-
饮用水消毒始于20世纪初[1],它能有效杀灭水中的微生物病原体, 大大降低了人们因饮水而感染痢疾、霍乱等水传播疾病而致死的几率, 是人类公共卫生领域的一次重大突破. 然而,在饮用水消毒工艺中,氯、氯胺等化学消毒剂可与水源水中的天然有机物(NOM)反应生成消毒副产物(DBPs)[2-4]. 毒理学和流行病学研究已经证明,饮用水DBPs具有细胞毒性和遗传毒性,并且与癌症、流产和出生缺陷等风险呈正相关[5-8]. 尽管研究人员已在饮用水中发现700多种DBPs[9],但已知DBPs均为分子量小于800 Da的低分子量DBPs,仍有超过50%的DBPs处于未知状态[10-11]. Kopfler等[12]通过超滤实验证明了高分子量DBPs的存在,且沙门氏菌突变试验表明DBPs中高分子量组分有显著的致突变作用,从而证实了高分子量DBPs的生物毒性. Zhang等[13-14]研究发现,高分子量氯代DBPs的平均分子量约为2000 Da且分子量分布高度分散. 由此可见,高分子量DBPs是DBPs的重要组成部分,亟需对其进行进一步探索.
目前对饮用水DBPs的检测识别主要集中于小分子DBPs,而对单个高分子量DBPs的检测与识别还非常缺乏. 气相色谱/质谱法(GC/MS)是目前对于DBPs常用的检测方法,但是GC/MS不适合鉴定极性/亲水/非挥发性衍生物,尤其不适合鉴定高分子量化合物[15]. 而液相色谱/质谱法(LC/MS)对无机盐耐受程度低[16],并且电喷雾电离等电离方式会产生多电荷离子[17],这又会给未知DBPs的识别带来很大挑战. 因此,研究高分子量DBPs的关键是选择合适的检测方法. 基质辅助激光解吸电离飞行时间质谱(MALDI-TOF MS)是测定高分子量物质的摩尔质量和摩尔质量分布的最有用的分析技术之一[18-20],并因其速度快,使用方便,灵敏度高,杂质耐受度较高,已被广泛应用在微生物、蛋白质、聚合物等大分子物质的检测领域[21-25]. MALDI是一种比电喷雾电离(ESI)更温和的电离技术,几乎只产生单电荷分子离子,能电离极性分子和非极性分子. MALDI-TOF MS在分析中能够保持高分子量化合物的完整[26],同时具有高分辨率质谱的优势,具有较高的质量精度和分辨率[24]. 因此MALDI-TOF MS对高分子量DBPs样品的识别具有巨大潜力.
MALDI-TOF MS的检测条件(如基质、阳离子化试剂、沉积方法、激光能量、检测模式等)应根据目标样品进行选择[27-28]. 尽管目前文献中已有一些样品制备和检测方法,但是由于高分子量DBPs的结构未知,且分子量分散、结构复杂,如何针对其性质找到最佳的样品制备方案和仪器条件仍然是一个挑战. 因此,筛选合适的基质、阳离子化试剂、仪器参数和点靶方法等,是基于MALDI-TOF MS的高分子量DBPs检测方法的关键. 在没有大量精确数据的情况下,识别未知物质是一项非常具有挑战性的工作. 仅使用低分辨率质谱,未知DBPs可能有许多分子式(分子量在800 Da以上的化合物则会有更多可能的分子式),且每个分子式都有多种可能结构. 高分辨率质谱可以提供精确的质荷比(通常是小数点后3位或4位),这大大缩小了分子式的选择范围[29]. MALDI-TOF MS作为一种高分辨率质谱(HRMS),凭借超高分辨率可以明确地分配每种物质的元素组成. MALDI-TOF MS还可以提供TOF/TOF串联质谱,分析碎片离子的组成,从而得到分子的结构信息. 因此MALDI-TOF MS为高分子量DBPs分子式和结构式的识别提供了可能.
本研究率先在高分子量DBPs的检测中引入了MALDI-TOF MS,建立了未知高分子量DBPs的检测方法,从检测模式、基质、激光强度、阳离子化试剂、点靶方法等进行优化,提高了未知高分子量DBPs的响应信噪比(S/N),改善了样品间测量的重现性;在检测基础上,通过高分辨质谱数据、同位素模式分析、TOF/TOF串联质谱提供的结构信息以及SciFinder数据库,建立了高分子量DBPs的分子式/结构式识别方法.
-
实验仪器:美国Bruker Daltonics公司UltrafleXtreme 基质辅助激光解析飞行时间质谱仪;美国FEI公司Quanta FEG 250 Environmental扫描电子显微镜; 美国Labacoco公司CentriVap离心真空浓缩机;美国Thermo Fisher Scientific公司Barnstead Micropure超纯水机.
实验所用试剂纯度均为质谱纯、色谱纯或优级纯. 超级2,5-二羟基苯甲酸(Super-DHB ,2,5-二羟基苯甲酸和 2-羟基-5-甲氧基苯甲酸的9:1混合物)、α-氰基-4-羟基肉桂酸(CHCA)、地蒽酚(DIT)、2,4,6-三羟基苯乙酮(THAP)、芥子酸(SA)、2-(4-羟基苯基偶氮)-苯甲酸(HABA)、3-吲哚丙烯酸(IAA)、可溶性淀粉购自Sigma-aldrich公司. 反-2-[3-(4-叔丁基苯基)-2-甲基-2-亚丙烯基]丙二腈(DCTB)、三氟乙酸钠(NaTFA)、氯化钾(KCl)购自Tokyo Chemical Industry公司. 三氟乙酸银(AgTFA)购自J&K Scientific公司. 次氯酸钠(NaClO)储备溶液购自Sigma-aldrich公司,并通过DPD亚铁滴定法进行标准化[30]. 本研究中使用的其他所有化学品均从Sigma-Aldrich公司购买.
-
为简化实验过程,本研究选择淀粉作为模型天然高分子量有机物,在实验室条件下进行消毒. 淀粉是分子结构为(C6H10O5)nŸH2O的聚合碳水化合物. 淀粉分子式为(C6H10O5)nŸH2O,广泛存在于藻类细胞、细胞外有机物中,是自然界中广泛存在的一种天然有机物[31-33]. 淀粉作为常用消毒场景下的模型化合物[31, 34-35],其组成简单,结构已知,已被广泛应用在MALDI-TOF MS检测中,因此是理想的高分子量DBPs的前体物.
为验证消毒样品中高分子量DBPs的产生,本研究中配制了以下模拟饮用水水源水水样:向1 L超纯水中加入可溶性淀粉(5 mg·L−1以C计)、NaHCO3(90 mg·L−1以CaCO3计)制备模拟原水样品. 然后,向模拟原水样品加入2.0 mg·L−1 NaClO(以Cl2计)进行氯化,并在20 ℃避光条件下无顶空接触24 h. 通过 DPD 亚铁滴定法测量每个模拟饮用水样品中的氯残留量,并立即用硫代硫酸钠终止反应. 将未消毒的模拟水样作为对照组,超纯水作为空白组. 3个实验组分别设置3个平行样品.
-
样品预处理对于解决污染问题以及使用MALDI-TOF MS来分析复杂的样品混合物很有帮助[36-37]. 首先利用离心真空浓缩机将1 L水样浓缩10 mL,随后将水样放入经过预处理的透析袋(美国MYM公司透析袋;截留分子量为500 Da)中脱盐. 水样脱盐程序基于先前的研究[38]. 脱盐完成后,使用离心真空浓缩机对水样进行再次浓缩至0.5 mL,得到浓缩水样.
-
所有质谱均使用配备355 nm Nd:YAG的Bruker Daltonics公司UltrafleXtreme MALDI-TOF MS质谱仪获得. 分子量检测范围设置为500—5000 Da,以排除由低质量离子产生的高强度信号,并覆盖模型化合物整个质量分布范围,研究质量范围为 800—5000 Da. 每个质谱是通过在样品的均匀区域上随机获得的,每个质谱总共有2000次激光射击. 使用flexAnalysis Version 3.4质谱软件处理质谱数据.
-
将DHB、THAP、CHCA、DIT、SA、DCTB、HABA和IAA分别称重并溶解在TA30(H2O:乙腈=70:30,V/V,含0.1% TFA)中,制成20 mg·mL−1溶液. 将阳离子化试剂称重并溶解在TA30中以制备5 mg·mL−1溶液. 以浓缩水样/基质/阳离子化剂为5/10/1(V/V/V)的混合比制备每一个质谱样品. 然后将3 μL样品沉积在MALDI靶板上,并在环境条件下干燥,分别进行反射-正离子、反射-负离子、线性-正离子、线性-负离子4种检测模式的不同激光强度下的检测. 确定基质种类、检测模式以及激光强度后,则进一步对基质浓度进行优化. 将不同浓度的基质溶液(浓度设置为0、5、10、20、30、40、50 mg·mL−1),与浓缩样品、5 mg·mL−1阳离子化试剂混合(负离子模式下不添加阳离子化试剂). 进行上述实验时,使用干点法[39]点靶,阳离子化试剂为5 mg·mL−1 NaTFA,检测器m/z范围设置为500—5000 Da.
-
阳离子化试剂的作用则是通过阳离子加合物提高分析物的灵敏度和响应强度[18]. 根据1.4.1节确定的基质、激光能量和检测模式,使用干点法将浓缩样品/基质/阳离子化剂按照5/10/1(V/V/V)分别与NaTFA、KCl和AgTFA混合. 确定最佳阳离子化试剂种类后,将最佳阳离子化试剂配制为不同浓度的溶液(浓度设置为0、0.5、1、2、5、10、20、50 mg·mL−1),与浓缩样品、20 mg·mL−1基质溶液混合,干点法制备质谱样品.
-
确定基质、激光能量、检测模式和阳离子化试剂后,本研究使用4种不同的沉积方法,同时确保等量的分析物和基质进行MALDI-TOF MS分析:(1)干点法(混合法),将浓缩水样和基质首先以体积比1:2混合,然后将3 μL所得样品点样到靶板上,自然风干;(2)薄层法(底层法),将2 μL基质施加到靶板上,干燥后将1 μL浓缩水样滴加在基质上并使其干燥;(3)种子层法,将1 μL基质施加到靶板上,自然风干后,将浓缩水样和基质等体积混合的2 μL样品点样到靶板上并让样品干燥;(4)三明治法(夹层法),将1 μL基质点样到靶板上,干燥后,将1 μL浓缩水样滴加到基质层上并使其干燥,最后将1 μL基质点样到样品上.
-
Quanta FEG 250 Environmental SEM用于评估不同点靶方式干燥后样品的表面分布. SEM图像是在高真空(130 Pa)下以200倍放大倍数获得的,电子束以10 kV电压和238 μA电流运行.
-
只在消毒水样(n=3)中重复出现、未消毒和空白水样中不存在的质谱出峰视为DBPs. 因此,仅根据存在和不存在而不是出峰的高度差异,来确定形成的DBPs. 根据高分子量DBPs的同位素模式,判断结构中是否存在卤素(即Cl),从而对其进行进一步识别. 对于质谱数据的处理,使用美国Bruker Daltonics公司开发的质谱分析软件flexAnalysis Version 3.4将特定的分子式分配给质谱中高分子量DBPs的分子离子. 分子式计算需要满足“氮原则”,元素个数约束需要根据分子量来确定,质量误差设为±10×10−6. 同时需要舍弃不切实际的C、H、O比例的分子式,只考虑C、H、O>0、0≤O/C≤1、0≤H/C≤2.5、双键当量(DBE)≥0的分子式[40]. 软件计算进行分子式赋值后,随后根据文献报道的可能反应产物以及SciFinder数据库进行合理性验证.
-
建立高质量和可靠的MALDI-TOF MS检测方法需要考虑许多因素,其中主要影响因素有基质(种类和浓度)、阳离子化试剂(种类和浓度)、激光能量、检测模式(线性/反射模式,正离子/负离子模式)以及点靶方式等[41]. 本研究比较了两个参数来评估高分子量DBPs的出峰效果:信噪比和变异系数(CV)[42]. 信噪比反映了质谱出峰信号的强弱,CV值则反映了结果的重现性的好坏. 两个参数都是根据消毒水样中出现的新峰信噪比之和计算的.
-
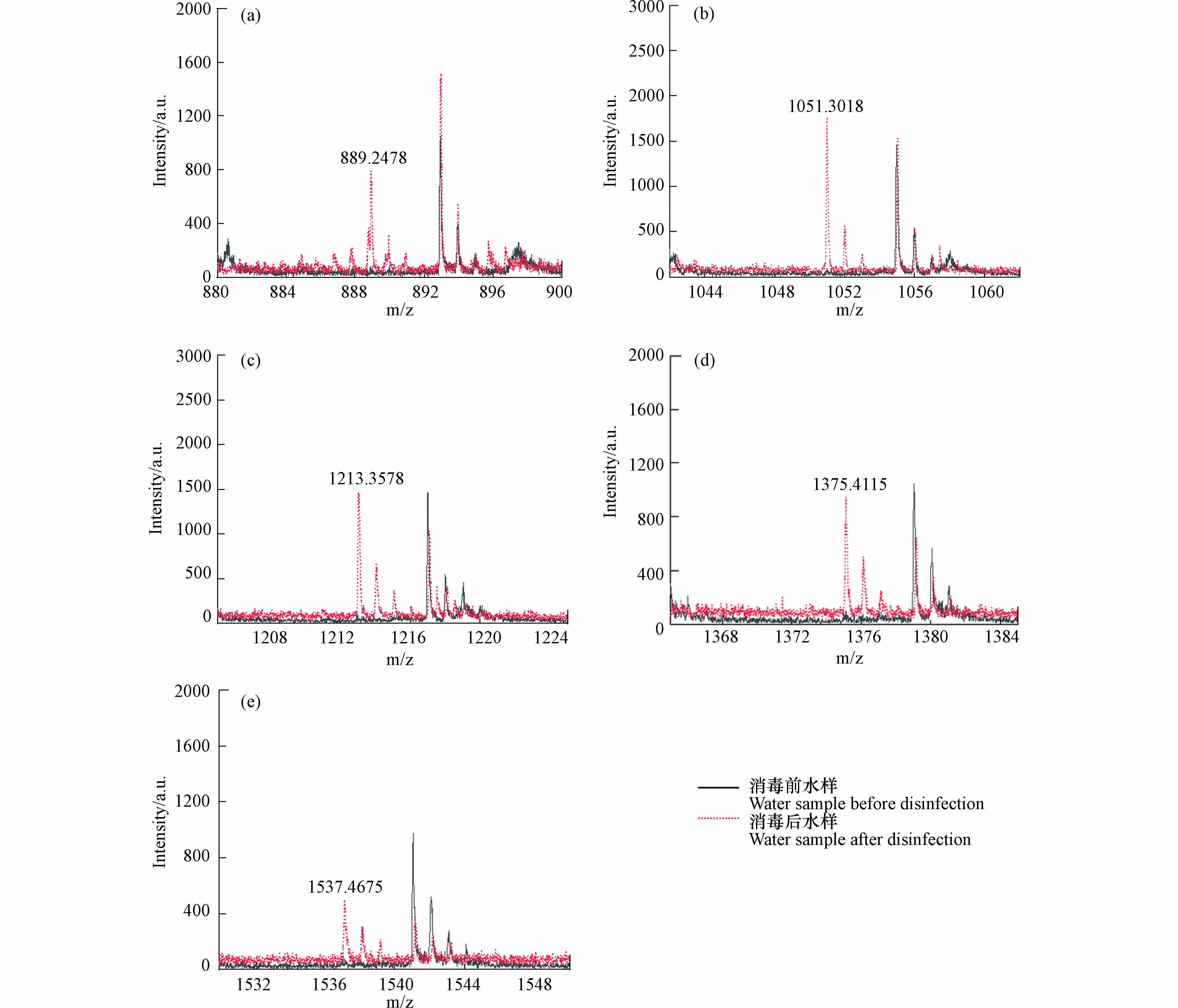
基质在MALDI分析中非常重要,因为它既是激光能量吸收剂,也是能量传输的工具. 此外,理想的基质应具有以下特性:在所用波长处具有高电子吸收、良好的真空稳定性、低蒸气压以及与分析物的良好互溶性,从而导致均匀的共结晶[18]. CHCA、DHB和THAP基质已被用于模型化合物淀粉的分析[43-45]. SA、DCTB、DIT、HABA和 IAA作为常用基质也被纳入基质筛选的范畴. NaTFA被用作基质比选阶段的阳离子化试剂. 表1显示了8种基质的检测新的DBPs的结果. 通过对消毒前后水样的逐一比对发现,以SA、DCTB、DIT、HABA和 IAA为基质未检测到新峰,表明这些基质不适合淀粉的高分子量DBPs分析,而DHB、CHCA和THAP均在反射-正离子模式下检测到了高分子量DBPs. 以DHB为基质,检测模式为反射-正离子模式时,高分子量DBPs出峰信噪比远高于THAP和CHCA,并且CV值更小,结果重现性更好,因此以DHB为基质,检测模式为反射-正离子模式时,高分子量DBPs出峰效果最好. 图1为以DHB为基质,检测模式为反射-正离子模式,激光强度设置为90%时,模拟水样消毒前后的全局对比图. 图2为m/z 889.2478、1051.3018、1213.3578、1375.4115和1537.4675的5种高分子量DBPs出峰情况.
高分子量DBPs在负离子模式下无法出峰可能是由于高分子量DBPs去质子化([M-H]-)的能力较弱. 此外,DHB作为基质时,高分子量DBPs信噪比远高于其他基质. 此外,通过将信噪比的标准偏差除以平均信噪比来计算它们的CV值,且使用DHB基质时,DBPs信噪比的CV值更小说明共结晶的均匀性更好,结果重现性更好. 然而这些峰的信噪比不高,信号重现性较差. 因此针对高分子量DBPs的MALDI-TOF MS检测方法亟待建立.
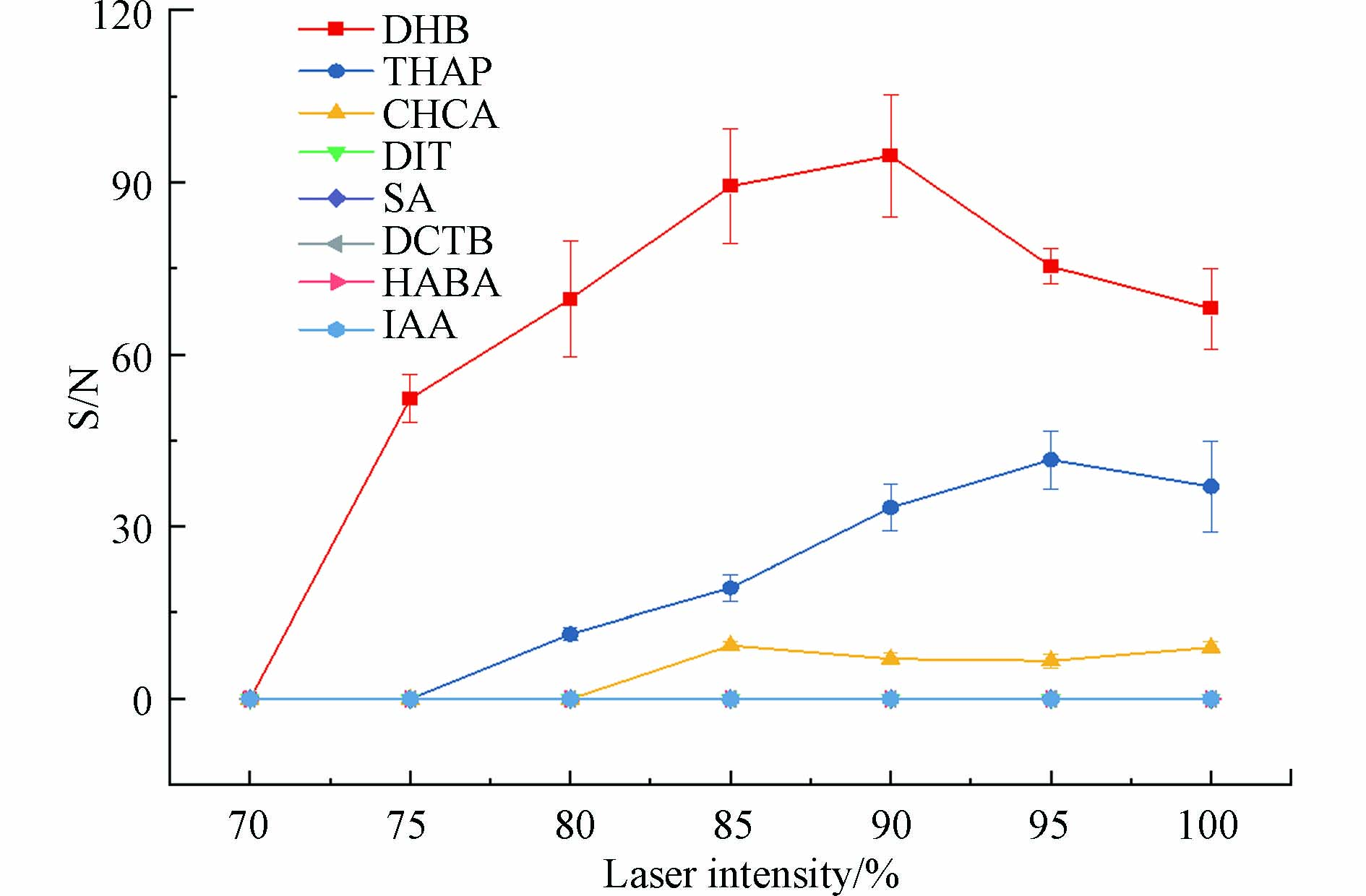
激光能量是决定解吸和阳离子化样品数量的一个关键因素,激光能量较低时,响应信噪比会显著降低或者无信号,过高的能量则可能会破坏样品,从而降低信噪比,而激光能量对信噪比影响的差异可归因于基质性质的差异[46-47]. 因此,本研究在各个检测模式下都进行了激光能量优化,只有在反射-正离子模式下可以检测到高分子量DBPs. 各基质在反射-正离子检测模式下,高分子量DBPs在不同激光能量下的信噪比如图3所示. 结果显示DHB作为基质,在各激光强度下,高分子量DBPs的信噪比均高于其他基质. DHB作为基质的情况下,在激光强度为90%时信噪比达到最高. 其原因可能是在90%激光强度以下时,激光能量不足,随着激光能量增加,电离水平提高,因而产生更高的信噪比峰[41],超过90%后,激光能量过高,在离子飞行过程中分子离子被破坏,增强了背景峰,从而导致信噪比降低.
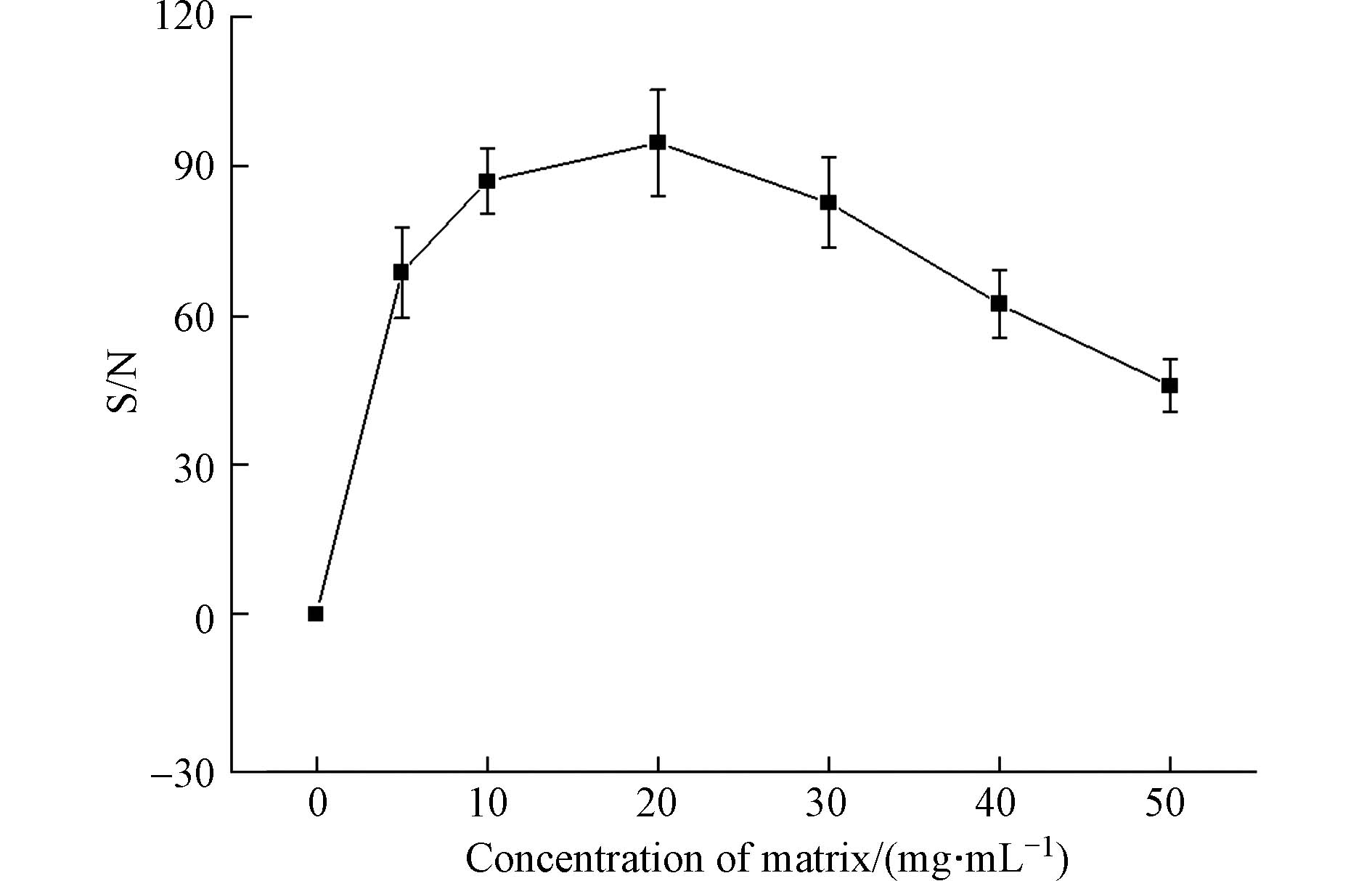
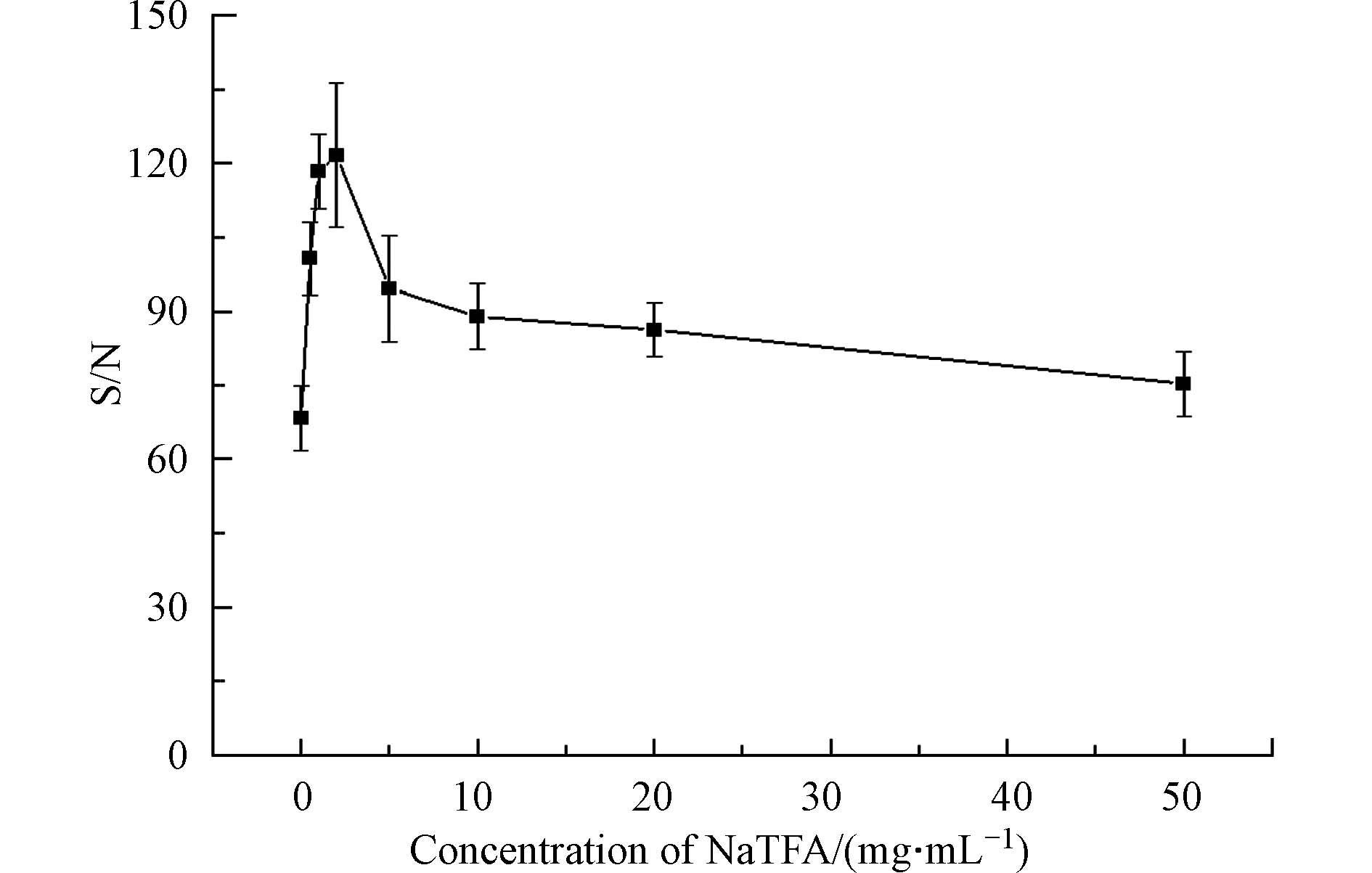
基质浓度直接影响基质与样品混合比例,因而可能会对分析物的响应产生影响[27, 41, 48]. 因此需要确定检测高分子量DBPs的DHB基质的最佳浓度. 检测模式为反射-正离子模式,激光强度为90%,阳离子化试剂为5 mg·mL−1 NaTFA,点靶方式为干点法. 如图4所示,在DHB浓度从0增加到50 mg·mL−1,高分子量DBPs的信噪比先升高,在DHB浓度为20 mg·mL−1时,达到最高信噪比,然后下降. 结果表明,DHB基质的浓度对高分子量DBPs响应信噪比有明显的影响,因此,选择20 mg·mL−1作为合适的DHB浓度.
-
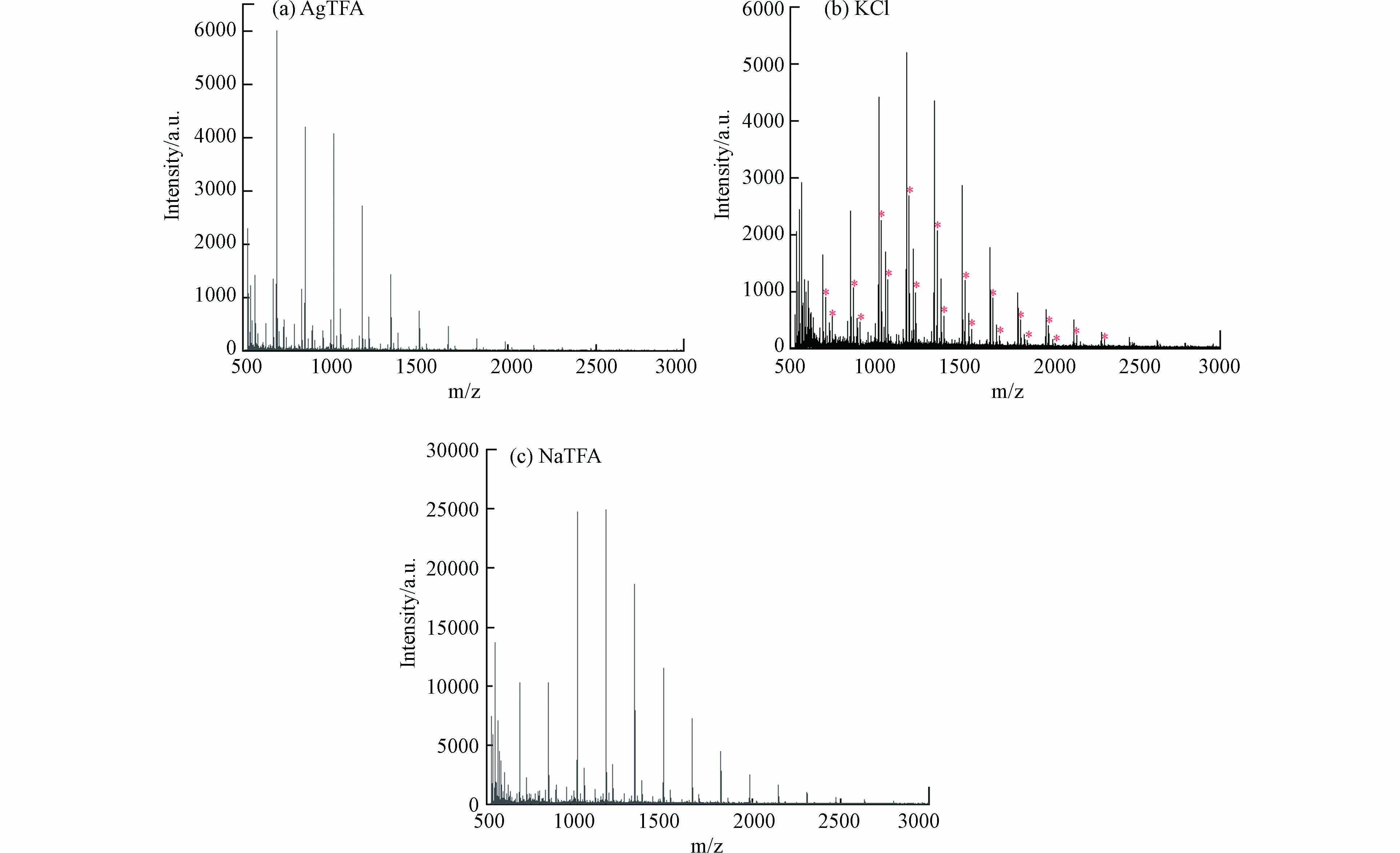
寡糖由于缺乏基本官能团及其高度亲水性,寡糖在包括MALDI-TOF MS在内的质谱分析中电离效率较低,在质谱分析中很难形成质子化离子,通常需要使用碱金属离子来实现的寡糖的离子化,形成碱金属缔合离子[49-51]. 本研究测试了3种阳离子化剂,包括NaTFA、KCl和AgTFA,用于分析消毒水样中产生的高分子量DBPs. 如图5(a)和(c)所示,当添加NaTFA或AgTFA时,高分子量DBPs出现在m/z 889、1051、1213、1375和1537处. 如图5(b)所示,添加KCl时,高分子量DBPs新增了m/z 905、1067、1229、1391、1553系列峰(图中标“*”),在这种情况下,m/z 889、1051、1213、1375和1537系列峰也能在质谱中被明显观察到,并且两个系列峰m/z对应相差约16,这与K和Na相对原子质量之差相同. 这表明m/z 889、1051、1213、1375和1537系列峰为加钠峰,而m/z 905、1067、1229、1391、1553系列峰为加钾峰. 添加NaTFA或添加AgTFA时,高分子量DBPs均以[M+Na]+离子的形式出现,并且添加NaTFA的样品高分子量DBPs响应信噪比更高,说明高分子量DBPs与银离子亲和力很弱,而易与钠离子结合. 而添加KCl时,高分子量DBPs则以[M+Na]+和[M+K]+离子的形式出现,质谱峰更加复杂,不利于质谱分析. 因此,NaTFA是分析高分子量DBPs的最佳阳离子化试剂.
随后,在反射-正离子模式,激光强度为90%,基质为20 mg·mL−1DHB,点靶方式为干点法的条件下,确定了NaTFA的最佳浓度. 如图6所示,NaTFA浓度在0到2 mg·mL−1范围内,响应信噪比迅速增加,然后在超过2 mg·mL−1后降低. 因此,选用了2 mg·mL−1作为NaTFA的最佳浓度. 因为消毒水样中钠离子的存在,所以即使不添加添加剂,仍然有一定强度的加钠峰. 随着盐浓度增加,高分子量DBPs信噪比先增高后降低. 这是因为较低浓度下钠离子对高分子量DBPs离子化有促进作用,而高浓度的钠离子又会使噪声升高,造成信噪比降低.
-
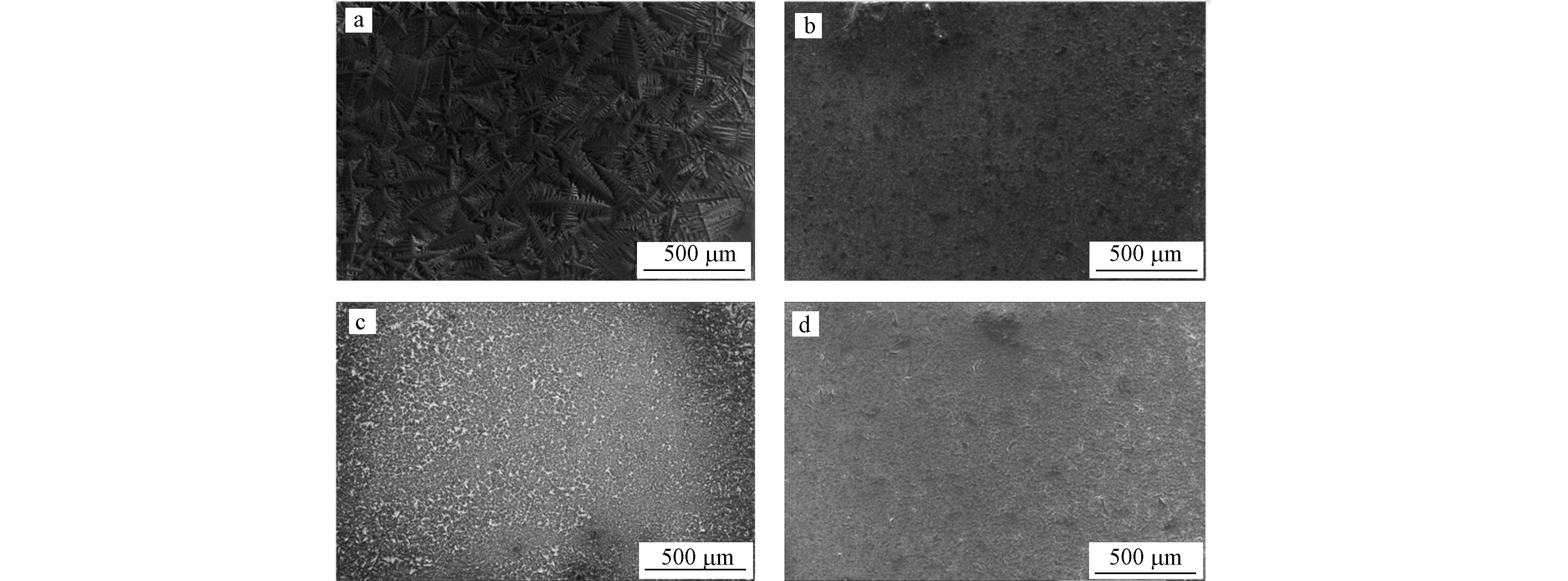
在前面实验确定的最佳实验条件的基础上,评估了干点法、薄层法、种子层法、三明治法等4种沉积方法的响应信噪比和结果重现性. 如图7所示,在200倍的扫描电镜下研究了4种不同沉积方法下,基质与样品的共结晶情况. 在使用干点法的情况下,观察到直径超过200 μm的晶体. 其他3种沉积方法的晶体均明显更小,结晶更加均匀. 在使用三明治法时,晶体的直径<50 μm,至少小了4倍. 使用三明治法的靶板上形成了更薄更均匀的样品和DHB的共结晶层. 由于产生更小的晶体有利于提高信号信噪比和结果重现性,因此三明治法的共结晶效果最好. 薄层法、种子层法更小晶体的形成可能是由于(1)薄层法、种子层法均有两次点靶过程,这使得大晶体可以再溶解和再结晶,这有利于形成更小的结晶;(2)此外,第一层基质干燥后,可以充当第二层样品分子的成核中心,增加了溶剂蒸发的速度,反过来阻止了大晶体的生长,并且这个过程使得样品更好地与基质结合,形成了更均匀分布的晶体层. 而三明治法经历了两次再溶解和再结晶过程,加强了上述两种效果.
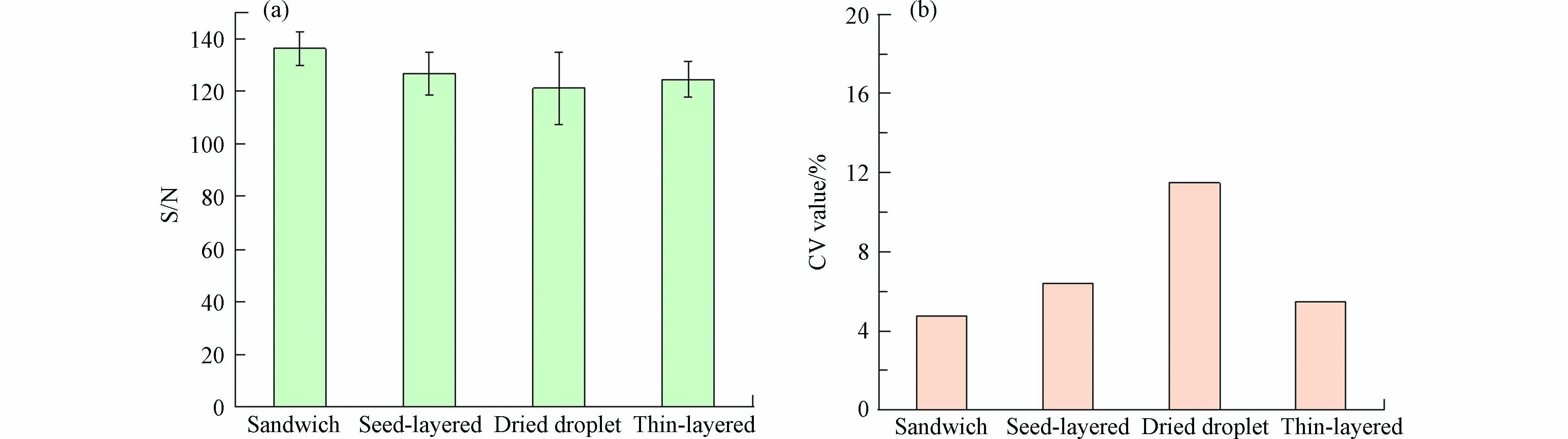
在反射-正离子模式,激光强度为90%,基质为20 mg·mL−1 DHB,阳离子化试剂为2 mg·mL−1 NaTFA条件下,计算了4种沉积方法的信噪比和CV值(n=10). CV值越小,数据越稳定. 如图8所示,在对高分子量DBPs的检测中,观察到4种沉积方法获得的响应信噪比为:三明治法>种子层法>薄层法>干点法,这表明三明治法获得了最好的信号响应,采用三明治法有助于提高检测方法灵敏度和降低检测限;而CV值则为三明治法<薄层法<种子层法<干点法,这说明使用三明治法的样品,分析物和基质共结晶更好,形成最佳的共结晶层,获得了最佳的结果重现性. 因此,对于高分子量DBPs,使用三明治法5种高分子量DBPs信噪比之和达到了136.2,CV值则为4.77%,优于其他点靶方法.
-
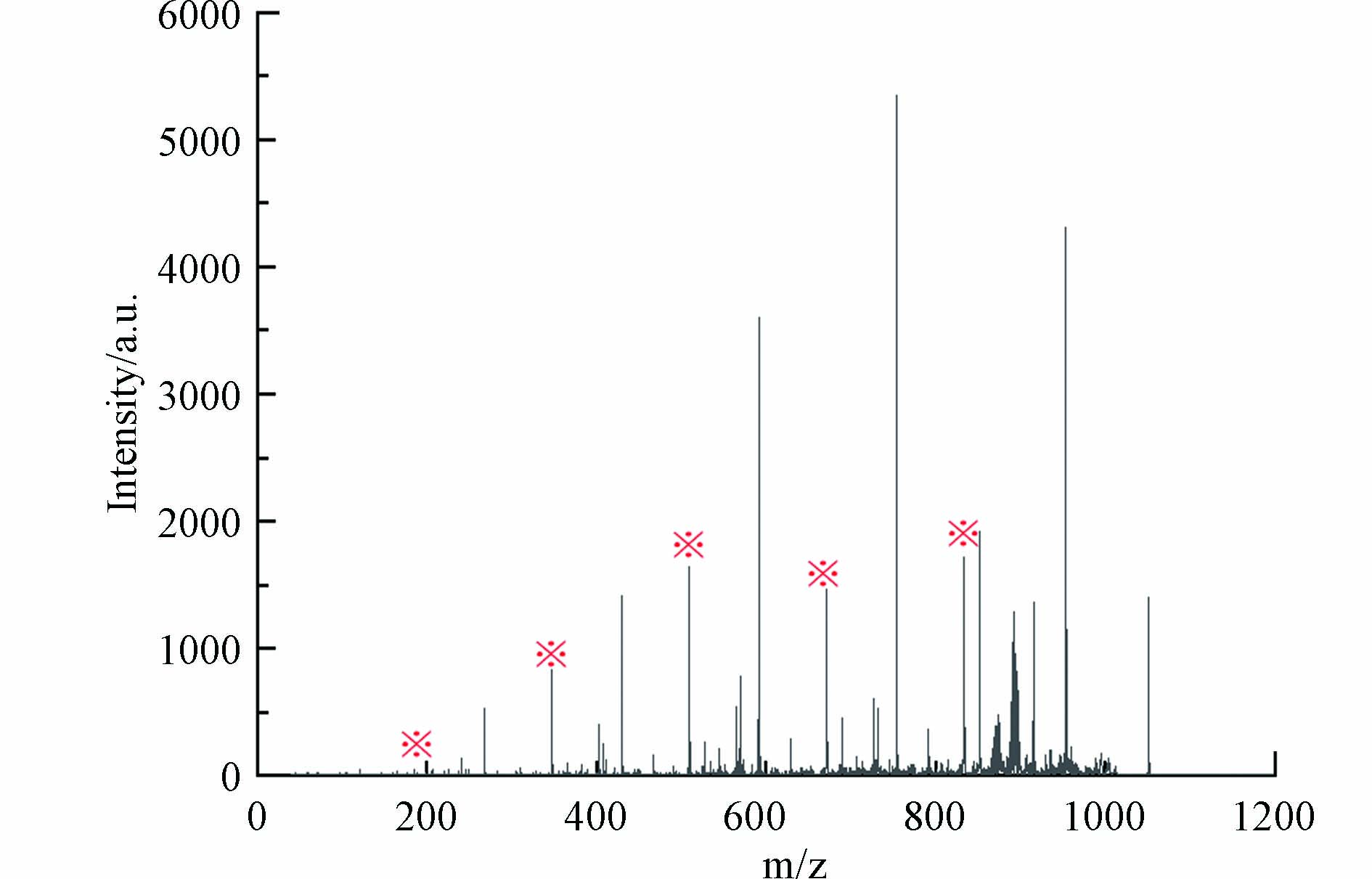
MALDI-TOF MS在消毒样品中发现m/z 889.2478、1051.3018、1213.3578、1375.4115和1537.4675共5种新形成的高分子量DBPs. 识别步骤如下,以m/z 1051处出峰为例.
-
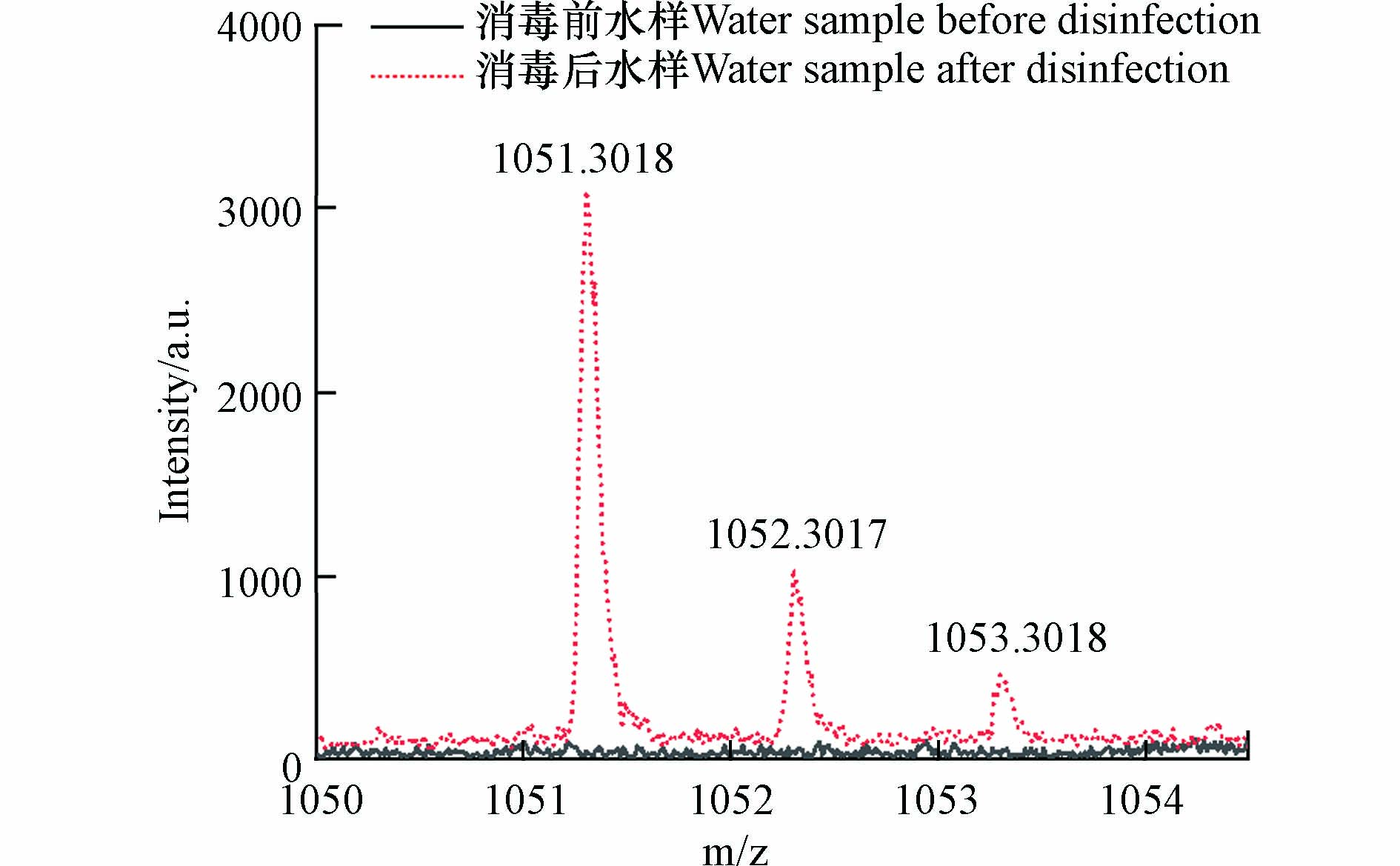
根据高分子量DBPs出峰(图9),其同位素峰与碳同位素出峰情况相符,不符合Cl的同位素出峰情况. 因此可判断消毒过程中未发生卤素取代反应,其分子式中不含Cl. 因此分子式分配的元素范围为 12C: 0—100, 1H: 1—200, 16O: 0—100, 23Na: 0—2.
-
以m/z 1051.3018峰为例,根据其碎片离子质谱图(图10),m/z 1051.3018母离子产生了一系列寡糖碎片离子(以※标记的峰),其中m/z 833碎片离子为五糖结构. 因此m/z 1051分子中存在五糖结构,由此可判断高分子量DBPs双键当量DBE≥5.
-
分子式计算中,设置质量误差为± 10×10−6,设置双键当量(DBE)≥ 5. 若要在地球物理化学环境中存在,分子式的C、H、O比例须满足:C、H、O > 0、0 ≤ O/C ≤ 1、0 ≤ H/C ≤ 2.5[40]. 根据MALDI-TOF MS提供的精确质量1051.3018以及上述筛选条件,得到分子式计算结果如表2所示.
-
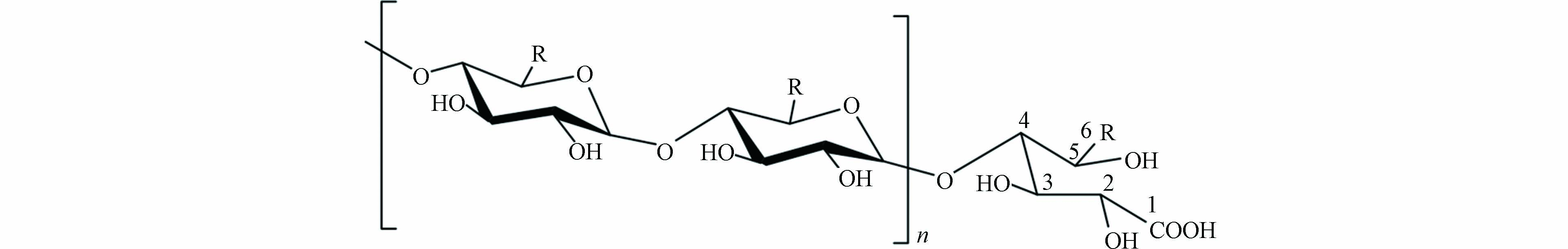
有文献报道,淀粉与次氯酸反应过程中,葡萄糖残基可被氧化为葡萄糖羧酸,高分子量DBPs可能为寡糖羧酸或寡糖羧酸钠[52]. 因此若消毒过程与文献中报道的反应相同,则可认为C36H61O32Na与C36H62O32(C36H61O32Na的酸)是同种物质. 过往文献中报道的可能产物结构见图11.
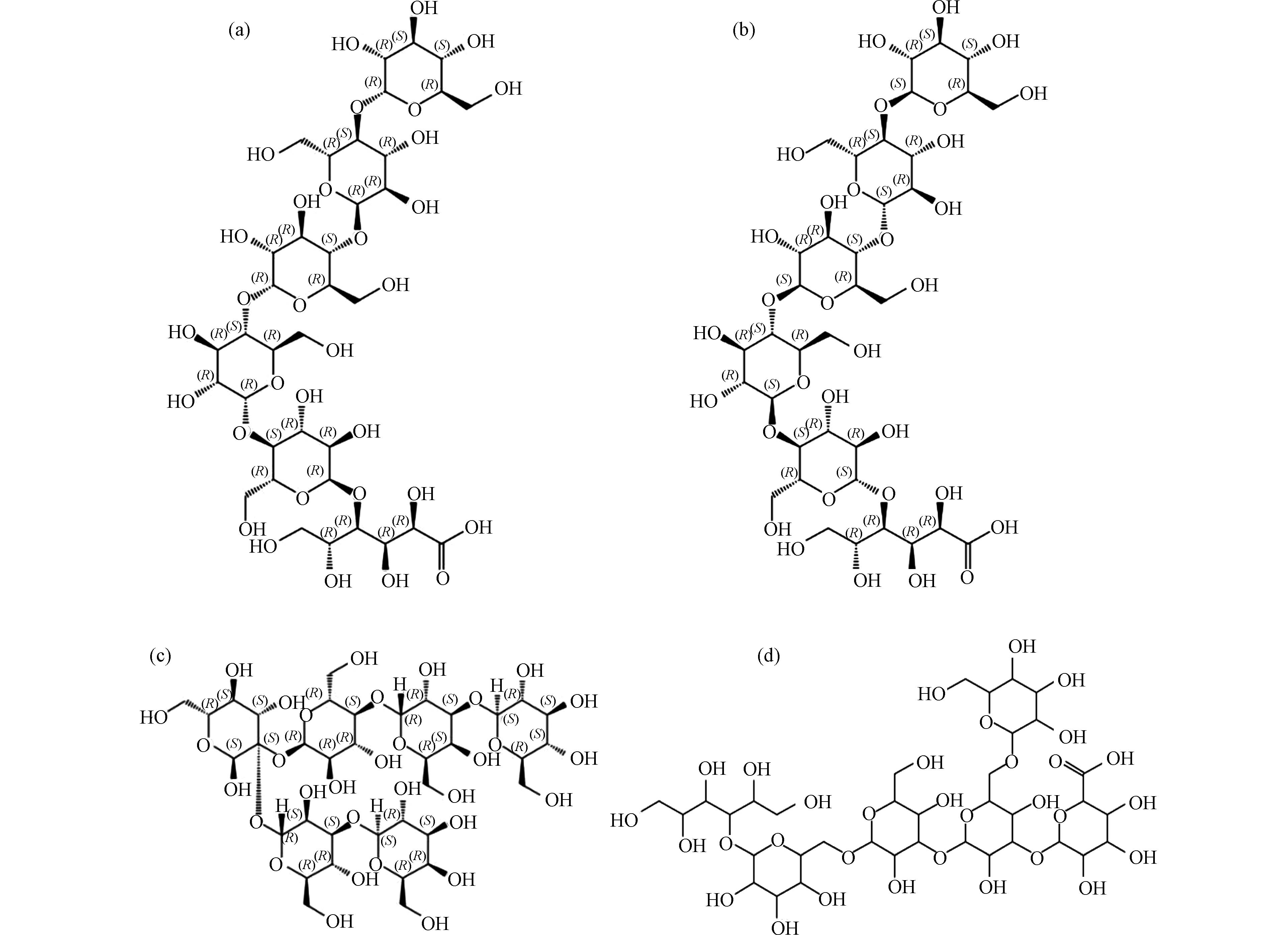
通过SciFinder数据库验证. 候选分子式C38H60O32、C40H61O29Na均未检索到对应物质,而C36H61O32Na(C36H62O32),得到了4种对应物质,其结构式如图12所示. 考虑到消毒前体物淀粉的结构,产物结构可排除图12(c)和(d)结构. 此外,图12(a)、(b)结构与文献中报道结构(图11)吻合. 因此可确定高分子量DBPs m/z 1051.3018的结构即为图12(a)或(b)结构.
同样的识别方法可以得到,5种高分子量DBPs分子式分别为C30H51O27Na、C36H61O32Na、C42H71O37Na、C48H81O42Na、C54H91O47Na(表3),并且通过数据库验证与以往研究证明5种新形成的高分子量DBPs同为羧酸糖类物质,新产生的高分子量DBPs的分子量相邻物质质量差约为162.1 Da,与己糖残基质量相同,因此可推测5种高分子量DBPs为相差若干个己糖残基的同类型物质.
在消毒过程中,淀粉中的还原端糖残基本身被氧化并开环,消毒模型化合物被氧化为寡糖醛酸或者寡糖羧酸钠. 因此,高分子量DBPs中未检测到含氯分子峰,可能是由于淀粉中糖苷键和还原端糖残基的存在,导致其消毒过程中更容易发生糖苷键断键、还原端糖残基开环和羟基-羧基转化,而不易发生卤素的取代反应.
-
(1)本研究引入MALDI-TOF MS,构建了针对高分子量DBPs的检测方法,并通过对消毒前后水样的质谱出峰逐一比对,在模拟饮用水中发现了5种新的高分子量DBPs.
(2)发现基质、检测模式、激光强度、阳离子化试剂和点靶方式均能对高分子量DBPs信噪比和信号重现性产生影响. 以DHB为基质,NaTFA为阳离子化试剂,三明治法为点靶方法,MALDI-TOF MS在反射-正离子模式、90%激光强度下,高分子量DBPs信噪比和信号重现性达到最优,信噪比达到136.2,CV值为4.77%.
(3)通过高分辨质谱提供的精确质量和同位素评价,TOF/TOF串联质谱提供的碎片离子特征以及SciFinder数据库验证,建立了高分子量DBPs的识别方法. 最终确定5种高分子量DBPs为相差若干己糖残基的寡糖羧酸钠类物质.
本研究基于MALDI-TOF MS的高分子量DBPs检测识别方法可为实际饮用水中复杂DBPs混合物的检测与鉴定提供新的技术支持.
饮用水中高分子量消毒副产物的检测识别方法
Detection and identification of high-molecular-weight disinfection byproducts in drinking water
-
摘要: 消毒是饮用水处理过程中的重要步骤,但普遍采用的氯系消毒剂在杀灭细菌病毒的同时,能产生大量具有“三致”效应的卤代消毒副产物(DBPs),严重威胁了饮用水的化学安全. 研究人员已在饮用水中陆续检测并识别出700多种DBPs,但这些已知DBPs均为分子量小于800 Da的低分子量DBPs,目前对高分子量DBPs的认识还较为有限. 本研究建立了基于基质辅助激光解吸电离飞行时间质谱(MALDI-TOF MS)的高分子量DBPs检测方法,发现在基质为2,5-二羟基苯甲酸,阳离子化试剂为三氟乙酸钠,使用三明治法点靶,反射-正离子模式,90%激光强度时,信噪比和信号重现性达到最优,信噪比之和达到了136.2,变异系数(CV)则为4.77%. 利用上述方法,在模拟饮用水中检测到5种新的高分子量DBPs. 在此基础上,通过同位素模式分析、TOF/TOF串联质谱和数据库验证,建立了针对未知高分子量DBPs的分子式/结构式识别方法,确定新的高分子量DBPs为寡糖羧酸类物质.
-
关键词:
- 高分子量消毒副产物 /
- 基质辅助激光解吸电离飞行时间质谱 /
- 阳离子化试剂 /
- 检测 /
- 识别.
Abstract: Disinfection is an important step in drinking water treatment process. However, disinfectants such as chlorine or chloramine can generate numerous halogenated disinfection byproducts (DBPs) with carcinogenicity, teratogenicity, and mutagenicity, which pose great threat to drinking water safety. More than 700 DBPs have been detected and identified in drinking water by researchers. The molecular weights of these known DBPs are all below 800 Da, and the understanding of high-molecular-weight DBPs is very limited. In this study, a detection method for high-molecular-weight DBPs was established based on matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). It was found that when the matrix was 2,5-dihydroxybenzoic acid, the cationization agent was sodium trifluoroacetate, the sandwich deposition method was used, the reflection-positive ion mode was applied, and the laser intensity was 90%, the highest signal intensity of high-molecular-weight DBPs could be obtained. At such condition, the signal reproducibility was maximized, the sum of the signal-to-noise ratio of new high-molecular-weight DBPs reached 136.2, and the coefficient of variation (CV) was 4.77%. Five new high-molecular-weight DBPs were detected in simulated drinking water using the above method. On such basis, a formula/structure identification method for unknown high-molecular-weight DBPs was established by isotopic pattern analysis, TOF/TOF tandem mass spectrometry, and database verification. With this method, the five new high-molecular-weight DBPs were determined to be oligosaccharide carboxylic acids. -

-
表 1 不同基质在四种检测模式下的高分子量DBPs出峰情况
Table 1. Peaks of high molecular weight DBPs in four detection modes using different matrices
基质
Matrix反射-正离子模式
Reflection-positive ion mode反射-负离子模式
Reflection-negative ion mode线性-正离子模式
Linear-positive ion mode线性-负离子模式
Linear-negative ion modeS/N CV/% S/N CV/% S/N CV/% S/N CV/% DHB 94.9 11.29 N.D. N.D. N.D. N.D. N.D. N.D. THAP 33.3 12.13 N.D. N.D. N.D. N.D. N.D. N.D. CHCA 7.0 14.28 N.D. N.D. N.D. N.D. N.D. N.D. SA N.D. N.D. N.D. N.D. N.D. N.D. N.D. N.D. DCTB N.D. N.D. N.D. N.D. N.D. N.D. N.D. N.D. DIT N.D. N.D. N.D. N.D. N.D. N.D. N.D. N.D. HABA N.D. N.D. N.D. N.D. N.D. N.D. N.D. N.D. IAA N.D. N.D. N.D. N.D. N.D. N.D. N.D. N.D. N.D. 未检出. N.D. not detected. 表 2 m/z 1051.3018的分子式计算结果
Table 2. The calculation result of the molecular formula of m/z 1051.3018
分子离子赋值
Molecular ion
assignment实际质荷比
Actual mass-to-
charge ratio理论质荷比
Theoretical mass-to-
charge ratio质量误差
Quality errorO/C H/C 双键当量
Double bond equivalent氮规则
Nitrogen rule[C38H60O32]Na + 1051.3018 1051.2960 5.517×10−6 0.842 1.579 8.5 Yes [C36H61O32Na]Na + 1051.3018 1051.2936 7.780×10−6 0.889 1.694 5.5 Yes [C40H61O29Na]Na + 1051.3018 1537.3088 6.658×10−6 0.725 1.525 9.5 Yes 表 3 高分子量DBPs分子式识别结果
Table 3. Molecular formula identification results of high molecular weight DBPs
分子离子赋值
Molecular ion
assignment实际质荷比
Actual mass-to-charge
ratio理论质荷比
Theoretical
mass-to-charge ratio质量误差
Quality errorO/C H/C 双键当量
Double bond
equivalent氮规则
Nitrogen rule[C30H51O27Na]Na + 889.2478 889.2408 7.872×10−6 1.700 0.900 4.5 符合 [C36H61O32Na]Na + 1051.3018 1051.2936 7.780×10−6 1.694 0.889 5.5 符合 [C42H71O37Na]Na + 1213.3578 1213.3464 9.396×10−6 1.690 0.881 6.5 符合 [C48H81O42Na]Na + 1375.4115 1375.3992 8.943×10−6 1.688 0.875 7.5 符合 [C54H91O47Na]Na + 1537.4675 1537.4529 9.496×10−6 1.685 0.870 8.5 符合 -
[1] NIEUWENHUIJSEN M J, TOLEDANO M B, EATON N E, et al. Chlorination disinfection byproducts in water and their association with adverse reproductive outcomes: A review [J]. Occupational and Environmental Medicine, 2000, 57(2): 73-85. doi: 10.1136/oem.57.2.73 [2] RICHARDSON S D, POSTIGO C. Drinking water disinfection by-products[M]//The Handbook of Environmental Chemistry. Berlin, Heidelberg: Springer Berlin Heidelberg, 2011: 93-137. [3] RICHARDSON S D, PLEWA M J, WAGNER E D, et al. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research [J]. Mutation Research/Reviews in Mutation Research, 2007, 636(1/2/3): 178-242. [4] 王志健, 胡霞林, 尹大强. 藻源有机质表征及消毒副产物生成潜能研究进展 [J]. 环境化学, 2021, 40(10): 2979-2991. doi: 10.7524/j.issn.0254-6108.2021033001 WANG Z J, HU X L, YIN D Q. Characterization and formation potential of disinfection by-products of algal organic matter: The critical review [J]. Environmental Chemistry, 2021, 40(10): 2979-2991(in Chinese). doi: 10.7524/j.issn.0254-6108.2021033001
[5] WAGNER E D, PLEWA M J. CHO cell cytotoxicity and genotoxicity analyses of disinfection by-products: An updated review [J]. Journal of Environmental Sciences, 2017, 58: 64-76. doi: 10.1016/j.jes.2017.04.021 [6] WRIGHT J M, EVANS A, KAUFMAN J A, et al. Disinfection by-product exposures and the risk of specific cardiac birth defects [J]. Environmental Health Perspectives, 2017, 125(2): 269-277. doi: 10.1289/EHP103 [7] LI X F, MITCH W A. Drinking water disinfection byproducts (DBPs) and human health effects: Multidisciplinary challenges and opportunities [J]. Environmental Science & Technology, 2018, 52(4): 1681-1689. [8] 刘晗, 邓琳. 饮用水中卤代硝基甲烷的分布特点、来源及毒性研究进展 [J]. 环境化学, 2022, 41(4): 1182-1192. doi: 10.7524/j.issn.0254-6108.2020112801 LIU H, DENG L. Research progress on distribution characteristics, source and toxicity of halonitromethanes in drinking water [J]. Environmental Chemistry, 2022, 41(4): 1182-1192(in Chinese). doi: 10.7524/j.issn.0254-6108.2020112801
[9] RICHARDSON S D, KIMURA S Y. Water analysis: Emerging contaminants and current issues [J]. Analytical Chemistry, 2020, 92(1): 473-505. doi: 10.1021/acs.analchem.9b05269 [10] RICHARDSON S D, TERNES T A. Water analysis: Emerging contaminants and current issues [J]. Analytical Chemistry, 2014, 86(6): 2813-2848. doi: 10.1021/ac500508t [11] YANG M T, ZHANG X R. Current trends in the analysis and identification of emerging disinfection byproducts [J]. Trends in Environmental Analytical Chemistry, 2016, 10: 24-34. doi: 10.1016/j.teac.2016.03.002 [12] KOPFLER F C, RINGHAND H P, COLEMAN W E, et al. Reactions of chlorine in drinking water, with humic acids and in vivo// Water Chlorination: Chemistry, Environmental Impact and Health Effects[M]. Chelsea, MI: Lewis Publishers, 1984: 161-173. [13] ZHANG X R, MINEAR R A. Formation, adsorption and separation of high molecular weight disinfection byproducts resulting from chlorination of aquatic humic substances [J]. Water Research, 2006, 40(2): 221-230. doi: 10.1016/j.watres.2005.10.024 [14] ZHANG X R, MINEAR R A, BARRETT S E. Characterization of high molecular weight disinfection byproducts from chlorination of humic substances with/without coagulation pretreatment using UF-SEC-ESI-MS/MS [J]. Environmental Science & Technology, 2005, 39(4): 963-972. [15] RICHARDSON S D. The role of GC-MS and LC-MS in the discovery of drinking water disinfection by-products [J]. Journal of Environmental Monitoring, 2002, 4(1): 1-9. doi: 10.1039/b105578j [16] ZHOU X T, MENG X J, CHENG L M, et al. Development and application of an MS ALL-based approach for the quantitative analysis of linear polyethylene glycols in rat plasma by liquid chromatography triple-quadrupole/time-of-flight mass spectrometry [J]. Analytical Chemistry, 2017, 89(10): 5193-5200. doi: 10.1021/acs.analchem.6b04058 [17] PAN C S, XU S Y, ZHOU H J, et al. Recent developments in methods and technology for analysis of biological samples by MALDI-TOF-MS [J]. Analytical and Bioanalytical Chemistry, 2007, 387(1): 193-204. doi: 10.1007/s00216-006-0905-4 [18] KARAS M, BACHMANN D, BAHR U, et al. Matrix-assisted ultraviolet laser desorption of non-volatile compounds [J]. International Journal of Mass Spectrometry and Ion Processes, 1987, 78: 53-68. doi: 10.1016/0168-1176(87)87041-6 [19] TANAKA K, WAKI H, IDO Y, et al. Protein and polymer analyses up to m/z 100 000 by laser ionization time-of-flight mass spectrometry [J]. Rapid Communications in Mass Spectrometry, 1988, 2(8): 151-153. doi: 10.1002/rcm.1290020802 [20] BALLATORE M B, BETTIOL M D R, VANDEN BRABER N L, et al. Antioxidant and cytoprotective effect of peptides produced by hydrolysis of whey protein concentrate with trypsin [J]. Food Chemistry, 2020, 319: 126472. doi: 10.1016/j.foodchem.2020.126472 [21] HEILBRONNER S, FOSTER T J. Staphylococcus lugdunensis: A skin commensal with invasive pathogenic potential [J]. Clinical Microbiology Reviews, 2020, 34(2): e00205-e00220. [22] BŁAŻEK K, BENEŠ H, WALTEROVÁ Z, et al. Synthesis and structural characterization of bio-based bis(cyclic carbonate)s for the preparation of non-isocyanate polyurethanes [J]. Polymer Chemistry, 2021, 12(11): 1643-1652. doi: 10.1039/D0PY01576H [23] NICOLAU R, LELOUP M, LACHASSAGNE D, et al. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) coupled to XAD fractionation: Method to algal organic matter characterization [J]. Talanta, 2015, 136: 102-107. doi: 10.1016/j.talanta.2015.01.011 [24] NAVALON S, ALVARO M, ALCAINA I, et al. Multi-method characterization of DOM from the Turia river (Spain) [J]. Applied Geochemistry, 2010, 25(11): 1632-1643. doi: 10.1016/j.apgeochem.2010.08.011 [25] DREISEWERD K, MÜTHING J, ROHLFING A, et al. Analysis of gangliosides directly from thin-layer chromatography plates by infrared matrix-assisted laser desorption/ionization orthogonal time-of-flight mass spectrometry with a glycerol matrix [J]. Analytical Chemistry, 2005, 77(13): 4098-4107. doi: 10.1021/ac048373w [26] RANKIN K, MABURY S A. Matrix normalized MALDI-TOF quantification of a fluorotelomer-based acrylate polymer [J]. Environmental Science & Technology, 2015, 49(10): 6093-6101. [27] KOOIJMAN P C, KOK S J, WEUSTEN J J A M, et al. Independent assessment of matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) sample preparation quality: A novel statistical approach for quality scoring [J]. Analytica Chimica Acta, 2016, 919: 1-10. doi: 10.1016/j.aca.2016.03.031 [28] KIMURA S Y, CUTHBERTSON A A, BYER J D, et al. The DBP exposome: Development of a new method to simultaneously quantify priority disinfection by-products and comprehensively identify unknowns [J]. Water Research, 2019, 148: 324-333. doi: 10.1016/j.watres.2018.10.057 [29] MCCRADY M H. Standard methods for the examination of water and waste-water (12th ed. ) [J]. American Journal of Public Health and the Nations Health, 1966, 56(4): 684. [30] WEI Y Y, LIU Y, MA L M, et al. Speciation and formation of iodinated trihalomethane from microbially derived organic matter during the biological treatment of micro-polluted source water [J]. Chemosphere, 2013, 92(11): 1529-1535. doi: 10.1016/j.chemosphere.2013.04.019 [31] ARTIFON V, ZANARDI-LAMARDO E, FILLMANN G. Aquatic organic matter: Classification and interaction with organic microcontaminants [J]. Science of the Total Environment, 2019, 649: 1620-1635. doi: 10.1016/j.scitotenv.2018.08.385 [32] LIU J L, LI X Y. Biodegradation and biotransformation of wastewater organics as precursors of disinfection byproducts in water [J]. Chemosphere, 2010, 81(9): 1075-1083. doi: 10.1016/j.chemosphere.2010.09.041 [33] ZHANG Q, LIU B, LIU Y. Effect of ozone on algal organic matters as precursors for disinfection by-products production [J]. Environmental Technology, 2014, 35(14): 1753-1759. doi: 10.1080/09593330.2014.881422 [34] HONG H C, MAZUMDER A, WONG M H, et al. Yield of trihalomethanes and haloacetic acids upon chlorinating algal cells, and its prediction via algal cellular biochemical composition [J]. Water Research, 2008, 42(20): 4941-4948. doi: 10.1016/j.watres.2008.09.019 [35] CHEN X G, KONG L, SU X Y, et al. Integration of ion-exchange chromatography fractionation with reversed-phase liquid chromatography-atmospheric pressure chemical ionization mass spectrometer and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for isolation and identification of compounds in Psoralea corylifolia [J]. Journal of Chromatography A, 2005, 1089(1/2): 87-100. [36] CHEN X G, HU L H, SU X Y, et al. Separation and detection of compounds in Honeysuckle by integration of ion-exchange chromatography fractionation with reversed-phase liquid chromatography-atmospheric pressure chemical ionization mass spectrometer and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis [J]. Journal of Pharmaceutical and Biomedical Analysis, 2006, 40(3): 559-570. doi: 10.1016/j.jpba.2005.07.043 [37] KRISTIANA I, TAN J, JOLL C A, et al. Formation of N-nitrosamines from chlorination and chloramination of molecular weight fractions of natural organic matter [J]. Water Research, 2013, 47(2): 535-546. doi: 10.1016/j.watres.2012.10.014 [38] ALMASOUD N, CORREA E, TRIVEDI D K, et al. Fractional factorial design of MALDI-TOF-MS sample preparations for the optimized detection of phospholipids and acylglycerols [J]. Analytical Chemistry, 2016, 88(12): 6301-6308. doi: 10.1021/acs.analchem.6b00512 [39] POSTIGO C, ANDERSSON A, HARIR M, et al. Unraveling the chemodiversity of halogenated disinfection by-products formed during drinking water treatment using target and non-target screening tools [J]. Journal of Hazardous Materials, 2021, 401: 123681. doi: 10.1016/j.jhazmat.2020.123681 [40] CAO D, HUANG H G, HU M, et al. Comprehensive characterization of natural organic matter by MALDI- and ESI-Fourier transform ion cyclotron resonance mass spectrometry [J]. Analytica Chimica Acta, 2015, 866: 48-58. doi: 10.1016/j.aca.2015.01.051 [41] SROKA-BARTNICKA A, CIESIELSKI W, LIBISZOWSKI J, et al. Complementarity of solvent-free MALDI TOF and solid-state NMR spectroscopy in spectral analysis of polylactides [J]. Analytical Chemistry, 2010, 82(1): 323-328. doi: 10.1021/ac9020006 [42] WANG J Q, ZHAO J, NIE S P, et al. Rapid profiling strategy for oligosaccharides and polysaccharides by MALDI TOF mass spectrometry [J]. Food Hydrocolloids, 2022, 124: 107237. doi: 10.1016/j.foodhyd.2021.107237 [43] KWON C, LEE S, JUNG S. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometric behavior of succinoglycan monomers, dimers, and trimers isolated from Sinorhizobium meliloti 1021 [J]. Carbohydrate Research, 2011, 346(14): 2308-2314. doi: 10.1016/j.carres.2011.07.023 [44] HUNG W T, WANG S H, CHEN Y T, et al. MALDI-TOF MS analysis of native and permethylated or benzimidazole-derivatized polysaccharides [J]. Molecules (Basel, Switzerland), 2012, 17(5): 4950-4961. doi: 10.3390/molecules17054950 [45] WETZEL S J, GUTTMAN C M, FLYNN K M, et al. Significant parameters in the optimization of MALDI-TOF-MS for synthetic polymers [J]. Journal of the American Society for Mass Spectrometry, 2006, 17(2): 246-252. doi: 10.1016/j.jasms.2005.11.007 [46] WETZEL S J, GUTTMAN C M, GIRARD J E. The influence of matrix and laser energy on the molecular mass distribution of synthetic polymers obtained by MALDI-TOF-MS [J]. International Journal of Mass Spectrometry, 2004, 238(3): 215-225. doi: 10.1016/j.ijms.2004.04.019 [47] LIN Y, HUANG X, LIU Q, et al. Thermal fragmentation enhanced identification and quantification of polystyrene micro/nanoplastics in complex media [J]. Talanta, 2020, 208: 120478. doi: 10.1016/j.talanta.2019.120478 [48] PARK E, YANG H, KIM Y, et al. Analysis of oligosaccharides in beer using MALDI-TOF-MS [J]. Food Chemistry, 2012, 134(3): 1658-1664. doi: 10.1016/j.foodchem.2012.03.069 [49] LING L, XIAO C S, MA Y, et al. 2-phenyl-3-(p-aminophenyl) acrylonitrile: A reactive matrix for sensitive and selective analysis of glycans by MALDI-MS [J]. Analytical Chemistry, 2019, 91(14): 8801-8807. doi: 10.1021/acs.analchem.9b01434 [50] LING L, JIANG L Y, CHEN Q R, et al. Rapid and accurate profiling of oligosaccharides in beer by using a reactive matrix via MALDI-TOF MS [J]. Food Chemistry, 2021, 340: 128208. doi: 10.1016/j.foodchem.2020.128208 [51] HAO J, LU J J, XU N Y, et al. Specific oxidation pattern of soluble starch with TEMPO-NaBr-NaClO system [J]. Carbohydrate Polymers, 2016, 146: 238-244. doi: 10.1016/j.carbpol.2016.03.040 [52] KATO Y, MATSUO R, ISOGAI A. Oxidation process of water-soluble starch in TEMPO-mediated system [J]. Carbohydrate Polymers, 2003, 51(1): 69-75. doi: 10.1016/S0144-8617(02)00159-5 -



 下载:
下载: