-
地下水中磷富集已成为湖泊富营养化的重要来源之一[1]。地下水中的无机磷主要以正磷酸盐阴离子(H2PO4−和HPO42-)的形式存在于地下水环境中[2]。近年来,许多研究表明,全球洪泛区地下水含水层中磷含量达0.05~1.2 mg·g−1[3]。然而,对于地下水中磷的污染过程尚不清晰[4]。
胶体是一种颗粒粒径在1~1 000 nm的分散体,在天然水域的地球化学循环中起着至关重要的作用[5]。课题组前期工作中研究了江汉平原地下水中铁锰胶体与磷的含量分布情况,发现了地下水中铁锰胶体与磷之间存在较强的耦合控制作用[6]。ELEANER等[7]研究铁胶体在深层地下水系统中形成和迁移,同样发现三价铁(氢)氧化物胶体能使无机盐发生迁移。土壤/沉积物中常见的铁锰矿物有水铁矿、针铁矿、二氧化锰、水钠锰矿[8],易从沉积物表面分离而下渗到地下水中,并以胶体的形式参与到地下水中磷的吸附、释放、迁移及转化[9]。然而,地下水环境中铁锰胶体对无机磷的吸附-脱附机制鲜有报道。
本研究对比探讨了常见的4种铁锰胶体(水铁矿、针铁矿、二氧化锰及水钠锰矿)对地下水中无机磷的吸附-脱附行为,考察了胶体质量浓度、时间、pH、离子强度及有机质对磷在铁锰胶体上吸/脱附影响,探究了其吸/脱附机制,为认识地下水中磷污染过程提供基础。
-
矿物制备:水铁矿和针铁矿制备参考GOLDBERG等[10]和ZHANG等[11]的方法。二氧化锰和水钠锰矿制备根据LI等[12]和ATKINS等[13]的方法。胶体悬浮液制备:分别称取0.05 g铁锰矿物于50 mL 离心管中,并向其中加入50 mL 1.0 mmol·L−1氯化钠溶液,然后将离心管水浴超声1 h后转入高速离心机中于6 200 r·min−1下离心54 min(基于斯托克斯沉降定律),以去除大于1 μm的颗粒。最后,将上清液转移至干净的烧杯中以备用,并根据差重法测得胶体母液质量浓度约为0.8 mg·L−1。
胶体表征:胶体粒径及Zeta电位使用纳米粒度电位仪(Malvern Zetasizer Nano ZS90,英国)测定;比表面积使用多通道全自动比表面积及孔隙度分析仪(Tristar II 3020 Plus,中国)测定;胶体吸附磷前后官能团的变化使用傅里叶变换红外光谱仪(Nicolet iS50,美国)分析;胶体的晶面结构采用X射线衍射仪(Bruker D8 Advance,德国)进行分析。
-
所有实验所用溶液均通入高纯N2(纯度99.999%)中30 min以排除水里的溶解氧,并调节pH近中性用以模拟地下水环境。
吸附动力学实验。先移取20 mL 100 mg·L−1铁锰胶体悬浮液于500 mL厌氧瓶中,然后加入400 mL 15 mg·L−1的NaH2PO4(以磷计)溶液,避光密封后,置于220 r·min−1恒温振荡器中振荡,每间隔一段时间取出2 mL样品过0.1 μm滤膜,采用钼蓝比色法测定上清液中磷的质量浓度,根据式(1)计算吸附量。
式中:qt为t时刻对磷的吸附量,mg·g−1;t为吸附反应时间,min;Ce和C0分别为反应前后磷的质量浓度,mg·L−1;v为吸附溶液体积,mL;m为胶体分散体质量,等于0.2 mg。
等温吸附实验:根据上述实验,改变磷溶液初始质量浓度,样品经振荡4 h后过膜,测定上清液中磷的质量浓度,并计算平衡时的吸附量(qe)。
影响因素实验:所有影响因素实验条件均与上述实验条件一致,不同的是,移取2 mL 100 mg·L−1铁锰胶体悬浮液于20 mL 15 mg·L−1的NaH2PO4溶液中。pH影响实验控制体系的pH 5~8;离子强度影响实验控制背景电解质浓度分别为1.0、10.0、100.0、100 0.0 mmol·L−1;在有机质影响实验中,分别向初始磷溶液中加入10、25、50 mg·L−1的腐殖酸和富里酸。
脱附实验:采用含有不同质量浓度的KCl和CaCl2的溶液作为洗脱液。制备磷饱和的4种铁锰胶体样品,将其过0.1 μm滤膜,用超纯水冲洗滤膜,收集滤膜上的固体,经冷冻干燥后即得磷饱和铁锰胶体分散体粉末。取1 g所得粉末加入到装有200 mL质量浓度为25、50、100 mg·L−1洗脱液的厌氧瓶中,避光密封后将其置于220 r·min−1恒温振荡器中振荡。计算吸附量,每间隔一段时间将取出的悬浮液样品过膜,测定上清液中磷的质量浓度,根据式(2)计算其脱附量。根据式(3)计算其解吸率。所有实验在常温下进行,每组实验设置平行实验。
式中:q1为磷在胶体上的吸附量,mg·g−1;q2为磷在胶体上的脱附量,mg·g−1;C为脱附后磷的质量浓度,mg·L−1;V为脱附溶液体积,mL;M为磷饱和胶体分散体质量,为1 g;η为磷在胶体上的解吸率,%。
-
所制备的水铁矿、针铁矿、二氧化锰、水钠锰矿胶体,其胶体特性如表1所示。由表1可知,2种铁胶体表面带正电荷,锰胶体表面带负电荷;铁胶体的粒径高于锰胶体,但锰胶体的比表面积高于铁胶体。
-
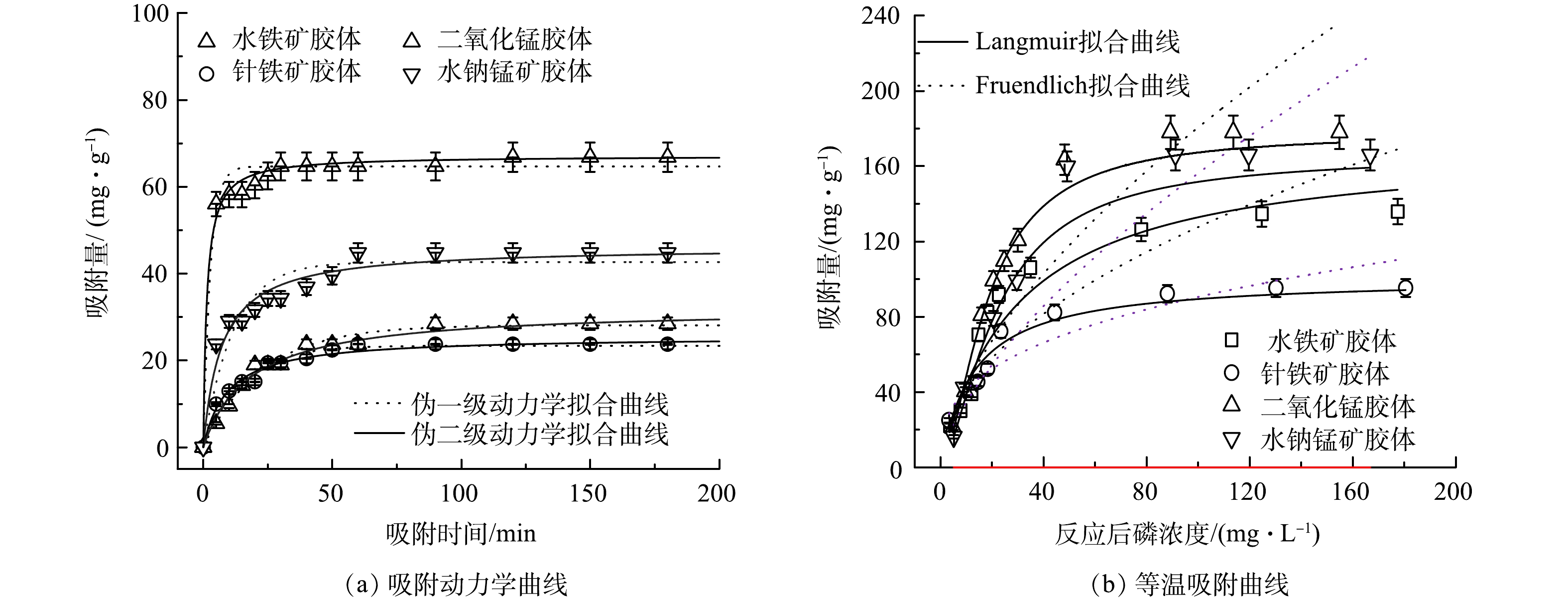
磷在不同铁锰胶体上的吸附动力学曲线如图1(a)所示。磷在4种铁锰胶体上的吸附可以分为2个过程:50 min内的快速吸附以及50 min后的缓慢吸附并在180 min内达到平衡的过程。磷在铁锰胶体上的饱和吸附量遵循二氧化锰胶体>水钠锰矿胶体>水铁矿胶体>针铁矿胶体,对应的最大吸附量分别为32.09、25.52、71.10、46.17 mg·g−1。远高于研究[14~15]报道的水铁矿、针铁矿、二氧化锰矿物上的磷最大吸附量(12.6、3.6、12.8 mg·g−1)。
采用伪一级吸附动力学模型和伪二级吸附动力学模型对动力学曲线进行拟合,结果如表2所示。由表2可知,磷在铁锰胶体表面的吸附过程同时存在物理吸附和化学吸附,但伪二级动力学比伪一级动力学模型更接近实验数据。且水铁矿胶体、针铁矿胶体、二氧化锰胶体和水钠锰矿胶体上的吸附速率分别为0.04、0.07、0.06、0.09 mg·(g·min)−1,表明锰胶体吸附量和吸附速率均高于铁胶体。
采用Langmuir和Freundlich吸附模型对等温吸附结果进行拟合,结果如图1(b)及表3所示。由表3可知,Langmuir模型能更好拟合实验数据,表明铁锰胶体吸附过程主要为单分子层吸附。4种铁锰胶体对磷的吸附量均随着初始磷浓度的增加而增加。这可能是因为高浓度的磷可提供必要的驱动力,从而克服了其在固液相之间的传质阻力[16]。
-
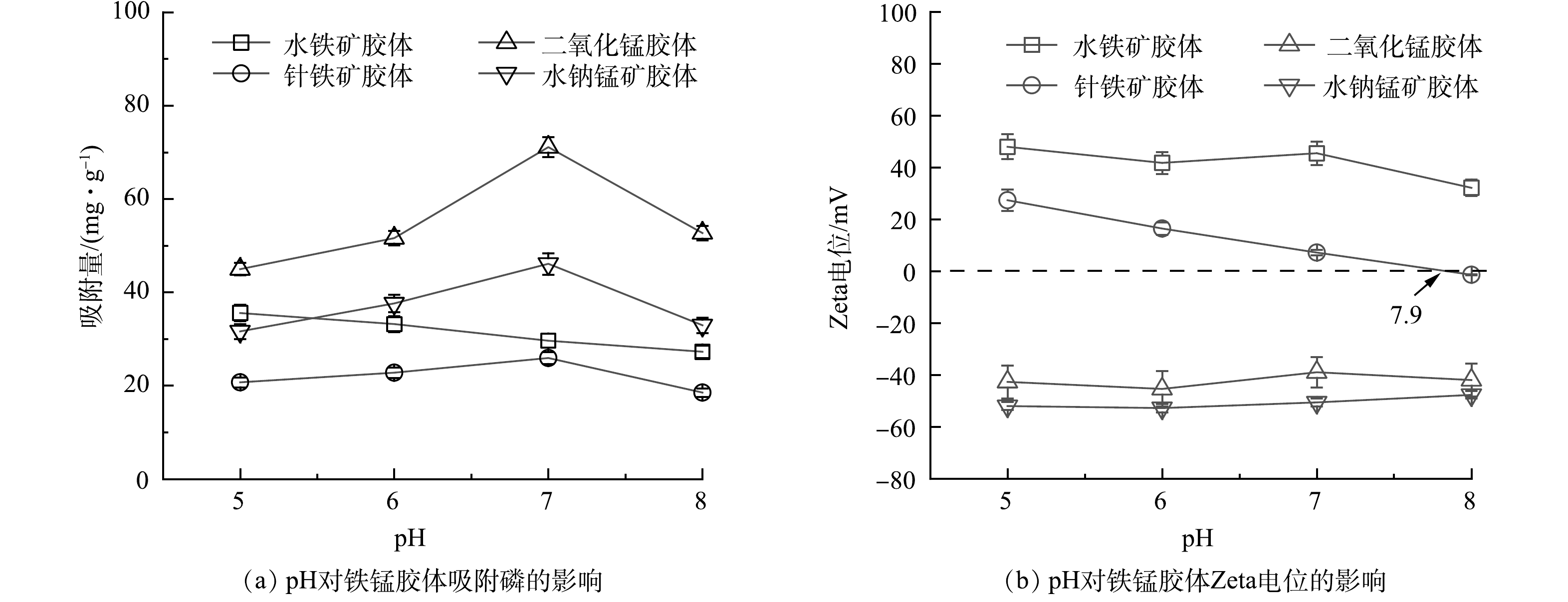
pH对四种铁锰胶体吸附磷的影响如图2所示。由图2(a)可知,针铁矿胶体、二氧化锰胶体、水钠锰矿胶体在pH=7时对磷的吸附能力最好,而水铁矿胶体对磷的吸附随着pH的增高而降低。由图2(b)可知,2种锰胶体在pH=5~8内的电位值恒为负。在相同pH下水钠锰矿胶体表面电荷密度高于二氧化锰胶体,二氧化锰胶体表面所吸附的阳离子(H+、Na+)较水钠锰矿胶体少,与磷酸根之间的静电阻力弱,因此,相同pH下二氧化锰胶体对磷的吸附量比水钠锰矿胶体对磷的吸附量大。而水铁矿和针铁矿的Zeta电位值随着pH的升高而逐渐降低,其等电点分别在7.9和9.2[17]左右。水铁矿胶体在较低pH下表现出较高的吸附性能,这是因为当pH小于8.0时,水铁矿胶体表面带正电荷,与磷酸根之间存在静电引力,随着pH的升高,表面电荷量较小且发生了电荷性质的逆转,从而造成了吸附量的降低。针铁矿胶体在pH=5~10内对磷的吸附量变化趋势与Zeta电位的变化趋势一致。
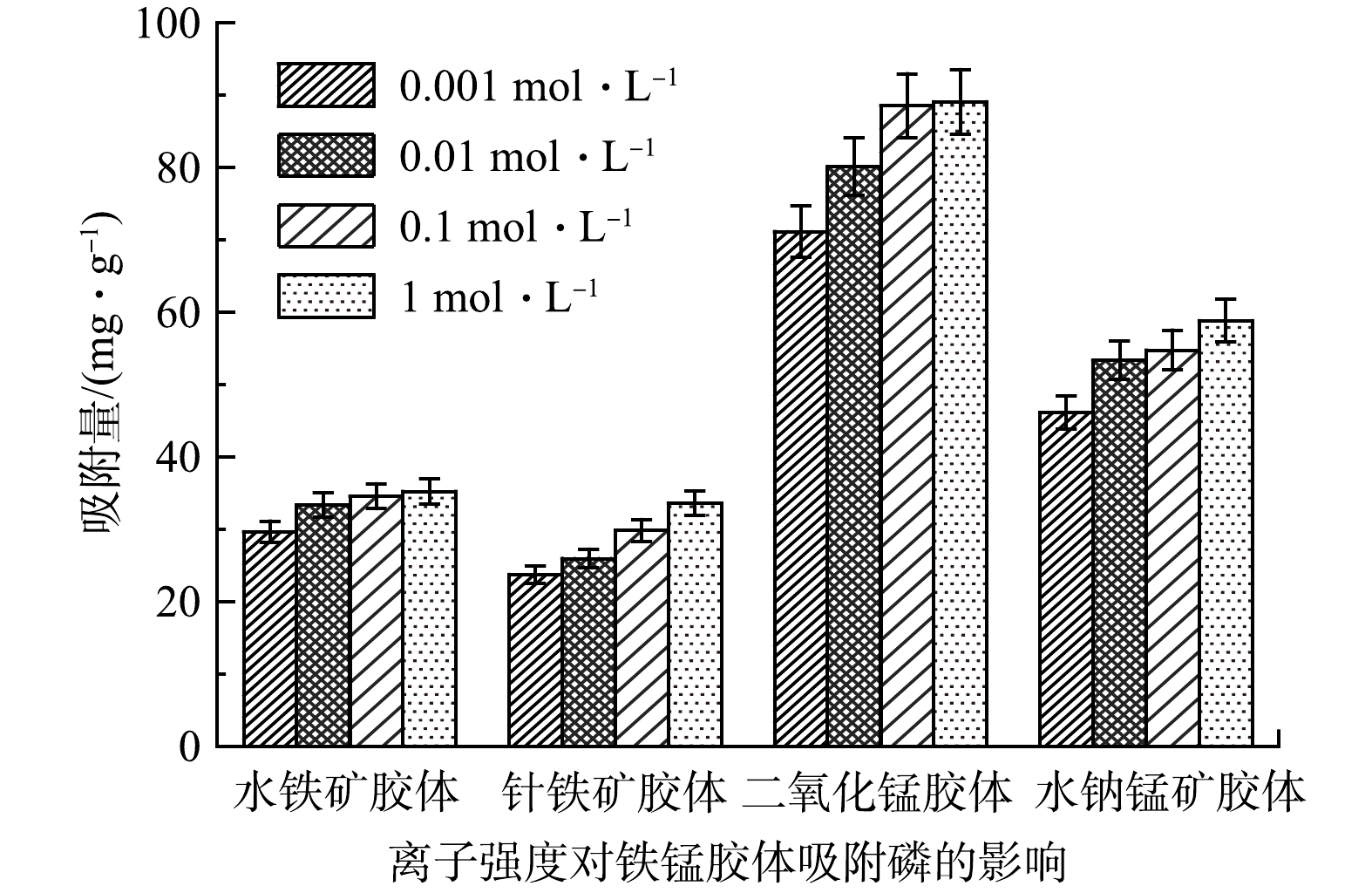
离子强度对4种铁锰胶体吸附磷的影响结果如图3所示。由图3可知,当离子强度从0.001 mol·L−1增加到1.000 mol·L−1时,水铁矿胶体、针铁矿胶体、二氧化锰胶体和水钠锰矿胶体对磷的吸附量分别上升了25.24%、27.61%、18.73%、41.54%。说明离子强度的增加能够促进铁锰胶体对磷的吸附,这是因为离子强度的增加减弱了磷酸根与胶体之间的静电斥力,从而有助于其吸附,MUSTUFA等[18]也观察到同样的实验现象。另外,离子强度的增加能够压缩胶体的双电层厚度,使胶体表面的活性位点更多的暴露在环境中,从而导致与更多的磷结合,促进了磷在铁锰胶体上的吸附[19]。
有机质对铁锰胶体吸附磷的影响如图4所示。由图4可知,磷在4种胶体表面的吸附量随有机质浓度的升高而下降,这与一些研究结果类似[20]。这可能是因为有机质会占据铁锰胶体表面的吸附位点从而降低了磷在铁锰胶体上的吸附[21]。水铁矿胶体、针铁矿胶体、二氧化锰胶体、水钠锰矿胶体在添加50 mg·L−1腐殖酸后对磷的吸附量分别由32.09、25.51、71.10和46.17 mg·g−1下降到10.53、6.30、21.19和17.78 mg·g−1;在添加50 mg·L−1富里酸后,4种铁锰胶体对P的吸附量分别从32.09、6.30、21.19和17.78 mg·g−1下降到18.5、6.64、22.29和18.72 mg·g−1。上述结果表明腐殖酸对磷在铁锰胶体上的吸附抑制作用高于富里酸,这可能是由于2种有机质所含基团的种类及数量不同所致。
-
不同电解质对磷从水铁矿胶体、针铁矿胶体、二氧化锰胶体、水钠锰矿胶体上的最大脱附效率如图5所示。由图5可知,磷从铁锰胶体上的脱附效率随着电解质浓度的升高而增加。当氯化钾和氯化钙质量浓度达到100 mg·L−1时,磷从水铁矿胶体、针铁矿胶体、二氧化锰胶体和水钠锰矿胶体上的脱附效率分别可达到72.47%和92.36%、59.11%和63.09%、67.38%和74.61%、82.91%和89.24%。以上结果表明铁胶体的脱附效率高于锰胶体,氯化钙对磷从铁锰胶体上的脱附影响高于氯化钠。这可能是因为钙离子更易夺取胶体表面吸附的磷酸根形成难溶解的磷酸钙沉淀(Ksp=2.0×10−29),从而使得磷酸根解吸,尤其在负电性较大的锰胶体表面,高价态阳离子易发生配位交换,从而更易导致磷的解吸。
-
为了进一步探究铁锰胶体吸附-脱附磷机理,用傅里叶变换红外光谱仪分析铁锰胶体吸附无机磷前后官能团的变化,其红外光谱图如图6所示。由图6可知,吸附无机磷后的水铁矿胶体、针铁矿胶体、二氧化锰胶体、水钠锰矿胶体分别在106 2.44、106 2.08和109 5.43、106 2.44、1064.96和109 5.43 cm−1处出现信号强烈的P—O键伸缩振动峰。水铁矿胶体、针铁矿胶体、二氧化锰胶体、水钠锰矿胶体分别在163 2.31、163 9.82、162 9.46、162 7.45 cm−1处对应的—OH弯曲振动峰发生横移。同时,水铁矿胶体和针铁矿胶体在138 4.02、135 7.10 cm−1处对应的—OH弯曲振动峰的强度在吸附后发生明显降低,峰值的减少说明—OH遭到破坏。这些现象均表明磷在水铁矿胶体、针铁矿胶体、二氧化锰胶体、水钠锰矿胶体上的吸附是通过与-OH进行结合。此外,有研究表明,磷酸基团的氧原子可以直接与表面Fe配位,使磷酸盐与水铁矿强结合形成双角双齿配合物[22]。由pH和浓度对磷酸盐吸附的影响可知,锰胶体吸附磷酸盐离子的过程是与固体表面带正电的基团络合,这与其他研究结果一致[18]。
pH发生变化时,磷酸根在实验设置组中以H2PO4−和HPO42-两种形式同时存在。已有报道证明,在pH=3~5(磷酸根的形态主要以H2PO4−存在)时,固体优先吸附双电荷的HPO42-,随着pH的升高,胶体表面对单电荷磷酸根吸附增多[15],不同电荷数的磷酸根都能被有效吸附。本研究条件下铁胶体表面电荷恒为正,锰胶体表面电荷恒为负。铁胶体表面正电荷促进了磷酸基团以静电吸引的方式吸附。但由于锰胶体比铁胶体拥有更大的比表面积从而暴露出更多的羟基吸附位点,对无机磷进行化学吸附作用,进而导致磷在锰胶体上的吸附量高于铁胶体。
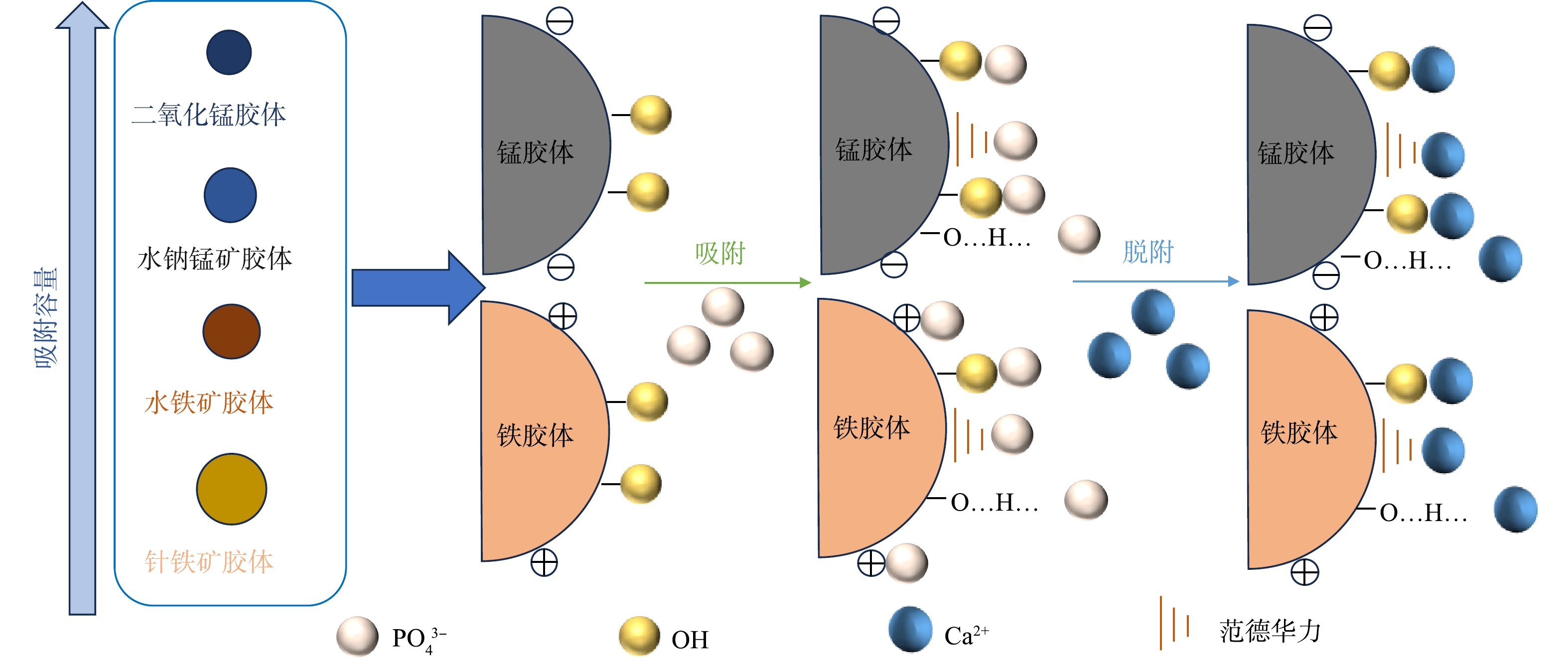
综上所述,铁锰胶体对磷的吸附/脱附机理图如图7所示。尽管铁锰胶体性质存在较大差异,但在近中性地下水环境中,铁锰胶体对磷的吸附主要通过:1)占据羟基活性位点的化学吸附作用;2)静电吸引作用;3)其他弱的吸附力作用(范德华力、氢键等)。磷从铁锰胶体表面的脱附主要受地下水电解质种类和浓度的影响,且高价阳离子更易与吸附的磷酸根发生配位交换,从而导致地下水磷的释放。
-
1) 水铁矿胶体、针铁矿胶体、二氧化锰胶体和水钠锰矿胶体对磷的吸附是一个快速的单分子层吸附过程,120 min内可达到吸附平衡,符合伪二级动力学模型和Langmuir等温吸附模型,其最大吸附量分别为磷32.09、25.52、71.10、46.17 mg·g−1。
2) pH值通过改变铁锰胶体表面的Zeta电位进一步影响磷在铁锰胶体上的吸附。当pH =7时有利于磷的吸附。由于离子强度的增加减弱了磷酸根与胶体之间的静电斥力,溶液离子强度升高可以促进四种铁锰胶体对磷的吸附。相反,有机质会竞争磷在铁锰胶体上的吸附位点从而抑制铁锰胶体对磷的吸附。
3) 磷从铁锰胶体的脱附随着电解质浓度的升高而增强,铁胶体的脱附效率高于锰胶体,且氯化钙的影响高于氯化钠。当氯化钙浓度达到100 mg·L−1时,磷从水铁矿胶体、针铁矿胶体、二氧化锰胶体和水钠锰矿胶体上的脱附率分别可达92.36%、63.09%、74.61%和89.24%。
铁锰胶体对地下水中无机磷的吸附-脱附特性
Adsorption and desorption properties of iron and manganese colloids to inorganic phosphorus in groundwater
-
摘要: 地下水中磷富集已成为水体富营养化的重要污染来源之一,但对于地下水中磷的污染过程认识不清。本研究对比了地下水中常见4种铁锰胶体(水铁矿胶体、针铁矿胶体、二氧化锰胶体和水钠锰矿胶体)对无机磷的吸附和脱附性能的差异。结果表明:铁锰胶体对磷的吸附主要是单分子层的化学吸附,锰胶体吸附量与吸附速率均大于铁胶体,中性pH和较高离子强度有利于铁锰胶体对磷的吸附,但有机质的存在会大大降低对磷的吸附量,尤其是针铁矿胶体受有机质的影响程度最大,其中腐殖酸影响比富里酸更大。同时,钙离子对磷从铁锰胶体上的脱附影响高于钠离子,且铁胶体的脱附效率高于锰胶体。以上研究结果为理解地下水中磷污染过程提供数据支撑。Abstract: Recent studies have shown that phosphorus enrichment in groundwater has become one of the important pollution sources of water eutrophication. However, the process of phosphorus pollution in groundwater is not clear. The differences of adsorption-desorption characteristics among four common iron and manganese colloids (ferrihydrite colloids, goethite colloids, manganese dioxide colloids and birnessite colloids) toward inorganic phosphoruswere compared. The results showed that phosphorus adsorption onto iron and manganese colloids was dominated by a monolayer and chemical process. The adsorption capacities and rates of manganese colloids were greater than those of iron colloids. The conditions of neutral pH and high ionic strength were favorable for the adsorption of phosphorus by iron and manganese colloids, while the presence of organic matter greatly decreased phosphorus adsorption capacity, especially the highest suppression effect occurred onto goethite colloids, and humic acid presented a stronger suppression than fulvic acid. Moreover, the phosphorus desorption were effected more seriously by Ca2+ ions than that by Na+ ones, and the desorption rate by iron colloids was higher than that by manganese ones. Therefore, above results can provide the data support for phosphorus pollution process in groundwater .
-
Key words:
- iron and manganese colloid /
- phosphorus /
- adsorption /
- desorption /
- underground water
-

-
表 1 实验中不同铁锰胶体物理化学特征
Table 1. Physical and chemical characteristics of different iron and manganese colloids in the experiment
胶体 Zeta电位/mV 粒径/nm 比表面积/(m2·g−1) 水铁矿胶体 45.5 287.5 135.3 针铁矿胶体 7.2 452.8 124.9 二氧化锰胶体 -38.9 161.5 180.2 水钠锰矿胶体 -50.5 150.4 142.4 表 2 铁锰胶体对磷的吸附动力学曲线拟合参数
Table 2. Fitting parameters of phosphorus adsorption kinetics on iron and manganese colloids
胶体 伪一级吸附动力学模型 伪二级吸附动力学模型 K1/min−1 qe/(mg·g−1) R12 K2/(mg·(g·min)−1) qe /(mg·g−1) R22 水铁矿胶体 0.02 28.12 0.982 0.04 32.09 0.984 针铁矿胶体 0.01 23.37 0.967 0.07 25.52 0.981 二氧化锰胶体 0.05 64.63 0.957 0.06 71.10 0.981 水钠锰矿胶体 0.04 42.68 0.964 0.09 46.17 0.989 表 3 铁锰胶体对磷的等温吸附线的拟合参数
Table 3. Fitting parameters of phosphorus adsorption isotherm on iron and manganese colloids
胶体 Langmuir模型 Freundlich模型 KL/(L·mg−1) Qm/(mg·g−1) RL2 KF n RF2 水铁矿胶体 0.02 141.86 0.960 10.58 3.23 0.840 针铁矿胶体 0.02 100.79 0.967 9.71 2.76 0.926 二氧化锰胶体 0.02 177.96 0.990 10.19 9.71 0.853 水钠锰矿胶体 0.02 165.96 0.970 12.27 7.80 0.856 -
[1] LIU L, OUYANG W, LIU H, et al. Drainage optimization of paddy field watershed for diffuse phosphorus pollution control and sustainable agricultural development[J]. Agriculture, Ecosystems & Environment, 2021, 308: 107238. [2] BORCH T, KRETZSCHMAR R, KAPPLER A, et al. Biogeochemical redox processes and their impact on contaminant dynamics[J]. Environmental Science & Technology, 2010, 44(1): 15-23. [3] LI Y, YU C, ZHAO B, et al. Spatial variation in dissolved phosphorus and interactions with arsenic in response to changing redox conditions in floodplain aquifers of the Hetao Basin, Inner Mongolia[J]. Water Research, 2022, 209: 117930. doi: 10.1016/j.watres.2021.117930 [4] 张嘉雯, 魏健, 刘利, et al. 衡水湖沉积物营养盐形态分布特征及污染评价[J]. 环境科学, 2020, 41(12): 5389-5399. doi: 10.13227/j.hjkx.202004237 [5] ZHANG J, LIANG X, JIN M, et al. Identifying the groundwater flow systems in a condensed river-network interfluve between the Han River and Yangtze River (China) using hydrogeochemical indicators[J]. Hydrogeology Journal, 2019, 27(7): 2415-2430. doi: 10.1007/s10040-019-01994-1 [6] HUANG S, CHEN L, Li J, et al. The effects of colloidal Fe and Mn on P distribution in groundwater system of Jianghan Plain, China[J]. Science of The Total Environment, 2023, 854: 158739. doi: 10.1016/j.scitotenv.2022.158739 [7] SPIELMAN-SUN E, BLAND G, WIELINSKI J, et al. Environmental impact of solution pH on the formation and migration of iron colloids in deep subsurface energy systems[J]. Science of the Total Environment, 2023: 166409. [8] JIANMIN Z. Ferrihydrite: Surface structure and its effects on phase transformation[J]. Clays and Clay Minerals, 1994, 42(6): 737-746. doi: 10.1346/CCMN.1994.0420610 [9] DEGUELDRE C, BENEDICTO A. Colloid generation during water flow transients[J]. Applied Geochemistry, 2012, 27(6): 1220-1225. doi: 10.1016/j.apgeochem.2012.01.017 [10] GOLDDBERG S, JOHNSTO C T. Mechanisms of arsenic adsorption on amorphous oxides evaluated using macroscopic measurements, vibrational spectroscopy, and surface complexation modeling[J]. Journal of Colloid and Interface Science, 2001, 234(1): 204-216. doi: 10.1006/jcis.2000.7295 [11] ZHANG G, QU J, LIU H, et al. Preparation and evaluation of a novel Fe–Mn binary oxide adsorbent for effective arsenite removal[J]. Water Research, 2007, 41(9): 1921-1928. doi: 10.1016/j.watres.2007.02.009 [12] LI Z, DING Y, XIONG Y, et al. Rational growth of various α-MnO 2 Hierarchical Structures and β-MnO 2 nanorods via a homogeneous catalytic route[J]. Crystal Growth & Design, 2005, 5(5): 1953-1958. [13] ATKINS A L, SHAW S, PEACOCK C L. Release of Ni from birnessite during transformation of birnessite to todorokite: Implications for Ni cycling in marine sediments[J]. Geochimica et Cosmochimica Acta, 2016, 189: 158-183. doi: 10.1016/j.gca.2016.06.007 [14] 邵兴华, 章永松, 林咸永, 等. 三种铁氧化物的磷吸附解吸特性以及与磷吸附饱和度的关系[J]. 植物营养与肥料学报, 2006(2): 2208-2212. [15] CHEN P, ZHOU Y, XIE Q, et al. Phosphate adsorption kinetics and equilibria on natural iron and manganese oxide composites[J]. Journal of Environmental Management, 2022, 323: 116222. doi: 10.1016/j.jenvman.2022.116222 [16] SCHOTTING R J, MOSER H, HASSANIZADE S M. High-concentration-gradient dispersion in porous media: experiments, analysis and approximations[J]. Advances in Water Resources, 1999, 22(7): 665-680. doi: 10.1016/S0309-1708(98)00052-9 [17] 李政辉. 针铁矿/水铁矿-有机质复合体对有机磷的吸附特征研究[D]. 武汉: 华中农业大学, 2022. [18] MUSTAFA S, ZAMAN M I, Khan S. pH effect on phosphate sorption by crystalline MnO2[J]. Journal of Colloid and Interface Science, 2006, 301(2): 370-375. doi: 10.1016/j.jcis.2006.05.020 [19] 李惠. 铝胶体和腐殖酸对铀在饱和石英砂中迁移影响研究[D]. 衡阳: 南华大学, 2021. [20] BORGGAARD O K, RABEN-LANGE B, Gimsing A L, et al. Influence of humic substances on phosphate adsorption by aluminium and iron oxides[J]. Geoderma, 2005, 127(3/4): 270-279. [21] 宋嘉慧, 皇甫小留, 何强, 等. t al0–2对重金属Tl(Ⅰ)的吸附效能及影响因素[J]. 中国给水排水, 2019, 35(9): 53-57. [22] HIEMTRA T, VAN RIEMSDIJK W H. A surface structural model for ferrihydrite I: Sites related to primary charge, molar mass, and mass density[J]. Geochimica et Cosmochimica Acta, 2009, 73(15): 4423-4436. doi: 10.1016/j.gca.2009.04.032 -



 下载:
下载: