-
随着世界人口的不断增加和经济发展需求的攀升,世界上越来越多的地区出现了缺水的现象[1]。2020年,全球超过20亿人生活在水资源紧张的国家,无法获得安全的饮用水,到2050年全球将有50亿人面临水资源短缺问题[2]。我国水资源总量虽然大,但我国人口众多,且水资源分布不平衡,导致我国约1/4的省份面临严重缺水问题。我国也是水资源污染严重的国家之一。中国的污废水排放量极大,2019年全国污水排放量554.65亿立方米,同比增长6.4%[3]。水污染加剧和水资源减少已成为制约经济社会发展的瓶颈,亟须发展水污染治理的新原理和新技术。
纳米材料与技术为高效、低成本的水环境中污染物去除开辟了新的途径[4],其在吸附[5-6]、催化[7-8]、氧化还原处理[9-11]和膜分离[12-14]方面的应用越来越多。纳米科技将对开发更清洁、更环保的水处理技术产生重大影响,如何基于纳米材料开发更高效的水处理技术是环境领域的热点问题[15]。与微米级或更大粒径的材料相比,纳米材料由于其具有较高的比表面积在吸附工艺中表现出卓越的水处理能力[16],如黏土[17]、沸石[18]、金属氧化物[19-20]、聚合膜[21-22]、多孔纳米纤维[23-24]和零价铁[25-26]等吸附材料在对重金属离子、有机物、染料和病原体等常见污染物的吸附过程中表现出了反应速度快、选择性高和去除能力强等优势[27-28]。尽管基础研究成果众多,但是纳米材料的工程化应用仍存在技术瓶颈,如何提高纳米材料的分散性、稳定性和安全性是纳米材料大规模应用的基础和前提[29-31]。
大量研究表明,将纳米材料负载在合适的基体材料上是解决上述问题的有效方法之一[32-33]。选用合适的基体物质可以将具有反应活性的纳米材料分隔在离散但接近的空间内,基体材料的选择性通道可以提供隐蔽的环境,不仅可以使分子扩散得到良好的控制,还能使纳米材料不受其他反应成分的干扰而保持活性[34-36]。用磁性物质作基体可以使得材料在磁场作用下快速从水介质中分离[37],与传统的过滤法相比,操作过程简单,且不容易引起堵塞或材料的流失[38]。限域空间内生长的纳米材料还显示出了优越的目标污染物净化功能[39-40]。本文综述了空间限域型纳米复合材料的合成方法,阐述了由多孔材料构成的限域空间内活性纳米颗粒尺寸、污染物富集对吸附过程的影响,总结了限域空间内物质分子微观结构和晶型等纳米材料表面特性,从分子水平揭示孔材料限域空间对吸附剂吸附性能、吸附反应过程、反应产物和吸附剂再生性能的作用机制,最后对空间限域型纳米复合材料在水处理领域的应用前景进行了展望,以期为纳米吸附剂制备、吸附过程的理论模拟、吸附剂和吸附作用的原位动态表征和纳米吸附剂的水处理应用等方面提供理论指导和技术支撑。
-
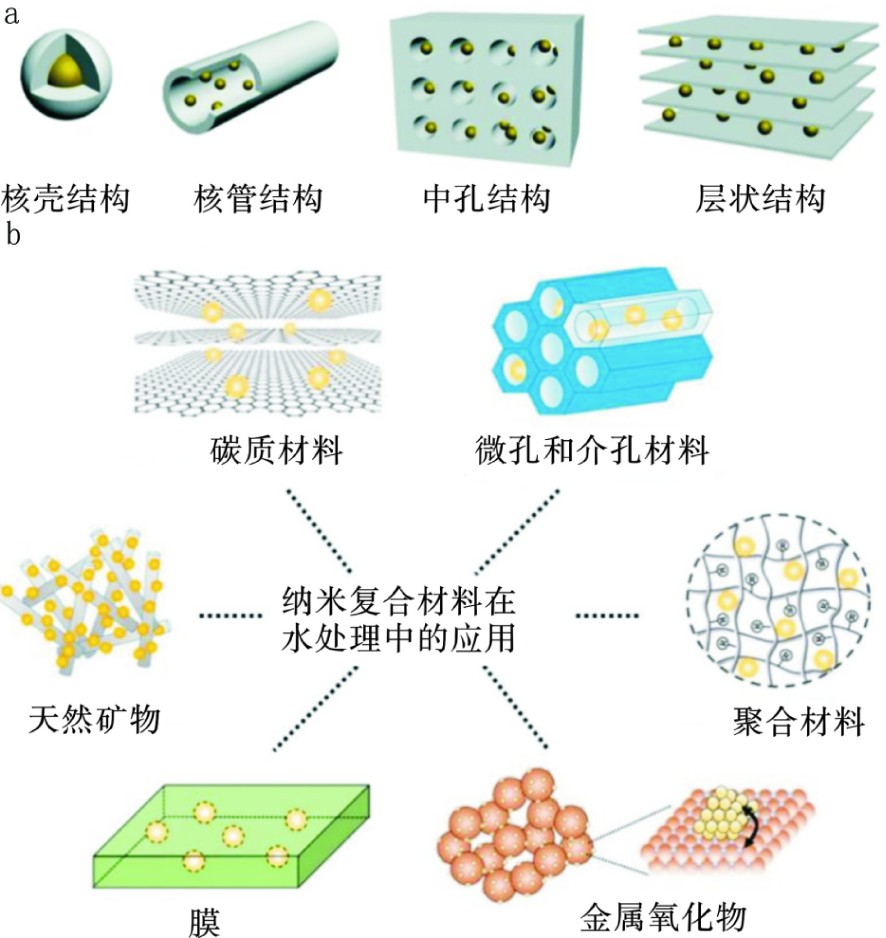
限域结构纳米复合材料的框架材料又称为基质材料,图1(a)可知,通常具有可调节和可加工的形状,主要是多孔结构,以形成不同的封装结构[41],如核-壳、核-管、介孔和层状结构。天然矿物、碳质材料、微孔和中孔材料、聚合物材料和金属氧化物等材料都可以被用作负载纳米粒子以进行水处理的基质材料[42],见图1(b)。空间限域型纳米复合材料的制备方法有浸渍法[43]、水热法[44]、溶胶-凝胶法[45]、原位合成法[46]、双溶剂法[47]和原子层沉积法[48]等。
浸渍法主要利用基质材料中的孔隙空间,纳米材料熔体通过毛细作用渗入孔隙,通过施加压力或抽真空促进浸渍过程[49-50],浸渍平衡后通过干燥和烘焙等工序后得到复合材料。采用该法时,纳米材料的化学反应性、熔体的黏度以及基体的浸润性等因素都会影响复合材料的性能。
水热法通常在带有聚四氟乙烯衬里高压釜等钢制压力容器中进行,基体材料和形成纳米颗粒所需的反应物混合在水溶液中在一定温度和压力下进行反应[51]。温度可以升高到水的沸点以上,达到蒸汽饱和压力。反应体系压力的大小由反应温度和加入高压釜的溶液量决定。水热法所得纳米颗粒晶体结构完整且不易团聚,是广泛用于合成复合材料的一种方法。
溶胶-凝胶法是在溶液或溶胶中加入纳米材料组分,经搅拌使之在液相中均匀分布,溶胶陈化后胶粒慢慢聚合,形成具有空间网络结构的凝胶,后经干燥或一定的温度热处理,压制、烧结即可形成复合材料[52]。目前该法所需成本一般较高,但随着需求的增加和生产规模的扩大,该法或成为未来复合材料合成的重要手段之一。
原位合成法是在基体中加入含有纳米相元素的化合物,控制其反应生成条件,使纳米颗粒在基体材料上原位生成[53]。该法可制作结构复杂的材料,成本低,有效避免了纳米晶体与人体的直接接触,是实现工业化生产最有前景的方法之一。
各合成方法的适用条件和优缺点,见表1。
-
限域空间内的纳米材料与传统纳米材料相比最显著的优点即为纳米颗粒的分散性好、稳定性强和纳米颗粒尺寸可调。首先,分散性好的纳米颗粒可以提供更多的有效吸附位点。DING et al[32]研究发现,当聚苯胺限制在的聚苯乙烯支架内时,支架的多孔结构使之表现出更多的作用位点,其对Cr(Ⅵ)的去除能力较分散的聚苯胺提高了5.4倍。其次,框架材料特定的尺寸空间限制了纳米颗粒的生长,可以通过选择合适的框架材料来准确调控纳米颗粒的粒径。在三维介孔网络中合成的钴铁氧体(CoFe2O4)颗粒粒径为6 nm左右,其对亚砷酸盐的吸附量高达252.8 mg/g[39],远高于CoFe2O4纳米聚集体(粒径10~30 nm,吸附量100 mg/g)[54]。除了利用框架材料本身的尺寸限制,还可以通过预先沉积法抑制活性纳米颗粒的浸出和再聚集。在MCM-41通道中预先沉积TiO2纳米点来合成的超细α-Fe2O3对多种阴离子污染物都表现出了优异的吸附性能,预先沉积的TiO2纳米颗粒将通道雕刻成的纳米格栅不仅通过空间限制将Fe2O3限制在超细尺寸,而且还抑制了它们在吸附过程中的浸出、迁移和热再生时的颗粒聚集[55]。除了可以显著提升吸附容量,限域空间纳米材料还表现出了高度的选择性。如,N,N-二水杨酸-4,5-二甲基亚苯基固定在介孔二氧化硅上制成的共轭纳米材料对Cu(Ⅱ)表现出高度选择性,在Ca2+、Pb2+和Mg2+等多种共存金属离子的存在下,材料对Cu(Ⅱ)离子的吸附率超过95%,且再生后无明显的吸附容量损失[56]。
-
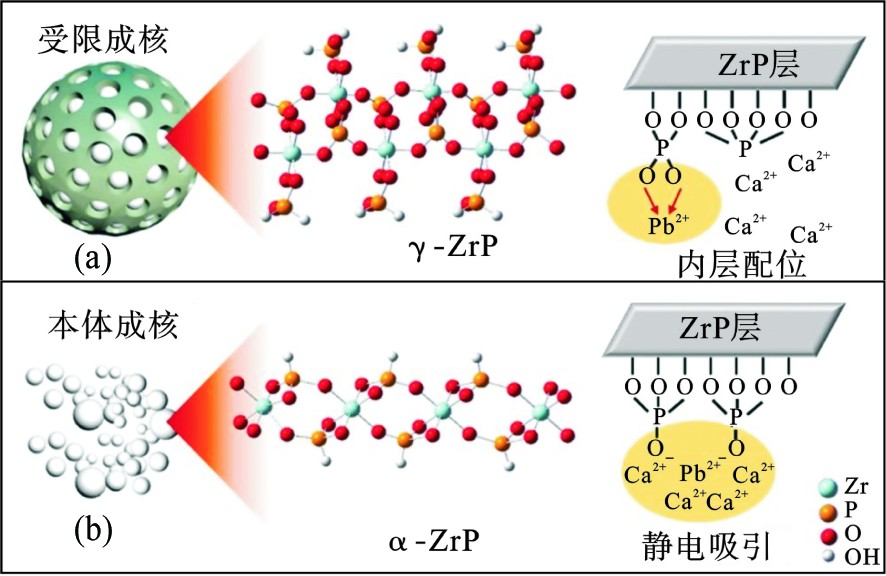
在尺寸仅为几个纳米的限域空间中,纳米颗粒的结晶行为与在敞开体系中差别很大,表现出晶体体积减小、晶型种类转化以及晶型稳定性变化等。以TiO2为例,热力学稳定性较差的锐钛矿是最活跃的相这点已成为人们的共识[57]。但当TiO2纳米颗粒的尺寸<14 nm时,锐钛矿型的TiO2纳米材料热力学稳定大大提升[58]。因此,可以通过将锐钛矿TiO2纳米颗粒的生长和聚集限制在该临界值以下,来确保其在高效去除污染物同时还能保持良好的稳定性。此外,以石墨烯为基底的氧化锆复合材料中,通过控制溶剂三乙醇胺的用量和反应时间,可得到不同晶相的氧化锆。其中水热反应器中三乙醇胺用量为0.1 mol/L反应9 h得到的氧化铬单斜晶相在吸附3-硝基-4-羟基苯胂酸(3-NHPAA)方面表现出色,具有更大的吸附容量和更高的pH适应范围[59]。磷酸锆在7.9 nm的孔道中生长成为热力学稳定的α相和亚稳态γ相的混合物,由于γ相的存在,材料吸附性能提高了10~90倍。与α相磷酸锆非特异性的静电作用不同,限域空间中生长的磷酸锆主要通过特异性较强的内层配位作用实现对重金属的吸附去除[60],见图2。不同晶型的氧化铁同样表现出与尺寸相关的特异性热力学稳定性[61],对于水处理中常见的氧化铁,纳米限域可作为一种有效的工具来稳定亚稳态的氧化铁多晶型物,亚稳态多晶型水铁矿与其他多晶型物相比,对砷酸盐[62]和镭[40]表现出更高的吸附能力。
除了晶型差异,限域空间还能调控纳米材料的主暴露晶面。将铂金纳米颗粒负载在氧化还原石墨烯上,球形的多面体铂金颗粒演化为(110)晶面为主的树枝状多聚体,改善了该材料循环使用的耐久性[63]。与主暴露晶面为(111)的常规Fe3O4纳米粒子相比,由3D石墨烯提供的纳米限域生成的具有优先暴露(400)晶面的Fe3O4纳米粒子对洛克沙胂的吸附能力由163.93提至454.48 mg/g [64]。限制在石墨烯网络中的ZnFe2O4纳米晶体对亚甲基蓝的高效光催化降解归因于(311)结晶面的暴露[65]。因此,纳米限域可作为一种有效的工具来控制纳米粒子活性晶面的暴露以获得所需的材料性能。
-
限域空间内的水相会表现出独特的结构、热力学和动力学特性,这些变化都不可避免地改变吸附材料对水相中污染物的吸附过程。当空间尺寸被限制在几个纳米甚至<1 nm时,水分子表现出不同于开放体系的独特结构和特性[66-68],其中氢键数目的减少[69]、离子水合被抑制[70]和介电常数的降低[71]将对污染物的吸附过程产生重要影响。
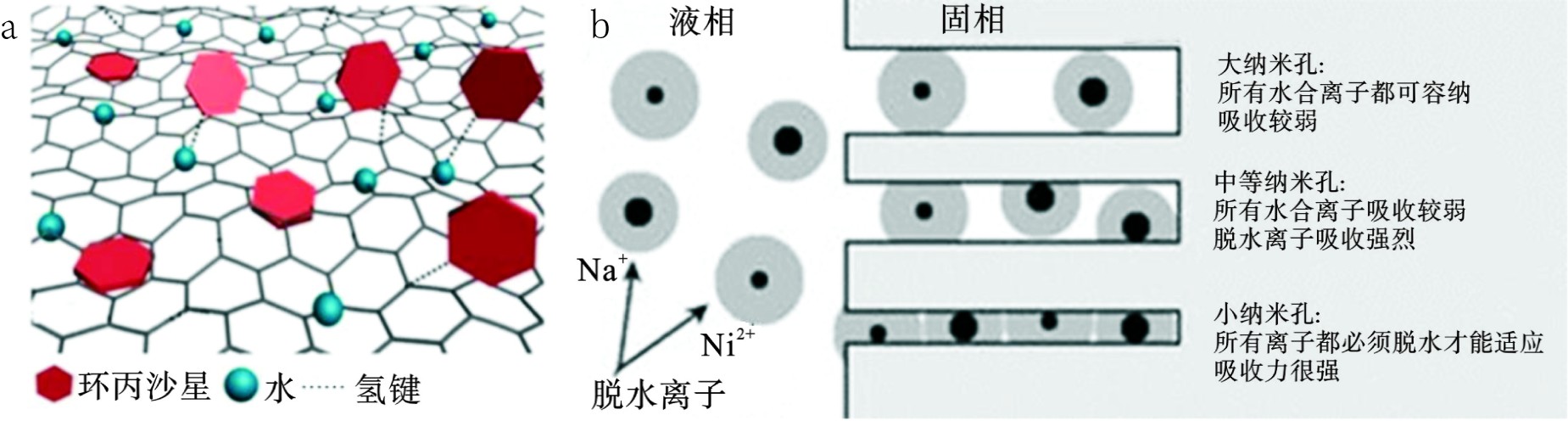
限域水分子大部分氢键是以扭曲的四面体结构或断裂的网络形式出现的,键的损失和相邻水分子的竞争减少使得水分子与一些有机污染物分子的氢键作用增强,从而使材料表现出更多地吸附位点和更强的吸附能力[72-73]。研究发现,相似的强氢键相互作用显著增强了多孔石墨烯材料对抗生素、染料和重金属离子等各类污染物的吸附能力[74-77],见图3(a)。此外,纳米限域下离子水合数和介电常数的降低会导致目标离子的脱水自由能垒下降[78-79],这种变化不可避免地影响其与吸附位点之间的相互作用,表现在材料吸附容量增加、选择性提高和离子水合状况变化导致吸附机制的转变。与没有纳米孔的沸石相比,具有纳米孔的沸石矿物对Na+、K+、Ca2+和Ni2+等阳离子的容量和选择性有所提高,这归因于纳米孔中促进脱水导致的吸附机制由离子交换到内球络合的转变[80-86]。图3(b)可知,当限域空间尺寸大于离子的水合直径时,所有的离子即使在水合的情况下也能进入孔中,并且以弱的外球吸附为主;在中型纳米孔中,一些离子必须脱水进入孔内限域环境,脱水后的离子以较强的内球吸附作用存在于孔道中;而在小的纳米孔中,所有的离子都必须脱水才能进入孔道,诱导材料与离子之间形成内球吸附,这可能对后续去除如F-和Cl-等强水合离子的研究具有重要指导意义[80]。
限域空间的水分子层在一些情况下表现出特殊的促吸附效应。带负电的黏土与碘离子因静电排斥作用通常几乎不发生化学反应,但是当碘离子进入层状黏土层间的时候,黏土层间的水分子可促进碘—钠离子对的形成,碘离子可以在钠盐介质中的黏土上发生吸附[87]。另有研究发现,黑皂膜(BSFs)内水芯厚度为2.7~6.2 nm,纳米封闭水分子的低介电常数有利于氢离子和氢氧根离子以离子对的形式存在,在质子转移反应中的电荷对无法有效形成,可作为缓冲介质响应外界pH的变化,可以抵抗酸/碱溶液[88]。因此,可利用限域空间内水分子的低介电常数来设计化学稳定性或反应活性更强的纳米颗粒,以拓展纳米材料的应用潜力和发展空间。
-
一般而言,限域空间由于强制将反应物分子限制在非常接近的位置并消除了在体相中普遍存在的扩散作用,反应动力学通常显著增强,化学反应速率明显提升。以碳纳米管(CNTs)材料为例,材料内部弯曲的石墨烯层形成了纳米通道,污染物分子在通道内都得到了富集,并且这种富集现象随着纳米管尺寸的减小变得更加明显,化学反应的速率也随之加快[89]。此外,由于内部表面性质的差异,限域空间会表现出对一些物质的优先富集,如,由于分子的性质和由石墨烯层形成的CNT的凹面和凸面的不同电子特性,CO与CNT内表面的相互作用比H2更强,在CNT内部优先富集,CO/H2的体积比明显高于CNT外部[90],这种分子的富集和比例的改变可以提供新的研究思路,即通过将化学反应限制在CNTs内部来调整反应速率和选择性。CNT通道的封闭疏水环境还可以加强反应过程中产物和反应物的分离,从而改变反应的平衡。以苯的羟基化为苯酚为例,苯在CNT通道内被富集,而产物苯酚则被选择性地排出通道[91]。此外,将反应物限制在限域空间内可以延长它们与吸附材料的接触时间[92],封装的纳米粒子因此表现出更高的活性[93]。因此,可以通过调控和优化复合纳米材料的结构,利用狭小空间的富集效应、内部孔道对物质的选择性以及反应物接触时间可调性等限域效应来研制和开发更高性能的水处理材料。
-
限域空间内吸附产物特异性的结晶行为会对吸附过程和水处理结果造成影响。环境中常见的Ca2+会大大影响水合氧化铁(HFO)对磷酸盐的吸附,图4(a)可知,材料表面羟基磷酸石的结晶会覆盖位点而阻止了材料进一步的吸附作用[94];但在介孔聚苯乙烯珠提供的纳米限域中,图4(b)可知,由于羟基磷灰石的独立结晶行为,Ca2+的存在促进了HFO纳米颗粒对磷酸盐的长期吸附,不影响HFO表面进一步吸附磷酸盐[95]。
通过水合氧化镧(HLO)去除磷酸盐的主要机制是内球络合,然而在SBA-15沸石、聚偏二氟乙烯膜、介孔二氧化硅和交联聚苯乙烯等各种基材的纳米限域下,HLO纳米粒子通过形成LaPO4·xH2O纳米晶体来固定磷酸盐[85, 96-97]。吸附机制转变的同时,材料卓越的稳定性、高吸附力以及快速的吸附动力学等特性都让限域型复合材料成为去除磷酸盐的更好选择。Zn(Ⅱ)在大块SiO2颗粒上的吸附产物主要为单齿、分角配合物,在小孔径(≤10 nm)的纳米多孔SiO2中与氧原子形成四面体配位,在大孔径(>10 nm)的SiO2中以Zn-O四面体和八面体配位的表面复合物的混合物存在[98]。可以看出,随着孔径减小,SiO2对Zn(Ⅱ)的吸附产生了不同的表面配合物。Cu(Ⅱ)在无孔SiO2上的吸附是一个吸热过程,Cu(Ⅱ)在表面络合之前经历脱水。相比之下,在纳米级SiO2孔隙中的吸附前仅发生部分脱水,并有大量的Cu-Cu多核复合物形成,这导致了一个整体的放热信号[99]。SiO2孔隙中的封闭促进了Cu-Cu的相互作用,导致Cu-Cu多核表面复合物的形成,Cu(Ⅱ)局部配位环境的差异导致了吸附过程的改变。
-
纳米材料及其技术的发展在水处理行业发展前景广阔,然而纳米材料在实际工程应用中仍存在易团聚、易流失、难回收、选择性较差等问题。基于纳米限域效应开发合成的纳米复合材料吸附剂是解决上述困难的有效途径之一。本文从限域空间下的水环境介质、吸附材料与吸附产物的特异结构和反应原理等多角度综述了限域结构纳米复合材料在水污染治理领域的最新研究成果,有望为拓展水污染治理领域纳米技术发展的新方向、推动纳米技术的实用化进程提供理论依据与方法参考。
在该领域还存在着较多有待解决的问题和广阔的发展空间,具体如下。
(1)反应机理研究还需要进一步深入。目前对于传统固液界面中污染物的形态与转化研究较为广泛,但对于发生在限域环境中的污染物形态与转化过程的研究则非常缺乏;如何利用限域效应实现对污染物吸附反应动力学、反应产物及吸附材料可再生性能的高效调控仍然没有解决;对于限域空间内的吸附反应来说,关键控制步骤究竟是限域空间内的吸附/解吸过程还是污染物进入限域空间的扩散尚没有定论。因此,需要应用实验室模拟和理论计算等多种手段来联合推进相关工作。
(2)真实环境体系下的应用。目前关于纳米复合材料在环境中的迁移与转化主要以实验室模拟为主,但在实际应用中,光照、温度、溶解氧和有机质等都可能会影响材料在水中的反应活性。哪些因素会造成复合材料中纳米材料的脱落、聚集和流失是纳米复合材料实际应用中亟待解决的问题。因此,在以后的研究中需要综合考虑多种环境参数,力求反映真实环境体系。另外,吸附剂的再生性能是其在工业生产中能否顺利实施的关键指标之一,限域空间对纳米材料的再生过程影响尚不明确,需要进一步的科学评估。
(3)材料的成本效益比和生态安全性。效益-成本比的经济分析始终是纳米复合材料应用于污水处理的重要研究内容。在对纳米复合材料开展规模化应用研究的同时,研究者们也需正视其可能存在的环境危害并采取积极的防控措施。例如纳米复合材料在水处理过程中可能存在残留,尤其部分材料所含的金属氧化物和有机聚合物会给环境安全和公共健康带来一定的潜在风险。因此迫切需要科学评估材料的环境与健康风险,给纳米材料的合理设计和安全应用提供科技支撑。
限域结构纳米复合材料及其吸附性能研究进展
Research progress of confined space-constructed environment nanocomposites and their adsorption properties
-
摘要: 纳米材料因其比表面积大和表面活性高,在水处理领域表现出了极具潜力的发展前景。利用空间限域结构来固定和分散纳米材料可有效解决纳米材料易团聚失活、操作分离困难和潜在环境风险等问题。文章综述了具有限域结构的纳米复合材料制备方法及其对水中污染物吸附性能的研究进展,从限域空间内纳米颗粒的尺寸调控与污染物的富集、限域空间中特异性的污染物分子结构和纳米材料晶体结构等多方面详细分析了纳米限域效应的环境行为及其对水环境中污染物去除的重要意义。根据分析可知,限域结构中的吸附机理、纳米复合材料在真实环境体系下的应用、材料的环境与健康风险等是未来该领域研究的重要方向和热点内容。Abstract: Nanomaterials show a great potential in the field of water treatment due to their large specific surface area and high surface activity. The use of spatially confined structures to fix and disperse nanomaterials can effectively solve the problems of easy agglomeration and deactivation of nanomaterials, difficulty in operation and separation, and potential environmental risks. In this paper, the preparation methods of nanocomposites with confined structures and their research progress on the adsorption performance of pollutants in water are reviewed. The environmental behavior of the nanoconfinement effect and its significance to the removal of water pollutants are analyzed in detail from several aspects, including the specific molecular structure of pollutants and crystal structures of nanomaterials in the confined space. The adsorption mechanism in the confined space, application of the confined structure nanocomposites in real environmental systems, and the environmental and health risks of materials are the key directions and hot topics of future research in this field.
-

-
表 1 各合成方法的适用条件和优缺点
Table 1. Applicable conditions, advantages and disadvantages of each synthesis method
-
[1] POKRAJAC L A, ABBAS A, CHRZANOWSKI W, et al. Nanotechnology for a sustainable future: Addressing global challenges with the international network4sustainable nanotechnology[J]. ACS Nano, 2021, 15(12): 18608 − 23. doi: 10.1021/acsnano.1c10919 [2] WHO. Drinking-water. [EB/OL]. [2022-02-29]. https://www.who.int/news-room/fact-sheets/detail/drinking-water. [3] 葛察忠. 基于污水处理成本全覆盖的价格机制探析[J]. 环境保护, 2021, 49(7): 38 − 42. doi: 10.14026/j.cnki.0253-9705.2021.07.007 [4] FALINSKI M M, TURLEY R S, KIDD J, et al. Doing nano-enabled water treatment right: sustainability considerations from design and research through development and implementation[J]. Environmental Science Nano, 2020, 7(11): 3255 − 3278. doi: 10.1039/D0EN00584C [5] NEMATI F, REZAIE M, TABESH H, et al. Cerium functionalized graphene nano-structures and their applications: A review[J]. Environmental Research, 2022, 208: 112685 − 112685. doi: 10.1016/j.envres.2022.112685 [6] ZULIANI A, BANDELLI D, CHELAZZI D, et al. Environmentally friendly ZnO/Castor oil polyurethane composites for the gas-phase adsorption of acetic acid[J]. Journal of Colloid and Interface Science, 2022, 614: 451 − 459. doi: 10.1016/j.jcis.2022.01.123 [7] TANG C, ZHANG R, LU W, et al. Fe-Doped CoP nanoarray: A monolithic multifunctional catalyst for highly efficient hydrogen generation[J]. Advanced Materials, 2017, 29(2): 1602441. doi: 10.1002/adma.201602441 [8] JIA Y, ZHANG L, GAO G, et al. A heterostructure coupling of exfoliated Ni-Fe hydroxide nanosheet and defective graphene as a bifunctional electrocatalyst for overall water splitting[J]. Advanced Materials, 2017, 29(17): 1700017. doi: 10.1002/adma.201700017 [9] WANG L, CHEN W, ZHANG D, et al. Surface strategies for catalytic CO2 reduction: from two-dimensional materials to nanoclusters to single atoms[J]. Chemical Society Reviews, 2019, 48(21): 5310 − 5349. doi: 10.1039/C9CS00163H [10] WANG J, WANG G, CHENG B, et al. Sulfur-doped g-C3N4/TiO2 S-scheme heterojunction photocatalyst for Congo Red photodegradation[J]. Chinese Journal of Catalysis, 2021, 42(1): 56 − 68. doi: 10.1016/S1872-2067(20)63634-8 [11] LI X, KANG B, DONG F, et al. Enhanced photocatalytic degradation and H-2/H2O2 production performance of S-pCN/WO2.72 S-scheme heterojunction with appropriate surface oxygen vacancies[J]. Nano Energy, 2021, 81: 105671. doi: 10.1016/j.nanoen.2020.105671 [12] ZHAO K, KANG S X, YANG Y Y, et al. Electrospun functional nanofiber membrane for antibiotic removal in water: Review[J]. Polymers, 2021, 13(2): 226. doi: 10.3390/polym13020226 [13] LI Y, WU Q, GUO X, et al. Laminated self-standing covalent organic framework membrane with uniformly distributed subnanopores for ionic and molecular sieving[J]. Nature Communications, 2020, 11(1): 599 − 607. doi: 10.1038/s41467-019-14056-7 [14] CHEN G, LI T, CHEN C, et al. Scalable wood hydrogel membrane with nanoscale channels[J]. Acs Nano, 2021, 15(7): 11244 − 11252. doi: 10.1021/acsnano.0c10117 [15] ELGARAHY A M, ELWAKEEL K Z, AKHDHAR A, et al. Recent advances in greenly synthesized nanoengineered materials for water/wastewater remediation: An overview[J]. Nanotechnology for Environmental Engineering, 2021, 6(1): 1 − 24. doi: 10.1007/s41204-020-00095-9 [16] HUA M, ZHANG S, PAN B, et al. Heavy metal removal from water/wastewater by nanosized metal oxides: A review[J]. Journal of Hazardous Materials, 2012, 211-212: 317 − 331. doi: 10.1016/j.jhazmat.2011.10.016 [17] ZHANG T, WANG W, ZHAO Y, et al. Removal of heavy metals and dyes by clay-based adsorbents: From natural clays to 1D and 2D nano-composites[J]. Chemical Engineering Journal, 2021, 420: 127574. doi: 10.1016/j.cej.2020.127574 [18] MUBARAK M F, MOHAMED A M G, KESHAWY M, et al. Adsorption of heavy metals and hardness ions from groundwater onto modified zeolite: Batch and column studies[J]. Alexandria Engineering Journal, 2022, 61(6): 4189 − 4207. doi: 10.1016/j.aej.2021.09.041 [19] RAJPUT A, SHARMA P P, YADAV V, et al. Synthesis and characterization of different metal oxide and GO composites for removal of toxic metal ions[J]. Separation Science and Technology, 2019, 54(3): 426 − 433. doi: 10.1080/01496395.2018.1500596 [20] GAO L, LI Y, XIAO M, et al. Synthesizing new types of ultrathin 2D metal oxide nanosheets via half-successive ion layer adsorption and reaction[J]. 2d Materials, 2017, 4(2): 025031. doi: 10.1088/2053-1583/aa5b1b [21] TURKCAN C, SOMTURK B, OZDEMIR N, et al. Quercetin adsorption with imprinted polymeric materials[J]. Journal of Biomaterials Science-Polymer Edition, 2019, 30(11): 947 − 960. doi: 10.1080/09205063.2019.1612727 [22] BRAEKEN L, BOUSSU K, VAN DER BRUGGEN B, et al. Modeling of the adsorption of organic compounds on polymeric nanofiltration membranes in solutions containing two compounds[J]. Chemphyschem, 2005, 6(8): 1606 − 1612. doi: 10.1002/cphc.200400624 [23] LI F, CHEN C, WANG Y, et al. Activated carbon-hybridized and amine-modified polyacrylonitrile nanofibers toward ultrahigh and recyclable metal ion and dye adsorption from wastewater[J]. Frontiers of Chemical Science and Engineering, 2021, 15(4): 984 − 997. doi: 10.1007/s11705-020-2000-3 [24] BAI Y, HUANG Z H, KANG F. Surface oxidation of activated electrospun carbon nanofibers and their adsorption performance for benzene, butanone and ethanol[J]. Colloids and Surfaces a-Physicochemical and Engineering Aspects, 2014, 443: 66 − 71. [25] ZHOU Y, WANG T, ZHI D, et al. Applications of nanoscale zero-valent iron and its composites to the removal of antibiotics: A review[J]. Journal of Materials Science, 2019, 54(19): 12171 − 12188. doi: 10.1007/s10853-019-03606-5 [26] LYE J W P, SAMAN N, NOOR A M M, et al. Application of nanoscale zero-valent iron-loaded natural zeolite for tetracycline removal process[J]. Chemical Engineering & Technology, 2020, 43(7): 1285 − 1296. [27] NAYERI D, MOUSAVI S A. Dye removal from water and wastewater by nanosized metal oxides - modified activated carbon: A review on recent researches[J]. Journal of Environmental Health Science and Engineering, 2020, 18: 1671 − 1689. doi: 10.1007/s40201-020-00566-w [28] M. SIDDEEG S, A. TAHOON M, BEN REBAH F. Simultaneous removal of calconcarboxylic acid, NH4+ and PO4U from pharmaceutical effluent using iron oxide-biochar nanocomposite loaded with pseudomonas putida[J]. Processes, 2019, 7(11): 800. doi: 10.3390/pr7110800 [29] ALVAREZ P J J, CHAN C K, ELIMELECH M, et al. Emerging opportunities for nanotechnology to enhance water security[J]. Nature Nanotechnology, 2018, 13(8): 634 − 641. doi: 10.1038/s41565-018-0203-2 [30] KELLER A A, LAZAREVA A. Predicted releases of engineered nanomaterials: from global to regional to local[J]. Environmental Science and Technology Letters, 2014, 1(1): 65 − 70. doi: 10.1021/ez400106t [31] MUELLER N C, NOWACK B. Exposure modeling of engineered nanoparticles in the environment[J]. Environmental Science & Technology, 2008, 42(12): 4447 − 4453. [32] DING J, PU L, WANG Y, et al. Adsorption and eeduction of Cr(VI) together with Cr(III) sequestration by polyaniline confined in pores of polystyrene beads[J]. Environmental Science & Technology, 2018, 52(21): 12602 − 12611. [33] FU H R, WANG N, QIN J H, et al. Spatial confinement of a cationic MOF: a SC–SC approach for high capacity Cr(Ⅵ)-oxyanion capture in aqueous solution[J]. Chemical Communications, 2018, 54(82): 11645 − 11648. doi: 10.1039/C8CC05990J [34] YANG H, FU L, WEI L, et al. Compartmentalization of incompatible reagents within pickering emulsion droplets for one-pot cascade reactions[J]. Journal of the American Chemical Society, 2015, 137(3): 1362 − 1371. doi: 10.1021/ja512337z [35] KLERMUND L, POSCHENRIEDER S T, CASTIGLIONE K. Biocatalysis in polymersomes: improving multienzyme cascades with incompatible reaction steps by compartmentalization[J]. ACS Catalysis, 2017, 7(6): 3900 − 3904. doi: 10.1021/acscatal.7b00776 [36] XU Z L, XIAO G W, LI H F, et al. Compartmentalization within self-assembled metal-organic framework nanoparticles for tandem reactions[J]. Advanced Functional Materials, 2018, 28(34): 1802479. doi: 10.1002/adfm.201802479 [37] MAO B, SIDHUREDDY B, THIRUPPATHI A R, et al. Efficient dye removal and separation based on graphene oxide nanomaterials[J]. New Journal of Chemistry, 2020, 44(11): 4519 − 4528. doi: 10.1039/C9NJ05895H [38] 张高生, 曲久辉, 刘会娟, 等. 活性炭/铁氧化物磁性复合吸附材料的制备及去除水中酸性橙Ⅱ的研究[J]. 环境科学学报, 2006, 26(11): 1763 − 1768. doi: 10.3321/j.issn:0253-2468.2006.11.002 [39] GEORGIOU Y, PAPADAS I T, MOUZOURAKIS E, et al. Mesoporous spinel CoFe2O4 as an efficient adsorbent for arsenite removal from water: high efficiency via control of the particle assemblage configuration[J]. Environmental Science:Nano, 2019, 6(4): 1156 − 1167. doi: 10.1039/C8EN01442F [40] CHEN M A, KOCAR B D. Radium sorption to iron (hydr)oxides, pyrite, and montmorillonite: implications for mobility[J]. Environmental Science & Technology, 2018, 52(7): 4023 − 4030. [41] LI S, GONG J. Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions[J]. Chemical Society Reviews, 2014, 43(21): 7245 − 7256. doi: 10.1039/C4CS00223G [42] QIAN J, GAO X, PAN B. Nanoconfinement-mediated water treatment: from fundamental to application[J]. Environmental Science & Technology, 2020, 54(14): 8509 − 8526. [43] ZHENG Y, HE P, FANG Y, et al. Hollow mesoporous silica supported PtIr bimetal catalysts for selective hydrogenation of phenol: significant promotion effect of iridium[J]. RSC Advances, 2017, 7(50): 31582 − 31587. doi: 10.1039/C7RA05653B [44] CHOI M, WU Z, IGLESIA E. Mercaptosilane-assisted synthesis of metal clusters within zeolites and catalytic consequences of encapsulation[J]. Journal of the American Chemical Society, 2010, 132(26): 9129 − 9137. doi: 10.1021/ja102778e [45] LU Z L, LINDNER E, MAYER H A. Applications of sol−gel-processed interphase catalysts[J]. Chemical Reviews, 2002, 102(10): 3543 − 3578. doi: 10.1021/cr010358t [46] PAN B, WU J, PAN B, et al. Development of polymer-based nanosized hydrated ferric oxides (HFOs) for enhanced phosphate removal from waste effluents[J]. Water Research, 2009, 43(17): 4421 − 4429. doi: 10.1016/j.watres.2009.06.055 [47] LIU C, LI J, QI J, et al. Yolk–shell Fe0@SiO2 nanoparticles as nanoreactors for fenton-like catalytic reaction[J]. ACS Applied Materials & Interfaces, 2014, 6(15): 13167 − 13173. [48] GEORGE S M. Atomic layer deposition: An overview[J]. Chemical Reviews, 2010, 110(1): 111 − 131. doi: 10.1021/cr900056b [49] 刘守新, 孙承林. 磁性椰壳活性炭的合成研究[J]. 新型炭材料, 2002, 17(1): 45 − 48. doi: 10.3321/j.issn:1007-8827.2002.01.011 [50] 单国彬, 张冠东, 田青, 等. 磁性活性炭的制备与表征[J]. 过程工程学报, 2004, 4(2): 141 − 145. doi: 10.3321/j.issn:1009-606X.2004.02.009 [51] 孙聆东, 付雪峰, 钱程, 等. 水热法合成CdS/ZnO核壳结构纳米微粒[J]. 高等学校化学学报, 2001, 22(6): 879 − 882. [52] 张健泓, 陈优生. 溶胶-凝胶法的应用研究[J]. 广东化工, 2008, 35(3): 47 − 49. doi: 10.3969/j.issn.1007-1865.2008.03.018 [53] 蔡利芳, 张永忠, 席明哲, 等. 原位合成法在材料制备中的应用及进展[J]. 金属热处理, 2005, 30(10): 1 − 6. doi: 10.3969/j.issn.0254-6051.2005.10.001 [54] ZHANG S, NIU H, CAI Y, et al. Arsenite and arsenate adsorption on coprecipitated bimetal oxide magnetic nanomaterials: MnFe2O4 and CoFe2O4[J]. Chemical Engineering Journal, 2010, 158(3): 599 − 607. doi: 10.1016/j.cej.2010.02.013 [55] SHEN G, PAN L, ZHANG R, et al. Low-spin-state hematite with superior adsorption of anionic contaminations for water purification[J]. Advanced Materials, 2020, 32(11): 1905988. doi: 10.1002/adma.201905988 [56] AWUAL M R. New type mesoporous conjugate material for selective optical copper(II) ions monitoring & removal from polluted waters[J]. Chemical Engineering Journal, 2017, 307: 85 − 94. doi: 10.1016/j.cej.2016.07.110 [57] LIU G, YANG H G, PAN J, et al. Titanium dioxide crystals with tailored facets[J]. Chemical Reviews, 2014, 114(19): 9559 − 5612. doi: 10.1021/cr400621z [58] CHEN X, MAO S S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications[J]. Chemical Reviews, 2007, 107(7): 2891 − 2959. doi: 10.1021/cr0500535 [59] ZOU S, DING B H, CHEN Y F, et al. Nanocomposites of graphene and zirconia for adsorption of organic-arsenic drugs: performances comparison and analysis of adsorption behavior[J]. Environmental Research, 2021, 195: 110752. doi: 10.1016/j.envres.2021.110752 [60] ZHANG X, SHEN J, PAN S, et al. Metastable zirconium phosphate under nanoconfinement with superior adsorption capability for water treatment[J]. Advanced Functional Materials, 2020, 30(12): 1909014. doi: 10.1002/adfm.201909014 [61] NAVROTSKY A, MAZEINA L, MAJZLAN J. Size-driven structural and thermodynamic complexity in iron oxides[J]. Science, 2008, 319(5870): 1635 − 1638. doi: 10.1126/science.1148614 [62] COUTURE R M, ROSE J, KUMAR N, et al. Sorption of arsenite, arsenate, and thioarsenates to iron oxides and iron sulfides: a kinetic and spectroscopic investigation[J]. Environmental Science & Technology, 2013, 47(11): 5652 − 5659. [63] YANG J, KANG D, JEON Y, et al. Sphere-to-multipod transmorphic change of nanoconfined Pt electrocatalyst during oxygen reduction reaction[J]. Small, 2019, 15(2): 1802228. doi: 10.1002/smll.201802228 [64] TIAN C, ZHAO J, ZHANG J, et al. Enhanced removal of roxarsone by Fe3O4@3D graphene nanocomposites: synergistic adsorption and mechanism[J]. Environmental Science:Nano, 2017, 4(11): 2134 − 2143. doi: 10.1039/C7EN00758B [65] YANG D, FENG J, JIANG L, et al. Photocatalyst interface engineering: spatially confined growth of ZnFe2O4 within graphene networks as excellent visible-light-driven photocatalysts[J]. Advanced Functional Materials, 2015, 25(45): 7080 − 7087. doi: 10.1002/adfm.201502970 [66] KYAKUNO H, MATSUDA K, YAHIRO H, et al. Confined water inside single-walled carbon nanotubes: global phase diagram and effect of finite length[J]. The Journal of Chemical Physics, 2011, 134(24): 244501. doi: 10.1063/1.3593064 [67] CHAKRABORTY S, KUMAR H, DASGUPTA C, et al. Confined water: structure, dynamics, and thermodynamics[J]. Accounts of Chemical Research, 2017, 50(9): 2139 − 2146. doi: 10.1021/acs.accounts.6b00617 [68] SOHRABI MAHBOUB M, FARROKHPOUR H. The dependence of the size of confined water fluid molecules on the radius of carbon nanotube[J]. Journal of Molecular Liquids, 2018, 266: 743 − 750. doi: 10.1016/j.molliq.2018.07.015 [69] ZHOU M, LI S, LU L, et al. The effect of surface wrinkles on the properties of water in graphene slit pores[J]. Molecular Simulation, 2020, 46: 604 − 615. doi: 10.1080/08927022.2020.1754411 [70] VARGHESE S, KANNAM S K, HANSEN J S, et al. Effect of hydrogen bonds on the dielectric properties of interfacial water[J]. Langmuir, 2019, 35(24): 8159 − 8166. doi: 10.1021/acs.langmuir.9b00543 [71] OLIVIERI J F, HYNES J T, LAAGE D. Confined water’s dielectric constant reduction is due to the surrounding low dielectric media and not to interfacial molecular ordering[J]. The Journal of Physical Chemistry Letters, 2021, 12(17): 4319 − 4326. doi: 10.1021/acs.jpclett.1c00447 [72] SUN Y, YU F, LI C, et al. Nano-/micro-confined water in graphene hydrogel as superadsorbents for water purification[J]. Nano-Micro Letters, 2019, 12(1): 1 − 14. [73] DE FRANCE K J, HOARE T, CRANSTON E D. Review of hydrogels and aerogels containing nanocellulose[J]. Chemistry of Materials, 2017, 29(11): 4609 − 4631. doi: 10.1021/acs.chemmater.7b00531 [74] SHEN Y, FANG Q, CHEN B. Environmental applications of three-dimensional graphene-based macrostructures: adsorption, transformation, and detection[J]. Environmental Science & Technology, 2015, 49(1): 67 − 84. [75] MA J, SUN Y, ZHANG M, et al. Comparative study of graphene hydrogels and aerogels reveals the important role of buried water in pollutant adsorption[J]. Environmental Science & Technology, 2017, 51(21): 12283 − 12292. [76] IGWEGBE C A, OBA S N, ANIAGOR C O, et al. Adsorption of ciprofloxacin from water: A comprehensive review[J]. Journal of Industrial and Engineering Chemistry, 2021, 93: 57 − 77. doi: 10.1016/j.jiec.2020.09.023 [77] CUKIERMAN A L, NUNELL G V, BONELLI P R. Removal of emerging pollutants from water through adsorption onto carbon-based materials[J]. Emerging and Nanomaterial Contaminants in Wastewater, 2019: 159 − 213. [78] LU C, HU C, RITT C L, et al. In Situ characterization of dehydration during ion transport in polymeric nanochannels[J]. Journal of the American Chemical Society, 2021, 143(35): 14242 − 14252. doi: 10.1021/jacs.1c05765 [79] NAZARI M, DAVOODABADI A, HUANG D, et al. Transport phenomena in nano/molecular confinements[J]. ACS Nano, 2020, 14(12): 16348 − 16391. doi: 10.1021/acsnano.0c07372 [80] SCHULTHESS C P, TAYLOR R W, FERREIRA D R. The nanopore inner sphere enhancement effect on cation adsorption: sodium and nickel[J]. Soil Science Society of America Journal, 2011, 75(2): 378 − 388. doi: 10.2136/sssaj2010.0129nps [81] March–April 2011 soil science society of america journal [J]. Soil Science Society of America Journal, 2011, 75(3): 1178-1178. [82] FERREIRA D R, SCHULTHESS C P, KABENGI N J. Calorimetric evidence in support of the nanopore inner sphere enhancement theory on cation adsorption[J]. Soil Science Society of America Journal, 2013, 77(1): 94 − 99. doi: 10.2136/sssaj2012.0140 [83] FERREIRA D R, SCHULTHESS C P. The nanopore inner sphere enhancement effect on cation adsorption: sodium, potassium, and calcium[J]. Soil Science Society of America Journal, 2011, 75(2): 389 − 396. doi: 10.2136/sssaj2010.0130nps [84] FERREIRA D R, SCHULTHESS C P, GIOTTO M V. An investigation of strong sodium retention mechanisms in nanopore environments using nuclear magnetic resonance spectroscopy[J]. Environmental Science & Technology, 2012, 46(1): 300 − 306. [85] ZHANG Y, PAN B, SHAN C, et al. Enhanced phosphate removal by nanosized hydrated La(III) oxide confined in cross-linked polystyrene networks[J]. Environmental Science & Technology, 2016, 50(3): 1447 − 1454. [86] MA J, YANG M, YU F, et al. Water-enhanced removal of ciprofloxacin from water by porous graphene hydrogel[J]. Scientific Reports, 2015, 5(1): 1 − 10. doi: 10.9734/JSRR/2015/14076 [87] MILLER A, KRUICHAK J, MILLS M, et al. Iodide uptake by negatively charged clay interlayers?[J]. Journal of Environmental Radioactivity, 2015, 147: 108 − 114. doi: 10.1016/j.jenvrad.2015.05.024 [88] LIAO K L, XU X Z, DU X Z. Unusual buffer action of free-standing nanoscopically confined water[J]. Journal of Colloid and Interface Science, 2010, 341(2): 280 − 285. doi: 10.1016/j.jcis.2009.09.063 [89] CHEN Z, GUAN Z, LI M, et al. Enhancement of the performance of a platinum nanocatalyst confined within carbon nanotubes for asymmetric hydrogenation[J]. Angewandte Chemie International Edition, 2011, 50(21): 4913 − 4917. doi: 10.1002/anie.201006870 [90] GUAN J, PAN X, LIU X, et al. Syngas segregation induced by confinement in carbon nanotubes: a combined first-principles and monte carlo study[J]. The Journal of Physical Chemistry C, 2009, 113(52): 21687 − 21692. doi: 10.1021/jp906092c [91] ZHANG H, PAN X, HAN X, et al. Enhancing chemical reactions in a confined hydrophobic environment: an NMR study of benzene hydroxylation in carbon nanotubes[J]. Chemical Science, 2013, 4(3): 1075 − 1078. doi: 10.1039/c2sc21761a [92] CHEN W, FAN Z, PAN X, et al. Effect of confinement in carbon nanotubes on the activity of fischer−tropsch iron catalyst[J]. Journal of the American Chemical Society, 2008, 130(29): 9414 − 9419. doi: 10.1021/ja8008192 [93] YUE H, ZHAO Y, ZHAO S, et al. A copper-phyllosilicate core-sheath nanoreactor for carbon–oxygen hydrogenolysis reactions[J]. Nature Communications, 2013, 4(1): 2339. doi: 10.1038/ncomms3339 [94] KUNASCHK M, SCHMALZ V, DIETRICH N, et al. Novel regeneration method for phosphate loaded granular ferric (hydr)oxide-a contribution to phosphorus recycling[J]. Water Research, 2015, 71: 219 − 226. doi: 10.1016/j.watres.2015.01.001 [95] ZHANG Y, SHE X, GAO X, et al. Unexpected favorable role of Ca2+ in phosphate removal by using nanosized ferric oxides confined in porous polystyrene beads[J]. Environmental Science & Technology, 2019, 53(1): 365 − 372. [96] CHEN L, LIU F, WU Y, et al. In situ formation of La(OH)3-poly(vinylidene fluoride) composite filtration membrane with superior phosphate removal properties[J]. Chemical Engineering Journal, 2018, 347: 695 − 702. doi: 10.1016/j.cej.2018.04.086 [97] CHEN L, LI Y, SUN Y, et al. La(OH)3 loaded magnetic mesoporous nanospheres with highly efficient phosphate removal properties and superior pH stability[J]. Chemical Engineering Journal, 2019, 360: 342 − 348. doi: 10.1016/j.cej.2018.11.234 [98] NELSON J, BARGAR J R, WASYLENKI L, et al. Effects of nano-confinement on Zn(II) adsorption to nanoporous silica[J]. Geochimica et Cosmochimica Acta, 2018, 240: 80 − 97. doi: 10.1016/j.gca.2018.08.017 [99] KNIGHT A W, ILANI-KASHKOULI P, HARVEY J A, et al. Interfacial reactions of Cu(Ⅱ) adsorption and hydrolysis driven by nano-scale confinement[J]. Environmental Science Nano, 2020, 7(1): 68 − 80. doi: 10.1039/C9EN00855A -



 下载:
下载: