-
白洋淀位于京津冀三角地带的中心地带,地理位置关键。其作为华北平原最大的淡水湖泊,对维护当地生态系统平衡和附近居民的居住环境有着重要的意义[1]。随着雄安新区的建立,白洋淀生态环境再次成为我国关注的焦点,进一步提升和改善白洋淀生态环境质量亦成为我们环保工作的重点。
水生植物依据其生活型不同,可划分为挺水植物、沉水植物、浮叶植物、漂浮植物和湿生植物。沉水植物因其大多处于水体以下界面,易受到流域水源、土壤的影响,使其在浅水湖泊恢复中作为初级生产者率先进行群落构建[2]。20世纪70年代初期,有研究[3]认为,沉水植物恢复的关键是改善生境限制因子(如降低过高的氮磷含量),但实验证明,高浓度营养盐并不是制约因素;随后,有研究提出基于水下光场变化的沉水植物恢复技术,并在后海[4]、太湖[5]和滇池[6]等湖泊进行了实际应用,取得了较好的效果。但目前大多以耐污种群恢复为主,较少涉及群落恢复,而群落相对种群有更强的稳定生态能力,因此,以群落恢复为目标可进一步提升生态稳定性;而且,生态恢复是一个长期持续性过程,目前恢复步骤虽表明其需要逐步落实[4-6],但大多并未明确提出包括耐污种、中等耐污种和敏感物种沉水植物多阶段恢复方案。
本研究在充分调研和分析白洋淀沉水植物历史现状演变的基础上,结合不同沉水植物种群耐污性和对光的敏感性特点,建立沉水植物阶段性恢复方案;根据白洋淀水下光场特点,对白洋淀沉水植物群落恢复区域适宜程度进行划分,提出不适宜恢复区域的关键生境恢复参数,进而为白洋淀淀区沉水植物群落整体恢复提供参考。本研究结果对于白洋淀以及我国北方浅水湖泊的生态恢复具有一定参考价值,并可直接支撑河北雄安新区生态建设。
-
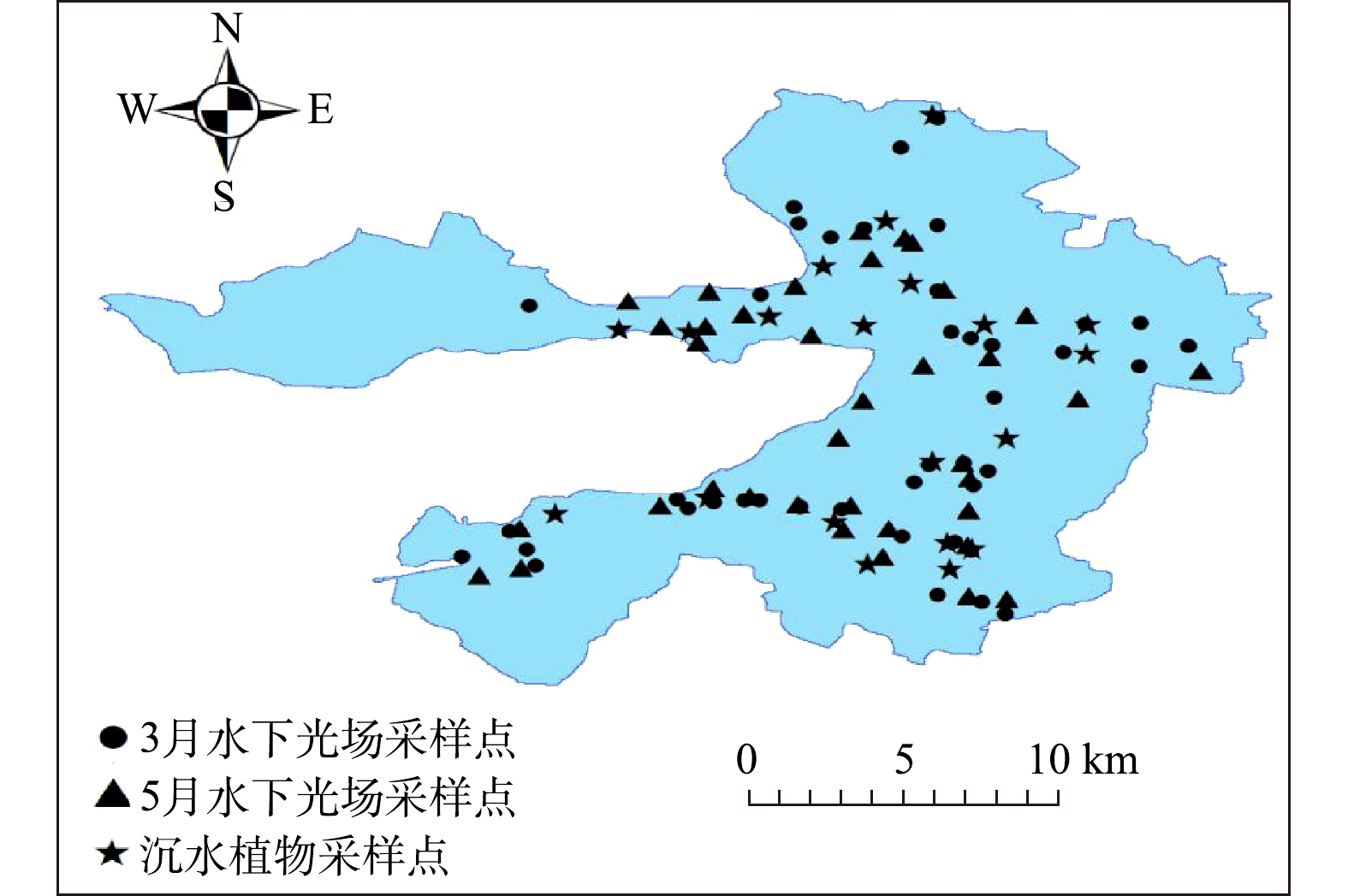
2019年3月和5月,在白洋淀布设43、37个点位(图1)并进行水下光场及水质调研,选择在晴好的天气,风浪小的区域进行采样,避免产生因天气改变、风浪而造成的误差。每个采样点现场监测透明度、水下光合有效辐射(photosynthetically active radiation,PAR)和实际水深,实验室测定总悬浮物(TSS)、叶绿素a (Chl.a)和溶解性有机碳(DOC)。利用30 cm的赛氏圆盘测定透明度;选用水下光量子仪(LI-COR 192SA)测定PAR;使用铅坠和带刻度的绳子测定水深;采集水样后,取0.5 L水样过滤,利用重量法将滤膜分析悬浮物;将0.5 L水样过滤,利用分光光度法取滤膜测定Chl.a;随后使用碳分析仪测定过滤后的清液中的DOC。2018年4月、7月和11月,布设20个采样点(图1)进行沉水植物调查。使用耙子(固定面积0.25 m2)采集沉水植物,后期在实验室完成测量记录工作。
-
沉水植物的光补偿点是指沉水植物光合作用产氧量与呼吸作用耗氧量相互抵消为0时对应的光辐射强度。对于湖泊生态环境管理,常将沉水植物昼夜光补偿点作为临界值[7]考虑。
实验在晴天的09:00—11:00进行。购买植物培养液,用NaOH调节pH至7.5,量取500 mL配置好的营养液,放入500 mL锥形瓶中备用。分别取5 g单物种黄花狸藻、光叶眼子菜、大茨藻,如果是多种植物,其中占比最大的植物按照5 g处理,其他植物根据比例计算得到具体样品重量(若物种比例过小,按照0.1 g处理)。用吸水纸吸去叶表面水分,分别称重记录后,放入装有培养液的备用锥形瓶中,用封口膜封住瓶口,将其放入人工气候箱,设定光照分别为0、11、12、20、33、38、43、50、61 μmol·(m2·s)−1。每隔0.5 h改变光强,同时利用便携式溶氧仪测定水中溶解氧含量。金鱼藻∶龙须眼子菜∶穗花狐尾藻比例为1∶2∶1,以此为基础,后期补种沉水植物,其占比与金鱼藻、穗花狐尾藻一致。
-
在生态学中,同一物种在特定时间占据一定空间的集体称为生物种群,而在该群落中具有绝对优势地位的种群称为优势种群,也称为优势种。优势度计算方法如式(1)所示。
式中:Y为优势度;ni为第i种沉水植物物种在监测区域的数量;N为在该监测区域出现的所有沉水植物数量总和;fi为第i种沉水植物在各个监测点位的出现频率。
优势度Y≥0.02的物种为优势种,一般优势度大于0.01的物种为主要优势种;大于0.1的物种为绝对优势种。
净光合速率是指植物光合作用积累的有机物,是总光合速率减去呼吸速率的值。对于光补偿点实验来说,沉水植物种群(群落)的净光合速率为含有沉水植物的锥形瓶中单位时间的净产氧量减去空白对照组的净产氧量,排除水体中藻类光合作用的影响,其具体计算方法如式(2)所示。
式中:R为净光合速率(以O2计),μmol·(g·h)−1;Q为沉水植物溶解氧含量,mg·L−1;QA为实验水样溶解氧含量,mg·L−1;t为同一光照培养时间,h;m为沉水植物种群(群落)的质量,kg。
对于浅水湖泊,其PAR随着水深的增加而降低,而沉水植物光补偿深度是指在该水深深度条件下,沉水植物光合作用与呼吸作用相平衡,不产生有机物。
将每个监测点的水深梯度与对应的PAR值进行回归分析,其回归方程的通式如式(3)所示。
式中:I0为在水面0 m处水下光量子仪测定的光合有效辐射值,μmol·(m2·s)−1;t为以水面0 m为基准向下的深度,m;It为水深在t处水下光量子仪测定的光合有效辐射值,μmol·(m2·s)−1;α为光衰减系数,m−1。
水下光场监测完毕后,得到每个监测点的水下PAR随深度的递减公式,随后将实验测定的沉水植物种群(群落)光补偿点代入It,即可得到每个监测点的沉水植物种群和群落的光补偿深度。
利用OrigiLab 2020对水下光场数据进行回归拟合;利用光合软件计算沉水植物光补偿点;利用ArcGIS 10.6中的反距离权重插值法,进行光衰减系数、TSS、Chl.a、DOC、光补偿深度空间分布分析和点位的确定;利用SPSS 20.0对光补偿深度与透明度进行回归拟合,利用R语言和OrigiLab 2020进行数据处理和绘图。
-
近60年(1958—2018年)白洋淀沉水植物调研的演变结果[8-14]见表1。可以看出,1958—2018年,白洋淀沉水植物由15种逐渐减少至11种。其中2018年淀区内沉水植物分别为,轮藻、穗花狐尾藻、龙须眼子菜、菹草、金鱼藻、黄花狸藻、轮叶黑藻、小茨藻、大茨藻、苦草和光叶眼子菜。曾出现的聚草、拟轮藻、丝网藻、马来眼子菜在2018年未发现,其中拟轮藻和丝网藻在20世纪80年代未见报道,聚草在20世纪90年代后未见报道,马来眼子菜在2011年存在,但2018年未发现。近38年,白洋淀沉水植物总生物量变化趋势复杂,1980年初,白洋淀沉水植物总生物量为6 359.97 g·m−2到1992年提高到7 741.69 g·m−2,但在2009年明显降低,仅为1 315.13 g·m−2,随后在2010年“十一五”专项调研中明显升高。但在2018年发现沉水植物总生物量降至2 411.6 g·m−2。因此,随着时间的推移,白洋淀沉水植物呈现种类减少、总生物量降低的趋势。
沉水植物因其扎根于湖泊沉积物,其植物体在水面下生存的特性使其对水质的污染程度较其他水生植物敏感,常作为指示生物[15]。通过样品采集和文献调研发现,在白洋淀现存沉水植物中,轮藻和小茨藻为敏感种,大茨藻、黄花狸藻为较敏感种,上述物种多分布在轻污染水域中;苦草、轮叶黑藻为中等耐污种;龙须眼子菜、金鱼藻、穗花狐尾藻、光叶眼子菜、菹草为耐污种,可生长在污染较大的水域中。近60年,白洋淀沉水植物优势物种随时间变化显著(表1)。2018年调研分析结果表明,金鱼藻,龙须眼子菜、菹草、穗花狐尾藻仍为优势物种,而黄花狸藻成为新增的优势物种。随着时间的推移,白洋淀沉水植物优势种逐渐由耐污种、中等耐污种和敏感物种的复合多样化,变为以耐污种为主的趋势,优势物种多样性降低,但耐污性能逐渐增强。
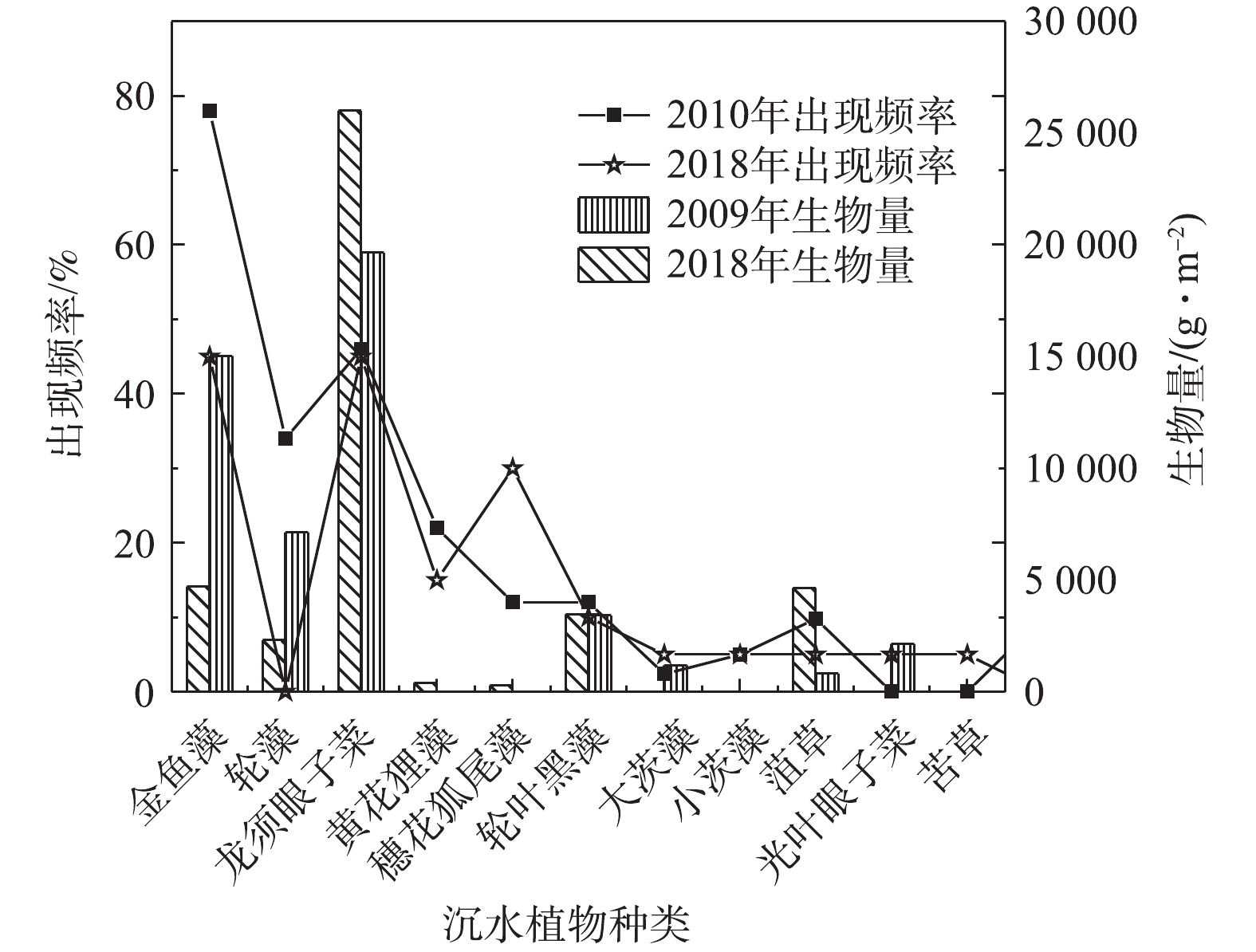
白洋淀不同沉水植物生物量在2009年和2018年对比分析结果见图2。可以看出:耐污性高的沉水植物生物量增加,龙须眼子菜生物量由19 648.3 g·m−2增长为26 008.96 g·m−2,菹草生物量由812 g·m−2增长为4 651 g·m−2;耐污性低的沉水植物生物量降低,轮藻生物量由7 140.2 g·m−2减少为2 306 g·m−2,大茨藻生物量由1 190 g·m−2减少为0.53 g·m−2。对白洋淀2010年8月与2018年7月不同沉水植物出现频率的分析结果见图2。可以看出,在8年内,马来眼子菜退化消失,而苦草重新出现。淀区主要植物组成与植物出现频率有较大变化。2010年,淀区内出现频率最高的为金鱼藻、轮藻和龙须眼子菜,分别为78%、34%和46%。其中轮藻为敏感种,而金鱼藻和龙须眼子菜为耐污种。2018年,沉水植物出现频率发生变化,出现频率最高的3种植物为金鱼藻、龙须眼子菜和穗花狐尾藻,分别为45%、60%和30%。与8年前相对比,耐污性高于金鱼藻的龙须眼子菜出现率增加,而作为敏感种的轮藻未有出现。
因此,基于优势度、出现频率的调研分析结果,初步选取7种沉水植物作为恢复物种,分别为3种耐污种(龙须眼子菜、金鱼藻和穗花狐尾藻)、2种中等耐污种(轮叶黑藻和苦草)和2种敏感物种(大茨藻和黄花狸藻)。考虑恢复初期水质较差,首先选取耐污种作为先锋物种,水质提升后,分阶段补充中等耐污种和敏感物种,进而形成稳定的群落。具体恢复方案分为2个阶段:在第1阶段,种植耐污种(金鱼藻+龙须眼子菜+穗花狐尾藻),使水质变好;在第2阶段,增加中等耐污种的种植(苦草+轮叶黑藻),最后补充敏感物种(大茨藻+黄花狸藻)。
-
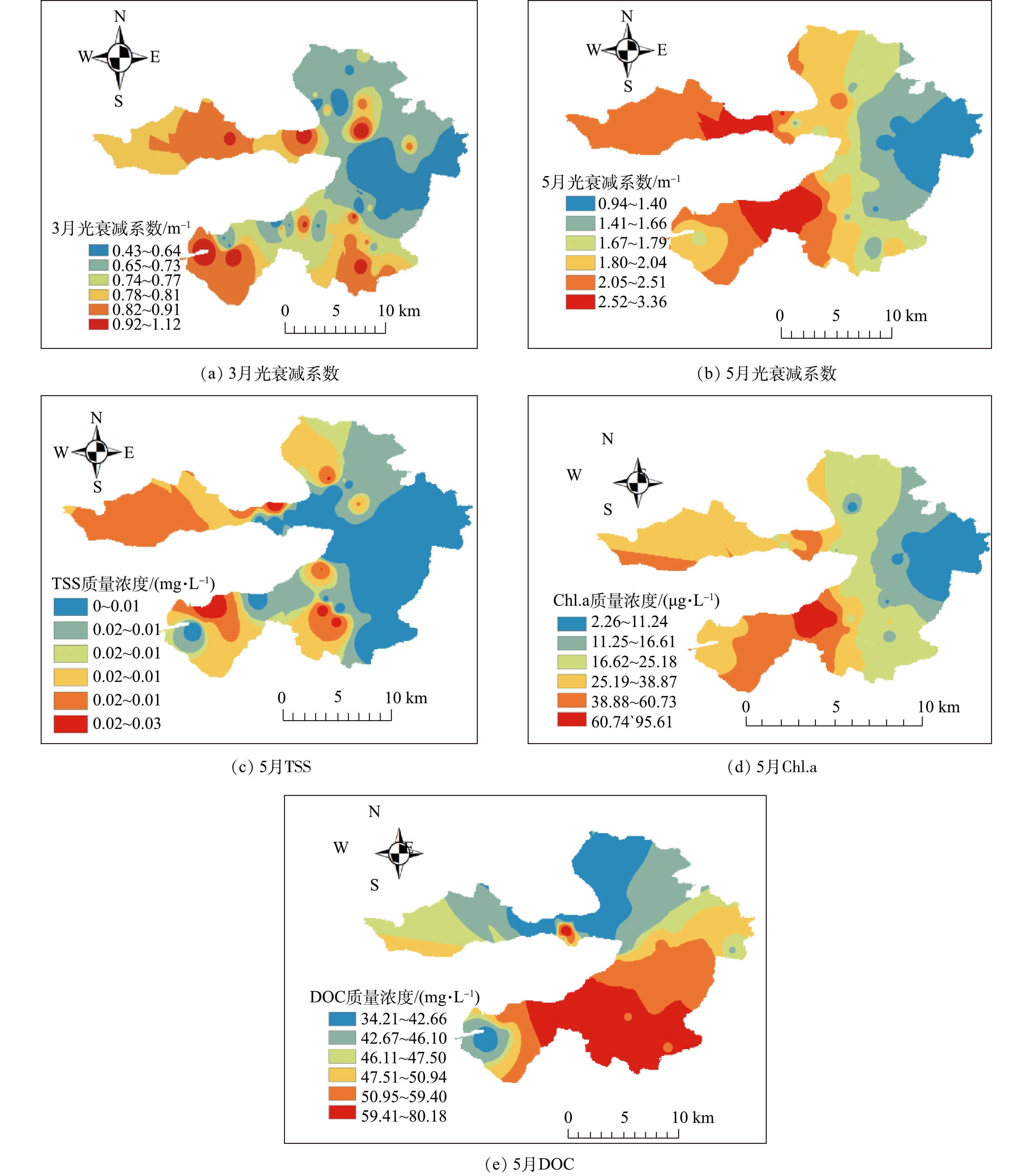
光衰减系数一般在湖泊光学中指光在水下随深度变化的衰减速率,衰减系数的高低可以反映出湖泊水体的清澈程度。本研究分别于2019年3月和5月对白洋淀进行水下光场调研,分别根据点位的不同水深的光合作用有效辐射(photosynthetically active radiation,PAR),同时利用式(3)计算光衰减系数,其空间分布结果如图3所示。可以看出:2019年3月,光衰减系数为0.426~1.121 m−1,变化极差为0.695 m−1,其中光衰减系数小于0.5 m−1的点位有6个,占所有点位的14.0%,处于0.5~1.0 m−1的点位有34个,占所有点位的79.0%,大于1.0 m−1的点位有3个,占所有点位的7.0%;2019年5月,光衰减系数为0.94 ~3.36 m−1,变化极差为2.42 m−1,其中光衰减系数小于1.0 m−1的点位有1个,占所有点位的2.7%,处于1.0~2.0 m−1的点位有23个,占所有点位的62.2%,处于2.0~3.0 m−1的点位有11个,占所有点位的29.7%,大于3.0 m−1的点位有2个,占所有点位的5.4%。这表明5月的光衰减系数较3月的高,对于白洋淀沉水植物恢复来说,最好选择在3月进行。低值区主要分布在东部退渔环湖区域、自然保护区域,如烧车淀、光淀张庄、枣林庄等区域。高值区主要分布在西部村庄聚集处、部分入淀河口处以及旅游观赏区,如南刘庄、端村、圈头等。
据报道[3],通常水中溶解物质、悬浮颗粒和浮游植物的变化会导致光衰减系数发生改变。因此,以5月光衰减系数和水样为例,分析各点位的Chl.a、TSS和DOC浓度,结果如图3所示。可以看出,Chl.a和TSS空间分布趋势大体为从西向东递减,与光衰减系数空间分布类似;而DOC空间分布趋势为从北向南递增。将Chl.a、TSS、DOC分别与光衰减系数进行相关性分析,结果表明光衰减系数和Chl.a具有较好的相关性(R2=0.507 8,P≤0.01),TSS其次(R2=0.174 5),而与DOC相关性较差(R2=0.010 5)。说明光衰减系数与Chl.a、TSS相关,其中Chl.a对光衰减的影响较大。其与太湖区域研究结果一致,太湖区域的光衰减系数同样受TSS和Chl.a的影响,但受TSS影响更为显著。这说明不同湖泊影响光衰减系数的因素大致相同,但影响程度不同,这可能是由于不同湖泊受人为干扰的来源不同,导致影响程度不同。太湖受船只与风浪的干扰较大,TSS含量为15~36 mg·L−1,TSS成为重要干扰因素;而白洋淀周边养殖场较多、村庄聚集,营养物质充足导致Chl.a含量为10~100 μg·L−1,成为主要影响淀区光衰减系数的主要因素,这与白洋淀5月温度上升,藻类生长旺盛,导致光衰减系数较3月大的结果一致。
-
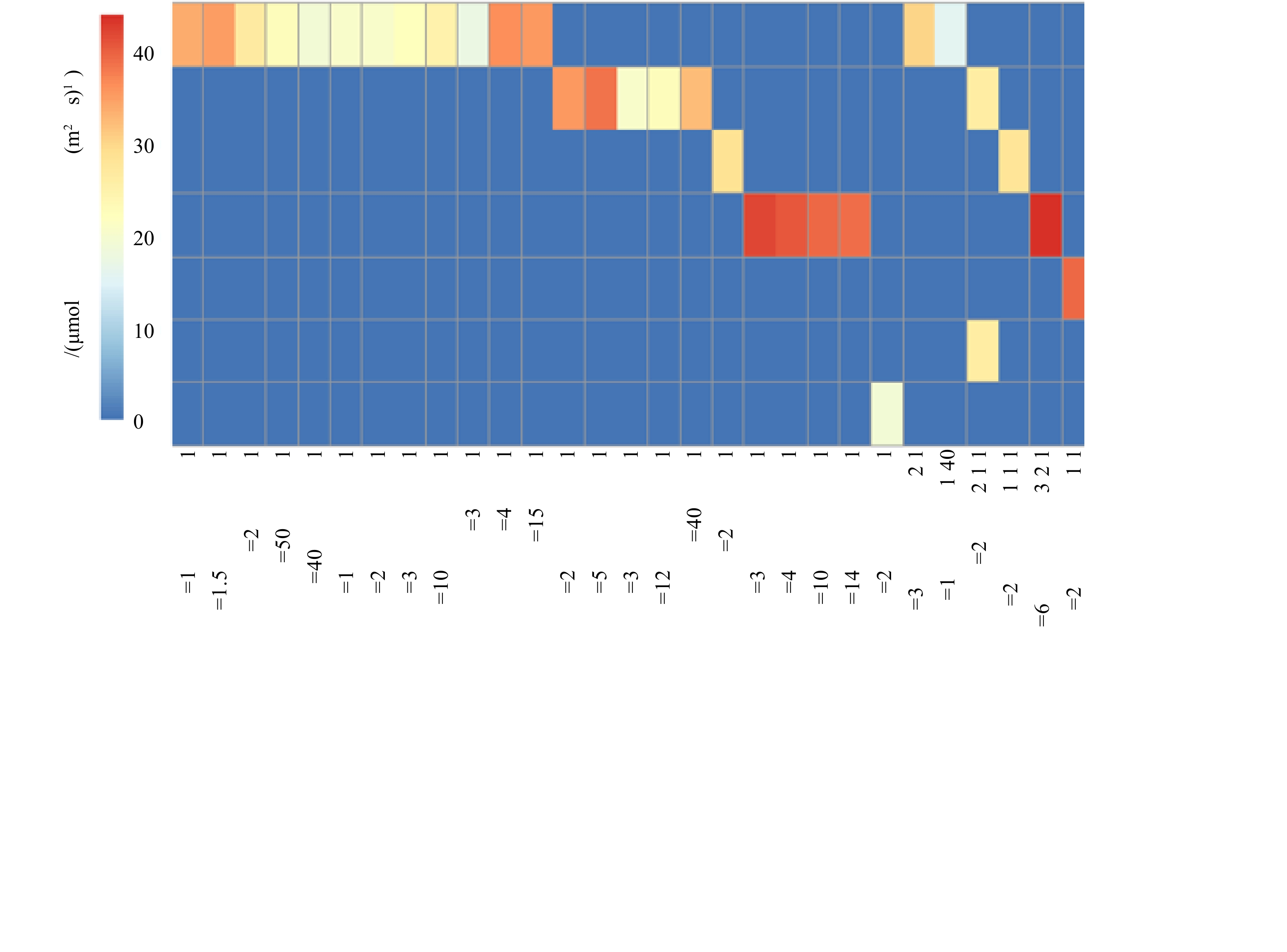
1)沉水植物的光补偿点。2018年,白洋淀沉水植物调研分析结果表明,目前沉水植物存在分别以龙须眼子菜、金鱼藻、穗花狐尾藻、菹草、光叶眼子菜、轮叶黑藻和黄花狸藻为优势种的群落类型。不同群落光补偿点分析结果见图4。可以看出,具有相同优势种的群落,即使物种比例不同,群落光补偿点区别不大;但是如果群落优势物种不同,群落的光补偿点相差较大。例如,龙须眼子菜+金鱼藻的搭配,以龙须眼子菜为优势种(2∶1和50∶1)的群落光补偿点的光强分别为22.46 μmol·(m2·s)−1和26.05 μmol·(m2·s)−1,以金鱼藻为优势种(1∶2和1∶5)的群落光补偿点的光强分别为35.01 μmol·(m2·s)−1和37.79 μmol·(m2·s)−1。
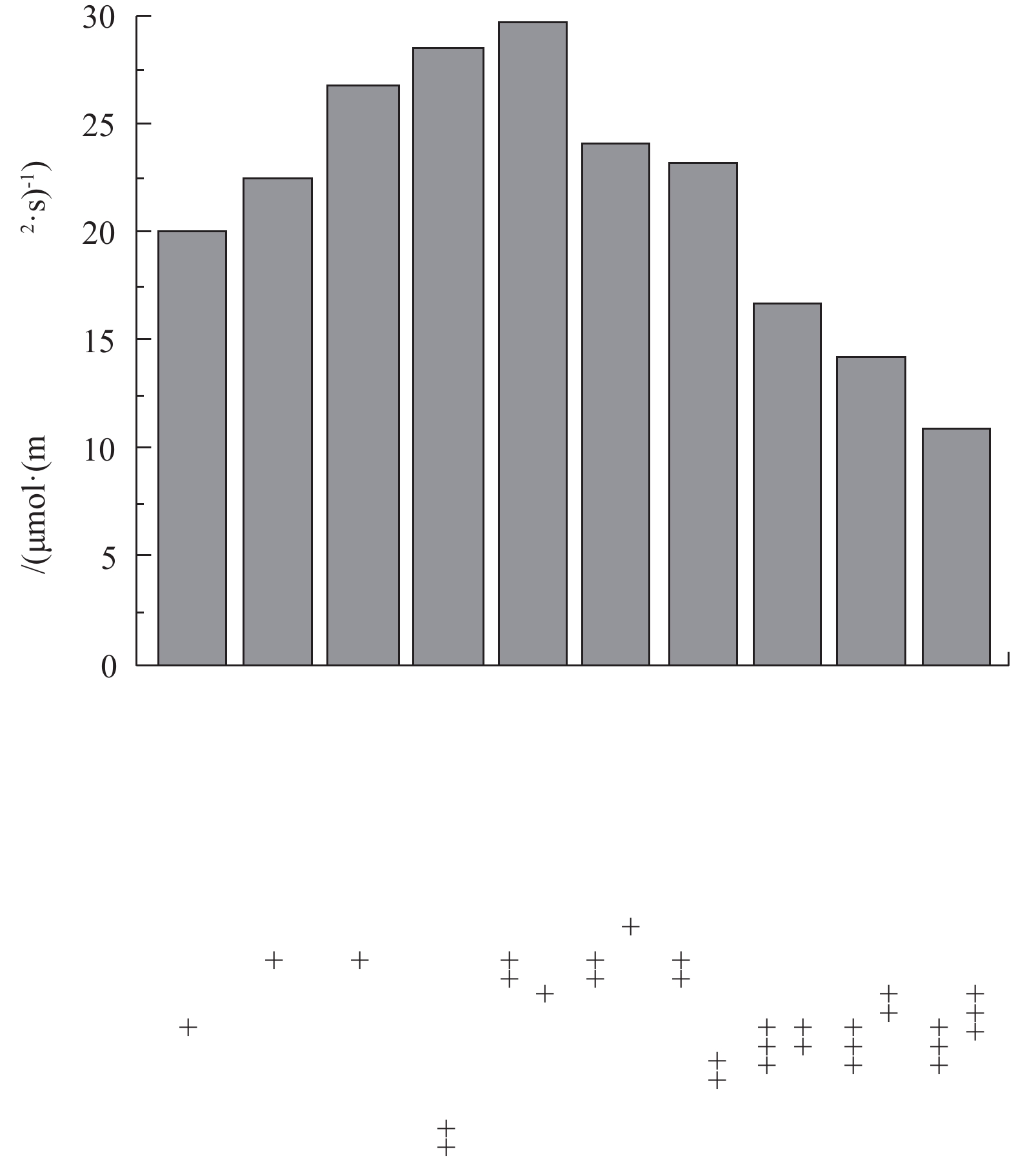
沉水植物种类不同,光补偿点具有较大差别。沉水植物按光补偿点强度从小到大排序为苦草<大茨藻<黄花狸藻<轮叶黑藻<龙须眼子菜<金鱼藻<穗花狐尾藻,即其适应弱光的能力按从大到小依次排序为苦草>大茨藻>黄花狸藻>轮叶黑藻>龙须眼子菜>金鱼藻>穗花狐尾藻。根据沉水植物2个阶段恢复方案搭配恢复物种,实验测得不同群落的光补偿点结果见图5。结果表明,第1阶段种植的耐污种(金鱼藻、龙须眼子菜和穗花狐尾藻),不同群落光补偿点大于20 μmol·(m2·s)−1,均高于该群落中任一单种沉水植物种群光补偿点,且以3种中的任意2种沉水植物形成的群落光补偿点小于3种沉水植物群落。这与报道的滇池、后海研究结果[3-5]相同,即群落较种群光补偿点高;随着水质改善,当加入轮叶黑藻后,群落光补偿点进一步增加,但当加入苦草、黄花狸藻和大茨藻后,因其光补偿点较低,更适应低光环境,导致群落出现它感效应[16-17],使得群落光补偿点[18]反而降低,即7种植物形成的群落光补偿点低于其他群落,如果仅依据该结果,即单纯依据群落光补偿点大小进行群落恢复,存在不合理性。因此,需要对群落光补偿点和植物耐污性进行综合考虑,进而建立多阶段恢复方案。
2)沉水植物种群(群落)光补偿深度与透明度[19]回归分析及应用。将沉水植物种群(群落)的光补偿点强度[20]代入方程,该方程为各个监测点水下光场水深与光合有效辐射的回归方程,可得到各个监测点位的上述沉水植物种群(群落)对应的光补偿深度。将其与透明度[3-5]进行拟合(表2),结果表明,7种沉水植物及不同群落光补偿深度与透明度呈显著相关(大部分R2>0.74)。因此,沉水植物种群(群落)光补偿深度与透明度回归方程式常用于针对目标水域现场测定透明度后,计算得到该区域沉水植物种群(群落)的光补偿深度。若沉水植物种群(群落)光补偿深度≥实际水深,表明沉水植物光合作用≥呼吸作用,有机物开始积累,沉水植物可以生存;若沉水植物种群(群落)光补偿深度<实际水深时,沉水植物光合作用<呼吸作用,不产生有机物,只消耗有机物,沉水植物无法生存。因此,表2中的光补偿深度与透明度关系式可用来快速判断目标区域沉水植物是否可以生存,对恢复方案的制定具有很重要的应用价值。
另外,当实际水深等于沉水植物群落光补偿深度时,利用沉水植物光补偿深度与透明度关系式,计算得到的透明度是该区域沉水植物恢复初期所需的最小值。只有当透明度达到该数值时,才能种植沉水植物[21-24];否则,无法满足沉水植物群落的需光要求,导致种植后无法存活。同时3月与5月2次光补偿深度与透明度的关系式表明,不同时期沉水植物恢复所需透明度不同,但5月沉水植物光补偿深度与透明度关系式可作为其沉水植物春季恢复所需最小透明度的代表式。
-
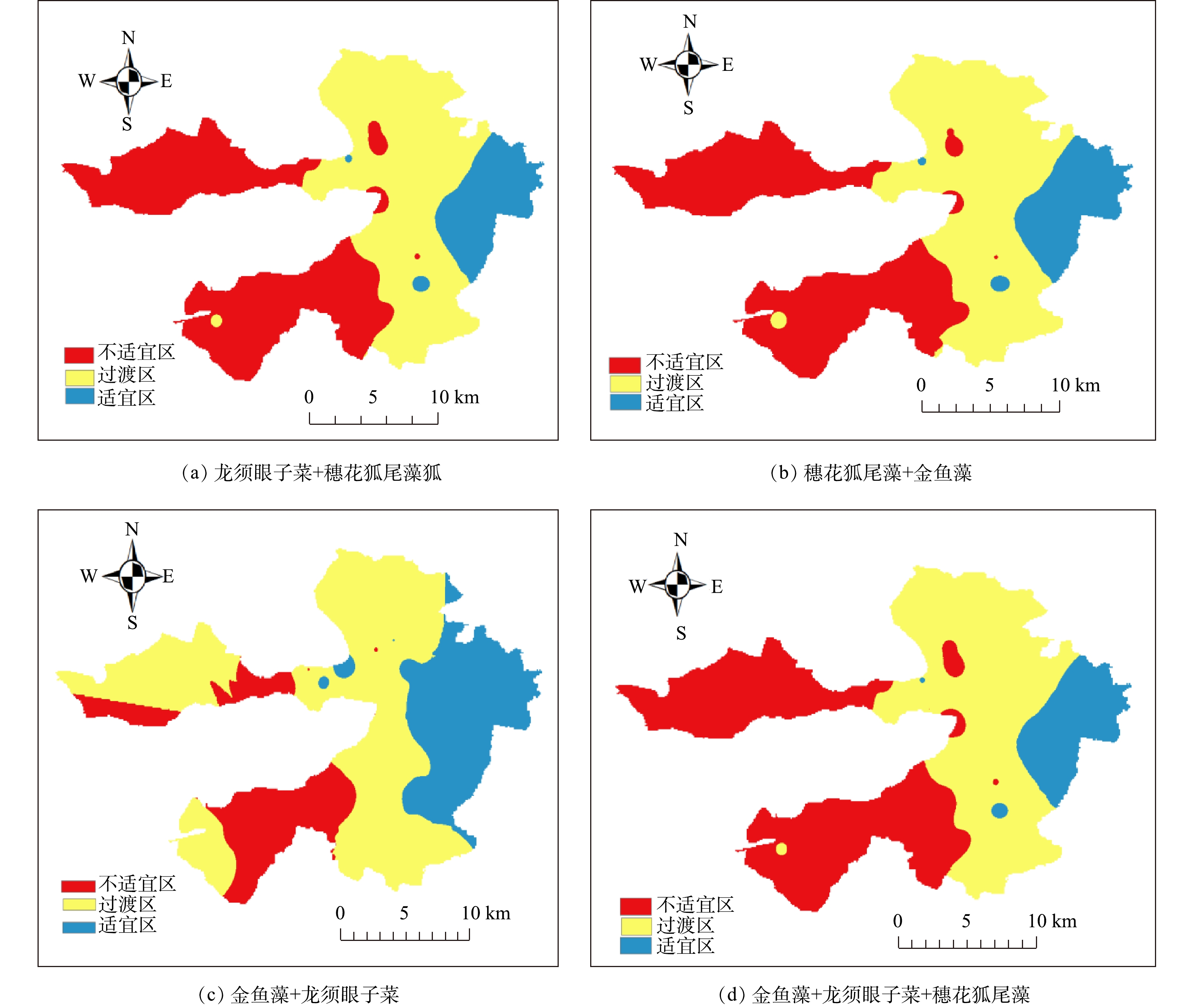
第1阶段恢复的耐污种群落光补偿深度与实际水深比值(Qi)的空间分布如图6所示。将Qi>1的区域作为沉水植物恢复的“适宜区”,可优选恢复;0.75<Qi<1作为沉水植物恢复的“过渡区”,只有通过适当的工程措施改善其透明度后才能恢复沉水植物;Qi<0.75作为沉水植物恢复的“暂不适宜区”,即需要加强改善透明度,才能考虑在该区域恢复[25]。由图6可以看出,沉水植物耐污种群落(龙须眼子菜+穗花狐尾藻、金鱼藻+龙须眼子菜、穗花狐尾藻+金鱼藻、金鱼藻+龙须眼子菜+穗花狐尾藻)的Qi均表现出由西到东递增的趋势,即在白洋淀西部为暂不适宜区,中部为过渡区,东部为适宜区,其Qi的衰减与光衰减系数由西到东递减的规律(图3)相反。这表明:在水体相对澄清区域(光衰减系数低),受到人为干扰较少,适宜沉水植物直接恢复,如枣林庄、烧车淀、光淀张庄等区域;在白洋淀中部,如圈头、赵北口等村庄聚集区,适宜通过工程措施将透明度提高才可种植耐污种群落;在白洋淀西部,如北刘庄、端村等村庄聚集区域,人为干扰较多,需要显著提高透明度,才可考虑恢复。透明度的主要影响因素是Chl.a和TSS[26-31],所以需要优选相应技术降低Chl.a和TSS,进而提高透明度,才能开展沉水植物的恢复。
根据2019年5月沉水植物光补偿深度与透明度关系式,以实际水深作为沉水植物光补偿深度,可以反推算出沉水植物恢复所需的最低的透明度及透明度恢复目标值,结果见表3。综合考虑水下光场、沉水植物耐污性和光敏感性,白洋淀沉水植物恢复方案技术路线见图7。据报道[6],当沉水植物种群光补偿深度≥实际水深,水域中可以有稀疏的沉水植物种群存活,当沉水植物群落光补偿深度≥实际水深时,水域中可以有沉水植物群落存活。因此,当实际水深等于沉水植物种群(群落)光补偿深度时,利用表2中5月光补偿深度与透明度关系式,可得到第1阶段各种群(群落)沉水植物恢复前的透明度目标值,其平均值可作为种群或群落恢复的透明度目标值。因此,针对白洋淀第1阶段沉水植物恢复 “过渡区”和“暂不适宜区”,可以通过工程措施将透明度提高至平均目标值,即可达到目标水域有稀疏群簇存活,进一步提升透明度,达到群落生长的透明度目标值后,可实现形成群落的目标。因此,该沉水植物恢复方案的考虑因素对北方湖泊生态治理具有借鉴意义。
-
1)白洋淀淀区沉水植物历年调研和2018年现状分析结果表明,白洋淀淀区现存11种沉水植物。结合沉水植物耐污性,确定沉水植物阶段性恢复方案为:在第1阶段,种植耐污种(金鱼藻+龙须眼子菜+穗花狐尾藻);在第2阶段,当水质变好后,增加中等耐污种(苦草+轮叶黑藻),最后补充敏感种(大茨藻+黄花狸藻)。当群落组成与优势物种确定时,植物比例对光补偿点影响不大。
2)白洋淀水下光衰减系数在2019年5月较2019年3月大,故对沉水植物的恢复工程最好在3月进行;同时,光衰减系数的空间分布均呈东低西高的趋势,且与Chl.a、TSS空间变化趋势一致,高值区存在于人为干扰较多区域,低值区则人为干扰较少。
3)沉水植物种群(群落)光补偿深度与透明度符合线性关系。针对淡水湖泊的恢复,可以通过对水体透明度的监测,从而计算出该区域群落生长的光补偿深度。当沉水植物种群(群落)光补偿深度≥实际水深,水体中有沉水植物种群(群落)出现;而当沉水植物种群(群落)光补偿深度<实际水深,水体中无法存活沉水植物。利用沉水植物种群(群落)光补偿深度和实际水深的比值评估第1阶段白洋淀修复区域,并通过前期光衰减系数与叶绿素a、悬浮物和DOC的相关性分析,确定可通过降低水体中叶绿素a和悬浮物含量,从而提高沉水植物光补偿深度,进而实现沉水植物群落恢复。
基于光补偿深度的白洋淀沉水植物修复技术
Remediation technology of submerged plants in Baiyangdian Lake based on light compensation depth
-
摘要: 沉水植物是浅水湖泊生态恢复的关键。为实现浅水湖泊沉水植物修复,对白洋淀淀区沉水植物历年变化和2018年现状进行了调研分析,并于2019年3月和5月测定了水下光场的变化;在考察水下光场、沉水植物耐污性和光敏感性的基础上,确定了沉水植物群落2个阶段(耐污种+中等耐污种和敏感种)恢复方案。结果表明:以2018年春、夏、秋3季调研的沉水植物群落为目标,测定的群落光补偿点和群落组成与优势物种的种类具有相关性,植物比例对其群落光补偿点的影响不大,如龙须眼子菜+金鱼藻的搭配,以龙须眼子菜为优势种(2∶1和50∶1)的群落光补偿点的光强分别为22.46 μmol·(m2·s)−1和26.05 μmol·(m2·s)−1,以金鱼藻为优势种(1∶2和1∶5)的群落光补偿点的光强分别为35.01 μmol·(m2·s)−1和37.79 μmol·(m2·s)−1;白洋淀水下光衰减系数呈现东低西高的趋势,且与叶绿素a和总悬浮物空间变化趋势一致,其中叶绿素的影响较大,高值区存在于人为干扰较多区域,低值区人为干扰较少;沉水植物种群(群落)光补偿深度与透明度符合线性回归模型,且其显著性较高(大部分R2>0.74);利用沉水植物种群(群落)光补偿深度和实际水深的比值评估第一阶段白洋淀修复区域,并通过前期光衰减系数与叶绿素a、悬浮物和DOC的相关性分析,确定可通过降低水体中叶绿素a和悬浮物含量,从而提高沉水植物光补偿深度,进而实现沉水植物群落恢复。以上研究结果可为白洋淀及北方湖泊的生态治理提供参考。Abstract: Submerged plants are the key to the ecological restoration of shallow lakes. In this study, submerged plants in Baiyangdian Lake area were sampled in 2018 and compared with its history. The changes of the underwater light field were also analyzed in March and May 2019. Considering the underwater light field, anti-pollution and light sensitivity of submerged plants, a two-phase restoration plan for submerged plant communities (stain-resistant + medium-tolerant and sensitive species) was determined. The results showed that the submerged plant community surveyed in the spring, summer and autumn of 2018 were taken as research target, the correlation between the measured light compensation point of the community and its composition and the dominant species were determined, and the plant proportion has slight effect on the light compensation point of its community. For example, the combination of P.pectinatus +Ceratophyllum, the light intensities of the community light compensation point with P.pectinatus as the dominant species (2∶1 and 50∶1) were 22.46 μmol·(m2·s)−1 and 26.05 μmol·(m2·s)−1, respectively. The light intensities of the community light compensation point with Ceratophyllum as the dominant species (1∶2 and 1∶5) were 35.01 μmol· (m2·s)−1 and 37.79 μmol ·(m2·s)−1, respectively. The results of the underwater light field showed the light attenuation coefficient had a trend of low values in the east and high values in the west. This spatial variation trend was similar to that of chlorophyll a and total suspended matter. Of which, the effect of chlorophyll was greater. The high-value regions had more human interference, while the low-value regions had less human interference. The depth of light compensation and the transparency of submerged plant populations (communities) showed significant linear positive correlation (most R2 values were higher than 0.74). The ratio of light compensation depth of submerged plant population (community) to actual water depth was used to evaluate the first stage of Baiyangdian restoration area, and through the correlation analysis of early light attenuation coefficient with chlorophyll a, suspended matter and DOC, it was determined that chlorophyll a and suspended matter content in the water body could be reduced, so as to increase the depth of light compensation of submerged plants, and then achieve the restoration of submerged plant communities. The above research results can provide a reference for the ecological management of Baiyangdian and northern lakes.
-

-
表 1 白洋淀不同水生植物种类及总生物量历年演变
Table 1. Evolution of different aquatic plant species and total biomass in Baiyangdian Lake
年份 物种
数量/
种物种名称 总生
物量/
(g·m−2)数据来源 优势物种 1958 15 轮藻、穗花狐尾藻、龙须眼子菜、菹草、金鱼藻、黄花狸藻、聚草、轮叶黑藻、小茨藻、大茨藻、苦草、拟轮藻、丝网藻、马来眼子菜、光叶眼子菜 — [8] 聚草、轮叶黑藻、小眼子菜、金鱼藻、苦草、龙须眼子菜、小茨草、菹草 1980 14 轮藻、穗花狐尾藻、龙须眼子菜、菹草、金鱼藻、黄花狸藻、聚草、轮叶黑藻、小茨藻、大茨藻、苦草、拟轮藻、丝网藻、光叶眼子菜 6 359.97 [9] 轮藻、光叶眼子菜、马来眼子菜、龙须眼子菜、聚草、轮叶黑藻和金鱼藻 1984 13 轮藻、穗花狐尾藻、龙须眼子菜、菹草、金鱼藻、黄花狸藻、聚草、轮叶黑藻、小茨藻、大茨藻、苦草、拟轮藻、马来眼子菜 — [10] 狐尾藻、金鱼藻、马来眼子菜、龙须眼子菜 1991 11 轮藻、穗花狐尾藻、龙须眼子菜、菹草、金鱼藻、黄花狸藻、轮叶黑藻、小茨藻、大茨藻、拟轮藻、马来眼子菜 — [11] 马来眼子菜、光叶眼子菜、龙须眼子菜、轮叶黑藻、范草和穗花狐尾藻和大茨藻 1992 11 轮藻、穗花狐尾藻、龙须眼子菜、菹草、金鱼藻、黄花狸藻、轮叶黑藻、小茨藻、大茨藻、拟轮藻、马来眼子菜 7 741.69 [12] 龙须眼子菜、五刺金鱼藻、轮叶黑藻、茨藻、穗状狐尾草和光叶眼子菜 2007 11 轮藻、穗花狐尾藻、龙须眼子菜、菹草、金鱼藻、黄花狸藻、轮叶黑藻、大茨藻、苦草、马来眼子菜 — [13] 金鱼藻、小茨藻、穗花狐尾藻和微齿眼子菜、龙须眼子菜、马来眼子菜 2009 8 轮藻、龙须眼子菜、菹草、金鱼藻、轮叶黑藻、大茨藻、苦草、黄花狸藻 1 315.13 2009年
实地调研金鱼藻、龙须眼子菜及轮藻 2010 9 轮藻、龙须眼子菜、菹草、金鱼藻、黄花狸藻、轮叶黑藻、小茨藻、大茨藻、马来眼子菜 48 760 [14] 金鱼藻、轮藻和龙须眼子菜 2018 11 轮藻、穗花狐尾藻、龙须眼子菜、菹草、金鱼藻、黄花狸藻、轮叶黑藻、苦草、小茨藻、大茨藻、光叶眼子菜 2 411.6 2018年
实地调研菹草、龙须眼子菜、金鱼藻、穗花狐尾藻和黄花狸藻 表 2 沉水植物光补偿深度与透明度回归方程式
Table 2. Regression equations of light compensation depth and transparency of submerged plants
沉水植物种群/群落 2019年3月 2019年5月 光补偿深度与
透明度关系模型R2 P 光补偿深度与
透明度关系模型R2 P 龙须眼子菜 Hc=2.91T+0.89 0.683 <0.01 Hc=1.94T+0.88 0.772 <0.01 金鱼藻 Hc=2.73T+0.56 0.629 <0.01 Hc=1.78T+0.79 0.767 <0.01 穗花狐尾藻 Hc=2.67T+0.46 0.607 <0.01 Hc=1.73T+0.76 0.764 <0.01 苦草 Hc=3.16T+1.33 0.707 <0.01 Hc=2.15T+0.99 0.779 <0.01 轮叶黑藻 Hc=2.92T+0.91 0.685 <0.01 Hc=1.95T+0.88 0.773 <0.01 黄花狸藻 Hc=2.75T+1.67 0.537 <0.01 Hc=2.07T+0.94 0.778 <0.01 大茨藻 Hc=2.79T+1.79 0.529 <0.01 Hc=2.01T+1.02 0.722 <0.01 龙须眼子菜+穗花狐尾藻 Hc=2.43T+0.74 0.479 <0.01 Hc=1.70T+0.74 0.764 <0.01 龙须眼子菜+金鱼藻 Hc=2.53T+1.03 0.509 <0.01 Hc=1.76T+0.97 0.715 <0.01 穗花狐尾藻+金鱼藻 Hc=2.46T+0.88 0.486 <0.01 Hc=1.65T+0.81 0.711 <0.01 金鱼藻+龙须眼子菜+穗花狐尾藻 Hc=2.44T+0.82 0.479 <0.01 Hc=1.63T+0.79 0.708 <0.01 金鱼藻+龙须眼子菜+穗花狐尾藻+轮叶黑藻 Hc=2.43T+0.78 0.475 <0.01 Hc=1.62T+0.79 0.71 <0.01 金鱼藻+龙须眼子菜+穗花狐尾藻+苦草 Hc=2.5T+0.98 0.475 <0.01 Hc=1.69T+0.83 0.713 <0.01 金鱼藻+龙须眼子菜+穗花狐尾藻+轮叶黑藻+苦草 Hc=2.52T+1.02 0.501 <0.01 Hc=1.71T+0.84 0.713 <0.01 金鱼藻+龙须眼子菜+穗花狐尾藻+轮叶黑藻+苦草+黄花狸藻 Hc=2.63T+1.34 0.522 <0.01 Hc=1.83T+0.92 0.716 <0.01 金鱼藻+龙须眼子菜+穗花狐尾藻+轮叶黑藻+苦草+大茨藻 Hc=2.68T+1.49 0.527 <0.01 Hc=1.89T+0.95 0.716 <0.01 金鱼藻+龙须眼子菜+穗花狐尾藻+
轮叶黑藻+苦草+黄花狸藻+大茨藻Hc=2.77T+1.74 0.529 <0.01 Hc=1.99T+1.01 0.722 <0.01 表 3 白洋淀沉水植物第1阶段恢复所需水体透明度目标值
Table 3. Water transparency requirements of the 1st stage restoration of submerged plant in Baiyangdian Lake
cm 水深 恢复沉水植物种群(群落)所需透明度目标值 龙须眼子
菜种群金鱼藻
种群穗花狐尾
藻种群稀疏
群簇群落1 群落2 群落3 群落4 群落5 200(实际) 58 68 72 66 74 59 72 74 70 300(实际) 109 124 129 121 133 115 133 136 129 400(实际) 161 180 187 176 192 172 193 197 189 500(实际) 212 237 245 231 251 229 254 258 248 600(实际) 264 293 303 287 309 286 315 320 307 252(平均) 85 97 102 94 105 88 104 106 101 注:群落1为龙须眼子菜+穗花狐尾藻群落;群落2为龙须眼子菜+金鱼藻群落;群落3为穗花狐尾藻+金鱼藻群落;群落4为金鱼藻+龙须眼子菜+穗花狐尾藻群落;群落5为拥有一定生物量的沉水植物群落。 -
[1] 朱金峰, 周艺, 王世新, 等. 白洋淀湿地生态功能评价及分区[J]. 生态学报, 2020, 40(2): 459-472. [2] 丁亚琴. 环境因子与沉水植物的关系[J]. 科技信息, 2012(3): 540-578. doi: 10.3969/j.issn.1001-9960.2012.03.409 [3] 李一平, 滑磊, 谈永锋, 等. 基于生物-光学模型的浅水湖泊水体透明度模拟研究[J]. 水力发电学报, 2013, 32(6): 127-134. [4] 王韶华, 赵德锋, 廖日红. 关于北京后海水体光照强度及沉水植物光补偿深度的研究[J]. 水处理技术, 2006, 32(6): 31-33. doi: 10.3969/j.issn.1000-3770.2006.06.008 [5] 宋玉芝, 王敏, 赵巧华, 等. 太湖沉水植物光补偿深度反演及影响因子分析[J]. 农业环境科学学报, 2011, 30(10): 2099-2105. [6] 任久长, 周红, 孙亦彤. 滇池光照强度的垂直分布与沉水植物的光补偿深度[J]. 北京大学学报(自然科学版), 1997, 33(2): 211-214. [7] 苏文华, 张光飞, 张云孙, 等. 5种沉水植物的光合特征[J]. 水生生物学报, 2004, 28(4): 391-395. doi: 10.3321/j.issn:1000-3207.2004.04.010 [8] 金相灿, 屠清瑛. 中国湖泊富营养化[M]. 北京: 中国环境科学出版社, 1990. [9] 童文辉. 白洋淀大型水生植物及其资源利用[J]. 华北农学报, 1984, 1(4): 72-80. [10] 张敏, 宫兆宁, 赵文吉, 等. 近30年来白洋淀湿地景观格局变化及其驱动机制[J]. 生态学报, 2016, 36(15): 4780-4791. [11] 田玉梅. 白洋淀水生植被[J]. 河北大学学报(自然科学版), 1995, 15(4): 59-66. [12] 赵芳. 白洋淀大型水生植物资源调查及对富营养化的影响[J]. 环境科学, 1995, 16(S1): 21-23. [13] 李峰, 谢永宏, 杨刚, 等. 白洋淀水生植被初步调查[J]. 应用生态学报, 2008, 19(7): 1597-1603. [14] 张帆. 白洋淀优势沉水植物对富营养化水体净化规律及影响效应的研究[D]. 保定: 河北农业大学, 2012. [15] 刘勇丽, 刘录三, 汪星, 等. 水生植物在河流健康评价中的应用研究进展[J]. 生态科学, 2017, 36(3): 207-215. [16] 陈淑芳. 植物化感作用影响因素的探讨[J]. 中国农学通报, 2009, 25(23): 258-261. [17] DONK E V, BUND W J V D. Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: Allelopathy versus other mechanisms[J]. Aquatic Botany, 2002, 72(1/2/3/4): 274. [18] ELLER F, ALNOEE A B, BODERSKOV T, et al. Invasive submerged freshwater macrophytes are more plastic in their response to light intensity than to the availability of free CO2, in air: Equilibrated water[J]. Freshwater Biology, 2015, 60(5): 929-943. doi: 10.1111/fwb.12547 [19] SQUIRES M M, LESACK L F W, HUEBERT D. The influence of water transparency on the distribution and abundance of macrophytes among lakes of the Mackenzie Delta, Western Canadian Arctic[J]. Freshwater Biology, 2002, 47(11): 2123-2135. doi: 10.1046/j.1365-2427.2002.00959.x [20] SNDERGAARD M, PHILLIPS G, HELLSTEN S, et al. Maximum growing depth of submerged macrophytes in European lakes[J]. Alessandro Oggioni. Hydrobiologia, 2013, 704(1): 165-177. doi: 10.1007/s10750-012-1389-1 [21] SCNDERGAARD M, JOHANSSON L S, LAURIDSEN T L, et al. Submerged macrophytes as indicators of the ecological quality of lakes[J]. Freshwater Biology, 2010, 55(4): 893-908. doi: 10.1111/j.1365-2427.2009.02331.x [22] NURMINEN L, HORPPILA J. Life form dependent impacts of macrophyte vegetation on the ratio of resuspended nutrients[J]. Water Research, 2009, 43(13): 3217-3226. doi: 10.1016/j.watres.2009.04.041 [23] LI E H, LI W, LIU G H, et al. The effect of different submerged macrophyte species and biomass on sediment resuspension in a shallow freshwater lake[J]. Aquatic Botany, 2008, 88(2): 126. [24] IBELINGS B W, PORTIELJE R, LAMMENS E H R R, et al. Resilience of alternative stable states during the recovery of shallow lakes from eutrophication: Lake veluwe as a case study[J]. Ecosystems, 2007, 10(1): 4-16. doi: 10.1007/s10021-006-9009-4 [25] BACHMANN R W, HOYER M V, CANFIELD D E. Evaluation of recent limnological changes at Lake Apopka[J]. Hydrobiologia, 2001, 448(1/2/3): 19-26. [26] SCHREIBER U, KLUGHAMMER C, JRG K. Assessment of wavelength-dependent parameters of photosynthetic electron transport with a new type of multi-color PAM chlorophyll fluorometer[J]. Photosynthesis Research, 2012, 113(1/2/3): 127-144. [27] ASAEDA T, SULTANA M, MANATUNGE J, et al. The effect of epiphytic algae on the growth and production of Potamogeton perfoliatus L. in two light conditions[J]. Environmental and Experimental Botany, 2004, 52(3): 225-238. doi: 10.1016/j.envexpbot.2004.02.001 [28] LUND-HANSEN L C. Diffuse attenuation coefficients Kd(PAR) at the estuarine North Sea Baltic Sea transition: Time-series, partitioning, absorption, and scattering[J]. Estuarine Coastal & Shelf Science, 2004, 61(2): 251-259. [29] KEMP W M, BATLESON R, BERGSTROM P, et al. Habitat requirements for submerged aquatic vegetation in chesapeake bay: Water quality, light regime, and physical-chemical factors[J]. Estuaries and Coasts, 2004, 27(3): 363-377. doi: 10.1007/BF02803529 [30] KURTZ J C, YATES D F, MACAULEY J M, et al. Effects of light reduction on growth of the submerged macrophyte Vallisneria americana and the community of root-associated heterotrophic bacteria[J]. Journal of Experimental Marine Biology and Ecology, 2003, 291(2): 199-218. doi: 10.1016/S0022-0981(03)00120-5 [31] FRENETTE J J, ARTS M T, MORIN J. Spectral gradients of downwelling light in a fluvial lake (Lake Saint-Pierre, St-Lawrence River)[J]. Aquatic Ecology, 2003, 37(1): 77-85. doi: 10.1023/A:1022133530244 -



 下载:
下载: