-
染料废水进入水体、土壤,会造成饮用水源污染、水生物丧失生命、生态景观恶化、环境质量下降等问题,对人类以及其他生物的生存产生极大的威胁[1]。目前常用于处理染料废水的方法主要有化学法、物理化学法和生物法等,其中以物理化学法中的吸附法应用比较广泛[2-3]。水凝胶作为一种新型的高分子功能材料,其内部含有多种水性基团如羟基、羧基等[4],且具有高膨胀倍率,对染料结晶紫(crystal violet,CV)具有较强的结合能力,水凝胶不仅拥有良好的网络结构[5],而且在水中溶胀又不溶于水[6],水凝胶吸附后极易与液相分离,很大程度上减轻后续的分离工作,是一种十分理想的吸附剂[7]。但水凝胶本身机械强度不高,易破碎,回用较为困难,而木质素具有丰富的官能团结构,且储量丰富、成本低、环境友好和可生物降解,被视为理想的有机聚合物组分[8]。有研究[9]表明,在水凝胶中引入木质素类聚合物,可以形成互穿网络结构,增加吸附位点,进而有效提高其机械强度和吸附效率。为了提高吸附材料的回收利用性,在吸附材料中引入磁性微粒,形成磁性吸附材料,外加磁场即可将其分离[10-11],且加入磁性微粒后,增大了吸附剂的活性位点和比表面积[12],从而更有利于吸附材料的吸附过程。
因此,本研究通过自由基接枝共聚和原位沉淀法制备磁性木质素磺酸钠水凝胶(Fe3O4@LS),探究在各种单因素条件下Fe3O4@LS对CV的吸附性能,并进行吸附动力学和吸附等温线拟合分析,考察其再生性能和共存离子对吸附性能的影响,并对Fe3O4@LS的吸附机理进行了分析。
-
木质素磺酸钠(LS)和N,N’-亚甲基双丙烯酰胺(MBA)均购自天津市光复精细化工研究所,丙烯酰胺(AM)购自天津市科密欧化学试剂有限公司,过硫酸钾(KPS)购自天津市北联精细化学品开发有限公司,N,N,N’,N’-四乙基乙二胺(TEMED)购自梯希爱(上海)化成工业发展有限公司,以上试剂纯度皆为分析纯。
-
称取0.400 g木质素磺酸钠(LS)、1.400 g丙烯酰胺(AM)和0.024 g交联剂(MBA)放入烧杯中加入20 mL蒸馏水,室温下搅拌溶解;称取0.220 g过硫酸钾(KPS)加入10 mL蒸馏水配制成溶液放入另外一个烧杯中。40 ℃下预热10 min后,将2个烧杯的溶液混合摇匀,加入0.050 mL催化剂(TEMED),搅拌均匀后快速倒入模具,在40 ℃下聚合10 min后,得到木质素水凝胶。洗净后,将水凝胶置于一定比例混合的FeSO4·7H2O和FeCl3·6H2O(1:1.4)混合溶液中浸泡8 h,洗去表面溶液后,置于50 mL1 mol·L−1 NaOH溶液中浸泡8 h,得到磁性水凝胶Fe3O4@LS [13]。洗净后在60 ℃烘箱中烘干至恒重,备用。参考本课题组前期关于Fe3O4@LS的表征结果[13],对Fe3O4@LS的结构进行分析。
-
在Fe3O4@LS吸附结晶紫(CV)模拟废水实验中,采用分光光度法测定583 nm处的吸光度A,利用标准曲线方程A=0.042 59+0.258 01C(R2=0.999 08)计算处理后水中剩余CV质量浓度。
探究负载磁性前后对吸附CV的影响;将0.05 g干Fe3O4@LS溶胀后加入不同pH(3、4、5、6、7、8、9、10)的50 mL CV(50 mg·L−1)溶液中,探究溶液pH对吸附的影响;将0.05 g干Fe3O4@LS溶胀后投入50 mL 50 mg·L−1 CV溶液中,在不同时间测定CV剩余浓度,探究吸附时间对吸附的影响;将不同质量的Fe3O4@LS投入50 mL 50 mg·L−1 CV溶液中,探究吸附剂用量对吸附的影响;将0.02 g干Fe3O4@LS溶胀后投入不同浓度的CV溶液中,探究CV初始浓度对吸附的影响。将0.02 g干Fe3O4@LS溶胀后投入50 mL 100 mg·L−1 CV溶液中,在不同温度下进行吸附,探究吸附温度对吸附的影响。以上每个数据点做3次实验,取平均值。
吸附动力学:取Fe3O4@LS置于100 mL具塞锥形瓶中,分别加入50 mL浓度为25、50、100 mg·L−1的CV 溶液,在298 K下,以150 r·min−1在恒温水浴振荡器中振荡吸附,每隔一段时间取上层清液测定吸光度以计算浓度,每个数据点做3次实验,取平均值。选择拟一阶(式(1))[14]、拟二阶(式(2))[15]以及内扩散动力学模型(式(3))[16]进行拟合。
式中:t为吸附时间,min;Qt和Qe分别代表吸附时间为t和吸附平衡时的吸附量,mg·g−1;k1为拟一级反应速率常数,min−1;k2为拟二级反应速率常数,g·(mg·min)−1;ki为粒子内扩散速率常数, mg·g−1·min−0.5;P为与边界层厚度相关的常数。通过以
$\text{ln}{\text{(}{Q}}_{\text{e}}{{-Q}}_{{t}}\text{)}$ 对t作图,k1和Qe的值分别对应为斜率和截距;k2和Qe可以由t/Qt对t作图,通过拟合曲线的截距和斜率计算所得。吸附等温线:取50 mL初始浓度为50~500 mg·L−1的CV溶液置于一系列样品瓶中,并调节溶液pH为7,加入Fe3O4@LS,分别在298、318、338 K温度下以150 r·min−1在恒温水浴振荡器中振荡吸附2 h。用磁铁分离Fe3O4@LS,测定吸光度计算浓度。每个数据点做3次实验,取平均值。采用Langmuir(式(4))[17]、Freundlich(式(5))[18]和Temkin(式(6))[19]这3种吸附等温线模型对实验所得数据进行拟合。
式中:Ce为吸附平衡时溶液中CV的浓度;Qm为饱和吸附量,mg·g−1;KL为吸附系数,L·mg−1;KF和n均为常数,n>1时,吸附为易吸附过程;α和β均为常数。以Ce/Qe对Ce作图,Qm和 KL为的值分别对应为截距和斜率;KF和n的值可以通过lnQe对lnCe作图,通过拟合曲线的截距和斜率计算所得。
为更进一步研究吸附的热力学性质,采用范特霍夫方程和Gibbs-Helmhotz方程[20]计算出不同温度下的吉布斯自由能(式(7))、焓变(式(8))和熵变(式(9))。
式中:∆G 为吉布斯自由能kJ·mol−1;∆H为焓变,kJ·mol−1;∆S为熵变,J·(mol·K)−1;R为气体常数,8.314 J·(mol·K)−1;T为绝对温度,K;KL为平衡常数,L·g−1。可由ln(Qe/Ce)对Qe进行拟合,截距为lnKL,通过lnKL对1/T作图,∆H和∆S分别对应为斜率和截距。
磁性能和解吸再生性能:采用美国LakeShore公司的VSM7307型振动样品磁强计进行Fe3O4@LS和Fe3O4的磁性能测定。在最佳的吸附条件下,将一定量Fe3O4@LS溶胀后投入50mL 100 mg·L−1 CV溶液中,调节pH=7,在298 K下恒温振荡吸附2 h后,取出,用去离子水洗净,置于50 mL无水乙醇或0.1 mol·L−1的NaOH中,静置解吸5 h,洗涤,干燥。连续进行数次吸附-解吸实验来探究Fe3O4@LS水凝胶的再生性能。
-
配制一系列含有不同 Na+、K+、Ca2+、Mg2+离子强度的CV混合溶液,保持CV浓度为100 mg·L−1。取混合溶液50 mL,调节溶液pH=6,投加0.05 g Fe3O4@LS,在25 ℃恒温水浴中以150 r·min−1的转速振荡吸附2 h,测定溶液中CV的含量,每个数据点做多次实验,取得平均值分别计算吸附量。
-
由本课题组前期研究成果[13]可知,Fe3O4能够成功负载到LS上,且在负载过程中,Fe3O4的晶体结构未发生改变。在吸附过程中,Fe3O4也可以作为吸附剂的活性位点,因此,水凝胶成功负载在Fe3O4上后,活性位点增加,使得Fe3O4@LS对CV的吸附性能大大提高。Fe3O4@LS呈现三维立体多孔结构,为分布较规整的蜂窝状孔洞,这些结构有利于活性位点的暴露,进而提高吸附剂的吸附性能。由BET表征结果(表1)可见,Fe3O4@LS材料具有介孔结构,Fe3O4@LS的比表面积为3.669 2 m2·g−1,高于LS的比表面积(2.342 2 m2·g−1)。这表明水凝胶负载Fe3O4后,Fe3O4@LS的比表面积增大,有利于提高吸附效率[21]。因此,Fe3O4在Fe3O4@LS中可提供大量的活性位点,并且Fe3O4@LS本身的较大比表面积使得活性位点更容易暴露,这些特点可使得Fe3O4@LS在吸附过程中展现出较好的吸附效果。
-
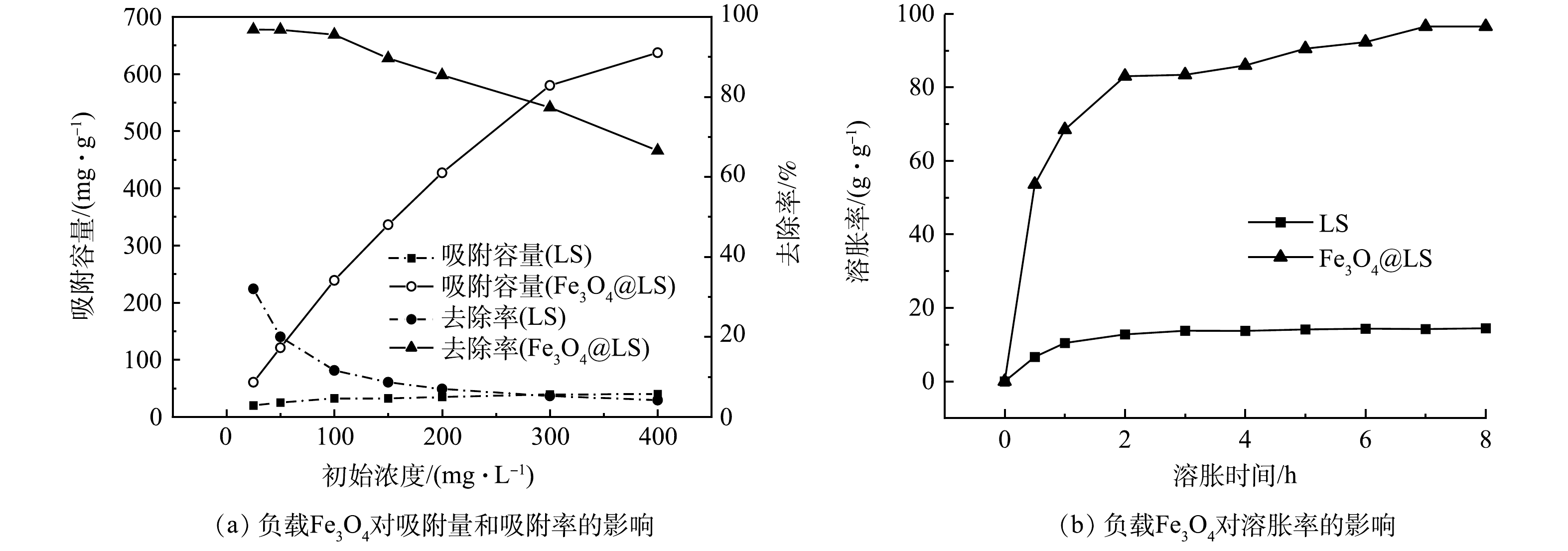
由图1(a)可以看出,在同等实验条件下,当Fe3O4负载到水凝胶上后(Fe3O4@LS),无论是对CV的吸附量还是去除率均明显高于LS。由图1(b)可以看出,Fe3O4@LS的溶胀率明显高于LS。导致2种吸附剂吸附性能差异的原因在于,Fe3O4@LS的内部有Fe3O4纳米粒子,而Fe3O4纳米粒子在提供活性位点的同时进而增加内部结构的孔隙,使得其溶胀率更高,溶胀后的体积更大,表面积也随之变大,进而提高吸附性能。
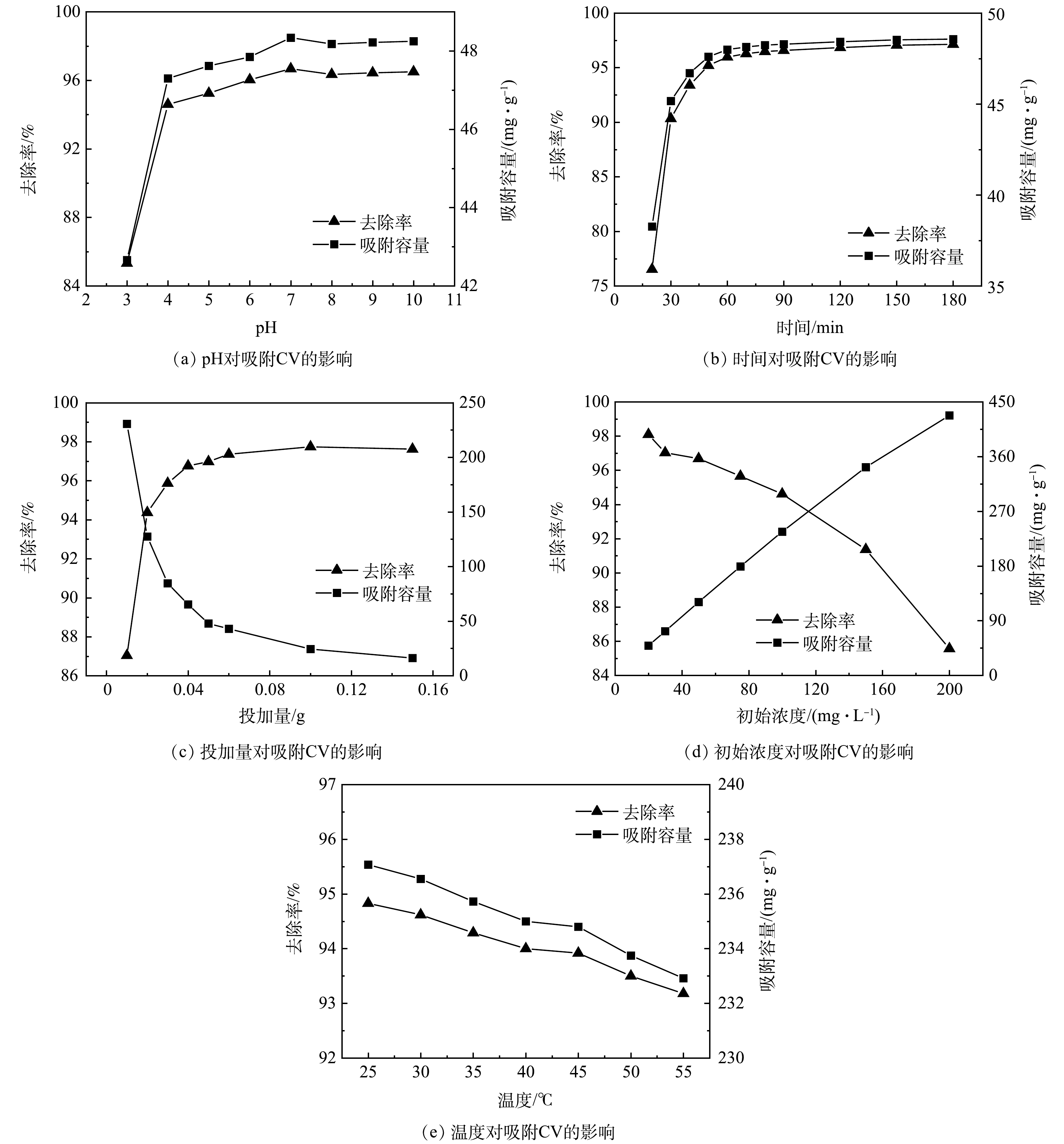
pH对吸附CV的影响结果如图2(a)所示。当pH由3上升到7时,吸附容量和CV的去除率均迅速上升,去除率在pH=7时达到最大值,为96.68%;当pH继续升高,Fe3O4@LS对CV的去除率和吸附量变化不大。当pH大于11时,CV会生成紫色絮状沉淀。因此,不考虑过高的pH对CV吸附的影响。有研究[13]表明,Fe3O4@LS的Zeta电位的等电位点约为3.2。当pH<3.2时,Fe3O4@LS表面带正电荷。因此,pH越低,静电斥力越大,对CV吸附越不利;当pH>3.2时,Fe3O4@LS表面的电负性增强,材料表面与CV之间也会产生静电吸引力,从而可增加吸附容量[22]。但当pH较低时,吸附效果较差,原因是吸附剂的表面官能团被质子化,从而削弱了吸附剂与CV的螯合作用[23]。同时,溶液中大量的H+会与CV形成竞争性活性位点[24],对CV的吸附有抑制作用。另外,Fe3O4@LS在酸性条件下体积会收缩,导致表面积变小,孔隙减少。因此,Fe3O4@LS在酸性条件下对CV的吸附效果较差。随着pH的增加,Fe3O4@LS表面的官能团逐渐去质子化,溶液中的H+减少,Fe3O4@LS的体积不再受影响。H+对活性位点竞争的影响减弱,相对活性位点增加。从而提高了对CV的吸附能力。
由图2(b)可以看出,吸附30 min Fe3O4@LS对CV去除率可达90%以上,在吸附的初始阶段,Fe3O4@LS提供了充足的吸附位点,CV有较高的概率与Fe3O4@LS中的众多活性位点有效接触。在80 min以后,吸附基本达到平衡,Fe3O4@LS对CV的去除率和吸附容量基本不再发生变化,Fe3O4@LS表面的活性位点大多数均已被占据,CV与吸附位点的接触概率大幅度降低,从而使吸附过程变得缓慢。
由图2(c)可知,Fe3O4@LS对结晶紫的去除率随Fe3O4@LS投加量的增加而增大。当Fe3O4@LS投加量为400 mg·L−1时,去除率达到94%以上,并且此时Fe3O4@LS对CV的吸附量可达127.4 mg·g−1。此后再增大Fe3O4@LS投加量,去除率的增加趋势不明显,吸附量大幅度下降。其原因可能是当CV浓度一定时,Fe3O4@LS投加量越大,可提供吸附的活性位点增多,对水中CV的去除率越大;但过量的Fe3O4@LS会使Fe3O4@LS重叠或聚合导致能利用的表面积和有效的吸附活性位点相应减少[25],而且单位质量吸附剂所吸附的CV总量也会降低。
如图2(d)所示,Fe3O4@LS的去除率随CV初始浓度升高而下降,吸附量随初始浓度升高而上升。在CV为100 mg·L−1的情况下,CV离子与吸附剂表面的吸附位点接触的概率增加,具有较高的去除率。CV浓度持续上升时,CV与Fe3O4@LS表面的活性位点接触概率增加,吸附量增加,但由于Fe3O4@LS的量一定,导致其表面的吸附位点有限,CV浓度上升时传质阻力提高。吸附也逐渐趋于饱和[26]。
由图2(e)可知,反应温度对于Fe3O4@LS对CV的吸附影响不大,在25 ℃时,吸附量达到237.08 mg·g−1,当温度升高时,去除率和吸附量均出现略微降低趋势。因为磁性水凝胶对结晶紫的吸附是放热过程,随着温度升高,部分磁性水凝胶可能出现脱附,使CV的吸附量降低。
-
选择拟一阶、拟二阶以及内扩散动力学模型开展吸附动力学研究,其中拟一阶动力学是假设其吸附受到扩散步骤的控制;拟二阶动力学模型是假设吸附速率由吸附剂表面未被占有的吸附空位数目的平方值决定,吸附过程受化学吸附机理的控制,这种化学吸附涉及到吸附剂与吸附质之间的电子共用或电子转移;而内扩散则描述吸附质在颗粒内部扩散的过程。
根据式(1)、式(2)、式(3)对实验所得数据进行拟合,拟合的动力学参数如表2、表3所示。在3种不同CV浓度下,对于Fe3O4@LS对CV的吸附过程,拟二级动力学模型的相关系数(R2>0.999)均明显高于拟一级动力学模型。因此,拟二级动力学方程能更好的描述Fe3O4@LS对CV的吸附过程,Fe3O4@LS对CV的吸附存在多个吸附阶段,其中物理吸附和化学吸附同时存在,以化学吸附为主。
-
根据式(4)、式(5)、式(6)对实验所得数据进行了拟合,拟合的参数如表4所示。由表4可知,在3种温度条件下,由Langmuir模型拟合的R2均大于0.96,大于Freundlich和Temkin模型。这说明Fe3O4@LS对CV的吸附过程符合Langmuir吸附模型。由此推测,Fe3O4@LS对CV的吸附属于单分子层吸附。在Langmuir等温吸附中,随温度的升高,吸附量和KL均有下降,表明低温有利于吸附的进行。在温度为298 K时,Fe3O4@LS对CV的吸附量最大,为617.28 mg·g−1。
-
为更进一步研究吸附的热力学性质,根据式(7)、式(8)、式(9)计算出不同温度下的吉布斯自由能变、焓变和熵变,结果如表5所示,磁性水凝胶吸附剂在不同温度下对CV吸附过程的∆G均小于零,说明吸附过程是自发进行的,并且∆G随着温度的升高而变大,说明高温不利于吸附的进行。∆H<0,说明吸附过程属于放热;∆S<0,说明吸附过程是熵值减小的过程。
-
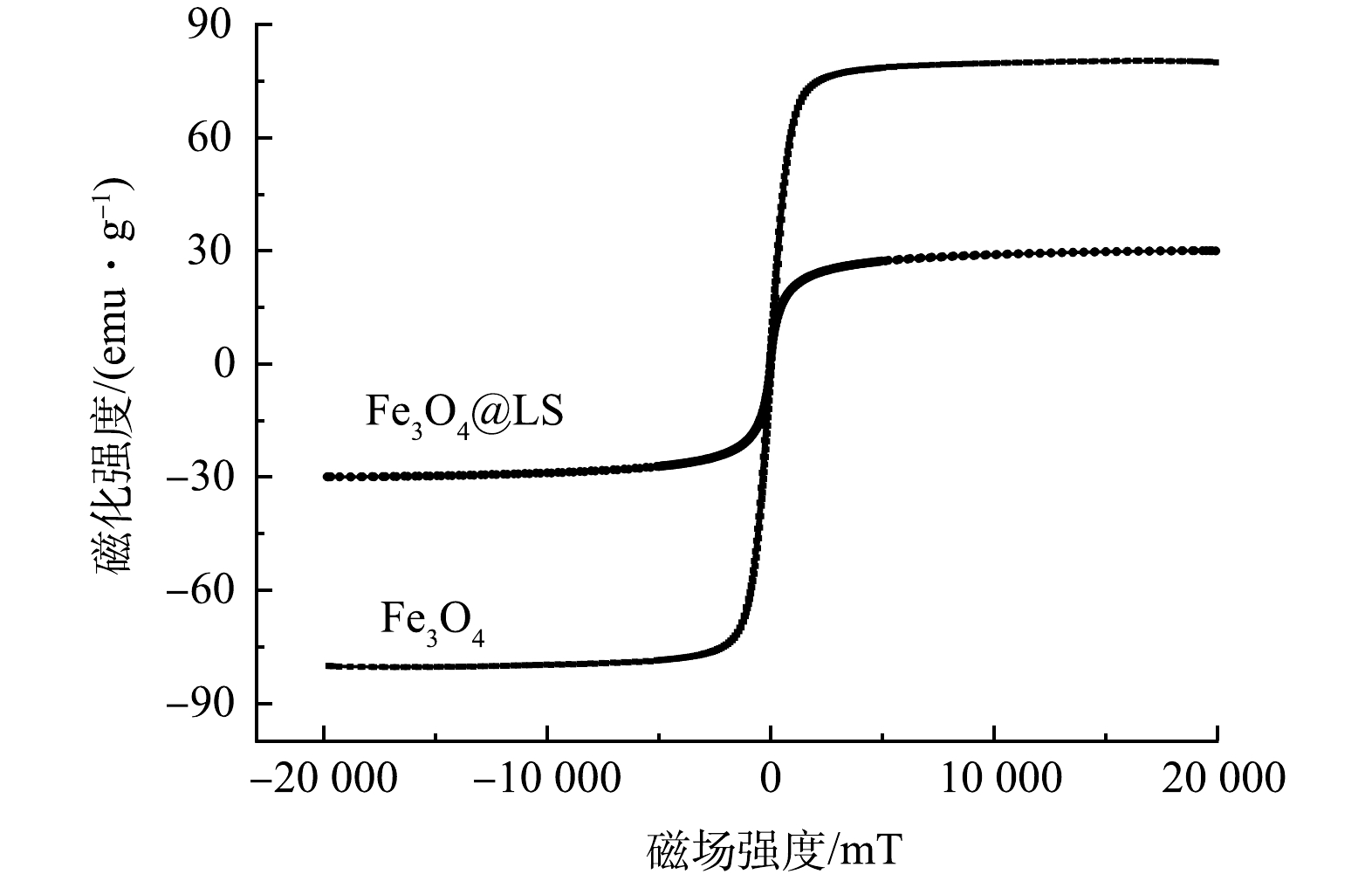
图3为在常温下,纯Fe3O4粉末与Fe3O4@LS的磁化曲线。由图3可见,Fe3O4@LS的饱和磁强(30 emu·g−1)约为纯Fe3O4粉末(80 emu·g−1)的38%。由磁化曲线可见,2种样品均未出现磁滞现象,而且剩磁和矫顽力均为0,表明利用原位沉淀法合成的Fe3O4@LS具有超顺磁性特征,对外加磁场具有良好的响应能力,能够在外界磁场的磁性作用下实现固液分离和回收利用,这样的性质对于吸附材料在实际应用中的快速固液分离及重复回收利用均具有重要意义。
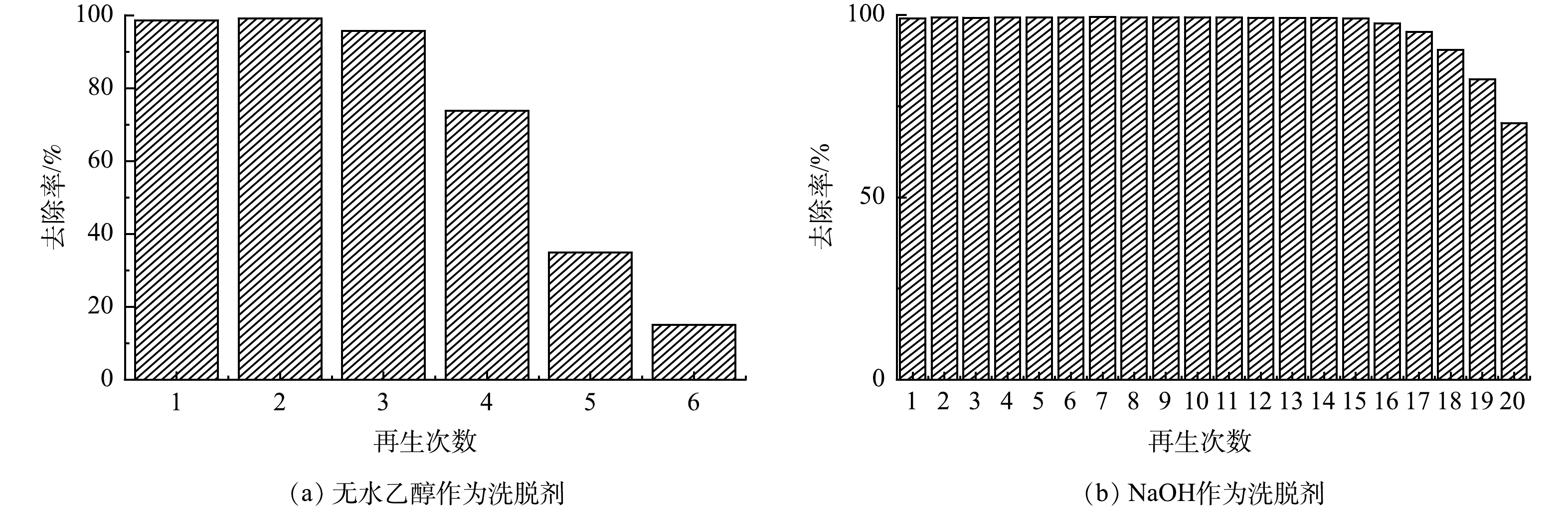
由CV溶液pH对吸附性能的影响(图2(a))可以看出,Fe3O4@LS适用于中性或碱性溶液作为洗脱液进行脱附,因此本实验选用无水乙醇和NaOH溶液作为洗脱剂。由图4(a)可知,采用无水乙醇作为洗脱剂,随着循环次数的增加,Fe3O4@LS在前3次再生的吸附效果较好,而在第4次洗脱后,Fe3O4@LS的吸附效果有所下降。其原因可能是因为洗脱循环次数过多,Fe3O4@LS结构被破坏[27],导致吸附位点逐渐减少。由图4(b)可知,采用NaOH溶液作为洗脱剂,当再生次数为15时,Fe3O4@LS去除率仍可达到98%以上,而Fe3O4@LS在多次溶胀-烘干的过程中有部分损失,从而导致再生次数大于15次后去除率有所下降。上述结果说明NaOH溶液可以有效地解吸出Fe3O4@LS吸附的CV,并且不会破坏Fe3O4@LS的内部结构,表明Fe3O4@LS具有较好的可循环利用性能。
-
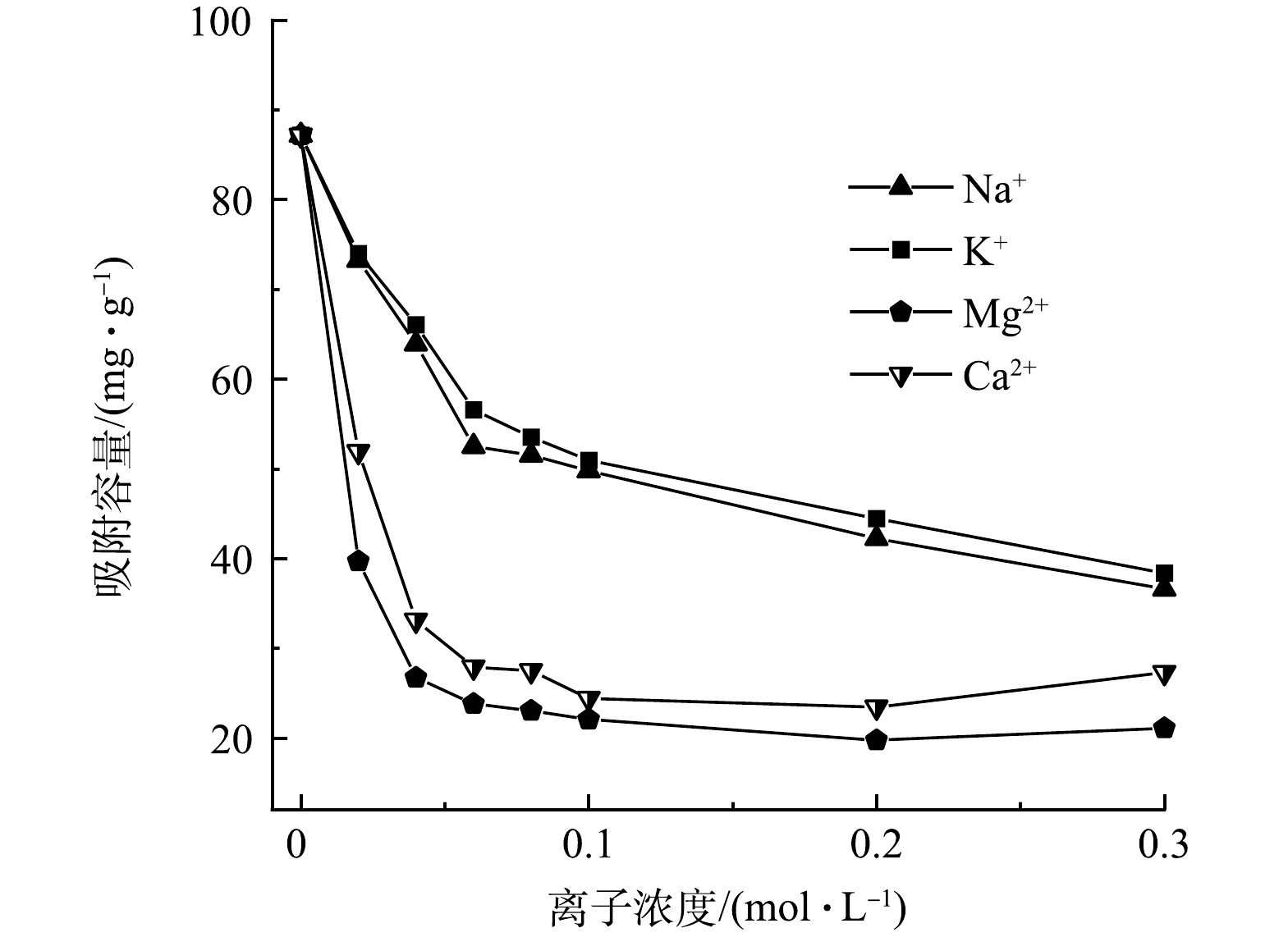
考虑到实际印染废水组分的复杂性,考察了金属离子对Fe3O4@LS吸附性能的影响。由图5可见,随着加入的Na+、K+、Ca2+和Mg2+浓度增加,Fe3O4@LS对CV的吸附量呈下降趋势,当未加入Na+、K+离子时,Fe3O4@LS对CV的吸附量为87.18 mg·g−1。当加入的Na+、K+、Ca2+和Mg2+离子浓度达到0.3 mol·L−1时,Fe3O4@LS对CV的吸附量降为36.6、31.37、21.1、27.3 mg·g−1。在相同浓度条件下,二价阳离子(Ca2+和Mg2+)的抑制作用强于一价阳离子(K+和Na+),抑制作用顺序为Mg2+> Ca2+> Na+> K+。在同类型离子共存的情况下,离子浓度越大,对CV的抑制作用越显著,对CV的吸附量越低。其原因可能是吸附剂的吸附顺序与离子的电荷数、水化能、离子半径和有效水化半径密切相关。一般情况下,电荷离子数量越多,半径越小,竞争吸附能力越强[28]。因此,在相同浓度下,二价离子(Ca2+和Mg2+)对CV的吸附抑制作用强于一价离子(K+和Na+)。如表6所示,当电荷数相同时,有效水化半径依次为Mg2+< Ca2+<Na+< K+。因此,对CV的吸附抑制顺序为Mg2+ > Ca2+ > Na+ > K+。
-
将本研究合成的磁性Fe3O4@LS水凝胶与其他文献中吸附CV所使用的材料进行对比,以最大吸附量作为对比依据。比较结果如表7所示,发现磁性Fe3O4@LS水凝胶对于CV的吸附性能明显优于其他材料,说明磁性Fe3O4@LS水凝胶在CV染料废水处理上具有极大的优势和应用前景。
-
1)相比于LS,Fe3O4@LS可提供更多的吸附位点,并且Fe3O4@LS具有比LS更大的比表面积,更有利于活性位点的暴露,这些优势有利于提高吸附性能。
2) Fe3O4@LS吸附结晶紫的最佳条件为:在25 ℃下、Fe3O4@LS投加量为400 mg·L−1、CV 废水pH=7.0、CV初始浓度为100 mg·L−1、吸附时间80 min,此条件下,对CV的平衡吸附量和去除率分别为237.08 mg·g−1和94.83%。
3) Fe3O4@LS对CV的吸附过程符合拟二级动力学方程及Langmuir吸附模型,Fe3O4@LS水凝胶对CV的吸附属于以化学吸附为主且为单分子层吸附。整个吸附过程是自发进行的,节能便利。
4)采用NaOH溶液作为洗脱剂,当再生次数为10时,Fe3O4@LS去除率仍可达到98 %以上,说明Fe3O4@LS具有很好的循环利用性能。
5)一价和二价金属离子均对CV的吸附有抑制作用,与Na+、K+离子相比,Ca2+和Mg2+离子对吸附的抑制作用更为明显,抑制顺序为Mg2+>Ca2+>Na+>K+,共存离子对吸附的影响与其化合价以及离子的水合半径等性质有关。
磁性木质素磺酸钠水凝胶对水中结晶紫的吸附性能
Adsorption performance of crystal violet in water by magnetic sodium lignonate hydrogel
-
摘要: 为了处理染料废水,本研究通过自由基接枝共聚和原位沉淀法制备了磁性木质素磺酸钠水凝胶(Fe3O4@LS),制备工艺简单环保,可实现木质素的高值化利用,且考察了Fe3O4@LS对水中结晶紫(CV)的吸附性能。结果表明,在25 ℃下,Fe3O4@LS投加量为400 mg·L−1、pH=7.0、CV初始浓度为100 mg·L−1、吸附时间80 min时,对结晶紫的平衡吸附量和去除率均达到最大,分别为237.08 mg·g−1和94.83%,Fe3O4@LS对CV的吸附过程符合拟二级动力学方程及Langmuir吸附模型;一价和二价金属离子均会抑制Fe3O4@LS对CV的吸附,Fe3O4@LS具有良好的磁响应性,使其便于回收;再生实验结果表明,Fe3O4@LS是一种具有循环使用潜力的优良生物质吸附材料;构效分析结果表明,Fe3O4@LS的优良吸附性能归因于其充足的活性位点以及丰富的孔洞结构。Abstract: The magnetic sodium lignosulfonate hydrogel (Fe3O4@LS) was prepared through free radical graft copolymerization and in-situ precipitation for the treatment of dye wastewater. The preparation process is simple and environmentally friendly, which could realize the high-value utilization of lignin. The adsorption performance of Fe3O4@LS to crystal violet (CV) in water were investigated. Results demonstrated that at 25 ℃, Fe3O4@LS dosage of 400 mg·L−1, pH 7.0, an initial CV concentration of 100 mg·L−1, and an adsorption time of 80 min, the equilibrium adsorption capacity reached the maximum value of 237.08 237.08 mg·g−1 with the highest CV removal rate of 94.83%. The adsorption behavior followed the pseudo-second-order kinetic equation and Langmuir adsorption model. Both monovalent and divalent metal ions could inhibit CV adsorption on Fe3O4@LS. Moreover, Fe3O4@LS displayed an excellent magnetic response facilitating easy recovery. Regeneration experiments confirmed that Fe3O4@LS is an outstanding biomass adsorbent material with recycling potential. The structure-activity analysis showed that the good adsorption performance of Fe3O4@LS was due to its abundant active sites and pore structures.
-
Key words:
- magnetism /
- lignin /
- hydrogel /
- adsorption /
- crystal violet
-

-
表 1 LS和Fe3O4@LS水凝胶的结构特征
Table 1. Structure characteristics of the LS and Fe3O4@LS hydrogel
样品 BET表面积/(m2·g−1) 孔隙体积/(cm3·g−1) 平均孔径/nm LS 2.342 2 0.003 975 7.792 3 Fe3O4@LS 3.669 2 0.009 593 10.746 2 表 2 不同浓度CV的动力学模型参数
Table 2. Kinetic model parameters of CV at different concentrations
CCV/
(mg·L−1)Qe,exp
(mg·g−1)拟一级动力学模型 拟二级动力学模型 k1/min−1 Qe,1/(mg·g−1) R2 k2/(g·(mg·min)−1) Qe,2/(mg·g−1) R2 25 60.590 00 0.043 25 30.609 00 0.958 60 0.169 85 63.091 00 0.999 04 50 121.120 00 0.048 25 88.218 00 0.980 10 0.169 08 126.103 00 0.999 13 100 242.090 00 0.042 03 142.124 00 0.973 80 0.166 88 251.880 00 0.999 60 表 3 不同浓度CV的内部扩散模型参数
Table 3. The parameters of internal diffusion model of CV at different concentrations
CCV/
(mg·L−1)内扩散模型参数 0~50 min 50~120 min 120~180 min Ki/(mg·(g·min0.5)−1) P R2 Ki/(mg·(g·min0.5)−1) P R2 Ki/(mg·(g·min0.5)−1) P R2 25 5.398 19.679 0.970 0.848 51.700 0.820 0.039 60.053 0.650 50 11.353 36.549 0.940 1.644 103.416 0.920 0.148 119.175 0.690 100 16.420 109.870 0.990 3.690 202.166 0.760 0.625 233.891 0.710 表 4 不同温度下等温吸附模型的参数
Table 4. Parameters of isothermal adsorption models at different temperatures
温度/K Langmuir参数 Freundlich参数 Temkin参数 Qm/(mg·g−1) KL/(L·mg−1) R2 KF/(mg·mg−1) n R2 α β R2 298 617.280 0 0.140 4 0.996 0 142.999 0 3.354 0 0.840 0 3.108 0 97.940 0 0.914 0 318 588.240 0 0.092 8 0.983 0 139.904 0 3.600 0 0.868 0 3.693 0 85.875 0 0.929 0 338 531.910 0 0.049 3 0.967 0 120.892 0 3.843 0 0.926 0 2.771 0 73.850 0 0.931 0 表 5 不同温度下吸附热力学参数
Table 5. Adsorption thermodynamic parameters at different temperatures
温度/K ∆G/(kJ·mol−1) ∆H/(kJ·mol−1) ∆S/(kJ·(mol·K)−1) 298 −8.399 −0.429 −1.019 318 −7.932 338 −5.507 表 6 共存离子的离子半径、水化半径和有效水化半径[28]
Table 6. Ionic radius, hydration radius and effective hydration radius of coexisting ions[28]
nm 离子 离子半径 水化半径 有效水化半径 Cd2+ 0.083 0.426 0.231 K+ 0.133 0.331 0.278 Na+ 0.098 0.358 0.207 Ca2+ 0.106 0.412 0.245 Mg2+ 0.078 0.428 0.199 表 7 不同材料吸附CV最大吸附量对比
Table 7. Comparison of maximum CV adsorption capacity of different materials
-
[1] 朱明新, 张进雨, 陈贝贝, 等. 磁性壳聚糖微球对酸性嫩黄G吸附行为的研究[J]. 工业水处理, 2023, 43(2): 61-67. [2] WANG T, XUE L, ZHENG L W, et al. Biomass-derived N/S dual-doped hierarchically porous carbon material as effective adsorbent for the removal of bisphenol F and bisphenol S[J]. Journal of Hazardous Materials, 2021, 416: 126126. doi: 10.1016/j.jhazmat.2021.126126 [3] PAL P, PAL A. Treatment of real wastewater: kinetic and thermodynamic aspects of cadmium adsorption onto surfactant-modified chitosan beads[J]. International Journal of Biological Macromolecules, 2019, 131: 1092-1100. doi: 10.1016/j.ijbiomac.2019.03.121 [4] GODIYA C B, LIANG M, SAYED S M, et al. Novel alginate/polyethyleneimine hydrogel adsorbent for cascaded removal and utilization of Cu2+ and Pb2+ ions[J]. Journal of Environmental Management, 2019, 232: 829-841. [5] ZHANG L, SU T, LUO Z R, et al. A graphene-based porous composite hydrogel for efficient heavy metal ions removal from wastewater[J]. Separation and Purification Technology, 2023, 305: 122484. doi: 10.1016/j.seppur.2022.122484 [6] MOHAMED A K, MAHMOUD M E. Nanoscale pisum sativum pods biochar encapsulated starch hydrogel: a novel nanosorbent for efficient chromium (Ⅵ) ions and naproxen drug removal[J]. Bioresource Technology, 2020, 308: 123263. doi: 10.1016/j.biortech.2020.123263 [7] LIU D, GU W Y, ZHOU W Q, et al. Magnetic Fe/carbon/sodium alginate hydrogels for efficient degradation of norfloxacin in simulated wastewater[J]. Journal of Cleaner Production, 2022, 369: 133239. doi: 10.1016/j.jclepro.2022.133239 [8] VANHOLME R, DEMEDTS B, MORREEL K, et al. Lignin biosynthesis and structure[J]. Plant Physiology, 2010, 153(3): 895-905. doi: 10.1104/pp.110.155119 [9] PARK D, KIM J W, SHIN K, et al. Bacterial cellulose nanofibrils-reinforced composite hydrogels for mechanical compression-responsive on-demand drug release[J]. Carbohydrate Polymers, 2021, 272: 118459. doi: 10.1016/j.carbpol.2021.118459 [10] ALMOMANI F, BHOSALE R, KHRAISHEH M, et al. Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP)[J]. Applied Surface Science, 2020, 506: 144924. doi: 10.1016/j.apsusc.2019.144924 [11] LIU X, GUAN J N, LAI G H, et al. Stimuli-responsive adsorption behavior toward heavy metal ions based on comb polymer functionalized magnetic nanoparticles[J]. Journal of Cleaner Production, 2020, 253: 119915. doi: 10.1016/j.jclepro.2019.119915 [12] KLAPISZEWSKI A, ZDARTA J, ANTECKA K, et al. Magnetite nanoparticles conjugated with lignin: A physicochemical and magnetic study[J]. Applied Surface Science, 2017, 422: 94-103. doi: 10.1016/j.apsusc.2017.05.255 [13] CHEN W, XIE H J, JIANG N, et al. Synthesis of magnetic sodium lignosulfonate hydrogel(Fe3O4@LS) and its adsorption behavior for Cd2+ in wastewater[J]. International Journal of Biological Macromolecules, 2023, 245: 125498. doi: 10.1016/j.ijbiomac.2023.125498 [14] JAVADIAN H, ANGAJI M T, NAUSHAD M. Synthesis and characterization of polyaniline/γ-alumina nanocomposite: A comparative study for the adsorption of three different anionic dyes[J]. Journal of Industrial & Engineering Chemistry, 2014, 20(5): 3890-3900. [15] ANSARI R, KEIVANI M B, DELAVAR A F. Application of polyaniline nanolayer composite for removal of tartrazine dye from aqueous solutions[J]. Journal of Polymer Research, 2011, 18(6): 1931-1939. doi: 10.1007/s10965-011-9600-z [16] NETHAJI S, SIVASAMY A. Adsorptive removal of an acid dye by lignocellulosic waste biomass activated carbon: Equilibrium and kinetic studies[J]. Chemosphere, 2011, 82(10): 1367-1372. doi: 10.1016/j.chemosphere.2010.11.080 [17] WANG X Y, CAI J H, ZHANG Y J, et al. Heavy metal sorption properties of magnesium titanate mesoporous nanorods[J]. Journal of Materials Chemistry A, 2015, 3(22): 11796-11800. doi: 10.1039/C5TA02034D [18] XU Q H, WANG Y L, JIN L Q, et al. Adsorption of Cu (Ⅱ), Pb (Ⅱ) and Cr (Ⅵ) from aqueous solutions using black wattle tannin-immobilized nanocellulose[J]. Journal of Hazardous Materials, 2017, 339: 91-99. doi: 10.1016/j.jhazmat.2017.06.005 [19] CHEN X Y, HOSSAIN M F, DUAN C Y, et al. Isotherm models for adsorption of heavy metals from water - a review[J]. Chemosphere, 2022, 307: 135545. doi: 10.1016/j.chemosphere.2022.135545 [20] MAO X Y, WANG L, GU S Q, et al. Synthesis of a three-dimensional network sodium alginate-poly(acrylic acid)/attapulgite hydrogel with good mechanic property and reusability for efficient adsorption of Cu2+ and Pb2+[J]. Environmental Chemistry Letters, 2018, 16(2): 653-658. doi: 10.1007/s10311-018-0708-9 [21] HUA Y Q, JIANG T T, WANG K, et al. Efficient pt-free electrocatalyst for oxygen reduction reaction: highly ordered mesoporous N and S co-doped carbon with saccharin as single-source molecular precursor[J]. Applied Catalysis B:Environmental, 2016, 194: 202-208. doi: 10.1016/j.apcatb.2016.04.056 [22] LI X Z, SHUAI K W, ZHANG Y R, et al. Removal of Cd2+ from wastewater to form a three-dimensional fiber network using si-mg doped industrial lignin-based carbon materials[J]. International Journal of Biological Macromolecules, 2023, 229: 62-69. doi: 10.1016/j.ijbiomac.2022.12.274 [23] FAN X B, WANG X H, CAI Y T, et al. Functionalized cotton charcoal/chitosan biomass-based hydrogel for capturing Pb2+, Cu2+ and MB[J]. Journal of Hazardous Materials, 2022, 423: 127191. doi: 10.1016/j.jhazmat.2021.127191 [24] HU X J, WANG J S, LIU Y G, et al. Adsorption of chromium (Ⅵ) by ethylenediamine-modified cross-linked magnetic chitosan resin: isotherms, kinetics and thermodynamics[J]. Journal of Hazardous Materials, 2011, 185(1): 306-314. doi: 10.1016/j.jhazmat.2010.09.034 [25] DEMIRBAS E, KOBYA M, SULAK M T. Adsorption kinetics of a basic dye from aqueous solutions onto apricot stone activated carbon[J]. Bioresource Technology, 2008, 99(13): 5368-5373. doi: 10.1016/j.biortech.2007.11.019 [26] 唐丹, 张丽青, 周波, 等. 米曲霉(Aspergillus oryzae)对Pb2+的吸附特性研究[J]. 环境科学与技术, 2013, 36(10): 161-167. [27] 王培, 牛丽丽, 陈灵智. 姜黄素-聚乙烯醇药物凝胶的制备及性能研究[J]. 中国塑料, 2023, 37(7): 34-40. [28] 谢厦, 徐应明, 闫翠侠, 等. 酸碱复合改性海泡石亚结构特征及其对Cd(Ⅱ)吸附性能[J]. 环境科学, 2020, 41(1): 293-303. [29] 陈勇, 程宁, 杨育兵, 等. 物理超声改性膨润土吸附结晶紫染料的性能研究[J]. 工业水处理, 2021, 41(9): 98-103. [30] 姬亚军, 李甜甜, 高培林, 等. 等级孔MFI分子筛的合成及高效吸附结晶紫性能研究[J]. 信阳师范学院学报(自然科学版), 2021, 34(2): 277-282. [31] 廖颖敏, 李晓慧, 蔡婷, 等. 改性榴莲壳对结晶紫的吸附性能研究[J]. 化工新型材料, 2019, 47(8): 228-232. [32] 褚淑祎, 杨敏, 肖继波, 等. 再力花残体活性炭的制备及对结晶紫的吸附[J]. 应用生态学报, 2013(6): 1693-1698. [33] 唐然肖, 李妍, 范胜男, 等. 核桃壳粉对阳离子染料结晶紫的吸附特性[J]. 河北大学学报(自然科学版), 2018, 38(3): 254-261. [34] 高美娜, 唐然肖, 翟焱洲, 等. 磁性介孔碳吸附模拟废水中的结晶紫[J]. 环境工程学报, 2015, 9(10): 4883-4889. [35] YI Y, TU G, YING G, et al. Magnetic biochar derived from rice straw and stainless steel pickling waste liquor for highly efficient adsorption of crystal violet[J]. Bioresource Technology, 2021, 341(1/2): 125743. [36] DRUZIAN S P, ZANATTA N P, BORCHARDT R K, et al. Chitin-psyllium based aerogel for the efficient removal of crystal violet from aqueous solutions[J]. International Journal of Biological Macromolecules, 2021, 179(2): 366-376. [37] 邓燕, 张艺潇, 朱红庆, 等. 磁性硅气凝胶对水中结晶紫的吸附作用[J]. 沈阳药科大学学报, 2018, 35(7): 587-592. -



 下载:
下载: