-
近年来,环境污染成为了遏制人类生存发展的关键问题之一,水体中难降解有机污染物尤其是新兴污染物(药物、个人护理产品、内分泌干扰物和消毒副产物等)严重威胁水生生态系统和人类的生命健康[1 − 4]. 因此,物理吸附[5]、生物处理[6]和高级氧化技术(AOPs)[7]等多种技术被广泛应用于废水中污染物的去除. 相比于其他方法,AOPs因其在去除有机污染物方面的巨大潜力而受到广泛关注,污染物可分解为低毒或无毒物质,甚至直接矿化成二氧化碳(CO2)和水(H2O)[8]. 传统的AOPs是以过氧化氢(H2O2)作为氧化剂,产生羟基自由基(·OH)降解有机污染物,基于硫酸根自由基(SO4•−)的高级氧化技术因其独特的优势被认为是一种很有前途的降解各种有机废水的处理方法. 与·OH相比,SO4•−具有更高的氧化还原电位、更强的选择性、更长的半衰期以及受pH值影响较小[9]. 通常,硫酸根自由基可通过活化过一硫酸盐(PMS)和过二硫酸盐(PDS)产生. 相比于PDS,PMS中的O-O键更容易被激活,有利于水中有机污染物降解. PMS活化方法多样,如加热[10]、紫外光[11]、碳材料[12]和过渡金属[13]等. 其中,以过渡金属为均相或非均相催化剂来活化PMS因其高效、低成本的优点成为近年来研究的热点.
过渡金属活化,包括Fe、Mn、Co、Ni、Ce、Cu等,在这些过渡金属中,铁元素由于低毒性、低成本以及优异的活化性能成为研究和使用最多的金属种类[14],但均相Fe2+/PMS体系存在催化剂回收困难、Fe2+/Fe3+转换率慢,易形成铁盐沉淀和造成二次污染等问题限制了其广泛应用[15],非均相单一铁元素催化剂也存在金属易团聚和金属离子浸出等问题[16]. 在单一铁元素的基础上,引入另外一种金属,制备成铁基非均相双金属催化剂. 首先,反应过程中金属离子浸出问题得到有效控制,延长了使用寿命,提高了催化剂的稳定性[16 − 17]. 其次,引入第二金属构建双金属催化剂可以有效地提高铁催化剂的催化性能,因为双金属的标准氧化还原电位的差异可以促进价态循环和电子转移,从而提高对PMS的活化性能[18 − 21]. 不同综述文献总结了铁基催化剂作为SR-AOPs活化剂在过硫酸盐高级氧化技术中的应用[22 − 25];Peng等[16]综述了尖晶石铁氧体及其复合物活化过一硫酸盐(PMS)降解有机污染物;Sun等[24]综述了尖晶石铁氧体纳米粒子及其纳米复合材料诱导过氧单硫酸盐活化去除有机污染物的应用;Kifle等[25]探讨了铁基双金属纳米结构异相活化过氧化物(过氧化氢、过一硫酸盐和过硫酸盐)用于污染水体的高效修复中的应用. 然而,针对不同铁基双金属催化剂修饰改性及其活化过一硫酸盐降解有机污染物机理的相关综述文献还相对较少.
本文概括了3种典型的铁基双金属催化剂的基本特征和其耦合PMS降解污染物的应用,并对铁基双金属催化剂常见的合成方法进行了介绍,梳理了铁基双金属催化剂的改性方法,简要分析铁基双金属催化剂活化PMS的机理. 最后,提出了铁基双金属催化剂在活化PMS有待深入研究的问题并对未来可能的发展方向进行展望,以期为将来铁基双金属催化剂耦合过一硫酸盐降解污染物的发展和应用提供有益的参考.
-
铁基双金属催化剂由于双金属的协同作用,双金属之间可以实现价态循环、循环活化PMS,促进低价离子再生,延长了催化剂的使用寿命. 双金属在其表面提供了更多反应活性位点,不同金属间也存在一定的配位作用,能够显著提高催化剂的催化活性和化学稳定性,还能一定程度降低金属浸出率. 此外,铁基双金属催化剂由于其不同的组分和结构展现出不同的理化性质,表现出不同的PMS活化能力. 目前,尖晶石铁氧体、铁基层状双金属氢氧化物和铁基双金属普鲁士蓝类似物催化剂,以其较强的活化过一硫酸盐能力和优异的空间结构被应用于耦合过一硫酸盐体系降解有机污染物.
-
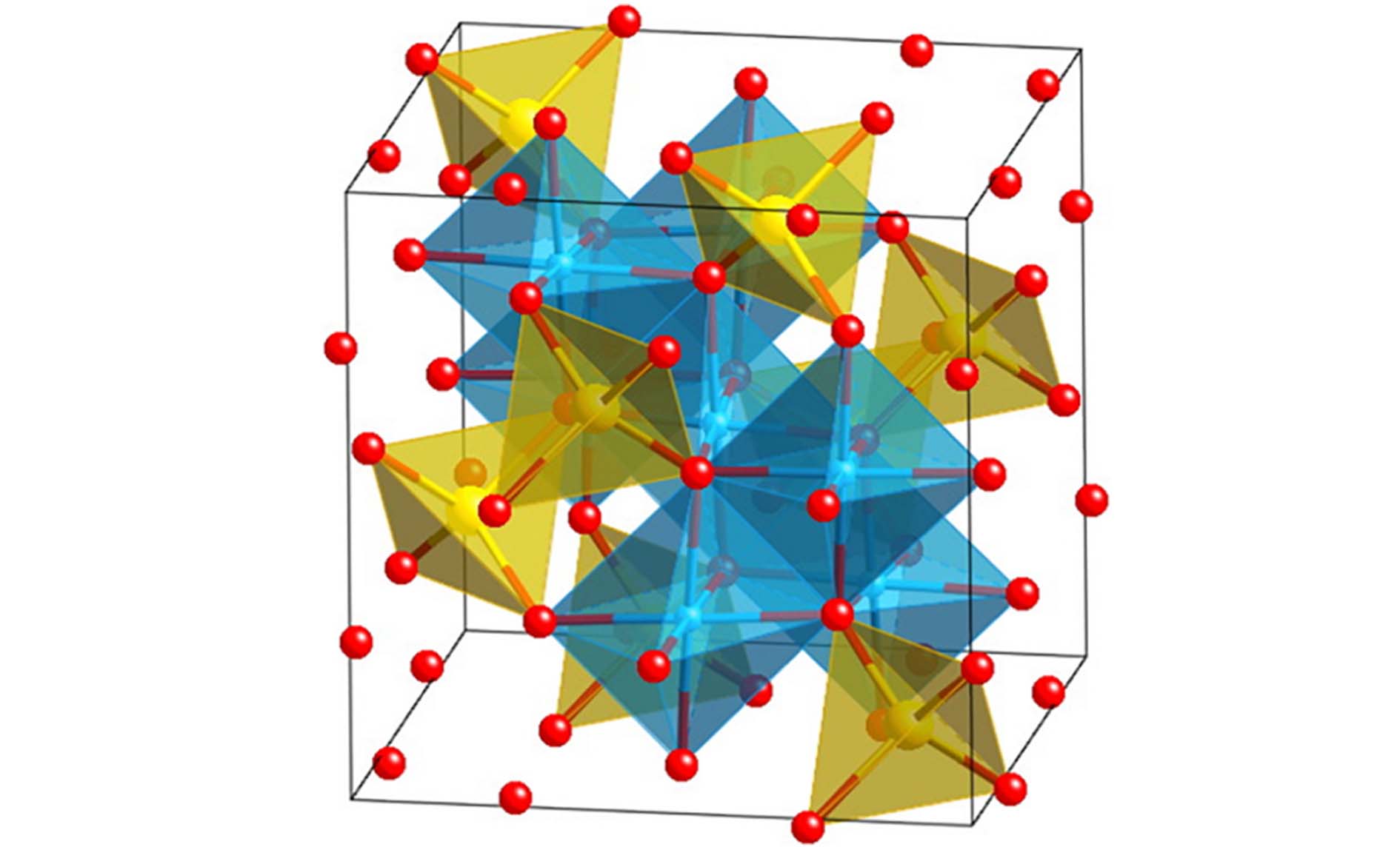
尖晶石铁氧体的化学式为MFe2O4,其中M表示二价过渡金属离子(如Co2+、Cu2+、Mn2+、Ni2+、Zn2+等). 根据晶体结构(图1)[26],尖晶石可分为三种类型[27]. 一是正尖晶石,M2+和Fe3+分别占据四面体和八面体的位置,常见的例子是ZnFe2O4. 二是反尖晶石,一半的Fe3+位于四面体位置,而M2+和另一半的Fe3+位于八面体位置,如NiFe2O4. 三是复尖晶石,M2+和Fe3+随机占据四面体和八面体位置,如MnFe2O4.
尖晶石铁氧体以其特殊的超顺磁性、热稳定性、宽光吸收频率、气敏性、电导率等优良理化性能,被广泛应用于环境、能源、化工、医药等领域[23, 28]. 在SR-AOPs中,MFe2O4作为PMS活化剂,表现出优异的催化性能、稳定的化学性质和耐腐蚀性,能有效地降解有机污染物. MFe2O4具有多孔结构[24],这增加了催化剂与反应物的接触面积,提高了催化性能. 尖晶石铁氧体存在双金属协同作用,M2+/M3+和Fe3+/Fe2+之间形成良性循环,加速了自由基的生成,有利于反应进行. Wang等[29]观察到CuO/PMS和Fe2O3/PMS体系对诺氟沙星去除率分别为28.0%和35.4%,而CuFe2O4/PMS对诺氟沙星去除率达到90%以上. 这表明双金属催化剂相比于单体具有更高的催化活性. 尖晶石铁氧体的物理化学性质(丰富的表面活性位点、高催化活性和易于修饰)依赖于其晶体学中金属阳离子的种类、含量、位置[30 − 32]. 由于M2+在晶体相中的活性不同,即使在相同的反应条件下,单组MFe2O4也具有不同的PMS催化活性. Ren等[33]采用溶胶-凝胶法制备了不同类型的MFe2O4(M = Co, Cu, Mn和Zn),观察到PMS的催化能力为CoFe2O4>CuFe2O4>MnFe2O4>ZnFe2O4. CoFe2O4与PMS耦合作用效果最好,这与CoFe2O4表面有丰富的羟基位点以及Co和Fe离子的协同作用最好有关. 研究表明,虽然CuFe2O4和MnFe2O4的催化活性不如CoFe2O4高,但Cu2+和Mn2+不像Co2+那样具有高毒性和潜在的致癌性[34 − 35].
-
层状双金属氢氧化物(LDHs)是由金属氧化物作为主体层板,阴离子/溶剂分子作为层间区域形成的类水滑石化合物,是一种特殊的层状二维(2D)结构材料,在(光)催化、吸附、电化学等方面得到了广泛的应用[36 − 37]. 近年来,LDHs耦合PMS降解水中有机污染物的研究日益增多,其衍生物活化PMS的潜力也被研究人员逐步开发. LDHs独特的结构在活化PMS时具备很多优势:(1)LDHs通常由共沉淀和水热法制备,操作简便,适合大规模工业应用;(2)比表面积大、活性位点丰富且分散;(3)多组分对称结构稳定,对金属离子起到良好的固定作用. 目前,已有大量研究利用铁基LDHs及其复合物活化PMS降解各类有机污染物,如染料、内分泌干扰素、抗生素等. 表1总结了近期铁基LDHs及其复合物活化PMS降解有机污染物的一些研究结果,如表1所示,铁基LDHs及其复合物处理染料和邻苯二甲酸二甲酯(内分泌干扰素)效果较好,而对十八胺、双酚A和抗生素类污染物的效率有限. 此外,铁基LDHs单体在反应过程中活性位点易流失和材料易聚集,影响其催化活性位点;同时体系中低价态的过渡金属容易被氧化成高价态的过渡金属,从而降低其催化活性[38]. 因此,一方面可以对材料进行改性修饰和制备复合材料,另一方面还可以在层间增加第三种金属形成铁基层状三金属氢氧化物(三元LDHs)及其衍生物以提升其处理有机污染物的效果[39]. 尽管LDHs的研究已经取得了一定的进展,但铁基LDHs作为耦合PMS催化剂的研究报道仍然非常有限.
-
普鲁士蓝类似物(PBAs)是由变价态的金属中心组成的形貌可调的三维金属(3D)开放框架,可以通过热处理形成金属氧化物,具有高表面积、结构可控和过氧化物酶活性高等优点,其复合材料及其衍生物被广泛应用于电化学能量转换和存储、电化学或生物传感器、吸附和芬顿高级氧化技术等领域[47]. 目前,已有报道将PBA材料应用于活化PMS高级氧化体系. 如Zhao等[48]证明了高自旋CoFe-PBA可以有效活化PMS降解有机污染物. Li等[49]则以CoFe-PBA作为前驱体,煅烧得到了尺寸和形貌可调的多孔FexCo3-xO4纳米笼,发现金属摩尔比对催化剂的制备有很大影响,优化后的催化剂在活化PMS降解有机污染物方面表现出优异的催化性能和较高的稳定性.
目前,利用铁基双金属PBA耦合PMS降解有机污染物的研究较少,多数研究集中在将其作前驱体或制备原材料,导致制备工艺步骤繁多,合成产率低. 如Zeng等[50]以CoAl-LDH为原料,采用自牺牲法合成了CoFe-PBA@CoAl-LDH纳米片,该材料用于活化PMS能高效降解磺胺甲噁唑,但制备工艺复杂繁多,周期较长. 虽然铁基PBAs单体具有高比表面积、易调整的孔隙率、自身结构稳定和易发生电子转移等优点,但其也存在金属-配位键不稳定、晶体结构易坍塌和水稳定性较低等缺陷. 因此,开发稳定高效的铁基PBAs作为非均相SR-AOPs催化剂有待深入的探索. Pi等[51]通过一步水热法制备CoFe-PBAs@rGO纳米复合材料,用于活化PMS体系降解盐酸左氧氟沙星,循环使用5次后,材料仍保持较高的催化活性和化学稳定性.
-
铁基双金属催化剂常用的合成方法包括浸渍热解法、溶胶-凝胶法、水热/溶剂热法、共沉淀法等,除了以上几种方法,还有模板法、自牺牲法等方法. 在实际应用中,这些方法通常被组合或改进,以获得性能更优的催化剂. 常见合成方法的优缺点归纳如表2.
催化剂的合成方法影响了其结构、形貌和催化活性等特性. 表3列出了不同合成方法制备的铁基双金属催化剂和其所对应的形貌、催化活性以及对目标污染物的去除率.
-
浸渍热解法是将载体浸渍在过渡金属盐溶液中,超声或搅拌一段时间后烘干除去剩余的液体,再将样品置于无氧或限氧条件下热解后即可制得催化剂. 浸渍热解法可利用已成型的材料(氧化硅、氧化铝、活性炭等)为载体,还能充分利用组分中的活性成分,热解过程中常因载体或活性成分的作用形成多孔的结构,常用于制备碳材料负载双金属纳米颗粒复合材料. Du等[52]以草酸、FeSO4·7H2O和MnSO4·H2O为原料制备磁性多孔p-Mn/Fe3O4. 研究发现草酸盐经高温热解分解为CO2和H2O,金属草酸盐同时转变为多孔金属氧化物,煅烧后的p-Mn/Fe3O4颗粒表面有丰富的孔隙和裂纹,呈立方体状. Xiao等[17]将尿素和榕树枝粉末浸渍在Ce(NO3)2·6H2O和Fe(NO3)3·9H2O溶液中,再经热解制得蜂窝状多孔结构的Fe-Ce双金属N掺杂生物炭,并用于活化PMS实现了高效降解甲硝唑.
-
溶胶-凝胶法是指在金属盐溶液中加入有机酸或鸡蛋清作为螯合剂,然后将混合物在水浴中蒸发形成凝胶,再将获得的固体进行煅烧的方法. Xu等[53]向硝酸铁和硝酸锰溶液中加入柠檬酸和氯化钠搅拌均匀,再用氨水调节溶液的pH至7左右,于100 ℃下干燥得到凝胶,300 ℃煅烧得到MnFe2O4@BC. 溶胶凝胶法一般可用于制备铁氧体/生物炭复合材料,该方法具有磁性颗粒结晶度高,分散性好等优点[58].
-
共沉淀法是指在溶液中混合两种或两种以上的阳离子,在一定温度下加入氢氧化钠或氢氧化铵溶液进行搅拌,通过调节pH值使其产生沉淀后,得到组分均匀的沉淀物,再将沉淀物洗涤、干燥或煅烧得到材料. Xu等[54]将Co(NO3)2·6H2O、Fe(NO3)3·9H2O加入到EG溶液中超声,加入NaOH调节pH使其沉淀,经水洗和醇洗后干燥400 ℃煅烧得到CoFe2O4-EG. 结果表明,CoFe2O4-EG对磺胺甲恶唑的去除率和矿化效分别超过99%和30.9%. 然而,共沉淀制备双金属NPs的过程中,可能导致纳米颗粒的团聚,从而影响材料的催化性能.
-
与共沉淀法相比,水热/溶剂热法具有相似的特点,即将两种金属盐的溶液逐滴引入另一种含碱的溶液从而制得材料. 水热合成法是指以水作为溶剂,在密闭不锈钢高压反应釜内,控制一定的温度和压强,物料经溶解和再结晶的制备方法. 溶剂热法是在水热法的基础上发展起来的,它与水热反应的不同之处在于以有机物代替水作为溶剂,有利于制备在水中难以合成、易于氧化或水解的材料. 近年来,许多学者通过水热/溶剂热法合成了稳定和高效的铁基双金属催化剂. 例如,Li等[55]通过水热法制备CoFe2O4材料用于活化PMS降解阿特拉津,30 min内对阿特拉津的去除率达到99%以上.Yang等[57]通过溶剂热和煅烧相结合的方法制备了具有多功能性的催化位点、稳定性高的新型磁性锰铁碳化物(mMFC)材料.
-
虽然铁基双金属催化剂表现出优异活化PMS性能,但仍存在纳米粒子团聚和金属离子浸出率较高的缺陷限制了其实际应用,科研工作者为了提高其催化性能,致力于对其进行改性研究. 目前,铁基双金属催化剂研究较多的改性方法有结构缺陷调控、复合改性、物理法强化等.
-
一般来说,在适当的温度下或还原气氛(H2)中煅烧使晶格中氧离子损失而引起氧缺陷. 在催化剂表面引入氧空位(OV)可以改善催化剂的电子结构,降低电阻,提高电子转移能力,促进PMS活化,从而提高催化剂的活性,还能促进双金属之间的价态循环,减少金属的浸出[59 − 60]. 此外,引入的表面氧空位可以作为电子供体和吸附活性位点,降低PMS自分解的能势垒[61]. Long等[62]的研究表明,OV是PMS的主要活性中心,通过提高了PMS的吸附能,有效提高了CoFe2O4纳米材料的催化活性. Zhou等[63]制备了富含OV的FeCo2O4-x纳米颗粒NPs(FCOVNPs) 耦合PMS 来评估其去除2,4-二氯苯酚的催化性能. 研究发现,体系最主要的活性物种1O2来源于OV与O2反应;OV有利于调控FCOVNPs的能带结构和电荷运动行为,显著促进了Co3+/Co2+和Fe3+/Fe2 +的氧化还原循环,也有利于SO4•−和·OH的产生.
-
碳材料(活性炭、生物炭、石墨烯、碳纳米管等)具有优良的化学稳定性和热稳定性、高比表面积、丰富的官能团和低成本,具有巨大的环境修复潜力[53, 64 − 66]. 铁基双金属催化剂独特的磁学性质导致其在实际应用过程中容易发生团聚,使催化剂的比表面积和活性位点数量减少以及催化活性的降低,将铁基双金属催化剂负载到碳材料上不仅可有效抑制纳米颗粒的聚集,确保催化剂的高分散,而且碳材料优异的电子转移能力能促进双金属之间的价态循环,使双金属循环再生,以上协同作用增强其活化PMS的能力. Ye等[43]制备了BC@CoFe-LDH复合材料. 结果表明,BC作为载体,抑制了CoFe-LDH的团聚,增大了比表面积和孔体积,有利于催化剂、PMS和邻苯二甲酸二甲酯之间的相互作用;引入CoFe-LDH后,BC中出现了更多的表面氧空位、缺陷和官能团,这将提高催化性能;BC的氧化还原活性基团( RAMs ),特别是还原的官能团,可以介导电子转移,加速Co(Ⅲ)和Fe(Ⅲ)的还原,从而增强CoFe-LDH的PMS活化. 得益于BC和CoFe-LDH之间的协同效应,BC-LDH对PMS活化表现出优异的催化活性和稳定性,BC-LDH/PMS体系对邻苯二甲酸二甲酯降解率明显优于CoFe-LDH/PMS体系. Dong等[67]利用密度泛函理论计算发现,锚定在氮掺杂碳纳米片上的CuFe2O4颗粒在其内部形成了一个内建电场,可调节电子转移过程,从而触发自由基和非自由基途径,具有丰富自由流动π电子的sp2杂化碳作为电子穿梭体加速了这种电子转移介导的非自由基过程.
-
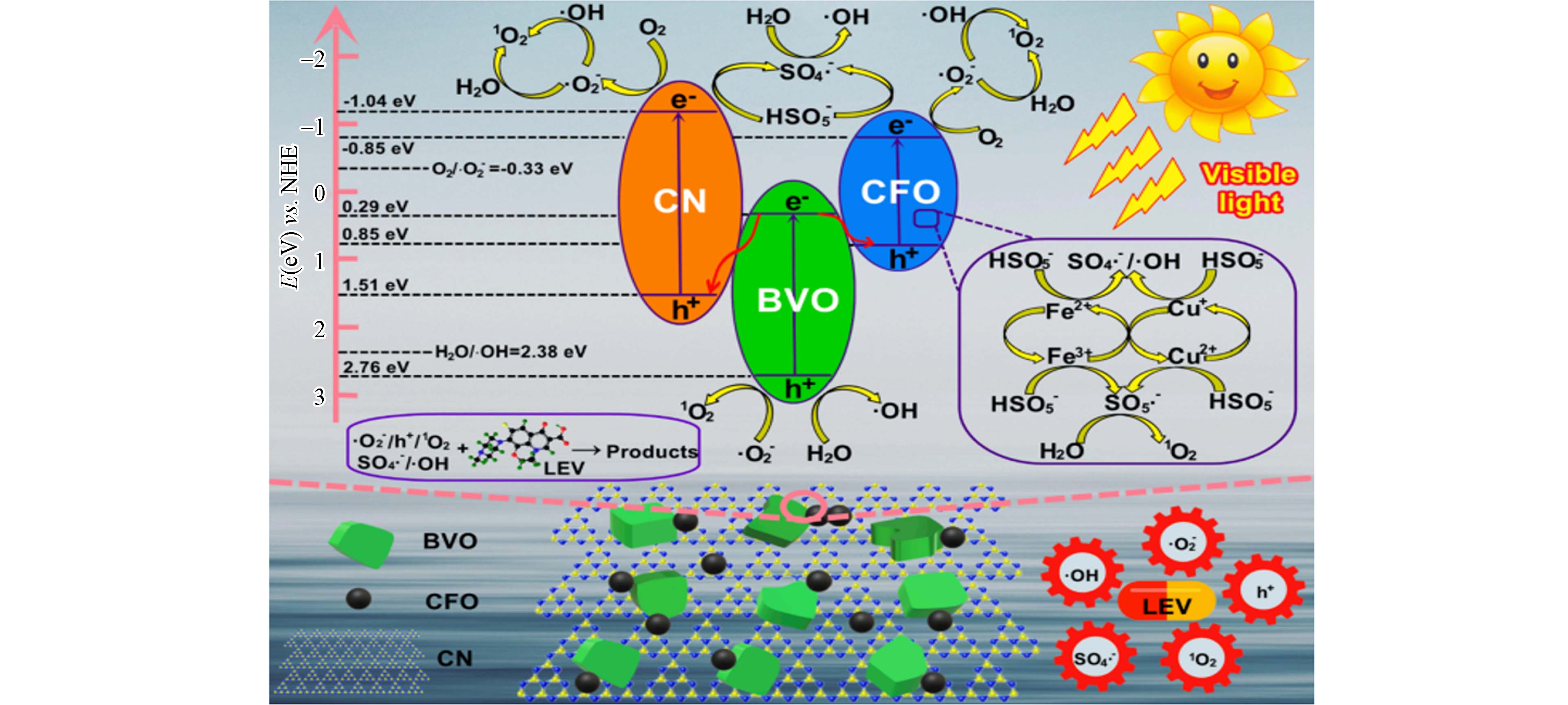
传统的半导体(TiO2、g-C3N4)存在比表面积较小,可见光吸收能力差和光生电子-空穴复合率高等缺陷,这些因素都会影响其催化性能和稳定性,限制其在废水处理中的实际应用[36]. MFe2O4和铁基LDHs本身也是一种的光催化剂,具有较窄的禁带宽度和较高的光化学稳定性[16, 68],而且它们的异质结构易于合成,促进光激发电子的传输和抑制电子-空穴复合的能力[69],已被广泛研究用于光催化降解污染物. 将铁基双金属催化剂与半导体材料耦合,这将有助于改善其结构性质,增强其光催化性能. Sun等[70]采用简单的共沉淀-煅烧法合成了漂浮型g-C3N4负载CuFe2O4催化剂,并将其应用于可见光照射下催化活化PMS降解抗生素,通过一系列表征手段发现材料表现出宽的光吸收范围,表现出良好的跨带结构,这可能会增强了光响应和加快电荷载流子的转移,提高光催化活性. Li等[71]通过水热法制备了一种新型的可回收的Z型g-C3N4/BiVO4/CuFe2O4(CN/BVO/CFO)异质结,其可见光下活化PMS降解左氧氟沙星机理见图2[71]. 结果表明,与单一的BVO、CN、CFO和二元CN/BVO相比,CN/BVO/CFO在可见光照射下对左氧氟沙星的光催化降解行为增强. 表征测试和机理分析表明,催化活性的提高归因于双Z型异质结构以及光催化和PMS活化之间的协同作用,使得多种活性物种的产生.
-
PMS的热、可见/紫外线、超声和电激活过程需要额外的能量,但将它们与铁基双金属活化技术耦合可以利用它们之间的协同效应,不仅能够减少能源和试剂消耗,而且显著提高有机污染物的降解效率和矿化率. Guo等[21]以普鲁士蓝类似物(PBA)为前驱体制备了碳基Cu-Fe氧化物(CuFeO@C),其在紫外可见光(UV-vis)照射下对洛美沙星的降解表现出高PMS活化性能. Pang等[72]发现在PMS/MnFe2O4/MW体系中,97%以上的对-硝基苯酚(PNP)在2 min内被降解. 单独使用PMS、MnFe2O4/MW或PMS/MW均未观察到PNP的明显降解,而当使用PMS/MnFe2O4时,对-硝基苯酚也只有少部分被降解,这说明了微波协同非均相活化技术的可行性. Xu等[73]构建EC(电化学)/PMS/CFO(CuFe2O4)耦合体系,将其与UV/H2O2、UV/PS、EC/H2O2/CFO和EC/PS/CFO等其他高级氧化体系进行比较,发现EC/PMS/CFO工艺对双氯芬酸的降解率和电能消耗都低于其他体系,研究发现电场的引入大大增强了CFO对PMS的活化作用,循环伏安测试和X射线光电子能谱表明,PMS和电场均加速了Fe(Ⅲ)/Fe(Ⅱ)和Cu(Ⅱ)/Cu(Ⅰ)的循环,促进了活性氧的产生. Xu等[74]发现在电场的驱动下延长了MnFe2O4活化PMS的寿命,这可能是由于Mn(Ⅱ)和Fe(Ⅱ)通过接受传递到阴极的电子而原位再生,并且在第7次运行中仍有超过80%的橙黄Ⅱ被去除.
-
铁基双金属催化剂活化PMS主要包括自由基途径和非自由基途径,非自由基途径有单线态氧、电子转移、高价金属三种机制. 研究发现铁基双金属材料中金属离子一般起到产生SO4•−的主导作用,复合催化剂中的碳材料则可以通过特殊的石墨化结构将电子传递,激活PMS生成活性自由基SO4•−和·OH. 此外,过一硫酸盐还可以通过自身的分解产生同样具有催化活性的单线态氧1O2. 体系中存在的高价金属具有强氧化性可以直接氧化分解有机污染物. 在实际活化降解过程中具体主导途径取决于诸多条件,包括催化剂、pH值和目标污染物等,在实验研究中应考虑到这些影响因素.
-
自由基途径是指催化剂中的过渡金属离子(Mn+)与PMS发生反应,使得PMS中的O—O结构断裂,然后产生自由基活性物种. 首先体系中存在的低价金属离子传递电子分解PMS产生SO4•−(式(1)和式(2)),SO4•−可与H2O和OH−反应生成·OH(式(3)和式(4)),最后由反应中存在的SO4•−和·OH共同降解有机污染物(式(5)). 然而,自由基主导途径降解有机污染物受到自由基与共存无机阴离子的副反应以及在反应过程中产生的自由基的自猝灭的严重限制.
此外,自由基途径也受到催化剂的失活问题的限制,铁离子氧化完全,无法再启动新一轮活化过硫酸盐的反应. 在铁基双金属催化剂中,由于铁与其他金属氧化还原电位的差异,其他金属离子可还原Fe3+,促进Fe2+再生(式(6)),这样不仅能降低金属离子的浸出,也是相比单一铁元素催化剂催化性能更好的原因之一.
-
非自由基途径是指过一硫酸盐进行自分解生成单线态氧1O2的过程、表面电子传递途径和高价金属直接氧化. 铁基双金属催化剂活化PMS产生1O2的过程,一般在碱性条件下,或在有碳基材料时发生. 碳材料中的酮基(C=O)有利于1O2的生成,铁基双金属催化剂的氧空位的存在能促进1O2和·O2−的产生. 与SO4•−和·OH相比,1O2氧化还原能力一般(E0 = 2.2 V),但对有机污染物具有较高选择性,且对pH、水基质和实际水体有较高适应能力[75]. Dong等[67]将CuFe2O4纳米颗粒锚定在氮掺杂碳纳米片上用于PMS活化,研究发现催化剂中的石墨N、sp2杂化结构以及C=O官能团被证明是1O2产生的主要位点. Zhou等[63]建立了以1O2为主导的非自由基过程降解2,4-二氯苯酚,研究发现OV与HSO5−反应生成的1O2在所有的反应过程中起着重要的作用(式(7)). 1O2可以通过PMS的自分解产生(式(8)和式(9)),·O2−可以是生成1O2的前体,Zhu等[76]证明了在晶体学锰氧化物存在下,通过·O2−生成1O2. 综上,活化PMS过程直接产生的·O2−和SO52−是生成1O2的中间产物,因此1O2浓度难以进行准确的定量分析,而且诱导1O2的生成机制众多,在研究中需要进行详尽的分析.
降解过程中电子转移方式有两种,一是污染物(电子给体)首先吸附到催化剂(传递介质)表面,然后电子从污染转移到PMS(电子受体),得到电子的PMS通过反应生成ROS降解污染物[54, 77],二是过一硫酸盐被吸附在催化剂上形成催化剂−过一硫酸盐络合物. 络合物具备比催化剂更高的氧化还原电位,当其超过污染物的氧化电位时,络合物从污染物中提取电子,实现对污染物的降解[78]. Li等[79]通过拉曼光谱证实了Co(Ⅱ)-PMS络合物的形成,结合自由基猝灭和 EPR 实验结果,证实了以CoFe2O4表面和溶液中的Co(Ⅱ)-PMS络合物是主要的反应活性物种.
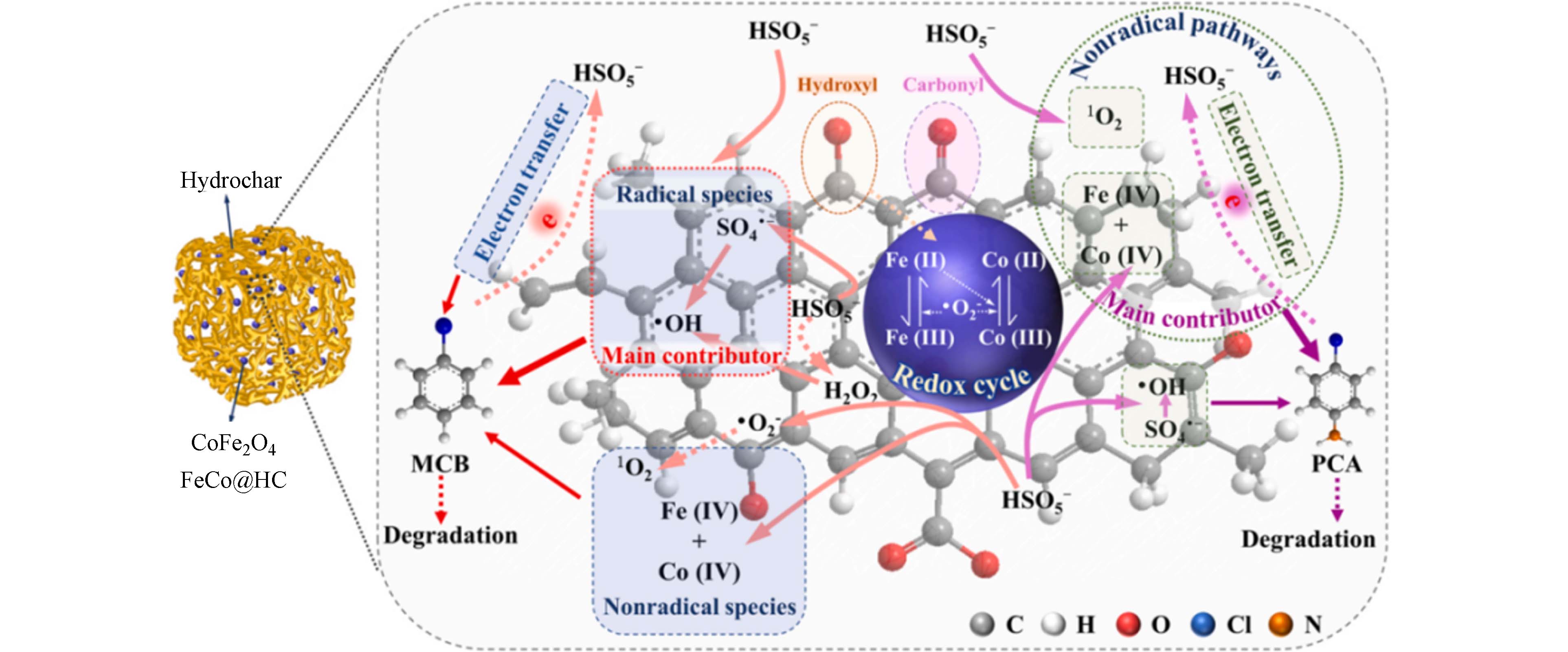
高价金属途径是指金属离子与过一硫酸盐反应过程中,除了会生成自由基外,还会产生更高价态的金属离子,同样对有机污染物有降解能力. 铁基双金属催化剂活化过一硫酸盐的反应中,金属离子也可能被氧化到更高的价态,高价态金属离子的氧化性使得体系中污染物有更多种降解路径. Zhang等[80]揭示了高价铁物种在Fe(Ⅵ)/PMS体系降解磺胺甲恶唑(SMX)过程中的重要作用,密度泛函理论计算表明,SMX更容易受到高价铁物种而非自由基引发的亲电攻击. Yang等[81]基于甲基苯基亚砜(PMSO)到甲基苯基砜(PMSO2)的选择性转化,确认了高价金属物种Fe(Ⅳ)/Co(Ⅳ)的存在,研究发现Fe(Ⅳ)/Co(Ⅳ)对污染物也有一定的降解作用. FeCo@HC140-10活化PMS产生Fe(Ⅳ)/Co(Ⅳ)(式(10)和式(11)),其反应机理如图3所示. 近年来,已开发用来预测Fe2+/PMS系统中Fe(Ⅳ)的形成和衰减的动力学模型[82],可更准确地判断高价铁离子驱动在反应中的贡献作用.
总之,自由基氧化性能较强,可以将有机底物进一步矿化为CO2和H2O. 而非自由基途径由于其氧化能力较弱,导致对目标污染物降解不彻底,但其优势在于其具有持久性且在基质/环境背景下抵抗有机/无机化合物的干扰能力较强. 因此,在对有机污染物降解时需要自由基和非自由基共同作用,同时要考虑不同的环境背景下主导以自由基或非自由基的主导路径降解有机污染物. 在今后研究中对这些特性应重视并加强探究和运用.
-
在综述中,简要介绍了铁基双金属催化剂的基本特征和其耦合PMS去除污染物的应用潜力,系统地介绍了常见铁基双金属催化剂的制备方法,并详细说明了铁基双金属催化剂的改性方法,围绕自由基途径、非自由基途径解析了铁基双金属催化剂活化PMS机理. 尽管铁基双金属催化剂在环境修复中的应用越来越多,然而大多数研究都是尖晶石铁氧体活化PMS降解污染物的应用,其他铁基双金属催化剂耦合PMS的研究和应用较少,而且仍有一些缺点需要克服,以下几个方面值得进一步加强研究.
1)铁基双金属催化剂虽然能弥补单一铁元素催化剂的不足,降低金属浸出率,提高催化活性,然而其稳定性和催化活性仍然有待提升. 要考虑在提升催化剂性能的同时,避免引入更多过渡金属引起二次污染,因此应聚焦于铁基双金属催化剂本身改性或将其与其他材料和其他PMS活化技术耦合,提高稳定性和催化活性,以应对日趋复杂的水污染问题.
2)开发具有优异催化性能的各种铁基双金属催化剂材料,制备材料的催化活性直接关系到催化反应能否顺利进行. 因此,设计和合成具有特定组分、结构和晶体形状的廉价、高催化活性的铁基双金属催化剂是一个不可忽视的问题. 这将有助于确定整个反应过程中的催化活性位点和发现催化机理.
3)铁基双金属催化剂活化作用机制,特别是双金属之间的协同作用以及铁基双金属复合材料各自耦合组分的协同作用,即载体自身结构与铁基双金属催化剂活化PMS性能之间的构效关系尚不清楚,仍然有待进一步研究.
4)目前铁基双金属催化剂/PMS高级氧化体系大多数研究都是在批歇反应器系统中进行模拟废水的去除,很少有针对实际废水或其他应用场景的研究,因此,应更多地考虑实际环境条件对性能和降解效果的影响,以证明该系统具有从实验室到工业规模应用的潜力.
铁基双金属催化剂耦合过一硫酸盐去除污染物的研究进展
Research progress on the coupling of iron based bimetallic catalysts with peroxymonosulfate for pollutants removal
-
摘要: 水中难降解有机物对人体健康和生态环境存在潜在威胁,开发稳定、高效和低成本的催化体系对去除水中难降解有机物具有重要意义. 基于硫酸根自由基的高级氧化技术(SR-AOPs)因其氧化能力和适应性受到广泛关注. 铁基双金属催化剂由于能快速活化过一硫酸盐(PMS)而被认为是有效的催化剂,它弥补了单一铁元素催化剂活化效率低和容易产生二次污染等缺陷. 本文综述了铁基双金属催化剂的基本特征,介绍了铁基双金属催化剂的合成方法,并说明其改性方法,接着探讨铁基双金属活化PMS的机理. 最后,指出铁基双金属催化剂活化过一硫酸盐降解有机污染物面临的挑战和未来可能的发展方向.Abstract: Organic compounds in water pose a potential threat to human health and the ecological environment. Developing a stable, efficient, and low-cost catalytic systems is of great significance for the removal of refractory organic compounds in water. Sulfate radical-based advanced oxidation processes (SR-AOPs) have received intensive attention due to their oxidation capacity and adaptability. Iron based bimetallic catalysts have been regarded as effective catalysts due to their quick activation of peroxymonosulfate (PMS), which makes up for the defects of low activation efficiency and formation of secondary pollution of single iron catalysts. This paper provides a detailed overview of the fundamental characteristics of iron-based bimetallic catalysts, and introduces the latest research progress in the synthesis method and modification method of iron-based bimetallic catalysts. Moreover, the mechanisms of iron based bimetallic activation of PMS are explored in detail. Finally, the challenges and potential future development directions of PMS activation with iron based bimetallic catalysts for the degradation of organic pollutants are also discussed.
-
Key words:
- iron based bimetal /
- peroxymonosulfate /
- organic pollutants /
- mechanism.
-

-
表 1 铁基层状双金属氢氧化物及其复合物活化过一硫酸盐降解有机污染物性能
Table 1. Summary of iron-based LDHs composites activated peroxymonosulfate for the degradation of organic pollutants
催化剂
Catalyst污染物
Target pollutant浓度/(mg·L−1)Concentration 反应条件
Reaction conditions去除率/%
Removal参考文献
ReferencesFe-Mn-LDH 十八胺 10 [Cat.] = 0.4 g·L−1、[PMS] = 0.4 g·L−1、pH = 1.8、t = 25 min 85 [20] FeAl-LDH 双酚A 20 [Cat.] = 0.2 g·L−1、[PMS] = 0.2 g·L−1、pH = 5.58、t = 60 min 60 [38] CoFeLa-LDOs 四环素 30 [Cat.] = 0.05 g·L−1、[PMS] = 10 mmol·L−1、pH = 5.4、t = 10 min、 90.1 [39] CoFe-LDH 酸性红27 200 [Cat.] = 0.1 g·L−1、[PMS] = 0.2 g·L−1、pH = 6.7、t = 15 min 96.7 [40] MnFe-LDH 酸性橙7 20 [Cat.] = 0.2 g·L−1、[PMS] = 0.2 g·L−1、pH = 6.1、t = 30 min 97.56 [41] FeCo-LDH 罗丹明B 20 [Cat.] = 0.2 g·L−1、[PMS] = 0.15 g·L−1、pH = 3.4、t = 10 min 100 [42] BC-CoFeLDH 邻苯二甲酸二甲酯 10 [Cat.] = 0.3 g·L−1、[PMS] = 0.3 g·L−1、pH = 6.8、t = 60 min 100 [43] La/FeNi-LDH 四环素 20 [Cat.] = 0.04 g·L−1、[PMS] = 0.2 mmol·L−1、t = 60 min 84 [44] FeMg-LDH/BC 多西环素 30 [Cat.] = 0.75 g·L−1、[PMS] = 0.75 g·L−1、pH = 7.0、t = 120 min 88.76 [45] FeNi-LDH@BC 多西环素 35 [Cat.] = 0.5 g·L−1、[PMS] = 0.75 g·L−1、pH = 4.5、t = 120 min 88.1 [46] 表 2 常见铁基双金属催化剂的合成方法的优缺点
Table 2. Advantages and disadvantages of synthesis methods for common iron based bimetal catalysts
表 3 铁基双金属催化剂的合成方法及形貌和催化性能
Table 3. Synthesis methods, morphology, and catalytic performance of iron based bimetallic catalysts
催化剂
Catalyst合成方法
Synthesis method形貌
Morphology污染物及浓度
Pollutant and concentration反应条件
Reaction conditions降解率/%
Degradation参考文献
ReferencesFe-Ce@N-BC 浸渍-热解法 蜂窝状片层多孔结构 [甲硝唑] =
0.010 g·L−1[PMS] = 2 mmol·L−1、[Cat.] =
0.75 g·L−1、pH = 5.74、t = 60 min97.5 [17] p-Mn/Fe3O4 浸渍-热解法 立方体多孔结构 [双酚A] =
0.1 mmol·L−1[PMS] = 2 mmol·L−1、[Cat.] =
0.2 g·L−1、pH = 7.0、t = 30 min100 [52] CuFe2O4 溶胶-凝胶法 典型的介孔结构 [碘海醇] =
1.0 mg·L−1[PMS] = 20 mg·L−1、[Cat.] =
50 mg·L−1、pH = 7.0、t = 15 min95.0 [35] MnFe2O4@BC 溶胶-凝胶法 MnFe2O4微球均匀地锚定
在BC的表面和多孔
结构上[双酚A] =
20 mg·L−1[PMS] = 0.2 g·L−1、[Cat.] = 0.2 g·L−1、
pH = 7.0、t = 30 min100 [53] CoFe-LDH 共沉淀法 层状结构 [酸性红27] =
200 mg·L−1[PMS] = 0.2 g·L−1、[Cat.] = 0.1 g·L−1、
pH = 6.7、t = 15 min96.7 [40] CoFe2O4-EG 共沉淀法 小尺寸的CoFe2O4颗粒
不均匀地分散在EG表面,
增加了EG表面的粗糙度[磺胺甲恶唑] =
10 mg·L−1[PMS] = 2 mmol·L−1、[Cat.] =
0.6 g·L−1、pH = 6.0 ± 0.3、t = 20 min>99 [54] CoFe2O4 水热法 纳米颗粒,平均尺寸约为13nm [阿特拉津] =
10 mg·L−1[PMS] = 8 mmol·L−1、[Cat.] =
0.4 g·L−1、pH = 6.3、t = 30 min>99 [55] Co-Fe/SiO2 溶剂热法 层状薄片状结构 [环丙沙星] =
10 mg·L−1[PMS] = 1.6 mmol (0.50 g·L−1)、[Cat.] = 0.2 g·L−1、pH = 7.0 ± 0.2、t = 10 min 98 [56] 新型磁性锰铁碳化物(mMFC) 溶剂热和煅烧
相结合的方法棒状结构 [尼泊金丁酯] = 25.74 μmol [PMS] = 2 mmol、[Cat.] = 125 mg·L−1、pH = 6.83 ± 0.20、t = 75 min 100 [57] -
[1] YE B, WU Q Y, WANG W L, et al. PPCP degradation by ammonia/chlorine: Efficiency, radical species, and byproducts formation[J]. Water Research, 2023, 235: 119862. doi: 10.1016/j.watres.2023.119862 [2] JIN X W, WANG Y Y, JIN W, et al. Ecological risk of nonylphenol in China surface waters based on reproductive fitness[J]. Environmental Science & Technology, 2014, 48(2): 1256-1262. [3] ABRAHAM D G, LIBERATORE H K, AZIZ M T, et al. Impacts of hydraulic fracturing wastewater from oil and gas industries on drinking water: Quantification of 69 disinfection by-products and calculated toxicity[J]. Science of The Total Environment, 2023, 882: 163344. doi: 10.1016/j.scitotenv.2023.163344 [4] JAIN M, KHAN S A, SHARMA K, et al. Current perspective of innovative strategies for bioremediation of organic pollutants from wastewater[J]. Bioresource Technology, 2022, 344: 126305. doi: 10.1016/j.biortech.2021.126305 [5] LIN Y Z, ZHONG L B, DOU S, et al. Facile synthesis of electrospun carbon nanofiber/graphene oxide composite aerogels for high efficiency oils absorption[J]. Environment International, 2019, 128: 37-45. doi: 10.1016/j.envint.2019.04.019 [6] AHMED M B, ZHOU J L, NGO H H, et al. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review[J]. Journal of Hazardous Materials, 2017, 323: 274-298. doi: 10.1016/j.jhazmat.2016.04.045 [7] AMOR C, FERNANDES J R, LUCAS M S, et al. Hydroxyl and sulfate radical advanced oxidation processes: Application to an agro-industrial wastewater[J]. Environmental Technology & Innovation, 2021, 21: 101183. [8] ZHAO Q X, MAO Q M, ZHOU Y Y, et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: A review on heterogeneous catalysts and applications[J]. Chemosphere, 2017, 189: 224-238. doi: 10.1016/j.chemosphere.2017.09.042 [9] HU P D, LONG M C. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications[J]. Applied Catalysis B:Environmental, 2016, 181: 103-117. doi: 10.1016/j.apcatb.2015.07.024 [10] LIU Y X, WANG Y, WANG Q, et al. Simultaneous removal of NO and SO2 using vacuum ultraviolet light (VUV)/heat/peroxymonosulfate (PMS)[J]. Chemosphere, 2018, 190: 431-441. doi: 10.1016/j.chemosphere.2017.10.020 [11] ALAYANDE A B, HONG S. Ultraviolet light-activated peroxymonosulfate (UV/PMS) system for humic acid mineralization: Effects of ionic matrix and feasible application in seawater reverse osmosis desalination[J]. Environmental Pollution, 2022, 307: 119513. doi: 10.1016/j.envpol.2022.119513 [12] DO MINH T, NCIBI M C, SRIVASTAVA V, et al. Gingerbread ingredient-derived carbons-assembled CNT foam for the efficient peroxymonosulfate-mediated degradation of emerging pharmaceutical contaminants[J]. Applied Catalysis B:Environmental, 2019, 244: 367-384. doi: 10.1016/j.apcatb.2018.11.064 [13] WANG J Q, HASAER B, YANG M, et al. Anaerobically-digested sludge disintegration by transition metal ions-activated peroxymonosulfate (PMS): Comparison between Co2+, Cu2+, Fe2+ and Mn2+[J]. Science of The Total Environment, 2020, 713: 136530. doi: 10.1016/j.scitotenv.2020.136530 [14] RASTOGI A, AL-ABED S R, DIONYSIOU D D. Effect of inorganic, synthetic and naturally occurring chelating agents on Fe(II) mediated advanced oxidation of chlorophenols[J]. Water Research, 2009, 43(3): 684-694. doi: 10.1016/j.watres.2008.10.045 [15] 张博, 黎素, 张扬, 等. 富氧空位MoO2强化Fe2+/过一硫酸盐体系降解四环素[J]. 环境科学学报, 2022, 42(11): 66-76. ZHANG B, LI S, ZHANG Y, et al. Oxygen vacancy-rich MoO2 strengthening the Fe2+/PMS system for the degradation of Tetracycline[J]. Acta Scientiae Circumstantiae, 2022, 42(11): 66-76 (in Chinese).
[16] PENG Y T, TANG H M, YAO B, et al. Activation of peroxymonosulfate (PMS) by spinel ferrite and their composites in degradation of organic pollutants: A Review[J]. Chemical Engineering Journal, 2021, 414: 128800. doi: 10.1016/j.cej.2021.128800 [17] XIAO K B, LIANG F W, LIANG J Z, et al. Magnetic bimetallic Fe, Ce-embedded N-enriched porous biochar for peroxymonosulfate activation in metronidazole degradation: Applications, mechanism insight and toxicity evaluation[J]. Chemical Engineering Journal, 2022, 433: 134387. doi: 10.1016/j.cej.2021.134387 [18] JI Q Q, LI J, XIONG Z K, et al. Enhanced reactivity of microscale Fe/Cu bimetallic particles (mFe/Cu) with persulfate (PS) for p-nitrophenol (PNP) removal in aqueous solution[J]. Chemosphere, 2017, 172: 10-20. doi: 10.1016/j.chemosphere.2016.12.128 [19] HUANG G X, WANG C Y, YANG C W, et al. Degradation of bisphenol A by peroxymonosulfate catalytically activated with Mn1.8Fe1.2O4 nanospheres: Synergism between Mn and Fe[J]. Environmental Science & Technology, 2017, 51(21): 12611-12618. [20] CHEN G, NENGZI L C, LI B, et al. Octadecylamine degradation through catalytic activation of peroxymonosulfate by FeMn layered double hydroxide[J]. Science of The Total Environment, 2019, 695: 133963. doi: 10.1016/j.scitotenv.2019.133963 [21] GUO R N, CHEN Y, NENGZI L C, et al. In situ preparation of carbon-based Cu-Fe oxide nanoparticles from CuFe Prussian blue analogues for the photo-assisted heterogeneous peroxymonosulfate activation process to remove lomefloxacin[J]. Chemical Engineering Journal, 2020, 398: 125556. doi: 10.1016/j.cej.2020.125556 [22] XIAO S, CHENG M, ZHONG H, et al. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review[J]. Chemical Engineering Journal, 2020, 384: 123265. doi: 10.1016/j.cej.2019.123265 [23] ZHAO G Q, ZOU J, CHEN X Q, et al. Iron-based catalysts for persulfate-based advanced oxidation process: Microstructure, property and tailoring[J]. Chemical Engineering Journal, 2021, 421: 127845. doi: 10.1016/j.cej.2020.127845 [24] SUN J W, WU T, LIU Z F, et al. Peroxymonosulfate activation induced by spinel ferrite nanoparticles and their nanocomposites for organic pollutants removal: A review[J]. Journal of Cleaner Production, 2022, 346: 131143. doi: 10.1016/j.jclepro.2022.131143 [25] KIFLE G A, HUANG Y, XIANG M H, et al. Heterogeneous activation of peroxygens by iron-based bimetallic nanostructures for the efficient remediation of contaminated water. A review[J]. Chemical Engineering Journal, 2022, 442: 136187. doi: 10.1016/j.cej.2022.136187 [26] KEFENI K K, MAMBA B B. Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment: Review[J]. Sustainable Materials and Technologies, 2020, 23: e00140. doi: 10.1016/j.susmat.2019.e00140 [27] ZHAO Q, YAN Z H, CHEN C C, et al. Spinels: Controlled preparation, oxygen reduction/evolution reaction application, and beyond[J]. Chemical Reviews, 2017, 117(15): 10121-10211. doi: 10.1021/acs.chemrev.7b00051 [28] QIN H, HE Y Z, XU P, et al. Spinel ferrites (MFe2O4): Synthesis, improvement and catalytic application in environment and energy field[J]. Advances in Colloid and Interface Science, 2021, 294: 102486. doi: 10.1016/j.cis.2021.102486 [29] WANG Y R, TIAN D F, CHU W, et al. Nanoscaled magnetic CuFe2O4 as an activator of peroxymonosulfate for the degradation of antibiotics norfloxacin[J]. Separation and Purification Technology, 2019, 212: 536-544. doi: 10.1016/j.seppur.2018.11.051 [30] KUSIGERSKI V, ILLES E, BLANUSA J, et al. Magnetic properties and heating efficacy of magnesium doped magnetite nanoparticles obtained by co-precipitation method[J]. Journal of Magnetism and Magnetic Materials, 2019, 475: 470-478. doi: 10.1016/j.jmmm.2018.11.127 [31] SUN B H, LI Q Q, ZHENG M H, et al. Recent advances in the removal of persistent organic pollutants (POPs) using multifunctional materials: a review[J]. Environmental Pollution, 2020, 265: 114908. doi: 10.1016/j.envpol.2020.114908 [32] LATIF A, SHENG D, SUN K, et al. Remediation of heavy metals polluted environment using Fe-based nanoparticles: Mechanisms, influencing factors, and environmental implications[J]. Environmental Pollution, 2020, 264: 114728. doi: 10.1016/j.envpol.2020.114728 [33] REN Y M, LIN L Q, MA J, et al. Sulfate radicals induced from peroxymonosulfate by magnetic ferrospinel MFe2O4 (M = Co, Cu, Mn, and Zn) as heterogeneous catalysts in the water[J]. Applied Catalysis B:Environmental, 2015, 165: 572-578. doi: 10.1016/j.apcatb.2014.10.051 [34] GUAN Y H, MA J, REN Y M, et al. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals[J]. Water Research, 2013, 47(14): 5431-5438. doi: 10.1016/j.watres.2013.06.023 [35] WANG S Y, CHEN Z L, YAN P W, et al. Enhanced degradation of iohexol in water by CuFe2O4 activated peroxymonosulfate: Efficiency, mechanism and degradation pathway[J]. Chemosphere, 2022, 289: 133198. doi: 10.1016/j.chemosphere.2021.133198 [36] KARIM A V, HASSANI A, EGHBALI P, et al. Nanostructured modified layered double hydroxides (LDHs)-based catalysts: A review on synthesis, characterization, and applications in water remediation by advanced oxidation processes[J]. Current Opinion in Solid State and Materials Science, 2022, 26(1): 100965. doi: 10.1016/j.cossms.2021.100965 [37] KOHANTORABI M, MOUSSAVI G, GIANNAKIS S. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants[J]. Chemical Engineering Journal, 2021, 411: 127957. doi: 10.1016/j.cej.2020.127957 [38] YE Q Y, WU J Y, WU P X, et al. Enhancing peroxymonosulfate activation of Fe-Al layered double hydroxide by dissolved organic matter: Performance and mechanism[J]. Water Research, 2020, 185: 116246. doi: 10.1016/j.watres.2020.116246 [39] LI X G, HOU T L, YAN L G, et al. Efficient degradation of tetracycline by CoFeLa-layered double hydroxides catalyzed peroxymonosulfate: Synergistic effect of radical and nonradical pathways[J]. Journal of Hazardous Materials, 2020, 398: 122884. doi: 10.1016/j.jhazmat.2020.122884 [40] WANG Z W, TAN Y N, DUAN X G, et al. Pretreatment of membrane dye wastewater by CoFe-LDH-activated peroxymonosulfate: Performance, degradation pathway, and mechanism[J]. Chemosphere, 2023, 313: 137346. doi: 10.1016/j.chemosphere.2022.137346 [41] HOU L H, LI X M, YANG Q, et al. Heterogeneous activation of peroxymonosulfate using Mn-Fe layered double hydroxide: Performance and mechanism for organic pollutant degradation[J]. Science of The Total Environment, 2019, 663: 453-464. doi: 10.1016/j.scitotenv.2019.01.190 [42] GONG C, CHEN F, YANG Q, et al. Heterogeneous activation of peroxymonosulfate by Fe-Co layered doubled hydroxide for efficient catalytic degradation of Rhoadmine B[J]. Chemical Engineering Journal, 2017, 321: 222-232. doi: 10.1016/j.cej.2017.03.117 [43] YE Q Y, WU J Y, WU P X, et al. Enhancing peroxymonosulfate activation by Co-Fe layered double hydroxide catalysts via compositing with biochar[J]. Chemical Engineering Journal, 2021, 417: 129111. doi: 10.1016/j.cej.2021.129111 [44] WANG R Y, SU S N, REN X H, et al. Polyoxometalate intercalated La-doped NiFe-LDH for efficient removal of tetracycline via peroxymonosulfate activation[J]. Separation and Purification Technology, 2021, 274: 119113. doi: 10.1016/j.seppur.2021.119113 [45] MA R, YAN X Q, MI X H, et al. Enhanced catalytic degradation of aqueous doxycycline (DOX) in Mg-Fe-LDH@biochar composite-activated peroxymonosulfate system: Performances, degradation pathways, mechanisms and environmental implications[J]. Chemical Engineering Journal, 2021, 425: 131457. doi: 10.1016/j.cej.2021.131457 [46] MI X H, MA R, PU X C, et al. FeNi-layered double hydroxide (LDH)@biochar composite for , activation of peroxymonosulfate (PMS) towards enhanced degradation of doxycycline (DOX): Characterizations of the catalysts, catalytic performances, degradation pathways and mechanisms[J]. Journal of Cleaner Production, 2022, 378: 134514. [47] WU X Y, RU Y, BAI Y, et al. PBA composites and their derivatives in energy and environmental applications[J]. Coordination Chemistry Reviews, 2022, 451: 214260. doi: 10.1016/j.ccr.2021.214260 [48] ZHAO C X, LIU B, LI X N, et al. A Co-Fe Prussian blue analogue for efficient Fenton-like catalysis: the effect of high-spin cobalt[J]. Chemical Communications, 2019, 55(50): 7151-7154. doi: 10.1039/C9CC01872G [49] LI X N, WANG Z H, ZHANG B, et al. FexCo3-xO4 nanocages derived from nanoscale metal-organic frameworks for removal of bisphenol A by activation of peroxymonosulfate[J]. Applied Catalysis B:Environmental, 2016, 181: 788-799. doi: 10.1016/j.apcatb.2015.08.050 [50] ZENG H X, DENG L, YANG K H, et al. Degradation of sulfamethoxazole using peroxymonosulfate activated by self-sacrificed synthesized CoAl-LDH@CoFe-PBA nanosheet: Reactive oxygen species generation routes at acidic and alkaline pH[J]. Separation and Purification Technology, 2021, 268: 118654. doi: 10.1016/j.seppur.2021.118654 [51] PI Y Q, MA L J, ZHAO P, et al. Facile green synthetic graphene-based Co-Fe Prussian blue analogues as an activator of peroxymonosulfate for the degradation of levofloxacin hydrochloride[J]. Journal of Colloid and Interface Science, 2018, 526: 18-27. doi: 10.1016/j.jcis.2018.04.070 [52] DU J K, BAO J G, LIU Y, et al. Facile preparation of porous Mn/Fe3O4 cubes as peroxymonosulfate activating catalyst for effective bisphenol A degradation[J]. Chemical Engineering Journal, 2019, 376: 119193. doi: 10.1016/j.cej.2018.05.177 [53] XU S Y, WEN L T, YU C, et al. Activation of peroxymonosulfate by MnFe2O4@BC composite for bisphenol A Degradation: The coexisting of free-radical and non-radical pathways[J]. Chemical Engineering Journal, 2022, 442: 136250. doi: 10.1016/j.cej.2022.136250 [54] XU M J, LI J, YAN Y, et al. Catalytic degradation of sulfamethoxazole through peroxymonosulfate activated with expanded graphite loaded CoFe2O4 particles[J]. Chemical Engineering Journal, 2019, 369: 403-413. doi: 10.1016/j.cej.2019.03.075 [55] LI J, XU M J, YAO G, et al. Enhancement of the degradation of atrazine through CoFe2O4 activated peroxymonosulfate (PMS) process: Kinetic, degradation intermediates, and toxicity evaluation[J]. Chemical Engineering Journal, 2018, 348: 1012-1024. doi: 10.1016/j.cej.2018.05.032 [56] ZHU S J, XU Y P, ZHU Z G, et al. Activation of peroxymonosulfate by magnetic Co-Fe/SiO2 layered catalyst derived from iron sludge for ciprofloxacin degradation[J]. Chemical Engineering Journal, 2020, 384: 123298. doi: 10.1016/j.cej.2019.123298 [57] YANG J C E, LIN Y, PENG H H, et al. Novel magnetic rod-like Mn-Fe oxycarbide toward peroxymonosulfate activation for efficient oxidation of butyl paraben: Radical oxidation versus singlet oxygenation[J]. Applied Catalysis B:Environmental, 2020, 268: 118549. doi: 10.1016/j.apcatb.2019.118549 [58] 梁锦芝, 许伟城, 赖树锋, 等. 磁性生物炭的制备及其活化过一硫酸盐的研究进展[J]. 环境化学, 2021, 40(9): 2901-2911. doi: 10.7524/j.issn.0254-6108.2021022301 LIANG J Z, XU W C, LAI S F, et al. Research progress on preparation and peroxymonosulfate activation of magnetic biochar[J]. Environmental Chemistry, 2021, 40(9): 2901-2911 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021022301
[59] ZHU Z Q, ZHANG Q, XU M J, et al. Highly active heterogeneous FeCo metallic oxides for peroxymonosulfate activation: The mechanism of oxygen vacancy enhancement[J]. Journal of Environmental Chemical Engineering, 2023, 11(1): 109071. doi: 10.1016/j.jece.2022.109071 [60] WU L Y, YU Y B, ZHANG Q, et al. A novel magnetic heterogeneous catalyst oxygen-defective CoFe2O4-x for activating peroxymonosulfate[J]. Applied Surface Science, 2019, 480: 717-726. doi: 10.1016/j.apsusc.2019.03.034 [61] XU Y S, ZHENG L L, YANG C, et al. Oxygen vacancies enabled porous SnO2 thin films for highly sensitive detection of triethylamine at room temperature[J]. ACS Applied Materials & Interfaces, 2020, 12(18): 20704-20713. [62] LONG X X, FENG C P, DING D H, et al. Oxygen vacancies-enriched CoFe2O4 for peroxymonosulfate activation: The reactivity between radical-nonradical coupling way and bisphenol A[J]. Journal of Hazardous Materials, 2021, 418: 126357. doi: 10.1016/j.jhazmat.2021.126357 [63] ZHOU Y B, ZHANG Y L, HU X M. Enhanced activation of peroxymonosulfate using oxygen vacancy-enriched FeCo2O4-x spinel for 2, 4-dichlorophenol removal: Singlet oxygen-dominated nonradical process[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2020, 597: 124568. doi: 10.1016/j.colsurfa.2020.124568 [64] MA Q L, NENGZI L C, LI B, et al. Heterogeneously catalyzed persulfate with activated carbon coated with CoFe layered double hydroxide (AC@CoFe-LDH) for the degradation of lomefloxacin[J]. Separation and Purification Technology, 2020, 235: 116204. doi: 10.1016/j.seppur.2019.116204 [65] CHEN L W, DING D H, LIU C, et al. Degradation of norfloxacin by CoFe2O4-GO composite coupled with peroxymonosulfate: A comparative study and mechanistic consideration[J]. Chemical Engineering Journal, 2018, 334: 273-284. doi: 10.1016/j.cej.2017.10.040 [66] WU D, ZHAO Y, XIA Q, et al. Bamboo-like nitrogen-doped carbon nanotubes on iron mesh for electrochemically-assisted catalytic oxidation[J]. Journal of Hazardous Materials, 2021, 408: 124899. doi: 10.1016/j.jhazmat.2020.124899 [67] DONG Z T, NIU C G, GUO H, et al. Anchoring CuFe2O4 nanoparticles into N-doped carbon nanosheets for peroxymonosulfate activation: Built-in electric field dominated radical and non-radical process[J]. Chemical Engineering Journal, 2021, 426: 130850. doi: 10.1016/j.cej.2021.130850 [68] SAHOO D P, NAYAK S, REDDY K H, et al. Fabrication of a Co(OH)2/ZnCr LDH “p-n” heterojunction photocatalyst with enhanced separation of charge carriers for efficient visible-light-driven H2 and O2 evolution[J]. Inorganic Chemistry, 2018, 57(7): 3840-3854. doi: 10.1021/acs.inorgchem.7b03213 [69] SAHOO D P, PATNAIK S, PARIDA K. An amine functionalized ZnCr LDH/MCM-41 nanocomposite as efficient visible light induced photocatalyst for Cr(VI) reduction[J]. Materials Today:Proceedings, 2021, 35: 252-257. doi: 10.1016/j.matpr.2020.05.526 [70] SUN Q N, WANG X J, LIU Y Y, et al. Visible-light-driven g-C3N4 doped CuFe2O4 floating catalyst enhanced peroxymonosulfate activation for sulfamethazine removal via singlet oxygen and high-valent metal-oxo species[J]. Chemical Engineering Journal, 2023, 455: 140198. doi: 10.1016/j.cej.2022.140198 [71] LI X F, CHEN T, QIU Y L, et al. Magnetic dual Z-scheme g-C3N4/BiVO4/CuFe2O4 heterojunction as an efficient visible-light-driven peroxymonosulfate activator for levofloxacin degradation[J]. Chemical Engineering Journal, 2023, 452: 139659. doi: 10.1016/j.cej.2022.139659 [72] PANG Y X, LEI H Y. Degradation of p-nitrophenol through microwave-assisted heterogeneous activation of peroxymonosulfate by manganese ferrite[J]. Chemical Engineering Journal, 2016, 287: 585-592. doi: 10.1016/j.cej.2015.11.076 [73] XU P, XIE S Q, LIU X, et al. Electrochemical enhanced heterogenous activation of peroxymonosulfate using CuFe2O4 particle electrodes for the degradation of diclofenac[J]. Chemical Engineering Journal, 2022, 446: 136941. doi: 10.1016/j.cej.2022.136941 [74] XU H D, QUAN X C, CHEN L. A novel combination of bioelectrochemical system with peroxymonosulfate oxidation for enhanced azo dye degradation and MnFe2O4 catalyst regeneration[J]. Chemosphere, 2019, 217: 800-807. doi: 10.1016/j.chemosphere.2018.11.077 [75] WU S H, YANG C P, LIN Y, et al. Efficient degradation of tetracycline by singlet oxygen-dominated peroxymonosulfate activation with magnetic nitrogen-doped porous carbon[J]. Journal of Environmental Sciences, 2022, 115: 330-340. doi: 10.1016/j.jes.2021.08.002 [76] ZHU S S, LI X J, KANG J, et al. Persulfate activation on crystallographic manganese oxides: Mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants[J]. Environmental Science & Technology, 2019, 53(1): 307-315. [77] LI Y, MA S L, XU S J, et al. Novel magnetic biochar as an activator for peroxymonosulfate to degrade bisphenol A: Emphasizing the synergistic effect between graphitized structure and CoFe2O4[J]. Chemical Engineering Journal, 2020, 387: 124094. doi: 10.1016/j.cej.2020.124094 [78] REN W, XIONG L L, NIE G, et al. Insights into the electron-transfer regime of peroxydisulfate activation on carbon nanotubes: The role of oxygen functional groups[J]. Environmental Science & Technology, 2020, 54(2): 1267-1275. [79] LI X H, ZHAO Z H, LI H C, et al. Degradation of organic contaminants in the CoFe2O4/peroxymonosulfate process: The overlooked role of Co(II)-PMS complex[J]. Chemical Engineering Journal Advances, 2021, 8: 100143. doi: 10.1016/j.ceja.2021.100143 [80] ZHANG X J, ZHU X B, LI H, et al. Combination of peroxymonosulfate and Fe(Ⅵ) for enhanced degradation of sulfamethoxazole: The overlooked roles of high-valent iron species[J]. Chemical Engineering Journal, 2023, 453: 139742. doi: 10.1016/j.cej.2022.139742 [81] YANG L, WEI Z F, GUO Z H, et al. Significant roles of surface functional groups and Fe/Co redox reactions on peroxymonosulfate activation by hydrochar-supported cobalt ferrite for simultaneous degradation of monochlorobenzene and p-chloroaniline[J]. Journal of Hazardous Materials, 2023, 445: 130588. doi: 10.1016/j.jhazmat.2022.130588 [82] WANG Z, QIU W, PANG S Y, et al. Further understanding the involvement of Fe(IV) in peroxydisulfate and peroxymonosulfate activation by Fe(II) for oxidative water treatment[J]. Chemical Engineering Journal, 2019, 371: 842-847. doi: 10.1016/j.cej.2019.04.101 -



 下载:
下载: