-
茜素黄R(alizarin yellow R,AYR),又名5-(4-硝基苯基偶氮)水杨酸,是一种水杨酸衍生物,属于水溶性阴离子偶氮类染料[1]. AYR被广泛用于化学检查中的pH指示剂和生物染色剂[2]. 由于其复杂的芳香族结构,稳定性强,降解难度大[3]. AYR降解过程中易形成二次污染物和有毒污泥,其在有氧和厌氧条件下都不可生物降解,传统废水处理工艺较难去除AYR[4]. 此外,AYR具有很高的毒性和致癌性,对生态系统和人类健康存在不利影响[1]. 因此,亟需找到一种能够高效去除水中AYR的技术.
迄今为止,吸附[5]、混凝[6]、膜过滤[7]、沉淀[8]和电化学处理[9]等已被证实能有效降解茜素染料,但对于AYR的去除鲜有报道. 近年来,基于硫酸根自由基(SO4−·)的过硫酸盐(persulfate,PS)高级氧化技术(Sulfate radical-based advanced oxidation processes,SR-AOPs)得到快速发展,与传统AOPs相比,SR-AOPs可产生选择性更高、半衰期更长的SO4−·[10]. PS不仅可以直接氧化降解污染物,还能够活化产生多种自由基[11],PS包括过一硫酸盐(Permonosulphate,PMS)和过二硫酸盐(Peroxodisulphates,PDS),PDS较PMS更稳定. 与·OH相比,SO4−·的氧化还原电位更高,即:E0(SO4−·/SO42−)=2.60—3.10 VNHE >E0(·OH/OH−)=1.90—2.70 VNHE. SR-AOPs优于传统AOPs[12],具体表现如下:(1)更高的自由基生成率[13];(2)激活的方法更广泛[14];(3)PDS运输和储存成本低廉[15].
微生物燃料电池(microbial fuel cell,MFCs)作为一种生物自发电装置受到广泛关注,常见的MFCs是由阳极室和阴极构组成,中间由质子交换膜(PEM)隔开[16]. 在阳极室微生物氧化有机物产生电子和质子;质子穿过PEM,而电子通过外部电路到达阴极室,从而形成完整的电回路[17]. MFCs处理含有偶氮染料的废水,是一种同时进行废水处理和能源生产的新兴技术. Liu等[18]使用偶氮染料作为MFCs的阴极电子受体,实验结果表明,在pH为3.0时,甲基橙(MO)、橙Ⅰ和橙Ⅱ等偶氮染料均可在被成功降解. Li等[19]对K2S2O8溶液的MFCs性能进行了评价,并与K3Fe(CN) 6溶液进行了比较,结果表明,PDS具有独特的自pH调节能力,可作为一种有效的阴极电子受体. Wang等[20]提出了一种在双室MFCs中投加K2S2O8-Fe2+体系作为新型阴极试剂,可有效提高阴极电位,K2S2O8-Fe2+物质的量比为2:1的MFC性能最佳. 综上所述,MFCs是一种有效的去除偶氮类染料的方式.
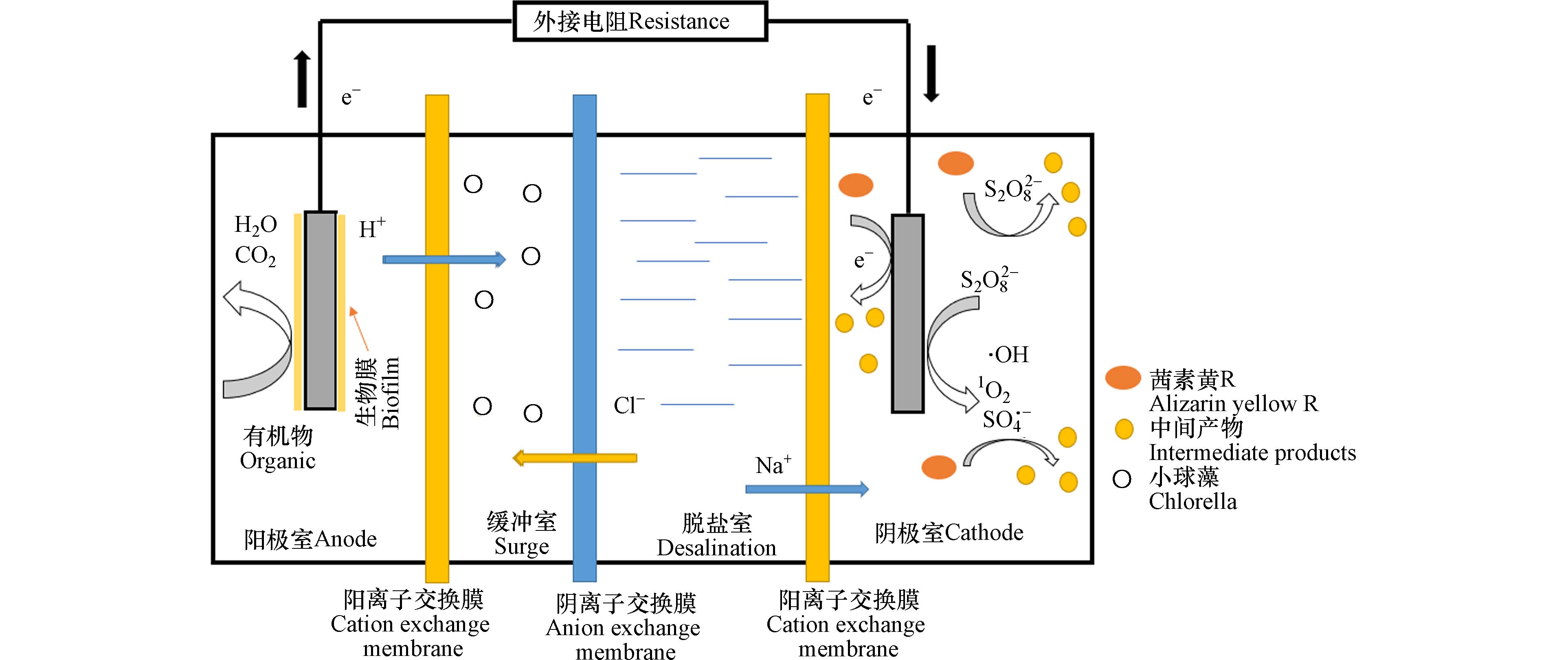
微生物脱盐燃料电池(microbial desalination fuel cells,MDCs)是在MFC的基础上发展演变而来,在阳极室与阴极室之间增加脱盐室,进行海水淡化[21]. MDCs使用阴阳离子交换膜取代PEM,成本降低[22]. 目前,使用PDS作为阴极电子受体与MDCs结合去除偶氮染料的报道较少. 本研究构建一个四室的MDCs,在阳极室和脱盐室中间增加缓冲室,阳极室产生的H+及脱盐室中的Cl-进入缓冲室,避免阳极室pH失衡,影响阳极微生物活性. 以PDS为阴极电子受体,提高AYR的脱色效率,同时达到去除阳极污染物及脱盐产电四位一体的效果. 本研究以AYR作为研究对象,对比了单独MDCs、单独PDS以及过硫酸盐阴极型MDCs去除水中AYR的效果,考察了不同溶液pH、PDS投加量、不同浓度共存阴离子以及有机物对降解效果的影响,、通过LC-MS检测结果分析了该工艺降解AYR的反应机理.
-
茜素黄R(C13H9N3O5)购自麦克林生化科技(上海)股份有限公司(表1). 过硫酸钠(Na2S2O8)、NaCl、Na2CO3、KNO3、KBr、H2SO4、NaOH、黄腐酸(FA)和牛血清蛋白(BSA)购自阿拉丁生化科技(上海)股份有限公司. 甲醇、叔丁醇、糠醇(HPLC级,纯度≥99.9%)购自麦克林生化科技(上海)股份有限公司. 其他所有化学品都是分析级及以上,购自国药控股化学试剂有限公司. 阴阳离子交换膜(中国绿合有限公司,Grion0011 V,横截面积100 cm2)、碳毡电极(B1B无防水层,50 mm×50 mm,E-Tech公司).
-
本研究采用MDCs四室构型,利用有机玻璃板制成密封的容器,尺寸为20 cm× 10 cm× 10 cm,阳极室、脱盐室及阴极室的有效容量均为200 mL,缓冲室有效容积为100 mL,顶部均设有进出水口,如图1所示. 阳极室使用的活性污泥取自于上海临港污水处理厂的二沉池,进行驯化培养2周后投入使用. 过硫酸盐阴极型MDCs反应装置于(25±2)℃室温下启动和运行,根据徐成龙等[23]的研究,经过阳极体积换算,以100 mg·L−1COD的葡萄糖培养基作为阳极的底物浓度,脱盐室使用15 g·L−1的NaCl溶液,缓冲室中小球藻的藻密度为2×107—3×107个·L−1. 通过向200 mL含有AYR(10 mg·L−1)反应溶液中加入一定剂量的过硫酸钠溶液,按预定的时间间隔进行取样,样品分析前,用甲醇进行淬灭. 随后进行AYR测定和降解产物分析. 所有测试均重复两次,数据图中的误差棒代表两次实验结果的标准偏差.
-
AYR的浓度采用使用UV-
2100 紫外可见分光光度计于λ=374 nm处测定吸光度. 采用总有机碳分析仪(TOC-LCPN,日本岛津)测定样品TOC的值. FA和BSA的三维荧光采用日立公司F-2700 型荧光分光光度仪进行测定,其中激发波长为250—600 nm,发射波长为220—550 nm,扫描间距均为5 nm,其扫描速率为1200 nm·min−1. 本研究以甲醇(MeOH)、叔丁醇(TBA)和糠醇(FFA)等3种不同的溶剂为淬灭剂. 使用电化学工作站(CHI660E,上海市辰华仪器有限公司)在0.2 V·s−1的扫描速度下测得电池的循环伏安曲线,在0.1 V·s−1的扫描速度下直接测得电池的线性伏安曲线. 将20 mmol·L−1的MeOH、TBA和FFA等分别加入到200 mL的反应体系中,通过考察MeOH、TBA和FFA对偶氮染料去除率的影响,间接推断出过硫酸盐阴极型MDCs体系中的自由基种类. LC-MS的流动相为水和甲醇,使用梯度洗脱:在15 min中,甲醇从10%升到100%,并且维持10 min,流速为0.2 mL·min−1,采用流动注射进样速率为0.3 μL·min−1,ESI源,正离子模式,质荷比扫描范围为50—400. -
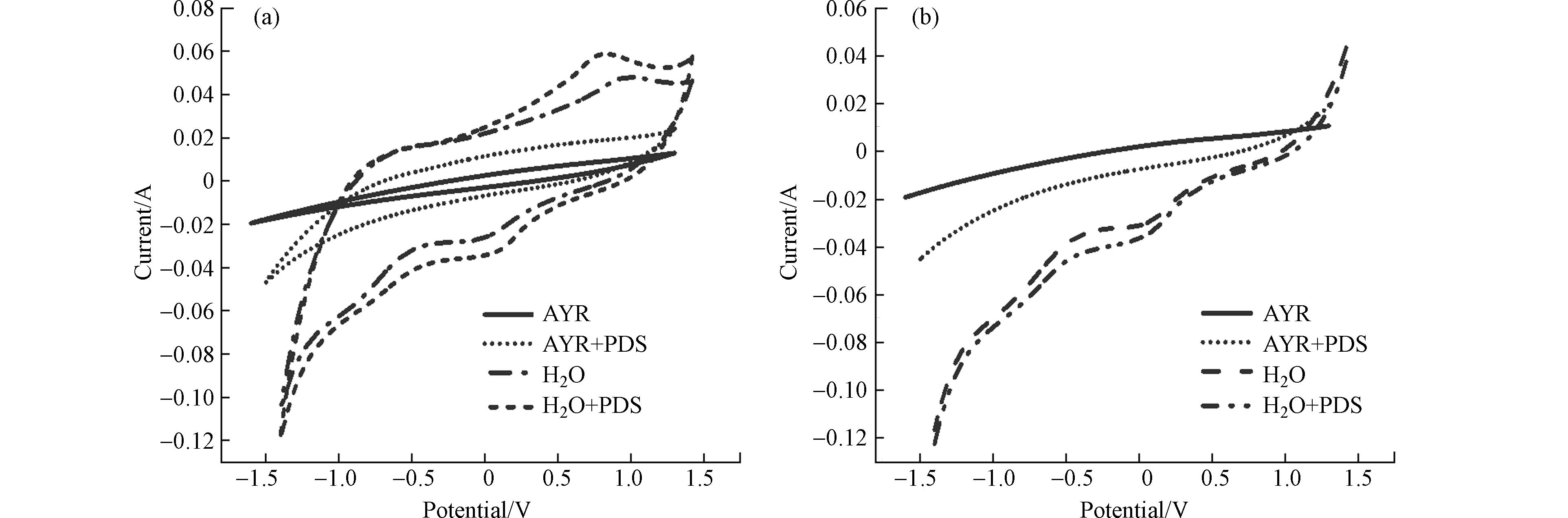
在过硫酸盐阴极型MDCs启动后,以间歇性模式运转,当电压下降到500 mV以下时,更换阳极底物溶液. 在进行了2周的培育之后,电压最大值可达到
1310 mV,为传统MDCs的两倍,分析原因有两个方面因素,一方面是因为阴极以PDS作为MDCs的电子受体,极大地提高了阴极的电极电势,与Li等[19]的研究结果较一致;另一方面,缓冲室的存在有效改善了阳极pH失衡的情况.从图2(a)可看出,阴极投加PDS后的循环伏安曲线,氧化还原峰增加,氧化还原峰越明显,说明电极表面电化学反应阻力越小,阳极的极化内阻也就越小[24]. 图2(b)在−1.5—1.5 V(vs.SHE)的扫描电压范围内,不同阴极得到的电流密度高低顺序如下:纯水+PDS≈纯水>AYR+PDS>AYR,随着电流从正往负,促进氧化还原反应的过电位逐渐升高,电流密度也逐渐增大,电流密度越高,说明其氧化还原特性越强[25], MDC中不同阴极组分的氧化还原能力的高低顺序依次为:纯水+PDS≈纯水>AYR+PDS>AYR. 即在MDCs的阴极投加PDS有利于阴极室中的氧化还原反应的进行,因此,AYR的氧化降解研究采用过硫酸盐阴极型MDCs展开.
-
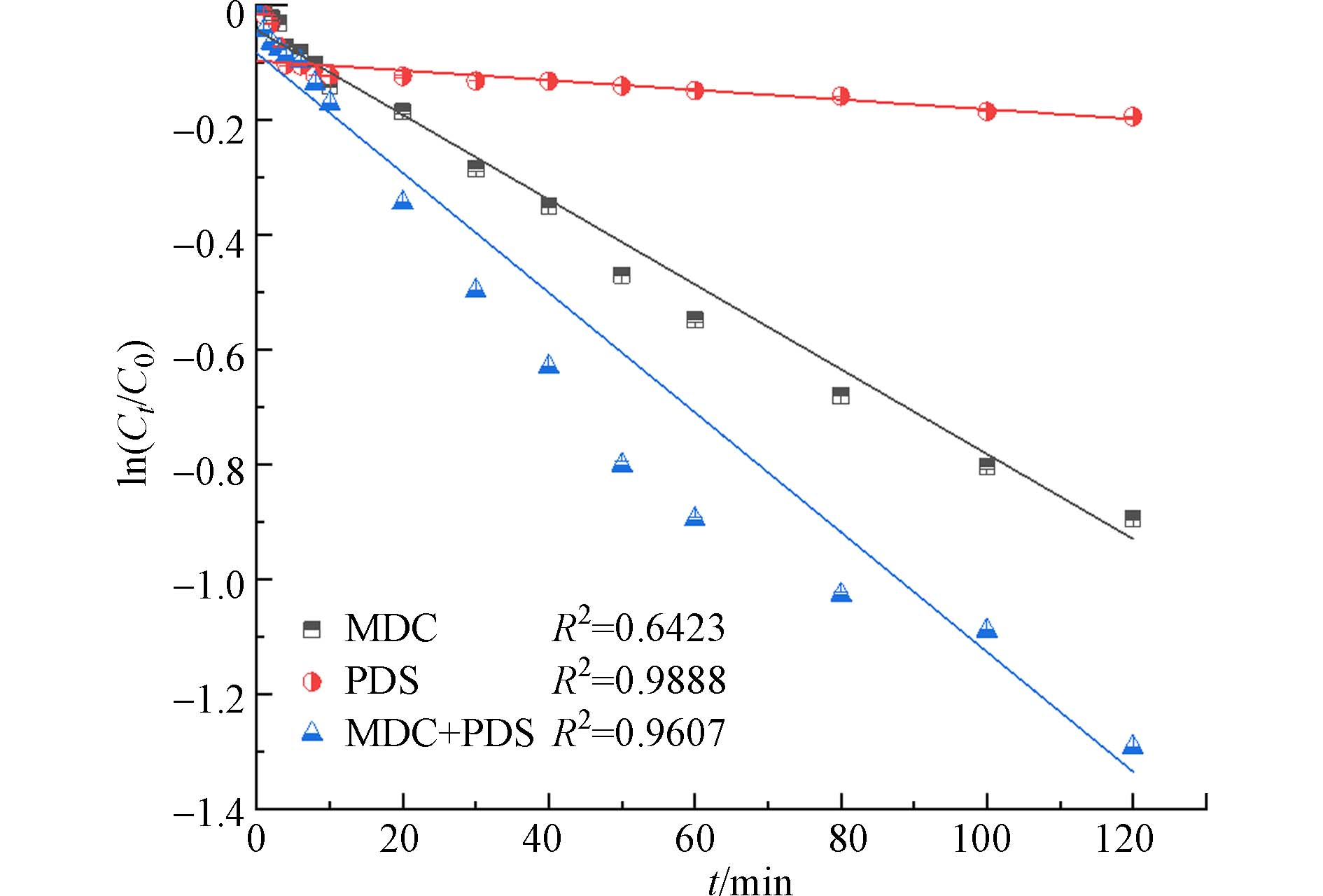
实验控制AYR浓度为10 mg·L−1,Na2S2O8的初始浓度为2 mmol·L−1,反应体系的初始pH值为3.00±0.02,温度为(25±2)℃,图3比较了AYR在单独MDCs、单独PDS及过硫酸盐阴极型MDCs工艺下的降解效果.
使用拟一级动力学反应模型,拟合过硫酸盐阴极型MDCs降解AYR的浓度变化,AYR的浓度和时间比值的对数有较好的线性关系,构建了AYR降解的动力学模型,如图3所示. 单独使用MDCs,单独使用PDS,以及过硫酸盐阴极型MDCs都遵循拟一级动力学模型(式1). 单独PDS对AYR的降解效果较差仅为17.6%,单独MDCs有较好的降解作用,去除率可达59.11%,过硫酸盐阴极型MDCs去除率为72.54%,表现出明显的协同增效作用,根据协同效应计算结果(式2),可以得到过硫酸盐阴极型MDCs对AYR的协同效应为1.27. AYR在3个不同反应中的降解速率kobs值分别为
0.0112 min−1、0.0077 min−1、0.0012 min−1,过硫酸盐阴极型MDCs降解AYR的速率最快,分别为单独MDCs和单独PDS的1.46倍和9.3倍.单独MDCs、单独PDS与过硫酸盐阴极型MDCs处理效果存在显著差异,其原因在于高氧化还原电位的SO4−·的作用. Na2S2O8加入反应体系后,会产生氧化还原电位为2.01 V的S2O82-,可对部分AYR直接降解[26]. MDCs体系去除AYR的机理是因为部分AYR得到电子,发生还原反应实现脱色. MDCs体系中阳极传递过来的电子,直接与AYR发生反应,断裂偶氮键(式3). 过硫酸盐阴极型MDCs体系对AYR协同效应是因为阴极中电子受体变为S2O82-,在该体系中不仅存在部分AYR得到电子断裂偶氮键和S2O82-自身的氧化作用,还因为S2O82-得到电子还原产生强氧化性的SO4−·等自由基(式4). SO4−·氧化还原电位较高且稳定性较强,可有效降解AYR.
为进一步探明不同体系对AYR的降解产生差异的原因,通过自由基淬灭实验和电子顺磁共振(EPR)确定过硫酸盐阴极型MDCs中的自由基种类. 由于SR-AOPs会产生高活性自由基,如羟基自由基(·OH)、硫酸盐自由基(SO4−·)和单线态氧(1O2)[27],因此在去除有机污染方面效率高且易于实施. 本研究分别选择甲醇(MeOH)、叔丁醇(TBA)、糠醇(FFA)来淬灭·OH、SO4−·以及1O2[28],结果如图4(a)所示. 淬灭实验证明了过硫酸盐阴极型MDCs体系中存在·OH、SO4−·以及1O2. EPR进一步验证过硫酸盐阴极型MDCs中的自由基种类,如图4(b)所示.
谱图中存在·OH、SO4−·以及1O2的峰,且自由基峰强随时间增长呈增加趋势. 这表明在反应过程中产生了·OH、SO4−·以及1O2,同时,反应后期存在的自由基量更大、更活跃. 与淬灭实验结果基本一致.
-
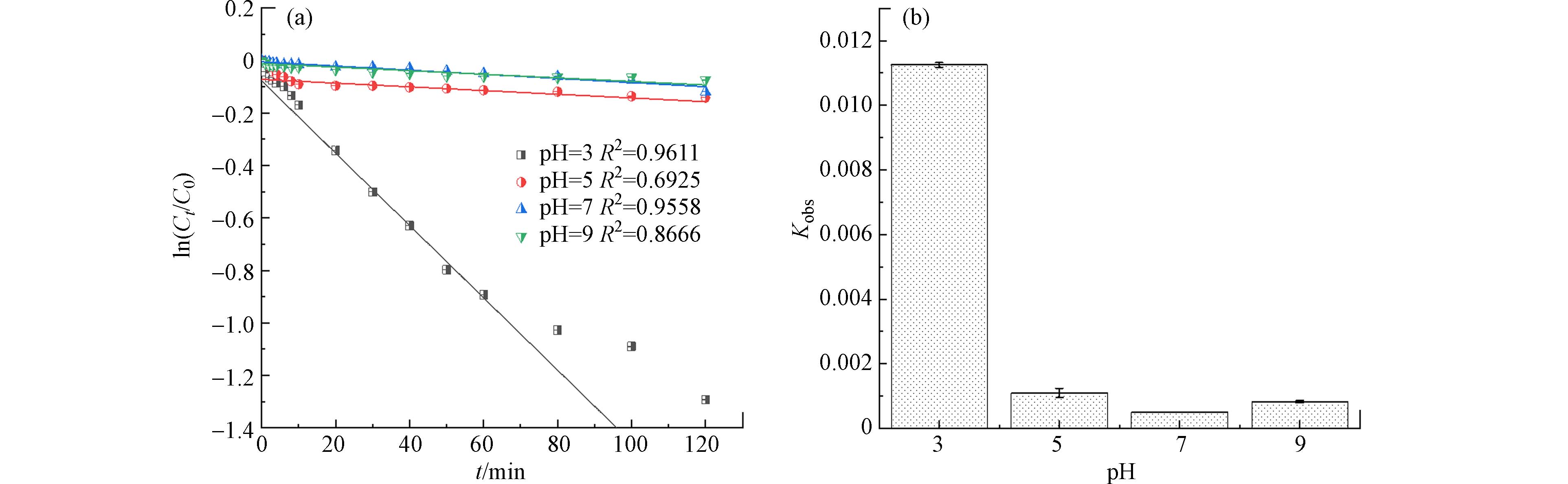
控制AYR的初始浓度为10 mg·L−1,Na2S2O8的初始浓度为2 mmol·L−1,温度(25±2)℃,初始pH通过10 mmol·L−1 NaOH和10 mmol·L−1 H2SO4调节,分别调节pH值为3.0、5.0、7.0、9.0,研究了不同初始pH值对AYR去除率的影响. 初始pH值对AYR的降解的影响如图5(a)所示,降解速率常数Kobs变化如图5(b)所示. 120 min后,在pH=3、pH=5、pH=7和pH=9条件下,AYR的降解效率分别达到72.54%、13.17%、11.19 %和7.13%. 其一级反应速率常数为
0.01125 min−1、0.0011min−1、0.0005 min−1、0.00085 min−1. 可以看出,pH=3时AYR降解效果最好,反应速率最快.从图5可以看出,中性及碱性条件不利于AYR的氧化降解,酸性条件有利于反应进行,强酸条件下效果最好. 研究结果表明[29],水中溶解氧的含量随着pH的降低会下降,与PDS竞争电子的氧气含量降低,PDS可以得到更多的电子生成SO4−·,进而提高AYR去除率. 反应过程如式(5)和式(6)所示.
-
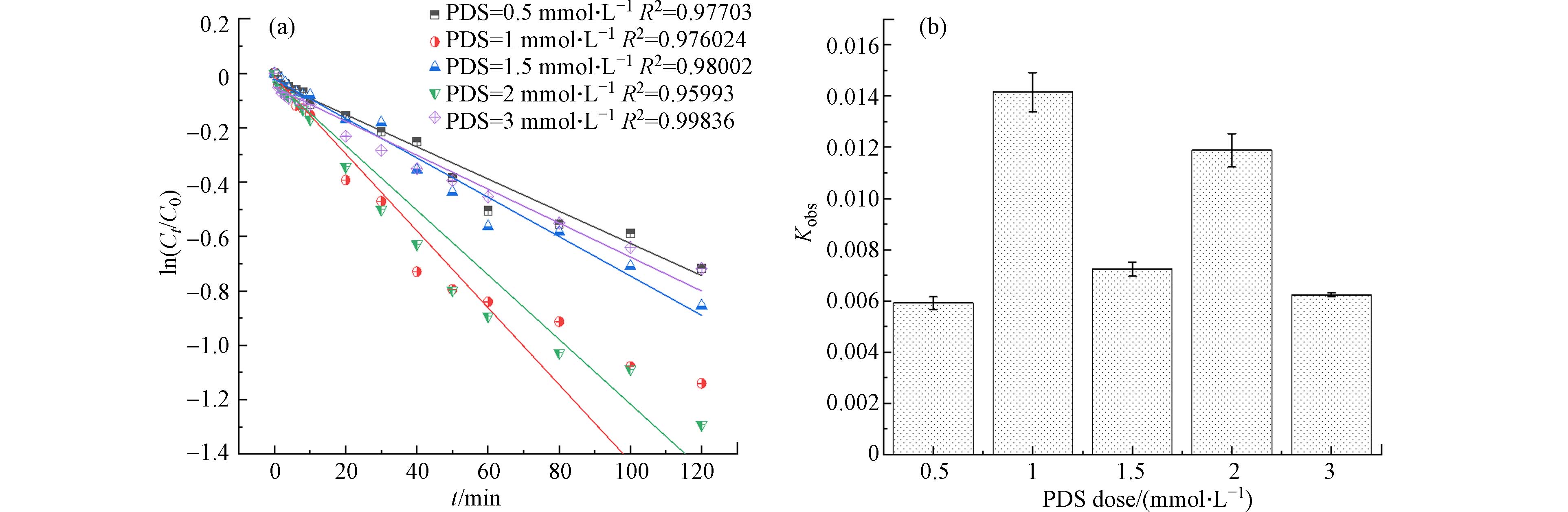
PDS是过硫酸盐阴极型MDCs中生成自由基的主要物质,因此,PDS的投加量在AYR的降解中发挥着重要的作用. 为了探究过硫酸盐阴极型MDCs组合工艺中PDS浓度对AYR降解的影响,通过投加不同浓度的PDS,使得电池阴极室溶液中氧化剂的浓度分别为0.5、1.0、1.5、2.0 、3.0 mmol·L−1,实验的AYR的初始浓度为10 mg·L−1,pH=3.0±0.02,温度为(25±2)℃,PDS投加量对AYR降解的影响如图6(a)所示,其降解速率常数Kobs变化如图6(b)所示.
从图6(a)可以看出,不同剂量的过硫酸盐对AYR的去除效率产生较大影响. 已有研究发现[30],增大PDS的浓度可以提高阴极电极电位,加快电子传递速率,进而加速了SO4−·的生成. AYR的去除率在PDS浓度为2 mmol·L−1时最高. 随着PDS浓度的增加,其生成的SO4−·浓度逐渐增加,AYR所接触的SO4−·增多,AYR去除率随之升高. 然而,在较高的PDS浓度情况下,活化生成的大量自由基(SO4−·为主)会在很短的时间内相互湮灭[31]. 因此,当PDS浓度过高时,一方面过量的SO4−·会发生自淬灭反应(式7),另一方面,过量的PDS会消耗SO4−·(式8),进而抑制AYR的去除.
如图6(b)所示,当PDS浓度为1 mmol·L−1时,Kobs为
0.01415 min−1,PDS浓度为2 mmol·L−1时其Kobs为0.01188 min−1. 可以发现PDS浓度为1 mmol·L−1时,AYR的降解速率常数最高. -
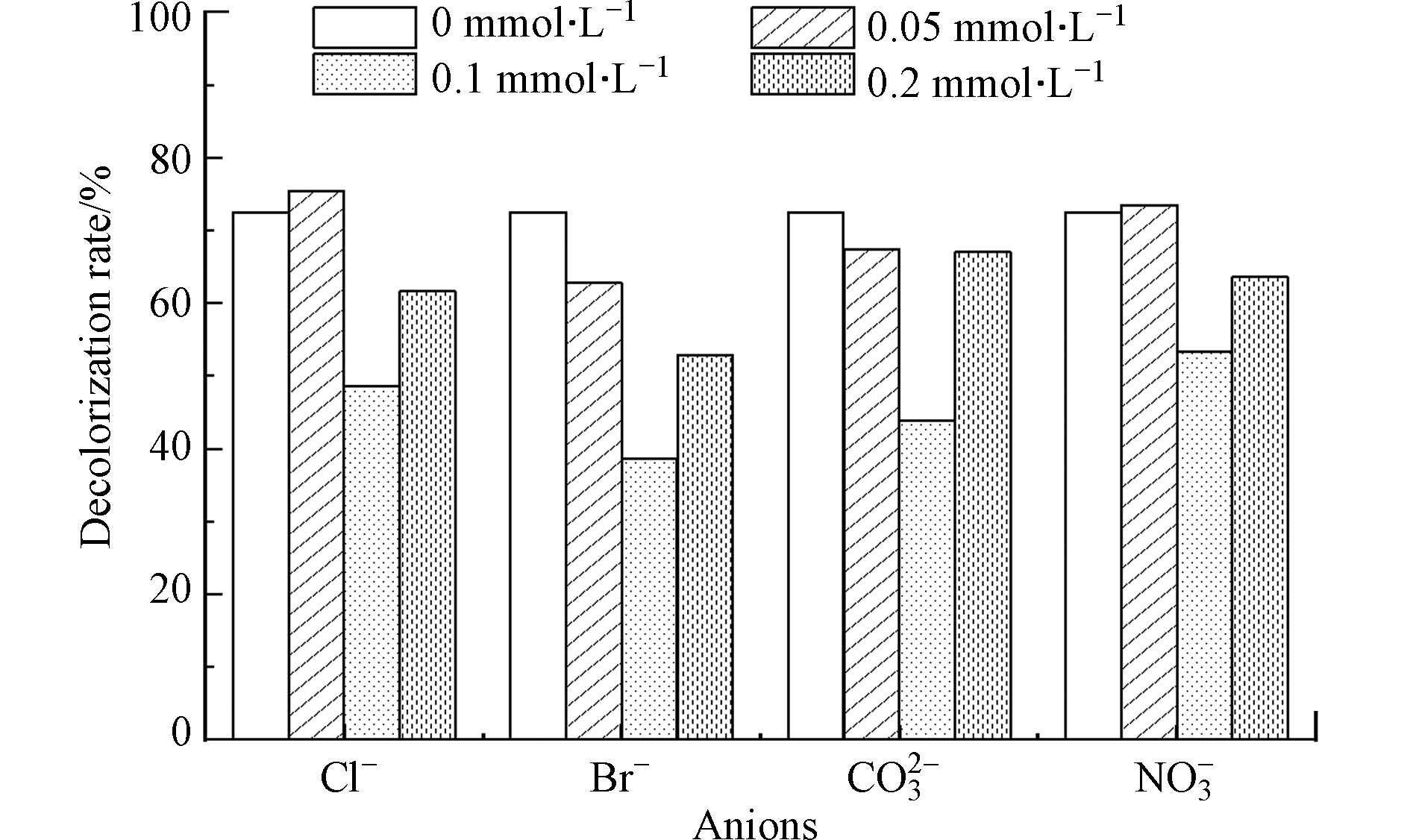
在染料的制备和使用的过程中,由于存在着大量的无机盐,造成了染料废水组分的复杂性,给其治理增加了难度,研究表明,Cl-、Br-、NO3-、CO32−等共存阴离子对过硫酸盐阴极型MDC去除有机污染物存在不同程度的影响[32],探究共存阴离子对AYR去除效果的影响具有重要意义. 实验设置AYR初始浓度为10 mg·L−1,PDS投加量为2 mmol·L−1,初始pH为3.00±0.02,温度为(25±2)℃. 向体系中投加不同浓度的无机阴离子(Br-、Cl-、NO3-、CO32−),考察其对过硫酸盐阴极型MDCs降解AYR的影响.
由图7可以看出,当Cl-、Br-、NO3-、CO32−无机阴离子的含量为0.2 mmol·L−1时,AYR的去除率分别为61.60%、52.85%、67.04%和63.59%. 随着Cl-、NO3-浓度的增加,AYR的去除均呈现先促进后抑制的作用. 在本研究中,较低浓度的Cl-对AYR的降解有促进作用,其原因在于,Cl-在水中可以提高其导电率,提高SO4−·的产率,从而加速其对AYR的降解. 高浓度的Cl-抑制AYR的去除,这与去除耐酸大红4BS的[33]研究相吻合,这是由于Cl-会消耗体系中SO4−·(式9),从而对污染物的降解产生影响. NO3-同理,NO3-消耗SO4−·转化为NO3·[34](式10).
反应体系中共存的CO32−和Br-对AYR去除率呈现先抑制后促进,但整体表现为抑制. 这是因为CO32−和Br-会和SO4−·反应,CO32−会消耗SO4−·,转化为CO3−·,如式(11),降低过硫酸盐阴极型MDCs体系内SO4−·浓度,进而影响AYR降解[35]. CO3−·是比SO4−·和HO·更具选择性的氧化剂,CO32−超过一定浓度后,会大量消耗SO4−·和HO·生成更多的CO3−·[36]. Br-可能会清除SO4−·以产生反应活性更小的溴自由基(式12),进而影响AYR降解. 有研究表明[37]UV/PDS降解二溴代乙酰胺中溴原子向溴酸盐转化率高达100%,随着Br-投加量的增加,Br-可与溴自由基进一步反应生成中间体HOBr/OBr—及溴酸盐等物质,进而降低了对SO4−·的消耗.
-
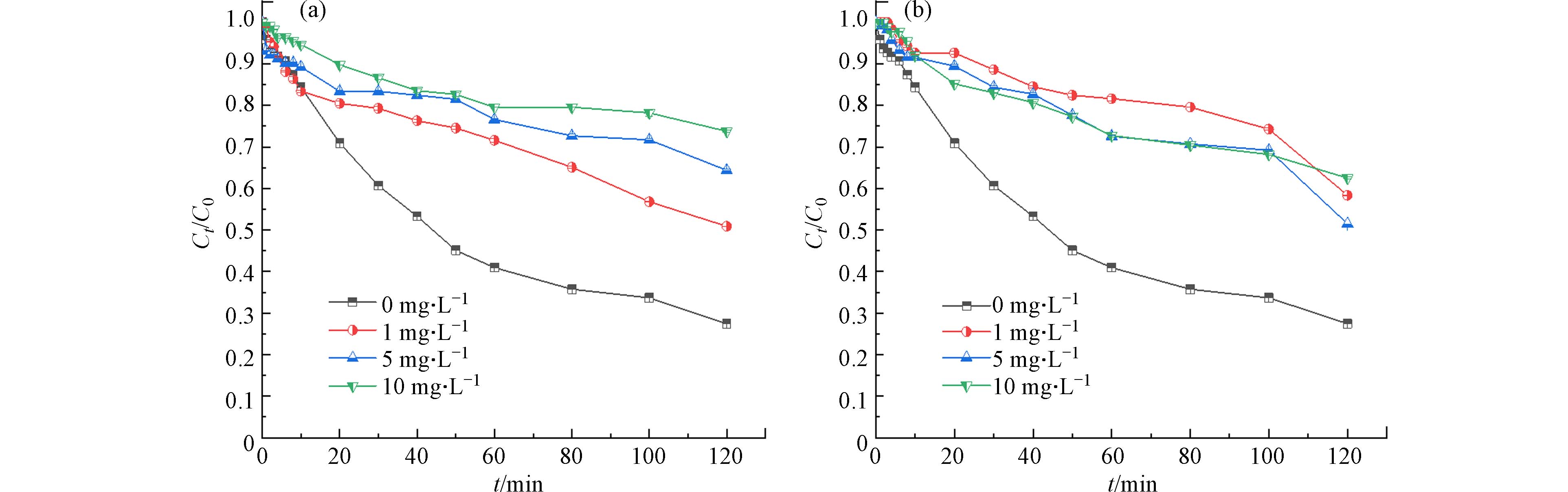
因为真实水体的组成更加复杂,为了探索天然有机物(NOM)对过硫酸盐阴极MDCs处理AYR的降解的影响,本研究选择了以腐殖酸为代表的天然有机质,其中黄腐酸(FA)是其中的重要组分,其含有羰基、羧酸、苯酚和羟基等,由于这些基团会争夺自由基,可能会阻碍AYR的去除. 实验设置AYR的浓度为10 mg·L−1,反应体系初始pH值为3.00±0.02,PDS的初始浓度为2 mmol·L−1,温度为(25±2)℃,图8(a)显示了不同浓度的FA对AYR降解的影响. 从图8(a)可见,AYR的降解效率随水中FA浓度的增加而有所降低,Kobs由0.05 min−1下降至
0.0025 min−1.本研究以牛血清蛋白(BSA)为水中蛋白质的代表,对其在不同浓度下对AYR降解的影响进行了研究,如图8(b)所示. 实验考察了AYR的初始浓度为10 mg·L−1,PDS的初始浓度为2 mmol·L−1,牛血清蛋白投加量对AYR的降解效果呈现抑制作用. BSA浓度为1 mg·L−1时抑制效果最显著,Kobs从
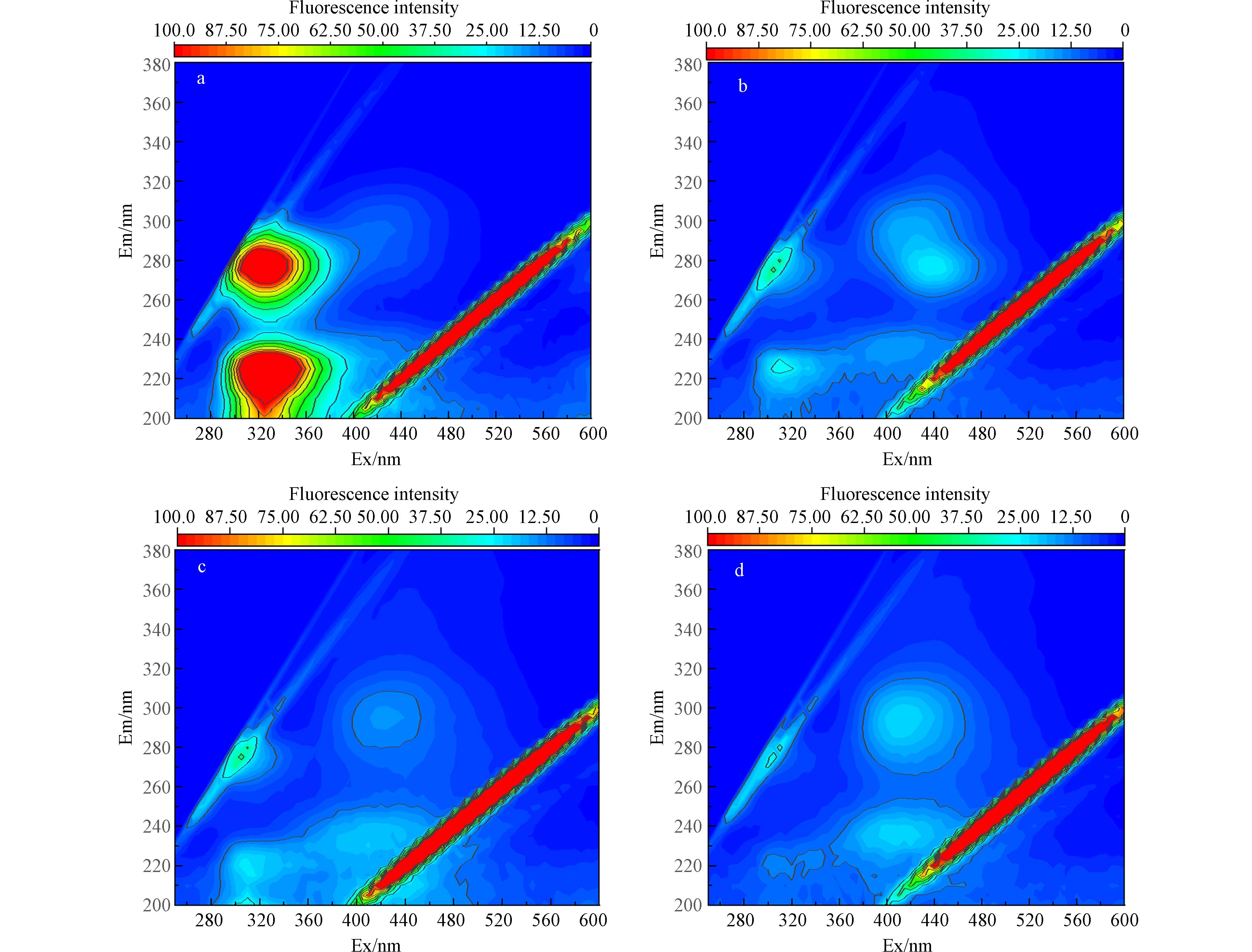
0.0112 min−1下降至0.0036 min−1.由于AYR基本没有荧光强度,而FA和BSA的荧光强度较显著,分别对含AYR+BSA及AYR+FA两种水样氧化前后分别进行了3D-Fluorescence. 从图9可以看出,FA、BSA均有显著的荧光变化. 在加入PDS后5 min,荧光强度会大幅度地降低,因此,过硫酸盐阴极型MDCs可以很好地去除水中的FA和BSA,这也说明了FA和BSA与AYR之间存在着争夺自由基的竞争关系.
-
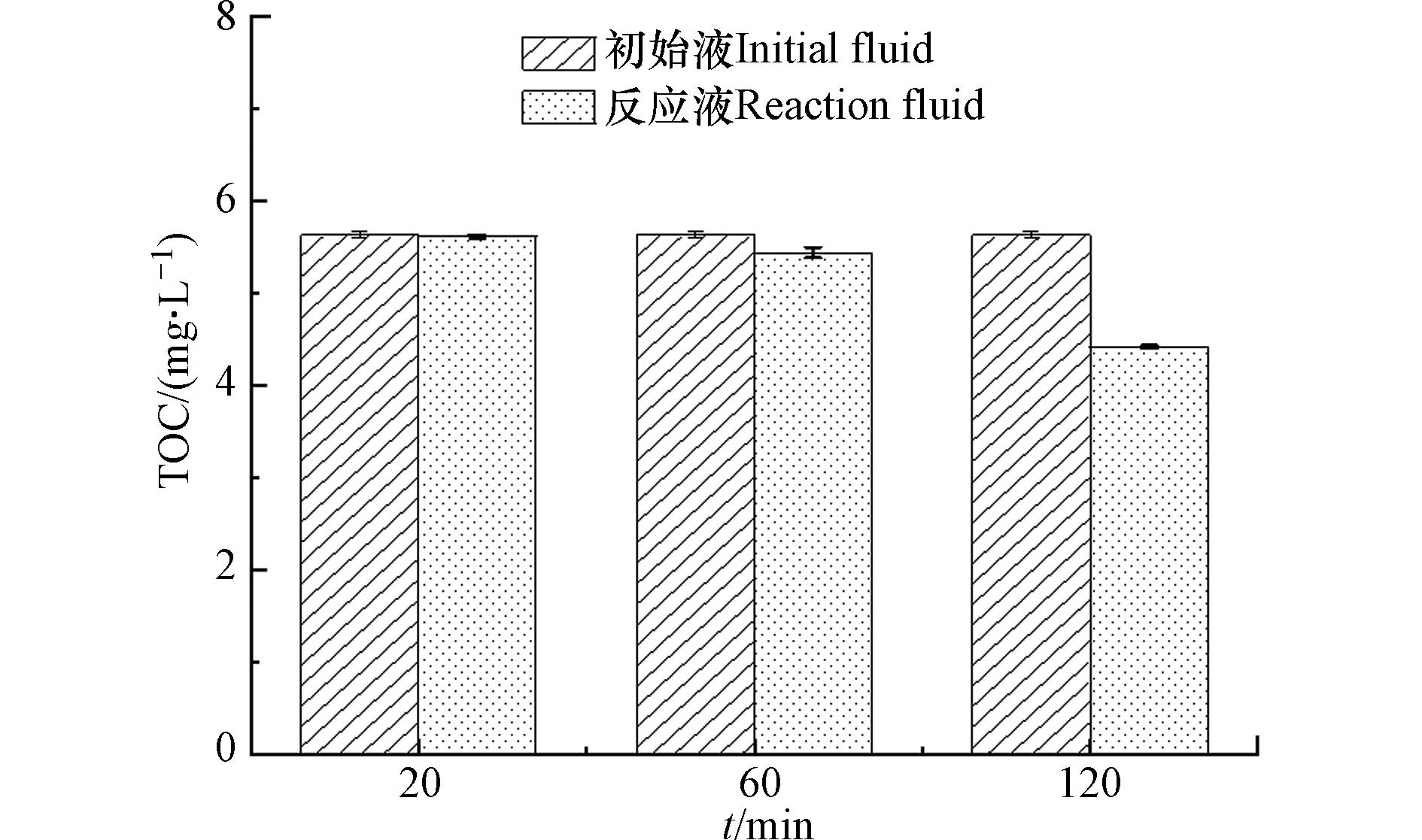
图10显示了过硫酸盐阴极型MDCs体系对AYR的TOC去除的影响. 结果显示,TOC在反应时间的20 min基本没有变化,60 min时TOC去除率仅为3.33%,120 min时反应液的TOC得到部分去除,TOC去除率仍较低为21.56%,说明AYR在反应过程中的矿化程度较低,随着反应的持续进行,AYR在降解过程中转化成其他的有机副产物.
-
由2.2.1可知,·OH、SO4−·以及1O2在AYR的降解中起到一定的作用,在过硫酸盐阴极型MDCs体系中,通过LC-MS检测分析得出以下中间产物,如表2所示.
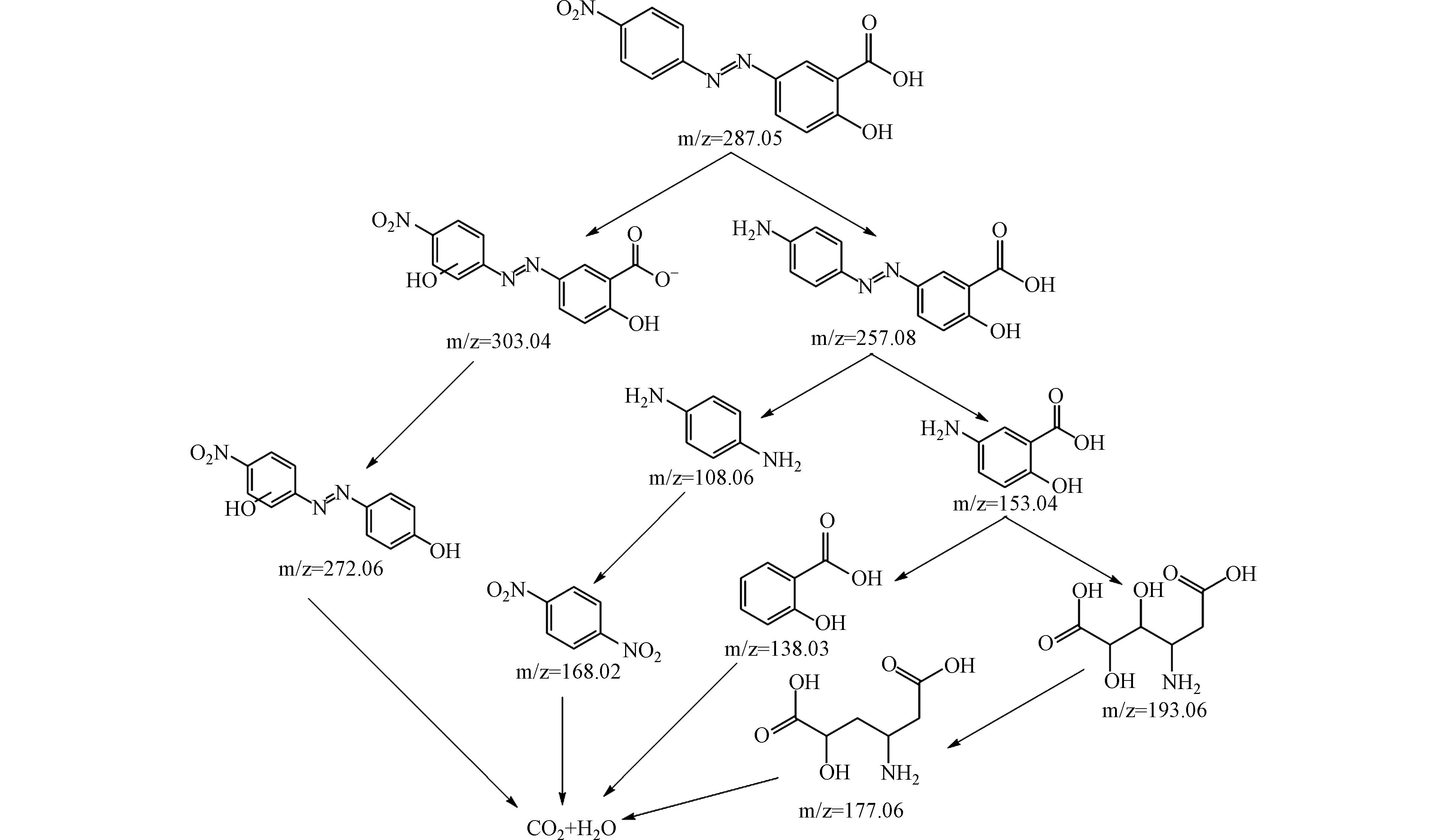
根据AYR的中间产物推测出降解路径如图11所示. 降解路径1是AYR发生取代反应,生成m/z为303的C13H9N3O6,然后在·OH、SO4−·以及1O2的作用下发生脱羧反应,生成m/z为272的C12H10N3O4,最后矿化为CO2和H2O[38].
降解路径2较复杂,AYR先发生还原反应m/z为257的C13H9N3O3,在·OH、SO4−·以及1O2的作用下断裂偶氮键,生成m/z为108的C6H8N2和m/z为153的C7H7NO3,C6H8N2发生氧化反应生成m/z为168的C6H4N2O4,最后矿化为CO2和H2O;C7H7NO3通过断裂苯环、脱氮等逐步被氧化降解,最后生成CO2和H2O[39].
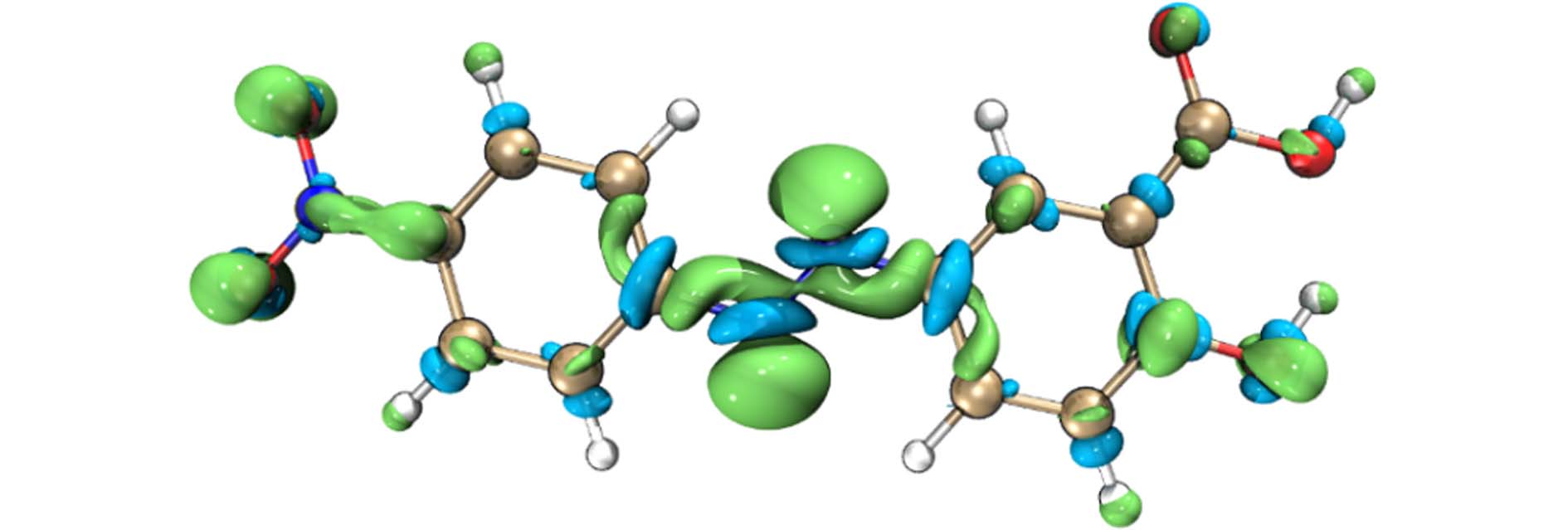
为了进一步探究AYR的降解机理,采用基于密度泛函理论(DFT)的福井函数(f 0)预测自由基攻击位点. 通过Gaussian 09W软件在b3lyp/6—31+G(d,p)理论水平上进行了AYR的几何优化计算,并使用了基于自然键轨道(NPA)分析的电子自旋密度;Multiwfn软件对福井函数(f 0)进行分析[40],并根据f 0数值验证自由基攻击AYR分子的区域选择性,如图12所示. 自由基总是倾向攻击f0值较高的反应位点[41],根据表3,N15(f 0 =
0.1273 )、N16(f 0 =0.1192 )、O21(f 0 =0.0628 )和O20(f 0=0.0624 )位置显示较高的f 0值,这表明这些位点可能受到活性物质的攻击,也就是说自由基会首先攻击碳氮双键和硝基,这与LC-MS产物分析得出的结论一致. 综上所述,AYR的主要降解机理包括偶氮键断裂、脱羧、苯环断裂及脱氮等多步反应. -
(1)与单独MDC和单独PDS氧化降解AYR相比,AYR在过硫酸盐阴极型MDCs反应体系中的去除率可达72.54%,其反应速率常数最高,分别为单独MDCs和单独PDS的1.46倍和9.3倍. 过硫酸盐阴极型MDCs可有效去除AYR.
(2)过硫酸盐阴极型MDCs体系去除AYR的最佳pH值为3;随着PDS投加量的逐渐增加(0.5— 3 mmol·L−1),AYR的降解速率在PDS剂量为1 mmol·L−1时达到最大;反应体系中共存的阴离子Cl-、Br-、NO3-、CO32−对AYR的降解存在不同程度的促进或抑制作用;天然有机物FA对AYR的降解具有较强的抑制效应,而有机物牛血清蛋白BSA对AYR的去除存在抑制作用,表现出先增大后减小的趋势.
(3)经过120 min的降解反应,TOC的去除率仍较低仅为21.56%,表明AYR氧化降解过程中产生了较多的中间产物;AYR的主要降解机理包括偶氮键断裂、脱羧、苯环断裂及脱氮等多步反应.
过硫酸盐阴极型微生物脱盐燃料电池降解茜素黄R
Degradation of alizarin yellow R using persulfate- cathode microbial desalination fuel cell
-
摘要: 茜素黄R(AYR)是一种广泛使用的阴离子偶氮类染料,长期存在于水环境中的AYR对生态系统和人类健康存在潜在威胁. 本研究在构建了一种过硫酸盐阴极型微生物脱盐燃料电池(MDCs)的基础上,系统研究了过硫酸盐阴极型MDCs降解AYR的动力学及机理. 分别探究了阴极初始pH值、过硫酸盐(PDS)投加量、共存阴离子和有机物等环境因素对AYR降解动力学的影响,同时分析了AYR的降解产物与降解机理. 结果表明,与单独MDCs和单独PDS相比,过硫酸盐阴极型MDCs能够快速高效地降解AYR,AYR的降解过程符合拟一级反应动力学模型. 在pH=3和PDS投加量为1 mmol·L−1时,AYR的降解速率常数(Kobs)最高;共存的阴离子对AYR的降解存在不同程度的促进或抑制效果;有机物腐殖酸及牛血清蛋白均抑制AYR的氧化降解. 反应120 min后,阴极反应液的TOC得到部分去除,AYR的主要降解机理包括偶氮键断裂、脱羧、苯环断裂及脱氮等多步反应.Abstract: Alizarin yellow R (AYR) is a commonly used anionic azo dye. The long-term presence of AYR in the aquatic environment poses a potential threat to ecosystems and human health. In this paper, on the basis of constructing a persulfate-cathode microbial desalination fuel cell (MDCs), the kinetics and mechanism of AYR degradation by persulfate-cathode MDCs were systematically investigated. The effects of environmental factors such as initial cathode pH, PDS dosage, coexisting anions, and organic matter on the kinetics of AYR degradation were investigated. Meanwhile the degradation products and mechanism of AYR were studied. The results demonstrated that persulfate-cathode MDCs can degrade AYR quickly and effectively compared with MDCs alone and PDS alone. The AYR degradation process followed pseudo first order reaction kinetics model. The degradation rate (Kobs) of AYR was highest with the pH=3 and 1 mmol·L−1 PDS dosage respectively. The coexisting anions had varying degrees of promoting or inhibiting effects on the degradation of AYR. Both organic humic acid and bovine serum protein inhibited the oxidative degradation of AYR. After 120 minutes of reaction, TOC was partially removed in the cathodic reaction solution, and multi-step reactions like azo bond breaking, decarboxylation, benzene ring breaking, and denitrification were the key mechanisms of AYR degradation.
-
Key words:
- alizarin yellow R /
- persulfate /
- microbial desalination fuel cell /
- kinetics /
- degradation mechanism.
-

-
表 1 茜素黄R的物化性质
Table 1. Physical and chemical properties of alizar in yellow R
名称
Compounds分子结构
Molecular structureCAS 分子量
Molecular weight溶解性
Solubility茜素黄R 
2243 -76-7287.20 溶于水和乙醇 表 2 AYR降解中间产物分析
Table 2. Analysis of AYR degradation intermediates
分子式
Molecular formula质荷比
Mass spectral(m/z)可能结构式
Possible structural formulaC13H9N3O5 287.05 
C13H9N3O3 257.08 
C13H9N3O6 303.04 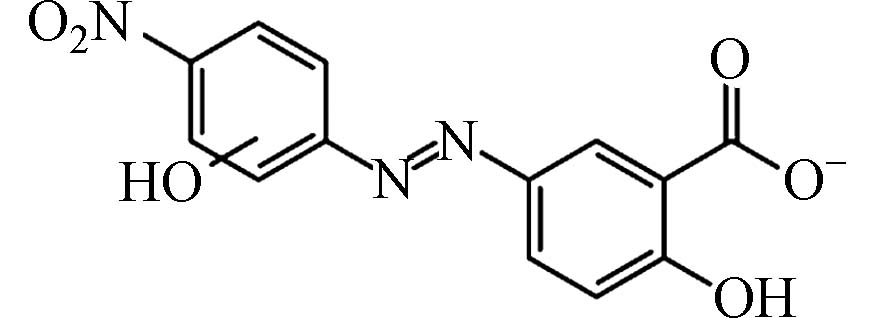
C12H10N3O4 272.06 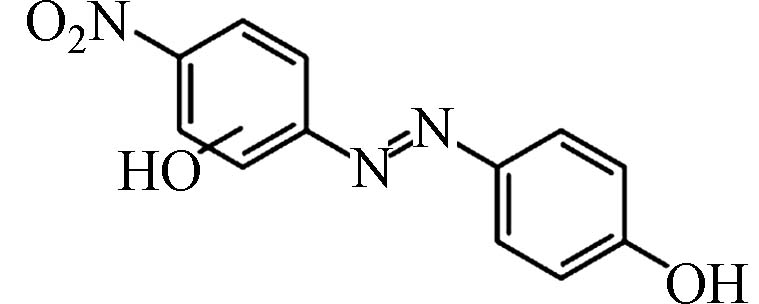
C7H7NO3 153.04 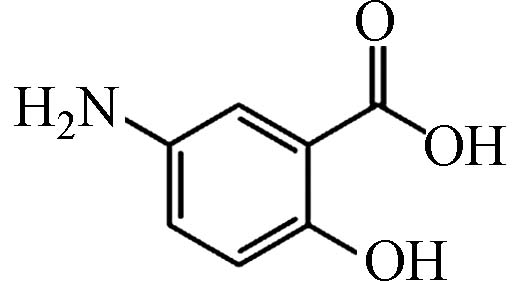
C6H8N2 108.06 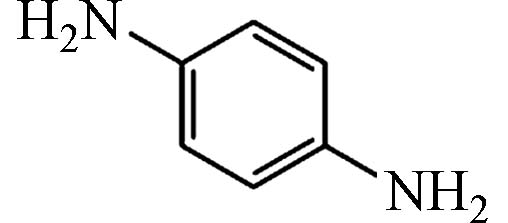
C7H6O3 138.03 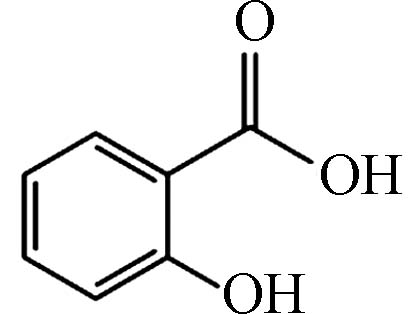
C6H8NO6 193.06 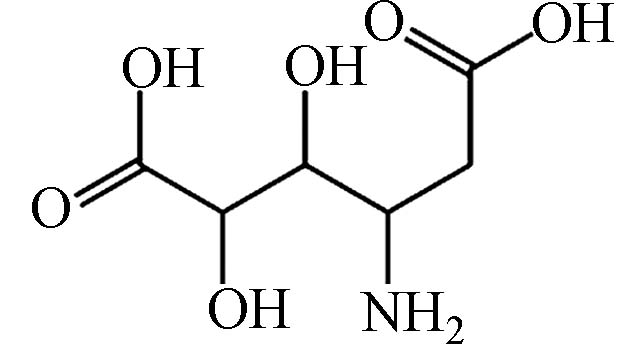
C6H4N2O4 168.02 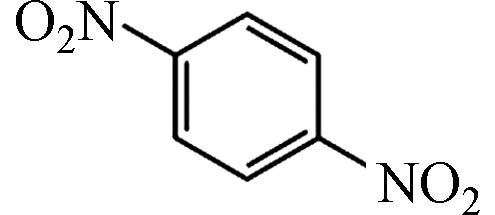
C6H9NO5 177.06 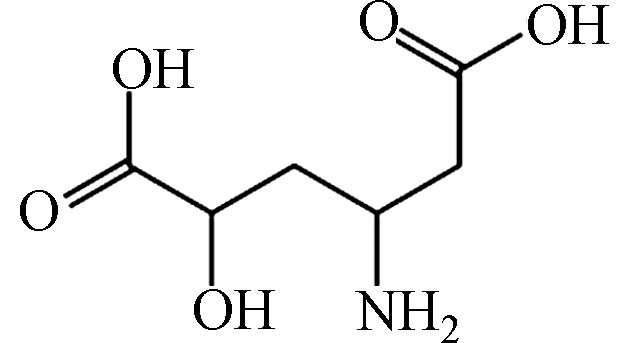
表 3 不同电子状态下AYR的Natural Population Analysis(NPA)电荷分布和计算的福井指数(f0)水平
Table 3. NPA charge distribution on AYR at different electron state and calculated Fukui index (f0) levels
原子
Atom序号
NumberqN qN+1 qN-1 f0 AYR N 15 − 0.0544 − 0.1456 0.109 0.1273 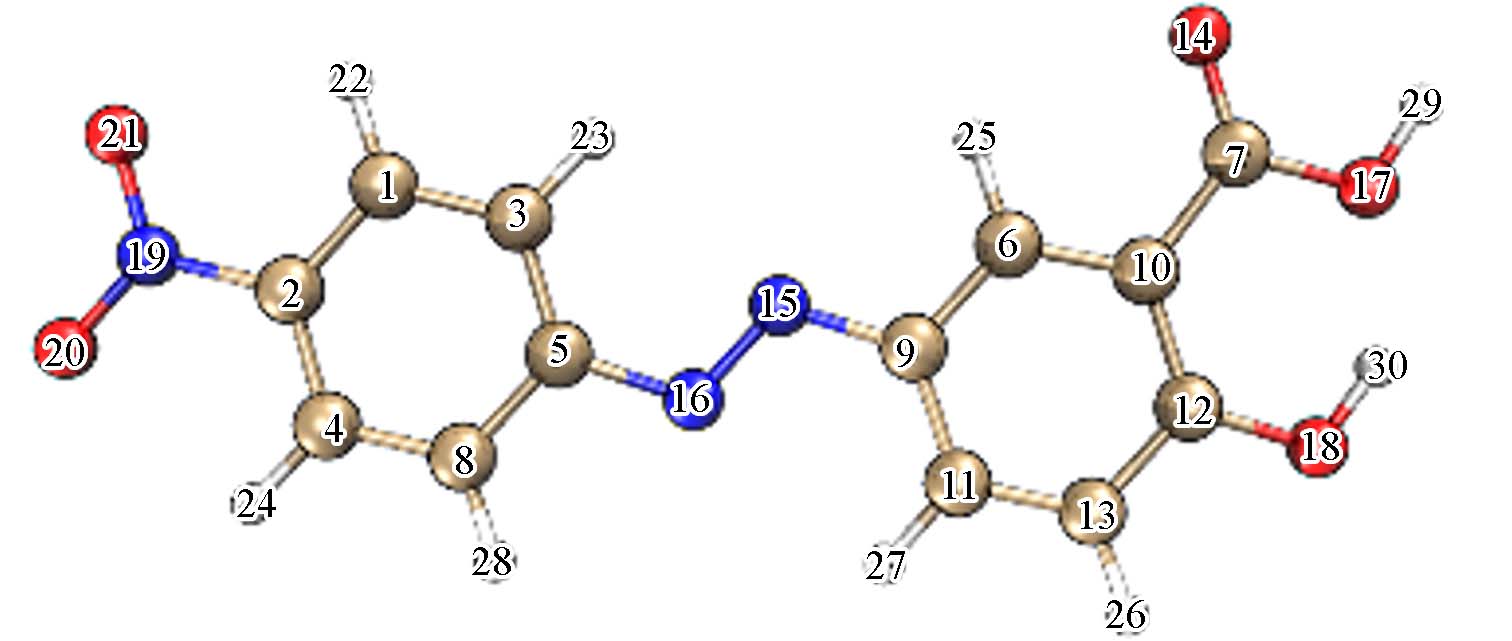
N 16 − 0.0723 −0.142 0.0963 0.1192 O 21 − 0.2092 − 0.2929 − 0.1672 0.0628 O 20 − 0.2101 − 0.2937 − 0.1689 0.0624 O 18 − 0.1803 − 0.2204 − 0.1321 0.0442 C 12 0.105 0.0603 0.1415 0.0406 C 2 0.0303 − 0.0092 0.0647 0.0369 C 4 − 0.0258 − 0.0651 0.0008 0.033 C 13 − 0.0476 − 0.0746 −0.009 0.0328 C 1 − 0.0248 − 0.0559 0.0032 0.0295 C 8 −0.029 − 0.0641 −0.006 0.029 O 14 − 0.2708 − 0.2972 − 0.2412 0.028 C 6 − 0.0125 − 0.0413 0.0123 0.0268 C 3 −0.031 − 0.0653 − 0.0135 0.0259 N 19 0.2446 0.2024 0.2538 0.0257 H 28 0.0559 0.035 0.0835 0.0243 C 11 − 0.0166 − 0.0485 − 0.0014 0.0236 C 10 − 0.0429 − 0.0624 − 0.0166 0.0229 H 22 0.0572 0.036 0.0794 0.0217 H 24 0.0575 0.0342 0.0776 0.0217 C 5 0.0398 0.0094 0.0518 0.0212 H 26 0.0537 0.0308 0.0708 0.02 H 23 0.0492 0.0293 0.0627 0.0167 H 29 0.2025 0.1869 0.2192 0.0162 C 9 0.0156 0.0077 0.0396 0.016 H 25 0.0562 0.0411 0.073 0.016 
H 27 0.0516 0.0352 0.0668 0.0158 H 30 0.1486 0.1345 0.1644 0.015 O 17 − 0.1468 − 0.1592 − 0.1324 0.0134 C 7 0.2063 0.1946 0.2174 0.0114 -
[1] AHMED A, USMAN M, YU B, et al. Efficient photocatalytic degradation of toxic Alizarin yellow R dye from industrial wastewater using biosynthesized Fe nanoparticle and study of factors affecting the degradation rate[J]. Journal of Photochemistry and Photobiology. B, Biology, 2020, 202: 111682. doi: 10.1016/j.jphotobiol.2019.111682 [2] LI Z L, SUN K, CHEN F, et al. Efficient treatment of alizarin yellow R contained wastewater in an electrostimulated anaerobic-oxic integrated system[J]. Environmental Research, 2020, 185: 109403. doi: 10.1016/j.envres.2020.109403 [3] YADAV N G, CHAUDHARY L S, SAKHARE P A, et al. Impact of collected sunlight on ZnFe2O4 nanoparticles for photocatalytic application[J]. Journal of Colloid and Interface Science, 2018, 527: 289-297. doi: 10.1016/j.jcis.2018.05.051 [4] TAN H B, ZHANG Y B, LI B W, et al. Preparation of TiO2-coated glass flat membrane and its photocatalytic degradation of methylene blue[J]. Ceramics International, 2023, 49(11): 17236-17244. doi: 10.1016/j.ceramint.2023.02.089 [5] HADI P, GUO J X, BARFORD J, et al. Multilayer dye adsorption in activated carbons-facile approach to exploit vacant sites and interlayer charge interaction[J]. Environmental Science & Technology, 2016, 50(10): 5041-5049. [6] HADADI A, IMESSAOUDENE A, BOLLINGER J C, et al. Aleppo pine seeds (Pinus halepensis Mill. ) as a promising novel green coagulant for the removal of Congo red dye: Optimization via machine learning algorithm[J]. Journal of Environmental Management, 2023, 331: 117286. doi: 10.1016/j.jenvman.2023.117286 [7] CUI Z X, TIAN S N, LIU X L, et al. Electrospinning preparation of TPU/TiO2/PANI fiber membrane with enhanced dye degradation and photocatalytic Cr(VI) reduction[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2023, 664: 131111. doi: 10.1016/j.colsurfa.2023.131111 [8] KUMAR P, THAKUR N, KUMAR K, et al. Photodegradation of methyl orange dye by using Azadirachta indica and chemically mediated synthesized cobalt doped α-Fe2O3 NPs through co-precipitation method[J]. Materials Today: Proceedings, 2023 [9] VENKATESAN S, CHEN Y Y, TENG H, et al. Enhanced adsorption on TiO2 photoelectrodes of dye-sensitized solar cells by electrochemical methods dye[J]. Journal of Alloys and Compounds, 2022, 903: 163959. doi: 10.1016/j.jallcom.2022.163959 [10] LIU H Z, BRUTON T A, DOYLE F M, et al. in situ chemical oxidation of contaminated groundwater by persulfate: Decomposition by Fe(Ⅲ)- and Mn(Ⅳ)-containing oxides and aquifer materials[J]. Environmental Science & Technology, 2014, 48(17): 10330-10336. [11] FANG G D, WU W H, LIU C, et al. Activation of persulfate with vanadium species for PCBs degradation: A mechanistic study[J]. Applied Catalysis B:Environmental, 2017, 202: 1-11. doi: 10.1016/j.apcatb.2016.09.006 [12] OH W D, DONG Z L, LIM T T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects[J]. Applied Catalysis B:Environmental, 2016, 194: 169-201. doi: 10.1016/j.apcatb.2016.04.003 [13] ANIPSITAKIS G P, DIONYSIOU D D. Radical generation by the interaction of transition metals with common oxidants[J]. Environmental Science & Technology, 2004, 38(13): 3705-3712. [14] LUTZE H V, BIRCHER S, RAPP I, et al. Degradation of chlorotriazine pesticides by sulfate radicals and the influence of organic matter[J]. Environmental Science & Technology, 2015, 49(3): 1673-1680. [15] GUERRA-RODRÍGUEZ S, RODRÍGUEZ E, SINGH D, et al. Assessment of sulfate radical-based advanced oxidation processes for water and wastewater treatment: A review[J]. Water, 2018, 10(12): 1828. doi: 10.3390/w10121828 [16] MAHMOODI NASRABADI A, MOGHIMI M. Experimental investigation of factors affecting the micro microbial fuel cells' main outputs[J]. Journal of Power Sources, 2023, 564: 232871. doi: 10.1016/j.jpowsour.2023.232871 [17] KOUAM IDA T, MANDAL B. Microbial fuel cell design, application and performance: A review[J]. Materials Today:Proceedings, 2023, 76: 88-94. doi: 10.1016/j.matpr.2022.10.131 [18] LIU L, LI F B, FENG C H, et al. Microbial fuel cell with an azo-dye-feeding cathode[J]. Applied Microbiology and Biotechnology, 2009, 85(1): 175-183. doi: 10.1007/s00253-009-2147-9 [19] LI J, FU Q, LIAO Q, et al. Persulfate: A self-activated cathodic electron acceptor for microbial fuel cells[J]. Journal of Power Sources, 2009, 194(1): 269-274. doi: 10.1016/j.jpowsour.2009.04.055 [20] WANG Y, NIU C G, ZENG G M, et al. Microbial fuel cell using ferrous ion activated persulfate as a cathodic reactant[J]. International Journal of Hydrogen Energy, 2011, 36(23): 15344-15351. doi: 10.1016/j.ijhydene.2011.08.071 [21] BORJAS Z, ESTEVE-NÚÑEZ A, ORTIZ J M. Strategies for merging microbial fuel cell technologies in water desalination processes: Start-up protocol and desalination efficiency assessment[J]. Journal of Power Sources, 2017, 356: 519-528. doi: 10.1016/j.jpowsour.2017.02.052 [22] 张慧超. 生物阴极微生物脱盐燃料电池驱动电容法深度除盐性能研究[D]. 哈尔滨: 哈尔滨工业大学, 2015. ZHANG H C. Biocathode microbial desalination cell driven capacitive deionization for salt water desalination[D]. Harbin: Harbin Institute of Technology, 2015 (in Chinese).
[23] 徐成龙. 微生物脱盐电池性能优化及处理盐碱地淋洗水的实验研究[D]. 上海: 上海海洋大学, 2020. XU C L. Performance optimization of microbial desalination cells and treatment of washing water in coastal saline-alkali soil[D]. Shanghai: Shanghai Ocean University, 2020 (in Chinese).
[24] 董容莉, 梁燃燃, 滕洪辉. 微生物燃料电池性能参数及其评价方法[J]. 广州化工, 2015, 43(4): 28-29,41. doi: 10.3969/j.issn.1001-9677.2015.04.012 DONG R L, LIANG R R, TENG H H. The performance parameters of microbial fuel cell and their evaluation method[J]. Guangzhou Chemical Industry, 2015, 43(4): 28-29,41 (in Chinese). doi: 10.3969/j.issn.1001-9677.2015.04.012
[25] 黎嘉仪, 骆海萍, 袁也, 等. 不同阴极对微生物燃料电池产电性能的影响比较[J]. 环境工程学报, 2014, 8(8): 3143-3148. LI J Y, LUO H P, YUAN Y, et al. Comparison in performance of microbial fuel cells using different cathodes[J]. Chinese Journal of Environmental Engineering, 2014, 8(8): 3143-3148 (in Chinese).
[26] 骆靖宇, 李学艳, 李青松, 等. 紫外活化过硫酸钠去除水体中的三氯卡班[J]. 中国环境科学, 2017, 37(9): 3324-3331. doi: 10.3969/j.issn.1000-6923.2017.09.015 LUO J Y, LI X Y, LI Q S, et al. Degradation of triclocarban aqueous solution through UV irradiation-activated sodium persulfate process[J]. China Environmental Science, 2017, 37(9): 3324-3331 (in Chinese). doi: 10.3969/j.issn.1000-6923.2017.09.015
[27] WANG X L, TONG Y P, FANG G D. Advances of single-atom catalysts for applications in persulfate-based advanced oxidation technologies[J]. Current Opinion in Chemical Engineering, 2021, 34: 100757. doi: 10.1016/j.coche.2021.100757 [28] WANG M M, WANG Y F, LI Y C, et al. Persulfate oxidation of tetracycline, antibiotic resistant bacteria, and resistance genes activated by Fe doped biochar catalysts: Synergy of radical and non-radical processes[J]. Chemical Engineering Journal, 2023, 464: 142558. doi: 10.1016/j.cej.2023.142558 [29] CHEN W S, HUANG C P. Mineralization of aniline in aqueous solution by electrochemical activation of persulfate[J]. Chemosphere, 2015, 125: 175-181. doi: 10.1016/j.chemosphere.2014.12.053 [30] 金春姬, 于辉, 刘明, 等. 利用过硫酸盐阴极型微生物燃料电池降解蒽醌燃料活性艳蓝的研究[J]. 中国海洋大学学报(自然科学版), 2015, 45(4): 85-94. JIN C J, YU H, LIU M, et al. Decolorization of an anthraquinone dye reactive brilliant blue KN-R in microbial fuel cells using ferrous catalyzed persulfate[J]. Periodical of Ocean University of China, 2015, 45(4): 85-94 (in Chinese).
[31] 冯俊生, 姚海祥, 蔡晨, 等. 微生物燃料电池电活化过硫酸盐降解甲基橙偶氮染料[J]. 环境科学研究, 2019, 32(5): 913-920. FENG J S, YAO H X, CAI C, et al. Microbial fuel cell electro-activated persulfate to degrade methyl orange azo dye[J]. Research of Environmental Sciences, 2019, 32(5): 913-920 (in Chinese).
[32] HAN J, ZENG H Y, XU S, et al. Catalytic properties of CuMgAlO catalyst and degradation mechanism in CWPO of methyl orange[J]. Applied Catalysis A:General, 2016, 527: 72-80. doi: 10.1016/j.apcata.2016.08.015 [33] 尹汉雄, 唐玉朝, 黄显怀, 等. 紫外光强化Fe(Ⅱ)-EDTA活化过硫酸盐降解直接耐酸大红4BS[J]. 环境科学研究, 2017, 30(7): 1105-1111. YIN H X, TANG Y C, HUANG X H, et al. Decolorization effect of direct fast scarlet 4BS by Fe (Ⅱ)-EDTA activated peroxodisulfate under ultraviolet light[J]. Research of Environmental Sciences, 2017, 30(7): 1105-1111 (in Chinese).
[34] FANG G D, DIONYSIOU D D, WANG Y, et al. Sulfate radical-based degradation of polychlorinated biphenyls: Effects of chloride ion and reaction kinetics[J]. Journal of Hazardous Materials, 2012, 227/228: 394-401. doi: 10.1016/j.jhazmat.2012.05.074 [35] 毕晨, 施周, 周石庆, 等. EGCG强化Fe2+/过硫酸盐体系降解金橙G的研究[J]. 中国环境科学, 2017, 37(10): 3722-3728. doi: 10.3969/j.issn.1000-6923.2017.10.013 BI C, SHI Z, ZHOU S Q, et al. Degradation of orange G by Fe2+/peroxydisulfate system with enhance of EGCG[J]. China Environmental Science, 2017, 37(10): 3722-3728 (in Chinese). doi: 10.3969/j.issn.1000-6923.2017.10.013
[36] 徐祥健. 基于羟基和硫酸根自由基的高级氧化技术降解有机污染物的研究[D]. 武汉: 武汉大学, 2019. XU X J. Hydroxyl radical-and sulfate radical-based advanced oxidation processes for the removal of organic pollutants[D]. Wuhan: Wuhan University, 2019 (in Chinese).
[37] 安琦, 刘建广. 基于羟基自由基或硫酸根自由基的高级氧化技术中溴酸盐形成与控制研究进展[J]. 净水技术, 2021, 40(12): 5-11,31. AN Q, LIU J G. Research progress of bromate formation and control in AOPs based on hydroxyl radical or sulfate radical[J]. Water Purification Technology, 2021, 40(12): 5-11,31 (in Chinese).
[38] 陈强, 王芳, 宋俊密, 等. 纳米TiO2光催化降解茜素黄R的反应机理与动力学[J]. 环境科学学报, 2009, 29(1): 175-180. doi: 10.3321/j.issn:0253-2468.2009.01.027 CHEN Q, WANG F, SONG J M, et al. Photocatalytic degradation of alizarin yellow R using TiO2 as catalyst: Mechanistic and kinetic investigations[J]. Acta Scientiae Circumstantiae, 2009, 29(1): 175-180 (in Chinese). doi: 10.3321/j.issn:0253-2468.2009.01.027
[39] ZHANG Y, HE P, JIA L P, et al. Ti/PbO2-Sm2O3 composite based electrode for highly efficient electrocatalytic degradation of alizarin yellow R[J]. Journal of Colloid and Interface Science, 2019, 533: 750-761. doi: 10.1016/j.jcis.2018.09.003 [40] ASOGWA F C, AGWAMBA E C, LOUIS H, et al. Structural benchmarking, density functional theory simulation, spectroscopic investigation and molecular docking of N-(1H-pyrrol-2-yl) methylene)-4-methylaniline as castration-resistant prostate cancer chemotherapeutic agent[J]. Chemical Physics Impact, 2022, 5: 100091. doi: 10.1016/j.chphi.2022.100091 [41] SHI J X, ZHANG B G, WANG W, et al. in situ produced hydrogen peroxide by biosynthesized Palladium nanoparticles and natural clay mineral for Highly-efficient Carbamazepine degradation[J]. Chemical Engineering Journal, 2021, 426: 131567. doi: 10.1016/j.cej.2021.131567 -



 下载:
下载: