-
硒是人体必需的微量元素,但若其在环境中浓度过高,将会造成污染[1]。在酸性矿山废水(acid mine drainage,AMD)中一般缺乏有机碳源[2],而AMD中的硒通常与其他重金属离子(如Cd(Ⅱ))共存,这类废水pH较低(pH为2.0~5.0)[3],硒和镉浓度可从几μg到几十mg。之前有研究者发现,酸性矿山废水中硒和镉浓度可同时达到53 mg·L−1和44 mg·L−1[4]。我国在《污水综合排放标准》(GB 8978-1996)中规定,总硒和镉的最大允许排放浓度均为0.1 mg·L−1[5]。因此,含硒、镉污染的废水必须进行适当处理。
与物理、化学方法相比,生物处理法具有成本低、节能环保的优点,具有良好的发展潜力。ESPINOSA-ORTIZ等[6]采用黄孢原毛平革菌,在葡萄糖(5 g·L−1)作为碳源的条件下,对pH为4.5的10 mg·L−1含Se(Ⅳ)废水进行了处理,结果表明,可溶性硒的去除率仅有70%。ZHANG等[7]研究了活性污泥SBR在限氧条件下对含硒含盐废水的生物处理效果,发现在28 ℃和pH为7.0~7.1的条件下,以醋酸盐(200~1 600 mg·L−1)为唯一碳源,98%以上的可溶性硒和72%以上的固体硒可被去除,且大部分亚硒酸盐被还原为硒元素且积累在污泥中,Proteobacteria (变形菌门)是除硒优势菌门。MAL等[8]的研究结果表明,在30 ℃和pH为7.3条件下,以乳酸钠(1 120 mg·L−1)作为碳源的厌氧颗粒污泥中,79 mg·L−1的Se(Ⅳ)在Cd(Ⅱ)(≤70 mg·L−1)存在下,7 d内Se(Ⅳ)可被完全还原,且在水相中发现了元素硒和CdSe纳米物质的存在。TAN等[9]通过上流式厌氧污泥床(UASB)对含硒、硝酸盐和硫酸盐的废水进行了处理,在pH为5.0和COD为2 g·(L·d)−1的条件下,总硒、溶解态硒和COD的去除率分别为43%、61%和20%,UASB运行不稳定,Campylobacteraceae和Desulfomicrobiaceae是UASB中优势菌,相对丰度分别为23%和10%。笔者此前使用UASB同步去除废水中的Se(Ⅵ)和Cd(Ⅱ),在pH为7.0、碳源为乳酸钠(1 800 mg·L−1)的条件下,总硒和镉的去除率分别为95.0%和99.2%[10]。
以上关于对废水中硒、镉去除的研究,大多是在pH中性或者高碳源条件下进行的。厌氧颗粒污泥具有可持留大量生物、沉降性能好、抗冲击能力强等诸多优点,因而受到学者们的广泛关注[11]。笔者此前的研究发现,厌氧颗粒污泥可在进水pH为中性及高浓度有机物的条件下,同步去除Se(Ⅵ)和Cd(Ⅱ)[10]。基于此,本研究在低碳源的条件下,进一步考察了厌氧颗粒污泥对酸性硒、镉废水的处理效果,并采用扫描电子显微镜和X射线能谱(SEM-EDS)分析了厌氧颗粒污泥的微观结构,利用X射线光电子能谱(XPS)分析了硒、镉等元素价态,通过高通量测序技术,解析了厌氧颗粒污泥中细菌、古菌的群落结构特征,以期为生物同步处理低碳源酸性含硒、镉废水提供参考。
全文HTML
-
亚硒酸钠、氯化镉、磷酸二氢钾、磷酸氢二钠、氯化铵、氯化钾、氯化钙、氯化镁、乳酸钠,分析纯;硝酸、盐酸,优级纯,以上所有试剂均购于衡阳宏进化工有限公司;硒标准溶液(1 000 µg·mL−1)、镉标准溶液(1 000 µg·mL−1),优级纯,购于南京化学试剂股份有限公司。PHS-3C型精密pH计,上海精密科学仪器;AUY220型电子天平,日本岛津公司;T.G.L-16gR型台式高速冷冻离心机,上海赫本田科学仪器有限公司;LGJ-50FG型真空冷冻干燥机,北京亚星仪科科技发展有限公司;EVO18型扫描电子显微镜,德国蔡司公司;AA-6300型原子吸收分光光度计,日本岛津公司;AFS-830型原子荧光光度计,北京吉天仪器有限公司。
-
接种的厌氧颗粒污泥取自某柠檬酸生产厂化工废水处理的UASB反应器。颗粒污泥外形为规则球形,强度高,泥水分离效果好,沉降性能好。其中,可挥发性悬浮物(VSS)大于60 g·L−1,菌泥有机含量VSS/TSS为0.7±0.1,有效污泥颗粒度60%~70%,沉降速率为30~150 m·h−1,颗粒直径0.5~4.0 mm,含水率90%。在实验前,用pH为7.5的磷酸盐缓冲溶液冲洗颗粒污泥3~4次,以去除残余化学物质。人工配置含硒、镉模拟废水,组分如下:17.29 mg·L−1 Na2SeO3(硒浓度为7.9 mg·L−1)、22.84 mg·L−1 CdCl2·2.5H2O(镉浓度为11.24 mg·L−1)、100 mg·L−1 KH2PO4、290 mg·L−1 NH4Cl、100 mg·L−1 Na2HPO4、50 mg·L−1 CaCl2·7H2O、50 mg·L−1 MgCl2、10 mg·L−1的FeSO4·7H2O。乳酸钠作为有机碳源配制COD为100~50 mg·L−1,进水pH为4.0。
-
将100 mL的厌氧颗粒污泥和900 mL的合成废水放入1 L的厌氧培养瓶中,以SBR方式进行去除实验。SBR每个周期包括5个步骤:10 min进水、5 h反应、30 min沉淀、5 min排水和15 min闲置,1个周期6 h。
根据进水COD值的不同,将SBR操作分为2个阶段(表1)。在第Ⅰ阶段进水COD为(100±9.1) mg·L−1;第Ⅱ阶段进水COD为(50±1.7) mg·L−1;进水Se(Ⅳ)和Cd(Ⅱ)浓度在2个阶段均保持稳定,2个阶段均设置3组平行实验。
-
利用SEM-EDS对厌氧颗粒污泥微观形态和元素组成进行分析[12]。利用XPS分析颗粒污泥在处理Se(Ⅳ)、Cd(Ⅱ)前后的元素组成及价态,结果采用Avantage软件进行分峰拟合分析,用Origin软件进行数据处理。
-
取接种厌氧颗粒污泥及2个阶段反应后的厌氧颗粒污泥样品分别进行均匀混合,利用E.Z.N.A. Soil DNA Kit试剂盒(OMEGA, BioTek, Winooski, VT, USA)提取基因组DNA,采用通用引物338F/806R扩增细菌16S rDNAV3~V4区片段[13],使用524F10extF/Arch958RmodR引物对古细菌进行PCR扩增[14],通过Illumina MiSeq PE平台进行高通量测序(上海美吉)。
获得的序列经过质控,以97%的相似水平划分操作分类单元(OTUs),利用Mothur软件计算微生物Alpha多样性指数,包括覆盖率、ACE指数、Chao指数、Shannon指数和Simpson指数。采用RDP classifier软件(2.6版本)将序列进行物种分类,统计每个样本在门(Phylum)、科(Family)或属(Genus)水平的群落组成与丰度。
-
在SBR运行期间,每周期运行结束后取10~20 mL水样进行总硒、四价硒、镉和COD的测定,为防止金属离子的吸附和沉淀,在测量前用浓度为1 mol·L−1的硝酸对液体样品进行酸化处理。总硒和四价硒采用氢化物发生——原子荧光法测定[15-16];镉采用原子吸收分光光度计(AAS-6630)测定;pH和COD分别使用精密pH计和标准重铬酸钾滴定法测定[17]。
1.1. 试剂和仪器
1.2. 厌氧颗粒污泥来源及实验废水
1.3. 实验装置及运行方式
1.4. 厌氧颗粒污泥特性
1.5. 微生物群落分析
1.6. 检测方法
-
在100个周期中,SBR对镉、总硒、四价硒和COD的去除效果如图1所示。在第Ⅰ阶段,镉的平均去除率可稳定在(96.67±2.70)%(图1(a))。总硒和四价硒的平均去除率在前16个周期较为稳定(图1(b)),分别为(97.07±3.17)%和(98.86±1.84)%;自17周期起,总硒和四价硒的去除率不稳定,且呈逐渐下降的趋势。第Ⅰ阶段结束时,总硒和四价硒的去除率分别下降至(54.47±0.62)%和(57.66±2.24)%。在第Ⅰ阶段,COD的去除率由最初几个周期(50%)逐渐升高到最末几个周期(98%左右,图1(c))。
第Ⅱ阶段,进水COD值为50 mg·L−1时,镉的去除率不断下降。到第55周期,镉的去除率降至(44.01±6.70)%;在55~100周期,镉的平均去除率仅为(40.94±6.22)%。这说明在进水COD为50 mg·L−1条件下,颗粒污泥去除镉效果不理想。反应器在运行41~48周期时,总硒和四价硒的平均去除率分别为(48.81±1.92)%和(51.57±2.15)%;在49周期,总硒和四价硒的去除率分别下降到(24.50±2.92)%和(30.96±5.39)%。本课题组前期的研究结果[10]表明,厌氧颗粒污泥在足够碳源条件下(1 800 mg·L−1,以乳酸钠计),对10 mg·L−1硒与2~5 mg·L−1镉可稳定去除的时间长达100 d。本研究在进水COD为100 mg·L−1时可短暂实现对硒、镉的高效去除,而长期运行及当COD值降至50 mg·L−1时,硒、镉去除率并不理想。因此,可推测在低碳源浓度下,部分异养菌碳源不足,影响了厌氧颗粒污泥整体的微生物活性,造成硒、镉去除率下降。
-
接种厌氧颗粒污泥为黑色(图2(a)),在去除Se(Ⅳ)和Cd(Ⅱ)第12周期起,黑色的厌氧颗粒污泥开始发生颜色变化,到第31周期厌氧颗粒污泥颜色从黑色整体变成了红色(图2(b)、图2(c))。以上颜色的变化表明有Se(0)生成,这说明厌氧颗粒污泥对Se(Ⅳ)有还原作用[18]。而厌氧颗粒污泥对Cd(Ⅱ)主要以生物吸附的方式去除[8]。
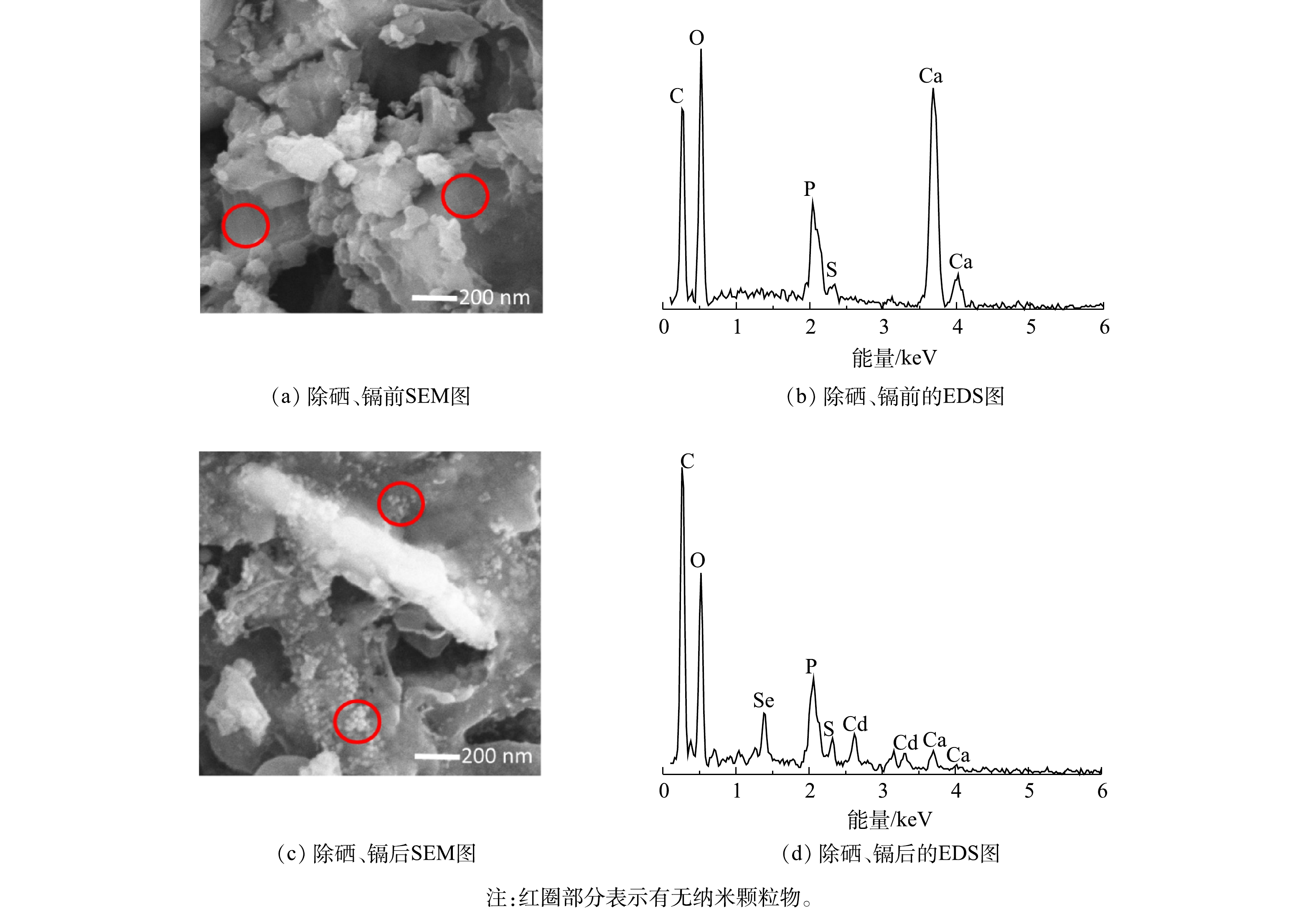
反应前后厌氧颗粒污泥微观形态和元素组成如图3所示。可以看到,接种颗粒污泥结构紧密,表面较为光滑,具有孔隙结构(图3(a))。在反应后,颗粒污泥表面孔道与反应前相比明显减少,出现了较多的纳米颗粒物质(图3(c)),这与之前的纳米硒(Se NPs)及纳米硒化镉(CdSe NPs)形态类似[19]。对应的能谱分析(EDS)结果表明,接种厌氧颗粒污泥中硒、镉元素低于检测限(图3(b)),在处理硒、镉废水后,2种元素含量分别为1.13%和1.34%(图3(d))。这证明了厌氧颗粒污泥对硒、镉具有去除作用。NANCHARAIAH等[19]研究结果表明,好氧颗粒污泥能将亚硒酸钠还原为Se(0)纳米球。在本研究中,厌氧颗粒污泥存在类似的纳米颗粒物,结合EDS结果可推测:Se(Ⅳ)被还原成Se(0)或者Se(−II),后者能够与Cd(Ⅱ)共沉淀形成CdSe纳米颗粒。
-
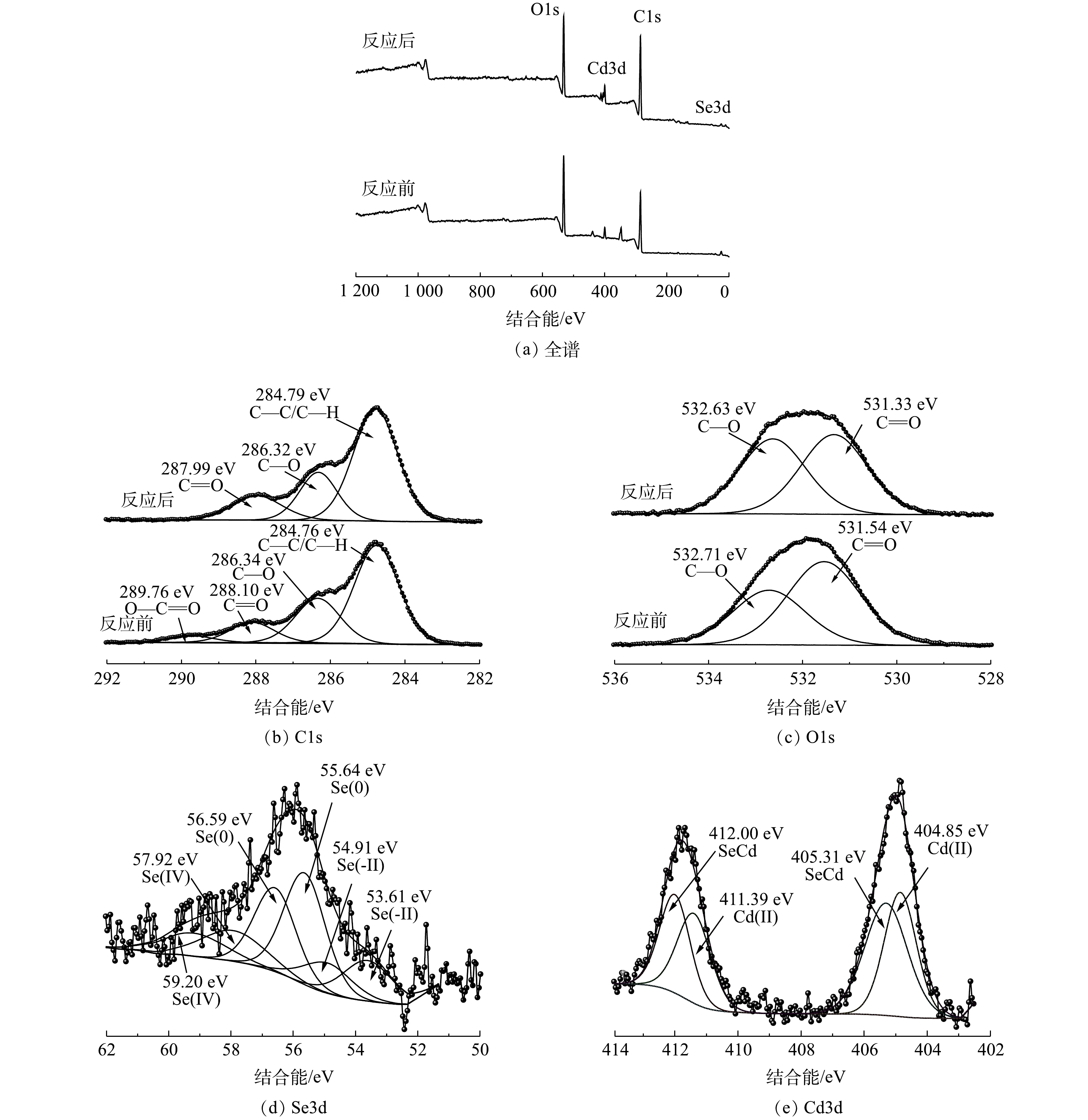
利用XPS分析在处理硒、镉前后厌氧粒污泥元素价态情况,结果如图4所示。全谱显示(图4(a)),反应后出现了在51.31~62.49 eV的Se3d峰和在402.50~414.09 eV处的Cd3d峰,这进一步为厌氧颗粒污泥捕获Se(Ⅳ)和Cd(Ⅱ)提供了证据。
厌氧颗粒污泥C1s精细谱图如图4(b)所示。C1s分峰拟合结果表明,存在C—C/C—H、C—O、C=O和O—C=O这4种化学键,在接种厌氧颗粒污泥中,其对应的结合能为284.76、286.34、288.10和289.76 eV。在处理含Se(Ⅳ)和Cd(Ⅱ)废水后,发现C=O和O—C=O结合能和峰面积发生了明显变化,这可能是Se(Ⅳ)和Cd(Ⅱ)与这些含氧官能团发生作用所致[20]。O1s谱图如图4(c)所示。C=O和C—O峰的结合能分别由接种厌氧颗粒污泥前的531.54 eV和532.71 eV转变到了处理后的531.33 eV和532.63 eV,峰面积也发生了相应的改变,这也说明含氧官能团参与了Se(Ⅳ)和Cd(Ⅱ)的去除。
图4(d)为反应后Se3d的精细谱分峰结果,Se3d可拟合为Se(−II)、Se(0)和Se(Ⅳ)峰。结合能为53.61 eV和54.91 eV处的峰值对应于Se(−II),这说明Se(Ⅳ)被还原成Se(−II),这也为CdSe纳米颗粒形成提供了可能[21]。结合能为55.64 eV和56.59 eV处的Se3d峰被认为是元素硒(Se(0))[22],结合能为57.92 eV和59.20 eV处的峰值对应于Se(Ⅳ)。半定量分析结果表明,污泥中Se(−II)、Se(0)和Se(Ⅳ)分别占22.93%、54.17%和22.89%,这说明大多数亚硒酸盐被厌氧颗粒污泥还原成元素Se(0)及Se(−II)。Cd3d的精细谱分峰结果如图4(e)所示。结合能位于405.31 eV和412.00 eV处的峰对应为CdSe,半定量分析结果表明,污泥中CdSe和Cd(Ⅱ)分别占52.76%和47.24%。这说明厌氧颗粒污泥能够生成纳米CdSe,这与SURESH等[23]之前报道的CdSe结合能一致。404.85 eV和411.39 eV处的Cd(Ⅱ)峰表明,厌氧颗粒污泥吸附了水中的Cd2+。此前作者也发现厌氧颗粒污泥具有吸附镉(5 mg·L−1)的能力[10]。
-
1)细菌多样性分析。由表2可知,原始污泥、第Ⅰ阶段和第Ⅱ阶段的细菌高通量测序均获得52 000条以上的有效序列,所有样本的覆盖率达到99.9%,能反映接近厌氧颗粒污泥中全部物种情况。微生物丰富度可通过ACE和Chao指数反映[24],与接种厌氧颗粒污泥相比,第Ⅰ、Ⅱ阶段样品的ACE指数分别由385.2下降到342.7和329.7;Chao指数分别由381.4下降到357.0和325.4。这表明处理低碳源硒、镉废水后,厌氧颗粒污泥中细菌丰富度下降。微生物多样性可采用Shannon指数(正相关)和Simpson指数(负相关)进行估算[25]。与原始污泥相比,第Ⅰ、Ⅱ阶段样品显示Shannon指数下降,Simpson指数增加,表明处理低碳源硒、镉废水后,厌氧颗粒污泥中细菌多样性下降。
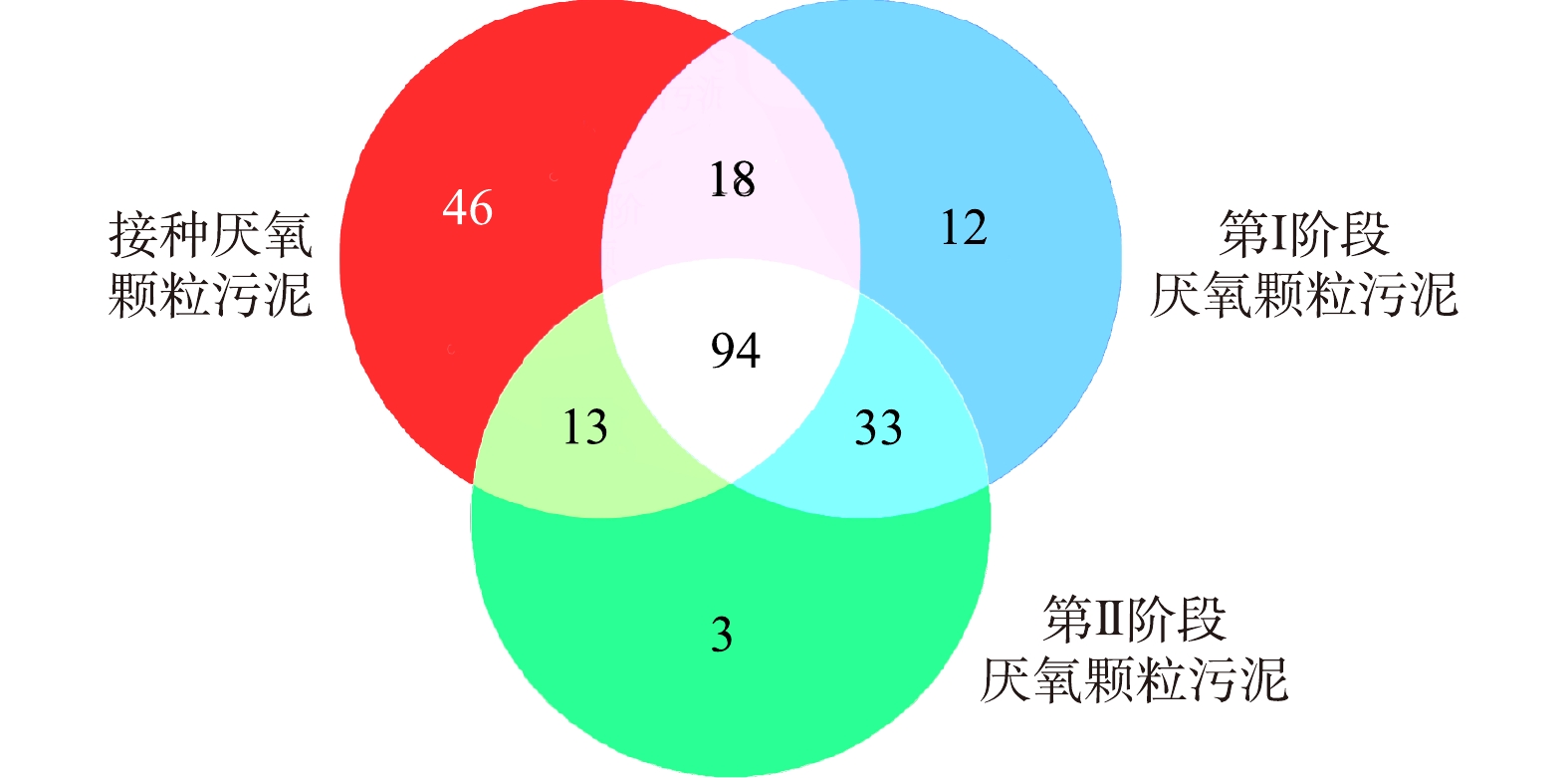
2)细菌共有及独有物种分析。接种厌氧颗粒污泥与2个阶段样品在属水平的Venn图如图5所示。接种厌氧颗粒污泥与第Ⅰ、Ⅱ阶段之间共有菌属分别为112种与107种;第Ⅰ、Ⅱ阶段间共有菌属为127种;而接种厌氧颗粒污泥、第Ⅰ、Ⅱ阶段样本各自独有的菌属分别为46、12和3种,三者共有的菌属为94种。这表明在不同碳源浓度下,细菌种类组成变化幅度较少,以共有菌属为主。
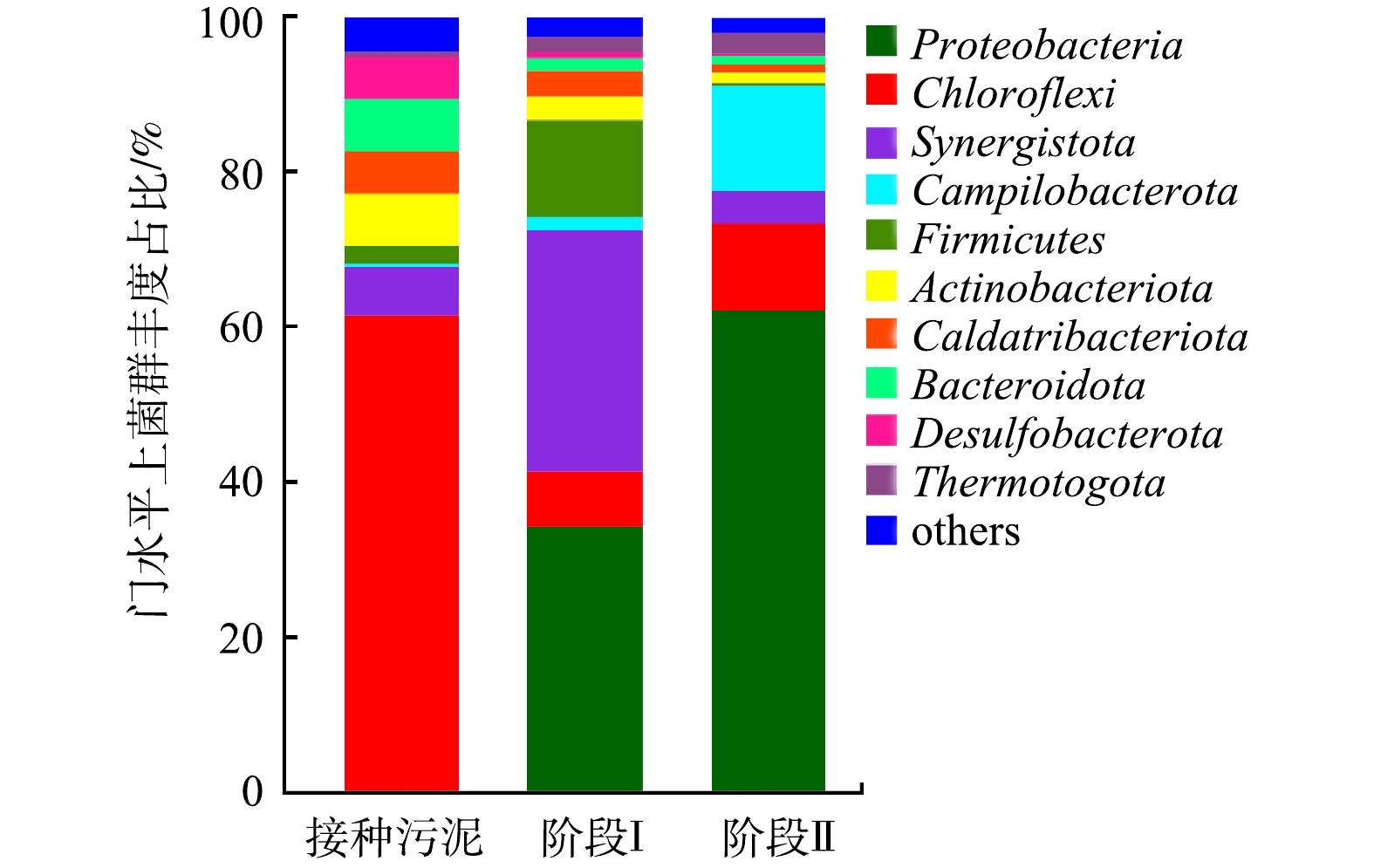
3)细菌群落结构解析。厌氧颗粒污泥中在门水平相对丰度超过1%的细菌群落组成见图6。在接种厌氧颗粒污泥中,以Chloroflexi(61.41%)、Bacteroidota(6.8%)、Actinobacteriota(6.76%)、Synergistota(6.29%)、Caldatribacteriota(5.45%)和Desulfobacterota(5.41%) 6种细菌含量较多。在处理低碳源酸性硒、镉废水后,Chloroflexi丰度急剧下降至7.15%~11.24%;Bacteroidota丰度下降至1.07%~1.62%;Actinobacteriota丰度急剧下降至1.49%~3.08%;Caldatribacteriota丰度下降至1.03%~3.33%;Desulfobacterota丰度下降至0.29%~0.75%。CHEN等[26]的研究结果表明,在长期高浓度重金属污染的黄河沉积物中,Chloroflexi的丰度极低,这表明Chloroflexi在重金属环境中较难存活。此外,以上6类细菌大多是异养菌,在低浓度碳源下生长受到影响。Synergistota丰度在反应第Ⅰ阶段上升至31.16%,第Ⅱ阶段下降至4.18%;Firmicutes丰度由接种污泥的2.26%上升至第Ⅰ阶段的12.48%,而后又下降至第Ⅱ阶段的0.31%,这说明他们对硒、镉具有一定的去除能力;但COD值由100 mg·L−1降低到50 mg·L−1后,其相对丰度受到影响。ZHANG等[7]曾报道Firmicutes为含硒(1~5 mmol·L−1)废水活性污泥处理中的优势菌,但它是在过量乙酸作碳源条件下富集形成的。Proteobacteria(0.06%)、Thermotogota(0.69%)和Campilobacterota(0.4%)在接种厌氧颗粒污泥中所占比例很低,但是处理低碳源酸性硒、镉废水之后,这3种菌的比例分别升高34.11%~62.17%、2.02%~2.62%和1.71%~13.61%。Proteobacteria是1种外膜由脂多糖组成的革兰氏阴性菌门,具有吸附Cd(Ⅱ)的能力[27]。有研究结果[28-29]也表明,Proteobacteria是去除硒酸盐和镉的优势菌。因此,Proteobacteria、Synergistota、Firmicutes、Thermotogota和Campilobacterota等是处理含硒、镉酸性废水的优势菌。
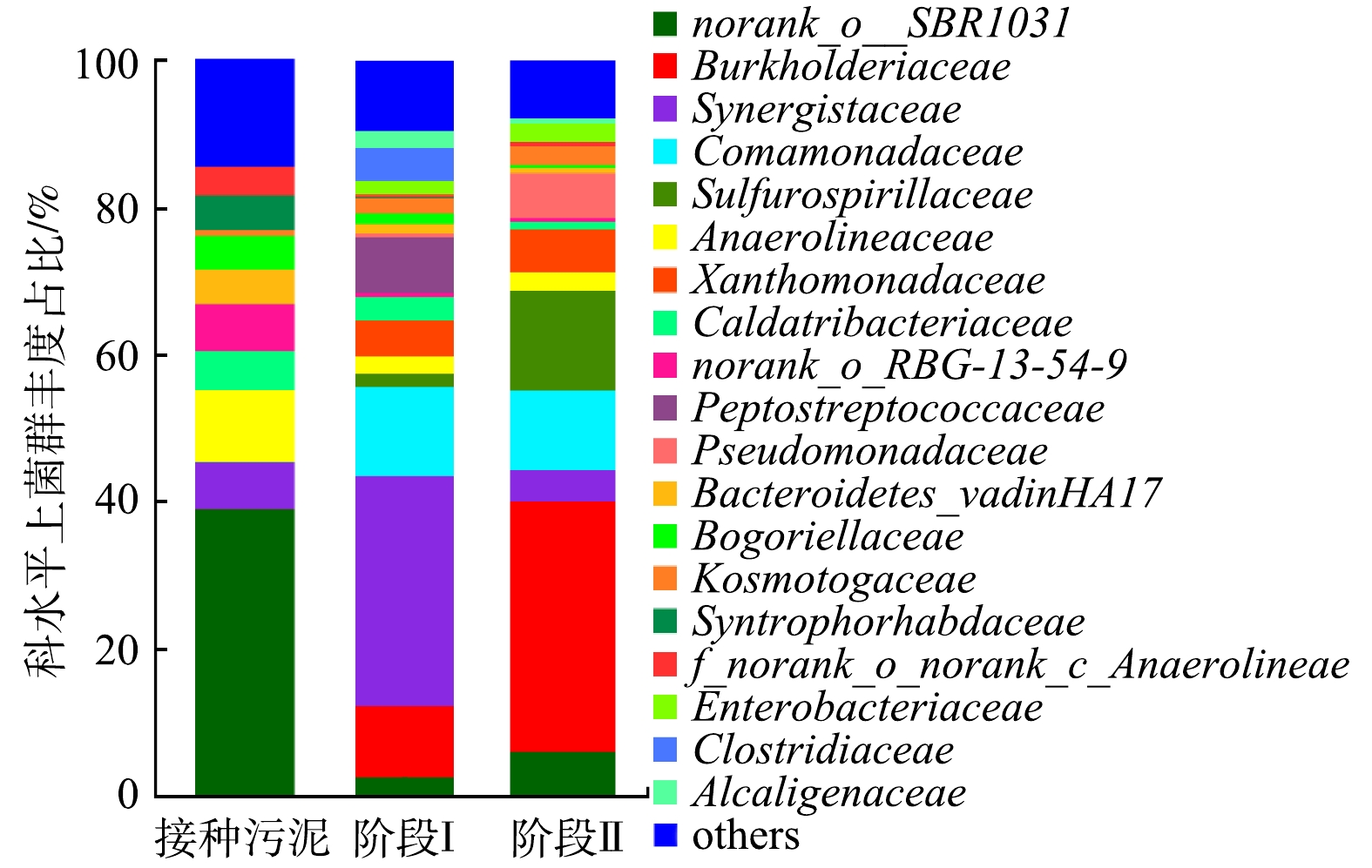
细菌在科(Family)水平上所占比例大于1%的群落组成及丰度如图7所示。与接种污泥相比,经过低碳源酸性硒、镉废水处理后,Burkholderiaceae、Synergistaceae、Comamonadaceae、Sulfurospirillaceae、Xanthomonadaceae和Pseudomonadaceae等相对丰度增加显著。KHOEI等[30]从土壤中分离出1株了Burkholderia(伯克霍尔德菌属),发现该菌能在96 h内将79 mg·L−1的亚硒酸盐还原为Se(0)。此外,DOURADO等[31]的研究结果表明,Burkholderia sp. SCMS54具有降低镉毒性的作用。DAI等[32]的研究中发现Pseudomonadaceae和Comamonadaceae可以去除高浓度的镉(20 mg·L−1)。Pseudomonadaceae对硒也具有较好的去除能力,LUSA等[33]的研究中发现Pseudomonas sp. strain T5-6-I能促进甘蓝对Se(Ⅳ)的吸收。推测在低碳源浓度下Burkholderiaceae、Comamonadaceae、Pseudomonadaceae、Synergistaceae、Sulfurospirillacea和Xanthomonadaceae对硒、镉有较好的去除作用,因此,对低碳源酸性含硒、含镉的废水,可通过富集这些菌进行生物强化处理。Synergistaceae和Comamonadaceae由接种的6.29%和0.03%分别升高至第Ⅰ阶段的31.16%和12.12%,而后降低至第Ⅱ阶段(进水COD 50 mg·L−1)的4.18%和10.84%;Peptostreptococcaceae和Clostridiaceae由接种的0.05%和0.02%分别升高至第Ⅰ阶段的7.46%和4.27%,而后降低至检测限以下(第Ⅱ阶段)。这表明Synergistaceae、Comamonadaceae、Peptostreptococcaceae和Clostridiaceae在低碳源(COD为50 mg·L−1)条件下不占优势。
4)古菌群落结构解析。在不同阶段厌氧颗粒污泥中丰度超过1%的古细菌在属(Genus)水平上群落组成如表3所示,Methanobacterium(67.53%)、Methanosaeta(27.40%)、norank_f__norank_o__norank_c__Bathyarchaeia(2.55%)和Methanolinea(1.12%)为接种厌氧颗粒污泥中的优势菌属。在第Ⅰ阶段末,Methanobacterium相对丰度降低到46.98%,但在第Ⅱ阶段末升高至80.49%。易敏等[34]发现,厌氧颗粒污泥中Cr (32.3 mg·kg−1)、As (95.1 mg·kg−1)和Zn (761.8 mg·kg−1)等重金属含量很高时,Methanobacterium是其中的优势菌。与接种污泥相比,Methanosaeta相对丰度在第Ⅰ阶段末期升高到51.00%,在第Ⅱ阶段末下降至16.56%,因此,这类古菌丰度受到低碳源(COD 50 mg·L−1)影响。HUANG等[35]的研究结果表明,在Cu(Ⅱ)和Cd(Ⅱ)短期暴露条件下,在厌氧生物反应器中处理2-氯酚时,Methanosaeta是主要的优势古细菌属之一。作者此前利用厌氧颗粒污泥短期去除浓度分别为10 mg·L−1和5 mg·L−1的Se(Ⅵ)和Cd(Ⅱ)时发现,Methanosaeta和Methanobacterium均为优势菌属[36]。这些研究结果说明,Methanosaeta和Methanobacterium具有较强的重金属耐受能力。一般酸性矿山废水特征为厌氧、低pH、多种重金属共存,厌氧颗粒污泥对此有较好的耐受能力,此前报道的厌氧颗粒污泥去除硒、镉等重金属废水大多是外加高碳源浓度[8-10]。本研究结果表明,通过驯化、富集出特定的细菌或古菌,能够适应低碳源、酸性硒、镉废水环境,这为酸性矿山废水低碳源处理提供了参考。
2.1. SBR运行效果
2.2. 厌氧颗粒污泥形态分析
2.3. 元素价态分析
2.4. 微生物群落结构特征解析
-
1)在进水COD为100 mg·L−1时,厌氧颗粒污泥对镉有较好的去除效果,平均去除率为(96.67±2.70)%;总硒和四价硒的平均去除率在前16个周期内较稳定,分别为(97.07±3.17)%和(98.86±1.84)%。当进水COD下降到50 mg·L−1时,厌氧颗粒污泥对硒、镉的去除率不高。
2)镉的去除以生物吸附为主,硒的去除主要以微生物的还原和吸附为主,且在微生物表面生成了硒(Se(0))和硒化镉(CdSe)纳米颗粒物。C—O、C=O和O—C=O等含氧官能团在硒、镉生物去除中发挥了重要作用。
3)在细菌门水平上,Proteobacteria(34.11%)、Synergistota(31.16%)和Firmicutes(12.48%)为优势菌;在科水平上,具有较强硒、镉去除能力的Burkholderiaceae、Comamonadaceae和Pseudomonadaceae丰度最高。具有较强重金属耐受能力的Methanosaeta和Methanobacterium在古菌中占优势地位。




 下载:
下载: