场规模超过897亿美元[1]。随着生产和应用不断扩大,纳米颗粒越来越多地被排放到环境中。
土壤是环境中纳米颗粒最重要的汇[2]。已有研究报道土壤中纳米颗粒的暴露改变土壤微生物群落、抑制植物根芽伸长和种子萌发以及导致蚯蚓的回避行为[3-5]。纳米颗粒可能通过物理损伤生物体细胞、诱发氧化应激反应和释放有毒物质等途径妨碍生理活性,造成生物毒性。此外,纳米颗粒可能被植物吸收或被土壤动物摄入,在生物体内转运、分布和富集,产生难以预测的毒性[6-7]。因此,从纳米颗粒的生物有效性和毒性方面,全面了解纳米颗粒如何对土壤生物产生不利影响非常必要。
一旦纳米颗粒进入环境,将不可避免地与共存污染物相互作用,产生一系列生物效应[8]。例如,添加1%纳米氧化铁使土壤中总石油烃的微生物降解率提高了11.0%[9]。Cui等[10]报道了添加生物炭和单壁碳纳米管(SWCNTs)明显抑制了沉积物中菲的微生物可降解性,使其矿化率显著下降9.1%—20.1%,且SWCNTs的抑制作用强于生物炭。研究表明,土壤中多壁碳纳米管(MWCNTs)的暴露降低了菲和3-甲基菲在玉米幼苗中的富集,但促进了化合物从根向茎叶的传输[11]。Li等[12]发现,向土壤中添加0.5%的碳纳米管(CNTs)导致六溴环十二烷在蚯蚓(E. fetida)和深土栖类蚯蚓(M. guillelmi)体内的富集量分别下降67.3%和47.4%。这些结果表明,纳米颗粒显著影响共存有机污染物的生物有效性,进而影响污染物被微生物降解、被植物吸收积累和在动物体内富集,从而改变其毒性和对人类潜在的健康风险。因此,研究纳米颗粒对共存有机污染物在土壤中的环境行为和归趋的影响有利于更准确地评价纳米颗粒的生物效应。
综上所述,研究被排放进入土壤的人工纳米颗粒与不同生物体的相互作用对于科学评估其环境效应和生态风险意义重大。本文基于微生物、植物和土壤动物的3个层面,对人工纳米颗粒的生物有效性和毒性进行了讨论。从微生物降解、植物积累和动物富集方面,探讨了纳米颗粒对共存有机污染物生物有效性的影响。
-
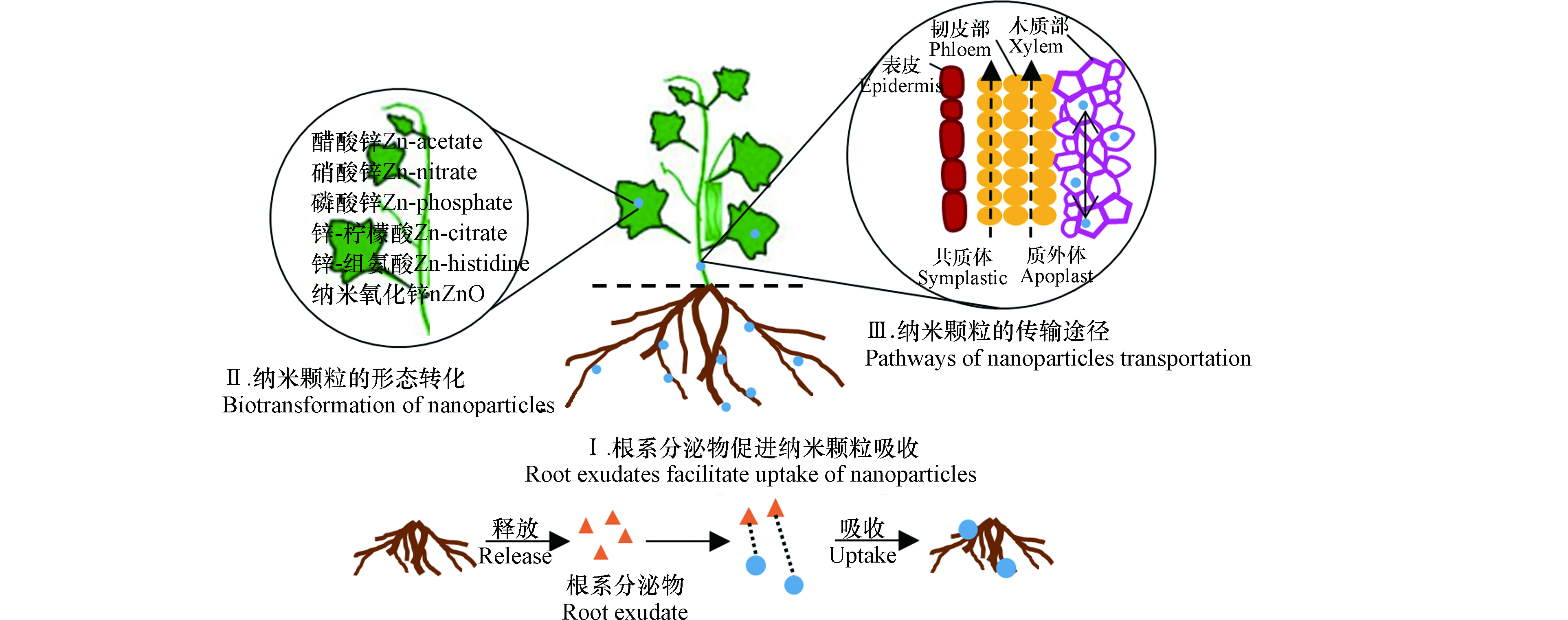
已有研究表明人工纳米颗粒能以颗粒形态被植物吸收且产生毒性效应。纳米颗粒有可能通过根/叶细胞的内吞作用、离子通道、水分子通道、与载体蛋白复合或者直接穿过细胞壁孔/膜,以及叶片表面的气孔和毛状体进入植物体[13-14]。Liu等[15]通过激光共聚焦显微镜观察到SWCNTs被吸收进入烟草(Nicotiana tobacum L.)BY-2细胞,且能携带异硫氰酸荧光素和DNA进入。利用14C放射性标记技术,Larue等[16-17]发现纳米TiO2和MWCNTs被小麦和油菜的根系吸收,并转移到叶片中,但并不会影响植物的发育和生理,比如不引起任何光合活性的改变,也不引起植物叶片的氧化胁迫。Kole等[18]发现富勒醇[C60(OH)20]在苦瓜叶柄、叶片、花和果实中的存在和积累,同样表明了碳纳米颗粒进入植物体的可能性。Miralles等[19]发现MWCNTs大部分吸附在苜蓿和小麦根系表面,仅在一个小麦根尖表皮中检测到MWCNTs,但并未进入内部组织。
植物对纳米颗粒的吸收受到纳米颗粒的粒径大小、化学组成和分散稳定性等理化性质以及受试植物的种类、大小等因素的影响[20-21]。纳米颗粒的暴露途径不同,植物对它们的吸收与毒性响应也有差别。Li等[22]比较了根系和叶面暴露后,大豆和水稻中对纳米Ag的吸收及植物毒性,发现无论是哪种暴露途径,均在叶片中检测到纳米Ag颗粒(36.0—48.9 nm),其粒径大于原始颗粒尺寸(17—18 nm)。但是,相同暴露浓度下,叶面暴露导致的纳米Ag在植物体内的积累量是根系暴露的17—200倍。并且,两种暴露途径引起的毒性效应有差异,根系暴露显著降低了植物生物量,而叶面暴露显著增加了叶片丙二醛(MDA)和H2O2含量。此外,植物根系分泌物也会影响纳米颗粒的生物有效性(图1)。例如,根尖分泌的小分子有机酸、氨基酸、多糖和黏液可以促进土壤中纳米颗粒的溶出和被植物吸收[23-24]。
植物吸收的纳米颗粒可能在其体内发生形态转化(图1)。例如,纳米ZnO被植物吸收后,在大豆幼苗体内发现醋酸锌、硝酸锌和Zn-柠檬水配合物等形态[25],在小麦体内观察到Zn-磷酸配合物[26],在豌豆体内发现Zn-柠檬酸、Zn-组氨酸等溶解态[27]。纳米银被根部吸收后生成AgCl、Ag2S、Ag-谷胱甘肽配合物和Ag-组氨酸配合物等[28]。
研究表明,纳米颗粒能够在植物体内传输,并转运到植物可食用部分,从而增大人体暴露的风险,该从根向茎叶的转运过程与韧皮部和木质部密切相关(图1)。Lin等[29]通过透射电镜观察到纳米C70被水稻根部吸收,并且以小团聚体形态出现在液泡和叶片细胞的细胞壁中。另外,在子代水稻茎叶中也检测到纳米C70,表明该纳米颗粒可被陆生植物代际传递。植物体内纳米颗粒的传输途径主要有两种(图1):①质外体途径,即纳米颗粒在细胞间隙间传输,转运进入木质部;②共质体途径,即纳米颗粒通过胞间连丝运输至木质部。Tripathi等[30]研究发现所有受试碳纳米颗粒都可能通过质外体途径传输,只有4.5 nm的碳量子点和SWCNTs(<10 nm)可以通过共质体途径传输,这是因为细胞间隙在50—60 nm范围内,细胞膜孔在10—150 nm的范围内,表明传输途径主要取决于纳米颗粒的尺寸大小。
-
研究表明,一些纳米颗粒(如纳米Au、Ag、CuO、ZnO、CNTs和C60等)能够以颗粒形态在土壤动物体内富集而致毒[31-33]。蚯蚓通过口腔摄入土壤颗粒,与土壤颗粒团聚或结合的纳米颗粒被消化吸收进入肠道,富集于脂肪组织中[34]。纳米Co颗粒在暴露7 d时被赤子爱胜蚓(Eisenia foetida)大量吸收,并且大部分在蚯蚓体内存留8周,只有不到20%被吸收的纳米Co会被排泄出来;运用闪烁技术和放射自显影技术,Oughton等[35]在赤子爱胜蚓的生精细胞、茧和血液中均检测到60Co。基于激光剥蚀电感耦合等离子体质谱和微-延展边X射线吸收精细结构技术,Unrine等[31]证实了纳米金以颗粒形态被赤子爱胜蚓吸收,在肠道上皮细胞以单个颗粒或者2—7个颗粒团聚方式存在。然而,Lapied等[36]将陆正蚓(Lumbricus terrestris)暴露于水中的纳米TiO2 7 d或者暴露于土壤和食物中0—100 mg·kg−1的纳米TiO2 2—8周后,未观测到明显死亡,而观测到蚯蚓的表皮、肠道上皮和嗜氯组织细胞凋亡频率显著升高,但是观测结果表明纳米TiO2并不能穿过肠上皮/促氯基质屏障进入蚯蚓体腔液,或者穿过表皮在蚯蚓肌肉中富集。不同研究中纳米颗粒的生物有效性有所差别,主要源于受试动物的生理特征、纳米颗粒的理化性质(如粒径大小、化学组成和分散稳定性)、暴露方式以及土壤条件等方面的差异[37-38]。值得注意的是,目前在纳米颗粒的生物吸收和生物毒性效应研究中,其暴露剂量远高于纳米颗粒的环境浓度。Li等[39]发现即使是在热点污染场地土壤中,MWCNTs在蚯蚓体内的富集系数仅为0.015 ± 0.004,表明该碳纳米颗粒不易被摄入。
-
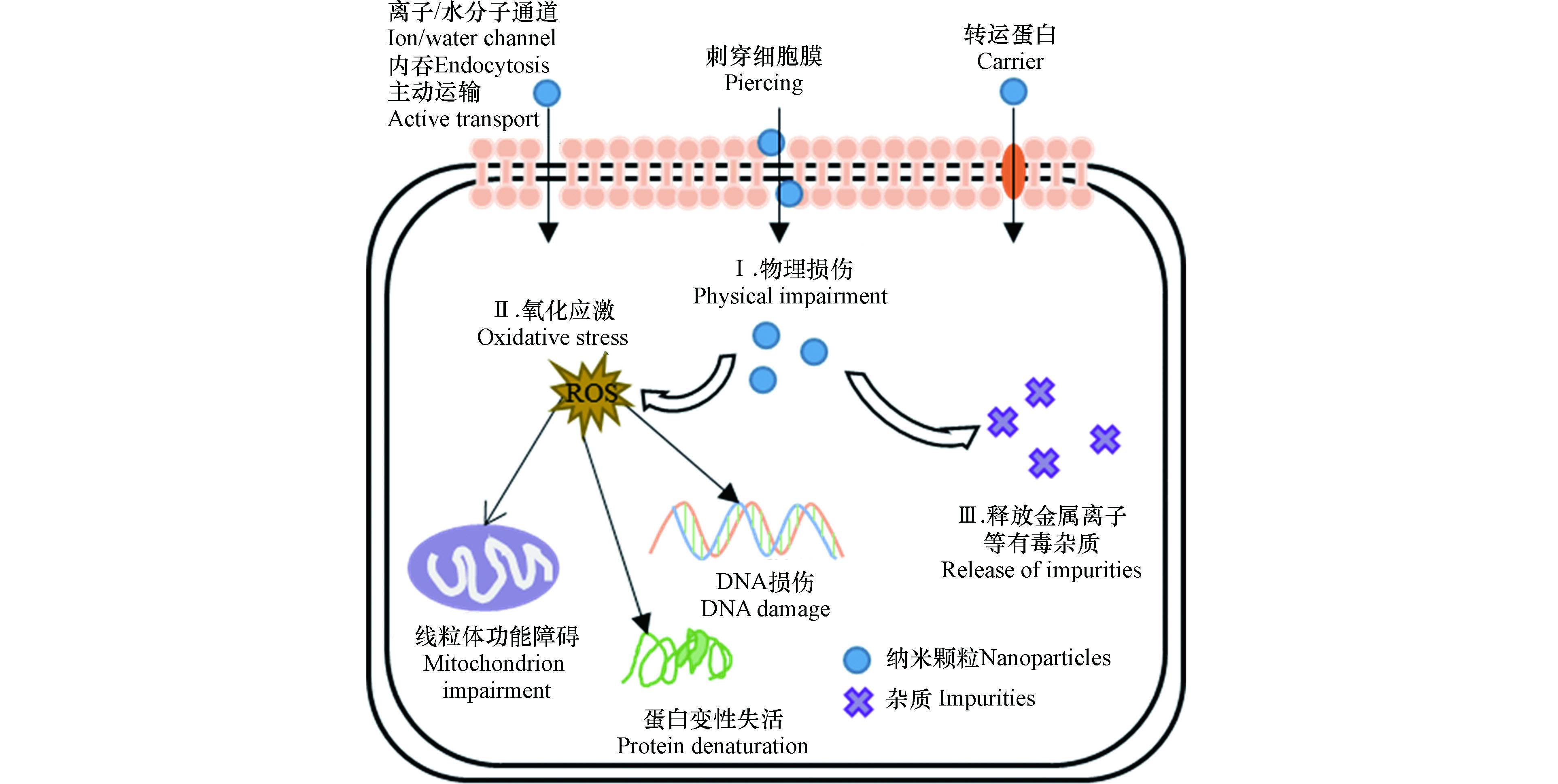
纳米颗粒的细胞毒性从附着于细胞表面开始,甚至能内化到细胞内,从而破坏细胞的结构和功能(图2)。当纳米颗粒的粒径小于10 nm时,可以直接通过细胞膜上的离子通道或者水分子通道进入细胞内;当纳米颗粒的粒径大于10 nm时,可以通过主动运输、吞噬作用、转运蛋白或者刺穿细胞膜/壁等方式进入细胞内[40-41]。纳米颗粒的主要致毒机制归纳为以下几个方面。
-
纳米颗粒物理附着在细胞表面,直接刺穿细胞膜或细胞壁,破坏细胞膜骨架,导致胞内物质渗漏。例如,由于碳纳米管的柱体结构和高纵横比,其锐利边缘容易刺穿细胞膜导致细胞死亡。Jiang等[42]提出带负电的MWCNTs粘附并被吞噬进入含有带正电脂质的细胞膜,说明纳米颗粒与细胞膜的相互作用由静电作用介导;然而,带负电的细胞膜脂质双层同样被带负电的MWCNTs损伤,表现在膜中磷脂被破坏流出,表明细胞膜损伤也可以发生在静电排斥条件下。C70对拟南芥根系的生长产生抑制作用,主要是由于C70进入拟南芥根系细胞内部导致细胞分裂紊乱、线粒体失活、微管组织被破坏以及生长激素的分泌受到干扰[43]。
-
纳米颗粒诱导细胞内产生过多活性氧自由基(ROS),如·OH、O2−、H2O2,使细胞内部产生氧化应激反应,造成氧化损伤,导致膜脂质氧化、蛋白质变性、线粒体损伤和DNA受损,干扰信号传递和基因转录,进而干扰细胞正常的代谢,最终导致细胞死亡。Begum和Fugetsu[44]研究发现0—80 mg·L−1石墨烯引起拟南芥T87细胞内ROS的生成,干扰线粒体的电子传递和能量转化,造成线粒体功能紊乱,并且造成严重的细胞核碎裂和细胞膜损伤,引发细胞凋亡。Patlolla等[45]发现纳米Ag诱导蚕豆细胞内ROS积累,损伤DNA,并阻碍受损DNA的修复,造成染色体畸变、基因组不稳定和分裂指数下降等毒害效应。
-
纳米颗粒释放金属离子、金属催化剂残留物和可溶性酸等有害杂质而致毒[46]。例如,金属及氧化物纳米颗粒(如纳米金、银、铜、氧化锌和氧化铜等)溶解释放相应的金属离子,导致细胞生长和繁殖毒性[47]。Karlsson等[48]发现纳米CuO的毒性大于碳纳米管,因为纳米CuO溶解释放的Cu2+增强其细胞毒性。
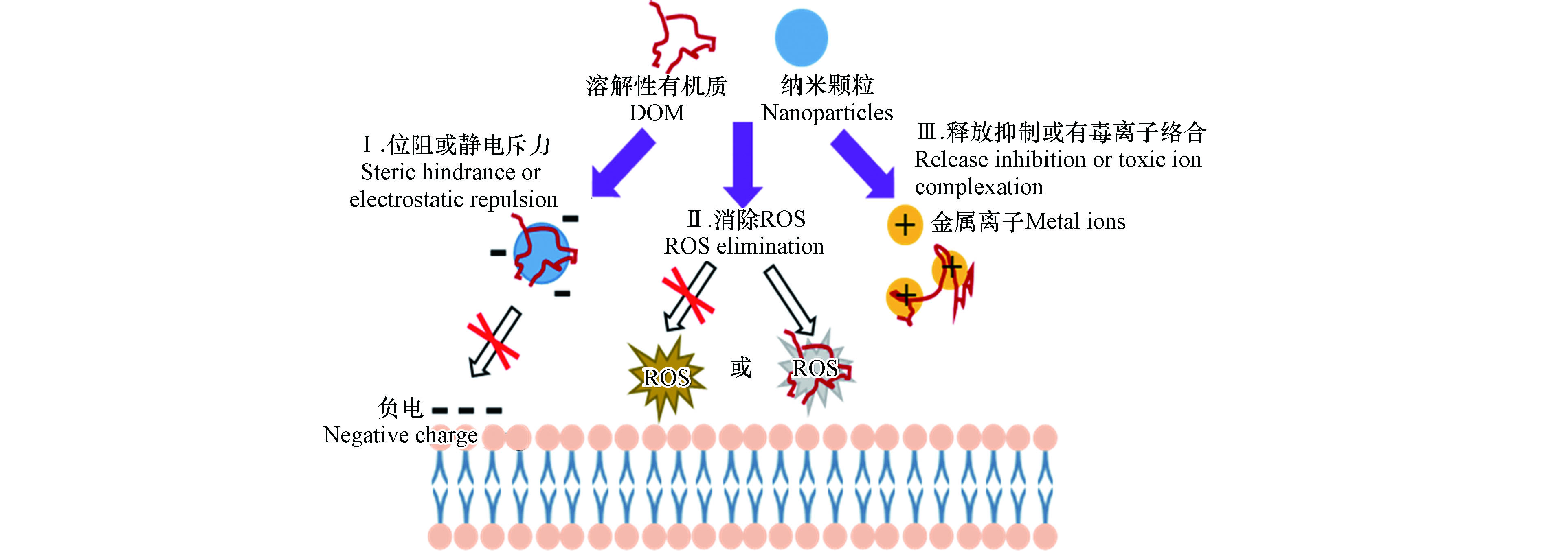
环境中广泛存在的腐殖酸、微生物胞外聚合物等天然有机质以及表面活性剂等人工有机物质可以减轻纳米颗粒的细胞毒性[49]。主要通过以下可能的机制(图3):①与纳米颗粒结合,通过静电或空间排斥,抑制纳米颗粒与细胞膜相互作用产生的直接接触损伤;②限制ROS的产生或者作为抗氧化剂与纳米颗粒诱导产生的ROS反应,抑制间接氧化损伤;③通过抑制金属离子的溶出释放或者与金属离子形成络合物,减弱溶出金属离子的毒性作用[50]。然而,有研究提出溶解性有机质的存在会加强纳米颗粒的细胞毒性。例如,Bai等[51]发现0.5 mg·mL−1非离子(TX100)和阳离子(CTAB)混合表面活性剂增强MWCNTs对金黄色葡萄球菌的杀伤性。0.125—1 mg·mL−1阴离子表面活性剂(AOT)修饰化MWCNTs增加变形链球菌的细胞裂解和死亡[52]。
-
微生物是主要的初级生产者和分解者,促进生态系统中物质和能量的循环。在纳米材料的生态毒理学研究中,对微生物的毒性效应研究较多,关注细胞和群落水平上的微生物响应(图4)。研究表明,纳米颗粒的种类和性质(包括颗粒尺寸、官能团构成、电荷情况和分散性)是影响其微生物毒性或抗菌性的关键因素[53]。例如,Liu等[54]研究发现石墨烯比氧化石墨烯的抑菌性更强,在40 mg·L−1石墨烯和氧化石墨烯中暴露2 h,革兰氏阴性大肠杆菌(E. coli)的存活率分别下降69.3%和47.4%。羟基和羧基修饰化SWCNTs在50 mg·L−1时显著抑制细菌生长,而MWCNTs在500 mg·L−1时仍未表现出明显的抑菌作用[55]。铁纳米材料对大肠杆菌的生长抑制作用与其化合价有关。纳米零价铁在较低浓度(70 mg·L−1)时就表现出显著的抑菌作用,抑菌率大于75%;纳米磁铁矿(Fe3O4)在700 mg·L−1时抑菌率达到85%,然而纳米磁赤铁矿(Fe2O3)在0—700 mg·L−1浓度范围内未表现出明显的抑菌性[56]。
纳米颗粒对土壤微生物群落的影响主要表现在微生物的生物量、群落的组成和结构以及胞外酶活性的改变(图4)[57]。Zhang等[58]报道了在300 mg·kg−1和3000 mg·kg−1MWCNTs的暴露下,土壤中细菌、真菌和降解功能基因nidA持有菌的生物量分别下降47.8%—60.7%、31.4%—71.6%和25.7%—59.9%。同样,Ren等[59]的研究显示石墨烯降低了土壤中一些与氮的生物地球化学循环相关的细菌种群(如硝化螺菌属和浮霉状菌属)的丰度,相反提高了节细菌属、地杆菌属和芽孢杆菌等与有机物降解相关的细菌种群的丰度。暴露于500—1000 mg·kg−1氧化石墨烯和300—1000 mg·kg−1SWCNTs 21 d后,土壤酶活性分别下降15%—50%[60]和8%—45%[61]。
Kumar等[62]研究了纳米银、纳米铜和纳米硅对北极土壤微生物群落的影响,发现纳米银抑制了土壤中大部分细菌的生长,尤其是对某些固氮细菌有显著抑制作用,而纳米铜和纳米硅对微生物群落的影响不大。研究发现,纳米TiO2和纳米ZnO均降低了土壤微生物的生物量,减少了土壤细菌群落的多样性,而相同剂量下,纳米ZnO的影响作用比纳米TiO2更加显著[63]。因此,纳米颗粒对土壤微生物群落的影响与其种类有关,受到不同纳米颗粒的物理结构、表面组成和颗粒尺寸的影响。土壤微生物群落对纳米颗粒的不同响应还源于纳米颗粒的暴露剂量和暴露时间以及土壤性质的不同。一般来说,纳米颗粒的浓度越高,对土壤微生物的影响作用更强。例如,在2000 mg·kg−1 MWCNTs下暴露61 d,土壤微生物的生物量无显著变化[64],但在5000 mg·kg−1剂量下仅暴露20 d,土壤微生物的生物量和酶活性受到显著抑制[65]。Wu等[66]报道称MWCNTs暴露下,土壤细菌群落的多样性和一些主要种群的相对丰度在短期内随着暴露时间的增加而不断变化,但许多种群在56 d的长期暴露后恢复到初始丰度。
-
植物是生态系统的生产者,也是主要的能量来源。大多数研究表明,纳米颗粒影响植物的生长发育、生理生化过程和基因,表现在对种子萌发、根和芽的生长、生物量和产量产生不利影响(图4)。Begum和Fugetsu[67]研究发现,暴露于125—1000 mg·L−1的碳纳米管,红菠菜(Amaranthus tricolor L)的根芽伸长、生物量、植株高度、叶片数目和面积均受到抑制。有研究提出,纳米颗粒通过堵塞细胞壁上微细孔隙和影响胞间连丝等物理作用影响植物体内的营养运输,从而抑制植物生长[68]。Geisler-Lee等[69]研究发现,纳米Ag进入拟南芥的根尖和根毛中,并在根尖根冠及小柱细胞中聚集,导致小柱初始细胞受损,影响细胞分裂,并诱导侧根根冠细胞脱落,从而抑制根毛生长。Lin和Xing[70]考察了5种纳米颗粒(纳米Al、Al2O3、Zn、ZnO和MWCNTs)对萝卜、生菜、油菜、黄瓜、玉米和黑麦草等6种植物生长的影响,发现暴露浓度高达2000 mg·L−1时,纳米Zn仅抑制黑麦草的种子萌发,纳米ZnO仅抑制玉米的种子萌发,这两种纳米颗粒抑制所有受试植物的根伸长;相反,其它纳米颗粒并未对种子萌发、根的生长和植物发芽产生显著抑制作用,表明不同纳米颗粒对不同植物表现出不同毒性[23]。有研究表明,纳米TiO2抑制了玉米根系的水分运输,从而抑制玉米叶片的蒸腾作用;然而,能够通过促进光吸收和电子传递速率,促进菠菜的光合作用,并提高其固氮能力[71]。Khodakovskaya等[72]甚至发现MWCNTs可以刺穿种皮,促进种子对水分的吸收,进而促进种子萌发和发芽。Wang等[73]同样发现氧化MWCNTs通过提高脱氢酶活性进而改变水通道蛋白,从而促进小麦幼苗(Triticum aestivum)的根伸长1.4倍。有研究报道富勒烯、C60和碳纳米管可以增加植物保水能力,提高生物量和果实产量达118%[74]。Kole等[18]同样发现碳纳米颗粒富勒醇[C60(OH)20]处理使得苦瓜(Momordica charantia)的生物量提高54%,水分含量提高24%,果实长度、数目和重量分别增加20%、59%和70%,果实产量提高128%;并且使得苦瓜中葫芦素B和番茄红素两种抗癌植物药的含量分别提高了74%和82%,苦瓜素和胰岛素两种抗糖尿病植物药的含量分别提高了20%和91%。因此,有学者提出纳米材料在促进农业生产方面具有巨大的应用前景。
纳米颗粒对植物的毒害作用引起细胞器损伤。例如,在氧化石墨烯的暴露下,植物出现质壁分离、细胞分裂受限、核染色质聚集和叶绿体类囊体丢失等一系列症状[75]。并且,氧化石墨烯抑制植物细胞内糖、氨基酸和肌醇的代谢,提高不饱和脂肪酸的代谢,这些变化与该碳纳米材料的细胞毒性相关。纳米颗粒的植物毒性还表现在改变植物根、叶形态上。例如,红菠菜在碳纳米管的暴露下,叶片呈黄色、卷曲萎蔫,细胞拉伸呈不规则化,表皮肿胀,气孔闭合[67]。纳米颗粒对植物细胞、组织和个体水平上以及形态上的影响作用除了与受试植物的种类密切相关,还随着纳米颗粒本身性质(如粒径大小、表面基团和比表面积等)的不同而存在差异[23,76-77]。例如,经表面官能团修饰后的碳纳米颗粒的植物毒性显著降低[74]。纳米颗粒的比表面积越大,越能促进活性氧产生,其植物毒性越强[78]。
-
土壤动物作为消费者在生态系统中发挥重要作用。典型代表蚯蚓是陆生生态系统的重要组成部分,能够改善土壤结构,富集污染物,对氮磷循环产生有利影响。纳米颗粒对土壤动物的有害影响表现在细胞、组织、个体甚至是种群水平上,具体引发细胞坏死凋亡、损伤组织器官、抑制生长发育和降低繁殖能力,甚至造成死亡(图4)。土壤中纳米ZnO和TiO2的暴露浓度为0.1—1000 mg·kg−1时,会降低陆正蚓(Lumbricus terrestris)体内抗氧化酶和纤溶酶活性,损害DNA和肠道细胞的线粒体;纳米TiO2还会引起蚯蚓表皮和肠上皮细胞的凋亡[36]。MWCNTs诱导的氧化应激引起细胞膜脂质过氧化,导致蚯蚓体内MDA含量升高[79];还会引起消化腺内过氧化氢酶活度减低[80]。Cailisi等[81]研究发现MWCNTs暴露引起蚯蚓免疫粒细胞溶酶体室增大,溶酶体膜失稳,金属硫蛋白浓度改变。蚯蚓(Lumbricus rubellus)暴露于15 mg·kg−1和154 mg·kg−1的C60,其HSP70基因的表达受到抑制,造成角质层损伤和表皮、肌肉的组织病变[82]。但蚯蚓会通过组织修复、下调免疫相关基因表达和改变代谢物等来适应纳米颗粒(如C60)的毒性[83],因而消耗更多的能量,导致生长速率减慢,体重减轻[81]。当线虫(Caenorhabditis elegans)暴露于不同浓度的纳米ZnO、Al2O3和TiO2 5 d,其体长明显缩短,虫卵数量和子代数均显著下降[84]。van der Ploeg等[85]报道了C60对蚯蚓个体和种群水平上的损害,发现暴露于C60 28 d后,粉正蚓(Lumbricus rubellus)亲代产茧量下降,子代生长率降低且死亡率升高,种群呈现年轻化趋势。
由于纳米颗粒自身性质、土壤类型、受试生物和暴露方式等方面的差异,纳米颗粒对土壤动物的影响作用不同。纳米颗粒的尺寸越小,毒性越大。纳米级的TiO2会使蚯蚓产生回避行为,而微米级TiO2不表现此效应[86]。粒径尺寸越小、亲水性越强的石墨烯使蚯蚓产生更显著的回避行为[87]。曹圣来等[88]的研究表明,水稻土中纳米CeO2和纳米ZnO对赤子爱胜蚓(Eisenia foetida)产生较强的毒性作用,诱导抗氧化系统产生显著性差异,从而引起MDA和蛋白质羰基含量的增加;而在红壤中这些纳米颗粒对赤子爱胜蚓的毒性效应相对较小。
-
纳米颗粒释放进入土壤对共存有机污染物的生物有效性和毒性的影响反映两者的复合环境效应。土壤中的纳米颗粒如何影响有机污染物的生物降解和生物富集如表1所示。
-
一般认为只有自由溶解态的有机化合物可以被微生物直接利用,而被土壤颗粒或纳米颗粒吸附的化合物必须首先解吸至液相(如土壤孔隙水)才能被微生物降解[89]。具有不同物理结构和官能团修饰的纳米颗粒与不同种类和性质有机污染物之间界面作用的强度、容量和主导机制以及关键影响因素均不同[90],致使有机污染物在纳米颗粒上的吸附/解吸行为不同,进而不同程度地影响污染物的微生物有效性。例如,碳纳米管对多环芳烃(PAHs)和阿特拉津具有很强的吸附,但该吸附是可逆的[91],这可能造成CNTs的强吸附性并不会对这些化合物的微生物有效性和降解产生负面影响。Zhang等[92]的研究表明,当CNTs浓度不超过25 mg·L−1,吸附在原始和氧化MWCNTs上的阿特拉津被Arthrobacter sp.完全降解。Shrestha等[93]观察到添加MWCNTs提高了有机质含量丰富的砂质粘壤土中PAHs的微生物有效性和降解效率,添加量为50 mg·kg−1和100 mg·kg−1时,PAHs残留浓度分别降低9.4%和21%。
大多数研究表明,纳米颗粒对有机污染物的吸附行为会降低污染物的微生物有效性。Towell等[94]观察到向土壤中添加富勒烯和CNTs降低了PAHs的有效态浓度,即其β-环糊精可提取量降低,因而PAHs的微生物矿化率降低。
Xia等[95]研究发现吸附在MWCNTs上菲的矿化率与其Tenax快、慢速解吸组分之和显著正相关,且随着MWCNTs表面积、微孔和介孔体积的增大而显著降低。Xia等[96]的研究表明经Agrobacterium矿化21天后,沉积物中菲的残留浓度随着MWCNTs微孔体积的增大而显著增加,并指出吸附在MWCNTs外表面和大孔中的菲分子可快速解吸至液相,容易被微生物利用,吸附在介孔和微孔中的可缓慢解吸,以较慢速率被微生物利用,而扩散进入颗粒内部或阻滞在微孔内的是难以解吸、不可降解的。Oyelami和Semple[97]发现向土壤中添加0.01%、0.1%、1%MWCNTs逐渐增强菲的吸附,减少有效态菲,从而其矿化速率和矿化量依序降低;但添加不同浓度的C60并不会影响土壤土著微生物对菲的代谢。该现象是由碳纳米颗粒不同的几何构型引起的,球形的C60易形成致密紧实的团聚体,菲优先吸附在其外表面,相对于MWCNTs管腔内吸附的菲生物可利用度高,导致添加C60土壤中菲的矿化强于添加MWCNTs的土壤。
然而最近的一些研究表明降解菌可以直接利用吸附在固体颗粒上的有机化合物[121-122]。Xia等[96]发现经5%MWCNTs改良后,沉积物中菲的XAD-2辅助解吸量和矿化量相当,然而在前期快速矿化阶段,菲的矿化速率显著高于其解吸速率。目前解释类似实验现象的作用机制包括(1)实验观测自由溶解态浓度(Cfree)或解吸动力学均是在无微生物条件下进行的,而微生物可能通过分泌生物表面活性剂类物质促进有机化合物的解吸,从而使更多的化合物被降解[123-124];(2)微生物可以物理附着于固体颗粒表面或通过形成生物膜,直接获取和高效利用吸附在颗粒上的有机化合物[125],该作用机制与纳米颗粒本身性质及降解菌种类均有关。Zhang等[98]比较吸附在活性炭、纳米石墨片、SWCNTs以及不同外径的原始、羟基和羧基修饰MWCNTs上菲的矿化,发现菲的Cfree相当时,亲水极性官能团修饰MWCNTs上菲的矿化率显著高于其它碳纳米材料,作者认为降解菌通过细胞附着直接利用吸附态菲,并通过环境扫描电镜清楚地观察到修饰化MWCNTs上附着的Mycobacterium。Shrestha等[93]报道称土壤中MWCNTs对PAHs的吸附降低其对低耐受菌种的有效性和毒性,高耐受菌种能够通过在PAHs-MWCNTs复合物表面增殖获取和利用吸附态PAHs,造成MWCNTs处理组与对照组的PAHs降解无显著差异。
-
土壤中1670 mg·kg−1的富勒烯C60对滴滴涕代谢物二氯二苯二氯乙烯(p,p’-DDE)在南瓜(Cucurbita pepo)根部和茎叶中的富集无显著影响[99]。但是,土壤中50、500、3000 mg·kg−1的单壁、多壁碳纳米管显著抑制菲被玉米幼苗根部吸收和富集,且随着添加剂量的增加,抑制作用增强,这是因为碳纳米颗粒对有机污染物的吸附固定作用降低了污染物的植物可利用性[11]。同理,Shen等[100]研究发现随着MWCNTs外径(<8 nm、20—30 nm、>50 nm)的增大,其对黄瓜幼苗中芘的富集的抑制作用减弱。Zhang等[101]也发现土壤中50—3000 mg·kg−1的MWCNTs显著降低芘和1-甲基芘在玉米幼苗根部和茎叶中的富集浓度,而表面活性剂十二烷基苯磺酸钠的存在削弱碳纳米管对植物富集OPs的抑制作用。同样,有研究发现由于纳米颗粒的强吸附性,土壤中500、1000、5000 mg·kg−1的MWCNTs显著降低了西葫芦、玉米、西红柿和大豆等4种农作物对氯丹和滴滴涕及其代谢物(DDX)的富集量达21%—80%[102]。在同一研究中,C60也显著抑制了玉米和西红柿对DDX的富集;然而却提高了西红柿和大豆对氯丹的富集量达34.9%。Ma和Wang[103]也发现添加2 mg·L−1和15 mg·L−1的C60纳米颗粒使得植物(Populus deltoides)对三氯乙烯的吸收分别增加-26%和82%。de la Torre-Roche 等[104]发现nC60的存在使得西葫芦、大豆和西红柿整个植株及根部对p,p’-DDE的累积量增加了30%—65%。土壤中的有机污染物与植物根系密切接触,尽管纳米颗粒的强吸附性阻碍有机污染物向根际迁移[106],或者其毒性减缓植物生长发育,降低植物的富集能力[107],从而显著降低有机污染物在植物体内的富集水平。但也有可能增加有机污染物在植物体内的积累,相关促进机制主要包括(1)纳米颗粒吸附并携带有机污染物进入植物[109-111];(2)纳米颗粒刺穿植物细胞,使有机污染更易进入植物细胞;(3)纳米颗粒促进植物生长发育,从而增加植物对有机污染物的富集能力。
-
关于纳米颗粒对土壤动物体内有机污染物富集的影响研究相对比较匮乏。Petersen等[112]研究了单壁和多壁碳纳米管对芘在赤子爱胜蚓(Eisenia fetida)体内富集和清除的影响,结果表明当土壤中CNTs的添加浓度达到0.3%时,芘的富集系数显著降低,清除速率显著加快。Zhang等[113]的研究发现碳纳米材料显著改变了典型食土蚯蚓(Metaphire guillelmi)体内菲的富集速率和程度,取决于碳纳米材料的种类和添加剂量。当土壤中MWCNTs的添加量为300 mg·kg−1时,增强被土壤吸附或锁定菲的释放和迁移,导致菲在蚯蚓体内的富集量提高30%—40%;而当添加量高达3000 mg·kg−1时,由于菲的吸附增强,蚯蚓体内菲的富集量降低23.6%—27.7%。蚯蚓对土壤中菲的最大富集量遵循C60>MWCNTs(>50 nm)>MWCNTs(<8 nm)的大小顺序,与这些颗粒对菲吸附能力的大小关系相反[113]。研究表明,向土壤中添加具有强吸附性的碳纳米颗粒(C60和MWCNTs)对菲和3-甲基菲在食土蚯蚓(Metaphire guillelmi)体内的富集无显著影响或者产生抑制作用,而使蚯蚓体内3,6-二甲基菲的富集量提高6.1%—25.9%,说明有机污染物的类型和性质也是影响其与纳米颗粒相互作用及吸收富集的重要因素[114]。Hu等[115]认为,吸附在CNTs上的有机污染物更容易进入动物体内,报道称MWCNTs能够作为载体,携带壬基酚刺穿肠道细胞而释放,提高壬基酚对蚯蚓的生物有效性。
纳米颗粒对有机污染物在土壤动物(如蚯蚓)体内富集的影响作用与蚯蚓吸收有机污染物的途径有关。蚯蚓从土壤孔隙水中通过皮肤被动吸收有机污染物,则富集受到纳米颗粒的影响作用较大;而通过直接摄取土壤颗粒,从胃肠道吸收,富集于脂肪中,该过程相对较少受到纳米颗粒的影响[12]。
-
尽管实际土壤环境中人工纳米颗粒的含量很低,现有研究往往都是高剂量暴露实验。但纳米颗粒的生物毒性毋庸置疑,其致毒机制比较清楚,对土壤生物造成细胞、组织、个体甚至群落层面的不利影响。
纳米颗粒对共存污染物生物有效性的影响根据生物体对有机污染物的摄取途径不同而有所不同。对于大多数主要从土壤孔隙水中吸收有机污染物的物种来说,土壤中的纳米颗粒会降低孔隙水中有机污染物的自由溶解态组分,从而降低污染物对生物的可利用性。然而,有机污染物对一些物种的暴露途径不是完全由孔隙水决定。比如某些微生物除了利用自由溶解态,可以通过物理附着直接获取和利用吸附态的有机污染物。典型土壤动物蚯蚓可以直接摄入土壤颗粒从肠道吸收有机污染物。这种情况下,纳米颗粒对有机污染物生物有效性(降解或富集)的影响作用不确定。目前关于纳米颗粒对生物体富集有机污染物的影响研究相对有限,且随着生物种类、有机污染物和纳米颗粒的类型以及浓度的不同,影响结果往往有所不同。例如,富勒烯C60使得西葫芦植株地上部分p,p’-DDE的富集量增加了29%,大豆地上部的p,p’-DDE减少了48%,而西红柿地上部的p,p’-DDE含量不受影响[104]。
关于土壤中纳米颗粒的生物效应研究取得了诸多进展,但仍有很多亟待解决的关键问题。
(1)由于缺乏工具和手段来定位和量化水、沉积物、土壤和生物体中痕量的人工纳米颗粒,有关纳米颗粒的环境影响研究受到了阻碍。尤其是在土壤这样非均相的复杂体系中,有大量的有机质、黏土矿物和阴阳离子等,增加了评价纳米颗粒毒性效应的难度。如何准确从土壤中提取、定量纳米颗粒并鉴定其在土壤中的形态是研究的重点方向之一。
(2)进入土壤的纳米颗粒与土壤有机质和矿物等相互作用,改变其表面形态、团聚性和化学活性等,影响其在土壤中的吸附、迁移等环境行为及各种生物效应。很多关于纳米颗粒的植物毒性及有效性的研究是在水培条件下进行,相应的研究成果外推到实际土壤存在差异和困难。进一步的研究应关注纳米颗粒在实际土壤中理化性质的变化对其生物毒性和生态风险的影响。
(3)关于微生物、植物和土壤动物与纳米颗粒的相互作用研究仍处于起步阶段。对于纳米颗粒能否进入生物体尚未取得统一结论,纳米颗粒在生物体内吸收、富集的具体过程和途径尚未完全清楚,纳米-生物界面上相互作用的分子机制仍存在大量知识空白,值得深入研究。这涉及到发展色谱、光谱和显微技术检测和定量生物样品中的纳米颗粒,尤其是在纳米颗粒的提取、样品人工处理、如何有效区分原始和转化态纳米颗粒方面方法的更新。
(4)关于纳米颗粒的生态风险研究越来越多,但关于纳米颗粒和有机污染物的复合毒性效应研究相对有限[126]。两者共存产生协同还是拮抗的毒性作用及相关分子机理亟待进一步研究。
(5)纳米颗粒具有高反应活性,在水土污染控制方面具有巨大的应用价值和潜力。例如纳米Fe3O4诱导与三氯吡啶醇(3, 5, 6-trichloro-2-pyridino,TCP)脱氯降解相关的细菌丰度显著增加,促使TCP的厌氧生物降解率提高15.71%[127]。如何开发低成本、高效率但低毒性的纳米修复材料也将是研究重点之一。
土壤中纳米颗粒的生物效应研究进展
Research progress on the biological effects of nanoparticles in soil
-
摘要: 纳米材料广泛应用于诸多行业,不可避免地导致大量纳米颗粒被排放到环境中。已有许多研究评估了纳米颗粒的生态环境效应和人体健康风险。然而,土壤中纳米颗粒对生物潜在的生态危害和风险仍不明确。重要的是,土壤生物通常同时暴露于纳米颗粒和其他污染物,如重金属和有机污染物。现有文献对真实土壤环境中纳米颗粒与共存有机污染物之间复杂的相互作用及相关生物效应的研究非常有限。本文综述了土壤中纳米颗粒的生物有效性和毒性,特别是纳米颗粒与微生物、植物和蚯蚓等不同生物的相互作用。此外,从不同生物响应水平上探讨了纳米颗粒释放对共存有机污染物生物有效性的影响。最后总结了目前纳米颗粒的生物效应与毒性研究中的不足,后续研究应重点关注纳米颗粒与污染物共暴露的复合毒性效应。Abstract: Widespread applications in various industries will inevitably result in the release of a substantial amount of nanoparticles (NPs) into the environment. Much research has been conducted to assess their health and environmental impacts. However, the potential ecological damages and risks of NPs for soil biota remain largely unclear. Importantly, soil organisms are generally co-exposed to both nanoparticles and additional contaminants, such as heavy metal and organic pollutants (OPs). The existing literature on the complex interactions of nanoparticles with co-existing OPs in real soil environment and the associated biological effects is rather limited. This paper presents an overview on the bioavailability and toxicity of NPs in the soil, and in particular, on their interactions with different organisms, such as microorganisms, plants and earthworms. Moreover, the effects of NPs release on the bioavailability of coexisting OPs to soil biota are also discussed at different level. The deficiencies of existing research on evaluating the biological effects and toxicity of NPs as well as the needs for future research on the joint toxicity of NPs-contaminant co-exposure are summarized.
-
Key words:
- nanoparticles /
- biotoxicity /
- organic pollutants /
- bioavailability /
- soil
-

-
表 1 纳米颗粒对有机污染物生物有效性的影响
Table 1. Mechanisms and possible effects of nanoparticles on the bioavailability of organic pollutants
纳米颗粒
Nanoparticles暴露 Exposure 污染物
Pollutants生物种类
Organism species生物降解/富集
Biodegradation/
Bioaccumulation机理
Mechanisms参考文献
References浓度
Concentration时间
TimeMWCNTs
O-MWCNTs5、25、
100 mg·L−124 h 阿特拉津 节杆菌属
(Arthrobacter sp.)25 mg·L−1 CNTs提高降解率20%
100 mg·L−1 CNTs降低降解率50%促进作用是CNTs促进细菌生长和降解基因的超表达;抑制作用归因于CNTs的毒性 [92] MWCNTs 25、50、
100 mg·kg−149 d PAHs(菲+芘)100 mg·kg−1 苜蓿根际微生物群落 有机质含量低沙壤土中无显著影响;有机质含量高砂质黏壤土中50 mg·kg−1和100 mg·kg−1CNTs提高降解率9.34%和21% CNTs对PAHs的吸附降低其对低耐受菌种的有效性和毒性;高耐受菌种获取和利用CNTs上吸附态PAHs [93] 富勒烯烟灰
SWCNTs
MWCNTs0.05、0.1、0.5% 14 d 14C-菲
14C-苯并[a]芘50 mg·kg−1假单胞菌属(Pseudomonad sp.) 降解率下降14.4%—80.2% 吸附降低生物有效态(β-环糊精可提取量) [94] MWCNTs 1000 mg·L−1 35 d 14C-菲578 mg·kg−1 嗜甲基菌(Methylophilus methylotrophus sp.) 降解率为2.38%—31.47% 吸附降低生物有效态(Tenax辅助可解吸量) [95] MWCNTs 5% 21 d 14C-菲 农杆菌属
(Agrobacterium sp.)老化21 d和28 d后,21 d降解率分别为90.9%和54.2% 吸附降低生物有效态(XAD-2辅助可解吸量)+降解菌直接获取利用吸附态污染物 [96] 富勒烯烟灰
MWCNTs C600.01、0.1、1% 14 d 14C-菲50 mg·kg−1 土著微生物 降解率下降43.7%—92.3%;
C60无显著影响降低生物有效态,但对微生物活性无
显著影响[97] SWCNTs
OH-/COOH-/MWCNTs50000 mg·L−1 30 d 14C-菲
14C-硝基苯
22 mg·kg−1分枝杆菌
(Mycobacterium vanbaalenii PYR-1)降解率下降31.6%—99% 降低生物有效态(PDMS测定Cfree)+降解菌物理附着于颗粒表面利用吸附态污染物 [98] C60 1670 mg·kg−1 21 d 二氯二苯二氯乙烯p,p’-DDE 南瓜(Cucurbita pepo) 无显著影响 DDE残留物的高风化性(>40 a)和新添加的C60限制了污染物-纳米颗粒的相互作用 [99] MWCNTs 1、10、100、
1000 mg·L−17 d 50 μg·L−1芘 黄瓜(Cucumis sativus L.) 根部富集量降低26.35%—99.23% 纳米颗粒的植物毒性削弱植物对污染物的吸收;吸附降低生物有效态 [100] MWCNTs 50、3000 mg·kg−1 28 d 1-甲基芘 玉米(Zea mays) 富集量降低21.7%—77.4%(芘)和68.6%—100%(1-甲基芘) 纳米颗粒对污染物的强吸附降低生物有效态 [101] MWCNTs 500、1000、
5000 mg·kg−128 d 氯丹
滴滴涕及代谢物DDx西葫芦(Cucurbita pepo)玉米(Zea mays)西红柿(Solanum lycopersicum)大豆(Glycine max) 富集量降低21%—28% 纳米颗粒对有机物的强吸附降低生物有效态 [102] C60 2、15 mg·L−1 4 d 三氯乙烯 美洲黑杨(Populus deltoides) 富集量分别增加−26%和82% 纳米颗粒吸附有机物后被植物吸收 [103] C60 40 mg·(12 g)−1蛭石 21 d 二氯二苯二氯乙烯p,p’-DDE 西葫芦
(Cucurbita pepo L.)
大豆(Glycine max L.)
土豆(Solanum lycopersicum L.)富集量增加30%—65% 纳米颗粒刺穿植物细胞使有机物易进入;吸附有机物后被植物吸收 [104] SiO2
有机蒙脱石10 g·kg−1 45 d 六氯苯
五氯苯水稻(Oryza sativa L.) 无显著影响 [105] TiO2 500、1000、
2000 mg·L−113 d 四环素5、10、
20 mg·L−1水稻(Oryza sativa L.) 富集量降低18.8%—62.5% 纳米颗粒的强吸附降低有机污染物的生物有效态 [106] 镍铁双金属纳米颗粒 30 g·kg−1 14 d 十溴联苯醚10 mg·kg−1 大白菜(Chinese cabbage) 富集量显著降低 纳米颗粒与污染物的联合毒性使植物生长发育减缓,富集能力减弱 [107] 零价铁nZVI 1000 mg·kg−1 28 d 硫丹 距花山姜(Alpinia calcarata)
圣罗勒(Ocimum sanctum)
柠檬草(Cymbopogon citratus)富集量显著降低,7 d内82%的硫丹被清除 纳米颗粒促进根际微生物生长 [108] Ag
CeO2100 mg·kg−1 30 d 吡虫啉10 mg·kg−1 西葫芦(Cucurbita pepo L.) 无显著影响或显著增加富集量 纳米颗粒与有机污染物结合被植物吸收 [109] TiO2
Ag
Al2O3
CNTs5% 25 d PAHs
有机氯农药OCPs
多溴联苯醚PBDE蕹菜(Ipomoea aquatica Forsk)
黄瓜(Cucumis sativus L.)
玉米(Zea mays L.)
菠菜(Spinacia oleracea L.)
南瓜(Cucurbita moschata)富集量显著增加 纳米颗粒吸附有机污染物后共同转移至植物组织 [110] Fe2O3 25 mg(Fe)·L−1 120 h 土霉素100 mg·L−1 水稻(Oryza sativa L.) 显著增加富集水平 纳米颗粒高比表面积吸附污染物,共同转移至植物组织 [111] SWCNTs
MWCNTs0.3、3.0 mg·g−1 28 d 芘 蚯蚓
(Eisenia fetida)0.3 mg·g−1时无显著影响;
3.0 mg·g−1时显著降低富集量低浓度纳米颗粒:芘的高吸附解吸速率导致富集不受影响;高浓度纳米颗粒:强烈吸附污染物降低其生物有效性和富集 [112] C60
MWCNTs300、3000 mg·kg−1 36 d 菲 蚯蚓
(Metaphire guillelmi)300 mg·kg−1时富集量增加30%—40%
3000 mg·kg−1时富集量降低23.6%—27.7%低浓度纳米颗粒增强被土壤吸附或锁定菲的释放和迁移,提高生物有效性;高浓度纳米颗粒增强菲的吸附,降低生物有效性 [113] C60
MWCNTs0.03%、0.3% 14 d 菲
2-甲基菲
3, 6-二甲基菲蚯蚓
(Metaphire guillelmi)不影响或降低菲和甲基菲的富集量;提高二甲基菲的富集量6.1%—25.9% 对菲和甲基菲的强吸附降低生物有效性;纳米颗粒吸附二甲基菲后被蚯蚓摄入,促进生物富集 [114] MWCNTs 0.1、1 g·kg−1 7 d 壬基酚10 mg·kg−1 蚯蚓
(Eisenia fetida)富集量增加 纳米颗粒携带有机物进入肠道细胞 [115] TiO2 500、2500 mg·kg−1 28 d 有机磷阻燃剂TDCIPP 5、
25 mg·kg−1蚯蚓
(Eisenia fetida)无显著影响 吸附TDCIPP的TiO2未被蚯蚓摄入 [116] Ag 500、1000、
2000 mg·kg−114 d 氯丹
二氯二苯二氯乙烯p,p’-DDE蚯蚓
(Eisenia fetida)富集量显著降低8.9%—92.9% 纳米银与污染物的联合毒性降低蚯蚓的富集能力 [117] CuO
ZnO10、50、
250 mg·kg−121 d 五氯硝基苯
100 μg·kg−1蚯蚓
(Eisenia fetida)富集量增大至3.13倍(CuO)和2.47倍(ZnO) 纳米颗粒造成体腔损伤 [118] CuO
ZnO10、50、
250 mg·kg−121 d 联苯菊酯
100 μg·kg−1蚯蚓
(Eisenia fetida)富集量增大至2.65倍(CuO)和3.32倍(ZnO) 纳米颗粒破坏蚯蚓的体腔,造成污染物容易进入 [119] ZnO 125、250 mg·kg−1 28 d 氯吡硫磷 20、
40 mg·kg−1蚯蚓
(Eisenia andrei)富集量显著增加45.5%—65.3% 纳米ZnO降低土壤对污染物的吸附和锁定,提高其生物有效性 [120] -
[1] JANKOVIĆ N Z, PLATA D L. Engineered nanomaterials in the context of global element cycles [J]. Environmental Science:Nano, 2019, 6(9): 2697-2711. doi: 10.1039/C9EN00322C [2] KELLER A A, LAZAREVA A. Predicted releases of engineered nanomaterials: From global to regional to local [J]. Environmental Science & Technology Letters, 2014, 1(1): 65-70. [3] LEFEVRE E, BOSSA N, WIESNER M R, et al. A review of the environmental implications of in situ remediation by nanoscale zero valent iron (nZVI): Behavior, transport and impacts on microbial communities [J]. Science of the Total Environment, 2016, 565: 889-901. doi: 10.1016/j.scitotenv.2016.02.003 [4] TRIPATHI D K, SHWETA, SINGH S, et al. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity [J]. Plant Physiology and Biochemistry, 2017, 110: 2-12. doi: 10.1016/j.plaphy.2016.07.030 [5] VELICOGNA J R, RITCHIE E E, SCROGGINS R P, et al. A comparison of the effects of silver nanoparticles and silver nitrate on a suite of soil dwelling organisms in two field soils [J]. Nanotoxicology, 2016, 10(8): 1144-1151. doi: 10.1080/17435390.2016.1181807 [6] RICO C M, MAJUMDAR S, DUARTE-GARDEA M, et al. Interaction of nanoparticles with edible plants and their possible implications in the food chain [J]. Journal of Agricultural and Food Chemistry, 2011, 59(8): 3485-3498. doi: 10.1021/jf104517j [7] HOU W C, WESTERHOFF P, POSNER J D. Biological accumulation of engineered nanomaterials: A review of current knowledge [J]. Environmental Science:Processes & Impacts, 2013, 15(1): 103-122. [8] REN X Y, ZENG G M, TANG L, et al. Effect of exogenous carbonaceous materials on the bioavailability of organic pollutants and their ecological risks [J]. Soil Biology and Biochemistry, 2018, 116: 70-81. doi: 10.1016/j.soilbio.2017.09.027 [9] BAGHAIE A H, JABARI A G. Effect of nano Fe-oxide and endophytic fungus (P. indica) on petroleum hydrocarbons degradation in an arsenic contaminated soil under barley cultivation [J]. Journal of Environmental Health Science and Engineering, 2019, 17(2): 853-861. doi: 10.1007/s40201-019-00402-w [10] CUI X Y, JIA F, CHEN Y X, et al. Influence of single-walled carbon nanotubes on microbial availability of phenanthrene in sediment [J]. Ecotoxicology, 2011, 20(6): 1277-1285. doi: 10.1007/s10646-011-0684-3 [11] WANG X L, LIU Y, ZHANG H Y, et al. The impact of carbon nanotubes on bioaccumulation and translocation of phenanthrene, 3-CH3-phenanthrene and 9-NO2-phenanthrene in maize (Zea mays) seedlings [J]. Environmental Science:Nano, 2016, 3(4): 818-829. doi: 10.1039/C6EN00012F [12] LI B, ZHU H K, SUN H W, et al. Effects of the amendment of biochars and carbon nanotubes on the bioavailability of hexabromocyclododecanes (HBCDs) in soil to ecologically different species of earthworms [J]. Environmental Pollution, 2017, 222: 191-200. doi: 10.1016/j.envpol.2016.12.057 [13] UZU G, SOBANSKA S, SARRET G, et al. Foliar lead uptake by lettuce exposed to atmospheric fallouts [J]. Environmental Science & Technology, 2010, 44(3): 1036-1042. [14] HATAMI M, KARIMAN K, GHORBANPOUR M. Engineered nanomaterial-mediated changes in the metabolism of terrestrial plants [J]. Science of the Total Environment, 2016, 571: 275-291. doi: 10.1016/j.scitotenv.2016.07.184 [15] LIU Q L, CHEN B, WANG Q L, et al. Carbon nanotubes as molecular transporters for walled plant cells [J]. Nano Letters, 2009, 9(3): 1007-1010. doi: 10.1021/nl803083u [16] LARUE C, PINAULT M, CZARNY B, et al. Quantitative evaluation of multi-walled carbon nanotube uptake in wheat and rapeseed [J]. Journal of Hazardous Materials, 2012, 227/228: 155-163. doi: 10.1016/j.jhazmat.2012.05.033 [17] LARUE C, LAURETTE J, HERLIN-BOIME N, et al. Accumulation, translocation and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp. ): Influence of diameter and crystal phase [J]. Science of the Total Environment, 2012, 431: 197-208. doi: 10.1016/j.scitotenv.2012.04.073 [18] KOLE C, KOLE P, RANDUNU K M, et al. Nanobiotechnology can boost crop production and quality: First evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia) [J]. BMC Biotechnology, 2013, 13: 37. doi: 10.1186/1472-6750-13-37 [19] MIRALLES P, JOHNSON E, CHURCH T L, et al. Multiwalled carbon nanotubes in alfalfa and wheat: Toxicology and uptake [J]. Journal of the Royal Society Interface, 2012, 9(77): 3514-3527. doi: 10.1098/rsif.2012.0535 [20] MALEJKO J, GODLEWSKA-ŻYŁKIEWICZ B, VANEK T, et al. Uptake, translocation, weathering and speciation of gold nanoparticles in potato, radish, carrot and lettuce crops [J]. Journal of Hazardous Materials, 2021, 418: 126219. doi: 10.1016/j.jhazmat.2021.126219 [21] LV J, CHRISTIE P, ZHANG S Z. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges [J]. Environmental Science:Nano, 2019, 6(1): 41-59. doi: 10.1039/C8EN00645H [22] LI C C, DANG F, LI M, et al. Effects of exposure pathways on the accumulation and phytotoxicity of silver nanoparticles in soybean and rice [J]. Nanotoxicology, 2017, 11(5): 699-709. doi: 10.1080/17435390.2017.1344740 [23] BEGUM P, IKHTIARI R, FUGETSU B. Graphene phytotoxicity in the seedling stage of cabbage, tomato, red spinach, and lettuce [J]. Carbon, 2011, 49(12): 3907-3919. doi: 10.1016/j.carbon.2011.05.029 [24] PRADAS del REAL A E, CASTILLO-MICHEL H, KAEGI R, et al. Fate of Ag-NPs in sewage sludge after application on agricultural soils [J]. Environmental Science & Technology, 2016, 50(4): 1759-1768. [25] HERNANDEZ-VIEZCAS J A, CASTILLO-MICHEL H, ANDREWS J C, et al. In situ synchrotron X-ray fluorescence mapping and speciation of CeO2 and ZnO nanoparticles in soil cultivated soybean (Glycine max) [J]. ACS Nano, 2013, 7(2): 1415-1423. doi: 10.1021/nn305196q [26] DIMKPA C O, LATTA D E, MCLEAN J E, et al. Fate of CuO and ZnO nano- and microparticles in the plant environment [J]. Environmental Science & Technology, 2013, 47(9): 4734-4742. [27] WANG P, MENZIES N W, LOMBI E, et al. Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata) [J]. Environmental Science & Technology, 2013, 47(23): 13822-13830. [28] WANG P, MENZIES N W, LOMBI E, et al. Silver sulfide nanoparticles (Ag2S-NPs) are taken up by plants and are phytotoxic [J]. Nanotoxicology, 2015, 9(8): 1041-1049. doi: 10.3109/17435390.2014.999139 [29] LIN S J, REPPERT J, HU Q, et al. Uptake, translocation, and transmission of carbon nanomaterials in rice plants [J]. Small, 2009, 5(10): 1128-1132. [30] TRIPATHI S, KAPRI S, DATTA A, et al. Influence of the morphology of carbon nanostructures on the stimulated growth of gram plant [J]. RSC Advances, 2016, 6(50): 43864-43873. doi: 10.1039/C6RA01163B [31] UNRINE J M, TSYUSKO O V, HUNYADI S E, et al. Effects of particle size on chemical speciation and bioavailability of copper to earthworms (Eisenia fetida) exposed to copper nanoparticles [J]. Journal of Environmental Quality, 2010, 39(6): 1942-1953. doi: 10.2134/jeq2009.0387 [32] LI D, FORTNER J D, JOHNSON D R, et al. Bioaccumulation of 14C60 by the earthworm Eisenia fetida [J]. Environmental Science & Technology, 2010, 44(23): 9170-9175. [33] HOOPER H L, JURKSCHAT K, MORGAN A J, et al. Comparative chronic toxicity of nanoparticulate and ionic zinc to the earthworm Eisenia veneta in a soil matrix [J]. Environment International, 2011, 37(6): 1111-1117. doi: 10.1016/j.envint.2011.02.019 [34] PETERSEN E J, HUANG Q G, WEBER J. Bioaccumulation of radio-labeled carbon nanotubes by Eisenia foetida [J]. Environmental Science & Technology, 2008, 42(8): 3090-3095. [35] OUGHTON D H, HERTEL-AAS T, PELLICER E, et al. Neutron activation of engineered nanoparticles as a tool for tracing their environmental fate and uptake in organisms [J]. Environmental Toxicology and Chemistry, 2008, 27(9): 1883-1887. doi: 10.1897/07-578.1 [36] LAPIED E, NAHMANI J Y, MOUDILOU E, et al. Ecotoxicological effects of an aged TiO2 nanocomposite measured as apoptosis in the anecic earthworm Lumbricus terrestris after exposure through water, food and soil [J]. Environment International, 2011, 37(6): 1105-1110. doi: 10.1016/j.envint.2011.01.009 [37] BACCARO M, van den BERG J H J, van den BRINK N W. Are long-term exposure studies needed?Short-term toxicokinetic model predicts the uptake of metal nanoparticles in earthworms after nine months [J]. Ecotoxicology and Environmental Safety, 2021, 220: 112371. doi: 10.1016/j.ecoenv.2021.112371 [38] COURTOIS P, RORAT A, LEMIERE S, et al. Ecotoxicology of silver nanoparticles and their derivatives introduced in soil with or without sewage sludge: A review of effects on microorganisms, plants and animals [J]. Environmental Pollution, 2019, 253: 578-598. doi: 10.1016/j.envpol.2019.07.053 [39] LI S B, IRIN F, ATORE F O, et al. Determination of multi-walled carbon nanotube bioaccumulation in earthworms measured by a microwave-based detection technique [J]. Science of the Total Environment, 2013, 445/446: 9-13. doi: 10.1016/j.scitotenv.2012.12.037 [40] 王震宇, 赵建, 李娜, 等. 人工纳米颗粒对水生生物的毒性效应及其机制研究进展 [J]. 环境科学, 2010, 31(6): 1409-1418. doi: 10.13227/j.hjkx.2010.06.018 WANG Z Y, ZHAO J, LI N, et al. Review of ecotoxicity and mechanism of engineered nanoparticles to aquatic organisms [J]. Environmental Science, 2010, 31(6): 1409-1418(in Chinese). doi: 10.13227/j.hjkx.2010.06.018
[41] SCHWAB F, ZHAI G S, KERN M, et al. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants - Critical review [J]. Nanotoxicology, 2016, 10(3): 257-278. doi: 10.3109/17435390.2015.1048326 [42] JIANG W, WANG Q, QU X L, et al. Effects of charge and surface defects of multi-walled carbon nanotubes on the disruption of model cell membranes [J]. Science of the Total Environment, 2017, 574: 771-780. doi: 10.1016/j.scitotenv.2016.09.150 [43] LIU Q L, ZHAO Y Y, WAN Y L, et al. Study of the inhibitory effect of water-soluble fullerenes on plant growth at the cellular level [J]. ACS Nano, 2010, 4(10): 5743-5748. doi: 10.1021/nn101430g [44] BEGUM P, FUGETSU B. Induction of cell death by graphene in Arabidopsis thaliana (Columbia ecotype) T87 cell suspensions [J]. Journal of Hazardous Materials, 2013, 260: 1032-1041. doi: 10.1016/j.jhazmat.2013.06.063 [45] PATLOLLA A K, BERRY A, MAY L, et al. Genotoxicity of silver nanoparticles in Vicia faba: A pilot study on the environmental monitoring of nanoparticles [J]. International Journal of Environmental Research and Public Health, 2012, 9(5): 1649-1662. doi: 10.3390/ijerph9051649 [46] BARBOLINA I, WOODS C R, LOZANO N, et al. Purity of graphene oxide determines its antibacterial activity [J]. 2D Materials, 2016, 3(2): 025025. doi: 10.1088/2053-1583/3/2/025025 [47] DIMKPA C O, MCLEAN J E, LATTA D E, et al. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat [J]. Journal of Nanoparticle Research, 2012, 14(9): 1125. doi: 10.1007/s11051-012-1125-9 [48] KARLSSON H L, CRONHOLM P, GUSTAFSSON J, et al. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes [J]. Chemical Research in Toxicology, 2008, 21(9): 1726-1732. doi: 10.1021/tx800064j [49] DENG R, LIN D H, ZHU L Z, et al. Nanoparticle interactions with co-existing contaminants: Joint toxicity, bioaccumulation and risk [J]. Nanotoxicology, 2017, 11(5): 591-612. doi: 10.1080/17435390.2017.1343404 [50] YU S J, LIU J F, YIN Y G, et al. Interactions between engineered nanoparticles and dissolved organic matter: A review on mechanisms and environmental effects [J]. Journal of Environmental Sciences, 2018, 63: 198-217. doi: 10.1016/j.jes.2017.06.021 [51] BAI Y, WANG C Y, GAO J J, et al. A study on dispersion and antibacterial activity of functionalizing multi-walled carbon nanotubes with mixed surfactant [J]. Journal of Surfactants and Detergents, 2015, 18(6): 957-964. doi: 10.1007/s11743-015-1729-z [52] BAI Y, PARK I S, LEE S J, et al. Effect of AOT-assisted multi-walled carbon nanotubes on antibacterial activity [J]. Colloids and Surfaces B:Biointerfaces, 2012, 89: 101-107. doi: 10.1016/j.colsurfb.2011.09.001 [53] MAURER-JONES M A, GUNSOLUS I L, MURPHY C J, et al. Toxicity of engineered nanoparticles in the environment [J]. Analytical Chemistry, 2013, 85(6): 3036-3049. doi: 10.1021/ac303636s [54] LIU S B, ZENG T H, HOFMANN M, et al. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress [J]. ACS Nano, 2011, 5(9): 6971-6980. doi: 10.1021/nn202451x [55] ARIAS L R, YANG L J. Inactivation of bacterial pathogens by carbon nanotubes in suspensions [J]. Langmuir, 2009, 25(5): 3003-3012. doi: 10.1021/la802769m [56] AUFFAN M, ACHOUAK W, ROSE J, et al. Relation between the redox state of iron-based nanoparticles and their cytotoxicity toward Escherichia coli [J]. Environmental Science & Technology, 2008, 42(17): 6730-6735. [57] SIMONIN M, RICHAUME A. Impact of engineered nanoparticles on the activity, abundance, and diversity of soil microbial communities: A review [J]. Environmental Science and Pollution Research, 2015, 22(18): 13710-13723. doi: 10.1007/s11356-015-4171-x [58] ZHANG H Y, WU F, CHEN W X, et al. Carbon nanomaterials differentially impact mineralization kinetics of phenanthrene and indigenous microbial communities in a natural soil [J]. NanoImpact, 2018, 11: 146-155. doi: 10.1016/j.impact.2018.08.001 [59] REN W J, REN G D, TENG Y, et al. Time-dependent effect of graphene on the structure, abundance, and function of the soil bacterial community [J]. Journal of Hazardous Materials, 2015, 297: 286-294. doi: 10.1016/j.jhazmat.2015.05.017 [60] CHUNG H, KIM M J, KO K, et al. Effects of graphene oxides on soil enzyme activity and microbial biomass [J]. Science of the Total Environment, 2015, 514: 307-313. doi: 10.1016/j.scitotenv.2015.01.077 [61] JIN L X, SON Y, YOON T K, et al. High concentrations of single-walled carbon nanotubes lower soil enzyme activity and microbial biomass [J]. Ecotoxicology and Environmental Safety, 2013, 88: 9-15. doi: 10.1016/j.ecoenv.2012.10.031 [62] KUMAR N, SHAH V, WALKER V K. Perturbation of an arctic soil microbial community by metal nanoparticles [J]. Journal of Hazardous Materials, 2011, 190(1/2/3): 816-822. [63] GE Y, SCHIMEL J P, HOLDEN P A. Evidence for negative effects of TiO2 and ZnO nanoparticles on soil bacterial communities [J]. Environmental Science & Technology, 2011, 45(4): 1659-1664. [64] SHAN J, JI R, YU Y J, et al. Biochar, activated carbon and carbon nanotubes have different effects on fate of 14C-catechol and microbial community in soil [J]. Scientific Reports, 2015, 5: 16000. doi: 10.1038/srep16000 [65] CHUNG H, SON Y, YOON T K, et al. The effect of multi-walled carbon nanotubes on soil microbial activity [J]. Ecotoxicology and Environmental Safety, 2011, 74(4): 569-575. doi: 10.1016/j.ecoenv.2011.01.004 [66] WU F, YOU Y Q, ZHANG X Y, et al. Effects of various carbon nanotubes on soil bacterial community composition and structure [J]. Environmental Science & Technology, 2019, 53(10): 5707-5716. [67] BEGUM P, FUGETSU B. Phytotoxicity of multi-walled carbon nanotubes on red spinach (Amaranthus tricolor L) and the role of ascorbic acid as an antioxidant [J]. Journal of Hazardous Materials, 2012, 243: 212-222. doi: 10.1016/j.jhazmat.2012.10.025 [68] MA X M, GEISER-LEE J, DENG Y, et al. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation [J]. Science of the Total Environment, 2010, 408(16): 3053-3061. doi: 10.1016/j.scitotenv.2010.03.031 [69] GEISLER-LEE J, WANG Q, YAO Y, et al. Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana [J]. Nanotoxicology, 2012, 7(3): 323-337. doi: 10.3109/17435390.2012.658094 [70] LIN D H, XING B S. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth [J]. Environmental Pollution, 2007, 150(2): 243-250. doi: 10.1016/j.envpol.2007.01.016 [71] ASLI S, NEUMANN P M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport [J]. Plant, Cell & Environment, 2009, 32(5): 577-584. [72] KHODAKOVSKAYA M V, de SILVA K, BIRIS A S, et al. Carbon nanotubes induce growth enhancement of tobacco cells [J]. ACS Nano, 2012, 6(3): 2128-2135. doi: 10.1021/nn204643g [73] WANG X P, HAN H Y, LIU X Q, et al. Multi-walled carbon nanotubes can enhance root elongation of wheat (Triticum aestivum) plants [J]. Journal of Nanoparticle Research, 2012, 14(6): 841. doi: 10.1007/s11051-012-0841-5 [74] HUSEN A, SIDDIQI K S. Carbon and fullerene nanomaterials in plant system [J]. Journal of Nanobiotechnology, 2014, 12: 16. doi: 10.1186/1477-3155-12-16 [75] HU X G, LU K C, MU L, et al. Interactions between graphene oxide and plant cells: Regulation of cell morphology, uptake, organelle damage, oxidative effects and metabolic disorders [J]. Carbon, 2014, 80: 665-676. doi: 10.1016/j.carbon.2014.09.010 [76] YIN L Y, CHENG Y W, ESPINASSE B, et al. More than the ions: The effects of silver nanoparticles on Lolium multiflorum [J]. Environmental Science & Technology, 2011, 45(6): 2360-2367. [77] HU X G, ZHOU Q X. Health and ecosystem risks of graphene [J]. Chemical Reviews, 2013, 113(5): 3815-3835. doi: 10.1021/cr300045n [78] YANG K, WAN J M, ZHANG S, et al. The influence of surface chemistry and size of nanoscale graphene oxide on photothermal therapy of cancer using ultra-low laser power [J]. Biomaterials, 2012, 33(7): 2206-2214. doi: 10.1016/j.biomaterials.2011.11.064 [79] ZHANG L J, HU C W, WANG W L, et al. Acute toxicity of multi-walled carbon nanotubes, sodium pentachlorophenate, and their complex on earthworm Eisenia fetida [J]. Ecotoxicology and Environmental Safety, 2014, 103: 29-35. doi: 10.1016/j.ecoenv.2014.01.041 [80] WANG J F, WAGES M, YU S Y, et al. Bioaccumulation of fullerene (C60) and corresponding catalase elevation in Lumbriculus variegatus [J]. Environmental Toxicology and Chemistry, 2014, 33(5): 1135-1141. doi: 10.1002/etc.2540 [81] CALISI A, GRIMALDI A, LEOMANNI A, et al. Multibiomarker response in the earthworm Eisenia fetida as tool for assessing multi-walled carbon nanotube ecotoxicity [J]. Ecotoxicology, 2016, 25(4): 677-687. doi: 10.1007/s10646-016-1626-x [82] van der PLOEG M J C, HANDY R D, HECKMANN L H, et al. C60 exposure induced tissue damage and gene expression alterations in the earthworm Lumbricus rubellus [J]. Nanotoxicology, 2013, 7(4): 432-440. doi: 10.3109/17435390.2012.668569 [83] LANKADURAI B P, NAGATO E G, SIMPSON A J, et al. Analysis of Eisenia fetida earthworm responses to sub-lethal C60 nanoparticle exposure using 1H-NMR based metabolomics [J]. Ecotoxicology and Environmental Safety, 2015, 120: 48-58. doi: 10.1016/j.ecoenv.2015.05.020 [84] WANG H H, WICK R L, XING B S. Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans [J]. Environmental Pollution, 2009, 157(4): 1171-1177. doi: 10.1016/j.envpol.2008.11.004 [85] van der PLOEG M J C, BAVECO J M, van der HOUT A, et al. Effects of C60 nanoparticle exposure on earthworms (Lumbricus rubellus) and implications for population dynamics [J]. Environmental Pollution, 2011, 159(1): 198-203. doi: 10.1016/j.envpol.2010.09.003 [86] MCSHANE H, SARRAZIN M, WHALEN J K, et al. Reproductive and behavioral responses of earthworms exposed to nano-sized titanium dioxide in soil [J]. Environmental Toxicology and Chemistry, 2012, 31(1): 184-193. doi: 10.1002/etc.714 [87] ZHANG H Y, VIDONISH J, LV W, et al. Differential histological, cellular and organism-wide response of earthworms exposed to multi-layer graphenes with different morphologies and hydrophobicity [J]. Environmental Pollution, 2020, 263: 114468. doi: 10.1016/j.envpol.2020.114468 [88] 曹圣来. 典型纳米金属氧化物对不同类型土壤中蚯蚓、小麦的毒性效应[D]. 南京: 南京大学, 2015. CAO S L. Toxicology of metal oxide nanoparticles on earthworm and wheat in different types of soils[D]. Nanjing: Nanjing University, 2015(in Chinese).
[89] JOHNSEN A R, WICK L Y, HARMS H. Principles of microbial PAH-degradation in soil [J]. Environmental Pollution, 2005, 133(1): 71-84. doi: 10.1016/j.envpol.2004.04.015 [90] ERSAN G, APUL O G, PERREAULT F, et al. Adsorption of organic contaminants by graphene nanosheets: A review [J]. Water Research, 2017, 126: 385-398. doi: 10.1016/j.watres.2017.08.010 [91] YANG K, XING B S. Desorption of polycyclic aromatic hydrocarbons from carbon nanomaterials in water [J]. Environmental Pollution, 2007, 145(2): 529-537. doi: 10.1016/j.envpol.2006.04.020 [92] ZHANG C D, LI M Z, XU X, et al. Effects of carbon nanotubes on atrazine biodegradation by Arthrobacter sp [J]. Journal of Hazardous Materials, 2015, 287: 1-6. doi: 10.1016/j.jhazmat.2015.01.039 [93] SHRESTHA B, ANDERSON T A, ACOSTA-MARTINEZ V, et al. The influence of multiwalled carbon nanotubes on polycyclic aromatic hydrocarbon (PAH) bioavailability and toxicity to soil microbial communities in alfalfa rhizosphere [J]. Ecotoxicology and Environmental Safety, 2015, 116: 143-149. doi: 10.1016/j.ecoenv.2015.03.005 [94] TOWELL M G, BROWNE L A, PATON G I, et al. Impact of carbon nanomaterials on the behaviour of 14C-phenanthrene and 14C-benzo-[a] pyrene in soil [J]. Environmental Pollution, 2011, 159(3): 706-715. doi: 10.1016/j.envpol.2010.11.040 [95] XIA X H, ZHOU C H, HUANG J H, et al. Mineralization of phenanthrene sorbed on multiwalled carbon nanotubes [J]. Environmental Toxicology and Chemistry, 2013, 32(4): 894-901. doi: 10.1002/etc.2125 [96] XIA X H, LI Y R, ZHOU Z, et al. Bioavailability of adsorbed phenanthrene by black carbon and multi-walled carbon nanotubes to Agrobacterium [J]. Chemosphere, 2010, 78(11): 1329-1336. doi: 10.1016/j.chemosphere.2010.01.007 [97] OYELAMI A O, SEMPLE K T. The impact of carbon nanomaterials on the development of phenanthrene catabolism in soil [J]. Environmental Science:Processes & Impacts, 2015, 17(7): 1302-1310. [98] ZHANG M, SHEN X F, ZHANG H Y, et al. Bioavailability of phenanthrene and nitrobenzene sorbed on carbonaceous materials [J]. Carbon, 2016, 110: 404-413. doi: 10.1016/j.carbon.2016.09.044 [99] KELSEY J W, WHITE J C. Effect of C60 fullerenes on the accumulation of weathered p, p'-DDE by plant and earthworm species under single and multispecies conditions [J]. Environmental Toxicology and Chemistry, 2013, 32(5): 1117-1123. doi: 10.1002/etc.2158 [100] SHEN X F, LI S L, ZHANG H Y, et al. Effect of multiwalled carbon nanotubes on uptake of pyrene by cucumber (Cucumis sativus L.): Mechanistic perspectives [J]. NanoImpact, 2018, 10: 168-176. doi: 10.1016/j.impact.2018.05.001 [101] ZHANG H Y, LIU Y, SHEN X F, et al. Influence of multiwalled carbon nanotubes and sodium dodecyl benzene sulfonate on bioaccumulation and translocation of pyrene and 1-methylpyrene in maize (Zea mays) seedlings [J]. Environmental Pollution, 2017, 220: 1409-1417. doi: 10.1016/j.envpol.2016.10.093 [102] de la TORRE-ROCHE R, HAWTHORNE J, DENG Y Q, et al. Multiwalled carbon nanotubes and C60 fullerenes differentially impact the accumulation of weathered pesticides in four agricultural plants [J]. Environmental Science & Technology, 2013, 47(21): 12539-12547. [103] MA X M, WANG C. Fullerene nanoparticles affect the fate and uptake of trichloroethylene in phytoremediation systems [J]. Environmental Engineering Science, 2010, 27(11): 989-992. doi: 10.1089/ees.2010.0141 [104] de la TORRE-ROCHE R, HAWTHORNE J, DENG Y Q, et al. Fullerene-enhanced accumulation of p, p'-DDE in agricultural crop species [J]. Environmental Science & Technology, 2012, 46(17): 9315-9323. [105] LIU C Y, JIANG X, MA Y C, et al. Pollutant and soil types influence effectiveness of soil-applied absorbents in reducing rice plant uptake of persistent organic pollutants [J]. Pedosphere, 2017, 27(3): 537-547. doi: 10.1016/S1002-0160(17)60349-7 [106] MA C X, LIU H, CHEN G C, et al. Effects of titanium oxide nanoparticles on tetracycline accumulation and toxicity in Oryza sativa (L. ) [J]. Environmental Science:Nano, 2017, 4(9): 1827-1839. doi: 10.1039/C7EN00280G [107] WU J, XIE Y Y, FANG Z Q, et al. Effects of Ni/Fe bimetallic nanoparticles on phytotoxicity and translocation of polybrominated diphenyl ethers in contaminated soil [J]. Chemosphere, 2016, 162: 235-242. doi: 10.1016/j.chemosphere.2016.07.101 [108] PILLAI H P S, KOTTEKOTTIL J. Nano-phytotechnological remediation of endosulfan using zero valent iron nanoparticles [J]. Journal of Environmental Protection, 2016, 7(5): 734-744. doi: 10.4236/jep.2016.75066 [109] de la TORRE ROCHE R, PAGANO L, MAJUMDAR S, et al. Co-exposure of imidacloprid and nanoparticle Ag or CeO2 to Cucurbita pepo (zucchini): Contaminant bioaccumulation and translocation [J]. NanoImpact, 2018, 11: 136-145. doi: 10.1016/j.impact.2018.07.001 [110] WU X, WANG W, ZHU L Z. Enhanced organic contaminants accumulation in crops: Mechanisms, interactions with engineered nanomaterials in soil [J]. Environmental Pollution, 2018, 240: 51-59. doi: 10.1016/j.envpol.2018.04.072 [111] BAO Y Y, MA C X, HU L, et al. Effect of individual and combined exposure of Fe2O3 nanoparticles and oxytetracycline on their bioaccumulation by rice (Oryza sativa L. ) [J]. Journal of Soils and Sediments, 2019, 19(5): 2459-2471. doi: 10.1007/s11368-018-2216-8 [112] PETERSEN E J, PINTO R A, LANDRUM P F, et al. Influence of carbon nanotubes on pyrene bioaccumulation from contaminated soils by earthworms [J]. Environmental Science & Technology, 2009, 43(11): 4181-4187. [113] ZHANG H Y, CHEN W X, SHEN X F, et al. Influence of multi-walled carbon nanotubes and fullerenes on the bioaccumulation and elimination kinetics of phenanthrene in geophagous earthworms (Metaphire guillelmi) [J]. Environmental Science:Nano, 2017, 4(9): 1887-1899. doi: 10.1039/C7EN00118E [114] ZHANG H Y, CHEN W X, ZHANG X Y, et al. Carbon nanomaterials differentially impact bioaccumulation and oxidative response of phenanthrene and methyl derivatives in geophagous earthworms (Metaphire guillelmi): A multi-contaminant exposure study [J]. Journal of Environmental Chemical Engineering, 2018, 6(5): 6537-6544. doi: 10.1016/j.jece.2018.10.007 [115] HU C W, CAI Y, WANG W L, et al. Toxicological effects of multi-walled carbon nanotubes adsorbed with nonylphenol on earthworm Eisenia fetida [J]. Environmental Science:Processes & Impacts, 2013, 15(11): 2125-2130. [116] ZHU Y, WU X Y, LIU Y X, et al. Synergistic growth inhibition effect of TiO2 nanoparticles and tris(1, 3-dichloro-2-propyl) phosphate on earthworms in soil [J]. Ecotoxicology and Environmental Safety, 2021, 208: 111462. doi: 10.1016/j.ecoenv.2020.111462 [117] MUKHERJEE A, HAWTHORNE J, WHITE J C, et al. Nanoparticle silver coexposure reduces the accumulation of weathered persistent pesticides by earthworms [J]. Environmental Toxicology and Chemistry, 2017, 36(7): 1864-1871. doi: 10.1002/etc.3698 [118] LI M, XU G H, GUO N, et al. Influences and mechanisms of nanoparticles on pentachloronitrobenzene accumulation by earthworms [J]. Environmental Science and Pollution Research, 2021: 1-9. [119] LI M, XU G H, YANG X T, et al. Metal oxide nanoparticles facilitate the accumulation of bifenthrin in earthworms by causing damage to body cavity [J]. Environmental Pollution, 2020, 263: 114629. doi: 10.1016/j.envpol.2020.114629 [120] GARCÍA-GÓMEZ C, BABÍN M, GARCÍA S, et al. Joint effects of zinc oxide nanoparticles and chlorpyrifos on the reproduction and cellular stress responses of the earthworm Eisenia andrei [J]. Science of the Total Environment, 2019, 688: 199-207. doi: 10.1016/j.scitotenv.2019.06.083 [121] YANG Y, HUNTER W, TAO S, et al. Microbial availability of different forms of phenanthrene in soils [J]. Environmental Science & Technology, 2009, 43(6): 1852-1857. [122] YANG Y, SHU L, WANG X L, et al. Effects of composition and domain arrangement of biopolymer components of soil organic matter on the bioavailability of phenanthrene [J]. Environmental Science & Technology, 2010, 44(9): 3339-3344. [123] CONGIU E, ORTEGA-CALVO J J. Role of desorption kinetics in the rhamnolipid-enhanced biodegradation of polycyclic aromatic hydrocarbons [J]. Environmental Science & Technology, 2014, 48(18): 10869-10877. [124] PACWA-PŁOCINICZAK M, PŁAZA G A, PIOTROWSKA-SEGET Z, et al. Environmental applications of biosurfactants: Recent advances [J]. International Journal of Molecular Sciences, 2011, 12(1): 633-654. doi: 10.3390/ijms12010633 [125] KUŚMIERZ M, OLESZCZUK P, KRASKA P, et al. Persistence of polycyclic aromatic hydrocarbons (PAHs) in biochar-amended soil [J]. Chemosphere, 2016, 146: 272-279. doi: 10.1016/j.chemosphere.2015.12.010 [126] CÁCERES-WENZEL M I, FUCHS J S, BERNASSANI F N, et al. Combined effects of goethite nanoparticles with metallic contaminants and an organophosphorus pesticide on Eisenia andrei [J]. Environmental Science and Pollution Research, 2020, 27(16): 20066-20075. doi: 10.1007/s11356-020-08547-0 [127] ZHANG C, WANG S H, LV Z, et al. NanoFe3O4 accelerates anoxic biodegradation of 3, 5, 6-trichloro-2-pyridinol [J]. Chemosphere, 2019, 235: 185-193. doi: 10.1016/j.chemosphere.2019.06.114 -



 下载:
下载: