-
液化天然气 (liquefied natural gas,LNG) 作为船用发动机替代燃料。LNG替代常规燃料,可使得PM和SOx的排放显著降低[1-2]。同时,LNG的碳氢比 (C/H) 低,相比柴油可减少约30%的CO2排放[3],其推广使用对于我国实现双碳目标、改善能源结构具有重要意义。然而,由于LNG发动机甲烷逃逸问题,其排放物中存在CH4。采用低压喷射的LNG发动机,CH4排放量一般为3.0~5.0 g·kWh−1 [4]。CH4是全球第二大温室气体,其全球增温潜势 (global warming potential,GWP) 是二氧化碳的21倍[5]。为控制LNG发动机CH4排放,2016年8月发布的《船舶发动机排气污染物排放限值及测量方法 (中国第一、二阶段) 》规定,根据不同机型及单缸排量,LNG发动机的CH4排放量不应超过1.0~2.0 g·kWh−1 [6]。
船用LNG发动机通常采取缸内稀薄燃烧,导致发动机排气温度较低 (250~400 ℃) ,同时排气为富氧状态,限制了三元催化的使用。目前,尾气中CH4的脱除主要依靠贵金属催化剂,但贵金属催化剂的成本高且低温活性 (<500 ℃) 和抗水性较差[7]。引入低温等离子体 (non-thermal plasma,NTP) 技术可提高催化剂低温活性和抗水性,同时NTP结合催化剂作用系统被证明可有效促进CH4催化还原NOx[8-15]。但由于NTP的加入、排气中N2和O2的存在,会导致额外NOx的生成,从而降低了系统中NOx的脱除效率,且其生成量随着放电功率的升高而显著增大[11]。NOx长期吸入会对人体内脏的功能造成严重伤害,甚至威胁生命安全[16-17]。此外,NOx也是造成光化学烟雾、雾霾和酸雨等有害天气的重要因素之一[18-19],故降低系统中NOx的生成是必要的。优化等离子体反应器结构参数可有效抑制NOx的生成[20-25]。赵如金等[21]用催化剂结合等离子体处理汽油机尾气发现,当放电间隙为2.5~3.5 mm时,3种污染物的去除率变化均小于3%,但放电间隙增大到4 mm时,脱除效率下降明显 (约10%) 。研究者在富氧条件下研究NTP反应器结构参数对NO氧化脱除的影响[23-24],以860
$ \times $ 10−6 NOx (92% NO+8% NO2) / 15% O2 / N2模拟柴油机尾气,发现增大电极直径使·O自由基生成效率增加,减少N2(X, v)和N生成,从而抑制了副反应的生成,提高了NOx氧化效率;而螺纹形电极表面电场强度远高于圆柱形电极,有利于N自由基生成,使得在富氧条件下,更易发生副反应生成NOx。针对低温等离子体脱除CH4的研究仍较少,为明确NTP结构参数对CH4脱除效率及NOx生成的影响,考虑实际LNG发动机尾气,本课题组在625
$ \times $ 10−6 NO/1820$ \times $ 10−6 CH4/10% H2O/6% O2/N2 (余) 气体组分下,针对单独低温等离子体脱除CH4、降低NOx (NO、NO2) 生成系统研究等离子体反应器电极结构参数对系统CH4脱除效率及NOx生成的影响及其机制,以期为提高NTP结合催化剂一体化处理CH4和NOx系统脱除效率提供参考。 -
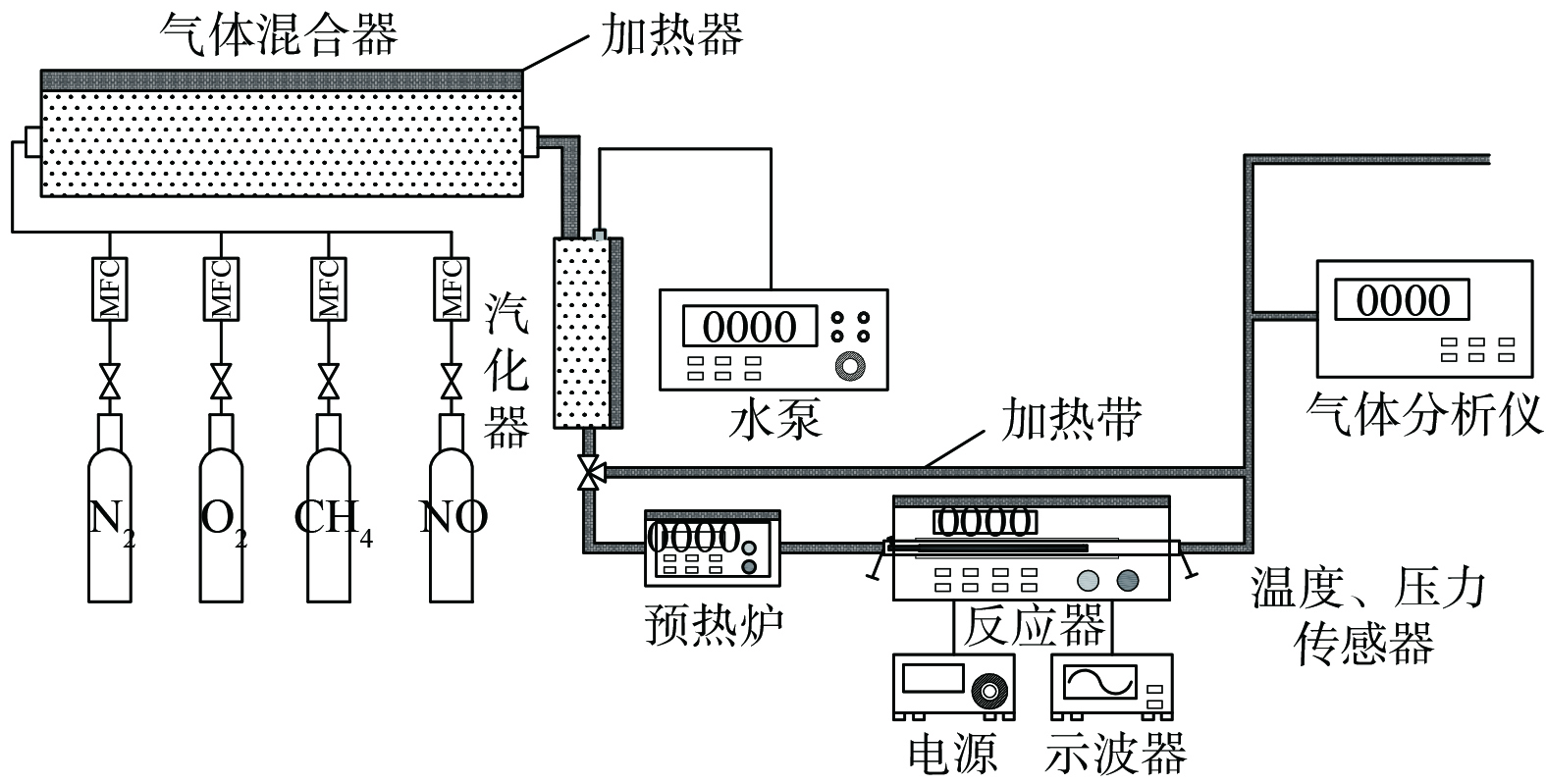
实验装置示意图如图1所示。模拟尾气的流量和各气体组分体积分数由质量流量控制器 (mass flow controller,MFC) 调节,水蒸气由水泵控制其体积分数调节和通入,所有气体组分通过混合器内充分混合后进入汽化器,完成模拟尾气的配制。随后反应气体进入配气气路或反应气路。反应气路装有预热炉和反应器,反应器包含加热炉与等离子体发生器。汽化器后的管路均缠有加热带维持气体温度。反应器前后均放置有温、压传感器,以测量反应前后气体的温度和压力。
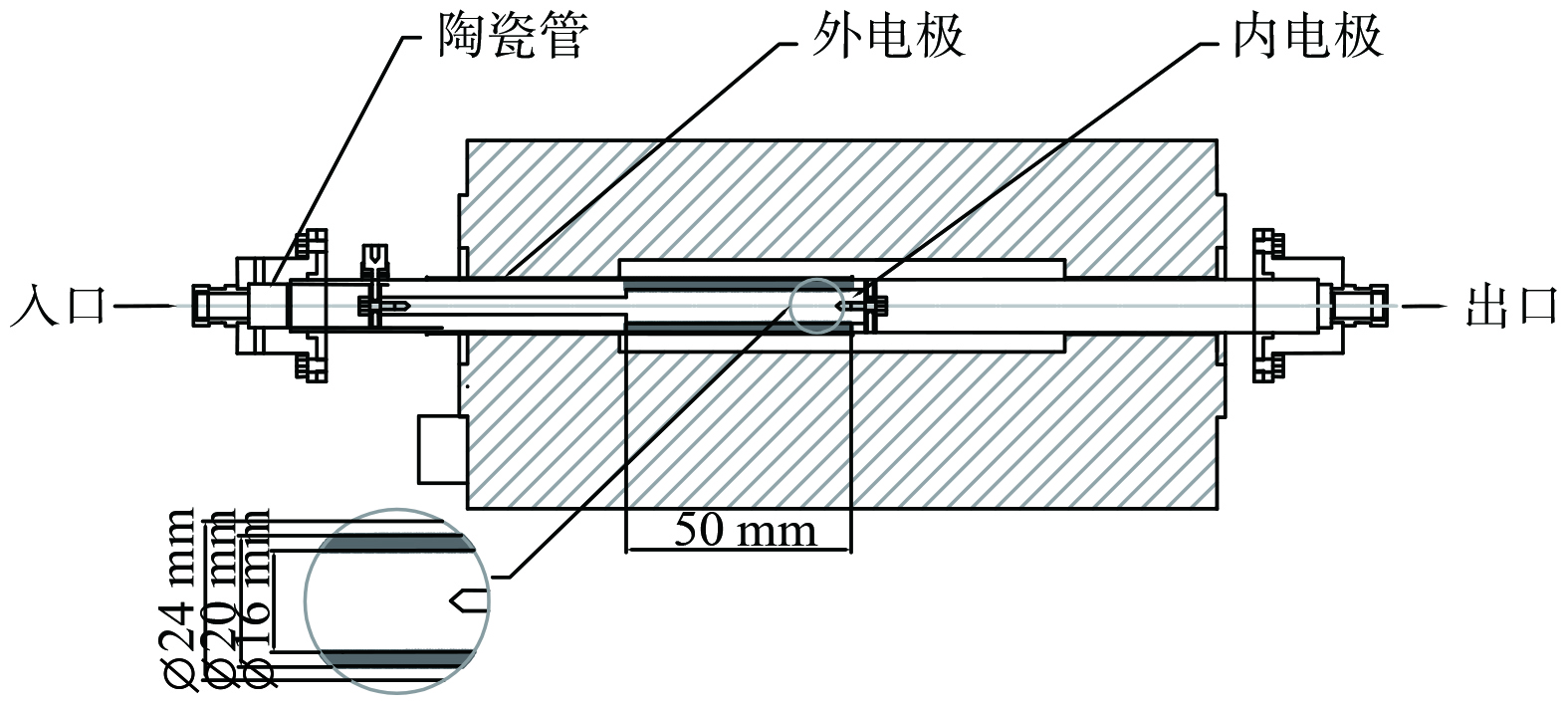
如图2所示,实验所用等离子体发生器为圆柱型介质阻挡放电器 (dielectric barrier discharge,DBD) ,由外电极、放电电极 (内电极) 以及绝缘介质组成,外接电源及示波器。阻挡介质为一个内径20 mm、外径24 mm的陶瓷管。陶瓷管外壁包裹铁网作为外电极,管内插入一根不锈钢棒作为放电电极。放电区间长度为50 mm。所用等离子体反应器电源为高压、高频交流电源型号为CTP2000K,由南京苏曼等离子体有限公司生产;电源频率变化范围为5~25 kHz,电源输出电压为0~25 kV,最大输出功率为500 W;电源输出电压测量采用型号为TEK P6015A的高压探头,最高电压20 kV,测量衰减倍数为1 000倍;所用数字示波器型号为TEK TBS1052B,采样频率1 GS/s,带宽50 MHz;所用气体分析仪型号为Antaris IGS,由美国Thermo Fisher Scientific公司生产。
实验所用N2、O2气体均为纯气,所用NO、CH4气体中N2的体积分数分别为1%、10%。为模拟实际LNG发动机尾气,将气体总流量恒定为3 300 mL·min−1;气体组分为10% H2O/6% O2/1 820×10−6 CH4/625×10−6 NO/ N2(余)。等离子体放电频率固定为10 kHz。模拟排气温度为400 ℃。放电电极直径分别为14、16、18 mm;电极形状为圆杆/螺纹;通过改变外电极长度来改变电极长度,电极长度分别为50、75、100 mm。
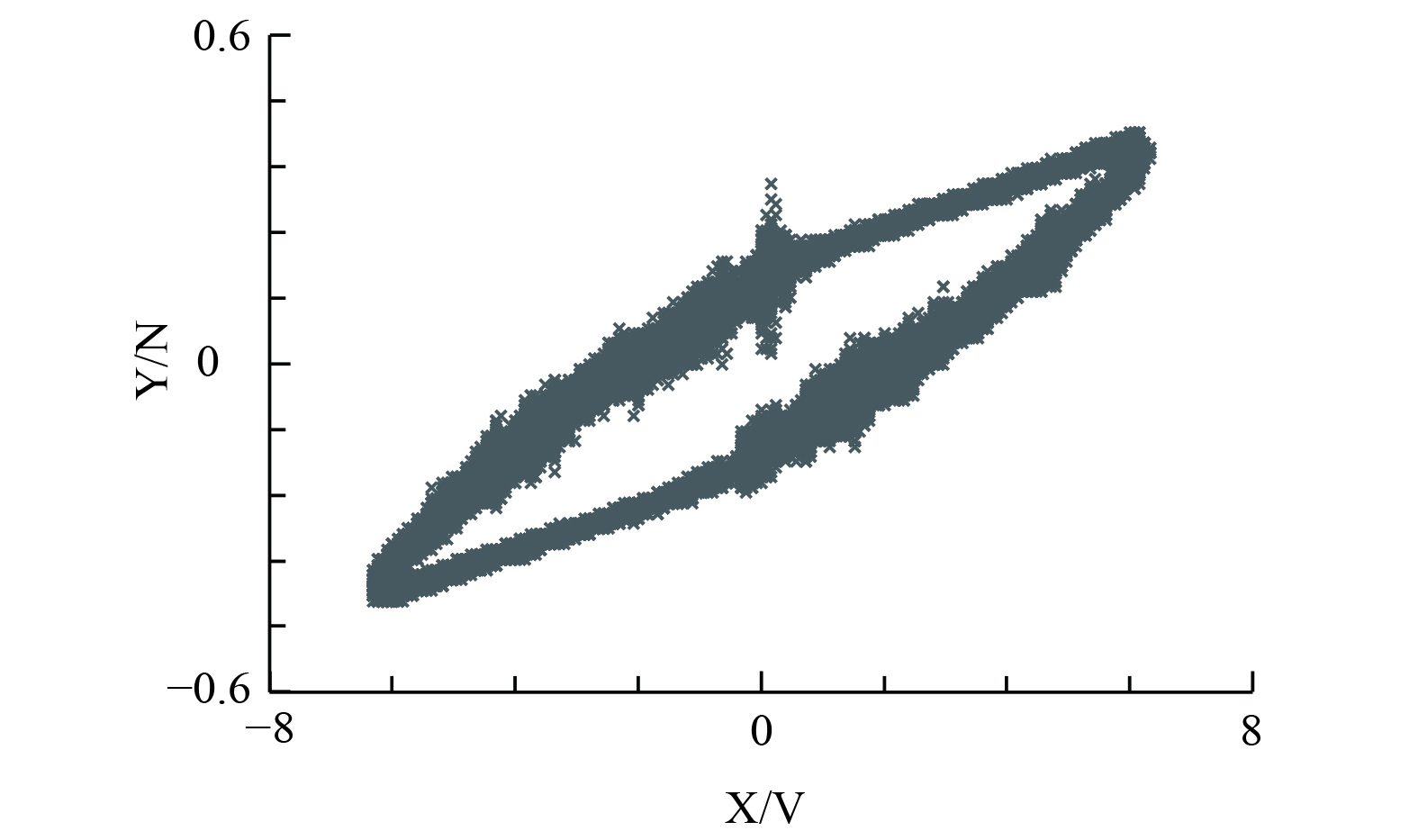
DBD反应器放电功率由放电电压 (即电源输出电压) 调整,通常利用李萨如图形法测量计算放电功率 (图3) 。图形围成的面积代表一个循环放电的放电能量,放电功率的计算公式见式 (1) 。
式中:
$ f $ 为放电频率;$ {C}_{\mathrm{m}}\mathrm{为} $ 与DBD反应器串联的电容,0.47 μF;$ {K}_{x} $ 为示波器X轴灵敏度,V/格;$ {K}_{y} $ 为示波器Y轴灵敏度,V/格;$ K $ 为高压探头衰减倍数,1 000∶1;$ A $ 为示波器中李萨如图形围成的面积。为评价DBD反应器能耗,定义放电能量密度[26] (the specific energy density,SED) 的公式为式 (2)。CH4的转化效率计算见式 (3) 。CO2的选择性计算见式 (4) 。
式中:
$ \mathrm{S}\mathrm{E}\mathrm{D} $ 为放电能量密度,J·L−1;$ P $ 为DBD放电功率,W;$ Q $ 为模拟尾气总流量,L·min−1。$ {\mathrm{\eta }}_{C{H}_{4}} $ 为CH4脱除效率;$ {\phi }_{{\mathrm{C}{\mathrm{H}}_{4}}_{\mathrm{i}\mathrm{n}}} $ 为反应器入口CH4体积分数;$ {{\phi }_{{\mathrm{C}{\mathrm{H}}_{4}}_{\mathrm{o}\mathrm{u}\mathrm{t}}}}_{} $ 为反应器出口CH4体积分数。$ {\mathrm{\alpha }}_{{CO}_{2}} $ 为CO2选择性;$ {\phi }_{{\mathrm{C}\mathrm{O}}_{\mathrm{o}\mathrm{u}\mathrm{t}}} $ 为反应器出口CO体积分数。 -
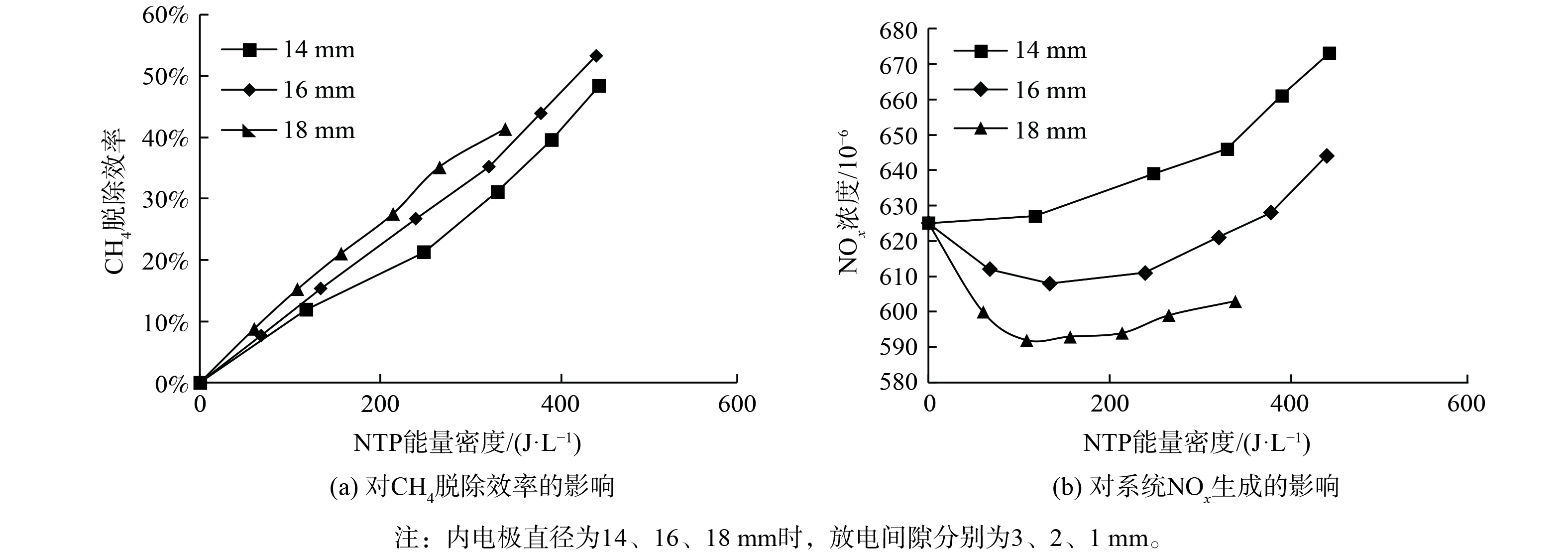
图4为NTP作用下内电极直径对系统CH4脱除效率、NOx生成的影响。随着能量密度的升高,CH4脱除效率呈线性增大,且内电极直径越大、CH4脱除效率越大。当内电极直径为16或18 mm时,在能量密度升高过程中,NOx体积分数先降低后升高;同时,增大内电极直径可减少副反应的发生,降低NOx生成。
增大内电极直径使得DBD反应器的气隙减小,从而在相同电源电压下提高了气隙内的折合场强E/N (电场强度与气体粒子数密度的比值) ,同时使E/N在放电间隙内分布更均匀[27]。折合场强对放电间隙内的碰撞反应及CH4氧化和NOx生成具有重要影响,CH4、O2和H2O解离生成的CH3、CH2、·O和·OH自由基,是促进CH4氧化的重要活性物质[12];同时,对于副反应,需考虑N2(A)、N2(X, v)及N自由基等活性物质,被认为是产生NO的重要反应物[28]。
为解释上述实验结果,对这些关键的活性物质的生成效率和折合场强E/N的关系进行计算。电子碰撞相关的等离子体化学反应效率计算参考式 (5) [29]。
式中:
$ k $ 为电子碰撞反应的速率常数;$ {v}_{\mathrm{d}} $ 为电子漂移速率;$ \mathrm{G}\mathrm{}‐\mathrm{v}\mathrm{a}\mathrm{l}\mathrm{u}\mathrm{e} $ 为每输入100 eV能量可发生的相关电子碰撞反应的次数,个。化学反应速率常数
$ k $ 表示单位时间单位体积发生的化学反应次数,计算参考式 (6) ;$ {\mathrm{v}}_{\mathrm{d}}\mathrm{E}/\mathrm{N} $ 表示单位时间单位体积内所消耗的能量。电子漂移速率$ {v}_{d} $ 可由$ {f}_{0} $ 计算参考式 (7) [30];电子平均能量$ \varepsilon $ 计算公式见式 (8) 。式中:
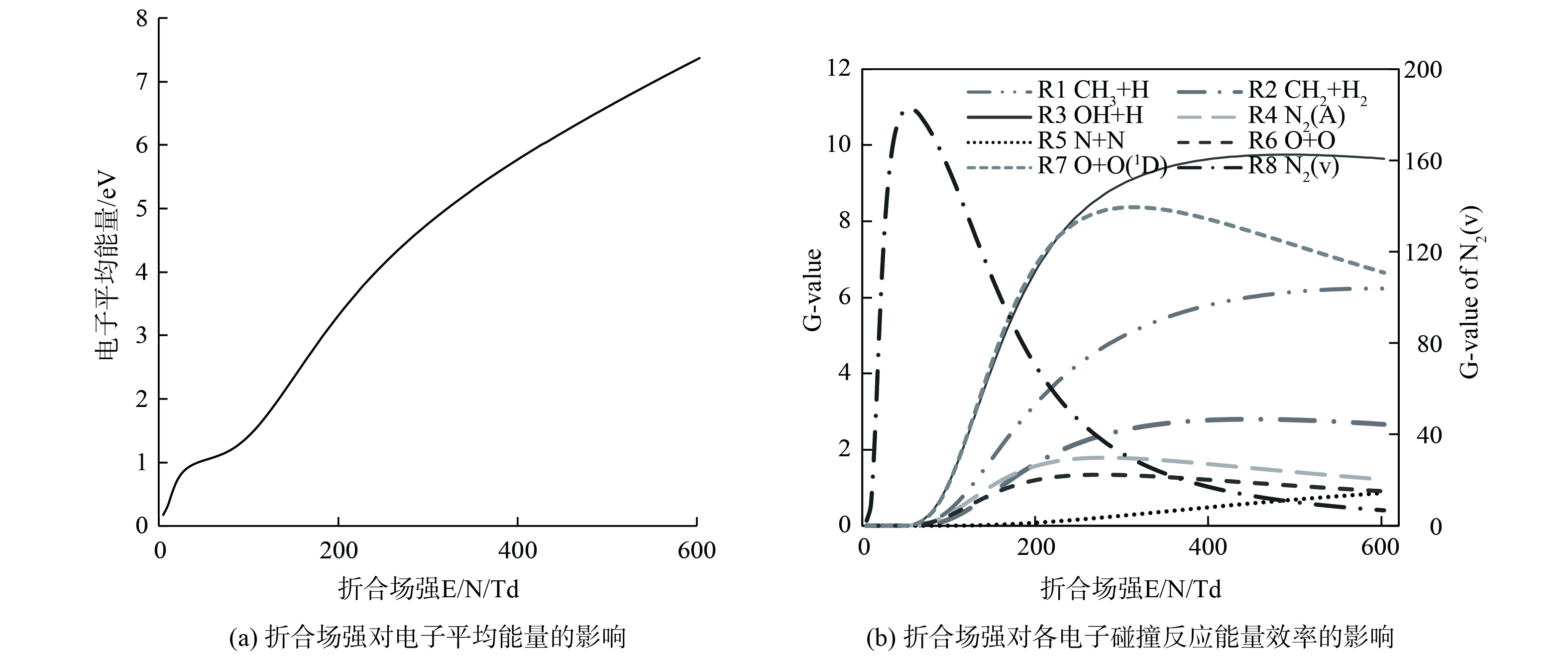
$ {\sigma }_{k} $ 为电子碰撞反应$ k $ 的碰撞截面;e为电子电荷;m为电子质量。电子碰撞截面数据通过LXCat获取[31-33]。$ Q $ 为有效总动量传递截面。$ \bar{\varepsilon } $ 为电子平均能量;$ {f}_{0} $ 为电子能量分布的各向同性部分。理论计算结果如图5所示。图5 (a)表明,增大放电电压,折合电场强度增大,使得电子平均能量升高,增加了放电间隙内的碰撞反应,促进了部分活性粒子的生成。图5 (b)展示了折合场强对各电子碰撞反应能量效率的影响,随着折合场强 E/N (100-250 Td) 的增大,
$ \mathrm{G}\mathrm{}‐\mathrm{v}\mathrm{a}\mathrm{l}\mathrm{u}\mathrm{e} $ 迅速减小,同时CH4、H2O及O2的离解反应式 (9~11) 和 (14~15) 的$ \mathrm{G}‐\mathrm{v}\mathrm{a}\mathrm{l}\mathrm{u}\mathrm{e} $ 显著提高。N2 (9.8 eV) 的离解能高于O2 (5.1 eV) ,故在初放电阶段,O自由基比N自由基更容易形成[34],O、OH和O3首先在DBD反应器中生成 (式 (11) 、 (16~18) ) ,促进CH4的氧化 (式 (19)~(23) ) [35]。随着SED的增加,E/N进一步增加 (E/N >250 Td) ,反应式 (13) 的$ \mathrm{G}‐\mathrm{v}\mathrm{a}\mathrm{l}\mathrm{u}\mathrm{e} $ 迅速增大。在富氧条件下,N自由基生成效率增加,与O、O2和O3发生副反应生成NO (式 (24)~(26) ) [36],从而导致NOx的生成。因此,内电极直径增大使得折合场强E/N增加,平均电子能量增大,增加了放电间隙内的碰撞反应,促进了CH4的解离及O、OH自由基的生成,从而促进CH4的氧化,增大了CH4的脱除效率[25,37-38]。此外,增大内电极直径使得气隙中E/N分布更均匀,抑制了N2(X, v)、N2(A)和N自由基的生成,亦减少了副反应 (式 (24)~(29) ) 的发生,使NOx的生成更少。
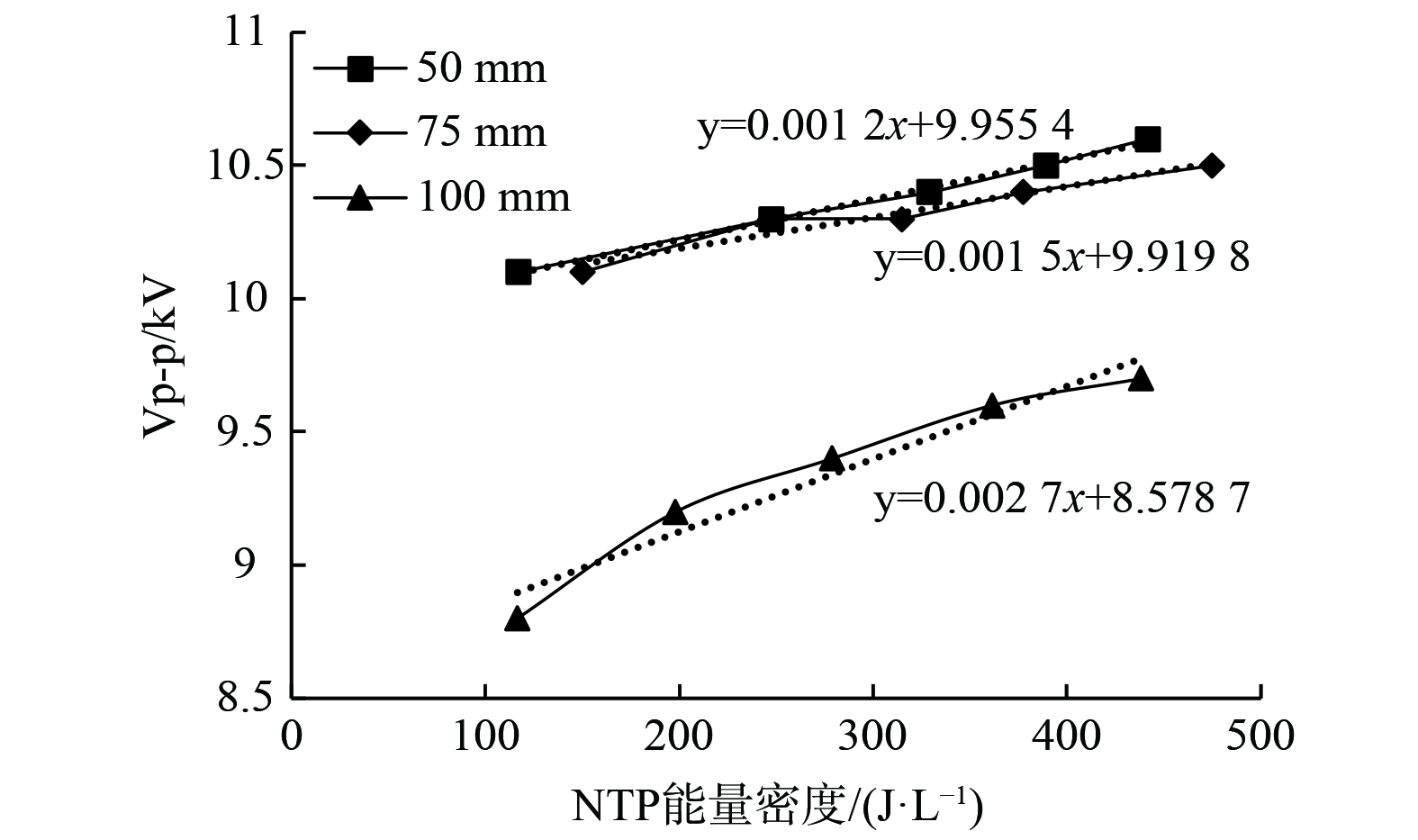
图6表示不同电极直径下SED和放电电压之间的关系,说明在相同的SED下,放电电压随内电极直径的增大而减小。
同轴圆柱形DBD反应器的击穿电压 (
$ {U}_{b} $ ) 可按式 (30) [39]计算。式中:
$ {U}_{\mathrm{b}} $ 表示击穿电压,V;P表示气压,Pa;d表示电极间的距离,mm;γ表示二次电子发射效率;A和B表示气体相关性。式 (30) 表明,增加内电极的直径可减少电极之间的距离 (d) ,从而降低击穿电压 (
$ {U}_{b} $ ) 使气体放电更容易。因此,间隙较小的DBD反应器具有更好的放电性能,在相同的SED下具有更高的CH4脱除效率。此外,根据汤森放电理论 (式 (31) ) ,放电电流随着气隙的增加呈指数增长[40-41]。
式中:I表示放电电流,A;
$ {I}_{0} $ 为阴极流出的初始电流,A;x为放电间隙,mm;$ \alpha $ 为电子碰撞电离系数。式 (31) 表明,间隙越大 (x) 系统具有较强的电子雪崩效应和较高的电流增长率。因此,如图6所示,电极直径较小的DBD反应器在SED增加期间的放电电压变化较小。这表明电极直径较小的DBD反应器需要消耗更多SED,以提高放电电压和E/N。此外,较强的电子雪崩效应会导致DBD反应器的热损失较大,这也增加了能量消耗。虽然电子雪崩作用会使得内电极直径小的反应器能耗增加,但也提高了放电电压和折合场强。然而,无论如何提高外加电压,内电极直径为14 mm和16 mm时的CH4脱除效率均低于内电极直径为18 mm时的CH4脱除效率,且NOx生成量也持续高于内电极直径为18 mm的反应器。因此,增大DBD反应器内电极直径可降低能量消耗,提高能量效率。
-
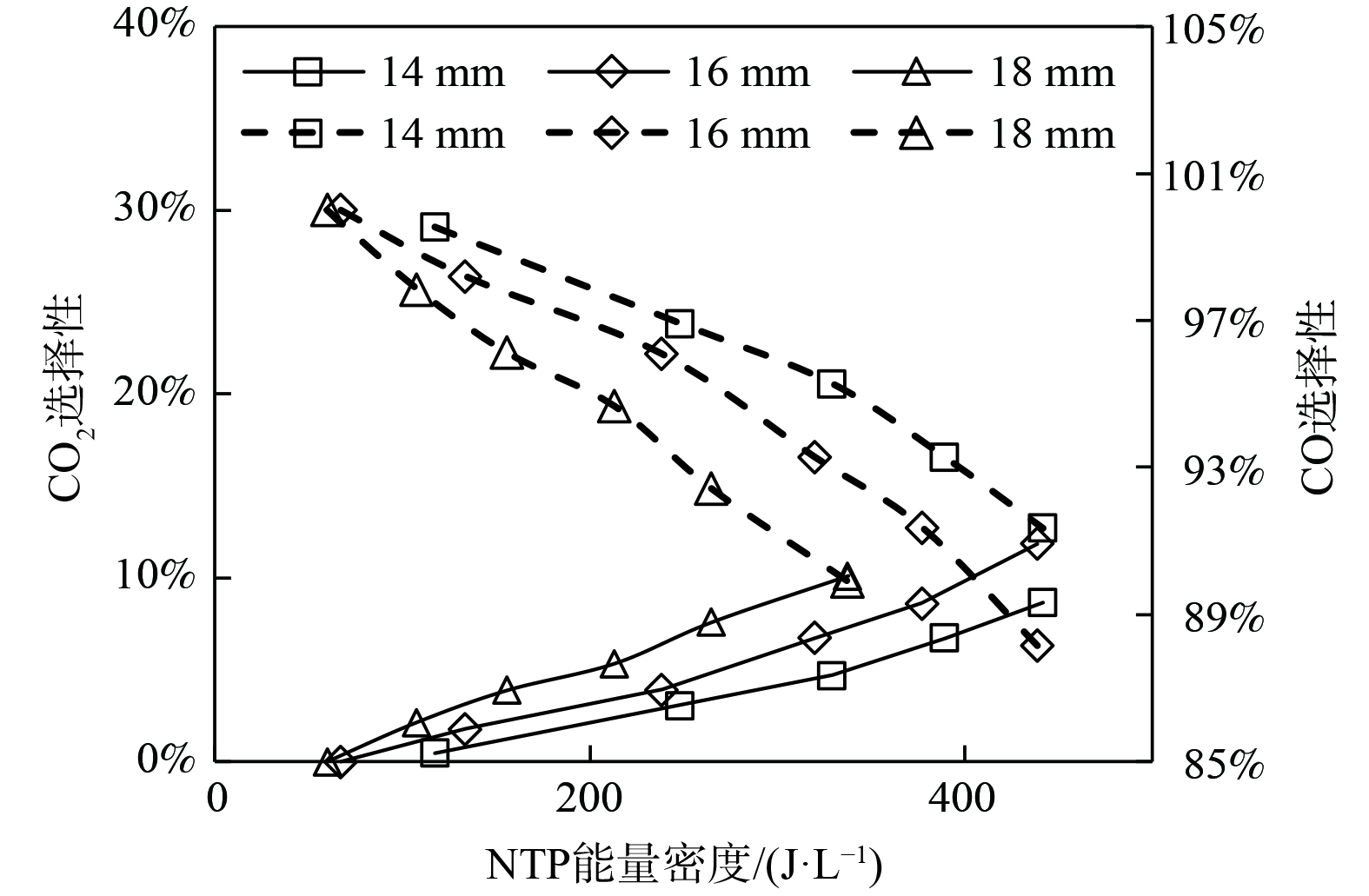
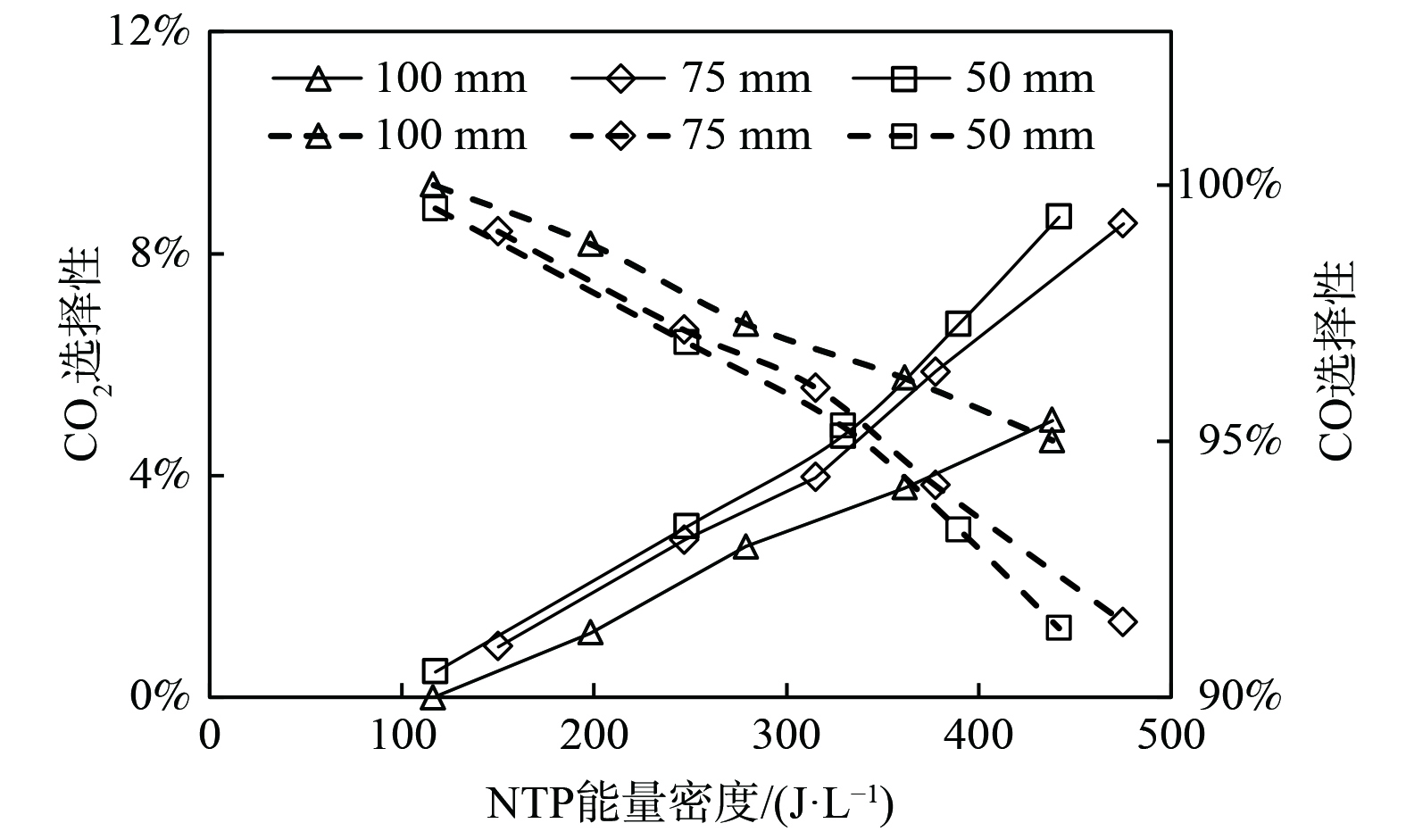
图7为NTP作用下内电极直径对CO2选择性的影响。随着能量密度的升高,CO2选择性呈线性增大,且内电极直径越大,CO2选择性越大。等离子体催化反应过程中,很少有碳的还原反应发生,几乎全部的CH4都被氧化为CO、CO2 [11]。增大能量密度,会使得反应器放电间隙内的折合场强增大,从而增加间隙内的氧化性活性粒子O(1D)、OH、HO2等,使CH4部分氧化反应产物CO及中间产物 CH2、CH更多地转化为 CO2 (式 (32)~(39) ) 。同时,增大内电极直径也使得折合场强和平均电子能量出现增大,从而促进CO等粒子的氧化。
-
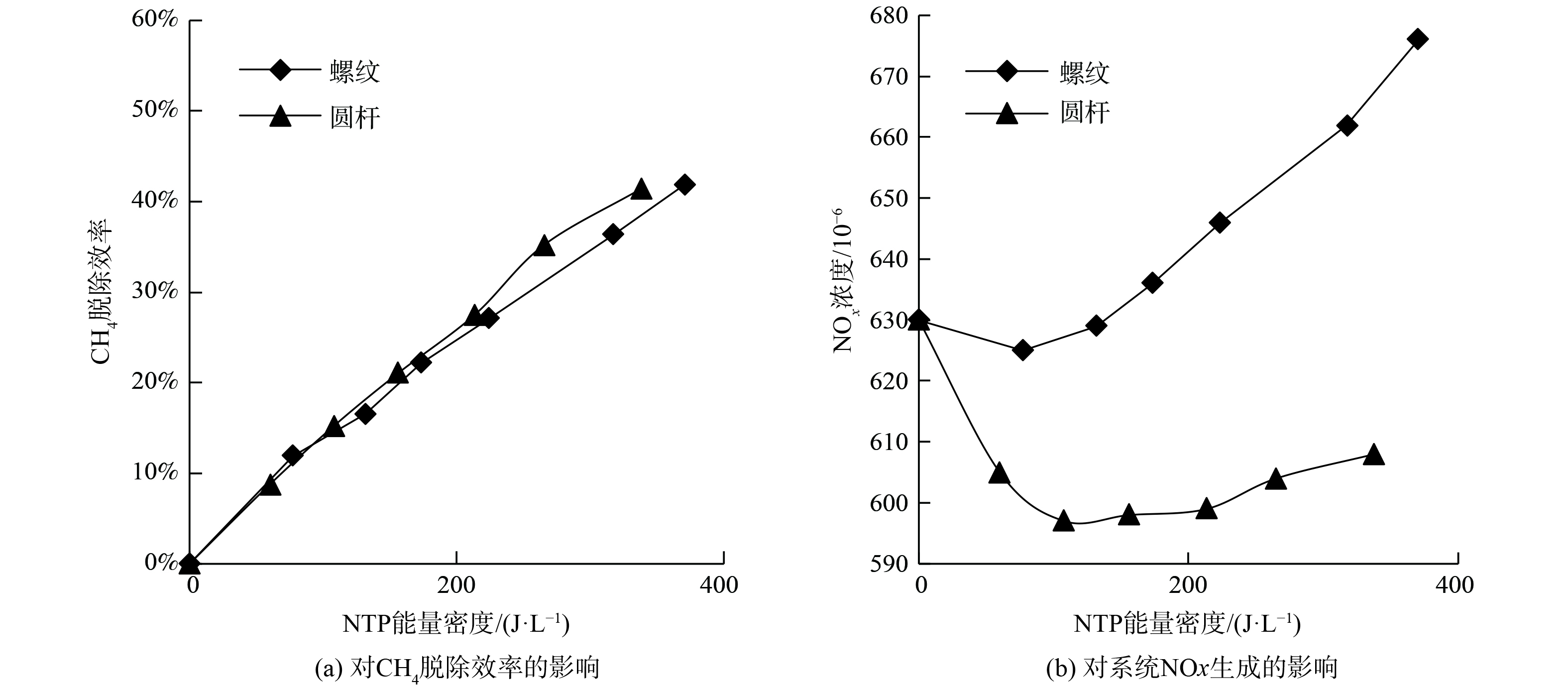
图8为NTP作用下内电极形状对CH4脱除效率和NOx生成的影响。随着能量密度从0升至500 J·L−1,相比于螺纹电极,使用圆杆电极时系统CH4的脱除效率更高,NOx生成更少。
为分析其机理,采用COMSOL模拟了不同内电极形状的DBD反应器气隙中电场强度E的分布 (内电极直径18 mm,放电间隙1 mm) 。所施加的电压为5 kV,刚玉陶瓷相对介电常数为9.8,气体的相对介电常数为1。图9表明,螺杆顶部附近的几何形状尖锐,容易使电荷堆积,电场强度 (E) 远高于杆电极的表面,且E/N在放电间隙内分布不均匀,促进了N自由基的生成[42],导致了NOx的生成。此外,过高E/N使·O自由基的生成效率降低,亦降低了CH4的氧化效率。因此,在富氧条件下,螺纹内电极DBD反应器的CH4脱除效率更低,发生副反应生成的NOx更多。
-
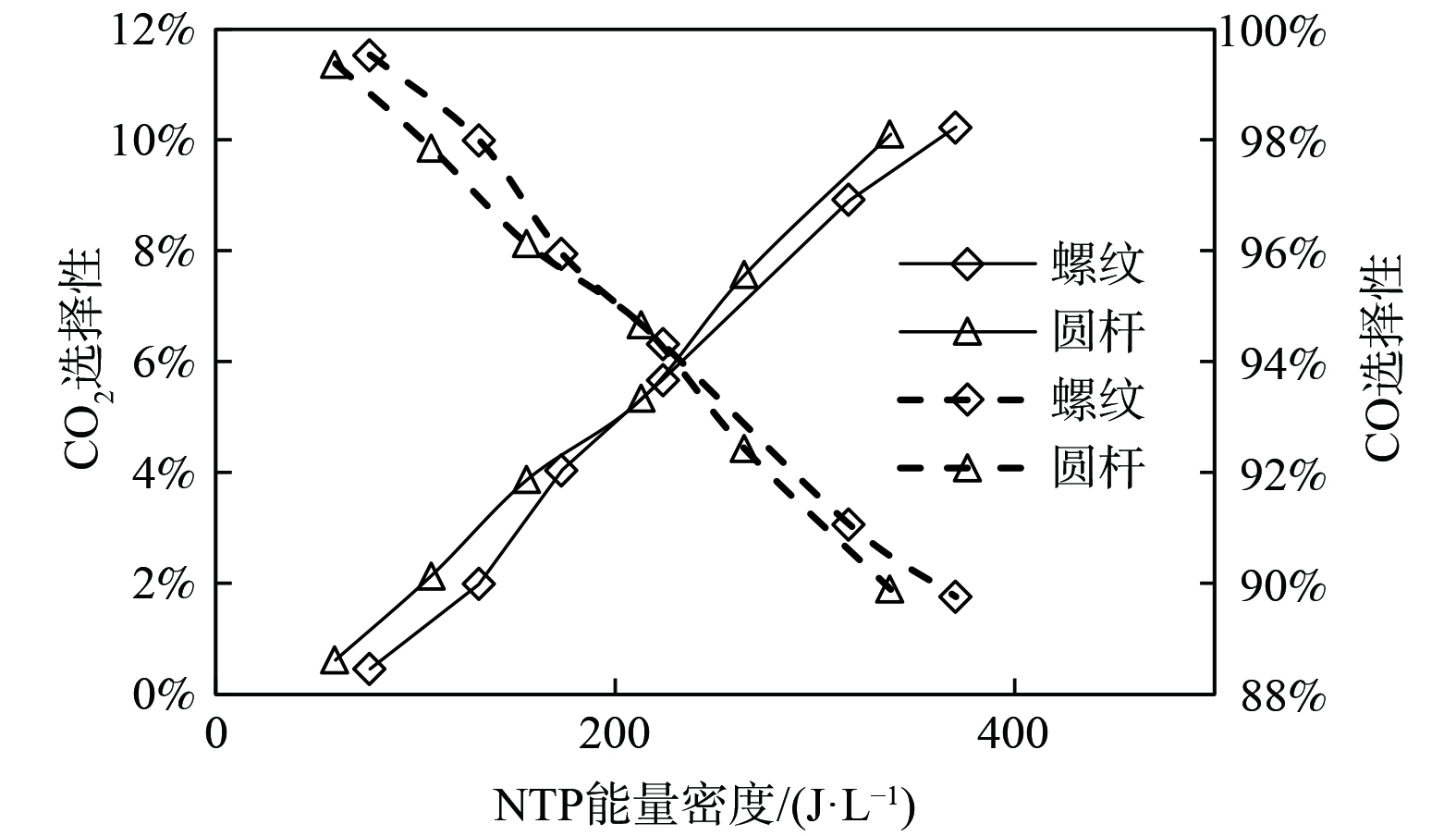
图10为NTP作用下内电极形状对CO2选择性的影响。内电极形状对CO2选择性的影响较小,相对于螺纹电极,使用圆杆电极的反应器CO2选择性高、CO选择性低。如2.2.1所述,螺纹电极间隙内场强分布不均匀,螺牙附近的折合场强过高,使得·O自由基的生成速率降低,从而抑制了CO2的生成。且螺纹的存在部分增大了放电间隙,降低了有效范围内的折合场强,亦减少了氧化性粒子的生成。
-
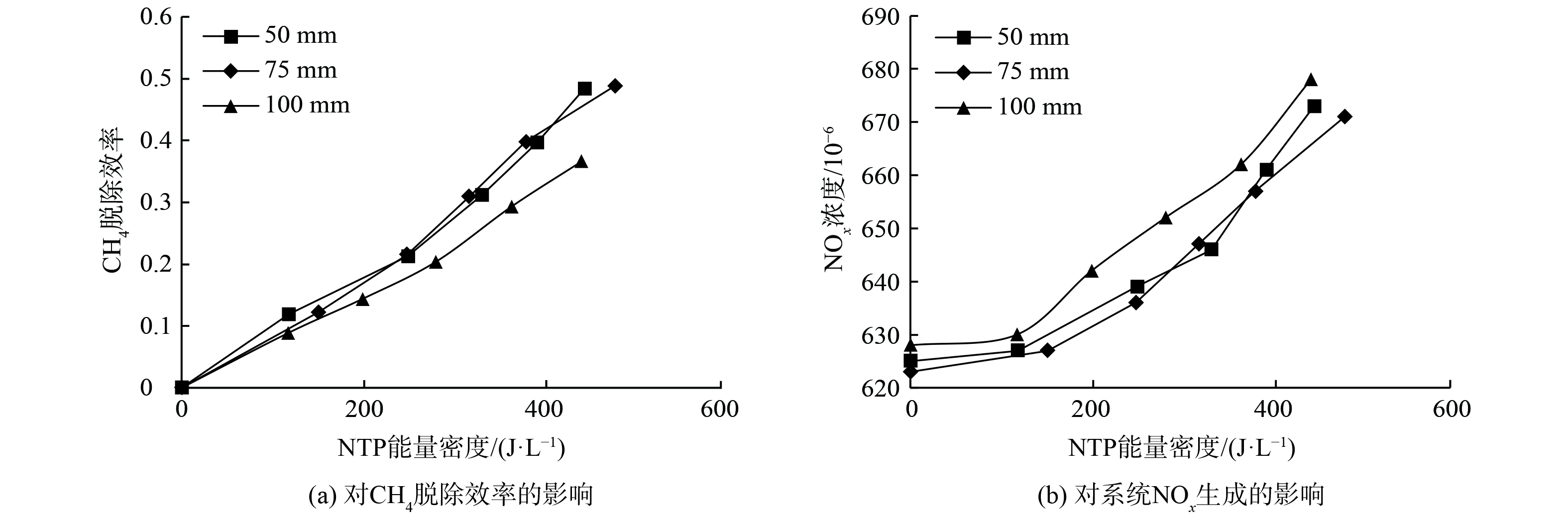
图11为NTP作用下电极长度对CH4脱除效率和NOx生成的影响。随着能量密度从0升至500 J·L−1,相比于长度为50 mm和75 mm的电极,使用100 mm电极时系统CH4的脱除效率更低,生成更多NOx。这与课题组关于电极长度对NO氧化脱除效率影响的研究结果在本质上是相似的。
电极长度为50 mm或75 mm时CH4脱除效率和NOx生成无明显差异。为解释电极长度过长 (100 mm) 导致CH4脱除效率降低、NOx增加的原因,图12展示了电极长度对DBD放电电压峰峰值的影响。对于不同电极长度的DBD反应器,放电电压随着能量密度的增加而增大,电极长度越长,电压增长速率越小。同时,在相同能量密度下,100 mm长度电极反应器的放电电压峰峰值明显小于75和50 mm长度电极反应器。这表明当能量密度相同时,100 mm电极长度反应器可获得的折合场强小,电子平均能量低。如图5所示,折合场强的降低使得CH4的分解反应 (R1~R2) 速率降低,同时氧化性活性粒子OH生成 (R7) 速率降低,使CH4的氧化效率减小。但是折合场强的减小反而增大了N2 (X,v) 生成速率 (R8) ,促进了副反应 (式 (14) ) 的发生,从而增大了NOx体积分数。另外,虽然电极长度增加导致放电区间体积增大,气体停留时间增长。但在富氧条件下,DBD放电区间内,氧化性自由基和气体振动激发态体积分数在几个放电周期内便达到平衡,此后随时间变化基本稳定[43],故增大停留时间对脱除效率的影响并不明显。因此,电极长度过长会导致CH4脱除效率降低、NOx增加,使反应器能耗增加。
-
图13为NTP作用下电极长度对CO2选择性的影响。随着能量密度从0升至500 J·L−1,电极长度为50 mm或75 mm时CO2和CO选择性无明显差异,使用100 mm电极时系统CO2的选择性更低。如2.3.1所述,在相同能量密度时,100 mm电极长度反应器放电间隙内的折合场强小,电子平均能量低,氧化性活性粒子少。因此,电极长度过长也会降低CO2选择性。
-
1) 增大内电极直径使得DBD反应器的气隙减小,从而在相同电源电压下提高了气隙的折合场强E/N,增加了平均电子能量,因此更容易产生O和OH自由基促进CH4氧化为CO2。同时,增大电极直径使得气隙中E/N变化减小,抑制了N2(X, v)和N自由基的生成,且抑制了副反应的发生。2) 螺纹电极的螺牙顶部附近的电场强度远高于圆杆电极的表面,促进了N自由基的生成,N和O自由基等反应会生成更多的NOx。3) 电极长度过长使可以获得的折合场强小,电子平均能量低,不利于氧化性活性粒子生成,却促进了N2(X,v) 的生成,导致CH4脱除效率降低、NOx增加。使用圆柱型内电极、增大内电极直径、避免使用过长电极,可提高CH4脱除效率和CO2选择性、减少NOx生成。
DBD结构对船用LNG发动机尾气CH4氧化脱除的影响
Effect of DBD structure on oxidative removal of CH4 from marine LNG engine exhaust
-
摘要: 为提高等离子体氧化CH4的脱除效率,同时避免放电过程中副产物NOx的生成,在模拟天然气 (LNG) 发动机排气中,研究了介质阻挡放电 (DBD) 反应器电极结构参数对CH4脱除效率、CO2选择性及NOx生成的影响及其机理。结果表明:增大电极直径使得气隙中折合场强E/N提高,增加了·O和·OH自由基的生成,促进了CH4的氧化,提高了CO2选择性;内电极直径的增大使E/N在气隙中分布更加集中,抑制了N2(X, v)和N自由基的生成,减少了副反应的发生。与圆杆电极相比,螺纹电极的螺牙顶部附近具有过高的电场强度,从而促进N自由基的生成并抑制·O自由基的产生,故圆杆电极比螺纹电极具有更高的CH4脱除效率及更低的NOx生成。电极长度过长降低了折合场强以及电子平均能量,不利于氧化性活性粒子生成,导致CH4脱除效率和CO2选择性降低,同时增加了N2(X,v) ,也使得NOx增加。本研究可为提高低温等离子体协同催化剂促进甲烷还原NOx系统的脱除效率,降低DBD反应器能耗提高能源利用率提供参考。Abstract: In order to improve the removal efficiency of CH4 by plasma and avoid the generation of the by-product—NOx, the influence of electrode structure parameters in the dielectric barrier discharge (DBD) reactor on CH4 removal efficiency and NOx generation, and its mechanism were studied under simulated liquified natural gas (LNG) engine exhaust. The results showed that the reduced field strength (E/N) in the air gap increased with the increase of inner electrode diameter. And the increase of E/N increased the formation of O and OH radicals, promoted the oxidation of CH4, and improved the CO2 selectivity; At the same time, the increase of the inner electrode diameter made the distribution of E/N more concentrated in the air gap, inhibited the formation of N2 (X, v) and N radicals, and reduced the occurrence of side reactions. Compared with the round rod electrode, the screw electrode had a higher electric field strength near the top of the thread, which promoted the generation of N free radicals and inhibited the generation of O free radicals. Therefore, the round rod electrode had a higher CH4 removal efficiency and lower NOx generation than the screw electrode. Excessive electrode length reduced the converted field strength and the average electron energy, which was not conducive to the generation of active particles, leading to the reduction of CH4 removal efficiency, the reduction of CO2 selectivity and the increase of NOx.
-
Key words:
- plasma /
- DBD /
- structure parameter /
- CH4 oxidative removal /
- NOx generation
-

-
-
[1] ÆSØY V, MAGNE E P, STENERSEN D, et al. LNG-fuelled engines and fuel systems for medium-speed engines in maritime applications[C]//SAE Technical Paper 2011-01-1998, 2011 [2] SCHINAS O B, BUTLER M A. Feasibility and commercial considerations of LNG-fueled ships[J]. Ocean Engineering, 2016, 122: 84-96. doi: 10.1016/j.oceaneng.2016.04.031 [3] INGEMAR N, MARCEL O. Development of a dual fuel technology for slow-speed engines[C]//第27届国际内燃机学会(CIMAC)大会论文集, 2013, 1-12 [4] LINDSTAD E, ESKELAND G S, RIALLAND A, et al. Decarbonizing maritime transport: The importance of engine technology and regulations for LNG to serve as a transition fuel[J]. Sustainability, 2020, 12(21): 1-19. [5] IPCC. Climate change 2014: Synthesis report [R/OL]. [2021-01-10].https://www.cma.gov.cn/2011xzt/2014zt/20141103/2014050703/201411/P020141113358178198444.pdf [6] 环境保护部会同质检总局, 船舶发动机排气污染物排放限值及测量方法(中国第一、二阶段): GB15097-2016 [S]. 北京. 中华人民共和国生态环境部, 2018 [7] SHI Y, PU J L, GAO L W, et al. Selective catalytic reduction of NOx with NH3 and CH4 over zeolite supported indium-cerium bimetallic catalysts for lean-burn natural gas engines[J]. Chemical Engineering Journal, 2020, 403(126394): 1-17. [8] ALVA E, PACHECO M, COLÍN A, et al. Nitrogen oxides and methane treatment by non-thermal plasma[J]. Journal of Physics Conference, 2015, 591(012052): 1-7. [9] HUU T P, GIL S, COSTA P D, et al. Plasma-catalytic hybrid reactor: Application to methane removal [J]. Catalysis Today, 2015: 86-92 [10] PAN H, GUO Y H, JIAN Y F, et al. Synergistic effect of non-thermal plasma on NOx reduction by CH4 over an In/H-BEA catalyst at low temperatures[J]. Energy Fuels, 2015, 29(8): 5282-5289. doi: 10.1021/acs.energyfuels.5b00864 [11] SHREKA M. 低温等离子体催化系统降低船用低压燃气发动机逃逸甲烷的研究[D]. 哈尔滨: 哈尔滨工程大学, 2019. [12] GHOLAMI R, STERE C, CHANSAI S, et al. Optimization of non-thermal plasma-assisted catalytic oxidation for methane emissions abatement as an exhaust aftertreatment technology[J]. Plasma Chemistry and Plasma Processing, 2022, 42(4): 709-730. doi: 10.1007/s11090-022-10253-3 [13] WEI L, PENG B, LI M, et al. Dynamic characteristics of positive pulsed dielectric barrier discharge for ozone generation in air[J]. Plasma Sci. Technol., 2016, 18(2): 147-156. doi: 10.1088/1009-0630/18/2/09 [14] 董冰岩, 李贞栋, 宿雅威, 等. 高压脉冲介质阻挡放电协同金属有机骨架材料催化剂去除氮氧化物的实验研究[J]. 电工技术学报, 2021, 36(13): 2740-2748. doi: 10.19595/j.cnki.1000-6753.tces.200891 [15] 王晓玲, 高远, 张帅, 等. 脉冲参数对介质阻挡放电等离子体CH4干重整特性影响的实验[J]. 电工技术学报, 2019, 34(6): 1329-1337. [16] INNES W. Effect of nitrogen-oxide emissions on ozone levels in metropolitan regions[J]. Environmental Science & Technology, 1981, 15(8): 904-912. [17] JEDRYCHOWSKI W, MAUGERI U, FLAK E. The effect of prolonged occupational exposure to the low concentrations of nitrogen oxides in combination with ammonia on chronic bronchitis and the lung function[J]. Giornale Italiano Di Medicina Del Lavoro, 1987, 9(3/4): 147-151. [18] XIE H W, ZHANG Y. The research status of acid rain[J]. Advanced Materials Research, 2013, 726-731: 4033-4036. doi: 10.4028/www.scientific.net/AMR.726-731.4033 [19] HUEBERT B. Computer modelling of photochemical smog formation [J]. 1974, 10(51): 644-645 [20] 王伟彬, 曹昌魁. 船舶减排满足IMO Tier Ⅲ 法规的探讨与实践[J]. 中国航海, 2017, 40(2): 108-111. doi: 10.3969/j.issn.1000-4653.2017.02.023 [21] 赵如金, 储金宇, 王瑞静, 等. 粉煤灰小球协同低温等离子体处理汽车尾气[J]. 高电压技术, 2008, 34(3): 517-520. doi: 10.13336/j.1003-6520.hve.2008.03.003 [22] 刘彤, 于琴琴, 王卉, 等. 等离子体与催化剂协同催化CH4选择性还原脱硝反应[J]. 催化学报, 2011, 32(9): 1502-1507. [23] 刘飞. 低温等离子体氧化NO的实验研究[D]. 武汉: 武汉理工大学, 2018 [24] CAI Y K, LU L, LI P. Study on the effect of structure parameters on NO oxidation in DBD reactor under oxygen-enriched condition[J]. Applied Sciences, 2020, 10(19): 2-16. [25] 米彦, 万佳仑, 卞昌浩, 等. 基于磁脉冲压缩的DBD高频双极性纳秒脉冲发生器的设计及其放电特性[J]. 电工技术学报, 2017, 32(24): 244-256. doi: 10.19595/j.cnki.1000-6753.tces.160818 [26] DENYSENKO I, YU M Y, XU S. Effect of plasma nonuniformity on electron energy distribution in a dusty plasma [J]. J. Phys. D: Appl. Phys. 2005, 38(3): 403-408 [27] CAI Y K, LV L, LU X P. The Effects of inner electrode diameter on the performance of dielectric barrier discharge reactor for desulfurization and denitrification[J]. IEEE Transactions on Plasma Science, 2021, 49(2): 786-793. doi: 10.1109/TPS.2021.3049126 [28] FRIDMAN A. Plasma Chemistry[J]. Plasma Chemistry, 2008, 29(1): 355-362. [29] PENETRANTE B M, HSIAO M C, MERRITT B T, et al. Pulsed corona and dielectric-barrier discharge processing of NO in N2[J]. Appl. Phys. Lett. 1996, 68, 3719-3721 [30] YOSHIDA K, GOTO S G, TAGASHIRA H, et al. Electron transport properties and collision cross sections in CF[J]. Journal of Applied Physics. 2002, 91(5), 2637-2647 [31] Community database, Available online: www. lxcat. net, retrieved on October 25, 2022.https://nl.lxcat.net/home/ [32] TRINITI database, Available online: www. lxcat. net, retrieved on June 26, 2020.https://nl.lxcat.net/home/ [33] Itikawa database, Available online: www. lxcat. net, retrieved on June 26, 2020.https://nl.lxcat.net/home/ [34] ZHAO G B, GARIKIPATI S, HU X, ARGYLE M, et al. The effect of oxygen on nonthermal-plasma reactions of dilute nitrogen oxide mistures in N2[J]. AIChE Journal. 2005, 51(6), 1813-1821 [35] KOSSYI I, KOSTINSKY A, MATVEYEV A, et al. Kinetic scheme of the non-equilibrium discharge in nitrogen-oxygen mixtures[J]. Plasma Sources Sci. Technol. 1992, 1(3), 207-220 [36] GUERRA V, SÁ P, LOUREIRO J. Role played by the N2 (A3Σu+) metastable in stationary N2 and N2-O2 discharges[J]. Journal of Physics D Applied Physics, 2001, 34(12): 1745-1755. doi: 10.1088/0022-3727/34/12/301 [37] PENG B, JIANG N, YAO X, et al. Experimental and numerical studies of primary and secondary streamers in a pulsed surface dielectric barrier discharge[J]. Journal of Physics D:Applied Physics, 2019, 52(32): 1-35. [38] 吴淑群, 董熙, 裴学凯, 等. 基于激光诱导荧光法诊断大气压低温等离子体射流中OH自由基和O原子的时空分布[J]. 电工技术学报, 2017, 32(8): 82-94. doi: 10.19595/j.cnki.1000-6753.tces.2017.08.009 [39] LATHAM R V. High Voltage Vacuum Insulation[M]. Academic Press, 1981 [40] NEMMICH S, TILMATINE A, DEY Z, HAMMADI N, et al. Optimal Sizing of a DBD Ozone Generator Using Response Surface Modeling[J]. Ozone: Sci. Eng. 2015, 37(1), 3-8 [41] NUR M. Plasma Technology Research and Its Applications: developing in the Faculty of Science and Mathematics Diponegoro University[C]// International Seminar on New Paradigm and Innovation on Natural Sciences and Its Application. 2013, 3 [42] SUN B, WANG T, YANG B, et al. Effect of Electrode Configuration on NO Removal in a Coaxial Dielectric Barrier Discharge Reactor[J]. Journal of Chemical Engineering of Japan. 2013, 46(11), 746-750 [43] WANG L N, ZHONG W L, ZHU A M, et al. Numerical simulation of OH and HO2 radicals in dielectric barrier discharge plasmas[J]. Acta Physico-Chimica Sinica, 2008, 24(8): 1400-1404. doi: 10.3866/PKU.WHXB20080813 -



 下载:
下载: