-
近年来,随着有色金属的开采与冶炼活动,矿区及其周围土壤出现重度的类金属砷 (As) 、锑 (Sb) 复合污染,大多数的Sb污染来源于熔炼,且经常和As污染同时发生[1–3],As、Sb污染土壤对人类健康和生态风险构成重大威胁,因此有必要针对As、Sb复合污染土壤进行修复治理。目前,固化/稳定化[4–5]、土壤淋洗[6]、电动修复[7]和植物修复[8]被普遍研究应用于修复类金属污染土壤,在这些修复技术中,土壤淋洗是修复As、Sb污染土壤的一种有效方法,因为该技术可以通过淋洗剂提取从土壤中永久去除目标金属[9]。在土壤淋洗技术中,关键在于选用一种高效、绿色的淋洗剂。目前,无机酸和盐 (HCl,H3PO4及其盐类) [6,10]、螯合剂如EDTA[11]以及强碱如NaOH[12],是常应用于修复类金属污染土壤的淋洗剂。然而,EDTA对于修复As、Sb等以氧阴离子形式存在于土壤中的金属并不是非常有效[13];采用常规的强酸、强碱作为土壤淋洗剂会严重破坏土壤结构[9];有研究表明,一些化学淋洗剂会造成土壤养分流失[14]。相较于化学试剂,微生物制剂由于其成本低且无二次污染更具应用前景[15]。微生物的某些代谢物,如有机酸和表面活性物质,可以有效去除重金属[16-17],因此可以考虑应用于土壤修复。黑曲霉(Aspergillus niger)由于其在生长代谢中可以产生大量的低分子有机酸,被应用于重金属污染土壤的淋洗修复[18],然而,单一的生物制剂应用于土壤修复中效率较低,如何提高生物制剂的修复效率已是当今研究的热点之一。
纳米零价铁 (nano zero-valent iron,nZVI) 由于具有比表面积大,强还原性和高反应活性等特点被广泛运用于环境污染的治理,利用nZVI增强淋洗剂的修复效率可能是一种有前景的方法。WANG等[19]发现nZVI能够增强低分子有机酸去除污染土壤中的Pb。CAO等[20]利用nZVI增强有机酸去除污染土壤中的重金属,结果表明,与单一有机酸相比,矿山土壤Cd、Pb和Zn的去除率分别提高了12.8%、24.9%和11.6%,农田土壤Cd、Pb和Zn的去除率分别提高了19.2%、18.1%和8.93%。目前,关于nZVI用于修复As、Sb污染土壤的研究较少,且鲜有研究nZVI联用微生物发酵液淋洗修复类金属污染土壤。
本研究以As、Sb复合污染土壤为对象,探究nZVI对黑曲霉发酵液的增强淋洗,并探究nZVI质量浓度、pH和淋洗时间对土壤中As、Sb去除效果的影响;最后,考察淋洗前后土壤中As、Sb赋存形态的变化情况。
-
供试土壤采于广西某锑矿区 (23°13′ N, 109°20′ E) 表层 (0~20 cm) 土壤,采集的样品剔除植物根系、石块等杂物后于通风阴凉处自然风干,过 10 目尼龙网筛混合均匀后储存备用。供试土壤的基本理化性质如表1所示。
-
黑曲霉菌株经马铃薯葡萄糖琼脂 (PDA) 培养基活化和传代,在30 ℃下培养7 d后用无菌水制成孢子悬浮液,经过计数和稀释制备出每毫升含2.5
$ \times $ 106 spores的孢子悬浮液,接种1 mL的孢子悬浮液至100 mL的纯培养基里面进行发酵。纯培养基成分[21]为每升含有0.5 g KCl、0.5 g NaCl、0.5 g MgSO4、0.02 g FeCl3、5 g蛋白胨和20 g蔗糖。经过前期实验,设定发酵条件为:发酵时间为6 d、初始pH为5、培养温度为30 ℃。结束发酵后将培养液经过滤纸过滤,得到黑曲霉发酵液 (FB) ,用于后续的淋洗实验。取FB进行稀释后,采用高效液相色谱仪测定其中的有机酸种类和质量浓度,色谱柱采用Inertsil ODS-SP 5 um 4.6
$ \times $ 150 mm,流动相为0.1%磷酸∶甲醇=96∶4,控制柱温为30 ℃,进样量为10 uL,进样速率为0.8 mL·min−1。测得FB的性质如表2所示。 -
1) nZVI质量浓度。FB中加入不同量的nZVI,配制成含nZVI为0、0.02、0.05、0.1、0.2、0.3、0.5、1 g·L−1的发酵液 (nZVI-FB) ,超声30 min后在室温下振荡3 h,调节pH为2.0后备用。取1.00 g风干过20目的污染土样于50 mL离心管中,分别加入10 mL配制的淋洗液,置于转速为220 r∙min−1的摇床中振荡提取3 h。振荡结束后对混合体系进行离心,离心机转速为10 000 r∙min−1,离心时长为5 min,离心结束后采用0.45 µm的滤膜对上清液进行过滤,通过原子荧光分光光度计检测溶液中的As、Sb质量浓度。每个处理设置3个重复。实验得到最优的nZVI质量浓度,后续实验均采用此nZVI质量浓度。
2) pH。称取1.00 g土样于50 mL离心管中,分别加入10 mL pH为1.0、3.0、5.0、7.0、9.0、11.0的nZVI-FB。采用0.1 mol·L−1 NaOH和0.1 mol·L−1的HNO3调节pH。淋洗实验的条件同上。每个处理设置3个重复。
3) 淋洗时间。称取1.00 g土样于50 mL离心管中,加入10 mL pH为1.0的 nZVI-FB,置于恒温摇床中振荡淋洗,转速设置为220 r∙min−1,温度设置为25 ℃,淋洗时间分别为5、15、30、60、90、120、180、240、300、360、480、600 min。淋洗实验的条件同上。每个处理设置3个重复。
-
土壤pH根据《土壤pH的测定》 (NY/T 1377-2007) 测定[22];土壤有机质根据《土壤检测第6部分:土壤有机质的测定》 (NY/T 1121.6-2006) 测定[23];土壤粒径分布采用激光粒度仪 (MAZ3000,英国马尔文仪器有限公司) 进行测定;土壤中As和Sb质量分数参考《土壤和沉积物 汞、砷、硒、铋、锑的测定 微波消解/原子荧光法》 (HJ680-2013) 测定[24]。消解液和淋洗液中As和Sb的质量浓度采用原子荧光分光光度计 (SA-20,北京吉天仪器有限公司) 测定。采用Wenzel提取法[25]对土壤中As和Sb的不同形态进行提取,提取的步骤如表3所示。
-
土壤中As和Sb的去除效率(RE)按照式(1)计算。
式中:Csolution为淋洗后溶液中的重金属质量浓度,mg·L−1;V为淋洗液的体积,mL;m为土壤的干重,g;Ctotal为供试土壤中重金属的质量分数,mg·kg−1。
-
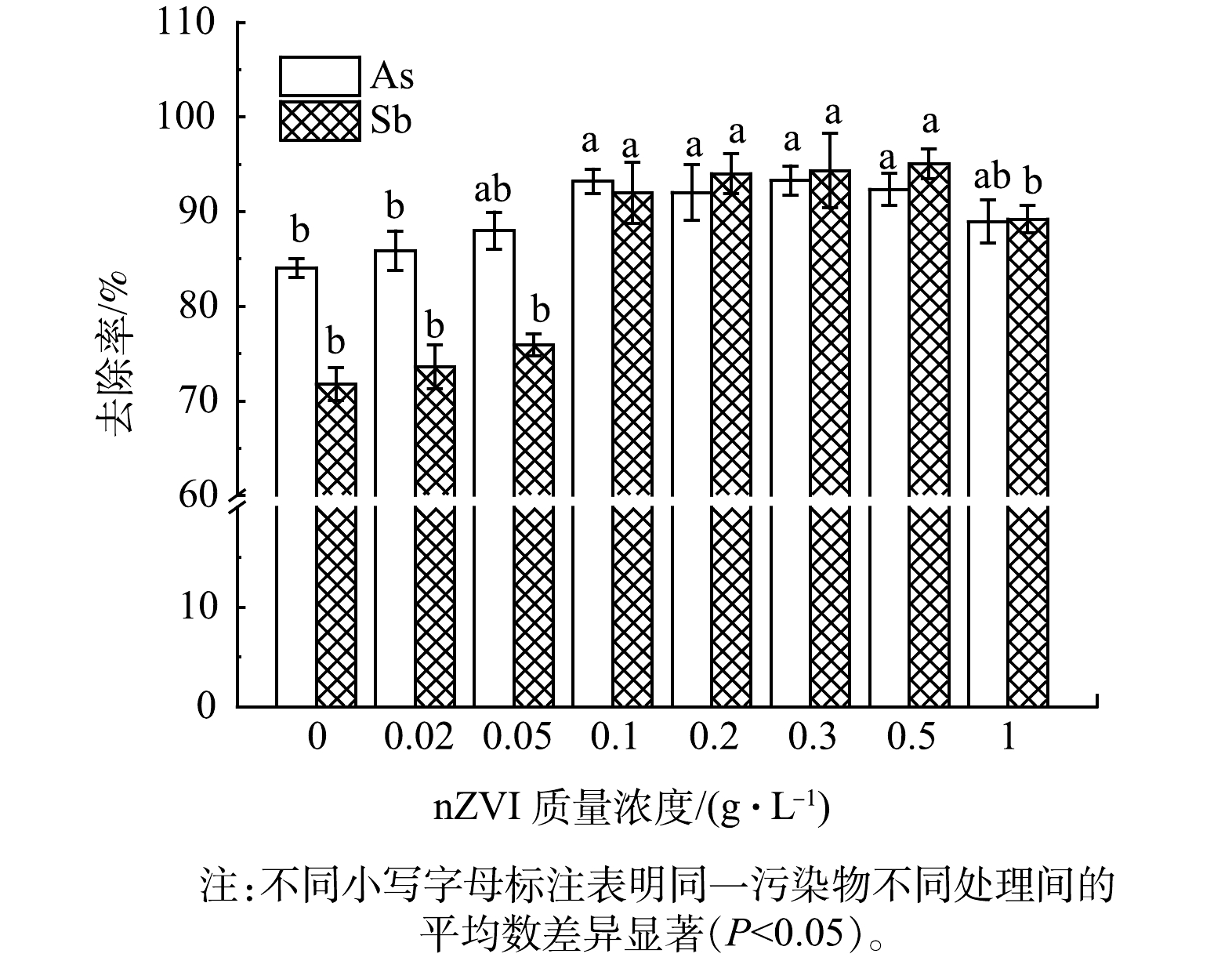
nZVI增强FB去除As和Sb的效果如图1所示。随着nZVI质量浓度的增加,As和Sb的去除率先显著提升而后降低。nZVI质量浓度在小于0.1 g·L−1时,其对增强FB去除As、Sb的效果显著增加 (P <0.05) ,进一步增加nZVI的质量浓度时 (0.1~0.5 g·L −1) ,As、Sb的去除率未有显著性差异的变化,增至1 g·L−1时效果显著下降 (P<0.05) 。过量添加nZVI可能由于其消耗了部分发酵液中的有机酸,造成其对As、Sb的效果下降。因此,结合增强效果和经济成本,最适宜的nZVI的质量浓度为0.1 g·L−1。
单一FB对于土壤中As、Sb的去除率分别为84.1%和71.8%,此时,土壤中剩余As和Sb的质量分数分别为155和148 mg·kg−1,其中As质量分数超过《土壤环境质量 建设用地土壤污染风险管控标准 (试 行) 》 (GB 36600-2018) [26]中第二类用地土壤污染风险筛选值(As为60 mg·kg−1、Sb为180 mg·kg−1),未达到修复目标。FB能够有效将土壤中As、Sb淋出,可能是由于其含有有机酸,且草酸质量浓度较高,草酸是一种有效溶出重金属或类金属的浸出试剂[27–29],且被证明能够通过溶解、还原和配位交换机制有效去除土壤中的As和Sb[30]。nZVI能够显著增强FB对土壤中As、Sb的淋出效果,nZVI质量浓度为0.1 g·L−1时,As和Sb的去除率分别提升了9.15%和20.2%,达到93.2%和92.0%,修复后土壤中As的质量分数为61.0 mg·kg−1,仍超过第二类用地标准,需要通过后续实验进一步优化提高其去除率。
-
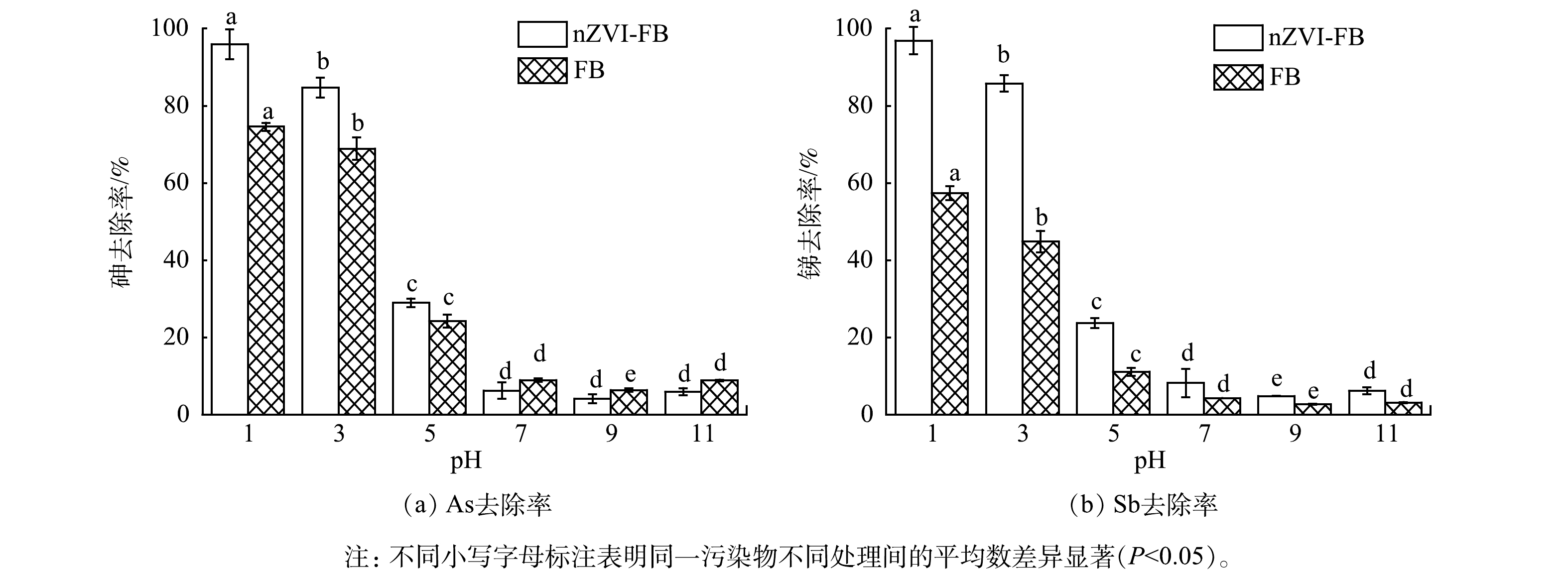
淋洗剂的pH是土壤淋洗修复技术在实践工程应用的一个重要的工艺参数,它不仅能够直接影响土壤胶体表面的重金属形态和移动性,还能决定淋洗过程中吸附解吸反应和沉淀溶解反应的动态平衡,从而影响淋洗剂与重金属之间结合的效率[31]。图2为FB和nZVI-FB 在不同pH (1.0~11.0) 下对土壤中As和Sb去除率的影响。结果表明,在酸性的条件下,nZVI-FB对As和Sb有着较好的去除效果,在淋洗液pH为1.0时,As和Sb的去除率分别达到96.0%和96.9%,pH增加后,淋洗效果显著下降。土壤中的As和Sb与铁氧化物结合密切,在酸性条件下,发酵液中的低分子有机酸尤其是草酸可能通过络合或H+交换与Fe(Ⅲ)发生反应,导致铁氧化物分解[32],进而解吸释放出As和Sb,酸性条件下有机酸与nZNI的络合作用会促进Fe(Ⅱ)的溶解[33]。pH为11.0时的淋洗效果较pH为9.0好,推测原因为在碱性的条件下,As、Sb的金属氧化物配合物稳定性降低,使得土壤中的As和Sb更容易释放。再者,可能是由于OH−与As、Sb的配位体置换反应以及在碱性条件下抑制As、Sb的再吸收[6,34]。
在酸性条件下 (pH为1.0、3.0、5.0) ,添加nZVI可以显著增强FB对As的去除,去除率分别提高了21.4%、15.8%、4.78%,但在中性和碱性条件下,nZVI均不能促进FB对As的去除,可能是由于在此pH范围内,nZVI未能充分溶于发酵液体系中,导致淋出的As重新吸附回固相中。在pH为1.0~11.0范围内,nZVI均能促进FB对Sb的去除,去除率提高了2.08%~41.0%。这表明,在酸性条件下,nZVI能较好地提高FB对As和Sb的淋洗效果且随着pH的增加而减弱。
-
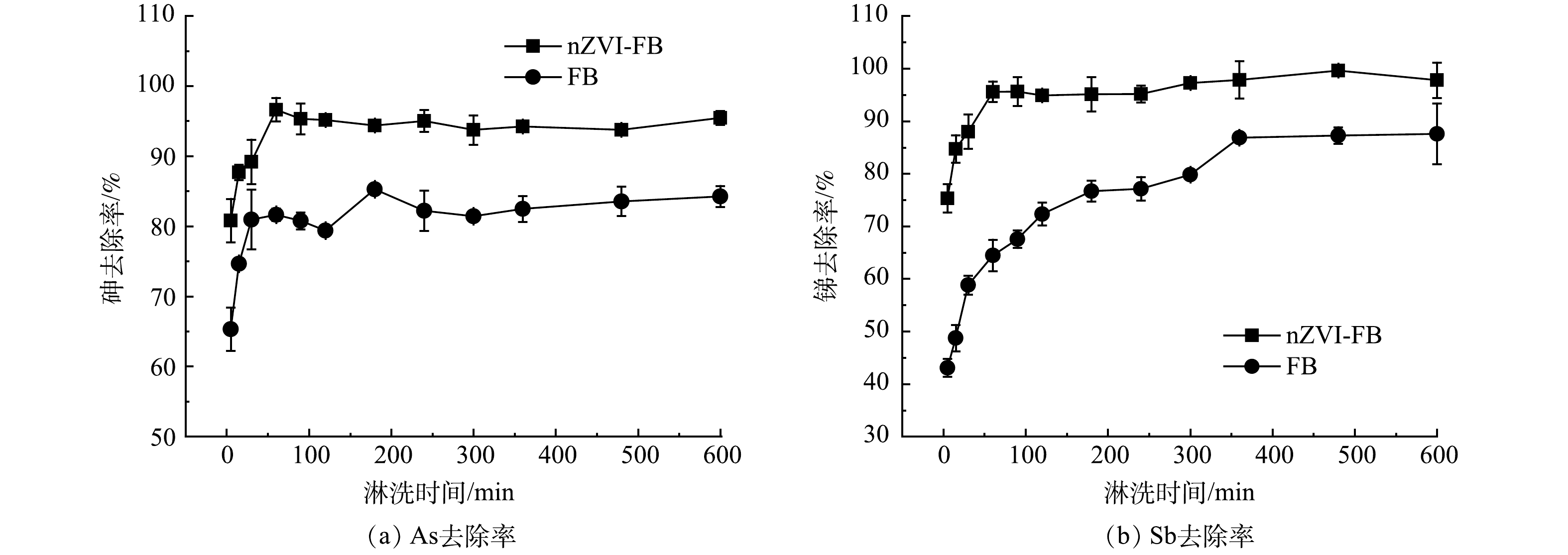
图3显示了FB和nZVI-FB在不同淋洗时间 (0~600 min) 下的土壤中As、Sb的去除率。结果表明,土壤中的As、Sb的淋出过程可以分为2个阶段,第一个阶段为快速去除阶段,0~60 min内,nZVI-FB能够快速的将土壤中的As、Sb淋出,对As和Sb的淋洗速率分别达到941和502 mg·kg−1·h−1,而FB在0~60 min内对土壤中As和Sb的淋洗速率仅为795和338 mg·kg−1·h−1。这表明,添加nZVI的发酵液不仅显著提高了对土壤中As、Sb的去除量,还提高了As、Sb的淋出速率,在较短的时间内能够从土壤中淋出大部分的As和Sb。第二个阶段为慢速去除阶段,60~600 min内,随着淋洗时间的延长,去除率缓慢增加并逐渐趋于一个稳定值。在快速去除阶段,淋洗剂主要将土壤表面或者是附着在无定型铁铝氧化物上的As和Sb淋洗去除,此过程较为容易,因而去除速率较快。而在后一反应阶段,去除对象主要为土壤颗粒上结合较为紧密的As和Sb,此部分的As和Sb难以被解吸出来,故去除相对较慢。nZVI-FB淋洗时间为60 min时,As和Sb的去除率达到96.6%和95.6%,此时土壤剩余的As和Sb的质量分数为32.7、23.3 mg·kg−1,达到《土壤环境质量 建设用地土壤污染风险管控标准 (试行) 》二类用地标准[26]。综上,选用60 min作为nZVI-FB淋洗修复As、Sb污染土壤的淋洗时间。
-
选取了拟一阶动力学方程、拟二级动力学方程和Elovich方程对nZVI-FB从污染土壤中解吸As、Sb的过程进行拟合,结果见表4。拟合结果的可行性和符合程度采用相关系数 (R2) 和均方根误差 (RMSE) 进行综合评价,拟合方程的R2越高、RMSE值越低,意味着建立的模型越符合。由表中可以看出,拟二级动力学方程有着较高的相关系数R2,能够较好的描述As、Sb的解吸行为,表明这一淋洗过程是一个以化学淋洗为主导的过程[35]。
-
As和Sb在土壤中存在着多种形态, As、Sb的生物毒性和其可利用性并不完全取决于总量,进入土壤的 As、Sb会与土壤中的矿物和胶体等物质发生一系列的物理化学反应[36],使其存在形态和迁移转化能力产生改变。
图4为淋洗前后土壤中As和Sb的形态分布情况。初始土壤中的As的赋存形态:F3(70.57%)>F2(13.31%)>F4(9.81%)>F5(6.18%)>F1(0.12%)。Sb的赋存形态:F3(60.28%)>F5(19.63%)>F4(17.24%)>F2(2.22%)>F1(0.64%)。经FB淋洗后的F3、F4、F5都显著降低 (P<0.05) ,其中F3的质量分数下降最多,FB和nZVI-FB去除F3对土壤中As和Sb总量的去除有着主要贡献,nZVI-FB去除F3在As和Sb各形态中的贡献占比分别为74.9%和63.3%。
相比于FB,nZVI-FB淋洗后土壤中各形态均有减少,其中F4质量分数大幅度降低,表明nZVI可以提高FB对土壤中较难淋出的晶质铁铝氧化物结合态的去除效率。nZVI使得淋洗液可以有效的破坏土壤中的晶型铁氧化物,提取结合其中的As和Sb,这种增强效果可能是由于nZVI加入体系后,能够与黑曲霉发酵液中的有机酸作用从而造成较强的还原环境,从而能够有效的还原溶解土壤中的铁氧化物[33]。初始土壤及经FB和nZVI-FB淋洗后的土壤中As的残渣态占比分别为6.18%、11.2%和16.4%,Sb的残渣态占比为19.6%、25.2%和34.8%,残渣态占比的增加是由于淋洗后其他迁移性较强的形态的总质量分数减少较多导致,这表明经nZVI-FB淋洗修复后土壤中的As和Sb环境风险显著降低。
-
1) nZVI对FB去除土壤中As、Sb有强化作用,其增强效果在强酸条件下最佳,且发酵液中添加nZVI后能加快As、Sb的淋出。综合经济成本和淋洗效率,nZVI-FB的最佳淋洗条件为nZVI质量浓度为0.1 g L−1、pH为1、淋洗时间为60 min,在该条件下,对As、Sb去除率分别为96.6%和95.6%,达到修复目标,且较单一发酵液对As、Sb的去除率分别提升了12.6%和23.8%。
2) 拟二级动力学方程适用于As和Sb的淋洗动力学过程 (R2>0.99) ,判断其淋出过程为化学淋洗过程。
3) nZVI-FB能够有效的去除土壤中As、Sb的铁铝氧化物结合态,且经nZVI-FB淋洗后,土壤中的As和Sb环境风险降低。
纳米零价铁与黑曲霉发酵液联用对砷锑污染土壤的淋洗修复
Washing remediation of arsenic and antimony contaminated soil by the combination of nano-zero-valent iron and Aspergillus niger fermentation broth
-
摘要: 土壤砷 (As) 、锑 (Sb) 污染对生态环境和人体健康有着潜在的风险,采用黑曲霉发酵液 (FB) 与纳米零价铁 (nZVI) 联用淋洗修复As、Sb污染土壤。通过振荡淋洗实验,探究nZVI强化FB淋洗去除As、Sb的效果及不同条件下对As、Sb淋洗效率的影响。结果表明,制备的FB对污染土壤中As和Sb有着较好的去除效果,去除率可达84.1%和71.8%;nZVI对FB去除As、Sb有强化作用,在nZVI质量浓度为0.1 g·L−1、pH为1和淋洗时间为60 min的条件下,其淋洗效果最佳,对As、Sb淋洗效率可达96.6%和95.6%,修复后的土壤达到《土壤环境质量 建设用地土壤污染风险管控标准 (试行) 》 (GB 36600-2018) 二类用地标准。nZVI-FB对土壤中As、Sb的解吸动力学符合拟二级动力学方程。nZVI-FB能够有效的提取土壤中As、Sb的铁铝氧化物结合态。本研究结果可为As、Sb复合污染土壤的淋洗修复提供参考。Abstract: Arsenic (As) and antimony (Sb) contamination in soils pose potential risks to the ecological environment and human health. Remediation of As and Sb contaminated soil by washing with the combination of Aspergillus niger fermentation broth (FB) and nano zero-valent iron (nZVI). The enhancement effect of nZVI combined with the broth in the soil washing process was studied, and the impacts of different conditions (such as pH, time, etc.) on the As and Sb washing efficiency were also investigated. The results showed that Aspergillus niger fermentation broth demonstrated good washing efficiencies on As and Sb in the contaminated soil, reaching 84.1% and 71.8%, respectively. The best elution effect was achieved at the nZVI addition of 0.1 g·L−1, pH 1 and 60 min of washing time, with the removal efficiency of 96.6% and 95.6% for As and Sb, and the As and Sb concentrations that remained in soil were lower than the standard value in Soil Environmental Quality, Risk Control Standard for Soil Contamination of Development Land in China. The kinetics of desorption of As and Sb from soil by nZVI-FB is consistent with the proposed secondary kinetic equation. The mixed washing agent can effectively extract the Fe/Al oxide bound state of As and Sb from the soil. The results of this study can provide a reference for the remediation of leaching of As and Sb contaminated soil.
-

-
表 1 供试土壤的基本理化性质
Table 1. Basic physicochemical properties of the test soil
土壤质地 粒径分布/% pH 有机质
/ (g·kg−1)As
/ (mg·kg−1)Sb
/ (mg·kg−1)Fe/% Al/% 砂粒
(>20 um)粉粒
(2~20 um)黏粒
(<2 um)砂质壤土 54.83 39.62 5.57 4.42 7.68 970.10 525.16 4.20 2.51 表 2 黑曲霉发酵液的性质
Table 2. Properties of Aspergillus niger fermentation broth
pH 草酸/ (g·L−1) 葡萄糖酸/ (g·L−1) 柠檬酸/ (g·L−1) 苹果酸/ (g·L−1) 1.39 8.44 1.15 0.43 1.05 表 3 Wenzel连续形态提取法
Table 3. Wenzel sequential extraction procedures
次序 形态 提取剂 提取步骤 1 非专性吸附态(F1) 25 mL 0.05 mol·L−1 (NH4)2SO4 室温振荡4 h,离心10 min,取上清液过滤 2 专性吸附态(F2) 25 mL 0.05 mol·L−1 NH4H2PO4 常温振荡16 h,离心10 min,取上清液过滤 3 无定型铁铝氧化物结合态(F3) 25 mL 0.2 mol·L−1草酸铵溶液 (pH=3.25) 黑暗中常温振荡4 h,离心10 min,取上清液过滤 4 晶质铁铝氧化物结合态(F4) 25 mL 0.2 mol·L−1草酸铵+0.1 mol·L−1
抗坏血酸溶液光环境下以96±3 ℃振荡30 min,离心10 min,
取上清液过滤5 残渣态(F5) 2 mL HCl+6 mL HNO3+2 mL HF 消解后测定 表 4 nZVI-FB对土壤中As、Sb的解吸动力学参数
Table 4. Desorption kinetic parameters of As and Sb in soil in the washing of the nZVI-FB
类金属 拟一级动力学方程 拟二级动力学方程 Elovich方程 R2 RMSE R2 RMSE R2 RMSE As 0.9923 22.28 0.9965 15.08 0.9885 27.16 Sb 0.9790 20.28 0.9948 10.17 0.9921 12.46 -
[1] ANAWAR H M, FREITAS M C, CANHA N, et al. Arsenic, antimony, and other trace element contamination in a mine tailings affected area and uptake by tolerant plant species[J]. Environmental Geochemistry and Health, 2011, 33: 353-362. doi: 10.1007/s10653-011-9378-2 [2] ASHLEY P M, CRAW D, GRAHAM B P, et al. Environmental mobility of antimony around mesothermal stibnite deposits, New South Wales, Australia and southern New Zealand[J]. Journal of Geochemical Exploration, 2003, 77(1): 1-14. doi: 10.1016/S0375-6742(02)00251-0 [3] HILLER E, LALINSKÁ B, CHOVAN M, et al. Arsenic and antimony contamination of waters, stream sediments and soils in the vicinity of abandoned antimony mines in the Western Carpathians, Slovakia[J]. Applied Geochemistry, 2012, 27(3): 598-614. doi: 10.1016/j.apgeochem.2011.12.005 [4] DOHERTY S J, TIGHE M K, WILSON S C. Evaluation of amendments to reduce arsenic and antimony leaching from co-contaminated soils[J]. Chemosphere, 2017, 174: 208-217. doi: 10.1016/j.chemosphere.2017.01.100 [5] ZHOU S, DU Y, FENG Y, et al. Stabilization of arsenic and antimony co-contaminated soil with an iron-based stabilizer: Assessment of strength, leaching and hydraulic properties and immobilization mechanisms[J]. Chemosphere, 2022, 301: 134644. doi: 10.1016/j.chemosphere.2022.134644 [6] JANG M, HWANG J S, CHOI S I. Sequential soil washing techniques using hydrochloric acid and sodium hydroxide for remediating arsenic-contaminated soils in abandoned iron-ore mines[J]. Chemosphere, 2006, 66(1): 8-17. [7] WANG L, LIN Z, CHANG L, et al. Effects of anode/cathode electroactive microorganisms on arsenic removal with organic/inorganic carbon supplied[J]. Science of the Total Environment, 2021, 798: 149356. doi: 10.1016/j.scitotenv.2021.149356 [8] FENG R, WANG X, WEI C, et al. The accumulation and subcellular distribution of arsenic and antimony in four fern plants[J]. International Journal of Phytoremediation, 2014, 17(4): 348-354. [9] DERMONT G, BERGERON M, MERCIER G, et al. Soil washing for metal removal: a review of physical/chemical technologies and field applications[J]. Journal of Hazardous Materials, 2007, 152(1): 1-31. [10] JHO E H, IM J, YANG K, et al. Changes in soil toxicity by phosphate-aided soil washing: effect of soil characteristics, chemical forms of arsenic, and cations in washing solutions[J]. Chemosphere, 2014, 119: 1399-1405. [11] QIU R, ZOU Z, ZHAO Z, et al. Removal of trace and major metals by soil washing with Na2EDTA and oxalate[J]. Journal of Soils and Sediments, 2009, 10: 45-53. [12] 陈寻峰, 李小明, 陈灿, 等. 砷污染土壤复合淋洗修复技术研究[J]. 环境科学, 2016(3): 1147-1155. doi: 10.13227/j.hjkx.2016.03.046 [13] WEI M, CHEN J, WANG X. Removal of arsenic and cadmium with sequential soil washing techniques using Na2EDTA, oxalic and phosphoric acid: Optimization conditions, removal effectiveness and ecological risks[J]. Chemosphere, 2016, 156: 252-261. doi: 10.1016/j.chemosphere.2016.04.106 [14] FAZLE BARI A S M, LAMB D, MACFARLANE G R, et al. Soil washing of arsenic from mixed contaminated abandoned mine soils and fate of arsenic after washing[J]. Chemosphere, 2022, 296: 134053. doi: 10.1016/j.chemosphere.2022.134053 [15] TANG A, LU Y, LI Q, et al. Simultaneous leaching of multiple heavy metals from a soil column by extracellular polymeric substances of Aspergillus tubingensis F12[J]. Chemosphere, 2021, 263: 127883. doi: 10.1016/j.chemosphere.2020.127883 [16] REN W X, LI P J, GENG Y, et al. Biological leaching of heavy metals from a contaminated soil by Aspergillus niger[J]. Journal of Hazardous Materials, 2009, 167(1): 164-169. [17] BOSSHARD P, BACHOFEN R, BRANDL H. Metal leaching of fly ash from municipal waste incineration by Aspergillus niger[J]. Environmental Science and Technology, 1996, 30(10): 3066-3070. doi: 10.1021/es960151v [18] ZHANG H, GAO Y, XIONG H. Removal of heavy metals from polluted soil using the citric acid fermentation broth: a promising washing agent[J]. Environmental Science and Pollution Research, 2017, 24(10): 9506-9514. doi: 10.1007/s11356-017-8660-y [19] WANG G, ZHANG S, XU X, et al. Efficiency of nanoscale zero-valent iron on the enhanced low molecular weight organic acid removal Pb from contaminated soil[J]. Chemosphere, 2014, 117: 617-624. doi: 10.1016/j.chemosphere.2014.09.081 [20] CAO Y, ZHANG S, ZHONG Q, et al. Feasibility of nanoscale zero-valent iron to enhance the removal efficiencies of heavy metals from polluted soils by organic acids[J]. Ecotoxicology and Environmental Safety, 2018, 162: 464-473. doi: 10.1016/j.ecoenv.2018.07.036 [21] 李广悦, 陶露, 孙静, 等. 黑曲霉浸铀过程中的形态特征及其对铀浸出的影响[J]. 稀有金属, 2019(10): 1085-1091. doi: 10.13373/j.cnki.cjrm.xy18080017 [22] 中国农业科学院农业质量标准与检测技术研究所, 中国农业科学院农业资源与农业区划研究所. 土壤pH的测定: NY/T 1377-2007 [S]. 北京: 中国农业出版社, 2007. [23] 全国农业技术推广服务中心, 中国农业科学院农业资源与农业区划研究所, 华中农业大学. 土壤检测. 第6部分: 土壤有机质的测定: NY/T 1121.6-2006 [S]. 北京: 中国农业出版社, 2006. [24] 宁波市环境监测中心. 土壤和沉积物 汞、砷、硒、铋、锑的测定 微波消解/原子荧光法: HJ 680-2013 [S]. 北京: 中国环境科学出版社, 2014. [25] WENZEL W W, KIRCHBAUMER N, PROHASKA T, et al. Arsenic fractionation in soils using an improved sequential extraction procedure[J]. Analytica Chimica Acta, 2001, 436(2): 309-323. doi: 10.1016/S0003-2670(01)00924-2 [26] 生态环境部南京环境科学研究所, 中国环境科学研究院. 土壤环境质量 建设用地土壤污染风险管控标准(试行): GB 36600-2018 [S]. 北京: 中国环境科学出版社, 2018. [27] LIU Q, TU T, GUO H, et al. High-efficiency simultaneous extraction of rare earth elements and iron from NdFeB waste by oxalic acid leaching[J]. Journal of Rare Earths, 2021, 39(3): 323-330. doi: 10.1016/j.jre.2020.04.020 [28] HU P, ZHANG Y, HUANG J, et al. Eco-friendly leaching and separation of vanadium over iron impurity from vanadium-bearing shale using oxalic acid as a leachant[J]. ACS Sustainable Chemistry and Engineering, 2018, 6(2): 1900-1908. doi: 10.1021/acssuschemeng.7b03311 [29] ZHOU J, ZHU N, LIU H, et al. Recovery of gallium from waste light emitting diodes by oxalic acidic leaching[J]. Resources, Conservation and Recycling, 2019, 146: 366-372. doi: 10.1016/j.resconrec.2019.04.002 [30] 谢冬燕, 曹斐姝, 陈建平, 等. 低分子有机酸对高浓度锑砷污染土壤的淋洗效率及机理研究[J]. 环境科学研究, 2023(1): 117-127. [31] YANG T, HODSON M E. Investigating the use of synthetic humic-like acid as a soil washing treatment for metal contaminated soil[J]. Science of the Total Environment, 2019, 647: 290-300. doi: 10.1016/j.scitotenv.2018.07.457 [32] LEE J C, KIM E J, KIM H W, et al. Oxalate-based remediation of arsenic bound to amorphous Fe and Al hydrous oxides in soil[J]. Geoderma, 2016, 270: 76-82. doi: 10.1016/j.geoderma.2015.09.015 [33] YUAN Y, WEI X, YIN H, et al. Synergistic removal of Cr (Ⅵ) by S-nZVI and organic acids: The enhanced electron selectivity and pH-dependent promotion mechanisms[J]. Journal of Hazardous Materials, 2022, 423: 127240. doi: 10.1016/j.jhazmat.2021.127240 [34] JANG M, HWANG J S, CHOI S I, et al. Remediation of arsenic-contaminated soils and washing effluents[J]. Chemosphere, 2005, 60(3): 344-354. doi: 10.1016/j.chemosphere.2004.12.018 [35] GUSIATIN Z M, KULIKOWSKA D, KLIK B. Suitability of humic substances recovered from sewage sludge to remedy soils from a former As mining area – a novel approach[J]. Journal of Hazardous Materials, 2017, 338: 160-166. doi: 10.1016/j.jhazmat.2017.05.019 [36] EHLERT K, MIKUTTA C, KRETZSCHMAR R. Effects of manganese oxide on arsenic reduction and leaching from contaminated floodplain soil[J]. Environmental Science and Technology, 2016, 50(17): 9251-9261. doi: 10.1021/acs.est.6b01767 -



 下载:
下载: