-
随着传统化石能源的快速消耗,大量的CO2被释放到大气中,随之而来的是越来越严重的能源和环境危机[1],因此通过光催化技术将CO2转化为具有高价值化学产品的方法已逐渐成为缓解能源危机和全球变暖的重要方法之一[2]。但由于C=O的断裂需要大量的能量并且在动力学上难以实现多电子反应过程[3],导致新型光催化剂的设计和合成实现CO2快速还原仍然面临巨大的挑战。
在以往的研究中,各种已开发的光催化剂主要分为两类,即均相催化剂和异相催化剂[4]。具有明确的单原子金属中心,并通过配位反应形成的分子配合物是目前应用最为广泛的均相光催化剂[5]。均相催化剂的光吸收、氧化还原电势和电子结构可以通过改变中心金属原子与各种有机配体的配位关系,从而调节其光催化活性和选择性来轻松实现[6]。近些年来一些廉价、低毒过渡金属铁、钴、镍配合物已被证实是降解有机染料以及CO2还原的有效光催化剂[7-8]。其中最为常见的Co(Ⅱ)离子不仅与多齿配体配位时会显示出良好的配位性[9-10],而且所形成得配合物具备较好的催化活性、产物选择性以及稳定性。另一方面,在共沸条件下将活性羰基(醛或酮)与伯胺缩合而形成的含有偶氮甲碱(−HC=N−)或亚胺(>C=N−)基团的席夫碱化合物因其灵活的配位方式与不同金属配位且由此形成的独特的光、电特性而受到研究者的广泛关注[11],因此将席夫碱化合物与钴盐反应后制备的配合物有望在光催化领域发挥效力。
本文首先合成了一种香豆素类席夫碱有机配体3-[(2-羟基-5-氯苯亚甲基)-氨基]-7-羟基香豆素(CHB),然后将配体CHB与过渡金属Co(Ⅱ)盐进行反应制备了一种单核离子型金属-有机小分子配合物CHB-Co,通过核磁共振氢谱(1H NMR)对配体的结构进行了表征,并通过质谱(MS)、元素分析、傅里叶变换红外光谱(FT-IR)以及紫外可见吸收光谱(UV-vis)表征了配合物的结构和光学特性。接着使用CHB-Co作为光催化剂,[Ru(phen)3](PF6)2为光敏剂,TEOA为牺牲剂探究了其光催化CO2还原性能,结果发现10 h后产生的CO的转换数(TON)和转换频率(TOF)分别为1468 h−1和146.8 h−1,选择性高达90%,最后通过电化学法和荧光分析法对该配合物光催化CO2还原的机理进行了系统地研究,为进一步推动该材料实现CO2资源化利用奠定了理论研究基础。
-
2,4-二羟基苯甲醛、乙酰甘氨酸试剂均购买于上海阿拉丁生化科技股份有限公司,六水合氯化钴(CoCl2·6H2O)购买于上海迈瑞尔化学技术有限公司,且使用前未经进一步提纯,有机试剂均购买于上海国药集团化学试剂有限公司,试剂使用前干燥处理;氯化三(2, 2′-联吡啶)钌(Ⅱ)六水合物([Ru(bpy)3]Cl2·6H2O)、TEOA购于西格玛奥德里奇(上海)贸易有限公司;三(1,10-菲咯啉)钌(Ⅱ)双(六氟磷酸盐)购自上海梯希爱化成工业发展有限公司(日本TCI独立子公司),实验所需试剂未经注明均为AR级;实验所用水为超纯水(电阻率大于 18 MΩ∙cm),所用二氧化碳、氩气均为高纯气体(99.999%)(马鞍山市晨虹气体有限公司)。
红外光谱采用美国Nicolet/Nexus-870 FT-IR型红外光谱仪(KBr压片,在4000—400 cm−1范围内)测试;核磁共振谱采用美国Varian 400 MHz型核磁共振光谱仪测定;采用美国Perkin-Elmer 2400 元素分析仪进行元素分析;采用日本日立公司Hitachi-700荧光光谱仪记录荧光光谱;采用日本岛津公司生产的UV-2700紫外可见分光光度仪记录紫外-可见光吸收光谱;采用德国Bruker生产MALDI-TOF质谱仪检测配合物分子量。使用泊菲莱PCX50B Discover多通道平行光催化反应系统进行光催化CO2还原反应,泊菲莱PL-MW 2000光辐射仪记录入射光功率;使用CHI650D型电化学工作站(上海辰华仪器有限公司)用于CHE-Co电化学分析;美国安捷伦公司生产的7820A气相色谱仪用于产物气体的分析。
-
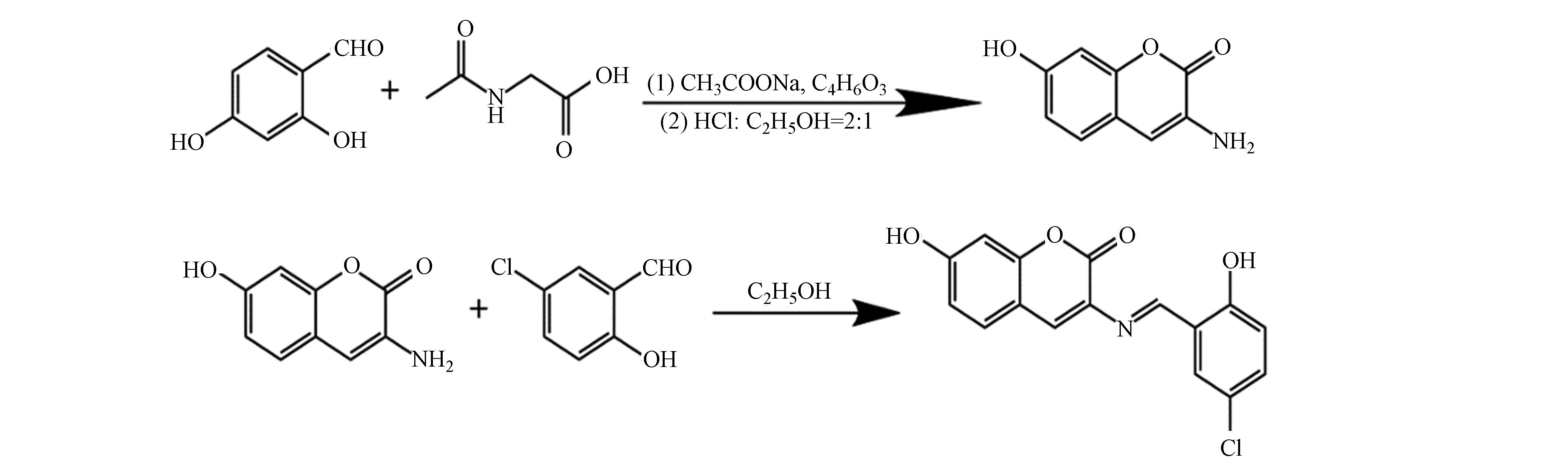
参考文献[12]改进后的合成方法合成CHB,准确称量2.76 g(20 mmol)2, 4-二羟基苯甲醛、2.34 g(20 mmol)乙酰甘氨酸和4.92 g(60 mmol)无水乙酸钠加入到200 mL圆底烧瓶中,再向烧瓶内加入50 mL乙酸酐,搅拌溶解后,加热回流8 h,冷却至室温后,再加入30 mL冰水,观察到大量沉淀生成,抽滤干燥后得到淡黄色固体。将上一步产物置于20 mL浓盐酸:乙醇(2∶1)的混合溶剂中于85 ℃条件下继续回流3 h,自然冷却,并用30% NaOH调节pH值至5—6之间,析出大量沉淀,过滤干燥后用无水乙醇重结晶得到3-氨基-7-羟基香豆素前驱体。
称取1.77 g 3-氨基-7-羟基香豆素(10 mmol)与1.56 g 5-氯水杨醛(10 mmol)溶于20 mL无水乙醇中,加热回流4 h,生成大量沉淀,冷却至室温后,将抽滤洗涤得到的固体置于真空干燥箱中于50 ℃干燥10 h,再将干燥后的粗产物使用无水乙醇重结晶得到纯净的香豆素席夫碱配体CHB(2.43 g),反应历程如图1所示。产率77%。1H NMR(400 MHz,DMSO)δ:12.74(s,1H),10.74(s,1H),9.26(s,1H),8.07(s,1H),7.75(d,1H),7.56(d,1H),7.43(d,1H),7.00(d,1H),6.83(d,1H),6.78(s,1H)。
-
参考文献[13]改进的方法合成配合物,准确称量31.3 mg CHB(0.10 mmol)置于50 mL的圆底烧瓶中,加入10 mL 甲醇溶液,使CHB完全溶解呈浅红色,再逐滴加入含有18.3 mg CoCl2·6H2O(0.05 mmol)的2.5 mL粉红色甲醇溶液,加热回流4 h,观察到溶液从浅红色逐渐转变为红棕色,结束反应,冷却溶液,过滤除去未反应的CHB,脱除溶剂,得到红棕色固体,抽滤并使用超纯水洗涤3次获得深红色固体粉末,最后使用无水乙醇重结晶得到27.7 mg红棕色晶状粉末(CHB-Co),产率为77%。元素分析计算值(%)CoC32H19N2O8.5Cl2:C 55.12, H 2.75, N 4.02;测定值(%):C 56.02, H 2.66, N 3.99。ESI-MS(m/z): 701.41。
-
采用带有密封橡胶垫圈的石英瓶作为光催化反应容器。光催化实验的步骤如下:首先,分别制备具有一定浓度的催化剂CHB-Co和光敏剂[Ru(phen)3]PF6的水-乙腈(V∶V=1∶4)溶液;其次,对密封的反应瓶进行抽真空置换高纯CO2操作3次,使瓶内充满CO2气体;然后,分别移取一定体积的催化剂、光敏剂溶液和牺牲剂三乙醇胺加入到反应池中,再加入一定体积的水-乙腈(V∶V=1∶4)混合溶液,使反应瓶内溶液的总体积为20 mL(其中催化剂的浓度为0.5 μmol·L−1,光敏剂的浓度为0.5 mmol·L−1,牺牲剂的浓度为0.3 M);继续对溶液中通入CO2气体鼓泡30 min,得到CO2饱和的溶液,密封好反应瓶盖;最后将石英反应瓶放入泊菲莱PCX50B Discover多通道平行光催化反应系统中进行光催化反应,该系统配置有5 W LED白光(λ=428 nm,90 mW·cm−2)光源。在光催化过程中,从反应瓶上部抽取气体并通过气相色谱仪进行分析。使用N2作为载气,柱箱、进样口和检测器的温度分别保持恒定在40、150、250 ℃。光催化反应至少重复3遍,取其平均值,以确保数据的准确性。
-
选用玻碳电极为工作电极,铂丝电极为对电极,Ag/AgCl(饱和KCl)电极为参比电极,在含有0.1 mol·L−1TBAPF6为支持电解质的水-乙腈(V∶V=1∶4)溶液中进行电化学实验。所有电位均以二茂铁(Fc0/+)为外标,并通过向测量的电位加上一定数值使其转换为参比NHE的电位值。测试之前先将玻碳电极分别用0.3 μm 和0.05 μm Al2O3抛光3 min以得到镜面,接着在乙醇和水中分别超声处理5 min,之后将玻碳电极置于空白缓冲介质中循环伏安扫描至稳定为止,最后用Ar或CO2吹扫15 min使电解质溶液充满Ar或CO2后再加入CHB-Co进行电化学实验研究。
-
转换数(TON)和转换频率(TOF)的计算公式如下:
式中,n(CO)代表生成CO的物质的量,n(cat)代表催化剂的物质的量,t为时间(s)。
由于将CO2还原为CO是两电子过程,故通过以下公式计算该过程的整体量子产率(ΦCO):
通过气相色谱仪测量得到气体样品中的物质的量,结合阿伏伽德罗常数 (6.022×1023),就可以获知CO的分子数;再利用测量反应容器内的入射光功率 (90 mW·cm−2) 来确定吸收的光子数(此时光的波长为428 nm,可见光的照射面积即光反应瓶底部面积为7.54 cm2),最终计算获得光照10 h后CO的量子产率。
-
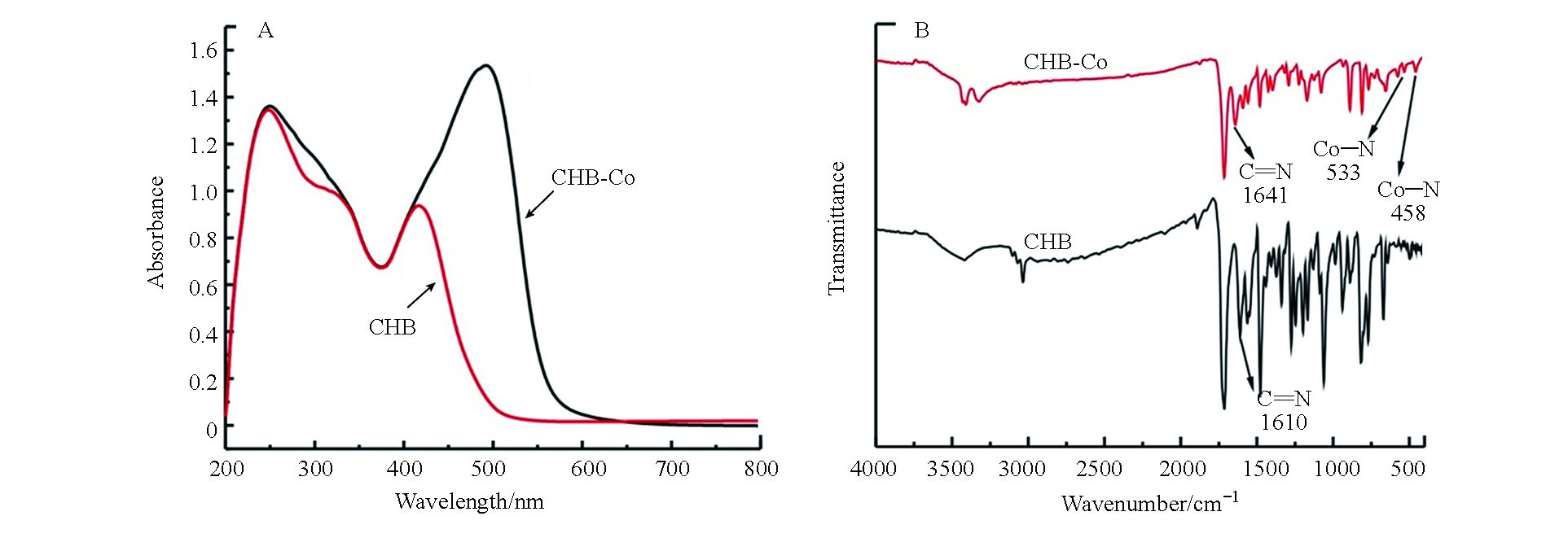
为检验催化剂是否合成成功,分别检测了CHB及CHB-Co的紫外可见吸收光谱,如图2A所示。配体CHB在251 nm和424 nm处具有明显的吸收峰,可归因于配体CHB的π-π*吸收,而催化剂CHB-Co则在251 nm同样具有吸收峰,并且在498 nm处出现了新的吸收峰,这归因于配合物分子内电荷转移跃迁吸收,即MLCT态跃迁吸收所导致,证实了金属钴中心与CHB配体成功配位。如图2B所示,CHB的红外光谱显示在1610 cm−1有一条谱带,归因于(C=N)的伸缩振动,当游离配体与金属离子配位后,它红移至更高的频率(1641 cm−1)[14]。CHB-Co的红外吸收光谱中,没有出现C=O和N-H的拉伸振动峰,表明可能发生了席夫碱缩合。通过新谱带533 cm−1和458 cm−1的存在可以确认发生了氮原子(Co–N)和氧原子(Co–O)的配位[15]。
-
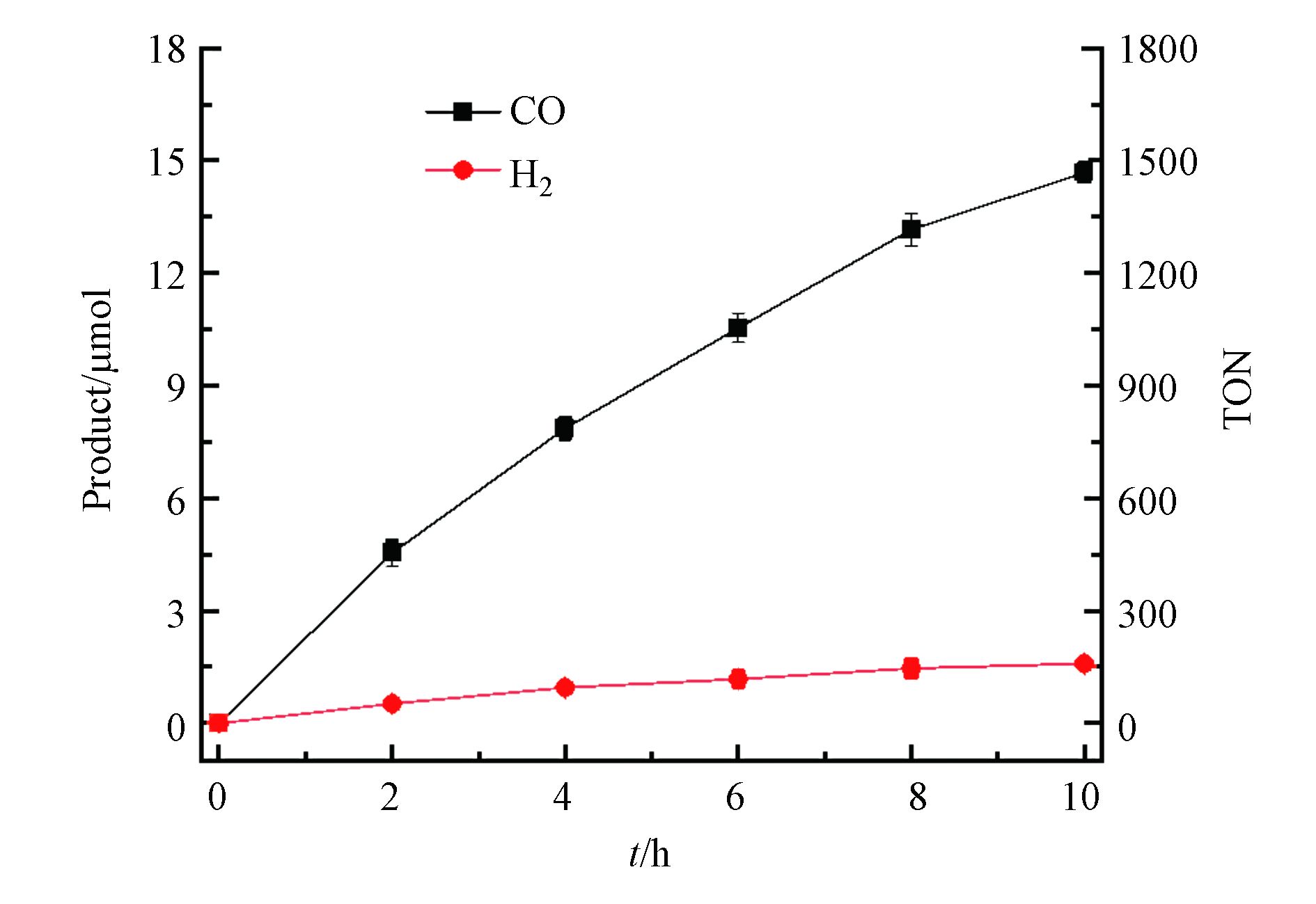
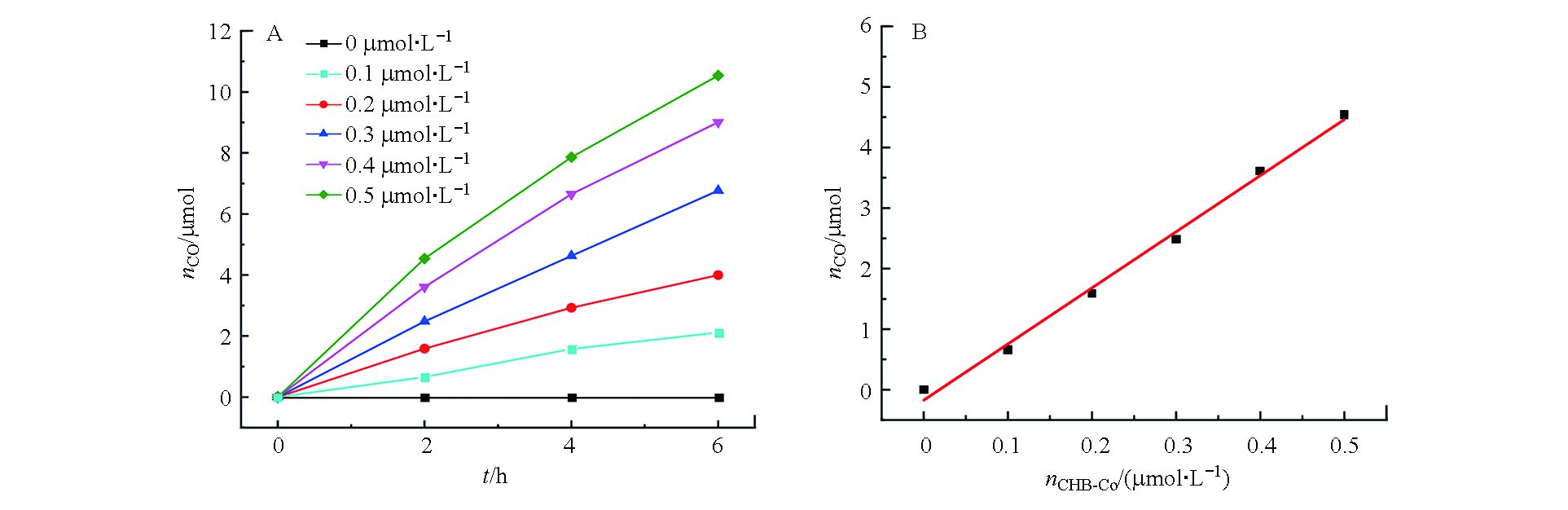
使用0.5 μmol·L−1配合物CHB-Co为光催化剂,0.5 mmol·L−1 [Ru(phen)3](PF6)2为光敏剂,0.3 mol·L−1三乙醇胺(TEOA)为牺牲剂研究了金属-有机配合物CHB-Co对CO2还原的光催化活性。在20 mL CO2饱和的H2O/CH3CN溶液(V∶V=1∶4)中通过LED灯模拟太阳能照射进行光催化实验,每隔2 h提取一次反应瓶上部的气体并通过气相色谱仪分析产生的气体成分和含量,如图3所示。当体系中使用催化剂CHB-Co,随着CO2光催化还原反应的进行,CO的释放量随着时间增长逐渐减慢,并在约10 h后增长停滞几乎达到饱和,经过计算,光照10 h后共计产生了14.68 μmol的CO和1.56 μmol的H2,相应的CO的转换数和转换频率分别为1468 h−1和146.8 h−1,对CO的选择性达到了90%,光催化反应的量子产率(ΦCO)经过计算为0.033%。
如表1所示,与一些已有的光催化CO2还原成CO的催化剂[16-19]相比,本文合成的光催化剂CHB-Co具备较高的TON和TOF值,同时表现出更好的选择性。
-
本实验通过更换反应条件研究了催化剂CHB-Co的光催化效果,如表2所示。在没有催化剂的情况下(Entry 2)会生成微量的CO和H2,这表明CO主要是由CHE-Co催化CO2还原反应产生而不是仅由[Ru(phen)3]2+催化生成。在没有光敏剂(Entry 3)、牺牲剂TEOA (Entry 4)或可见光(Entry 5)的对照实验中,均无CO生成,表明这些组分对于光催化过程中CO2转化为CO必不可少。众所周知,在氩气氛下无CO2源(Entry 6)的情况下肯定无法观察到CO的形成,表明在(Entry 1)中形成的CO来自CO2的还原而不是CHB-Co或光敏剂/牺牲还原剂的分解。另外使用CoCl2·6H2O代替CHB-Co进行光催化反应,发现CO和H2的产量显著降低(Entry 7),表明配合物的形成改善了Co(Ⅱ)盐的催化活性。
-
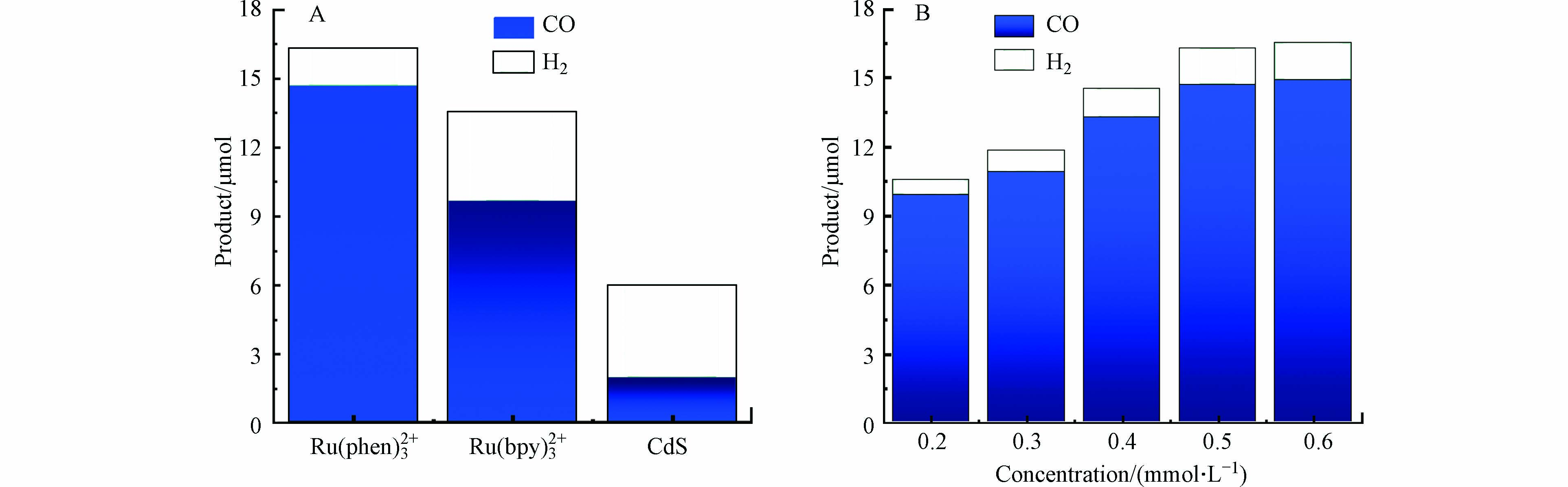
光敏剂是光催化系统的重要组成部分,不同的光敏剂在受光照后具有不同的还原电势,其还原电势过正可能会难以驱动催化剂还原CO2,过负又会产生副反应,为了优化光催化的活性和选择性,所以筛选了3种相同浓度(0.5 mmol·L−1)的光敏剂[Ru(bpy)3]Cl2、[Ru(bpy)3]Cl2和CdS来对比同样测试条件下将CO2光催化还原为CO的效果。经过对比发现与[Ru(bpy)3]Cl2和CdS相比,[Ru(phen)3](PF6)2作光敏剂时产物CO的产量和选择性更高(图4A)。由于光敏剂涉及与催化剂之间会发生电子转移,因此光敏剂的浓度会对光催化结果产生影响,研究催化剂浓度固定为0.5 μmol·L−1的情况下,将光敏剂[Ru(phen)3]2+的浓度从0.2 mmol·L−1逐渐改变至0.6 mmol·L−1,结果发现光催化产生的CO量逐渐增加,推测可能是由于光敏剂浓度增加后,其吸收的光能量逐渐随之增加所致,考虑到成本等原因,光敏剂[Ru(phen)3](PF6)2浓度选择为0.5 mmol·L−1(图4B)。
牺牲剂的作用是还原光敏剂转化为激发态后所产生的空穴,而不同的牺牲还原剂由于还原能力不同对应着不同的还原电势。当光敏剂固定时,选择与光敏剂相匹配的牺牲剂对于催化剂活性的影响很大,本文以[Ru(phen)3](PF6)2作为光敏剂,选择3种常见的牺牲剂TEOA、TEA以及异丙醇开展研究,可以观察到当选择TEOA作为牺牲剂时,CHB-Co的光催化性能最好(图略)。
-
分别采用3种浓度的CHB-Co进行光催化实验,结果表明,保持光敏剂和牺牲剂的浓度为最佳值的情况下,使用0.5 μmol·L−1 CHB-Co作为催化剂光照10 h后产生了14.68 μmol CO,CO的TON值为1468,TOF值为146.8 h−1,量子产率为0.033%,选择性为90%。当将CHB-Co的浓度提高到1.0 μmol·L−1时,将会产生19.34 μmol CO,CO的TON值为967,TOF值为96.7 h−1,量子产率为0.044%,选择性下降为88%。结果表明CHB-Co在相对较高的浓度下具有高效的催化性能,但是选择性下降。另一方面,将CHB-Co浓度降低至0.1 μmol·L−1会产生更大的TON和TOF值(分别为1685 h−1和168.5 h−1),但是CO的产量过低,因此本体系选择0.5 μmol·L−1浓度的催化剂进行光催化实验。
催化剂CHB-Co的稳定性或耐受性是光催化CO2还原中的一个重要因素。水-乙腈(V∶V=1∶4)溶液中,模拟光照射10 h后,CO的生成速度明显降低,系统的失活可能与催化剂或光敏剂的光降解有关。通过紫外可见光谱法研究可知,光照10 h后,催化剂CHE-Co的吸光度几乎没有变化(图略),而光敏剂[Ru(phen)3]2+的吸光度明显下降(图略),推测在光催化过程中,催化剂是稳定的,催化体系的失活与光敏剂的光降解有关。此外通过连续的催化实验表明,添加新鲜当量的[Ru(phen)3](PF6)2可以重新激活CO2光催化还原过程,然而重新加入催化剂CHE-Co和/或牺牲剂TEOA却不能使已经接近停止的反应再继续进行,表明CHB-Co是该光催化系统中稳定的均相催化剂。
-
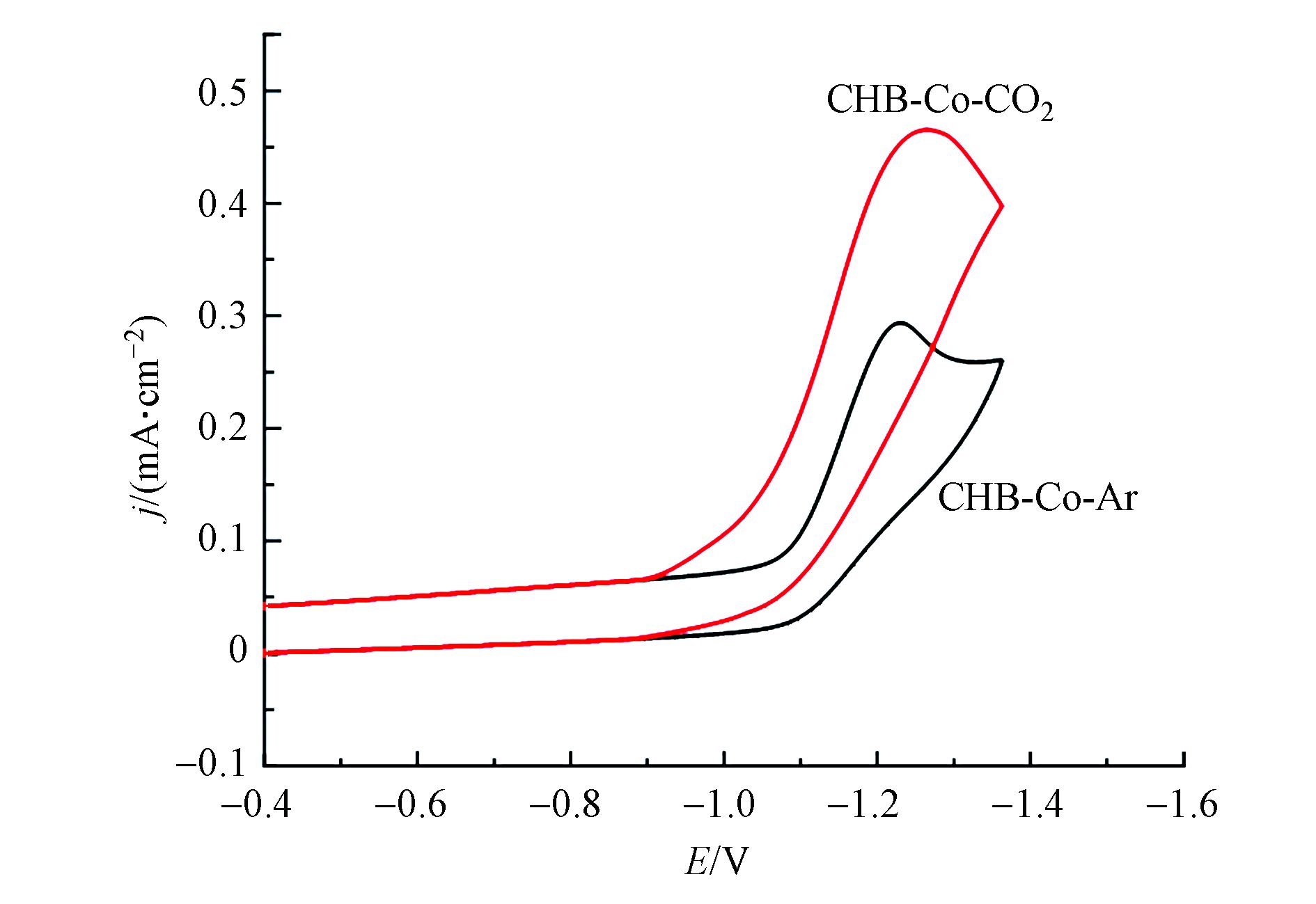
为了探寻光催化反应机理,通过循环伏安法(CV)在含支持电解质0.1 mol·L−1 TBAPF6的水-乙腈(V∶V=1∶4)溶液中研究了CHB-Co的电化学性质,如图5所示,在氩气氛下,CHB-Co在E=−1.10 V(vs NHE)时显示出Co(Ⅱ)/Co(Ⅰ)不可逆的还原过程。此外,在CO2气氛下电流在电位为−1.27 V(vs NHE)处显著增加,并且看出CO2氛围下配合物CHB-Co还原反应的起始电位Eonset为−0.89 V(vs NHE)时就显示出电流增强的电催化趋势,比光敏剂[Ru(phen)3](PF6)2的电位值(−1.12)[20]更正,因此配合物CHE-Co有望驱动电子从光敏剂中的钌中心转移至催化剂,从而极大地促进了光催化CO2向CO的转化[21]。
在其他条件不变的情况下,进行了CO生成的动力学研究,如图6所示。当催化剂浓度从0至0.5 µmol·L−1范围内变化时,CO的产量随之增大而增加(图6A),并且反应2 h后生成CO的物质的量与加入催化剂的浓度呈线性关系,求得的线性方程为nCO= 9.27C催–0.14,相关系数r为0.9970(图6B),表明CO2还原的限速步骤涉及单分子催化剂CHE-Co的单个Co位点[18]。
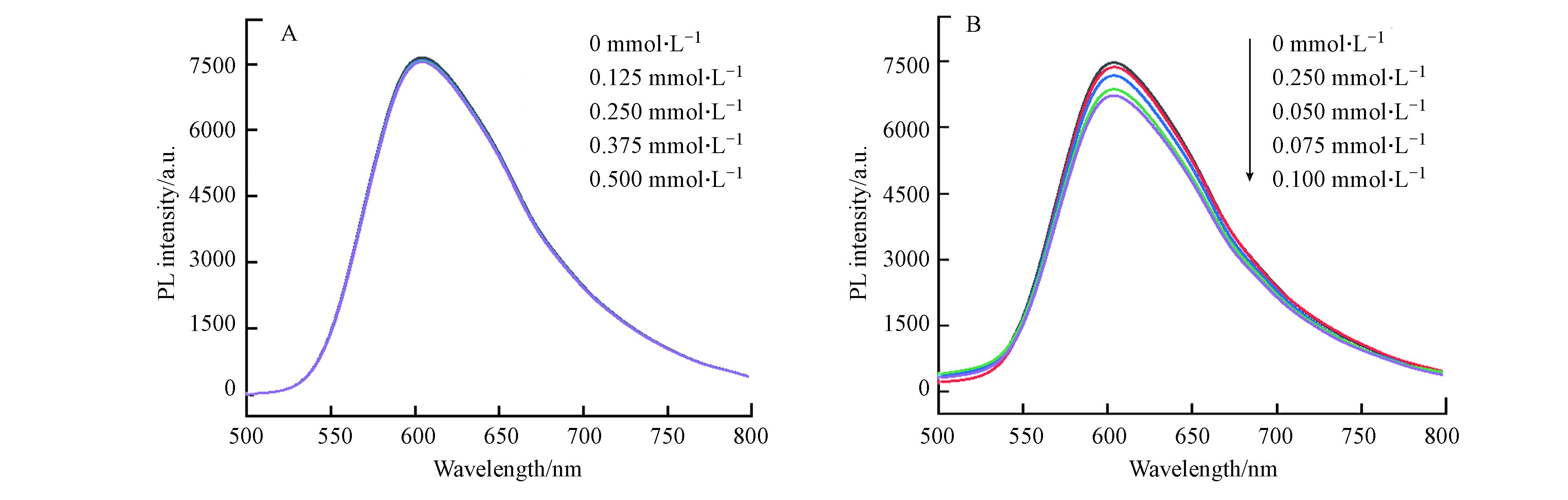
另外为了更好地理解此催化体系中的光诱导电子转移,通过荧光实验对CHE-Co光催化CO2还原的机理进行了进一步的探究。在加入CHB-Co和TEOA的条件下进行了激发态[Ru(phen)3]2+*的猝灭实验(图7)。结果表明在激发波长为450 nm处激发时,[Ru(phen)3]2+在591 nm处发出强烈的荧光,当向0.05 mmol·L−1光敏剂中分别加入0、0.125 、0.250 、0.375 、0.5 mmol·L−1 TEOA时,[Ru(phen)3](PF6)2在591 nm处的发射强度几乎保持不变。而当向体系中分别加入0、0.025、0.050、0.075、0.100 mmol·L−1催化剂时,[Ru(phen)3](PF6)2的荧光发射强度随着加入催化剂浓度的增大而减弱,即[Ru(phen)3]2+*有效地被CHB-Co氧化猝灭[22-23],证明光敏剂[Ru(phen)3](PF6)2和催化剂CHE-Co之间发生了电子转移[24]。
-
本文以2, 4-二羟基苯甲醛为起始原料合成了一种香豆素类席夫碱有机配体CHB,再将CHB与金属钴(Ⅱ )盐通过常温液相反应制备了一种新型的离子型金属-有机分子配合物CHB-Co并进行了表征,接着研究了模拟太阳光照射下CHB-Co光催化CO2还原的性能。在优化光敏剂、牺牲剂的种类和浓度以及催化剂的浓度等条件之后发现,0.05 μmol·L−1配合物CHB-Co在以0.5 mmol·L−1 [Ru(phen)3]2+为光敏剂,0.3 mol·L−1 TEOA为牺牲剂的H2O/CH3CN溶液 (V∶V=1∶4)中具有很好的光催化CO2还原性能,主要产物CO的产量为14.68 μmol,TON值达1468,TOF值为146.8 h−1,选择性高达90%。通过不同光照时间下催化剂的紫外可见吸收光谱以及补加光敏剂的实验验证光催化过程中催化剂CHB-Co稳定性较强。最后通过电化学测试、动力学及荧光猝灭实验研究了CHB-Co的光催化机理,证实催化剂与光敏剂之间发生有效的电子传递,从而催化CO2发生还原反应,该研究结果为设计开发新型高效光催化还原CO2体系提供了科学依据和实验基础。
3-[(2-羟基-5-氯苯亚甲基)-氨基]-7-羟基香豆素钴配合物用于光催化还原CO2
The study of 3-[(2-hydroxy-5-chloro-benzylidene)-amino]-7- hydroxy-coumarin cobalt complex for photocatalytic reduction of CO2
-
摘要: 采用2,4-二羟基苯甲醛为起始原料合成了一种香豆素类席夫碱有机配体3-[(2-羟基-5-氯苯亚甲基)-氨基]-7-羟基香豆素CHB,接着将该配体与二价钴金属盐配位制备了新型的离子型金属-有机分子配合物CHB-Co,采用多种手段分别表征了配体和配合物的结构特征,然后研究了席夫碱类配合物CHB-Co对CO2还原的可见光催化活性。结果表明,优化各项实验条件后,配合物CHB-Co在H2O/CH3CN溶液 (V∶V=1∶4) 中光催化CO2还原的主要产物CO的产量为14.68 μmol,转换数(TON)值达1468,转换率(TOF)值为146.8 h−1,选择性高达90%。通过不同光照时间下催化剂与光敏剂的紫外可见吸收光谱实验结果验证光催化过程中催化剂CHB-Co稳定存在,光敏剂的光降解是造成光催化体系停滞的主要原因。电化学实验结果显示CO2氛围下配合物CHB-Co发生还原反应的起始电位位于−0.89 V(vs NHE),表明CHB-Co足以驱动电子从光敏剂的金属中心转移到催化剂,催化剂与光敏剂之间发生了有效的电子传递,从而催化CO2发生还原反应。通过荧光光谱和荧光猝灭实验可知,牺牲剂不会对激发态光敏剂产生猝灭效应,而催化剂可以,并且随着催化剂浓度的增大,其对激发态光敏剂的猝灭效应也逐渐增强,表明催化剂CHB-Co与光敏剂之间能够较好地进行电子传输,从而展现出该材料在减轻环境污染和CO2资源化利用方面的潜力。Abstract: While 2, 4-dihydroxy benzaldehyde was chosen as starting material, a coumarin Schiff-base ligand 3-[(2-hydroxy-5-chloro-benzylidene)-amino]-7- hydroxy -coumarin CHB was prepared. Subsequently, a novel cobalt Schiff base ionic metal-organic molecular complexes CHB-Co was synthesized and then characterized by various methods. The photocatalytic activities of this cobalt Schiff base complexes for visible-light-driven CO2 reduction were studied in detail. The experimental results show that CHB-Co possess excellent photocatalytic efficiency for CO2 reduction in H2O/CH3CN solution (V∶V=1∶4) under the optimum conditions. The yield of the main product CO was 14.68 µmol with the turnover number (TON) value of 1468 and the turnover frequency (TOF) value of 146.8 h−1. The selectivity was up to 90%. The results of UV-Vis absorption spectra of the catalyst and the photosensitizer under different illumination time verified that the catalysts CHB-Co was stable in the photocatalytic process, and the photodegradation of the photosensitizer was the main reason for the stagnation of the photocatalytic system. In contrast, electrochemical result showed that the initial potential of CHE-Co was −0.89 V (vs NHE), indicating that it is sufficient to drive electron transfer from the metal center of the photosensitizer toward the catalyst, and effective electron transfer occurred between the catalyst and the photosensitizer in the process of photocatalytic conversion of CO2. The fluorescence spectra and fluorescence quenching experiments showed that the sacrificial agent didn’t have quenching effect on the excited photosensitizer, while the catalyst did. With the increase of catalyst concentration, the quenching effect on the excited photosensitizer was gradually enhanced, suggesting that electron transport can be carried out between the catalyst and the photosensitizer. Therefore, above results show the perspective highlights of this material in reducing environmental pollution and the utilization of CO2 resources.
-
Key words:
- cobalt Schiff-base complex /
- synthesis /
- photocatalytic reduction of CO2 /
- mechanism.
-

-
表 1 不同催化剂光催化CO2为CO转化结果
Table 1. Results of photocatalytic CO2 to CO conversion with different catalysts
催化剂
Catalysts光敏剂
Photosensitizer转换数
TONCO转化频率/ h−1
TOFCO选择性 /%
Selectivity参考文献
LiteraturesCo-bipy CdS 4.1 0.5 88 16 Fe-CB CdS 1220 152.5 85 17 [Co(NTB)CH3CN](ClO4)2 $\rm Ru(phen)_3^{2+} $ 1179 115.2 97 18 Co-ZIF-67 $\rm Ru(bpy)_3^{2+} $ 112 224 66.7 19 CHB-Co $\rm Ru(phen)_3^{2+} $ 1468 146.8 90 本文 表 2 CHB-Co在不同控制变量下的光催化CO2还原结果
Table 2. Photoinduced CO2 reduction results by CHB-Co under different conditions
条目
EntryCHB-Co添加量 / (µmol·L−1)
AddedCO产量/µmol
Yield of COH2产量/µmol
Yield of H2转换数TONco 转换频率/h−1
TOFcoCO 选择性/ %
Selectivity of CO1 0.5 14.68 1.52 1468 146.8 90 2 0 trace trace 0 0 0 3 0.5 0 0 0 0 0 4 0.5 0 0 0 0 0 5 0.5 0 0 0 0 0 6 0.5 0 0.014 0 0 0 7 0 2.96 0.14 296 29.6 95 -
[1] KONDRATENKO E V, MUL G, BALTRUSAITIS J, et al. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes [J]. Energy & Environmental Science, 2013, 6(11): 3112. [2] RAO H, SCHMIDT L C, BONIN J, et al. Visible-light-driven methane formation from CO2 with a molecular iron catalyst [J]. Nature, 2017, 548(7665): 74-77. doi: 10.1038/nature23016 [3] LIU Y Y, YANG Y M, SUN Q L, et al. Chemical adsorption enhanced CO2 capture and photoreduction over a copper porphyrin based metal organic framework [J]. ACS Applied Materials & Interfaces, 2013, 5(15): 7654-7658. [4] ZHANG J H, WEI M J, WEI Z W, et al. Ultrathin graphitic carbon nitride nanosheets for photocatalytic hydrogen evolution [J]. ACS Applied Nano Materials, 2020, 3(2): 1010-1018. doi: 10.1021/acsanm.9b02590 [5] GAO C, WANG J, XU H X, et al. Coordination chemistry in the design of heterogeneous photocatalysts [J]. Chemical Society Reviews, 2017, 46(10): 2799-2823. doi: 10.1039/C6CS00727A [6] BERARDI S, DROUET S, FRANCÀS L, et al. Molecular artificial photosynthesis [J]. Chemical Society Reviews, 2014, 43(22): 7501-7519. doi: 10.1039/C3CS60405E [7] WANG J, ZHONG Y Y, BAI C, et al. Series of coordination polymers with multifunctional properties for nitroaromatic compounds and CuII sensing [J]. Journal of Solid State Chemistry, 2020, 288: 121381. doi: 10.1016/j.jssc.2020.121381 [8] CHAI X M, HUANG H H, LIU H P, et al. Highly efficient and selective photocatalytic CO2 to CO conversion in aqueous solution [J]. Chemical Communications, 2020, 56(27): 3851-3854. doi: 10.1039/D0CC00879F [9] XUE X F, LIU Y Q, LIU Q, et al. Four novel coordination polymers based on flexible 1, 4-bis(1, 2, 4-triazol-1-ylmethyl)benzene ligand: Synthesis, structure, luminescence and magnetic properties [J]. Journal of Cluster Science, 2019, 30(3): 777-787. doi: 10.1007/s10876-019-01539-2 [10] BI Q Q, WANG J W, LV J X, et al. Selective photocatalytic CO2 reduction in water by electrostatic assembly of CdS nanocrystals with a dinuclear cobalt catalyst [J]. ACS Catalysis, 2018, 8(12): 11815-11821. doi: 10.1021/acscatal.8b03457 [11] JIANG J H, LEI Y H, LI X, et al. New cobalt(II) Schiff base complex: Synthesis, characterization, DFT calculation and antimicrobial activity [J]. Inorganic Chemistry Communications, 2021, 127: 108350. doi: 10.1016/j.inoche.2020.108350 [12] DURGAPAL S D, SONI R, SOMAN S S, et al. Synthesis and mesomorphic properties of coumarin derivatives with chalcone and imine linkages [J]. Journal of Molecular Liquids, 2020, 297: 111920. doi: 10.1016/j.molliq.2019.111920 [13] IRFAN R M, JIANG D C, SUN Z J, et al. Enhanced photocatalytic H2 production on CdS nanorods with simple molecular bidentate cobalt complexes as cocatalysts under visible light [J]. Dalton Transactions, 2016, 45(32): 12897-12905. doi: 10.1039/C6DT02148D [14] KEYPOUR H, SHAYESTEH M, REZAEIVALA M, et al. Synthesis and characterization of a series of transition metal complexes with a new symmetrical polyoxaaza macroacyclic Schiff base ligand: X-ray crystal structure of cobalt(Ⅱ) and nickel(Ⅱ) complexes and their antibacterial properties [J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2013, 101: 59-66. doi: 10.1016/j.saa.2012.09.048 [15] SHAHABADI N, KASHANIAN S, DARABI F. DNA binding and DNA cleavage studies of a water soluble cobalt(Ⅱ) complex containing dinitrogen Schiff base ligand: The effect of metal on the mode of binding [J]. European Journal of Medicinal Chemistry, 2010, 45(9): 4239-4245. doi: 10.1016/j.ejmech.2010.06.020 [16] CHAI Z G, LI Q, XU D S. Photocatalytic reduction of CO2 to CO utilizing a stable and efficient hetero–homogeneous hybrid system [J]. RSC Advances, 2014, 4(85): 44991-44995. doi: 10.1039/C4RA08848D [17] GUO J H, DAO X Y, SUN W Y. An iron-nitrogen doped carbon and CdS hybrid catalytic system for efficient CO2 photochemical reduction [J]. Chemical Communications, 2021, 57(16): 2033-2036. doi: 10.1039/D0CC07692A [18] OUYANG T, HOU C, WANG J W, et al. A highly selective and robust Co(Ⅱ)-based homogeneous catalyst for reduction of CO2 to CO in CH3CN/H2O solution driven by visible light [J]. Inorganic Chemistry, 2017, 56(13): 7307-7311. doi: 10.1021/acs.inorgchem.7b00566 [19] QIN J N, WANG S B, WANG X C. Visible-light reduction CO2 with dodecahedral zeolitic imidazolate framework ZIF-67 as an efficient co-catalyst [J]. Applied Catalysis B:Environmental, 2017, 209: 476-482. doi: 10.1016/j.apcatb.2017.03.018 [20] WESTHUIZEN D, ESCHWEGE K G, CONRADIE J. Electrochemistry and spectroscopy of substituted [Ru(phen)3]2+ and [Ru(bpy)3]2+ complexes [J]. Electrochimica Acta, 2019, 320: 134540. doi: 10.1016/j.electacta.2019.07.051 [21] LIU D C, HUANG H H, WANG J W, et al. Highly efficient and selective visible-light driven CO2-to-CO conversion by a co(Ⅱ) homogeneous catalyst in H2O/CH3CN solution [J]. ChemCatChem, 2018, 10(16): 3435-3440. doi: 10.1002/cctc.201800727 [22] CHAN S L F, LAM T L, YANG C, et al. A robust and efficient cobalt molecular catalyst for CO2 reduction [J]. Chemical Communications, 2015, 51(37): 7799-7801. doi: 10.1039/C5CC00566C [23] OUYANG T, HUANG H H, WANG J W, et al. A dinuclear cobalt cryptate as a homogeneous photocatalyst for highly selective and efficient visible-light driven CO2 reduction to CO in CH3CN/H2O solution [J]. Angewandte Chemie (International Ed. in English), 2017, 56(3): 738-743. doi: 10.1002/anie.201610607 [24] SHIMODA T, MORISHIMA T, KODAMA K, et al. Photocatalytic CO2 reduction by trigonal-bipyramidal cobalt(Ⅱ) polypyridyl complexes: The nature of cobalt(I) and cobalt(0) complexes upon their reactions with CO2, CO, or proton [J]. Inorganic Chemistry, 2018, 57(9): 5486-5498. doi: 10.1021/acs.inorgchem.8b00433 -



 下载:
下载: