-
近年来,工业排水的成分日益复杂,传统的废水处理技术难以高效处理水体中的难降解污染物。高级氧化技术(advanced oxidation technologies, AOTs)是随着水处理技术发展与进步出现的新型技术,其特点是通过各种过程产生羟基自由基(·OH)及其他活性氧物种氧化环境中的难降解有机污染物,促使有机物最终氧化降解为CO2、H2O及其他无机离子,其具有减少甚至消除二次污染的优点。常用的处理难降解有机废水的高级氧化技术有Fenton法[1-2]、光催化法[3-4]、超临界水氧化法[5]、超声法[6-7]、低温等离子体法[8]等。介质阻挡放电等离子体(dielectric barrier discharge plasma,DBDP)作为低温等离子体的一种[9],是一种将绝缘介质插入放电空间的气体放电形式,由于放电电极与介质壁紧贴,放电电场强,可以产生高浓度的活性氧物质,包括O3、H2O2、·OH、·O、O2·−等,其中·OH的氧化性最强,是降解难降解有机污染物的主要物质。然而,该技术存在着能量利用率低、放电体系激发的光能和其他能量不能够被充分利用等缺点。因此,研发更高效的处理方法是该技术的主要发展方向之一。
已有研究表明,催化剂的添加可以实现对低温等离子体体系中不同物化效应的正向激活。陈颖[10]采用DBDP水上等离子协同TiO2 光催化术对17β-雌二醇进行了降解,处理30 min后,单独DBDP体系对17β-雌二醇去除率仅为72%,而向DBDP体系中投加TiO2光催化剂后17β-雌二醇的去除率提高了22%。王慧娟等[11]以酸性橙 II 作为目标污染物,建立了脉冲放电/活性炭协同脱色体系。在反应60 min后,脉冲放电/活性炭体系中染料的脱色效率较单独脉冲放电体系提高30%。SHEN等[8]研究了Fe2+协同脉冲放电降解苯酚的过程,结果表明,加入Fe2+比未加Fe2+时的苯酚降解率提高了24%。以上研究表明,低温等离子体协同催化技术可以应用于废水的处理,提高了废水处理的效率。
纳米氧化锌(ZnO)作为一种过渡金属氧化物,同TiO2半导体光催化材料类似,以高稳定性、价廉、无毒等优势在光催化领域里倍受青睐[12-13]。有研究证明,ZnO不仅可以催化O3生成·OH,进而以更快的速度氧化有机物[14-15],还可以催化H2O2生成·OH,有研究[16]发现,同等条件下的ZnO/H2O2光化学体系较TiO2/H2O2光化学体系对有机物具有更优的降解效果。结合DBDP作用过程紫外光、O3和H2O2各物理和化学效应共存的优势,本研究考虑将纳米ZnO引入DBDP反应体系,协同处理水体有机污染物,验证其催化作用及效果。与纳米TiO2的单纯光催化作用相比,纳米ZnO不仅可以充分利用DBDP体系的光效应,还可以与DBDP体系中产生的H2O2及O3作用,形成多效催化,促使放电体系中有更多的·OH生成,达到提高难降解有机污染物的处理效果的目的,同时提高DBDP体系的能量利用效率。
基于此,本研究以BPA这一典型的环境内分泌干扰物为目标污染物,建立了DBDP/ZnO协同水处理体系。考察了DBDP/ZnO协同体系中催化剂的添加量、载气种类、溶液初始pH对BPA降解率及能量利用效率的影响,同时考察了不同自由基捕获剂的对BPA降解的影响,以及体系中COD的去除和BPA溶液UV-vis光谱的变化,以期为该协同体系的进一步应用提供实验基础。
全文HTML
-
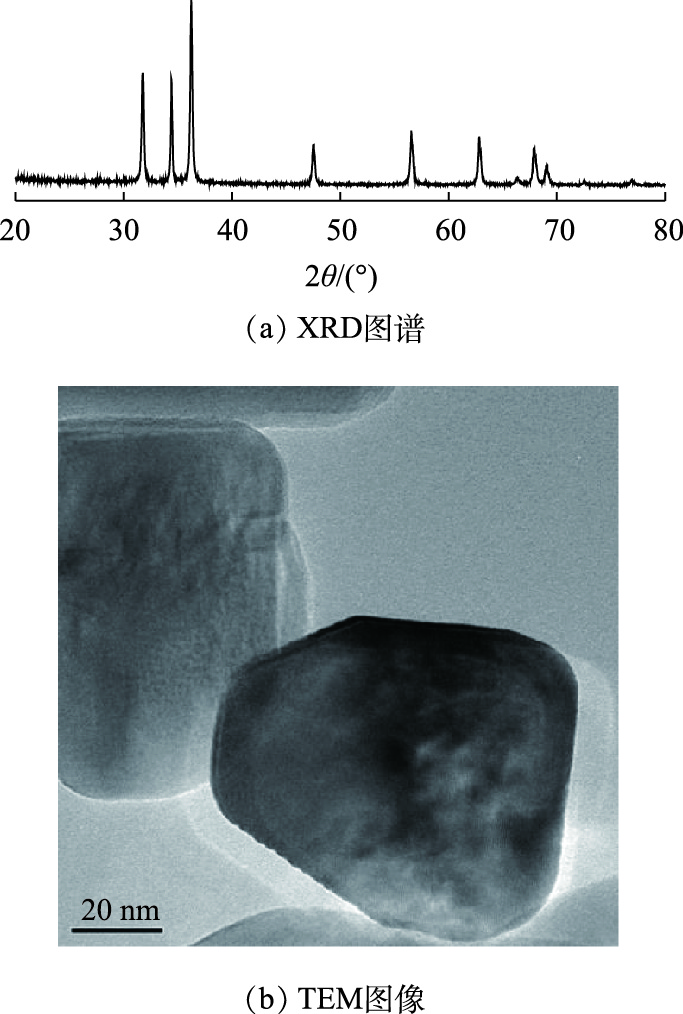
研究使用BPA(分析纯)配制目标模拟废水。实验中所需纳米ZnO采用水热合成法制备,制备方法如下[17]:以无水乙醇为溶剂,配制0.1 mol·L−1乙酸锌溶液以及0.8 mol·L−1氢氧化钠溶液。将40 mL氢氧化钠溶液用滴定管缓慢滴入40 mL乙酸锌溶液中,同时进行磁力搅拌至乳白色溶液。将得到的溶液移至100 mL的聚四氟乙烯内胆中并密封,在烘箱中150 ℃保温2 h后取出,冷却至室温后,离心得到沉淀物,用无水乙醇洗涤所得产物4次。置于60 ℃烘箱内干燥12 h得到纳米ZnO产物。所制备的纳米ZnO产物表征结果如图1所示。图1(a)为水热法制备的纳米氧化锌的XRD图谱,其中纳米氧化锌在31.7°、34.4°、36.5°、47.5°、56.6°、62.9°、67.9°、69.1°、72.6°处均存在明显的晶面峰,分别对应(100)、(002)、(101)、(102)、(110)、(103)、(112)、(201)、(004)晶面,制备的纳米ZnO的峰与JCPDS卡上列出的ZnO(00-036-1415)的标准图一致,这表明制备的ZnO具有六方纤锌矿结构,其中 (101) 的峰强度最高,证明ZnO晶体是沿着(101)方向生长的;没有多余杂质峰,表明制备的ZnO纯度较高。图1(b)为ZnO的TEM图像,可见所制备的样品为片状,直径约为80 nm,具有多边形边界,厚度约为40 nm。
-
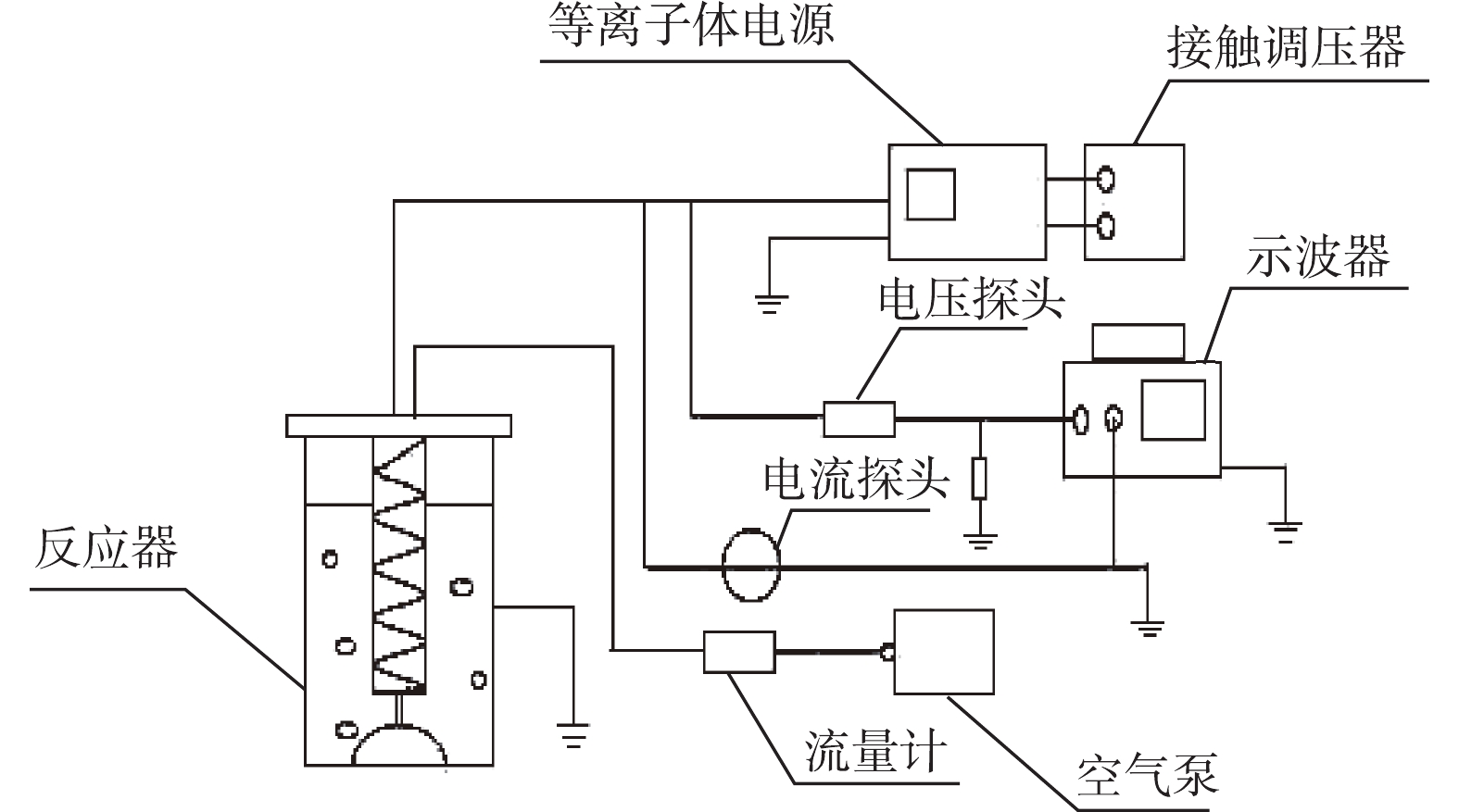
实验系统如图2所示,实验系统由供电系统、电气检测系统、载气系统、反应系统4个部分构成。供电系统包括低温等离子体实验电源、接触调压器。电气检测系统由示波器、电压探头、电流探头组成。载气系统包括气泵以及流量计。
反应系统的构成如图3所示,反应器的主体是一个透明玻璃圆筒,通过紧缠在玻璃圆筒外部的铝箔胶带连接接地电极,弹簧状不锈钢(线径4 mm)为高压电极,紧贴高压电极的注射器状石英玻璃管(壁厚2 mm、内径40 mm)为介质。在实验过程中,气体由进气口进入,在石英管进行放电后,经由与石英管相连的曝气石释放到目标水体中。曝气过程有利于催化剂在反应器内部的均匀分布。
-
实验所用目标废水由BPA和去离子水配制,实验时溶液的初始浓度为20 mg·L−1,经测量初始pH为6.8,单次实验所用废水的体积为500 mL,每次放电总时间为40 min,每隔10 min取样2 mL测定液相BPA残留浓度。溶液pH由pH计(Orion 828)测定;使用岛津-2600紫外分光光度计对废水进行全波段扫描;污染物的可生化性由COD测量(连华科技5B-3A)进行检测;使用Waters 2489高效液相色谱仪测定降解过程中残留的BPA浓度,计算BPA的降解率,所使用的液相条件为:流动相为70%的乙腈(色谱纯)和30%的超纯水,以1 mL·min−1的流速通过Waters C18色谱柱,设置检测波长为276 nm。本研究所有结果均测定3次,取平均值作为最终实验结果,以保证实验的准确性。本研究根据式(1)计算能量利用效率G50。
式中:G50 为能量利用效率,g·(kWh)−1;C0 为反应物初始质量浓度,g·L−1;t50 为反应物被降解50%时所用的时间,h;E为相对能量密度,表示等离子体废水处理装置单位体积内所注入的能量,kW·L−1,可根据式(2)进行计算。
式中:P为电源系统的功率,kW;V为装置处理的溶液体积,L。
式中:f为输出电压频率,Hz;C为实验电源内部取样电容,F;S为利萨如图形面积。
1.1. 实验材料
1.2. 实验装置
1.3. 实验方法
-
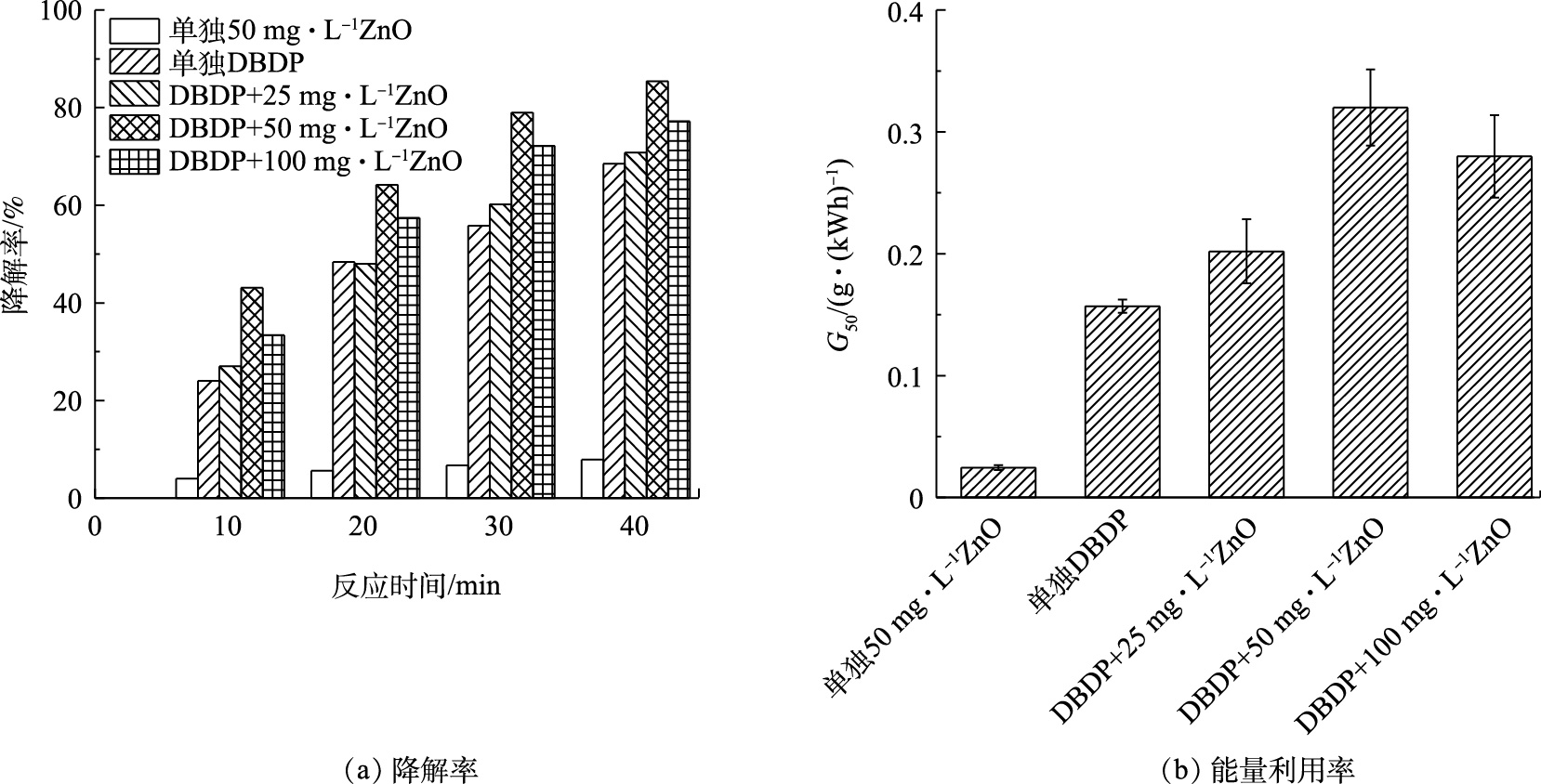
研究首先对比考察了放电电压为5 kV、载气气体为空气、进气量为0.5 L·min−1时,不同浓度的催化剂对BPA降解率以及体系能量利用率的影响,同时对比了单独DBDP作用和单独纳米ZnO (50 mg·L−1)吸附对体系中BPA的降解率和能量利用效率的影响,结果如图4所示。由图4(a)可见,随着放电处理时间的延长,在DBDP单独作用体系和DBDP/ZnO协同体系中,BPA的降解整体呈上升趋势,且协同体系较单独的DBDP体系对BPA降解率更高。当处理时间为40 min时,单独DBDP体系与单独ZnO吸附体系的降解率分别为68.5%和7.9%,在催化剂浓度为25、50、100 mg·L−1时,所对应的BPA降解率分别为70.8%、85.4%和77.2%。在催化剂添加量为50 mg·L−1时,BPA的降解率达到最大,与未添加催化剂的体系相比,降解率提高了17.0%。图4(b)归纳了相同条件下,不同浓度的催化剂对系统的能量利用率[18]的影响规律。结果表明,催化剂的添加显著提高了系统的能量利用率,当ZnO添加浓度为50 mg·L−1时的能量利用率最高,为0.32 g·(kWh)−1, 而在单独DBDP体系中的G50仅为0.157 g·(kWh)−1,这是由于在介质阻挡放电过程中产生O3、H2O2等活性离子,纳米ZnO既可以催化O3、H2O2生成氧化性更强的·OH[14],同时也可以利用体系中产生的紫外光进行光催化作用生成·OH[19],使整个系统的能量利用率提高,从而达到更好的处理效果。然而,当催化剂浓度继续提高至100 mg·L−1时,催化效果反而降低,且系统的能量利用率下降至0.28 g·(kWh)−1,与50 mg·L−1相比,降低了0.04 g·(kWh)−1。这可能由2个原因导致的:一是由于催化剂添加量过大时,多余的催化剂产生团聚,使催化剂的效果降低,从而降低了污染物的去除率以及系统的能量利用率[20-21];二是当催化剂浓度增加后,溶液中的悬浮颗粒增多,导致紫外光的透射性降低,进而阻碍了等离子体通道内光效应对ZnO光催化活性的诱导,最终导致BPA降解率下降[22]。因此,适量催化剂的添加有利于提高DBDP体系的能量利用率,有利于污染物的去除。
-
在介质阻挡放电体系中,气体在放电空间被电离,从而产生活性粒子。因此,载气种类会极大的影响到放电体系中活性氧物质的种类及数量。考察了载气分别为氧气、空气、氮气时BPA降解率的变化。研究过程中ZnO的添加浓度为50 mg·L−1、电压为5 kV、载气量为0.5 L·min−1。图5反映了不同载气种类下BPA的降解效果。
添加催化剂的协同体系中的BPA降解率变化结果如图5(a)所示。可以看出,在氧气条件下,在处理时间为20 min时,BPA的降解率达到了100%,与相同处理时间的空气和氮气条件下分别提高了35.8%和78.2%。而根据图5(b)可知,当载气种类为氧气时,G50为1.82 g·(kWh)−1,其远远高于空气(0.32 g·(kWh)−1)和氮气条件(0.098 g·(kWh)−1)下的能量利用效率。
由此可见,载入气体的氧分子越多,系统的能量利用效率越高,降解效果越好。这是由于当氧分子载入放电系统时,氧分子与高能电子碰撞产生·O(式(4))。
·O既可以氧化BPA分子,也可以在体系中转化为O3,对有机物产生降解的作用,而在有催化剂存在的体系,纳米ZnO催化剂可以将O3转化成氧化性更强的活性氧粒子,提高系统中高效活性粒子的浓度[15]。因此,氧气系统的氧化性更强,催化效果更好,BPA的降解率更高。空气中的氧气含量约占21%,所以空气条件下的降解效果次之。而当载入气体为氮气时,降解40 min后,BPA的降解率仅为34.6%,这是因为在介质阻挡放电通道内,氮气更不易分离,N2在放电过程中的分离过程[23-24]如式(5)所示。
当氮气作为载气时,体系中的氧化物质主要为·N,氧化能力更弱,因此,在氮气体系中BPA的降解率最小。空气中的氧气含量约占21%,氮气占70%,所以在空气条件下的能量利用率及BPA降解率介于氧气和空气之间。
-
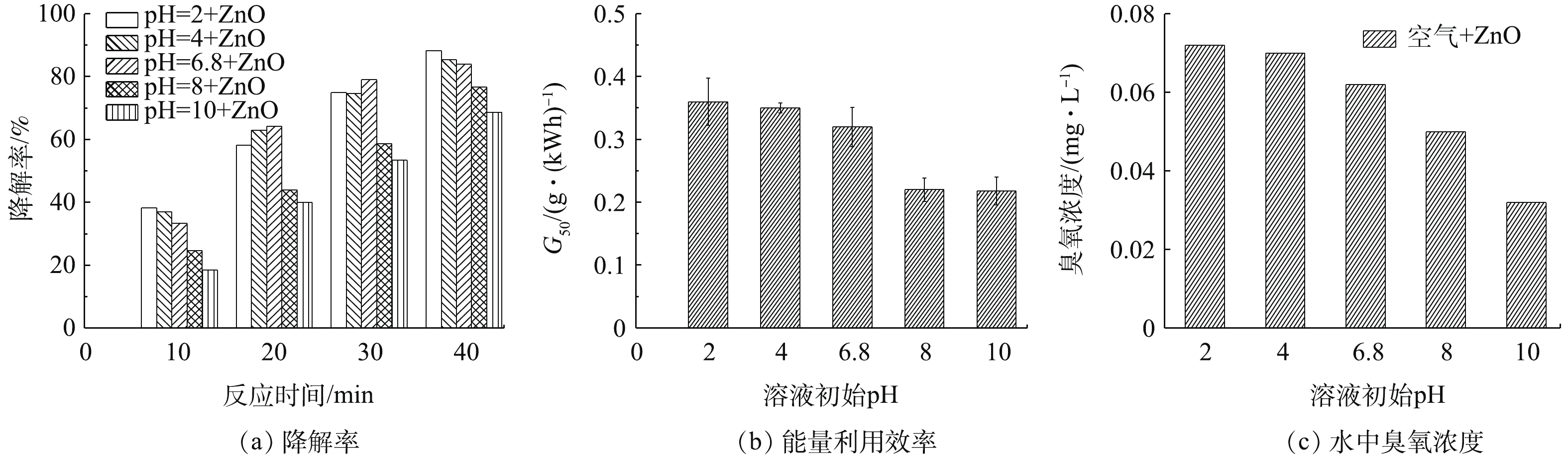
溶液初始pH影响DBDP体系中活性物质的产生。实验选取电压为5 kV,载气为空气,进气量为0.5 L·min−1,纳米ZnO浓度为50 mg·L−1,实验选取pH分别为2、4、6.8、8、10,考察不同pH下BPA的降解效果。DBDP与纳米ZnO协同系统中,溶液初始pH对BPA降解率的影响如图6所示。由图6(a)可以看出,相对于中性和碱性溶液,酸性溶液更有利于BPA的降解,当pH为2、4、6.8、8、10时对应的BPA降解率分别为88.3%、85.4%、83.9%、76.7%和68.6%。当pH=2时,比pH=10的条件下BPA的降解率高出19.7%。图6(b)为pH对DBDP/ZnO体系的能量利用率的影响。结果表明,随着pH的增大,系统的能量利用率逐渐减小。此外,测定了DBDP/ZnO体系中,放电40 min时的臭氧浓度,当溶液初始pH=2时,臭氧浓度为0.072 mg·L−1,且随着pH的增大,臭氧浓度逐渐降低,当pH=10时,水体中的臭氧浓度仅为0.032 mg·L−1,如图6(c)所示。这主要是因为介质阻挡放电可以产生大量的臭氧,臭氧在酸性条件下溶解性较强,更加稳定,特别是臭氧能快速的破坏苯环结构,使BPA的浓度降低[25]。此外,在碱性条件下,反应过程中会产生碳酸根离子等中间产物,可以对自由基进行捕收,导致降解BPA分子的活性物质浓度大大减少。
-
在介质阻挡放电的过程中会产生自由基降解水中的污染物,有研究[26-27]证明,在水中过氧化氢与·OH生成的HO2·会导致O2·–的形成。而O2·–与·OH、HO2·可以反应生成1O2,如式(6)~式(9)所示。
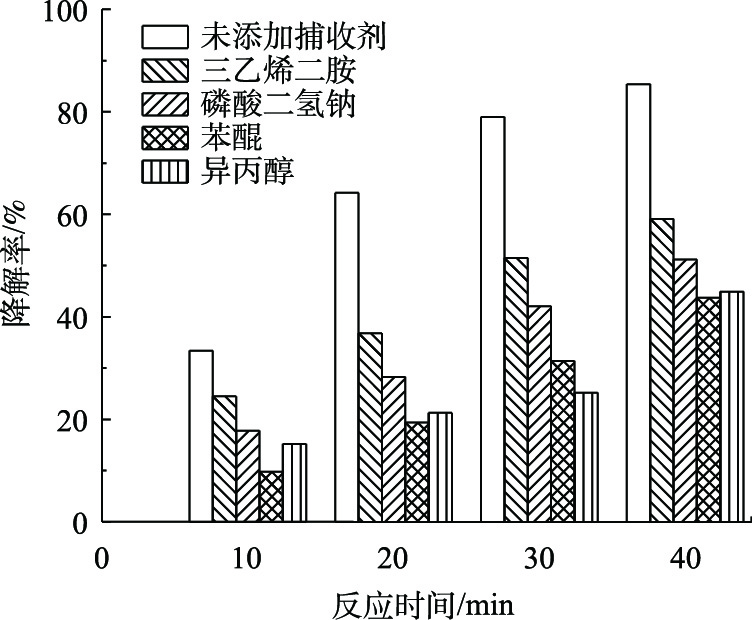
为了证明在BPA过程中起作用的自由基,本研究使用三乙烯二胺、磷酸二氢钠、苯醌和异丙醇分别作为1O2、电子、O2·–、·OH的捕获剂。考察了在DBDP/纳米ZnO体系中,捕获剂的添加对BPA降解率的影响。添加的捕获剂浓度均为1.0 mmol·L−1,实验结果如图7所示。
与未添加捕获剂相比,捕获剂的添加均导致BPA的降解率降低,由图7可知,在降解40 min后,三乙烯二胺、磷酸二氢钠、苯醌和异丙醇的添加分别使得BPA的降解率降低了26.3%、34.2%、41.7%、40.5%。这说明1O2、电子、O2·–、·OH均在DBDP/ZnO体系降解有机物的过程中起重要作用。
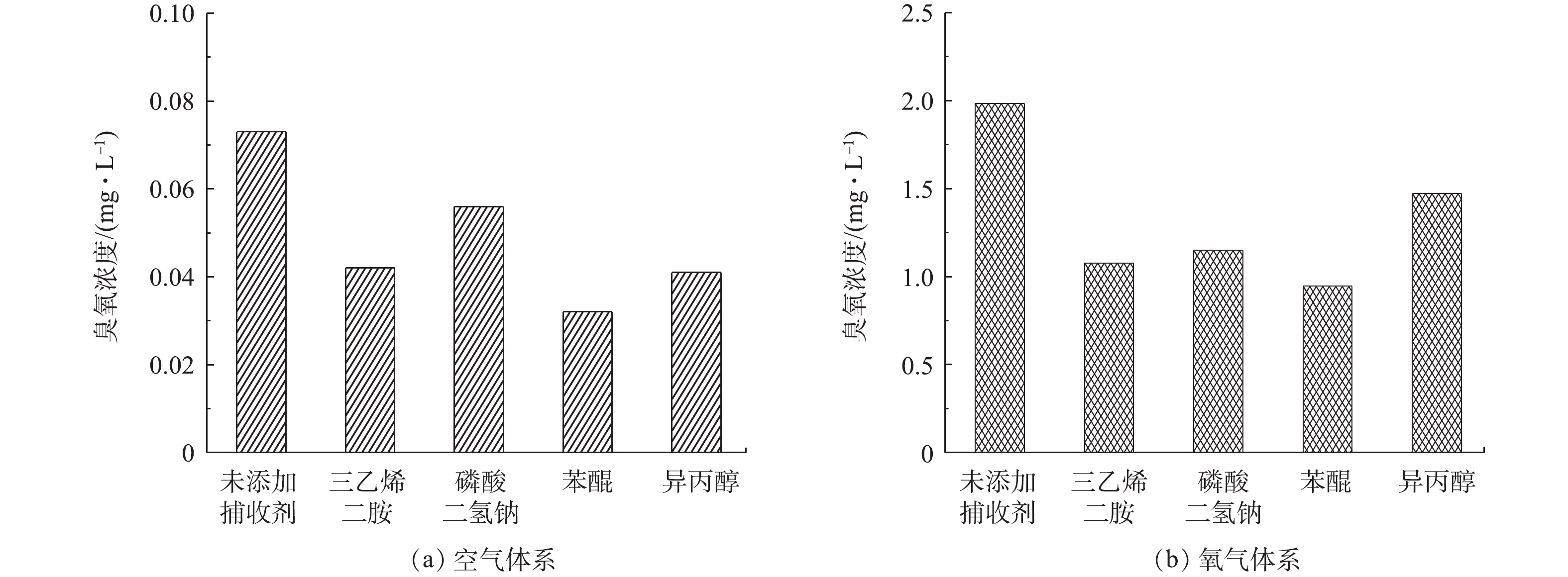
为了进一步说明系统中活性氧物质的影响,对相同操作条件下的臭氧浓度进行检测,结果如图8所示。在未添加捕获剂时,空气体系下降解40 min后的臭氧浓度为0.073 mg·L−1,氧气体系下的臭氧浓度远远高于空气体系,为1.984 mg·L−1。在添加捕获剂之后,40 min后的臭氧浓度均有所下降,进一步证明了1O2、电子、O2·–、·OH是DBDP/ZnO体系的关键活性物质。
-
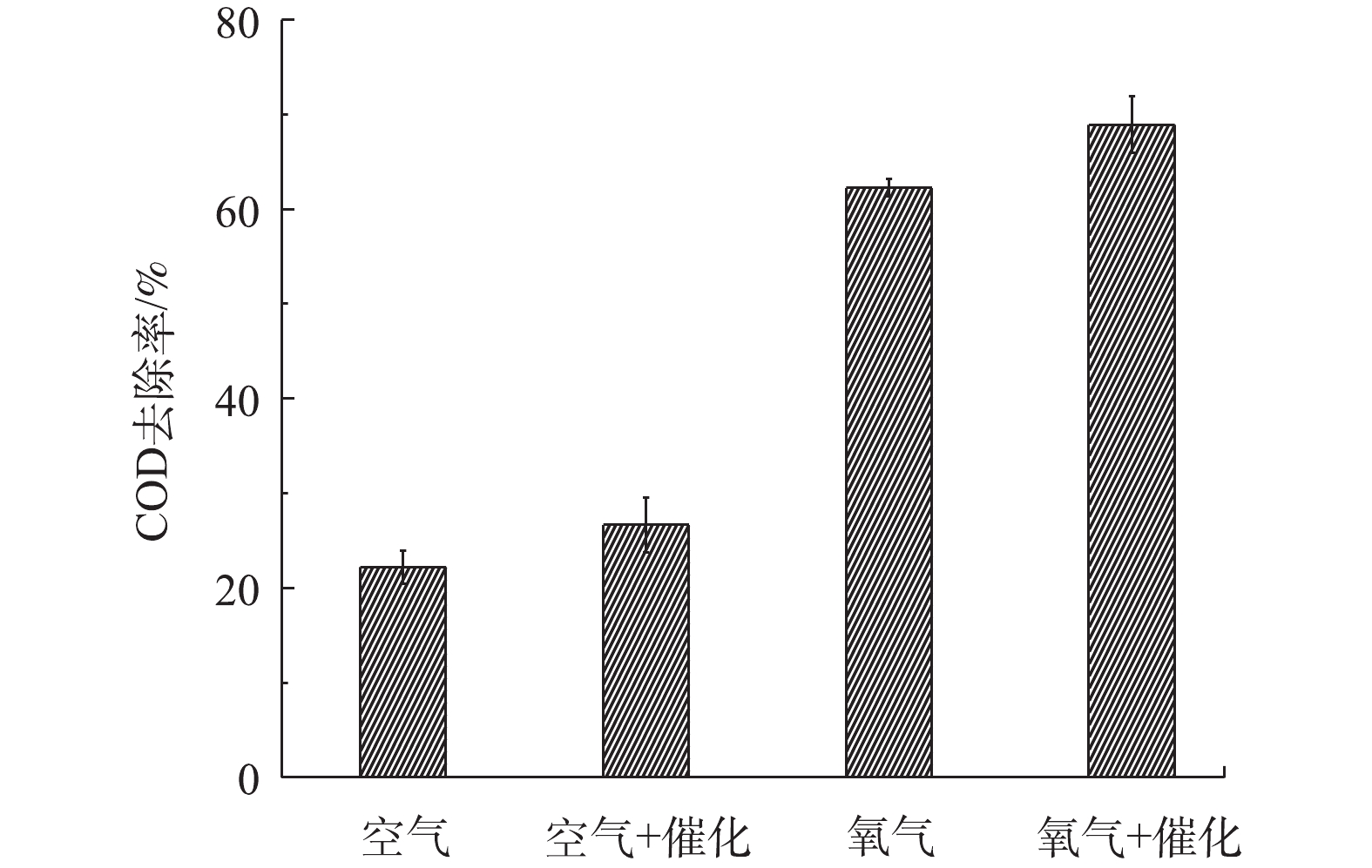
在介质阻挡放电降解BPA时,会产生各种中间产物,中间产物继续降解生成H2O和CO2。溶液COD值可以表示水体的可生化性。图9为40 min放电处理后,不同反应体系中BPA溶液的COD去除率。以空气为载气时,单独DBDP体系和DBDP/ZnO体系中BPA溶液的COD去除率分别为22.2%和26.7%,而以O2为载气的2种反应体系中COD去除率分别为62.2%和68.9%。由此可见,添加催化剂可以提高BPA溶液的可生化性,与空气为载气的体系相比,氧气载气体系中可产生更多的活性氧物种,从而促成BPA更有效的降解,进而提高了BPA溶液的可生化性。
-
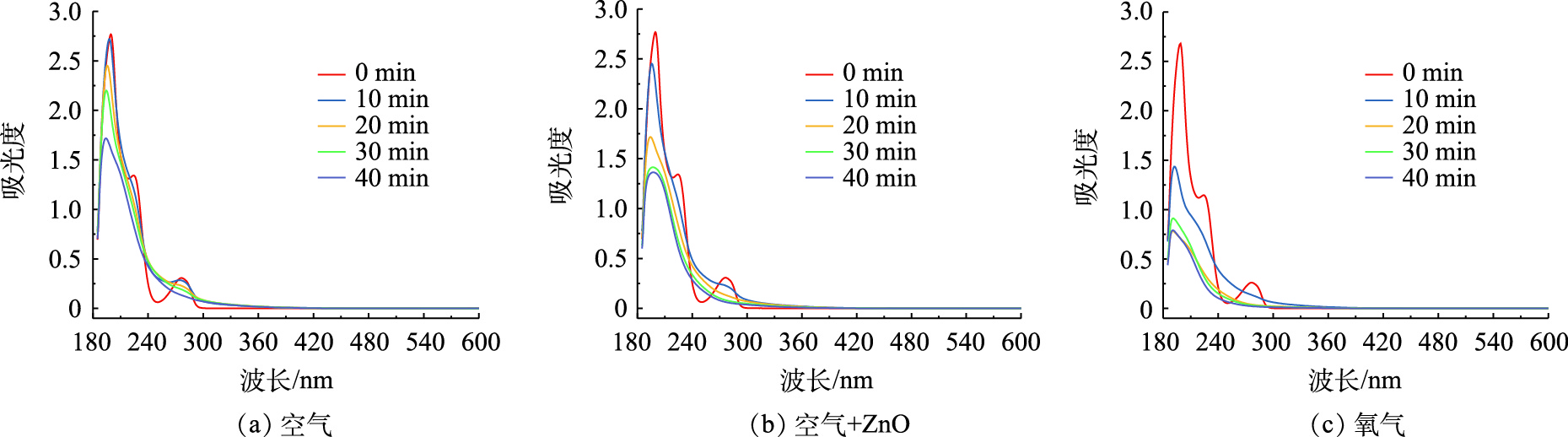
为了更系统的表现BPA的降解过程,使用紫外-可见全波段扫描仪对BPA的降解过程进行检测,图10分别表示空气条件、空气/纳米ZnO条件、氧气条件下BPA降解过程的紫外-可见全波段扫描图。
BPA的特征峰出现在276 nm处,其强度随着处理时间的延长而逐渐降低,在空气条件下,处理40 min后特征峰几乎消失,这证明BPA被降解,而在降解过程中,240~260 nm的吸收峰有所增加,这可能是由于生成了新的降解中间产物,许多官能团在230~300 nm处具有吸收峰,如共轭双键、苯环和不饱和羰基[28-29]。因此,在240~260 nm处吸收峰强度的增加标明产生了对苯二酚、对异丙基苯酚等物质。在以空气为载气的协同体系中,放电处理20 min后,不仅BPA的特征峰逐渐降低,240~260 nm处的中间产物峰也逐渐减少,表明苯环正在开裂,证明纳米ZnO催化剂的添加有利于污染物的完全降解。
在氧气条件下,当处理时间20 min时,BPA特征峰已经完全消失,说明BPA已被完全降解。中间产物峰同样趋于平滑,表明在氧气载气体系中,活性氧物质产量更为丰富,可以将BPA以及降解产生的中间体完全降解,进而生成CO2和H2O。
2.1. 催化剂浓度的影响
2.2. 载气种类的影响
2.3. pH的影响
2.4. 自由基捕获剂的影响
2.5. COD的去除
2.6. BPA降解过程的紫外全波段扫描
-
1) DBDP与纳米ZnO结合对水中BPA降解具有协同效应,表明该方法具有处理难降解有机废水的可行性。纳米ZnO在DBDP体系中的最佳添加浓度为50 mg·L−1。
2) 通过向体系内通入不同种类的载气类型,发现当O2为载气更有利于BPA降解,其次是空气,N2最差。
3) 较低的溶液初始pH有利于BPA的降解,而较高的pH对BPA的降解有抑制作用。
4) 1O2、电子、O2·–、·OH是协同过程中降解BPA的主要活性物质。
5) 相较于未添加催化剂,添加纳米ZnO和载氧气条件有利于BPA的降解和提高BPA溶液的可生化性。



 下载:
下载: