-
含磷废水排入收纳水体,易导致水中磷元素含量超标造成水体富营养化现象[1]。吸附法因其高效、清洁和可回收等众多优点在除磷技术中占据重要地位。相关研究[2]表明,稀土元素能够在低浓度下与磷生成不易溶解的络合物,具备超高的亲磷能力。镧基吸附剂已在许多研究中展现出优异的除磷能力[3],而铈基吸附剂的报道相对较少。事实上,铈(Ce)是储量最丰、价格最便宜的稀土元素,对于阴离子展现出良好的吸附性能,具有强大的除磷潜力[4],但单一采用Ce及其氧化物会面临一系列的问题,如吸附时易团聚、价格昂贵、吸附后回收困难及吸附点位不均等[5]。因此,寻找一种合适的载体可以弥补铈作为除磷吸附剂的不足。
在自来水厂的水质净化过程中,不可避免地会产生净水污泥(drinking water treatment residues, DWTR)。DWTR含水率高,表面附着羟基,具有大量Si、Al、O、Fe元素以及一定的孔道结构与层状结构。过往对于DWTR的处理方式主要为焚烧、填埋和弃海,这些处理方法回收效率低、回收成本高[6]。为响应可持续性发展战略,利用DWTR的特性制备除磷吸附剂可以进一步推进其资源化再利用。近几十年,研究者们采用热处理、酸碱盐改性以及亲和元素负载等方式改性DWTR,用以吸附废水中的无机物、有机物和毒害物质[7-9]。
本研究采用煅烧-碱浸-铈负载联合改性DWTR的方式制备了一种新型除磷吸附剂,弥补了DWTR孔道结构连通性差、Si元素占据点位、亲和基团不足以及稀土元素易团聚等缺点。考察了Ce/泥负载质量比、投加量、pH以及共存离子对材料吸附除磷效能的影响,采用SEM、XRD、BET、XPS、FT-IR探究了材料的吸附特征,对磷酸盐吸附过程动力学、吸附等温线及颗粒扩散模型的拟合,探究了材料的循环再生能力,且根据上述所得结果推测了可能的吸附机理。
-
本研究中所使用的化学药剂均为分析级。主要试剂磷酸二氢钾(KH2PO4)购于天津科密欧化学试剂有限公司,氯化铈(CeCl3)、氢氧化钠(NaOH)购于上海阿拉丁生化科技股份有限公司。净水污泥取自中国苏州某自来水厂。
-
取浓缩池沉淀净水污泥脱水烘干,粉碎后过150目筛,得到原净水污泥(DWTR)。将DWTR置于马弗炉中700 ℃下煅烧4 h,制得热处理后的净水污泥(RDWTR)。将RDWTR于1.5 mol·L−1的NaOH溶液浸泡6 h,过滤洗涤烘干后研磨过筛,得热碱改性的净水污泥(RJDWTR)。
在碱性条件下(pH>10)通过共浸渍法将质量分数为10%~60%的Ce负载于净水污泥上,搅拌2 h后,过滤洗涤烘干后研磨过筛,得不同负载比下热碱铈顺次改性的净水污泥(RJDWTR@10%~60%Ce)。
-
材料表面性质由扫描电子显微镜和能谱仪( SEM-EDS,日立S - 4800,日本岛津公司)、X射线衍射仪(XRD,布鲁克D2 PHASER,德国)、傅里叶红外光谱仪(FT-IR, Thermo Scientific Nicolet 6700,美国)、x射线光电子能谱仪(XPS, Thermo Scientific K-Alpha,美国)以及全自动比表面及孔隙度分析仪(BET, 麦克TriStar II 3020, 美国)进行观察分析。溶液中金属离子浓度由电感耦合等离子体发射光谱仪(ICP-OES, Thermo Fisher iCAP PRO,美国)确定。
-
实验所需磷酸盐溶液均由磷酸二氢钾与去离子水配置而成,采用1 mol·L−1 HCl和1 mol·L−1 NaOH溶液调节pH,吸附实验在250 mL带塞锥形瓶中完成。吸附结束后,溶液通过0.45 μm微孔膜过滤,滤液采用钼酸铵分光光度法测定残留磷酸盐质量浓度(以P计)。实验结果取3组平行实验均值,吸附量和去除率根据式(1)和式(2)计算。
式中:qe 为吸附量,mg·g−1;C0为初始磷的质量浓度,mg·L−1;Ce 为吸附后磷的质量浓度,mg·L−1;V为溶液体积,L;m为吸附剂质量,g;R为去除率,%。
1)吸附效果因素影响实验。设定初始磷酸盐质量浓度为10 mg·L−1,在25 ℃、150 r·min−1、0.6 g·L−1投加量和240 min的条件下,考察Ce/泥质量分数分别为10%、20%、30%、40%、50%和60%的RJDWTR@Ce对磷的吸附效果,比选出最优吸附材料进行后续实验。初始pH影响实验条件同负载量实验,将溶液初始pH分别调至2、3、4、5、6、7、8、9、10和11,检测不同初始pH对RJDWTR和RJDWTR@Ce吸附磷酸盐的影响。投加量实验在最适初始pH条件下进行,设计投加量分别为0.1、0.2、0.3、0.4、0.5、0.6、0.7和0.8 g·L−1。在最适初始pH和投加量的条件下,调节溶液体积为100 mL,分别于5、10、20、30、40、60、90、120、240、480、720、1 440、2 160和2 880 min时取样,检测接触时间对2种材料吸附效果的影响。在最适pH、投加量和接触时间条件下,将CO32− 、SO42−、Cl−、NO3−、F−及HA分别以0、50、100和150 mg·L−1的质量浓度加入到溶液中,探究共存离子及HA对2种材料吸附磷酸盐的影响。在最佳条件下,控制温度恒定,分别调节C0为10、30、50、100、150和200 mg·L−1,探究初始浓度对磷酸盐去除的影响。
2)吸附动力学实验。采用拟一级动力学(式(3))、拟二级动力学(式(4))及颗粒扩散模型(式(5))对实验数据进行拟合。采用Langmuir吸附等温线模型(式(6))和Freundlich吸附等温线模型(式(7))对数据进行吸附等温线拟合。
式中:qt 为t时刻吸附量,mg·g−1;qe为平衡吸附量,mg·g−1;k1为拟一级动力学反应速率常数,min−1;k2为拟二级动力学反应速率常数,g·(mg·min)−1;kd 为粒子内扩散速率常数,mg·g−1·min1/2;C 为与边界层厚度密切相关的常数,mg·g−1。
式中:Qm为最大时刻吸附量,mg·g−1;Qe 为平衡吸附量,mg·g−1;Ce为平衡时磷质量浓度,mg·L−1;KL为Langmuir模型,L·mg−1、KF为Freundlich模型常数,(mg·g−1)·(L·mg−1)1/n;n为Freundlich等温方程数。
4)循环再生实验。首次吸附后采用1 mol·L−1 氢氧化钠溶液为解吸剂,以固液比1 g·L−1将材料脱吸附240 min,解吸完成后使用去离子水将材料冲洗至中性,烘干过筛后进行下一次吸附。以上步骤分别进行5个解吸-吸附循环。
-
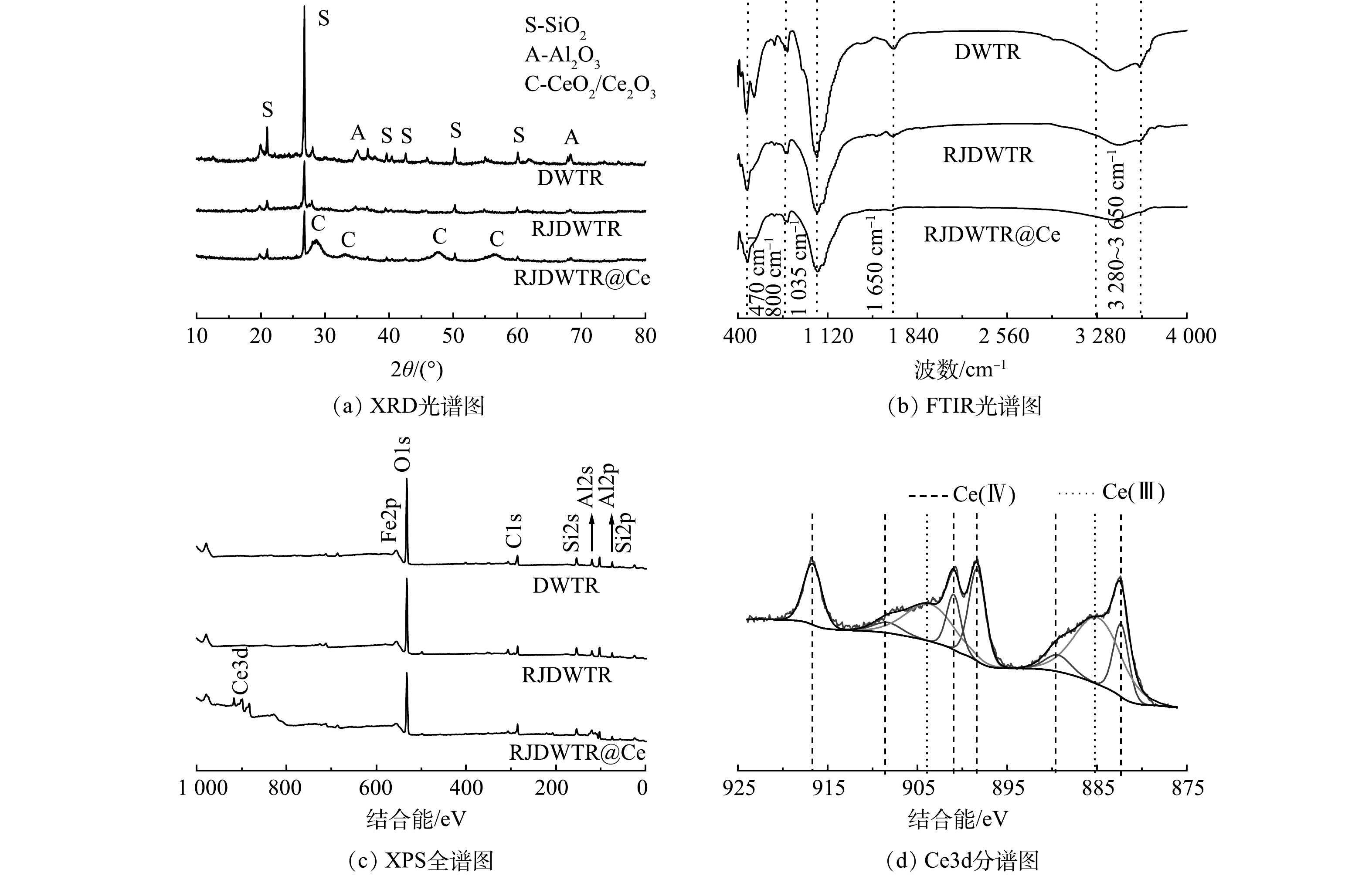
DWTR、RJDWTR与RJDWTR@40%Ce的表面形貌如图1(a)~(c)所示。可见,经煅烧和碱改性后,材料表面变得粗糙,由原先致密的结构变得松散层状化,这是由于高温煅烧致使DWTR内部的水分蒸发,同时部分孔道结构坍塌;随后采用强碱浸泡,导致DWTR内部分元素的晶体形态遭到破坏[10],洗脱的Si、Al元素为材料表面带来更大的孔隙结构。Ce负载后可以观察到材料表面出现更多松散状颗粒物。经微波消解测量后,RJDWTR@40%Ce上实际Ce含量为12.1%。
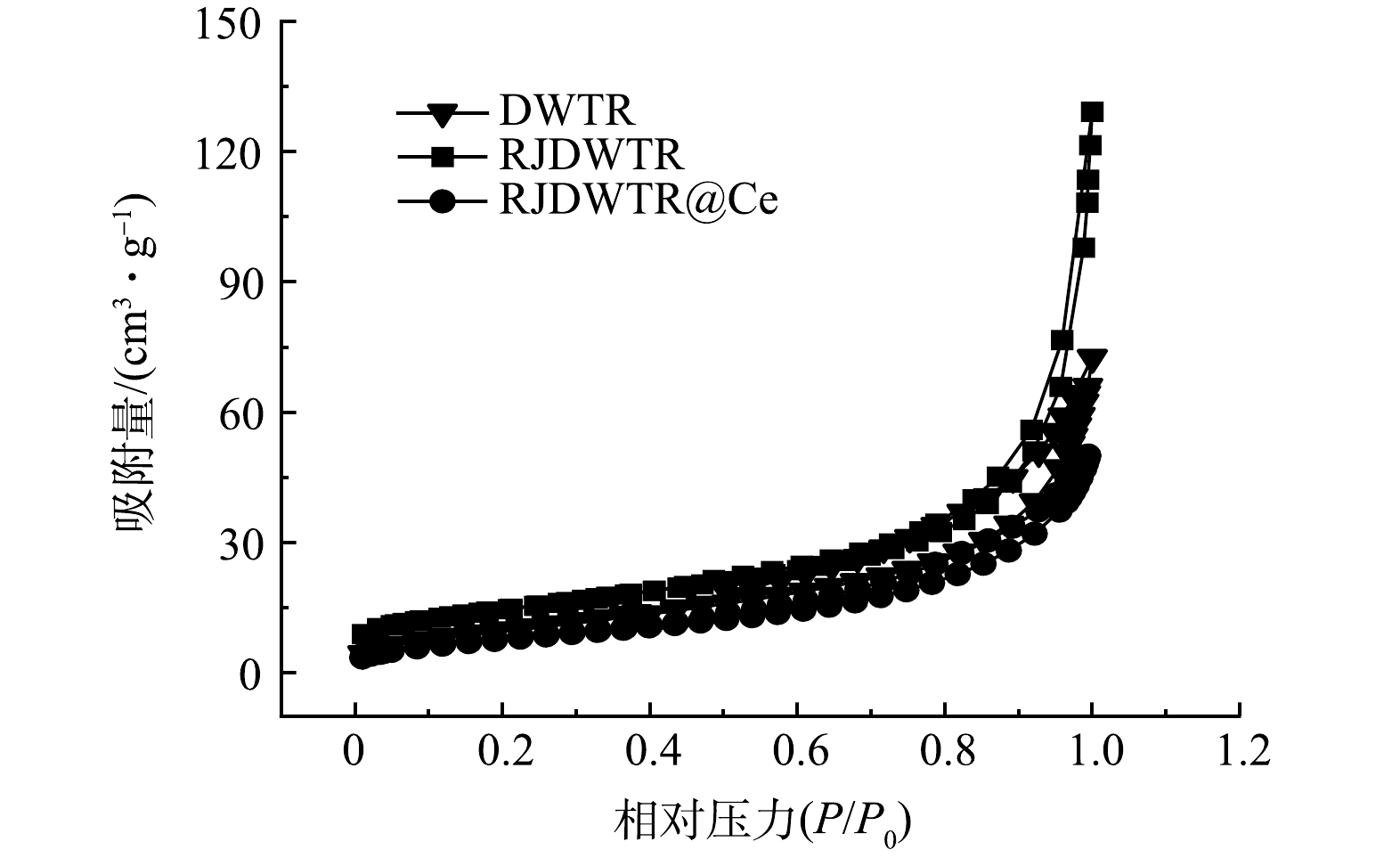
通过N2吸附-脱附实验确定了DWTR、RJDWTR与RJDWTR@10%~60%Ce的吸附物理特性。如表1所示,经煅烧和碱处理后,材料的吸附物理特性大幅度上升,但随着铈负载浓度的上升,材料的比表面积和孔隙体积呈现下降的趋势。这是由于铈的氧/氢氧化物在RJDWTR的孔道结构中沉淀,堵塞了孔隙空间,负载的Ce含量与吸附物理特性间存在协同效应,适当的亲和点位和比表面积能够保障吸附性能的有效性。改性后,孔体积与平均孔径的比值降低,这证实在改性过程中DWTR的微孔发生了坍塌[11]。由图2可知,DWTR、RJDWTR与RJDWTR@40%Ce的等温线均属于Ⅳ型等温线,RJDWTR@40%Ce的滞回环明显减小,表明其内部有利于磷酸盐吸附的中孔得到改善。
DWTR、RJDWTR与RJDWTR@40%Ce的XRD图谱见图3(a)。改性后,DWTR上的Si、Al元素在强碱的作用下由晶体结构转化为非晶相结构,以离子的形式(SiO2−、AlO2−)从RJDWTR上脱附[12],RJDWTR@40%Ce出现了Ce相关氧化物的晶体衍射强度[13]。在图3(b)中,470~530、800和1 035 cm−1的峰分别为Al—O、O—Si—O和Al—OH的吸收峰[14],经改性后,这3个吸收峰均有所减弱,推测是由于高温煅烧破坏羟基的结构影响了与之相连的离子,另一方面强碱参与了元素的洗脱。1 650 cm−1及3 440~3 650 cm−1的宽峰为—OH吸收峰[15],由于材料表面的水分因改性流失,—OH的吸收峰减弱。利用XPS分析改性前后材料元素的组成和价态(图3(c))。改性后,材料表面Si、Al元素有所降低,同时出现了Ce3d的收缩峰强。将Ce3d进行分峰拟合(图3(d)),处于885.2 eV/903.9 eV的峰是Ce (III)化学态,882.4 eV/901.0 eV、889.6 eV/908.5 eV和898.4 eV/ 916.8 eV的峰属于Ce (IV)化学态[16],证实了Ce的加载。
-
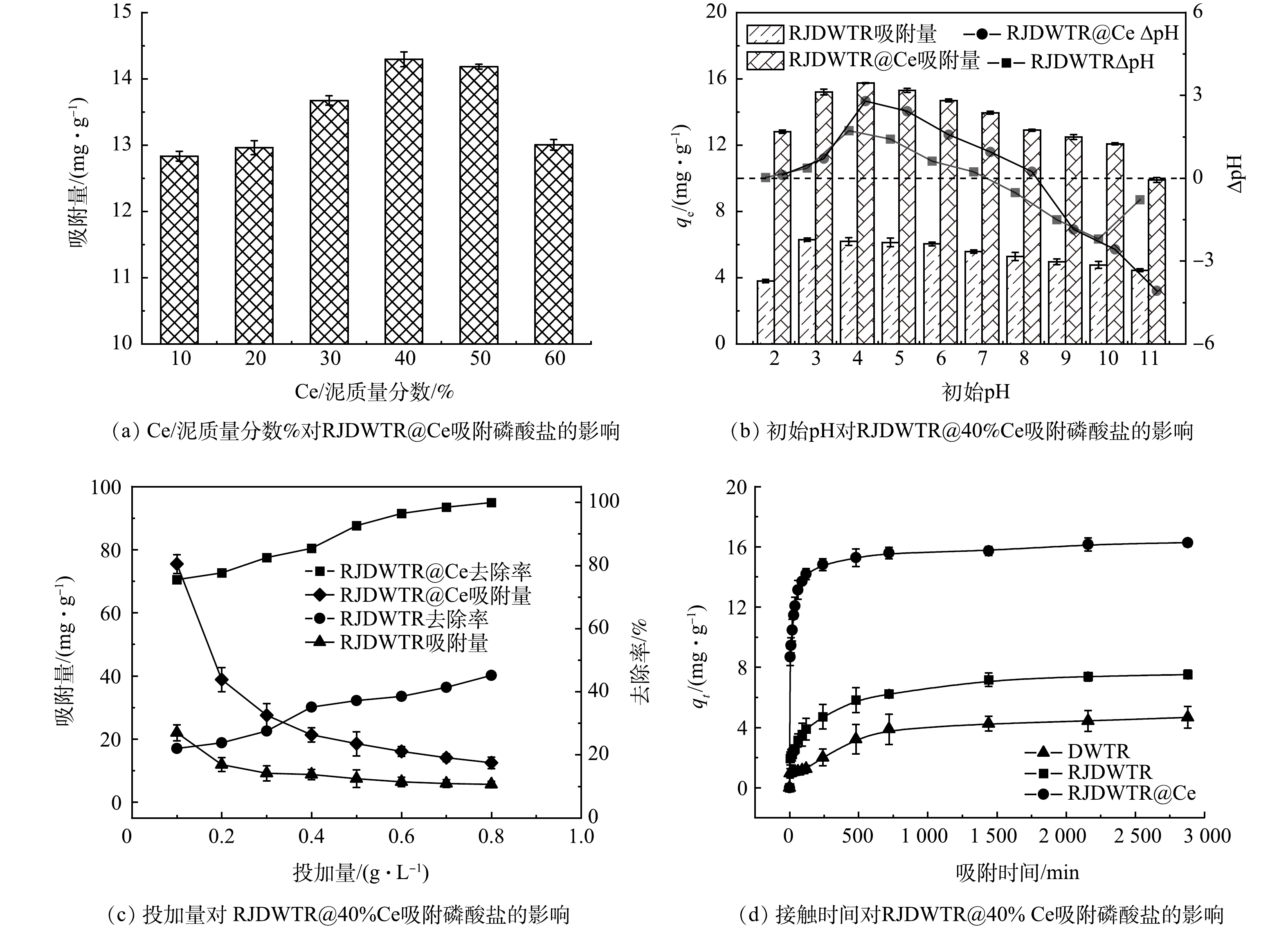
1) Ce/泥质量分数对吸附性能的影响。通过调整Ce/泥质量分数,探究Ce负载量对RJDWTR@Ce吸附磷酸盐的影响。如图4(a)所示,随着Ce负载量的提升,材料对磷的吸附效果逐渐提升,当Ce/泥质量分数为40%时,RJDWTR@Ce对磷酸盐的吸附量最大,为14.3 mg·g−1,随后负载量继续增加,吸附量开始下降。由表1推测,低负载量时Ce含量的提升可为RJDWTR@Ce提供更多的活性点位,但随着负载量持续提升,过量Ce(OH)3和CeO2堵塞孔道结构,吸附活性位点减少[17]。故后续吸附实验采用RJDWTR@40%Ce作为吸附剂。
2)初始pH对吸附性能的影响。在初始pH 2~11内的RJDWTR与RJDWTR@40%Ce吸附磷酸盐的结果见图4(b)。RJDWTR在初始pH为3时对磷酸盐的吸附量最大(6.31 mg·g−1),RJDWTR@40%Ce在较为宽泛的pH(2~10)范围内均能稳定吸附磷酸盐(13 mg·g−1以上),在初始pH为4时达到峰值(15.75 mg·g−1)。一般来说,水溶液中磷的存在形式、污染物表面电荷以及金属基吸附剂脱质子作用都与溶液pH密切相关。当pH小于等电点时,材料表面呈现质子化,带有负电荷的磷酸盐会在静电引力的作用下被带有正电荷的吸附剂捕获[18],由ΔpH推测,RJDWTR与RJDWTR@40%Ce的等电点分别为8.11和7.32。当初始pH=4时,溶液中磷酸盐主要以H2PO4−的形态存在,此形态下的磷酸盐易于与材料结合;当pH>7.32时,磷酸盐以HPO42−与PO43−为主,材料表面脱质子化,带有更高负电荷的磷酸盐会与材料表面产生更强的静电斥力,抑制吸附进程,同时碱性条件下高浓度共存的OH−也会与磷酸盐竞争吸附点位,因此,碱性条件下材料对磷酸盐的吸附能力大大降低。此外,由于吸附与解吸的过程相逆,再生实验中可以考虑采用强碱溶液作为再生液。
3)投加量对吸附性能的影响。如图4(c)所示,当吸附剂投加量由0.1 g·L−1增至0.3 g·L−1时,RJDWTR@40%Ce对磷酸盐的去除率稳步提升,由75.54%增至82.53%;当吸附剂投加量达到0.6 g·L−1时,磷酸盐去除率达到96.47%,此时溶液中所含磷酸盐残留小于0.5 mg·L−1,达到国家一级排放A标准;继续增大吸附剂投加量后,材料对磷酸盐的去除率及吸附量的变化减缓,说明溶液中已存在足够丰富的磷酸盐吸附位点。RJDWTR在0.6 g·L−1投加量时,对磷酸盐的吸附量为6.42 mg·g−1,去除率为38.51%。综合考虑各因素,后续实验中选取0.6 g·L−1为最适吸附剂投加量。
4)接触时间对吸附性能的影响。为近一步考察改性对材料吸附性能的影响,考察了不同接触时间下DWTR、RJDWTR与RJDWTR@40%Ce对磷酸盐吸附的影响。如图4(d)所示,在前120 min内3种吸附剂对磷酸盐为快速吸附,自240 min时吸附开始减缓,此时3种材料的吸附量分别为2.01、4.71和14.82 mg·g−1,RJDWTR@40%Ce已吸附90.25%的磷酸盐。随后,时间持续增加,吸附速率近一步减缓,吸附量逐渐达到饱和,至2 880 min时,吸附已达平衡,DWTR、RJDWTR与RJDWTR@40%Ce的平衡吸附量分别为4.67、7.52和16.27 mg·g−1,RJDWTR@40%Ce的平衡吸附量约为DWTR的3.5倍。
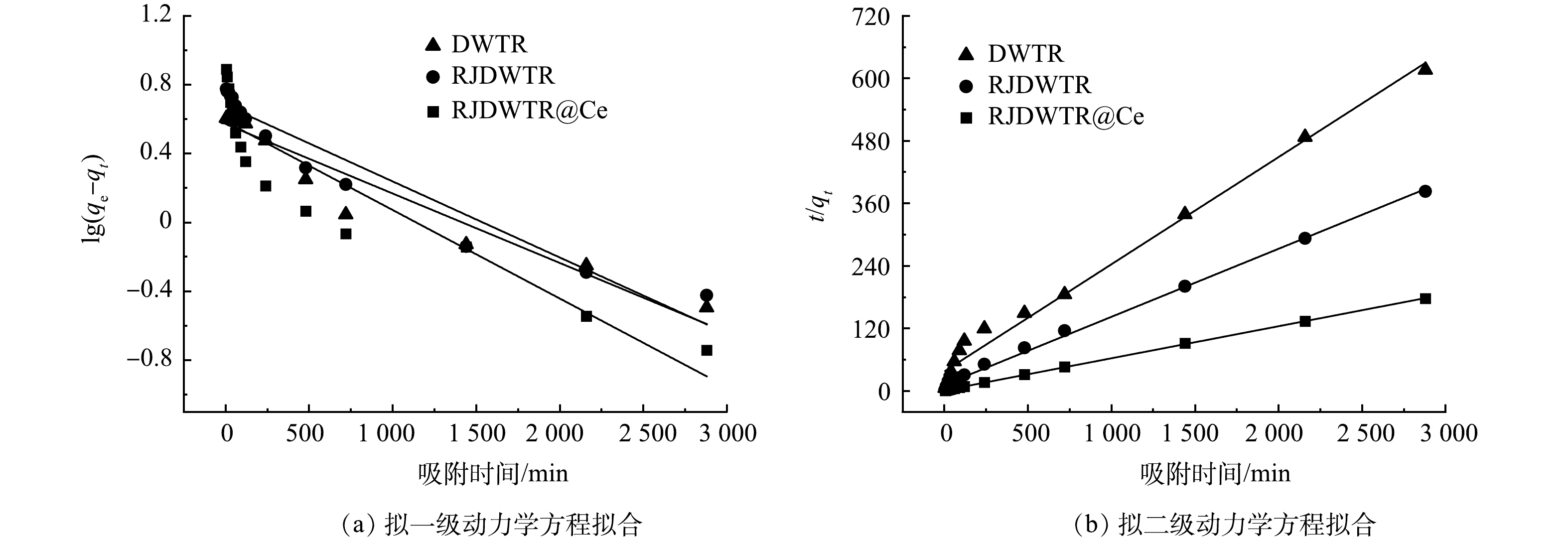
5)吸附动力学。对DWTR、RJDWTR与RJDWTR@40%Ce吸附磷酸盐的过程进行动力学拟合,拟合结果如图5及表2所示。由表2可以看出,3种材料的拟二级动力学拟合系数均高于拟一级动力学,计算所得qe基本与实验数据吻合,说明拟合结果可靠,吸附过程可能以化学吸附为主,磷酸盐在吸附剂上形成了化学键[19]。通过对比不同吸附材料的R2与k1/2发现,RJDWTR与RJDWTR@40%Ce的化学吸附占比高于DWTR,吸附速率更快。由图6和表3的颗粒扩散模型拟合结果可见,吸附过程分为3个阶段,k值越小,空间位阻效应越大,DWTR的快速吸附位于第2阶段,RJDWTR与RJDWTR@40%Ce的快速吸附位于第1阶段,这表明改性后的材料拥有了短时间内快速吸附的能力;同时,各段C值均不为0,代表可能还有边界层效应的参加,内扩散并不是唯一的限速步骤[20]。
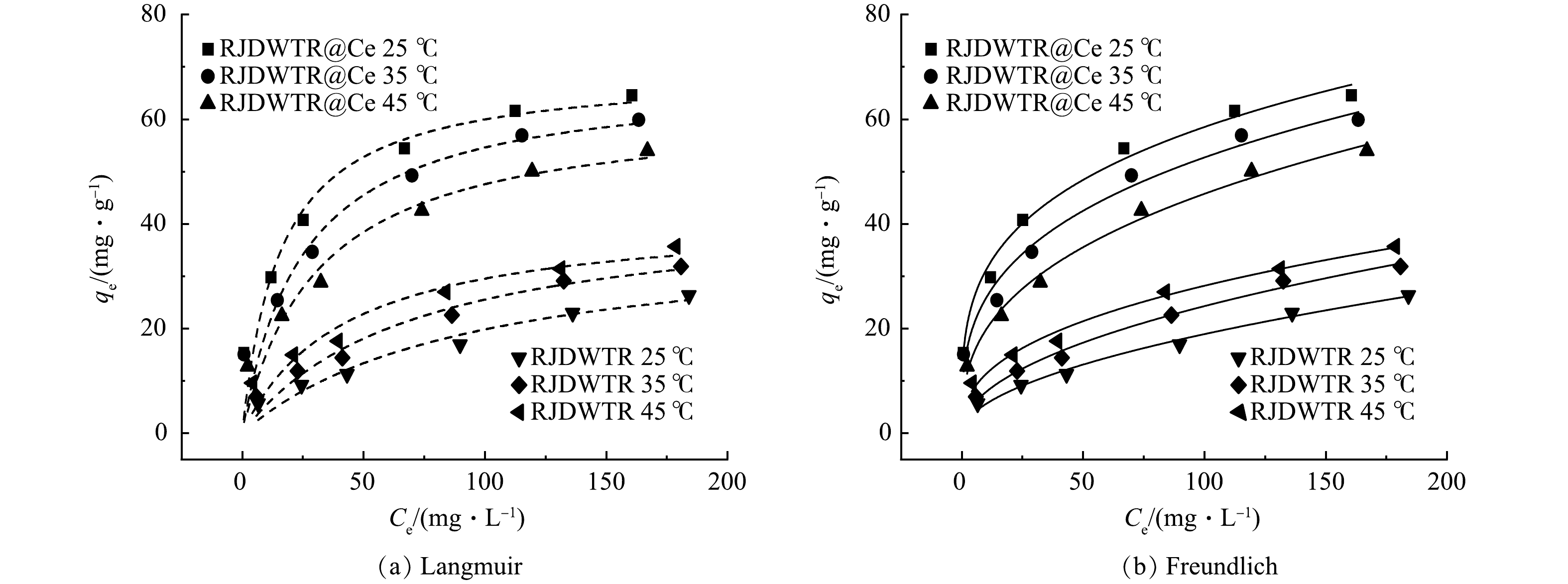
6)吸附等温线。由图7和表4可见,Freundlich等温线模型(R2=0.979~0.992)可以更好地描述RJDWTR和RJDWTR@40%Ce对磷酸盐的吸附过程。这说明在吸附过程中,材料表面的官能团不均一,磷酸盐以多层的形式被吸附[12]。此外,RJDWTR@40%Ce的吸附强度值1/n为0~1,表明吸附条件良好,并且可能存在协同吸附[21],代表对吸附有利的KF值随着温度的升高而减小,说明升温可能不利于反应的进行。25 ℃下,Langmuir等温线模型R2为0.950,拟合具备一定的参考性,方程拟合最大吸附量为69.43 mg·g−1。相较于RJDWTR,负载Ce后的材料具有更大的Qm、KF和n,说明负载总体上提升了材料的吸附性能。由表5可见,与不同方式制备的除磷吸附剂相比,RJDWTR@40%Ce表现出优异的除磷效果。
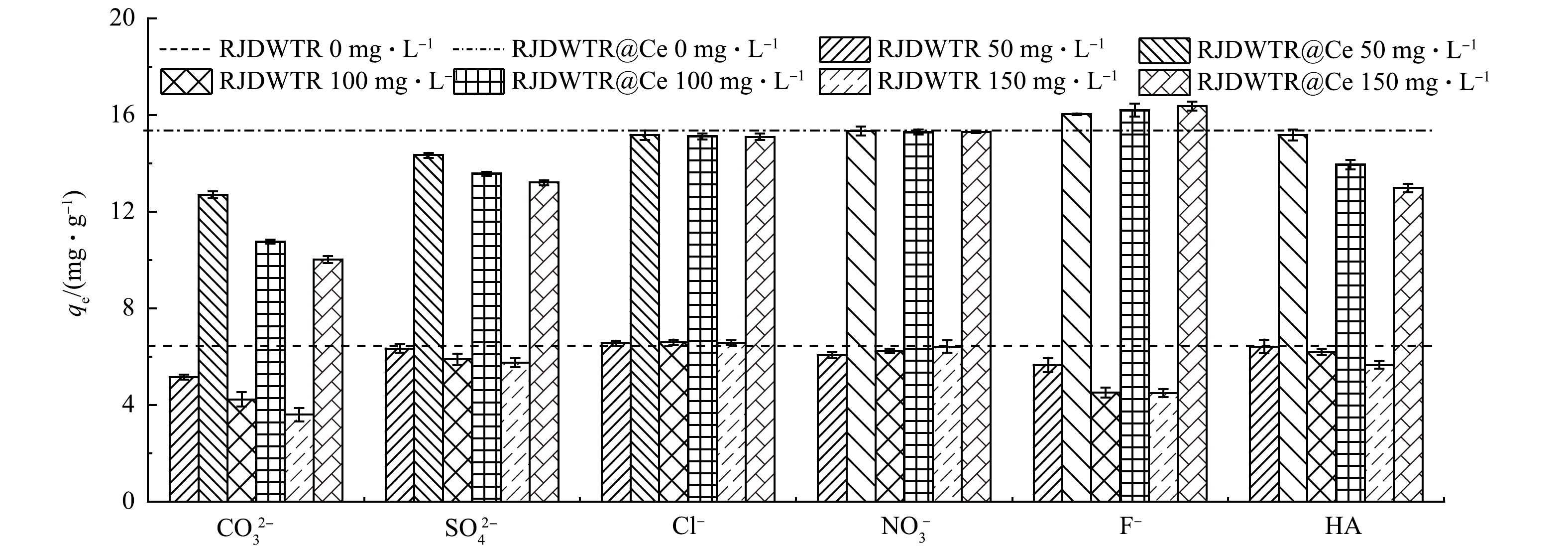
7)共存离子对吸附性能的影响。实际水体往往组分复杂多变,优异的吸附剂需要具备良好的抗干扰性。初始pH=4时,溶液中磷酸盐存在形式以H2PO4−为主。由图8可见,当共存离子质量浓度由0 mg·L−1升至150 mg·L−1时,Cl−与NO3−的加入并没有对RJDWTR和RJDWTR@40%Ce捕获H2PO4−产生明显的影响,这是由于Cl−与NO3−所带电荷量相对较低,同时与吸附剂多形成外球表面配合物[31],对以内球络合机制为主的磷酸盐吸附没有较大影响。当溶液中存在CO32−、SO42−及HA时,2种材料对磷酸盐的吸附量呈现不同程度地减少,CO32−、SO42与磷酸盐在结构上具有相似性,同时带有更高的负电荷量,能够与H2PO4−竞争质子化吸附剂上的吸附点位;而HA是一种天然有机高分子化合物,表面带有负电荷,与吸附剂结合后将会降低其所带的正电荷量,还能够通过空间位阻效应影响吸附剂与磷酸盐的结合[32]。在F−的共存下,RJDWTR对H2PO4−的吸附量显著降低,但对RJDWTR@40%Ce吸附H2PO4−起到促进作用,并随着F−质量浓度的提升,促进效果有所增强。众所周知,F−具有很强的电负性,易于与质子化的吸附剂结合,并且吸附点位与磷酸盐类似,总体上会抑制吸附剂对磷酸盐的吸附。对于RJDWTR@40%Ce展现出的不同结果,可能有以下2点原因:Ce对于P具有强亲和力,当RJDWTR负载Ce后,Ce-OH能够抵抗F−的影响优先与H2PO4−结合[33];F−质量浓度的增加可提高溶液中的离子强度,促进离子的热运动过程,使溶液中的H2PO4−更易移动至吸附剂表面。总得来说,RJDWTR@40%Ce对于磷酸盐的吸附具有良好的抗干扰性,在干扰离子质量浓度远大于磷酸根质量浓度的情况下,仍然展现出较好的效果。
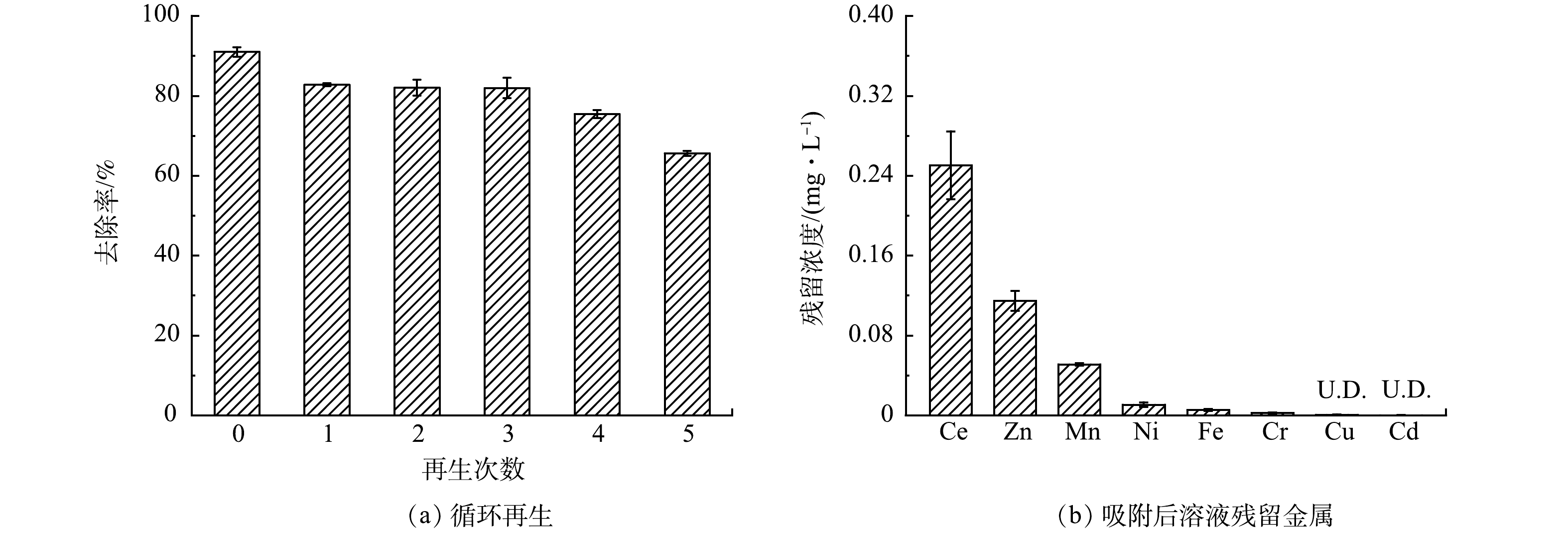
8)RJDWTR@40%Ce的再生性能。能够在实际中应用的吸附剂除具有高效性外,还应具备稳定性和安全性。有研究[34]表明,强碱溶液富集的OH−能够取代材料中的磷酸盐,并将其洗脱到再生液中。结合碱性条件下RJDWTR@40%Ce对于磷酸盐的结合显著减弱,本研究采用强碱溶液再生RJDWTR@40%Ce。由图9(a)可见,首次吸附后RJDWTR@40%Ce对磷酸盐的去除率为90.99%,经5次解吸-吸附循环后,磷酸盐去除率为65.59%,这表明RJDWTR@40%Ce具备一定的再生性与稳定性,但在解吸过程中存在部分吸附位点的损失,导致解吸后吸附容量未能完全恢复。另外,对吸附后滤液的残留金属进行了采集(图9(b)),残留Ce含量为0.25 mg·L−1,其余各重金属含量均低于国家污水综合排放标准。上述结果证明RJDWTR@40%Ce的使用安全可靠,不会为处理水带来二次污染,RJDWTR@40%Ce作为一种高效、经济和清洁的除磷吸附剂具备广泛应用的潜力。
-
根据2.2初始pH的探究,得到了RJDWTR@40%Ce的等电位点以及碱性条件下吸附量降低的结论,可以推测磷酸盐在材料表面的吸附机制包含静电引力的作用:带有正电荷的材料吸引溶液中带有负电荷的磷酸盐,通过静电作用将一部分磷酸盐固定在吸附剂表面。但在强碱条件下,RJDWTR@40%Ce的吸附能力仍显著高于RJDWTR,这说明吸附过程中还存在其他机制,且这种机制与Ce有关。
为近一步分析材料对磷酸盐的吸附机理,对吸附磷酸盐后的RJDWTR@40%Ce-P进行了XPS图谱采集,结果如图10(a)所示。吸附后,XPS图谱中出现了P2p的衍射峰,表明磷酸盐已被成功吸附至材料表面,将P2p轨道进行分峰拟合(图10(b)),被捕获的磷酸盐峰值为133.18 eV,与KH2PO4的标准峰值(134.0 eV)具有约0.82 eV的负位移差,这代表在吸附的过程中磷酸盐与RJDWTR@40%Ce之间可能形成了化学键[35]。对吸附前后的Ce3d衍射峰进行对比(图10(c)),吸附后Ce3d的结合能略有提升(约为0.28 eV),这说明Ce3d价带上发生了电子转移,Ce与磷酸盐形成了某种络合物[36]。吸附过程中,RJDWTR@40%Ce表面存在不饱和Ce(Ⅲ)的活性位点,可以水解产生大量Ce-OH,由于Ce的高亲磷性,Ce-OH与溶液中的磷酸盐通过配体交换的方式完成磷酸盐结合以及羟基释放。有研究[19]表明,稀土元素可以与磷酸盐形成多种内球配合物,推测在RJDWTR@40%Ce吸附磷酸盐的过程中发生了如式(8)和式(9)的化学反应。RJDWTR@40%Ce与RJDWTR@40%Ce-P的O1s分峰拟合图谱结果见图10(d),吸附前材料表面具有的氧形态为表面活性氧、晶格氧与部分H2O;吸附后,材料表面的晶格氧与H2O有所降低,表面活性氧的峰值攀升、含量增加,这是由于RJDWTR@40%Ce与磷酸盐完成羟基置换后,生成Ce-O-P,P=O和P-OH (其中O均以表面活性氧形式存在)[37];同时部分水分子也与磷酸根离子通过配体交换形成P-OH,造成H2O峰值降低。
综上所述,当RJDWTR@40%Ce投加进溶液后,磷酸盐通过分子热运动和静电吸引的方式来到材料表面,在静电引力和配体交换的机制下被RJDWTR@40%Ce吸附,此过程主要涉及-OH与Ce-OH基团。
-
1) RJDWTR@Ce吸附磷酸盐最佳条件为:制备Ce/泥质量分数为40%,实际负载分数为12.1%,初始pH为4,投加量为0.6 g·L−1,接触时间为1 440 min。
2)吸附动力学与吸附等温线模型拟合结果表明,RJDWTR@40%Ce对磷酸盐的吸附以多分子层的化学吸附为主,颗粒内扩散与边界层效应为主要限速因素,最大吸附量为69.43 mg·g−1。
4)共存阴离子Cl−、NO3−对RJDWTR@40%Ce吸附磷酸盐几乎没有影响,CO32−、SO42−和HA通过竞争吸附点位以及空间位阻作用对吸附有抑制作用,F−通过增强离子强度促进吸附的进行。RJDWTR@40%Ce经5次循环再生后解吸率为72.08%。
4) RJDWTR@40%Ce去除磷酸盐的机理为静电引力与配体交换,主要通过材料表面的Ce-OH与磷酸基团发生交换形成内球络合物。
铈改性净水污泥除磷效能与机理分析
Performance and mechanism analysis of phosphorus removal by cerium-modified drinking water treatment residual
-
摘要: 磷酸盐含量是控制水环境质量的重要标准之一,吸附是一种高效、清洁和经济的除磷技术。采用铈改性净水污泥吸附去除磷酸盐,考察了铈负载量、投加量、pH、共存离子等因素对吸附磷酸盐的影响,探讨了可能的吸附机理及吸附材料循环再生能力。结果表明,铈改性净水污泥吸附磷酸盐过程符合拟二级动力学和Freundlich吸附等温线,最大吸附量为69.43 mg·g−1,吸附速率受内扩散、边界层效应等多重因素的限制。在Cl−、NO3−、CO32−、SO42−等共存离子干扰下,铈改性净水污泥具备选择性吸附磷酸盐的能力。在进行5次吸附-解吸循环后,吸附材料对磷酸盐的去除率下降了25.4%。吸附机制主要为磷酸盐与羟基以及含铈基团的静电吸引和配体交换。Abstract: Phosphate content is one of the important criteria for controlling the quality of the water environment, and adsorption is an efficient, clean, and economical technique for phosphate removal. In this study, cerium-modified drinking water treatment residual was used to remove phosphate by adsorption, and the effects of cerium loading, dosage, pH, and coexisting ions on phosphate adsorption were investigated to explore the adsorption mechanism and the cyclic regeneration capacity of adsorbent materials. The results showed that the phosphate adsorption process of cerium-modified drinking water treatment residual was consistent with the proposed secondary kinetics and Freundlich adsorption isotherm, and the maximum adsorption capacity was 69.43 mg·g−1. The adsorption rate was controlled by multiple factors, such as internal diffusion and the boundary layer effect. The cerium-modified drinking water treatment residual had the ability to selectively adsorb phosphate at the interference of co-existing ions such as Cl−, NO3−, CO32−, SO42−, etc. After five adsorption-desorption cycles, the removal rate of phosphate by the adsorbent material decreased by 25.4%. The adsorption mechanism is mainly electrostatic attraction and ligand exchange between phosphates and hydroxyl groups as well as cerium-containing groups.
-

-
表 1 DWTR、RJDWTR与RJDWTR@10%~60%Ce的吸附物理特性
Table 1. Physical properties of DWTR, RJDWTR and RJDWTR@10%~60%Ce for adsorption
吸附剂 比表面积/
(m2·g−1)孔径/
nm孔体积/
(cm3·g−1)DWTR 35.411 10.934 0.098 RJDWTR 52.141 14.417 0.165 RJDWTR@10%Ce 44.615 11.736 0.126 RJDWTR@20%Ce 38.811 11.155 0.102 RJDWTR@30%Ce 30.013 10.706 0.09 RJDWTR@40%Ce 28.645 10.156 0.073 RJDWTR@50%Ce 26.4 9.499 0.058 RJDWTR@60%Ce 22.196 8.046 0.045 表 2 磷酸盐在DWTR、RJDWTR与 RJDWTR@40%Ce上的吸附动力学参数
Table 2. Adsorption kinetic parameters of phosphate on DWTR, RJDWTR and RJDWTR@40%Ce
吸附剂 qe,ecp 拟一级动力学 拟二级动力学 /(mg·g−1) k1/min−1 qe,1/(mg·g−1) R2 k2/(g·(mg·min)−1) qe,2/(mg·g−1) R2 DWTR 4.995 0.000 9 1.77 0.941 0.001 1 4.862 0.987 RJDWTR 7.895 0.00 10 1.973 0.937 0.001 5 7.659 0.997 RJDWTR@40%Ce 16.45 0.00 12 1.797 0.851 0.003 5 16.263 0.999 表 3 磷酸盐在DWTR、RJDWTR与 RJDWTR@40%Ce上的颗粒内扩散模型参数
Table 3. Intra-particle diffusion modeling parameters of phosphate adsorption on DWTR, RJDWTR and RJDWTR@40%Ce
吸附剂 第1阶段 第2阶段 第3阶段 kd1 Cd1 Rd12 kd2 Cd2 Rd22 kd3 Cd3 Rd32 /(mg·g−1·min1/2) /(mg·g−1) — /(mg·g−1·min1/2) /(mg·g−1) — /(mg·g−1·min1/2) /(mg·g−1) — DWTR 0.024 0.908 0.932 0.169 0.583 0.996 0.027 3.216 0.984 RJDWTR 0.222 1.337 0.960 0.151 2.333 0.981 0.022 6.333 0.994 RJDWTR@40%Ce 0.724 7.266 0.980 0.086 13.363 0.968 0.035 14.454 0.917 表 4 磷酸盐在RJDWTR和RJDWTR@40%Ce上的吸附等温线参数
Table 4. Phosphate adsorption isotherm parameters on RJDWTR and RJDWTR@40%Ce
吸附剂 温度/ ℃ Langmuir模型 Freundlich模型 Qm
/(mg·g−1)KL
/(L·mg−1)R2 KF
/(mg·g−1) ·(L·g−1)1/n1/n R2 RJDWTR 25 38.364 0.010 7 0.909 1.649 0.53 0.989 35 40.539 0.014 2 0.894 2.550 0.489 4 0.991 45 41.945 0.023 8 0.933 4.531 0.396 8 0.987 RJDWTR@40%Ce 25 69.425 0.063 3 0.950 16.587 0.273 7 0.990 35 68.270 0.039 9 0.953 12.623 0.310 3 0.979 45 62.464 0.032 0 0.912 8.533 0.364 6 0.992 表 5 除磷吸附剂对比
Table 5. Comparison of phosphorus removal by adsorbents
吸附剂 最大吸附量/
(mg·g−1)初始P质量浓度/
(mg·L−1)参考文献 粒状羟基铁 8.99 5 [22] 改性钢渣与水泥 21.70 1~50 [23] La改性粉煤灰 24.90 10~200 [24] 磁性Fe-Zr
二元氧化物13.65 0~100 [25] La-Zr双金属改性磁吸附剂 49.07 50 [26] 茶渣载纳米银
活性炭13.62 10~100 [27] La2(CO3)3改性
微纤维34.2 10~200 [28] 铈改性锂硅粉 10.89 — [29] 氢氧化铈纳米
复合材料36.45 30 [30] RJDWTR 41.95 10~200 本研究 RJDWTR@40%Ce 69.43 10~200 本研究 -
[1] 黄镁宁, 宁寻安, 张建易, 等. 漫水河清远流域磷污染特征及富里酸对沉积物释磷的影响[J]. 环境工程学报, 2022, 16(5): 1549-1557. doi: 10.12030/j.cjee.202112023 [2] CETINER Z S, WOOD S A, GAMMONS C H. The aqueous geochemistry of the rare earth elements. Part XIV. The solubility of rare earth element phosphates from 23 to 150 ℃[J]. Chemical Geology. 2005, 217(1/2): 147-169. [3] HE Q, ZHAO H, TENG Z, et al. Phosphate removal and recovery by lanthanum-based adsorbents: A review for current advances[J]. Chemosphere. 2022, 303: 134987. doi: 10.1016/j.chemosphere.2022.134987 [4] SHAN S, ZHANG T, WANG W, et al. Magnetite/hydrated cerium (III) carbonate for efficient phosphate elimination from aqueous solutions and the mechanistic investigation[J]. Chemical Engineering Journal. 2021, 425: 128894. doi: 10.1016/j.cej.2021.128894 [5] YU Y, YU L, KOH K Y, et al. Rare-earth metal based adsorbents for effective removal of arsenic from water: A critical review[J]. Critical reviews in environmental Science and Technology. 2018, 48: 1127-1164. doi: 10.1080/10643389.2018.1514930 [6] 余杰, 鱼红霞, 杜义鹏, 等. 城市垃圾焚烧厂直接掺烧城市污泥处置技术及其污染控制[J]. 环境工程学报, 2020, 14(11): 3155-3161. doi: 10.12030/j.cjee.202001003 [7] 何李文泽, 陈钰, 孙飞, 等. 镧改性净水污泥水热炭对水体中磷的吸附特性及底泥内源磷的固定[J]. 环境科学, 2023, 44(6): 3288-3300. doi: 10.13227/j.hjkx.202207114 [8] LIAN J, ZHOU F, CHEN B, et al. Enhanced adsorption of molybdenum (VI) onto drinking water treatment residues modified by thermal treatment and acid activation[J]. Journal of Cleaner Production. 2019, 244(1): 118719. [9] LI Y, ZHANG Y, SU F, et al. Adsorption behaviour of microplastics on the heavy metal Cr (VI) before and after ageing[J]. Chemosphere. 2022, 302: 134865. doi: 10.1016/j.chemosphere.2022.134865 [10] 张玉妹, 韩乙萱, 魏杰, 等. 碱改性净水污泥对水中氨氮的吸附效能研究[J]. 环境科学学报, 2014, 34(10): 2484-2490. doi: 10.13671/j.hjkxxb.2014.0648 [11] TANG Y, CHEN Z, WEN Q. Magnetic powdery acrylic polymer with ultrahigh adsorption capacity for atenolol removal: Preparation, characterization, and microscopic adsorption mechanism[J]. Chemical engineering journal. 2022, 446: 137175. doi: 10.1016/j.cej.2022.137175 [12] TANG L, YU J, PANG Y, et al. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal[J]. Chemical Engineering Journal. 2018, 336: 160-169. doi: 10.1016/j.cej.2017.11.048 [13] ALMáši M, ZELEňáK V, OPANASENKO M, et al. Ce (III) and Lu (III) metal–organic frameworks with Lewis acid metal sites: Preparation, sorption properties and catalytic activity in Knoevenagel condensation[J]. Catalysis Today. 2015, 243: 184-194. doi: 10.1016/j.cattod.2014.07.028 [14] YANG H, ZENG G, LIU Y, et al. Study on adsorption and recovery utilization of phosphorus using alkali melting-hydrothermal treated oil-based drilling cutting ash[J]. Journal of Environmental Management. 2023, 332: 117373. doi: 10.1016/j.jenvman.2023.117373 [15] 徐晋, 马一凡, 姚国庆, 等. KOH活化小麦秸秆生物炭对废水中四环素的高效去除[J]. 环境科学, 2022, 43(12): 5635-5646. doi: 10.13227/j.hjkx.202201253 [16] HE J, XU Y, SHAO P, et al. Modulation of coordinative unsaturation degree and valence state for cerium-based adsorbent to boost phosphate adsorption[J]. Chemical Engineering Journal. 2020, 394: 124912. doi: 10.1016/j.cej.2020.124912 [17] WANG Y, XIE X, CHEN X, et al. Biochar-loaded Ce3+-enriched ultra-fine ceria nanoparticles for phosphate adsorption[J]. Journal of Hazardous Materials. 2020, 396(8): 122626. [18] LIU X, WANG Y, SMITH R L, et al. High-capacity structured MgO-Co adsorbent for removal of phosphorus from aqueous solutions[J]. Chemical Engineering Journal. 2021, 426(21): 131381. [19] 李迎春, 董良飞, 仝驰, 等. 稀土改性凹凸棒土对低浓度磷的吸附性能[J]. 环境工程学报, 2021, 15(10): 3214-3222. doi: 10.12030/j.cjee.202106129 [20] GUPTA N, SAIFUDDIN M, KIM S, et al. Microscopic, spectroscopic, and experimental approach towards understanding the phosphate adsorption onto Zn–Fe layered double hydroxide[J]. Journal of Molecular Liquids. 2020, 297(1): 111935. [21] 宋志伟, 卿卓霖, 钱锋, 等. 海藻酸钠/锆@钙水凝胶的制备及其对磷的吸附研究[J]. 环境科学学报报, 2022, 42(3): 151-161. [22] PAPPER R A, COUPERTHWAITE S J, MILLAR G J. Re-use of waste red mud: Production of a functional iron oxide adsorbent for removal of phosphorous[J]. Journal of Water Process Engineering. 2018, 25: 138-148. doi: 10.1016/j.jwpe.2018.07.006 [23] LI J, WU B, ZHOU T, et al. Preferential removal of phosphorus using modified steel slag and cement combination for its implications in engineering applications[J]. Environmental Technology & Innovation. 2018, 10: 264-274. [24] ASAOKA S, KAWAKAMI K, SAITO H, et al. Adsorption of phosphate onto lanthanum-doped coal fly ash—Blast furnace cement composite[J]. Journal of Hazardous Materials. 2020, 406(2): 124780. [25] LONG F, GONG J L, ZENG G M, et al. Removal of phosphate from aqueous solution by magnetic Fe–Zr binary oxide[J]. Chemical Engineering Journal. 2011, 171(2): 448-455. doi: 10.1016/j.cej.2011.03.102 [26] LIN X, XIE Y, LU H, et al. Facile preparation of dual La-Zr modified magnetite adsorbents for efficient and selective phosphorus recovery[J]. Chemical Engineering Journal. 2021, 413: 127530. doi: 10.1016/j.cej.2020.127530 [27] TRINH V T, NGUYEN T M P, VAN H T, et al. Phosphate adsorption by silver nanoparticles-loaded activated carbon derived from tea residue[J]. Scientific Reports. 2020, 10: 3634. doi: 10.1038/s41598-020-60542-0 [28] YUAN J, ZHU Y, WANG J, et al. Preparation and application of Mg-Al composite oxide/coconut shell carbon fiber for effective removal of phosphorus from domestic sewage[J]. Food and Bioproducts Processing. 2021, 126(22): 293-304. [29] LIN W L, GU J C, WANG W Y, et al. Adsorption of phosphorus by Ce-modified Lithium Silica Fume[J]. Applied Mechanics & Materials. 2013, 368-370(III): 687-691. [30] YANG W, SHI X, DONG H, et al. Fabrication of a reusable polymer-based cerium hydroxide nanocomposite with high stability for preferable phosphate removal[J]. Chemical Engineering Journal. 2021, 405: 126649. doi: 10.1016/j.cej.2020.126649 [31] YANG Q, WANG X, LUO W, et al. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge[J]. Bioresource Technology. 2017, 247: 537-544. [32] WEI X, SUN Y, PAN D, et al. Adsorption properties of Na-palygorskite for Cs sequestration: Effect of pH, ionic strength, humic acid and temperature[J]. Applied Clay Science. 2019, 183: 105363. doi: 10.1016/j.clay.2019.105363 [33] HE J, XU Y, XIONG Z, et al. The enhanced removal of phosphate by structural defects and competitive fluoride adsorption on cerium-based adsorbent[J]. Chemosphere. 2020, 256(3): 127056. [34] LV N, LI X, et al. Phosphorus removal from wastewater using Ca-modified attapulgite: Fixed-bed column performance and breakthrough curves analysis[J]. Journal of Environmental Management. 2023, 328: 116905. doi: 10.1016/j.jenvman.2022.116905 [35] GU Y, XIE D, MA Y, et al. Size modulation of zirconium-based metal organic frameworks for highly efficient phosphate remediation[J]. ACS Applied Materials & Interfaces. 2017, 9(37): 32151-32160. [36] DU M, ZHANG Y, WANG Z. La-doped activated carbon as high-efficiency phosphorus adsorbent: DFT exploration of the adsorption mechanism[J]. Separation and Purification Technology. 2022, 298: 121585. doi: 10.1016/j.seppur.2022.121585 [37] MIN X, WU X, SHAO P, et al. Ultra-high capacity of lanthanum-doped UiO-66 for phosphate capture: Unusual doping of lanthanum by the reduction of coordination number[J]. Chemical Engineering Journal. 2019, 358: 321-330. doi: 10.1016/j.cej.2018.10.043 -




 下载:
下载: