-
羟胺作为重要的活性氮化合物,在地球氮循环中起着至关重要的作用[1-3]. 大量研究表明多种氨氧化微生物催化的氨氧化[4-5],氮固化[6]、硝化和反硝化[7]等过程中均有羟胺化合物的产生,为了深入了解羟胺在地球氮循环中的作用,研究羟胺在环境中的迁移转化是十分必要的.
NOM作为环境中普遍存在的复杂有机混合物,包含羟基、酮基、羧基等多种活性基团[8-9],对多种含氮化合物的环境迁移转化有着重要影响. 例如,环境中的苯胺化合物可以共价结合NOM分子[10];氨作为农业土壤氮肥中含量最丰富的一种形式,可与NOM结合而被称之为氨固化[11]; 3,4-二氯苯胺是敌草隆除草剂降解过程中产生的主要代谢物,它能够不可逆地与NOM结合,从而降低其毒性[12]. 在NOM与上述含氮化合物的相互作用过程中,NOM的醛、酮官能团被认为是主要的作用位点.
羟胺也可以与羰基反应生成肟类产物[13],已有多篇研究报道了羟胺与NOM的相互作用以探究NOM对羟胺的吸收作用[14]或测定含羰基的NOM组分[15]. Thorn等通过液体15N NMR光谱法对羟胺衍生的NOM产物进行了表征,证明肟类化合物是其主要衍生物[16]. 然而,由于NOM组成极其复杂,传统的方法很难分离羟胺衍生的NOM组分从而获得更全面的分子信息. 因此,尽管羟胺衍生的NOM的分子组成对环境化学家更好地理解有机质对全球氮循环中羟胺转化的影响具有重要意义,但到目前为止尚未见报道.
FTICR-MS,因其具有超高的质量检测准确度和分辨率,目前已成为表征NOM[17]及其消毒副产物[18]分子组成的重要分析工具. 通过对NOM中存在的小分子有机物的精确质量数测定,从而获得其分子式信息,在此基础上,通过各种可视化图分析,可进一步区分NOM分子所属化合物种类,如脂类、木质素类、鞣酸类、稠环芳烃类等,还可进一步分析化合物的疏水性、芳香度、不饱和度等性质[19-20]. 基于此,本研究采用FTICR-MS对羟胺衍生的NOM产物进行分子表征,采用15N同位素标记的羟胺作为起始反应物,以保证所得到的15N同位素标记羟胺衍生NOM产物能排除NOM中其他组分的干扰,从而被特异性地识别检测;同时考察了时间对羟胺与NOM反应的影响.
-
15N标记盐酸羟胺 (H215NOH.HCl, >98%) 购置于 Sigma-Aldrich 公司(Steinheim, Germany),NOM标准品购置于美国腐殖酸学会,超纯水制备于Milli-Q system (Millipore, Milford, MA, USA),甲醇 (色谱纯) 购置于Fisher Scientific 公司(Fair Lawn, N.J., USA),其他试剂均为国产分析纯试剂.
称取2.0 mg NOM标准品溶解于100 mL超纯水中制备成20.0 mg·L−1 NOM储备液. 取10 mL NOM储备液4份,分别加入50倍质量过量的15N标记盐酸羟胺,在室温条件分别振荡反应2、4、8、10 h,然后,采用全自动固相萃取装置(睿科公司,厦门)和安捷伦Bond Elut PPL固相萃取柱((0.5 g/6 mL, Varian, Palo Alto, CA, U.S.A.)对上述样品进行固相萃取以去除残留的15N标记盐酸羟胺并富集浓缩样品[21],具体操作步骤如下:(1)用甲酸调节反应样品 pH至2.0;(2)在固相萃取装置上安装好PPL柱,先后用 12 mL 甲醇(色谱纯)和 12 mL 0.1%的甲酸水溶液淋洗活化柱子;(3)PPL柱缓慢加入10 mL样品,样品以1 mL·min−1 的流速通过固相萃取柱以富集目标化合物;(4)样品流完后加入 12 mL 0.1% 甲酸水溶液淋洗柱子,以除去不保留成分;(5)氮吹干燥柱子,然后用 12 mL 甲醇洗脱,收集洗脱液;(6)氮吹洗脱液至甲醇完全挥发,FTICR-MS测定前用 1 mL甲醇溶解.
-
FTICR-MS(Solarix 15T, Infinity cell,Bruker Daltonik, GmbH, Bremen, Germany)配备电喷雾/基质辅助激光解吸双离子源(ESI/MALDI). 本研究均采用ESI 源进行样品离子化,进样方式采用直接连续进样,进样流速为120 μL·h−1;离子源喷针电压设置为 4.0 kV,样品采用负离子宽谱扫描模式进行分析,离子累积时间设置为0.06 s,四百万字节时域采集瞬变模式,每个样品采集300扫描并叠加(每个样品重复测定3次); 质量扫描范围(m/z)100—1600. 仪器测定样品前,采用10 mmol·L−1 甲酸钠(50%异丙醇水溶液)校准液进行外标校准,样品测定后获得数据采用自制的NOM中常见已知CHO分子式列表作为内标校准文件进行进一步的内标校准,校准后的质量误差均小于百万分之0.5. 质谱数据采用Data Analysis软件进行分析 (Bruker Daltonics, version 5.3).
-
采用Data Analysis (Bruker Daltonics, version 5.3)软件对所得数据进行质谱峰确认和可能分子式匹配,分子式匹配条件如下:,确认质谱峰信噪比(S/N)大于等于4,质量检测误差值小于等于百万分之0.5,仅考虑C、H、O、N、S元素,元素个数范围设置如下:12C (0—∞), 1H (0—∞), 16O (0—∞), 14N (0—3 ), 15N (0—2 ), and 32S (0—1);进一步采用如下规则[22] 对得到的可能分子式进行进一步筛选:氢碳比值(H/C)范围: 0.3<H/C<2.2,氧碳比值范围(O/C):O/C<1.2,不饱和度(DBE)值需为整数且小于40,分子式组成满足偶氮规则,同时H、C、N元素个数满足:H<=2C+2+N,如果一个质核比(m/z)离子有多个可能分子式时,采用杂元素之和最小规则[23]筛选出正确分子式(即选择N、S元素个数之和最小的分子式是正确的),保留3次重复测定结果中共同鉴定到的分子式结果并转换成Excel表数据进行作图分析.
根据已有文献报道,计算每个分子式的不饱和度减氧元素个数值(DBE-O)[24],修正芳香指数(AI-mod) [25];根据AI-mod 和 H/C 值范围将所得分子式分子划分为4类[26]: 第一类(Group 1)属于燃烧源稠环多环芳烃类化合物 (AI_mod > 0.66), 第二类(Group 2)属于维管植物源多酚类化合物 (0.66 ≥AI_mod > 0.5), 第三类(Group 3)属于高不饱和酚类化合物(AI_mod ≤ 0.50 and H/C <1.5), 第四类(Group 4)属于脂肪族化合物 (2.0 ≥ H/C ≥ 1.5).
-
羟胺作为活性氮化合物可直接与NOM中的含羰基分子反应形成多种羟胺衍生化NOM产物[16],然而,由于NOM中本身含有的含氮化合物同分异构体的存在,导致羟胺衍生化NOM产物很难被FTICR-MS特异性识别检测. 本研究采用15N同位素标记羟胺作为反应物,当与NOM发生反应后形成15N同位素标记羟胺衍生化NOM产物,而NOM本身含氮分子的15N同位素峰通常掩盖在FTICR-MS的噪音信号以下,不会对15N同位素标记羟胺衍生化NOM产物的质谱信号造成干扰,从而能在FTICR-MS检测时特异性识别此类产物. 实验结果如图1 所示. 以质谱图标称m/z 389和m/z 460为例,可以看到,当NOM与15N同位素标记羟胺在室温条件下反应10 h后,与原NOM样品的质谱图相比(图1 A、C),明显有15N同位素标记羟胺衍生化NOM产物峰出现(图1 B、D),且这些质谱峰不受NOM中其他离子峰干扰,产物分子式种类主要包括CHO15N1, CHO15N2, CHOS115N1 和 CHON115N1等4类. 以上实验结果表明,当NOM与15N同位素标记羟胺反应10 h后,会明显产生15N同位素标记羟胺衍生化NOM产物,且这些产物峰可以被FTICR-MS特异性检测识别.
-
为了进一步分析羟胺衍生化NOM产物的分子组成,对整个质谱数据依据1.3节部分描述进行分子式匹配和筛选,得到所有检测到的15N同位素标记羟胺衍生化NOM产物的分子式结果. 分析结果如图2所示,共鉴定到2137个15N同位素标记羟胺衍生化NOM产物分子式(图2B),其中,包括CHO15N1分子式1346个(约占鉴定总数的63%),CHO15N2分子式194个(约占鉴定总数的9%),CHON115N1分子式221个(约占鉴定总数的10%),CHOS115N1分子式376个(约占鉴定总数的18%). 而对于反应前的NOM样品,共鉴定到CHO分子式2902个(约占鉴定总数的51%),CHON1分子式929个(约占鉴定总数的17%),CHOS1分子式1831个(约占鉴定总数的32%). 通过比较分析,可以看到,约40%以上的NOM中的CHO分子组分可以与15N同位素标记羟胺发生反应形成CHO15N1羟胺衍生NOM产物,而仅有低于7%的NOM中的CHO分子组分可以形成CHO15N2羟胺衍生NOM产物;对于NOM中的CHON1和CHOS1分子组分,仅有约23.7%的CHON1和约20.7%的CHOS1分子组分可与羟胺反应形成相应的CHON115N1 和CHOS115N1产物. 据此,可以推测,这些与羟胺反应的NOM分子中很可能至少含有一个羰基基团. Baluha等的研究表明,苏万尼河富里酸中约39%的CHO分子组分含有一到两个醛基或者酮基基团[27],此研究结果为本研究的推测提供了支持证据. 而形成CHO15N2羟胺衍生物的数目较少的原因可能是由于空间位阻、氢键作用或者电荷效应等导致NOM的CHO分子很难与两个羟胺分子连续发生反应.
通过Van Krevelen (VK)图分析,可以进一步推测化合物的理化属性和分子种类,通过AI_mod 和 H/C值的范围可以将化合物划分为4类[26] (如图2C、D所示). 可以看到,15N同位素标记羟胺衍生化NOM产物绝大部分属于维管植物源多酚类(Group 2)和高不饱和酚类化合物(Group 3)(图2D);与原始NOM样品的VK图(图2C)比较发现,NOM组分在VK图上的点分布范围几乎完全覆盖羟胺衍生化NOM产物组分的点分布,表明羟胺衍生化NOM产物组分的H/C 和O/C值与原NOM组分相比没有发生较大变化,对这一结果的可能解释是羟胺衍生化NOM产物很可能是通过羟胺与NOM组分所含的羰基发生肟化反应形成[16],因为发生肟化反应时,羟胺衍生化NOM产物分子式与原NOM分子相比H元素个数仅增加了1,O元素个数无变化,同时增加了1个15N元素. 为了进一步证明此推测,基于肟化反应,根据羟胺衍生化NOM产物分子式还原出相应的NOM反应物分子式,如果被还原出的分子式在原NOM样品中被鉴定到,则被定义为母-子分子对,同时认为此产物应该是通过肟化反应产生的. 通过数据分析发现(结果如图3A所示),对于CHO15N1羟胺衍生化NOM产物,可以找到1160个母-子分子对(约占总数的86.1%),对于CHO15N2羟胺衍生化NOM产物,共鉴定到172个母-子分子对 (约占总数的86.4%),对于CHON115N1羟胺衍生化NOM产物,共鉴定到198个母-子分子对 (约占总数的89.6%),对于CHOS115N1羟胺衍生化NOM产物,共鉴定到357个母-子分子对 (约占总数的94.9%). 此结果表明,86%以上的羟胺衍生化NOM产物很可能是通过肟化反应形成.
通过对上述鉴定到的羟胺衍生化NOM产物的母分子的VK图分析发现(图3B、C、D),H/C值范围在0.4—1.4同时O/C值范围在0.3—0.9的NOM CHO分子,H/C值范围在0.6—1.1同时O/C值范围在0.5—0.75的NOM CHON1分子,以及H/C值范围在0.7—1.5同时O/C值范围在0.42—0.9的NOM CHOS1分子更容易与15N同位素标记羟胺反应形成单肟产物,且这些分子大部分属于维管植物源多酚和高不饱和酚类化合物;而H/C 值范围在0.6—1.2同时O/C值范围在0.3—0.7的NOM CHO分子可以与羟胺形成双肟产物. 其他被检测到的NOM分子,如H/C值大于1.4或者O/C值大于0.9的NOM CHO分子,H/C值大于1.1或者O/C值大于0.75的NOM CHON1分子,以及H/C 值大于1.5或者O/C值大于0.9的NOM CHOS1分子,在此实验条件下不与羟胺发生反应形成肟化产物. 此实验结果表明,低H/C值和O/C值NOM组分可能具有较高的羟胺反应活性. 低H/C值和O/C值意味着分子具有较高的不饱和度和较低的氧化度,据此可以推测,这些分子可能含有更多的羰基基团和较少的羧基基团,从而导致其对羟胺的反应活性较高.
-
为了进一步研究NOM组分对羟胺的反应活性,实验考察了反应时间对15N同位素标记羟胺衍生化NOM产物的影响,过量的羟胺与NOM在室温条件下分别反应0、2、4、8、10 h后,分别过固相萃取柱进行浓缩萃取并进行FTICR-MS检测. 本研究主要关注CHO15N1类羟胺衍生NOM产物,由于此类产物的占比较高. 所得质谱结果的部分质谱图如图4所示,可以看到,有一些15N同位素标记羟胺衍生化NOM产物的峰强度会随反应时间的增加而逐渐增大(图4A),对应其NOM反应物的峰强度随时间延长而降低(图4D),这一结果较容易解释,即随着反应时间的延长,反应物的量会随着产物量的增加而降低;有一些产物峰强度随反应时间延长没有发生明显变化(图4B),而对应NOM反应物的峰强度却随反应时间延长而降低(图4E),这一结果较为异常,正常情况下,反应物峰强度应该不会发生明显变化,造成此异常结果的原因可能是此类NOM反应物除了生成CHO15N1类产物,还有可能发生了其他化学反应,具体原因有待进一步研究验证;有一些产物峰强度随反应时间增加而降低(图4C),对于其NOM反应物的峰强度在反应2 h后几乎均降低到质谱噪音峰强度水平(图4F),这一结果表明此类NOM反应物在反应2 h后已经反应完全,对应产物峰强度在2 h后进一步降低,推测此类产物有进一步转化为其他产物的可能性. 总之,对于15N同位素标记羟胺衍生化NOM产物而言,在反应10 h后,产物峰强度几乎不再变化. 此结果表明,15N同位素标记羟胺与NOM的反应10 h后可能已达到平衡. CHO15N1类羟胺衍生化NOM产物的形成具有高度的时间依赖性,很可能是由于NOM组分对羟胺的反应活性不同所造成的.
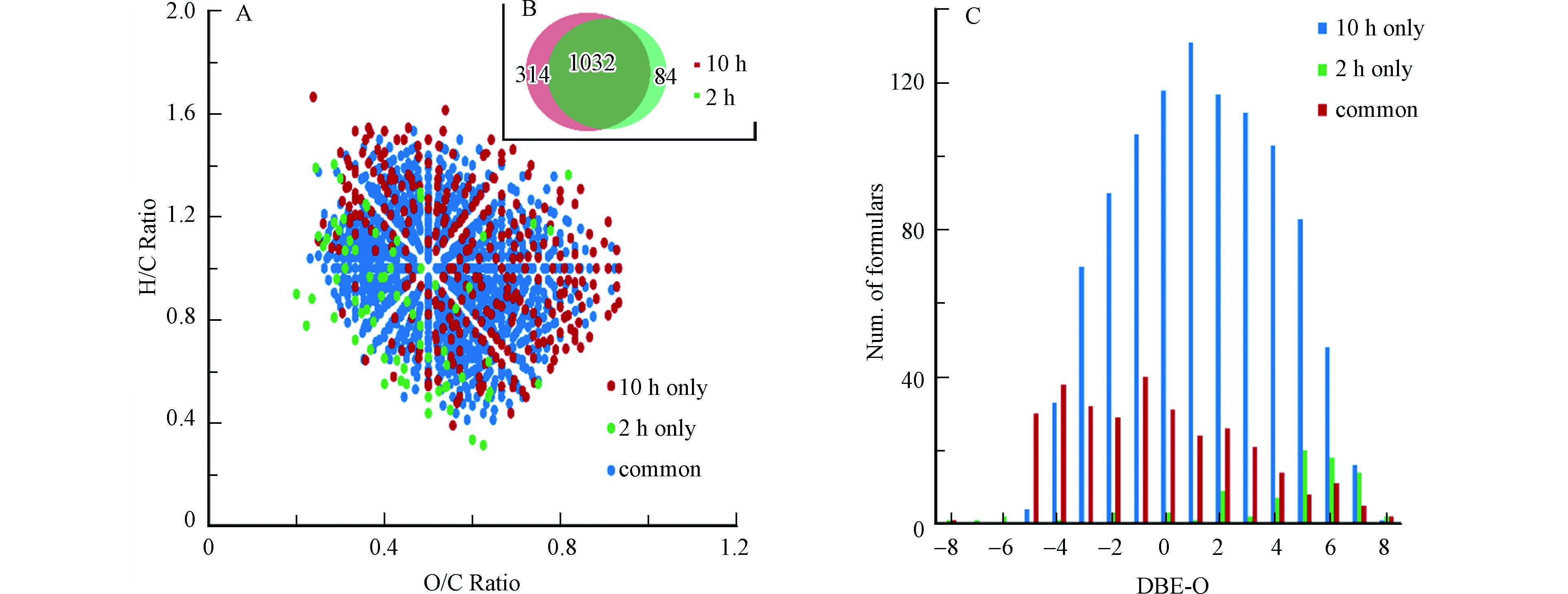
本研究进一步比较了反应2 h和10 h的CHO15N1产物的分子组成,结果如图5所示. 可以看到,反应2 h单独鉴定到的CHO15N1类分子式数目为84个,而10 h单独鉴定到314个,两个时间点共同鉴定到的分子式数目为1032个(图5B);VK图结果(图5A)显示,大部分2 h单独鉴定到的分子的O/C值较低,而大部分10 h单独鉴定到的分子的O/C值较高;DBE-O对分子式数目图[24](图5C)显示,10 h单独鉴定到的分子中,DBE-O值偏负的分子数目占比明显高于2 h单独鉴定到的分子,DBE-O值偏负表明分子中氧个数大于双键和环的个数[28]. 此结果表明,一些高氧化且饱和的NOM的CHO化合物对羟胺可能具有较低的反应活性,只有当延长反应时间时才可能形成羟胺衍生化NOM产物.
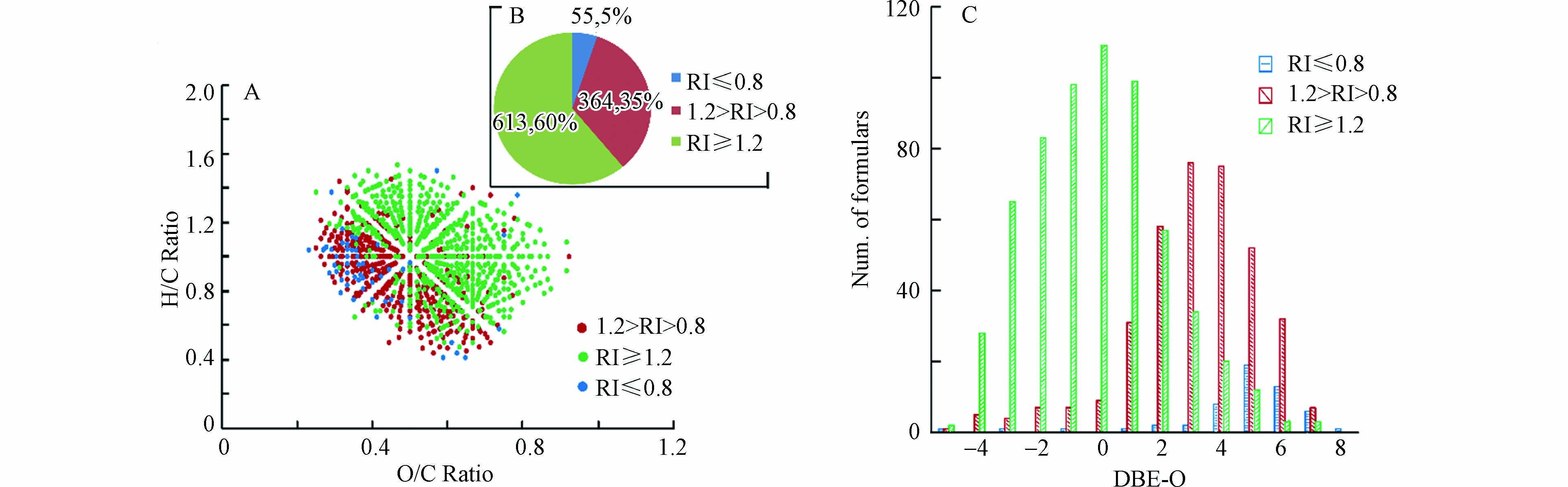
对于反应2 h和10 h共同鉴定到的CHO15N1产物的分子,为了考察反应时间对其影响,同一产物离子在10 h的质谱峰强度除以其在2 h的质谱峰强度被定义为该离子的相对峰强度(RI),为了减小测定误差的影响,RI值小于等于0.8被定义为该离子的峰强度在反应10 h时明显小于反应2 h的峰强度,代表该类产物在反应10 h的产量低于其在2 h 的产量;RI值大于0.8且小于1.2被定义为该离子的峰强度在反应10 h时与其反应2 h的峰强度无明显差异,代表该类产物在反应10 h与在2 h 的产量相当;RI值大于等于1.2被定义为该离子的峰强度在反应10 h时明显大于其反应2 h的峰强度,代表该类产物在反应10 h的产量大于其反应2 h的产量. 通过分析发现,对于1032个共同鉴定到的CHO15N1产物的分子(结果如图6B所示),RI值大于等于1.2的分子式有613个(约占总数的60%),RI值在0.8—1.2之间的分子式有364个(约占总数的35%),而RI值小于等于0.8的分子式仅有55个(约占总数的5%). 此结果表明,有60%共同鉴定分子的产量随反应时间的延长而增大,说明产生此类产物的NOM反应物可能由于反应活性较弱,并不能在较短反应时间内与羟胺反应完全,而延长反应时间会增加羟胺衍生NOM产物的产量;有35%共同鉴定分子的产量随反应时间的延长无明显变化,说明产生此类产物的NOM反应物可能由于反应活性较强,在较短反应时间内便能与羟胺反应完全形成单肟化产物或随时间延长进一步形成其他产物;而仅有5%共同鉴定分子的产量随反应时间的延长而降低,说明产生此类产物的NOM反应物可能含有多个羰基基团,随反应时间延长可能进一步生成多肟化产物或其他不稳定产物,从而导致这些单肟化产物随反应时间延长而产量下降.
VK图和DBE-O对分子式数目图结果(图6A、C)显示,RI值小于1.2的共同鉴定分子大部分具有较低的O/C值且DBE-O值偏正的分子式数目较多,而RI值大于等于1.2的共同鉴定分子大部分具有较高的O/C值且DBE-O值偏负的分子式数目较多,此结果说明高氧化度高饱和的NOM CHO分子对羟胺的反应活性相对较弱,需要较长的反应时间才能反应完全,而低氧化度高不饱和的NOM CHO分子对羟胺的反应活性相对较强,其反应完全时间较短甚至有些可以生成其他衍生产物.
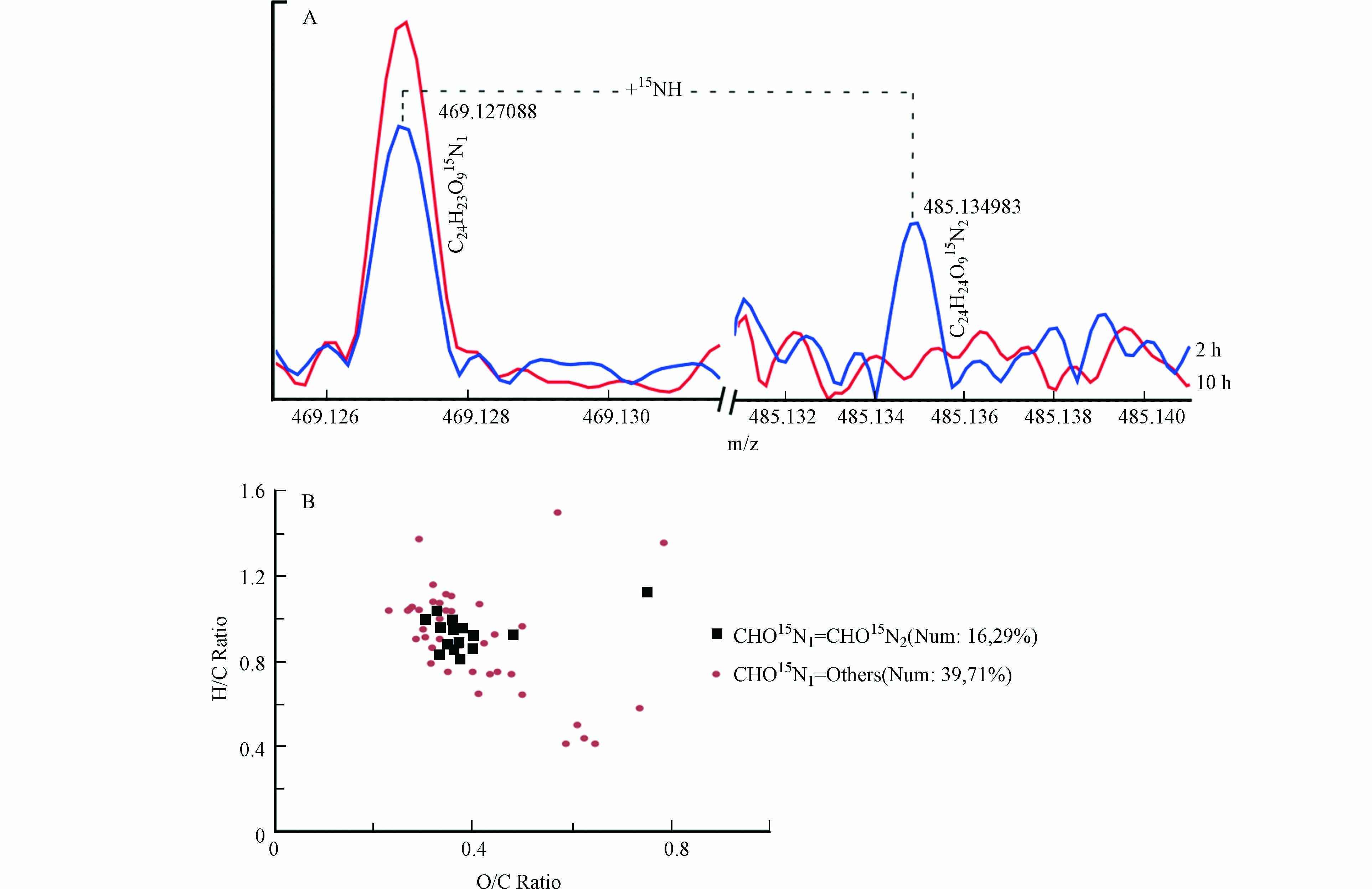
事实上,RI值小于等于0.8的共同鉴定分子的进一步肟化产物确实在此研究实验中被检测到,图7A是检测质谱结果的一个举例;约有29%的此类分子可以进一步与羟胺反应形成双肟化产物,而其他71%的分子可能并不稳定或转化成其他产物(图7B).
-
羟胺作为地球氮循环过程中重要的活性氮化合物,可以与环境中普遍存在的NOM发生反应,从而转化成其他含氮产物. 本研究采用FTICR-MS超高分辨质谱结合15N同位素标记技术全面表征了羟胺与NOM在自然条件下发生反应的产物分子信息,共鉴定到2000多个羟胺衍生化NOM产物,主要包括CHO15N1、CHO15N2、CHOS115N1和CHON115N1 等4类分子产物,其中大部分产物可能属于维管植物源多分和高不饱和酚类化合物. 86%以上的产物可能是通过肟化反应形成,数据可视化分析表明,高氧化高饱和的NOM CHO化合物可能与羟胺的反应活性较弱,而低氧化高不饱和度的CHO化合物与羟胺的反应活性较强,其中部分可形成双肟化产物或其他产物. 本研究揭示了NOM不同组分对羟胺的反应活性以及相应产物的分子信息,可为深入探究羟胺在地球氮循环过程中的迁移转化和相应转化机制研究提供更全面的基础信息.
傅里叶变换离子回旋共振质谱分子表征15N同位素标记羟胺衍生化天然有机质
Molecular characterization of 15N-labelled hydroxylamine-derivatized natural organic matter by FTICR-MS
-
摘要: 羟胺化合物是地球氮循环过程中的重要中间产物. 天然有机质(NOM)作为环境中普遍存在的复杂有机混合物,对羟胺的环境转化有重要影响. 本研究采用傅里叶变换离子回旋共振质谱(FTICR-MS)在分子层面表征羟胺与NOM的相互作用产物,采用15N同位素标记羟胺作为反应物以排除NOM本身存在的化合物的质谱峰干扰,特异性识别15N同位素标记羟胺衍生化NOM的质谱信号;同时考察了反应时间对NOM与羟胺反应活性的影响. 结果表明,NOM与过量15N同位素标记羟胺在室温条件反应下10 h,可以鉴定到2137个15N同位素标记羟胺衍生化NOM产物分子式,其中包括,1个15N同位素标记羟胺分子与1个仅含C、H、O元素的NOM分子反应形成的产物分子式1346个(CHO15N1,约占产物分子式总数63%),两个15N同位素标记羟胺分子与一个仅含C、H、O元素的NOM分子反应形成的产物分子式194个(CHO15N2,约占产物分子式总数9%),一个15N同位素标记羟胺分子与一个仅含C、H、O和一个S元素的NOM分子反应形成的产物分子式376个(CHOS115N1,约占产物分子式总数18%),一个15N同位素标记羟胺分子与一个仅含C、H、O和一个N元素的NOM分子反应形成的产物分子式221个(CHON115N1,约占产物分子式总数10%). 这些羟胺衍生化NOM产物主要属于维管植物源多酚类和高不饱和酚类化合物,86%以上的产物很可能通过羟胺与NOM分子所含的羰基基团发生肟化反应形成. CHO15N1类产物的形成具有高度的时间依赖性,高氧化高饱和度的NOM化合物需要较长的反应时间才能形成羟胺衍生化NOM产物,表明此类化合物与羟胺的反应活性较弱;而低氧化高不饱和度的NOM化合物仅需要较短的时间便能形成羟胺衍生化产物,且随着反应时间延长,其中的部分化合物可以进一步生成多肟化产物或不稳定的其他产物,此结果表明这类NOM化合物对羟胺的反应活性较强. 本研究采用FTICR-MS首次在分子水平全面表征了羟胺与FTICR-MS相互作用产物信息,可为羟胺在地球氮循环过程中的迁移转化机制研究提供全新视角和信息.
-
关键词:
- 15N同位素标记羟胺 /
- 傅里叶变换离子回旋共振质谱 /
- 天然有机质 /
- 羟胺衍生化天然有机质.
Abstract: Hydroxylamine is a crucial intermediate in the natural nitrogen cycle. Natural organic matter (NOM), as a very complicated heterogeneous mixture widely existing in the environment, may affect the transformation processes of hydroxylamine. Here, Fourier transform ion cyclotron resonance mass spectrometry (FTICR-MS) is used for molecular characterization of hydroxylamine derivatized-NOM. 15N-labelled hydroxylamine is applied to specifically discriminate 15N-labelled hydroxylamine derivatized-NOM from other interferences original existing in NOM. The effect of reaction time on the reactivity of NOM to hydroxylamine is investigated. A total of 2137 molecular formulas of the derivatives are obtained including CHO15N1 (1346, 63%), CHO15N2 (194, 9%), CHOS115N1 (376, 18%) and CHON115N1 (221, 10%). The 15N-labelled hydroxylamine derivatized-NOM are mainly assigned to vascular plant-derived polyphenols and highly unsaturated and phenolic compounds. More than 86% of the derivatives appear to be formed by oximation reaction. The generation of CHO15N1 derivatives is highly time dependent. More oxidized and saturated NOM CHO compounds need long time to form the hydroxylamine derivatives, indicating they appear to be less liable to react with hydroxylamine, while less oxidized and more unsaturated NOM CHO compounds could form the hydroxylamine derivatives in short time, and some of them even could generate multi-oximed products or other unstable products within longer time, which suggests they may have high reactivities to hydroxylamine. This is the first report on the comprehensively molecular characterization of the hydroxylamine derivatized-NOM by FTICR-MS and can provide new insight into the research on migration and transformation mechanism of hydroxylamine in the nitrogen cycle. -

-
图 1 NOM样品FTICR-MS质谱图
Figure 1. Mass spectra of NOM at nominal m/z 389 (A) and m/z 460 (C); Mass spectra of NOM reacting with -labelled hydroxylamine in 10 h, at nominal m/z 389 (B), at nominal m/z 460(D). Molecular formulas of the detected 15N- labelled-hydroxylamine derivatized-NOM components were listed under the spectra
图 2 NOM与15N标记羟胺反应前(A)后(B)鉴定到的分子式数目和占比图;NOM样品(C)和NOM与15N标记羟胺反应10 h后(D)鉴定分子式的Van Krevelen图
Figure 2. Number and percentage of molecular formulas identified in NOM (A) and NOM reacting with 15N-labelled hydroxylamine in 10 h (B); Van Krevelen diagrams of the identified formulas in NOM (C) and NOM reacting with 15N- labelled hydroxylamine in 10 h (D)
图 3 (A)基于肟化反应的母子-分子对个数和百分比图;(B)NOM中CHO分子以及与羟胺反应10 h形成CHO15N1 和 CHO15N2产物的CHO分子的VK图,(C)NOM中CHON1分子以及与羟胺反应10 h形成CHON115N1产物的CHON1分子的VK图,(D)NOM中CHOS1分子以及与羟胺反应10 h形成CHOS115N1产物的CHOS1分子的VK图
Figure 3. (A) Number and percentage of the precursor-product molecule pairs based on oximation reaction; (B) Van Krevelen diagrams of CHO formulas in NOM, precursors of CHO15N1 and CHO15N2 formulas obtained in NOM reacting with 15N-labelled hydroxylamine in 10 h; (C) Van Krevelen diagrams of CHON1 formulas in NOM and precursors of CHON115N1 formulas obtained in NOM reacting with 15N- labelled hydroxylamine in 10 h; (D) Van Krevelen diagrams of CHOS1 formulas in NOM and precursors of CHOS115N1 formulas obtained in NOM reacting with 15N- labelled hydroxylamine in 10 h
图 4 15N同位素标记羟胺与NOM反应0, 2, 4, 8 和 10 h后的FTICR-MS质谱图(A)m/z 411.142检测的质谱峰,(B)m/z 499.064检测的质谱峰, (C)m/z 469.127检测的质谱峰, (D) m/z 395.134检测的质谱峰, (E) m/z 483.056检测的质谱峰, (F) m/z 453.119检测的质谱峰
Figure 4. Mass spectra of NOM reacting with 15N-labelled hydroxylamine in 0, 2, 4, 8 and 10 h (A) the detected peak at m/z 411.142, (B) at m/z 499.064, (C) at m/z 469.127, (D) at m/z 395.134, (E) at m/z 483.056, (F) at m/z 453.119
图 7 (A)15N同位素标记羟胺与NOM反应2、10 h后的FTICR-MS质谱图m/z 469.127和m/z 485.134;(B)形成双肟化产物或者其他产物的RI值小于等于0.8的共同鉴定CHO15N1产物的VK图
Figure 7. (A) Mass spectra of NOM reacting with 15N-labelled hydroxylamine in 2 and 10 h at m/z 469.127 and m/z 485.134; (B) Van Krevelen diagrams of formulas with RI lower than 0.8 formed dioxime products and other products
-
[1] TANAKA M. Occurrence of hydroxylamine in lake waters as an intermediate in bacterial reduction of nitrate [J]. Nature, 1953, 171(4365): 1160-1161. [2] WHITE C J, LEHNERT N. Is there a pathway for N2O production from hydroxylamine oxidoreductase in ammonia-oxidizing bacteria? [J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(51): 14474-14476. doi: 10.1073/pnas.1617953114 [3] VAJRALA N, MARTENS-HABBENA W, SAYAVEDRA-SOTO L A, et al. Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine Archaea [J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(3): 1006-1011. doi: 10.1073/pnas.1214272110 [4] CARANTO J D, LANCASTER K M. Nitric oxide is an obligate bacterial nitrification intermediate produced by hydroxylamine oxidoreductase [J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(31): 8217-8222. doi: 10.1073/pnas.1704504114 [5] STEIN L Y. Insights into the physiology of ammonia-oxidizing microorganisms [J]. Current Opinion in Chemical Biology, 2019, 49: 9-15. doi: 10.1016/j.cbpa.2018.09.003 [6] PETHICA B A, ROBERTS E R, WINTER E R S. Role of hydroxylamine in biological fixation of nitrogen [J]. Nature, 1949, 163(4141): 408. [7] VERSTRAETE W, ALEXANDER M. Heterotrophic nitrification in samples of natural ecosystems[J]. Environmental Science & Technology. 1973, 7: 39-42. [8] SUTTON R, SPOSITO G. Molecular structure in soil humic substances: The new view [J]. Environmental Science & Technology, 2005, 39(23): 9009-9015. [9] PICCOLO A. The supramolecular structure of humic substances [J]. Soil Science, 2001, 166(11): 810-832. doi: 10.1097/00010694-200111000-00007 [10] THORN K A, PETTIGREW P J, GOLDENBERG W S, et al. Covalent binding of aniline to humic substances. 2. 15N NMR studies of nucleophilic addition reactions [J]. Environmental Science & Technology, 1996, 30(9): 2764-2775. [11] THORN K A, MIKITA M A. Ammonia fixation by humic substances: A nitrogen-15 and carbon-13NMR study [J]. Science of the Total Environment, 1992, 113(1/2): 67-87. [12] ALBERS C N, BANTA G T, HANSEN P E, et al. Effect of different humic substances on the fate of diuron and its main metabolite 3, 4-dichloroaniline in soil [J]. Environmental Science & Technology, 2008, 42(23): 8687-8691. [13] PEARSON D E, KEATON O D. Lethargic reactions. I. the preparation of hindered oximes [J]. The Journal of Organic Chemistry, 1963, 28(6): 1557-1558. doi: 10.1021/jo01041a028 [14] NELSON, D W. Transformations of hydroxylamine in soils[J]. Proceedings of the Indian Acade of Science. 1977, 87: 409-413. [15] SCHNITZER M, SKINNER S I M. A polarographic method for the determination of carbonyl groups in soil humic compounds [J]. Soil Science, 1966, 101(2): 120-124. doi: 10.1097/00010694-196602000-00008 [16] THORN K A, ARTERBURN J B, MIKITA M A. Nitrogen-15 and carbon-13 NMR investigation of hydroxylamine-derivatized humic substances [J]. Environmental Science & Technology, 1992, 26(1): 107-116. [17] STENSON A C, MARSHALL A G, COOPER W T. Exact masses and chemical formulas of individual Suwannee River fulvic acids from ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectra [J]. Analytical Chemistry, 2003, 75(6): 1275-1284. doi: 10.1021/ac026106p [18] LAVONEN E E, GONSIOR M, TRANVIK L J, et al. Selective chlorination of natural organic matter: Identification of previously unknown disinfection byproducts [J]. Environmental Science & Technology, 2013, 47(5): 2264-2271. [19] KIM S, KRAMER R W, HATCHER P G. Graphical method for analysis of ultrahigh-resolution broadband mass spectra of natural organic matter, the van Krevelen diagram [J]. Analytical Chemistry, 2003, 75(20): 5336-5344. doi: 10.1021/ac034415p [20] HUGHEY C A, HENDRICKSON C L, RODGERS R P, et al. Kendrick mass defect spectrum: A compact visual analysis for ultrahigh-resolution broadband mass spectra [J]. Analytical Chemistry, 2001, 73(19): 4676-4681. doi: 10.1021/ac010560w [21] HAN R X, LV J T, LUO L, et al. Molecular-scale investigation of soil fulvic acid and water-extractable organic matter by high-resolution mass spectrometry and 1H NMR spectroscopy [J]. Environmental Chemistry, 2019, 16(2): 92. doi: 10.1071/EN18124 [22] KOCH B P, DITTMAR T, WITT M, et al. Fundamentals of molecular formula assignment to ultrahigh resolution mass data of natural organic matter [J]. Analytical Chemistry, 2007, 79(4): 1758-1763. doi: 10.1021/ac061949s [23] KUJAWINSKI E B, LONGNECKER K, BLOUGH N V, et al. Identification of possible source markers in marine dissolved organic matter using ultrahigh resolution mass spectrometry [J]. Geochimica et Cosmochimica Acta, 2009, 73(15): 4384-4399. doi: 10.1016/j.gca.2009.04.033 [24] GONSIOR M, PEAKE B M, COOPER W T, et al. Photochemically induced changes in dissolved organic matter identified by ultrahigh resolution Fourier transform ion cyclotron resonance mass spectrometry [J]. Environmental Science & Technology, 2009, 43(3): 698-703. [25] KOCH B P, DITTMAR T. From mass to structure: An aromaticity index for high-resolution mass data of natural organic matter [J]. Rapid Communications in Mass Spectrometry, 2006, 20(5): 926-932. doi: 10.1002/rcm.2386 [26] KELLERMAN A M, DITTMAR T, KOTHAWALA D N, et al. Chemodiversity of dissolved organic matter in lakes driven by climate and hydrology [J]. Nature Communications, 2014, 5: 3804. doi: 10.1038/ncomms4804 [27] BALUHA D R, BLOUGH N V, del VECCHIO R. Selective mass labeling for linking the optical properties of chromophoric dissolved organic matter to structure and composition via ultrahigh resolution electrospray ionization mass spectrometry [J]. Environmental Science & Technology, 2013, 47(17): 9891-9897. [28] STENSON A C. Reversed-phase chromatography fractionation tailored to mass spectral characterization of humic substances [J]. Environmental Science & Technology, 2008, 42(6): 2060-2065. -




 下载:
下载: