-
电容去离子(capacitive deionization,CDI)因其具有低能耗和对环境影响小的优点而备受关注[1-3]。然而,传统的CDI存在吸附容量低和间歇运行的缺点。为克服这些缺陷,2013年由JEON等[4]首次提出了流动电极电容去离子(flow electrode capacitive deionization,FCDI)的技术。活性炭[5-6]、炭黑(carbon black,CB)[7]以及碳纳米管[8-10]等多孔碳材料被用作FCDI中的流动电极,而不是传统CDI中的固定电极。带电离子在电场下迁移,然后储存在表面带有相反电荷的流动电极中。由于不断有再生的流动电极补充到电极室,因此,FCDI能够进行连续脱盐[11-12]。然而,关于流动电极孔径分布对FCDI脱盐性能的影响的研究较少。在先前的研究中提到,增加流动电极的微孔面积可以有效地改善CDI电极的脱盐性能[13]。此外,也有研究[14]表明,较高的介孔率使离子更容易的进入电极/电解质界面。因此,流动电极的孔径分布对FCDI的脱盐性能的影响机制仍存在争议。
在本研究中,使用具有不同孔径和相同导电性的4种炭黑作为流动电极,比较了孔径分布对FCDI脱盐性能的影响,使用X射线光电子能谱(XPS)、傅里叶变换红外光谱(FT-IR)、X射线衍射(XRD)、拉曼光谱(Raman)和电化学阻抗谱(EIS)对流动电极的电子传递能力进行了表征,在2种操作模式下对FCDI脱盐效果进行了评估,并且使用吸附-解吸实验探究了微孔和介孔对离子的吸附和解吸速率的影响,以期为设计和选择具有最佳脱盐效果的流动电极提供参考。
-
使用18.2 MΩ·cm的超纯水(Millipore)和Na2SO4(国药集团化学试剂有限公司)制备,2 g·L−1的Na2SO4作为流动电极的电解液和FCDI的进水。具有不同比表面积和孔径分布的4种炭黑(VXC、ECP、ECP600和BP2000)被用作流动电极材料(VXC和BP2000购自美国Cabot,ECP和ECP600购自日本Lion)。流动电极是通过在电解液中加入1%(质量分数)的炭黑来制备的。流动电极和进水的体积分别为50 mL和60 mL。流动电极的分散液被连续搅拌12 h以确保均匀混合。
-
实验中所有的FCDI装置均相同,包括2个15 cm×15 cm的PVC塑料板作为装置的底板。使用2个石墨板作为集流体,集流体上刻有21个深度和宽度均为2 mm的S形流道。使用1对阴/阳离子交换膜(CEM-CSE-2,AEM-AGU)使离子可以选择性的通过。为了防止水的泄漏以及电极之间的直接接触,使用空心硅胶垫插入离子交换膜之间作为密封层,离子交换膜的有效面积为36 cm2。电极室分别位于离子交换膜的两侧(图2(a))。
FCDI脱盐系统包括脱盐装置、蠕动泵、电导率仪和直流电源。使用蠕动泵(BT100-2J)将溶液连续泵入FCDI装置。在脱盐过程中,使用三通道直流电源(IT6322A),以1 V的恒定电压为设备连续供电,并实时监测外部电路的电流。使用电导率仪(DDSJ-308F)连续监测进水溶液的电导率。为了保证准确性,所有的脱盐实验均重复3次。
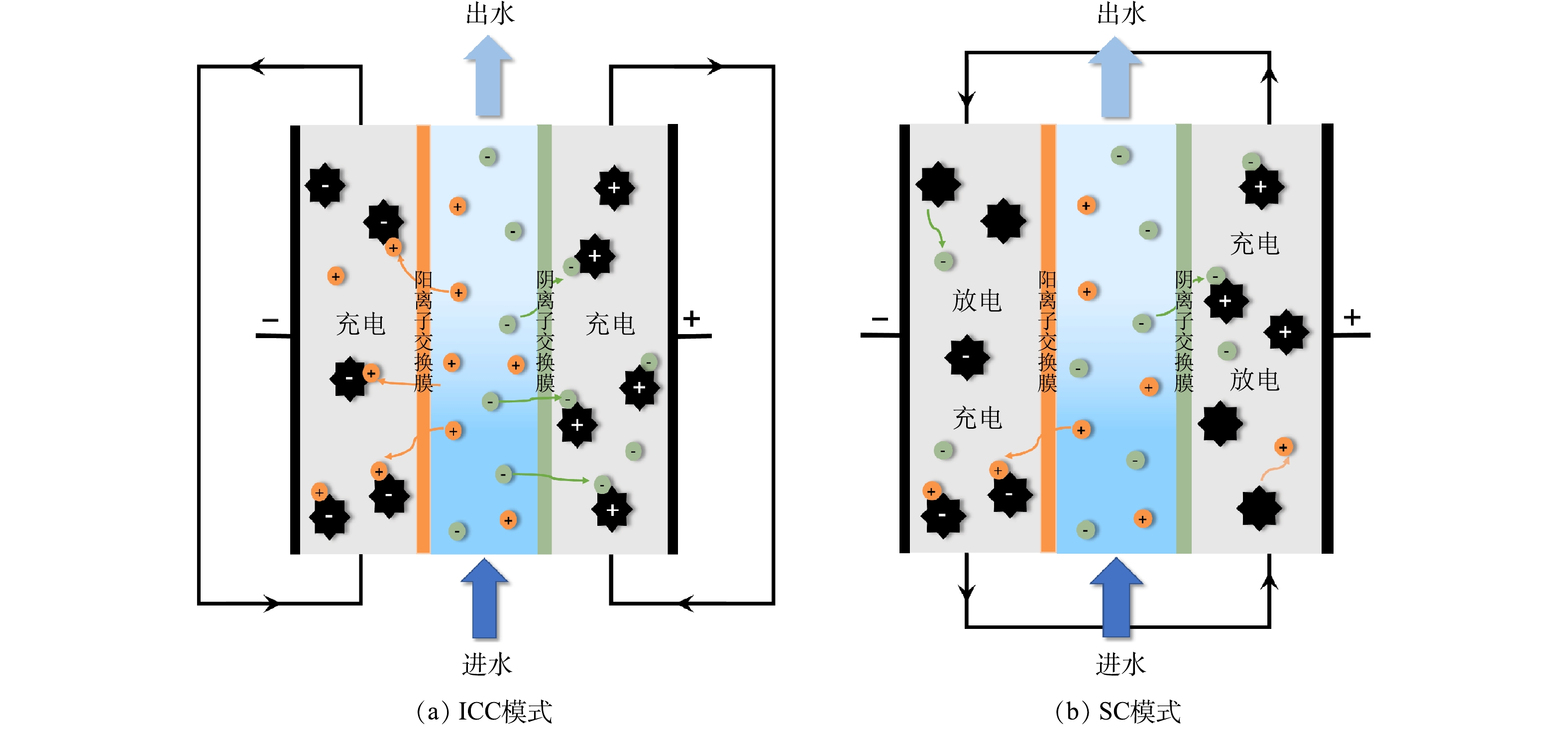
常见的FCDI操作模式包括单循环(single cycle,SC)和隔离封闭循环(isolated closed-cycle,ICC),它们适用于不同的盐分离情况。在ICC模式下,如图1(a)所示,流动电极在阴极和阳极分别循环,脱盐过程中离子吸附占主导地位,以实现溶液中的阴离子和阳离子的分离。在流动电极吸附容量达到饱和后,电压被逆转以实现流动电极的再生。在SC模式下,如图1(b)所示,流动电极在阴极和阳极循环,同时进行离子吸附和解吸的过程,因此,流动电极实现连续再生与更新。
-
4种炭黑的比表面积及孔径分布使用氮气吸附-解吸仪(ASAP 2460)测定,其中比表面积使用Brunauer-Emmett-Teller法计算,孔径分布参数使用Barrett-Joyner-Halenda法计算,外表面积使用t-plot法计算。
炭黑样品的表面元素组成使用能量色散X射线光谱仪(Escalab250Xi)在2×10−6 Pa真空度下进行测定,结合能以C1s峰(284.8 eV)为标准进行了标定。晶体结构使用X射线衍射仪(D8 Advance)进行表征。炭黑样品的石墨化程度是通过共聚焦拉曼显微镜(inVia Qontor)获得,拉曼光谱是使用532 nm激光的拉曼光谱仪采集的,中心光谱范围为1 500 cm−1,激光功率为1%,累计5次。官能团组成使用傅里叶变换显微红外光谱仪(Nicolet iN10MX)进行分析,光谱扫描范围为4 000~500 cm−1,分辨率为4 cm−1,扫描64次。炭黑的粒度由激光粒度分析仪测试得到(Mastersizer 3000),将4种炭黑分散在超纯水中,保持搅拌将高速转动使浆液混匀,使用自动进样器将浆液送入装置,从而得到炭黑的粒度分布曲线。在液氮温度(77 K)下,用氮气吸附脱附仪测定了样品的比表面积。为保证测试结果准确,在测试之前,样品在真空(10−4 MPa)下于300 ℃下脱气24 h。
炭黑的电化学阻抗谱使用电化学工作站(Interface1000)进行测定,使用三电极体系实物装置如图2(b)。在超声条件下,将聚偏氟乙烯(PVDF)溶解在N, N-二甲基乙酰胺中,按照PVDF:CB=1:9的比例分别加入4种炭黑,制成炭黑浆液。浆液加热搅拌至粘稠,并滴涂在导电玻璃上以制备成固定电极用于电化学阻抗谱的测试。扫描频率范围为1 000 kHz至0.1 Hz。在开路电压条件下,在5 mV的低振幅电压下记录流动电极的阻抗谱,并得到它们的接触电阻和电子转移电阻。
-
在所有的脱盐实验中,对电压、装置内电流和进水的电导率进行实时监测和记录,根据单位时间脱盐量,计算出脱盐性能。脱盐量根据式(1)进行计算。
式中:R为脱盐量,μmol·(cm2·min)−1;C0为进水质量浓度,g·L-1;ɳ为去除率,%;V为进水体积,L;t为运行时间,min;Aeff为离子交换膜的有效面积,cm2;M为Na2SO4的摩尔质量,g·mol−1。
-
炭黑样品的N2吸附-脱附曲线见图3(a),ECP和ECP600的吸附脱附曲线是典型的介孔等温线。孔径分布曲线如图3(b)所示。可见,4个炭黑样品均有丰富的介孔和微孔结构,BP2000主要以0.2 nm和1.2 nm的微孔形式存在,ECP600则以3 nm的介孔形式存在。此外,由激光粒度仪测定VXC、ECP、ECP600和BP2000的粒径均在47~100 μm,4种炭黑流动电极的主要得分布形式分别为59、64、61和69 μm的团聚体。同时,使用SEM表征了4种炭黑流动电极的表面形貌(图4)。可见,炭黑粉末表面呈均匀分布,且通过计算得到4种炭黑粉末的粒径基本在0.1 μm左右,4种炭黑粉末表面形貌没有明显区别。由于粒径极小,发生了明显的团聚现象,这与激光粒度仪的测试结果一致。
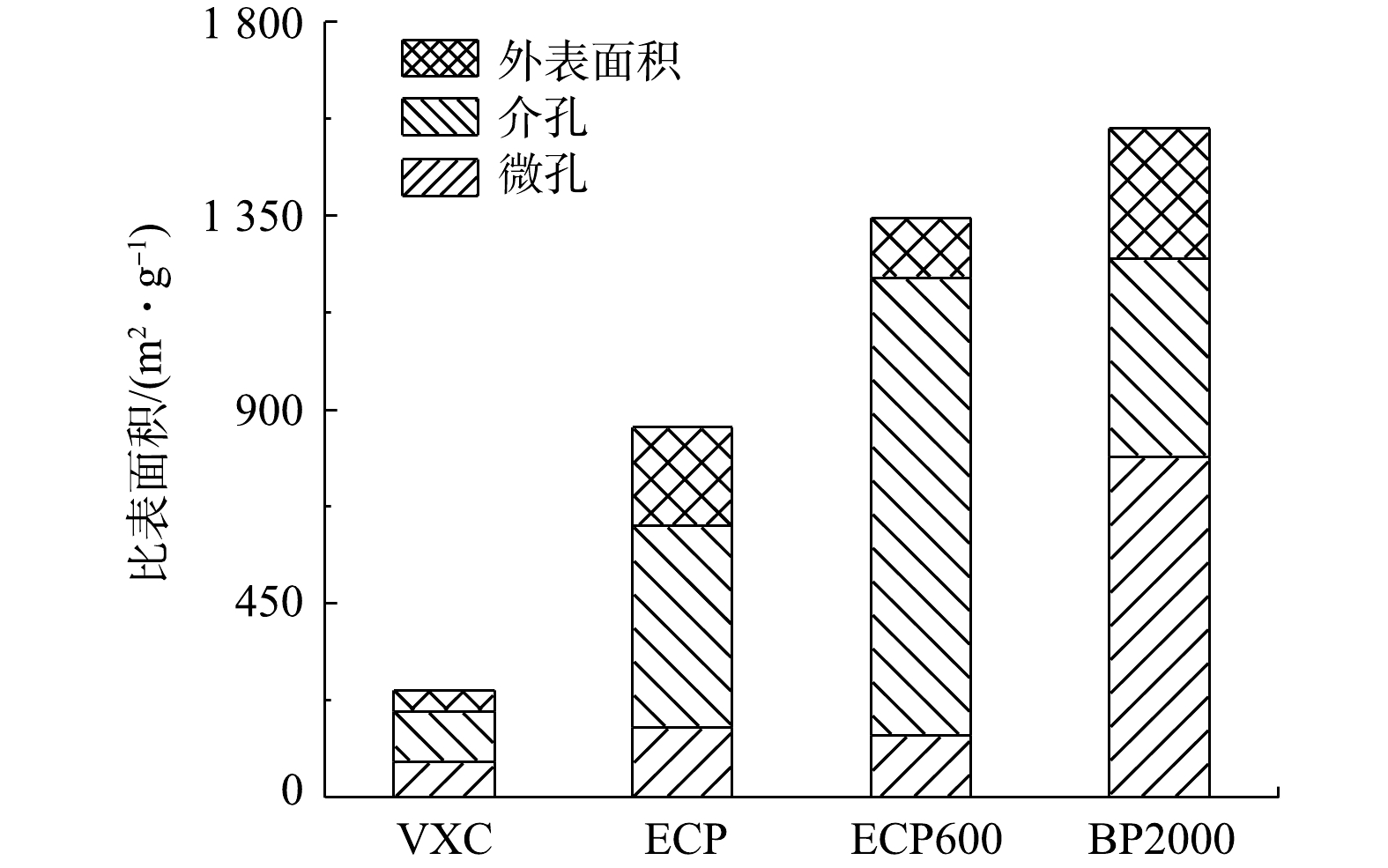
炭黑的比表面积由介孔面积、微孔面积与外表面积3部分组成。由图5可见,4种炭黑的比表面积由大到小为:BP2000(1 552.96 m2·g−1)、ECP600(1 343.84 m2·g−1)、ECP(858.84 m2·g−1)和VXC(249.07 m2·g−1)。ECP600和BP2000分别具有最大的介孔和微孔面积。VXC、ECP和ECP600的微孔面积相对接近(分别为83.77、161.94和143.80 m2·g−1)。ECP的介孔面积是VXC的4倍,ECP600的介孔面积是ECP的2倍。值得一提的是,BP2000和ECP的介孔面积比较接近(分别为459.36 m2·g−1和468.41 m2·g−1),而BP2000的微孔面积是ECP的4倍。4种类型炭黑的孔径分布和比表面积有所不同。后续进一步比较在2种操作模式下使用4种炭黑流动电极时FCDI的脱盐性能。
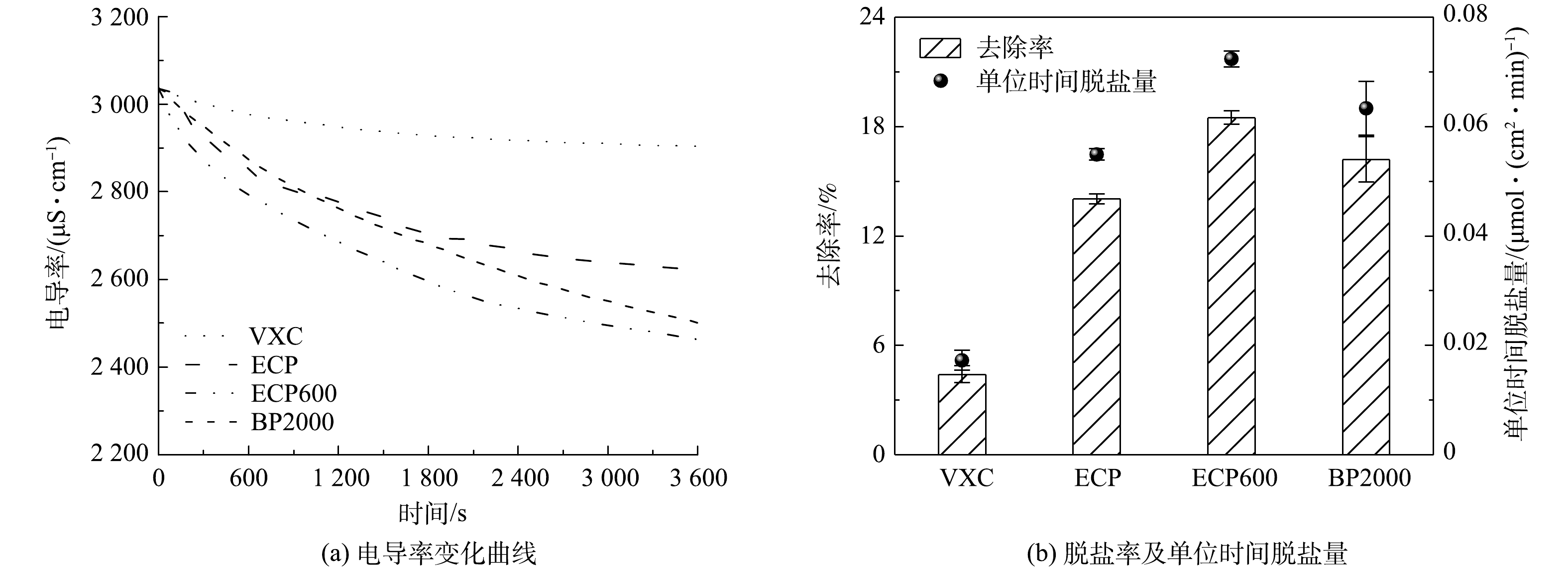
在ICC模式下,比较了4种炭黑作为流动电极时FCDI的脱盐性能。如图6(a)所示,在1 V电压条件下,当使用ECP600作为流动电极时,FCDI的脱盐率可以达到18.5%,高于其他3种炭黑流动电极。同时,与脱盐性能最差的VXC相比,使用ECP600作为流动电极时,FCDI的单位时间脱盐量提高了300%,达到0.07 μmol·(cm2·min)−1(图6(b))。结果表明,在ICC模式下,ECP600作为流动电极可使FCDI获得最佳脱盐效果。
在SC模式下进行了3 h的脱盐实验,SC模式与ICC模式下的脱盐效果明显不同。如图7(a)所示,在SC模式下,使用具有最大的比表面积和微孔面积的BP2000作为流动电极时,FCDI的脱盐率仅为7.9%,在4种炭黑流动电极中最低。当ECP600被用作流动电极时,FCDI的脱盐率能够达到63.3%,高于其他3种炭黑流动电极。并且相对于脱盐性能最差的BP2000而言,脱盐量可以达到0.08 μmol·(cm2·min)−1,提高了700%,(图7(b))。结果表明,在SC模式下,当具有大量介孔的炭黑流动电极时,FCDI的脱盐性能最好;当使用具有大量微孔的流动电极时,FCDI的脱盐性能最差。此外,在SC模式下,BP2000作为流动电极时,FCDI的脱盐效果远远低于ICC模式。这可能是因为在SC模式下流动电极不断地再生,而BP2000存在大量微孔使流动电极不能及时再生,导致流动电极的吸附容量下降。
为了阐明流动电极的孔径分布对脱盐性能的影响,利用IBM SPSS统计软件分析了流动电极的孔径分布与FCDI在ICC和SC模式下的脱盐性能之间的相关性。选择皮尔逊系数作为多个相关因素的双变量相关分析的相关系数,用来衡量多个因素之间的相关程度(表1)。
在ICC模式下,炭黑流动电极的介孔面积(P<0.01)[15],比表面积(P<0.01)和外表面积(P<0.05)与脱盐效果表现出高度的相关性,其对应的皮尔逊相关系数r分别为0.842、0.918和0.650(表1)。在ICC模式下,微孔面积和FCDI的脱盐效果之间没有明显的相关性(P>0.05)。结果表明,在ICC模式下,FCDI的去除效率与流动电极的比表面积呈正相关,且介孔面积是决定FCDI脱盐性能的主导因素[16]。
在SC模式下,炭黑流动电极的比表面积(P>0.05)和外表面积(P>0.05)与FCDI的脱盐性能之间的相关性不显著。介孔面积与FCDI的脱盐性能表现出显著正相关(P<0.05),r=0.583。而微孔面积显示出显著负相关(P<0.01),r=−0.725。因此,在SC模式下,FCDI的脱盐性能随着流动电极介孔面积的增加而提高,随着微孔面积的增加而降低。
-
在FCDI的脱盐过程中主要存在电子传递和离子迁移2个过程。因此,为了探索离子迁移对脱盐性能的影响,需要在电子传递能力一致的条件下进行分析。一般认为,多孔碳材料的电子传递能力主要来自2个方面:由表面具有氧化还原能力的官能团产生的间接电子传递和由碳基体产生的直接电子传递。为了测试炭黑流动电极的电子传递能力,使用XPS和FT-IR对间接电子传递能力进行表征;使用XRD和拉曼光谱对直接电子传递能力进行表征。
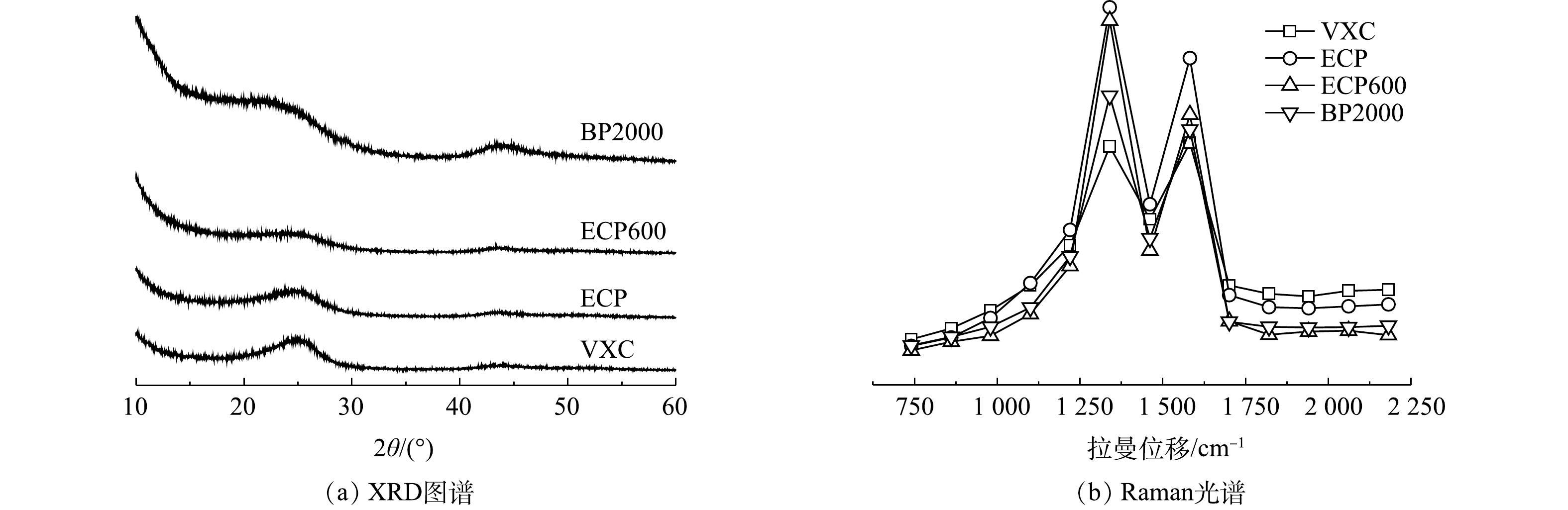
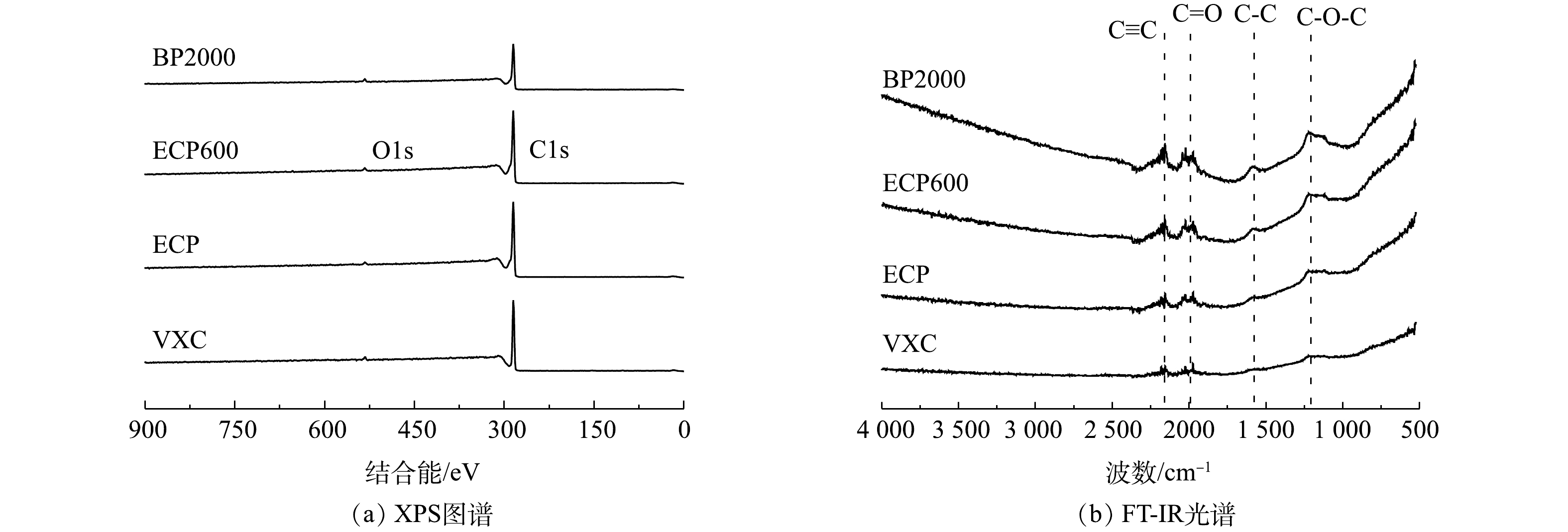
1)碳基质的直接电子转移。炭黑可以通过碳基质直接实现电子传递,这可以用其石墨化程度来表示[17-18]。炭黑的晶体结构如图8(a)所示。4个炭黑样品在2θ=26.2°处表现出相对清晰的峰,这是由于石墨的(002)面的衍射[19-20]。这一结果与拉曼光谱结果一致(图8(b))。在1 300 cm−1和1 580 cm−1处分别观察到明显的D和G峰[21]。D峰代表C原子晶体的缺陷,G峰代表C原子sp2杂化的面内拉伸振动。D波段和G波段综合强度的比例值(ID/IG)越大[22-23],C原子晶体的缺陷越多,炭黑的石墨化程度越高[24-25]。4个炭黑样品的ID/IG值分别为1.03、1.14、1.17和1.13,表明其通过碳基体进行直接电子传递的能力比较接近。
2)间接电子传递。具有氧化还原活性的表面官能团是炭黑主要的电活性成分[26]。图9(a)为炭黑的典型XPS元素图谱。C1s光电子峰占主导地位,次要的信号峰表明在样品表面区域还存在氧元素(O1s)[27-28]。4个炭黑中的碳元素和氧元素都很丰富,且比例接近[29]。此外,炭黑样品的表面官能团使用FT-IR进行表征。如图9(b)所示,在2 200 cm−1和1 980 cm−1的C≡C和C=O伸缩振动表明芳香碳和羧基的存在[30]。分别在1 600 cm−1和1 250 cm−1出现C—C和C—O—C的骨架振动吸收峰,表明醚的存在。图谱中未检测到醌和苯醌等电活性基团[31-32]。因此,4种炭黑流动电极主要以直接电子传递为主且传递能力接近。
通过分别表征表面官能团间接电子传递和碳基体的直接电子传递能力,观察到4个炭黑流动电极的电子传递能力基本相同,电子传递能力的差异并不是4种炭黑流动电极脱盐性能不同的主要原因。因此,使用炭黑作为流动电极时,FCDI的脱盐效果主要受离子迁移(受流动电极的孔径分布控制)的影响。
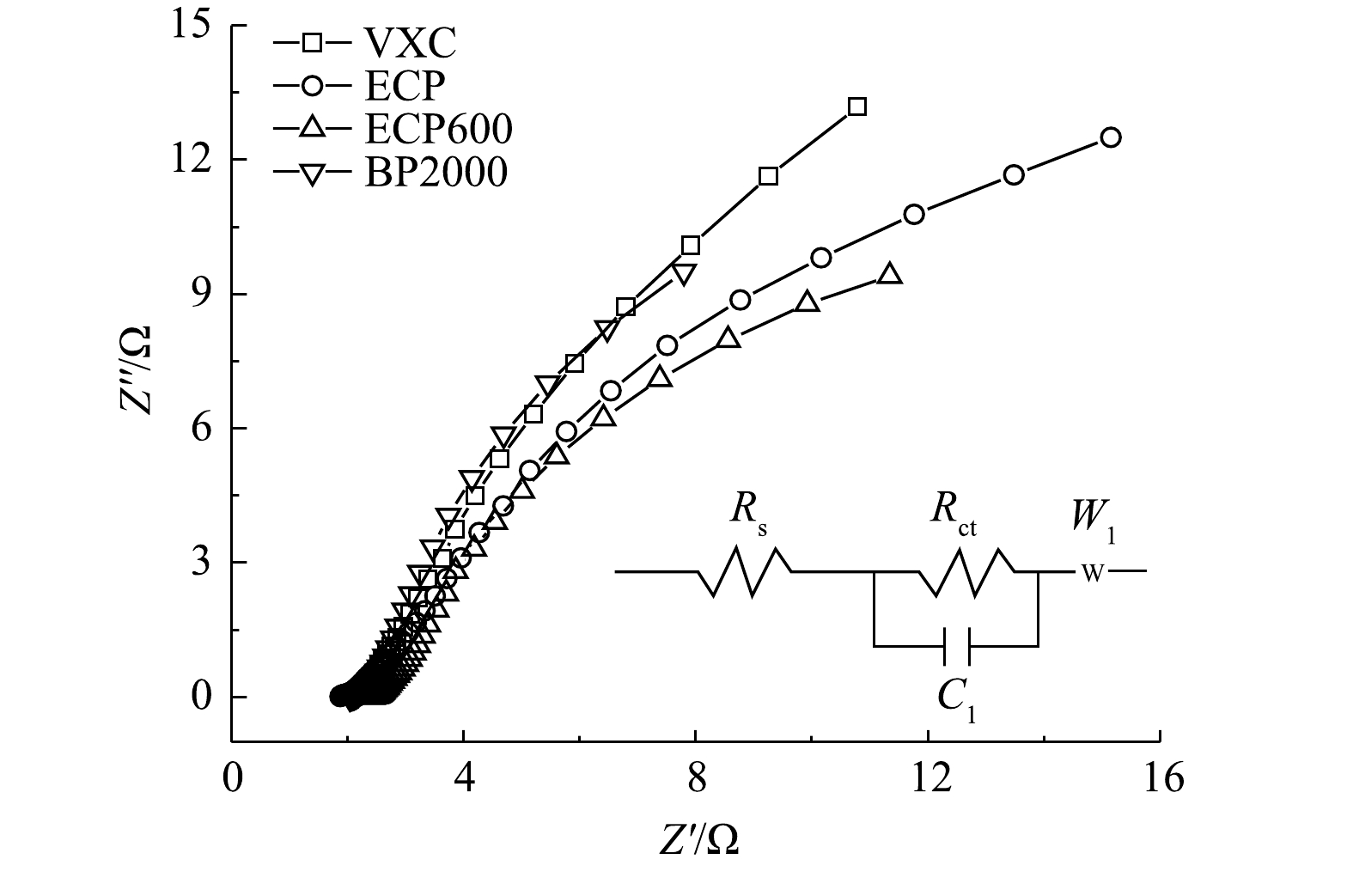
3)炭黑的导电性。由炭黑的奈奎斯特图(图10)可见,曲线与X轴的交点是炭黑样品的欧姆电阻(Rs)[33-35],高频区域的半圆直径代表炭黑和集流体之间的电荷转移电阻,也被称为接触电阻(Rct)[36-37]。欧姆电阻和接触电阻值由等效电路计算得到。VXC、ECP、ECP600和BP2000作为流动电极的Rs非常接近,分别为2.07、2.28、2.07和1.89 Ω。这说明炭黑样品具有相似的电阻。
-
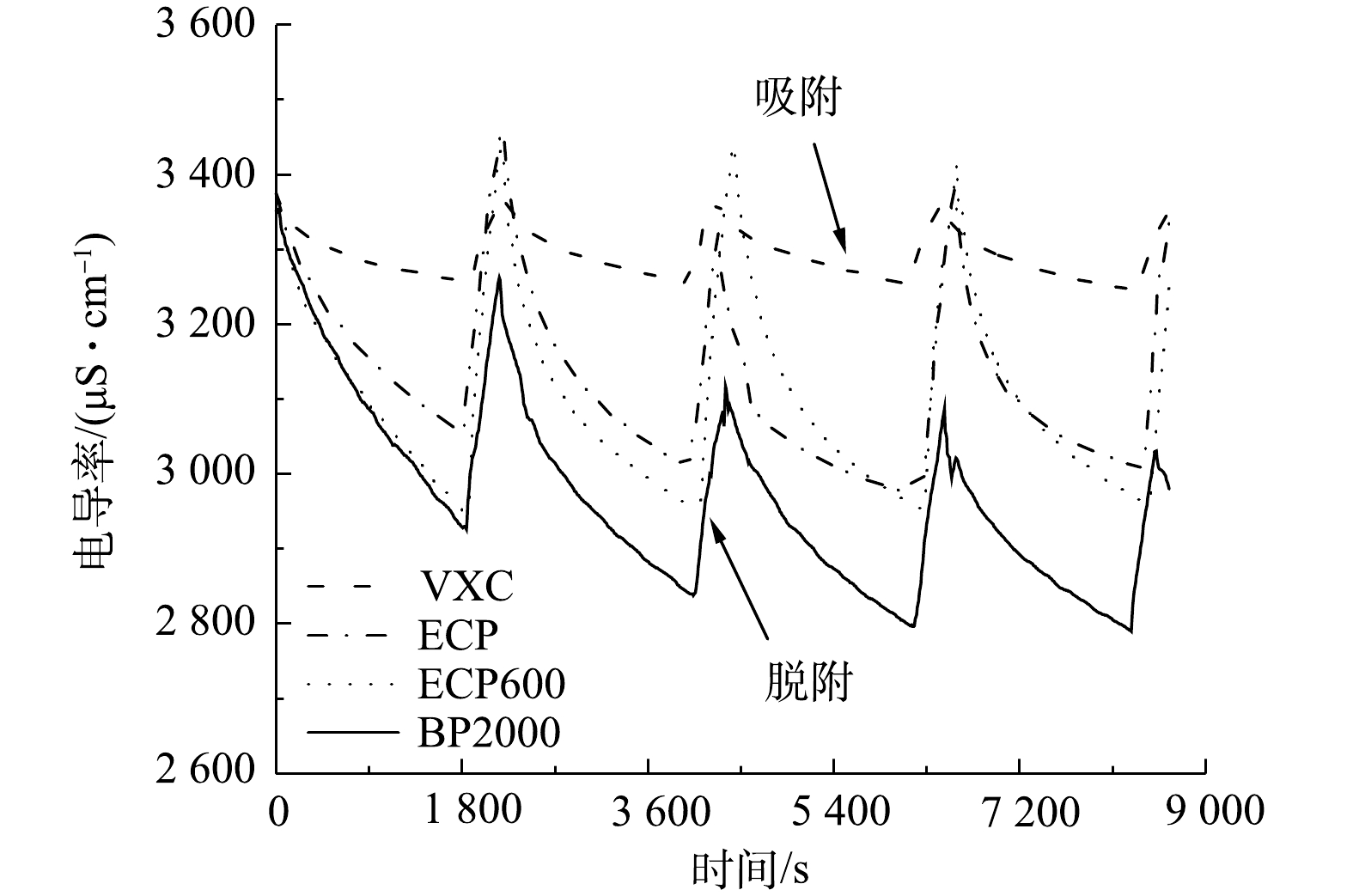
为了阐述流动电极的孔径分布对FCDI中离子吸附和解吸的影响,进行了充电-放电循环实验。电压在充电30 min后迅速反转,此时被吸附的离子从流动电极表面解吸,解吸时间为5 min。1次充电和放电为1个周期,每个样品进行4个周期的实验。
当VXC、ECP和ECP600作为流动电极时,每个循环中盐溶液的吸附和解吸量均非常接近。如图11所示,在每个充电-放电循环后,电导率的变化是完全可重复的,表明介孔型流动电极的再生与更新具有显著的稳定性,而BP2000作为流动电极时,电导率变化曲线呈明显的下降趋势。
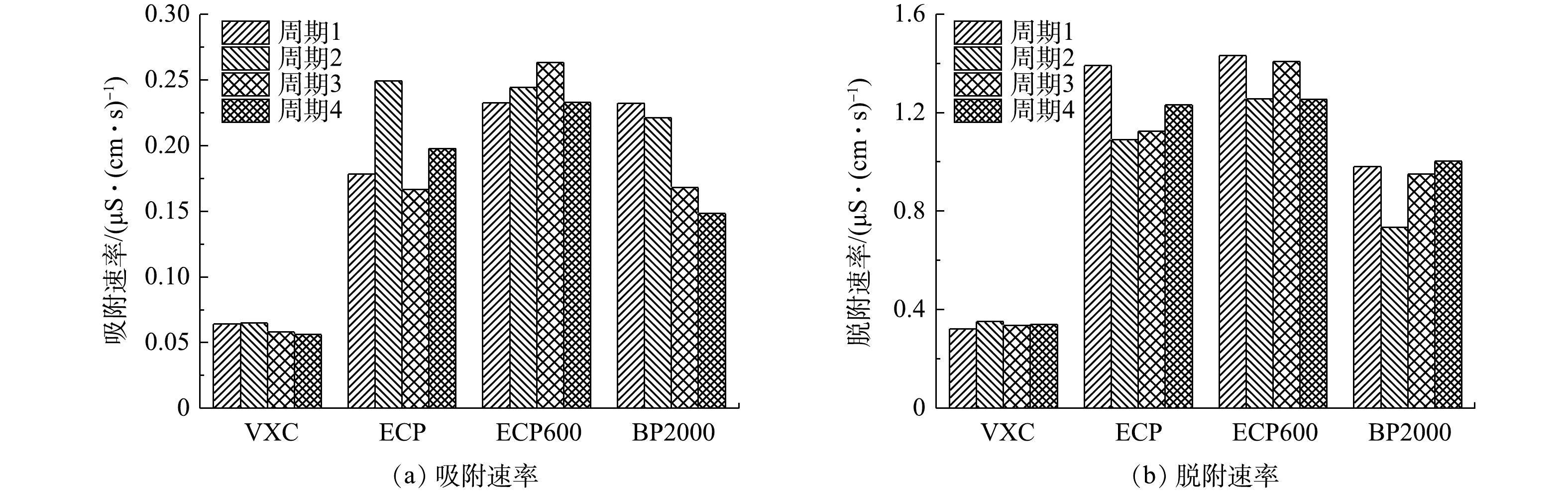
吸附速率如图12(a)所示。当以微孔为主的BP2000作为流动电极时,第1个循环的充电效率是4种流动电极中最高的,达到0.23 μS·(cm·s)−1。但如图12(b)所示,BP2000作为流动电极时,放电效率低于ECP和ECP600,仅为0.95 μS·(cm·s)−1,并且在所有4个循环中,解吸量均低于吸附量,导致充电效率在4个循环过程中逐渐下降。以上结果证明,由于较小的微孔尺寸和较长的吸附-解吸路径,很难实现快速解吸。流动电极的再生因微孔数量过多而受到限制,导致脱盐效果下降。
结合上述结果可以得出以下结论:在ICC模式下,ECP600被用作流动电极时,FCDI呈现出最佳的脱盐性能。高比表面积使ECP600作为流动电极具有很高的吸附容量。由于通过介孔的离子吸附路径比微孔的短,ECP600在1 h内可吸附更多的离子。
在SC模式下,流动电极同时进行离子的吸附和解吸,可确保流动电极一直拥有良好的脱盐性能。然而,离子的脱附过程明显受到微孔的孔径较小和迁移路径较长的限制。在长期运行过程中,流动电极不能快速再生,限制了FCDI的脱盐性能。因此,在SC模式下,BP2000作为流动电极时,FCDI的脱盐性能很差。相反的是,ECP600和ECP均是以介孔为主,孔径较大,吸附-解吸路径较短。离子在流动电极表面迅速地进行吸附和解吸过程。经过长期的脱盐过程,流动电极的更新率仍然得到保证,吸附容量依然保持在较高水平。
-
1)不同孔径分布的流动电极在不同运行模式下的脱盐效果差异明显。在ICC运行模式下,使用介孔面积最大的炭黑作为流动电极时,FCDI的脱盐率最高,微孔面积最大的炭黑流动电极次之。而在SC运行模式下,微孔面积最大的炭黑作为流动电极时,FCDI的脱盐效果骤降,去除率仅为7.9%。
2) 4种炭黑流动电极基本不存在间接电子传递,均以直接电子传递为主。同时,孔径分布存在非常明显的差异,ECP600和ECP是以介孔结构为主导,而BP2000则是微孔结构为主导。
3)在ICC模式下,FCDI的脱盐性能与流动电极的比表面积呈正相关,并以介孔面积为主导因素。
4)在SC模式下,FCDI的脱盐性能与介孔面积呈正相关,与微孔面积呈明显的负相关,即脱盐性能随着流动电极介孔面积的增加而提高,随着微孔面积的增加而降低。
5)在脱盐过程中微孔结构会抑制了离子的解吸,流动电极不能快速再生,从而降低了吸附容量。因此,具有大量微孔的BP2000在SC模式下的脱盐效果远低于其他3种流动电极,具有介孔结构的流动电极能够避免这个缺点。
炭黑流动电极的孔径分布对流动电极电容去离子脱盐性能的影响
Effect of pore size distribution of carbon black flow electrode on the desalination of flow electrode capacitive deionization
-
摘要: 流动电极电容去离子(flow electrode capacitive deionization,FCDI)主要依靠流动电极的电吸附来实现离子去除。而其中流动电极的孔径分布是影响FCDI的脱盐性能的重要因素。为此,选择4种具有高导电性的炭黑(carbon black,CB)作为流动电极,考察了流动电极的孔径分布对FCDI脱盐性能的影响。结果表明,在隔离闭合循环(isolated closed-cycle,ICC)模式下,脱盐性能与流动电极的比表面积呈正相关(r=0.918),并主要受介孔面积的影响。在单循环(single cycle,SC)模式下,FCDI的脱盐性能与介孔面积呈正相关(r=0.583),与微孔面积呈负相关(r=-0.725)。当使用介孔面积最大的炭黑作为流动电极时,离子在流动电极表面的解吸速率升高了53%,FCDI的脱盐率提高了702%。吸附-解吸实验结果表明,由于微孔存在尺寸小、吸附-解吸路径长等缺点,使被流动电极吸附的离子难以被快速解吸,从而抑制了在SC模式下流动电极的再生。介孔可强化FCDI在2种操作模式下的脱盐性能;而微孔却抑制了SC模式下的离子解吸,从而降低了FCDI的脱盐性能。以上研究结果可为流动电极的设计和选择提供参考。Abstract: Flow electrode capacitive deionization (FCDI) mainly relies on the electro-adsorption of the flow electrode to realize ion removal. Among them, the pore size distribution of flow electrode is an important factor affecting the desalination performance of FCDI. Therefore, four kinds of carbon black (CB) with high conductivity were selected as flow electrodes, and the influence of pore size distribution of flow electrodes on the desalination performance of FCDI was investigated. The results showed that in the isolated closed-cycle (ICC) mode, the desalination performance was positively correlated with the specific surface area of the flow electrode (r=0.918), and was mainly affected by the mesoporous area. In the single cycle (SC) mode, the desalination performance of FCDI was positively correlated with mesopore area (r=0.583) and negatively correlated with micropore area (r=-0.725). When carbon black with the largest mesoporous area was used as the flow electrode, the desorption rate of ions on the surface of the flow electrode increased by 53%, and the desalination rate of FCDI increased by 702%. The results of adsorption-desorption experiments showed that due to weakness of micropores such as small size and long path for adsorption-desorption, the rapid desorption for the ions absorbed by the flow electrode was difficult, which inhibited the regeneration of the flow electrode in SC mode. Mesopores could strengthen the desalination performance of FCDI in two operation modes, while micropore limited the desorption of ions in SC mode and the desalination performance of FCDI was weakened. The results can provide a reference for design and selection of the flow electrode.
-

-
表 1 流动电极的比表面积和孔径分布与FCDI脱盐性能的相关性
Table 1. Correlation between specific surface area and pore distribution on FCDI desalination performance
运行模式 微孔/(m2·g−1) 介孔/(m2·g−1) 比表面积/(m2·g−1) 外表面积/(m2·g−1) r P r P r P r P ICC 0.391 0.209 0.842** 0.001 0.918** 0 0.650* 0.022 SC −0.725** 0.008 0.583* 0.047 −0.073 0.822 −0.279 0.379 注: r是皮尔逊相关系数,P是显著性指数,*表示P<0.05水平上显著;**表示P<0.01水平上显著。 -
[1] PORADA S, WEINSTEIN L, DASH R, et al. Water desalination using capacitive deionization with microporous carbon electrodes[J]. ACS Applied Materials & Interfaces, 2012, 4: 1194-1199. [2] SUSS M E, PORADA S, SUN X, et al. Water desalination via capacitive deionization: What is it and what can we expect from it?[J]. Energy & Environmental Science, 2015, 8: 2296-2319. [3] WANG T Y, LIANG H, BAI L M, et al. Adsorption behavior of powdered activated carbon to control capacitive deionization fouling of organic matter[J]. Chemical Engineering Journal, 2020, 384: 10. [4] JEON S I, PARK H R, YEO J G, et al. Desalination via a new membrane capacitive deionization process utilizing flow-electrodes[J]. Energy & Environmental Science, 2013, 6: 1471-1475. [5] CHANG J J, DUAN F, CAO H B, et al. Superiority of a novel flow-electrode capacitive deionization (FCDI) based on a battery material at high applied voltage[J]. Desalination, 2019, 468: 9. [6] LIANG P, SUN X L, BIAN Y H, et al. Optimized desalination performance of high voltage flow-electrode capacitive deionization by adding carbon black in flow-electrode[J]. Desalination, 2017, 420: 63-69. doi: 10.1016/j.desal.2017.05.023 [7] MA J J, ZHANG C Y, YANG F, et al. Carbon black flow electrode enhanced electrochemical desalination using single-cycle operation[J]. Environmental Science & Technology, 2020, 54: 1177-1185. [8] CAI Y M, ZHAO X T, WANG Y, et al. Enhanced desalination performance utilizing sulfonated carbon nanotube in the flow-electrode capacitive deionization process[J]. Separation and Purification Technology, 2020, 237: 12. [9] CHO Y, YOO C Y, LEE S W, et al. Flow-electrode capacitive deionization with highly enhanced salt removal performance utilizing high-aspect ratio functionalized carbon nanotubes[J]. Water Research, 2019, 151: 252-259. doi: 10.1016/j.watres.2018.11.080 [10] SASI S, MURALI A, NAIR S V, et al. The effect of graphene on the performance of an electrochemical flow capacitor[J]. Journal of Materials Chemistry A, 2015, 3: 2717-2725. doi: 10.1039/C4TA05785F [11] BIAN Y H, CHEN X and REN Z Y J. pH dependence of phosphorus speciation and transport in flow-electrode capacitive deionization[J]. Environmental Science & Technology, 2020, 54: 9116-9123. [12] LUO T, ABDU S and WESSLING M. Selectivity of ion exchange membranes: A review[J]. Journal of Membrane Science, 2018, 555: 429-454. doi: 10.1016/j.memsci.2018.03.051 [13] CHAO L M, LIU Z Y, ZHANG G X, et al. Enhancement of capacitive deionization capacity of hierarchical porous carbon[J]. Journal of Materials Chemistry A, 2015, 3: 12730-12737. doi: 10.1039/C5TA01036E [14] BAROUD T N and GIANNELIS E P. High salt capacity and high removal rate capacitive deionization enabled by hierarchical porous carbons[J]. Carbon, 2018, 139: 614-625. doi: 10.1016/j.carbon.2018.05.053 [15] DURICKI D A, SOLEMAN S and MOON L D F. Analysis of longitudinal data from animals with missing values using SPSS[J]. Nature Protocols, 2016, 11: 1112-1129. doi: 10.1038/nprot.2016.048 [16] SHI R Y, LIU W T, LIAN Y H, et al. Phytotoxicity of polystyrene, polyethylene and polypropylene microplastics on tomato (Lycopersicon esculentum L. )[J]. Journal of Environmental Management, 2022, 317: 11. [17] OH S Y, SON J G, LIM O T, et al. The role of black carbon as a catalyst for environmental redox transformation[J]. Environmental Geochemistry and Health, 2012, 34: 105-113. doi: 10.1007/s10653-011-9416-0 [18] XU W Q, PIGNATELLO J J and MITCH W A. Role of black carbon electrical conductivity in mediating hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine (RDX) transformation on carbon surfaces by sulfides[J]. Environmental Science & Technology, 2013, 47: 7129-7136. [19] FENG H B, CHENG R, ZHAO X, et al. A low-temperature method to produce highly reduced graphene oxide[J]. Nature Communications, 2013, 4: 7. [20] LEE S M, LEE S H and ROH J S. Analysis of activation process of carbon black based on structural parameters obtained by XRD Analysis[J]. Crystals, 2021, 11: 11. [21] GONZALEZ-GONZALEZ R B, GONZALEZ L T, MADOU M, et al. Synthesis, purification, and characterization of carbon dots from non-activated and activated pyrolytic carbon black[J]. Nanomaterials, 2022, 12: 19. [22] DE MENDONCA F G, DA CUNHA I T, SOARES R R, et al. Tuning the surface properties of biochar by thermal treatment[J]. Bioresource Technology, 2017, 246: 28-33. doi: 10.1016/j.biortech.2017.07.099 [23] NATH B C, MOHAN K J, BARUA R, et al. Dimensionally integrated alpha-MnO2/Carbon black binary complex as platinum free counter electrode for dye sensitized solar cell[J]. Journal of Photochemistry and Photobiology A:Chemistry, 2017, 348: 33-40. doi: 10.1016/j.jphotochem.2017.06.041 [24] JIANG F H, CAO D F, HU S X, et al. High-pressure carbon dioxide-hydrothermal enhance yield and methylene blue adsorption performance of banana pseudo-stem activated carbon[J]. Bioresource Technology, 2022, 354: 12. [25] RAJA M, ANGULAKSHMI N and STEPHAN A M. Sisal-derived activated carbons for cost-effective lithium-sulfur batteries[J]. Rsc Advances, 2016, 6: 13772-13779. doi: 10.1039/C5RA25850B [26] SUN T R, LEVIN B D A, GUZMAN J J L, et al. Rapid electron transfer by the carbon matrix in natural pyrogenic carbon[J]. Nature Communications, 2017, 8: 1-12. doi: 10.1038/s41467-016-0009-6 [27] BRON M, RADNIK J, FIEBER-ERDMANN M, et al. EXAFS, XPS and electrochemical studies on oxygen reduction catalysts obtained by heat treatment of iron phenanthroline complexes supported on high surface area carbon black[J]. Journal of Electroanalytical Chemistry, 2002, 535: 113-119. doi: 10.1016/S0022-0728(02)01189-0 [28] PAPIRER E, LACROIX R, DONNET J B, et al. XPS study of the halogenation of carbon-black. 2. chlorination[J]. Carbon, 1995, 33: 63-72. doi: 10.1016/0008-6223(94)00111-C [29] STROHMEIER B R, PIASECKI J D, BUNKER K L, et al. XPS and FESEM/STEM surface characterization of activated carbon, carbon black, and carbon nanotubes[J]. Microscopy and Microanalysis, 2010, 16: 442-443. doi: 10.1017/S1431927610059635 [30] KEILUWEIT M, NICO P S, JOHNSON M G, et al. Dynamic molecular structure of plant biomass-derived black carbon (biochar)[J]. Environmental Science & Technology, 2010, 44: 1247-1253. [31] NOPAWAN RATASUK M A N. Characterization and quantificationof reversible redox sites in humic substances[J]. Environmental Science & Technology, 2007, 41: 7844-7850. [32] YANG P, JIANG T, CONG Z, et al. Loss and increase of the electron exchange capacity of natural organic matter during its reduction and reoxidation: the role of quinone and nonquinone moieties[J]. Environmental Science & Technology, 2022, 56: 6744-6753. [33] DENNISON C R, BEIDAGHI M, HATZELL K B, et al. Effects of flow cell design on charge percolation and storage in the carbon slurry electrodes of electrochemical flow capacitors[J]. Journal of Power Sources, 2014, 247: 489-496. doi: 10.1016/j.jpowsour.2013.08.101 [34] QU Y T, BAUMANN T F, SANTIAGO J G, et al. Characterization of resistances of a capacitive deionization system[J]. Environmental Science & Technology, 2015, 49: 9699-9706. [35] YANG S, PARK H R, YOO J, et al. Plate-shaped graphite for improved performance of flow-electrode capacitive deionization[J]. Journal of the Electrochemical Society, 2017, 164: E480-E488. doi: 10.1149/2.1551713jes [36] GU X Y, YANG Y, HU Y, et al. Nitrogen-doped graphene composites as efficient electrodes with enhanced capacitive deionization performance[J]. RSC Advances, 2014, 4: 63189-63199. doi: 10.1039/C4RA11468J [37] YASIN A S, JEONG J, MOHAMED I M A, et al. Fabrication of N-doped & SnO2-incorporated activated carbon to enhance desalination and bio-decontamination performance for capacitive deionization[J]. Journal of Alloys and Compounds, 2017, 729: 764-775. doi: 10.1016/j.jallcom.2017.09.185 -




 下载:
下载: