-
近年来,锂电池 (LIBs) 因其高能量密度、高电压和良好的循环性能等优点,在交通运输和大规模能源存储行业广泛应用。据估计,LIBs的产量将以59%的增长速率从2012年的1.07×104 t增加到2025年的4.64×105 t[1],这导致大量的废旧LIBs亟待处理处置。然而这些废旧LIBs含有高值Li (5%~7%) 、Ni (5%~10%) 、Mn (5%~11%) 、Co (5%~25%) 等[2],其金属含量比自然界矿石中金属含量还高,可以作为二次能源物质加以利用。同时,随着LIBs需求量不断增加,可利用金属资源越来越稀缺[3],LIBs原材料价格不断上涨,而与开采和提炼原材料金属相比,回收废旧LIBs中的金属可以节省大量的能源。此外,废旧LIBs若不被有效处理,其含有的重金属可能会对环境产生不利影响[4]。因此,从废旧LIBs中回收有价金属不仅是保护环境的迫切需求,还是缓解资源短缺、节约生产成本的有效途径。
目前,从废旧LIBs中浸出有价金属是金属回收的一个重要环节,其浸出方法包括化学浸出和生物浸出[5]。化学浸出一般利用强酸或碱将固体废物中金属离子浸出,但该方法消耗大量的化学试剂且需要热量促进反应进行[6]。与化学浸出相比,生物浸出法因能量投入低、成本低廉、操作简单、浸出过程绿色环保等潜在优势而受到越来越多的关注[7-8];而且,微生物可以不断产生H2SO4和细胞的接触机制使浸出具有更好性能[9]。生物浸出废旧LIBs的方法可分为一步生物浸出法及两步生物浸出法。在一步生物浸出方法中,微生物与废旧LIBs一起接种在微生物培养基中,其中微生物培养和生长以及金属浸出同时发生[10]。这种浸出方式较为简单,但由于LIBs中存在的金属离子对微生物生长和活性产生不利影响,生物浸出效率可能会受到限制[11],Co、Li的浸出率均分别低于60%和80%[12-14]。为了解决上述问题,有学者开始采用两步生物浸出法浸出废旧LIBs,即微生物在培养基中生长达到活性较强、生长速度较快的对数增长期,然后添加废旧LIBs开始浸出,以抵御金属离子对微生物生长的不利影响,避免微生物的延滞期延长[15-17],有效地减少废旧LIBs对微生物的毒性作用,Co、Li的浸出率可以分别达到70%及90%以上[8, 18-19]。因此,运用两步生物浸出废旧LIBs具有一定的优势。然而,对于两步生物浸出,浸出微生物活性较强、生长速度较快的对数增长期会维持一定的时间,可分为对数增长期的前、中、后期,而不同时期的微生物活性有一定差异,但在微生物对数增长期的不同时期加入废旧LIBs对金属浸出率的影响鲜少报道。因此,选择微生物活性最强、生长速度最快的时期投加废旧LIBs以抵御加入固体废弃物对微生物生长的不良影响,也许可以简单有效地提高金属浸出率,优化两步生物浸出。
此外,选择活性高的浸出微生物是进一步提高金属浸出率的重要因素。目前,已发现40余种微生物可用于生物浸出[20],而在生物浸出废旧LIBs的研究中应用最广泛的是氧化亚铁硫杆菌(Acidithiobacillus ferrooxidans)、氧化亚铁钩端螺旋菌(Leptospirillum ferrooxidans)和氧化硫硫杆菌 (Acidithiobacillu thiooxidans)[19],这些细菌具有嗜酸、以CO2为碳源,利用铁/硫作为能源物质的特点。此外,也有利用真菌浸出废旧LIBs[10],但利用真菌进行浸出LIBs所需时间比嗜酸菌长且需要外加碳源,能耗较高[7]。因此,选择嗜酸菌浸出LIBs更有利。同时,XIN等[9]研究发现,具有铁硫氧化能力的生物浸出体系有较高的浸出效率。因此,本研究选取具有铁硫氧化能力且应用较广泛的A. ferrooxidans作为浸出微生物。
本研究采用两步生物浸出法对废旧LIBs进行浸出,并探究在A. ferrooxidans对数增长期的前、中和后期向浸出体系中投加废旧LIBs对金属浸出率的影响和作用机制,以期通过优化两步生物浸出,获得更高的金属浸出率,为生物浸出废旧LIBs的工业化提供参考。
-
浸出原料。本实验使用的废旧LIBs由广东深圳某废旧电池回收厂提供,该废旧LIBs已经过放电及初步破碎处理。
实验菌种。本实验选用A. ferrooxidans DX作为浸出微生物,该菌由中南大学教育部生物冶金实验室提供。
实验药品。硫酸 (H2SO4) 、硫酸铵 ((NH4)2SO4) 、氯化钾 (KCl) 、磷酸氢二钾 (K2HPO4) 、硝酸钙 (Ca(NO3)2) 、硫粉 (S0) 、七水合硫酸亚铁 (FeSO4·7H2O) 。所有化学试剂均为分析纯,实验用水为超纯水。
实验仪器。电热恒温鼓风干燥箱 (DHG-9240A,上海一恒科学仪器有限公司) ;恒温振荡器 (HZQ-X300C,上海一恒科学仪器有限公司) ;真空干燥箱 (DZF-6050,上海一恒科学仪器有限公司) ;荧光相差显微镜 (CX43,日本奥林巴斯公司) ;数字pH计 (STARTER 2100,美国奥豪斯仪器有限公司) ;紫外可见分光光度计 (UV-2100,北京瑞利分析仪器有限公司) ;原子吸收分光光度计 (AAS,AA-6880,日本岛津公司) ;扫描电子显微镜-能量色散x射线光谱 (SEM-EDS,MIRA LMS,捷克泰思肯电镜公司) ;X射线衍射 (XRD,Smartlab 9KW,日本理学公司) ;X射线电子能谱 (XPS,Scientific K-Alpha,美国赛默飞世尔科技有限公司) 。
-
1) 材料的预处理及表征。将LIBs碎片过200目筛,获得LIBs粉末。然后用蒸馏水洗涤[21],以去除黏附的电解质和粘合剂。随后在电热恒温鼓风干燥箱中烘干[22],并对样品的形貌、物相和元素组成进行分析。 同时,采用王水微波消解LIBs粉末,并用AAS测定目标金属质量浓度。
2) 浸出微生物的培养。在250 mL锥形烧瓶中,将10 mL初始浓度为108 cells·mL−1 的A. ferrooxidans菌液添加到90 mL的无铁9k培养基[23],培养基初始pH为1.85,并加入44.7 g·L−1 FeSO4·7H2O和5 g·L−1 S0[8, 24]作为能源物质。最后,将锥形瓶置于30 ℃和170 r·min−1的恒温振荡器中培养,定期测定pH、细菌数、Fe2+和Fe3+质量浓度以观察浸出菌生长状况,以确定A. ferrooxidans对数增长期的前、中和后期。
3) 生物浸出实验。在上述的培养条件下,对A. ferrooxidans进行培养,当培养至对数增长期的前、中、后期,分别向体系加入10 g·L−1废旧LIBs开始浸出,从而构建了A、B和C浸出体系,以探究废旧LIBs在对数期的投加时间对金属浸出的影响。浸出过程中,测定pH、细菌数、Fe2+和Fe3+质量浓度及目标金属质量浓度,以上实验重复不少于2次。浸出结束后,从浸出体系中离心收集固体物质,并用蒸馏水洗涤[21],放入40 ℃真空干燥箱干燥24 h获得浸出渣。通过分析浸出前后废旧LIBs的表面形貌、物相及元素价态变化,揭示A. ferrooxidans浸出废旧LIBs的机理。
-
采用荧光相差显微镜通过血细胞计数法测定细菌数;利用数字pH计测定pH;运用紫外分光光度计采用5-磺基水杨酸法[25]和邻菲罗啉法[26]分别测定浸出液中Fe3+和Fe2+质量浓度;采用AAS测定目标金属质量浓度;采用SEM-EDS、XRD和XPS分别分析浸出前后材料的形貌与元素分布、物相和价态变化。
-
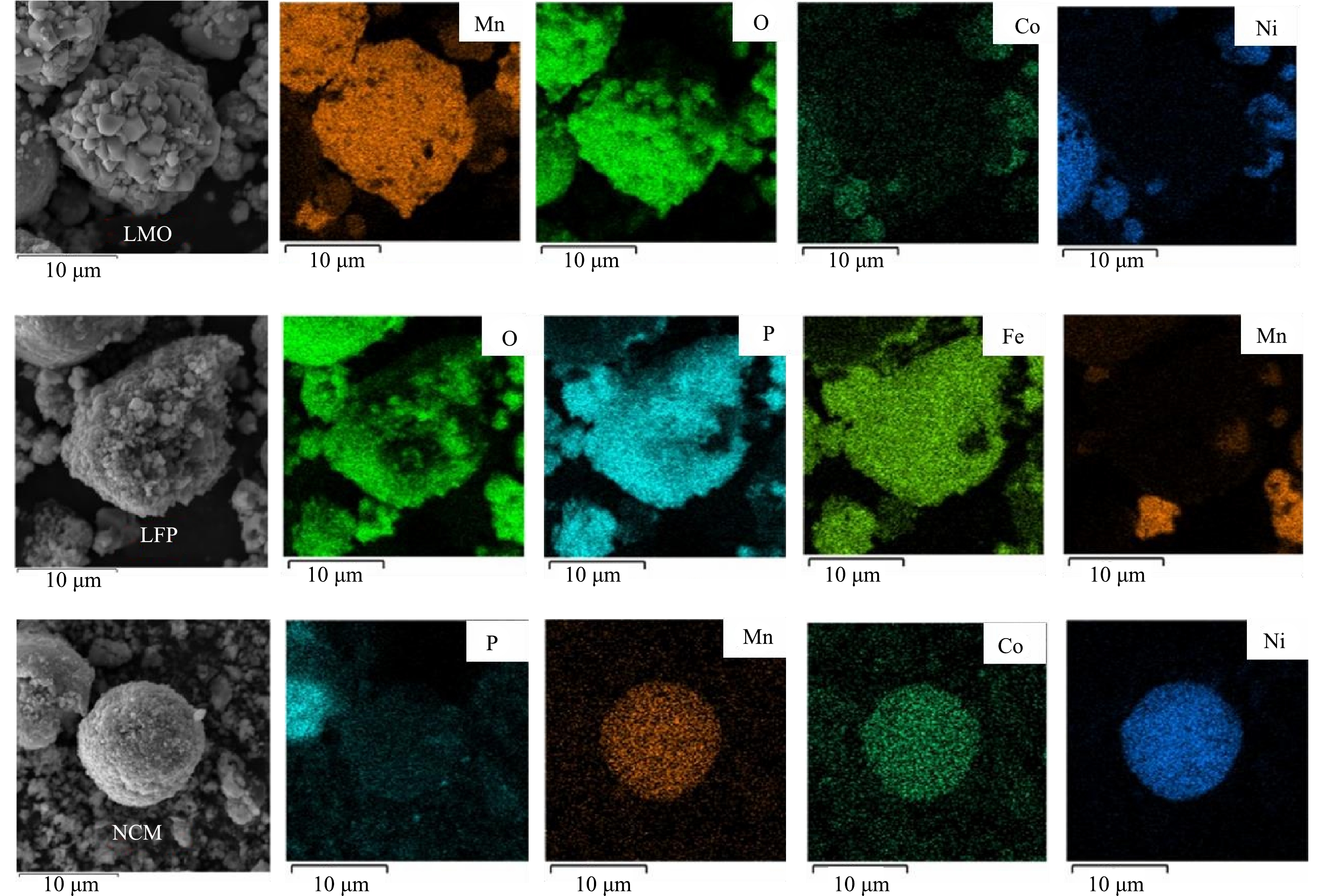
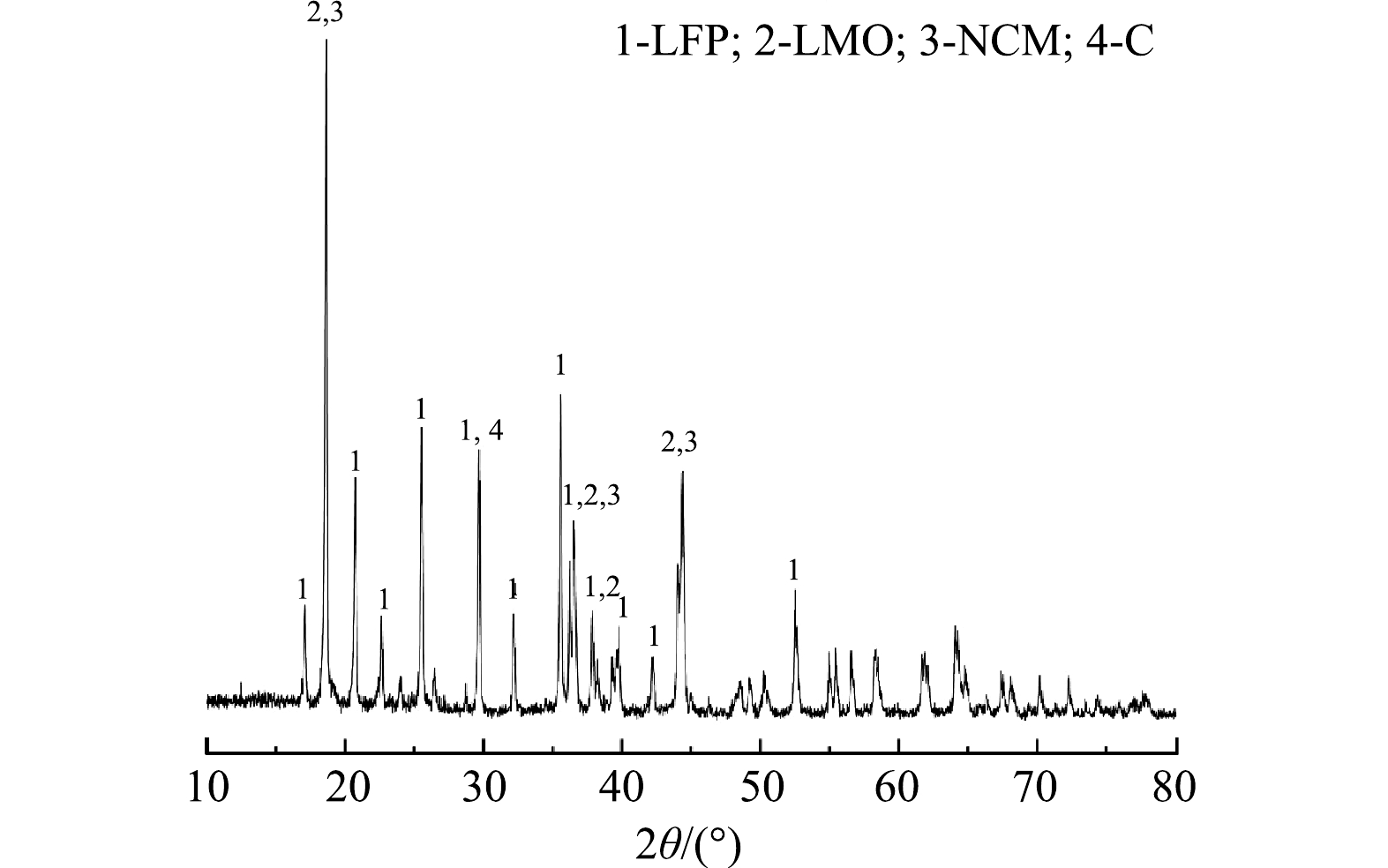
1) 原料分析。利用XRD对浸出材料的物相组成进行分析,如图1所示。结果表明,废旧LIBs含有LiNixCoyMn1–x–yO2 (NCM) 、LiFePO4 (LFP) 、LiMn2O4 (LMO) 多种阴极材料以及阳极材料石墨 (C) 。同时,利用SEM-EDS对原料的形貌及元素分布 (图2) 进一步分析,可以观察到,原料中有多种形貌:尖晶石型的LMO[27]、橄榄石型的LFP[28]和球状的NCM[29]。因此可了解到,该材料由C、LMO、LFP、NCM等组成。因此,本研究以Li、Ni、Co和Mn作为浸出实验的目标金属。化学分析结果表明,废旧LIBs中含有2.95% Co、8.53% Mn、10.59% Ni、3.66% Li以及17.04% Fe,证实该种废旧LIBs具有较高的回收价值。
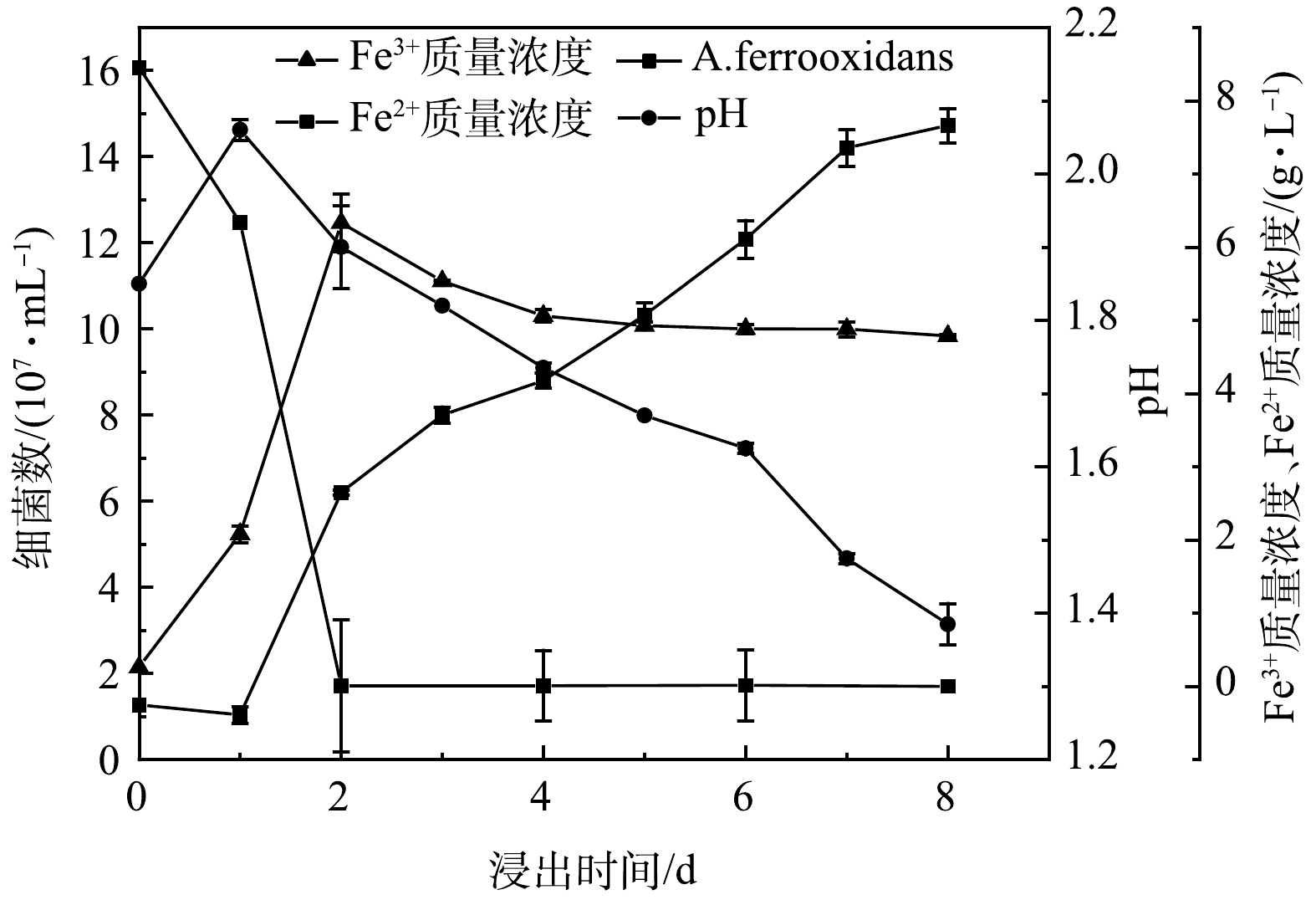
2) A. ferrooxidans的生长特性。为了了解微生物在浸出过程中发挥的作用及更好地选择投加废旧LIBs的时间,分析了浸出菌的生长特性,结果如图3所示。A. ferrooxidans将Fe2+氧化成Fe3+过程中可获得生长所需的能源能量,导致Fe2+和H+消耗 (式(1)) 。因而在0~1 d,pH呈上升趋势,而Fe2+质量浓度不断降低[30]。随着A. ferrooxidans利用S0产生生物硫酸 (式(2)) 及氧化形成的Fe3+不断水解、与9k培养基中的K2HPO4及(NH4)2SO4生成K+/NH4+Fe3(SO4)2(OH)6沉淀,促进H+的产生 (式(3)、式(4)) [31],pH开始下降。同时,Fe3+也呈先增加后减少趋势。而从图3可知,细菌数量呈现“S”型变化趋势。因此,2~6 d为A. ferrooxidans的对数增长期,并分别以2、4和6 d作为对数增长期的前、中、后期。然而,目前废旧LIBs浸出多数在对数期加入到浸出体系,但究竟在对数期哪个阶段投加LIBs获得更好的浸出效率,需要进一步的探究。
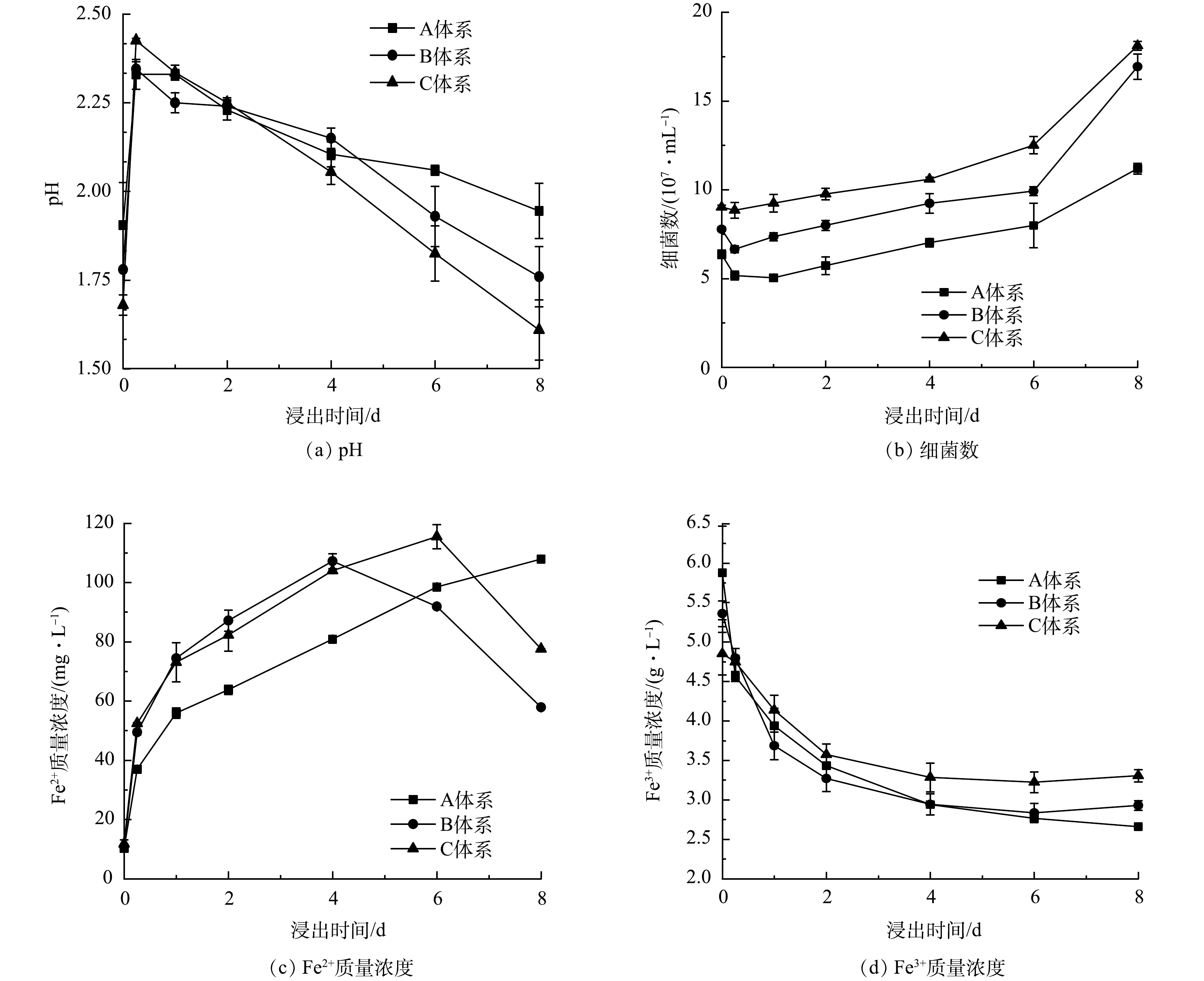
3) 浸出废旧LIBs的过程分析。在对数增长期不同时期投加废旧LIBs后,浸出过程中可能发生的反应由式(1)~式(7)表示,浸出体系中pH、细菌数、Fe3+以及Fe2+质量浓度的变化如图4所示 (以加入废旧LIBs作为浸出实验的0点,下同) 。当废旧LIBs投加到浸出体系后,pH在0~0.25 d急剧增加 (图4(a)) 。这可能是因为LIBs的碱性[8]及金属的溶出会导致H+消耗。但pH在1~8 d不断下降。这是因为, A. ferrooxidans能通过氧化S0促进生物酸产生 (式(2)) 。同时, Fe3+会发生水解、或形成K+/NH4+Fe3(SO4)2(OH)6促进H+释放 (式(3)、式(4)) ,进一步加速pH下降。对于不同时期投加废旧LIBs,体系中pH的变化趋势不同。在2~8 d,C体系中保持最低pH (2.25~1.61) ,明显低于A体系 (2.23~1.95) 和B体系 (2.23~1.7) 。因此,采用两步法浸出废旧LIBs时,在对数期后期加入废旧LIBs,浸出体系可以产生更多的生物酸。
废旧LIBs浸出过程中细菌数量的变化如图4(b)所示。当废旧LIBs加入后,细菌浓度在0~0.25 d呈下降趋势。这可能是因为pH迅速上升以及释放的重金属和电池中的电解质对A. ferrooxidans的生长产生不利影响[32]。而在0.25~8 d,细菌数量逐渐增加,表明A. ferrooxidans对不利的生长环境具有适应性,可以继续保持自身活性,产生生物酸。对于不同时期投加废旧LIBs,C体系中的A. ferrooxidans适应能力大于A和B体系。而且,浸出过程中C体系中细菌量显著高于A和B体系。因此,在微生物对数期后期添加LIBs,细菌对浸出体系的适应性更好,可以保持较高的细菌浓度。
废旧LIBs生物浸出过程中Fe2+质量浓度的变化如图4(c)所示。当加入废旧LIBs后,在生物酸的攻击作用下,LIBs中LFP会发生溶解,释放Fe2+,导致Fe2+质量浓度逐渐增加 (式(5),式(6)) 。对于不同时期加入废旧LIBs,体系中Fe2+的产生速度不同。B体系中Fe2+的产生速度快于A和C体系,但是C体系可以实现更多Fe2+产生,且浸出的Fe2+可以将废旧LIBs中难溶解的Ni(Ⅲ)、Mn(Ⅳ)、Co(Ⅲ)还原成易溶解的Ni(Ⅱ)、Mn(Ⅱ)、Co(Ⅱ)[33] (式(7)) ,同时也可作为A. ferrooxidans生长的能源物质 (式(2)) ,促进浸出菌生长。因此,在对数期后期加入LIBs,A. ferrooxidans可以促进LIBs更多的Fe2+释放,有望促进金属浸出。
对于Fe3+,在0~4 d,Fe3+质量浓度呈显著下降趋势,这与Fe3+的水解、沉淀释放H+有关,且该反应有利于浸出体系维持较低pH,为废旧LIBs中金属的溶出提供H+。在4~8 d,Fe3+质量浓度趋于稳定,这说明在此过程中pH的下降主要是由于浸出微生物的产酸作用。
综上所述,与前期和中期投加LIBs的体系相比,在对数增长期后期加入废旧LIBs,浸出体系中细菌数量最高、Fe2+和生物酸的产生量最多,为LIBs中金属的溶出创造了有利的条件。
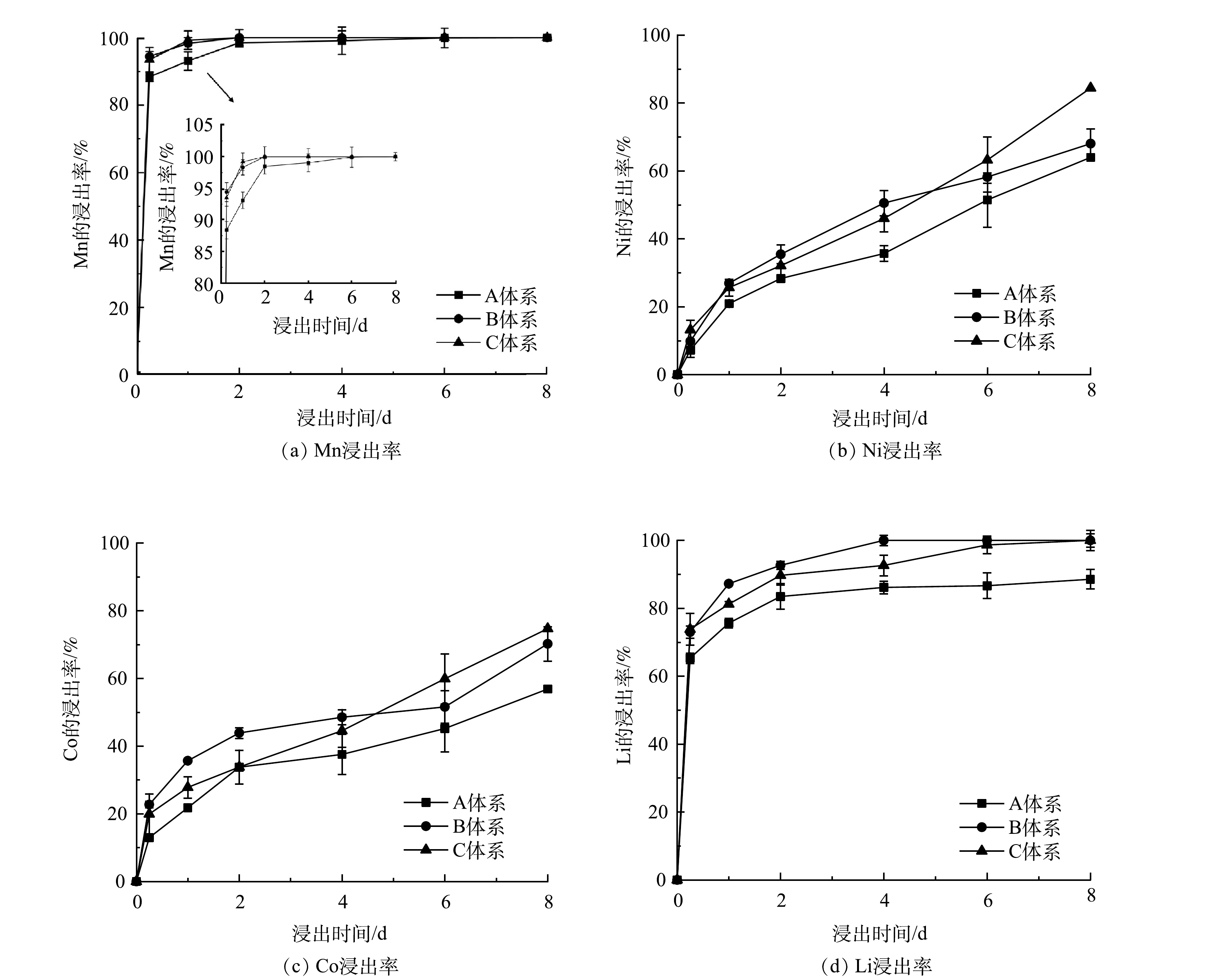
4) 不同投加时间对浸出效果的影响。图5显示了在A. ferrooxidans对数增长期的不同时期投加废旧LIBs的金属浸出率。从图5中可知,Mn、Li较容易浸出,而Ni、Co较难浸出。但是在对数期不同时期投加废旧LIBs,对金属浸出率有明显影响。其中,对Mn的影响最小,其次是易溶解金属Li,影响较大的是浸出率较低的Ni和Co。对于具有酸溶性的Li,生物浸出8 d后,B和C体系均能完全浸出,而A体系浸出率仅为88.56%。这可能是由于A. ferrooxidans在对数期前期耐受性较差、细菌量较低和产酸能力较弱,pH下降不明显 (图4) ,导致Li未能完全浸出。而对于浸出率较低的Ni和Co,生物浸出8 d后,C体系中Ni和Co的浸出率高于A和B体系。这是由于生物浸出过程中,C体系中细菌量最高,可以促进产生更多的H+释放,促进更多的Fe2+产生,而Fe2+和H+可以同时攻击废旧LIBs,导致金属释放[7]。此外,高细菌量能分泌更多的胞外聚合物 (EPS) 黏附在废旧LIBs上,并吸附Fe3+、Fe2+,在细胞-EPS-LIBs界面形成高质量浓度的Fe3+、Fe2+,对浸出固体有更强的侵蚀作用[34],更好地利用Fe2+还原浸出。综上所述,运用微生物两步浸出废旧LIBs过程中,在对数增长期不同时期加入废旧LIBs对金属浸出率有明显影响,且后期加入废旧LIBs可以实现高效的金属浸出率。
-
1) 形貌分析。利用SEM对生物浸出过程中固体形貌变化进行分析,深入了解浸出微生物对浸出固体的作用效果 (图6) 。在微生物对数期不同时期添加LIBs,浸出体系中LIBs的形貌具有明显的差异。生物浸出4 d后,A、C体系均无LMO形貌,C体系浸出渣中的橄榄石LFP结构消失,而A体系的浸出渣中仍能观察到块状的LFP (图6(a)、图6(d)) ,说明在微生物对数期后期加入废旧LIBs,A. ferrooxidans能更快地浸出LFP,释放Fe2+,这可作为还原剂及细菌生长的能源物质,提高浸出效率。生物浸出8 d后,2个体系的生物浸出渣均不存在LFP形貌的物质,表明LFP均完全浸出 (图6(b)、图6(e)) 。但2个体系浸出渣中NCM的结构有明显差别 (图6(c)、图6(f)) 。在A体系中,NCM球状结构的内部较光滑,无明显裂缝,仅有细小孔洞,而C体系浸出渣中的NCM内部变得多孔且粗糙、有明显的腐蚀痕迹,这表明C体系中微生物对NCM结构有更强的破坏能力。这是因为,在微生物对数期后期加入LIBs,A. ferrooxidans对浸出体系的适应性更强,产生更多的生物酸,攻击LIBs,导致其结构破坏。总之,A. ferrooxidans对废旧LIBs中阴极材料浸出的难易程度不同,LMO最容易被浸出,其次到LFP,而球状的NCM结构最为稳定,难以浸出;对于不同时期加入废旧LIBs,后期加入废旧LIBs时,A. ferrooxidans能较快浸出LFP中Fe2+,且对材料结构破坏更明显,可以促进LIBs中金属的溶出。
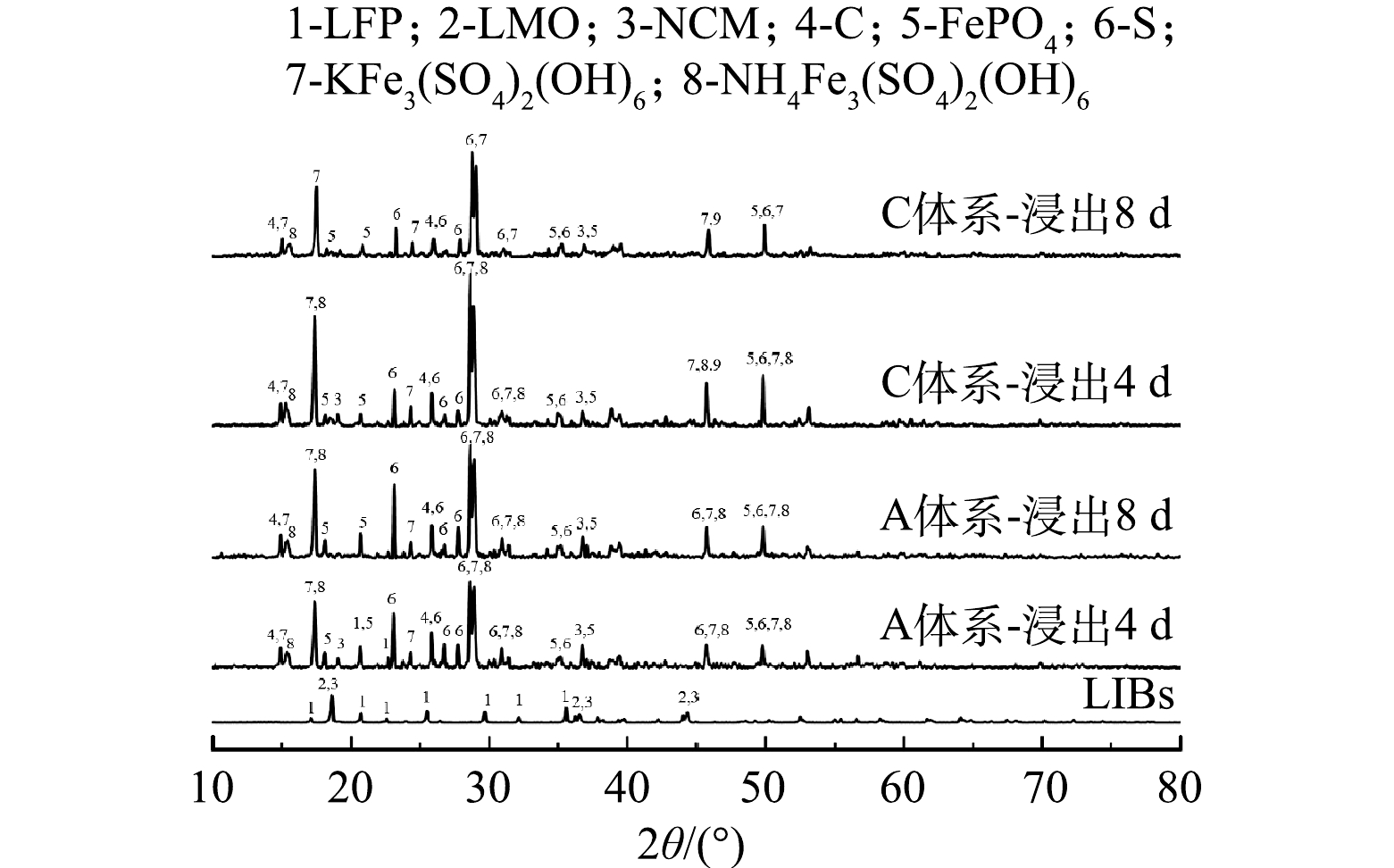
2) 物相分析。通过XRD分析对数增长期的不同时期加入废旧LIBs在浸出过程中固体物相的变化,探究不同时期投加废旧LIBs的浸出效果 (图7) 。观察发现,浸出原料以LFP、LMO、NCM为主,以及部分C,而浸出渣以K+/NH4+Fe3(SO4)2(OH)6、S0、C、FePO4为主,以及部分NCM。与浸出原料相比,LFP和LMO的特征峰均消失,其中LMO特征峰的消失均仅需4 d。这说明,A. ferrooxidans率先浸出LMO,且在不同时期加入废旧LIBs对LMO的浸出影响不大。然而对于对数增长期不同时期加入废旧LIBs,浸出渣的物相有明显差异。生物浸出4 d后,A体系的浸出渣可观察到LFP的特征峰,而C体系的浸出渣中未发现该谱峰。这说明,在微生物对数期后期向浸出体系加入废旧LIBs时,微生物能在较短时间内完全浸出LFP,产生Fe2+,从而为LIBs中金属的还原提供电子以及细菌生长提供能源物质;但生物浸出8 d后,A和C体系的浸出渣中仍然存在NCM的特征峰,而且C体系的NCM的特征峰强度低于A体系。该结果表明,A. ferrooxidans作用下未能实现NCM完全浸出,但在微生物对数期后期加入废旧LIBs,A. ferrooxidans对NCM浸出效果较好。综上所述,A. ferrooxidans会优先促进废旧LIBs中LMO浸出,其次到LFP,NCM最难浸出。而在对数期不同时期添加废旧LIBs,会影响A. ferrooxidans对LFP和NCM的浸出,在后期添加LIBs时,A. ferrooxidans对LFP和NCM的浸出作用更大,可以促进更多的金属离子释放。
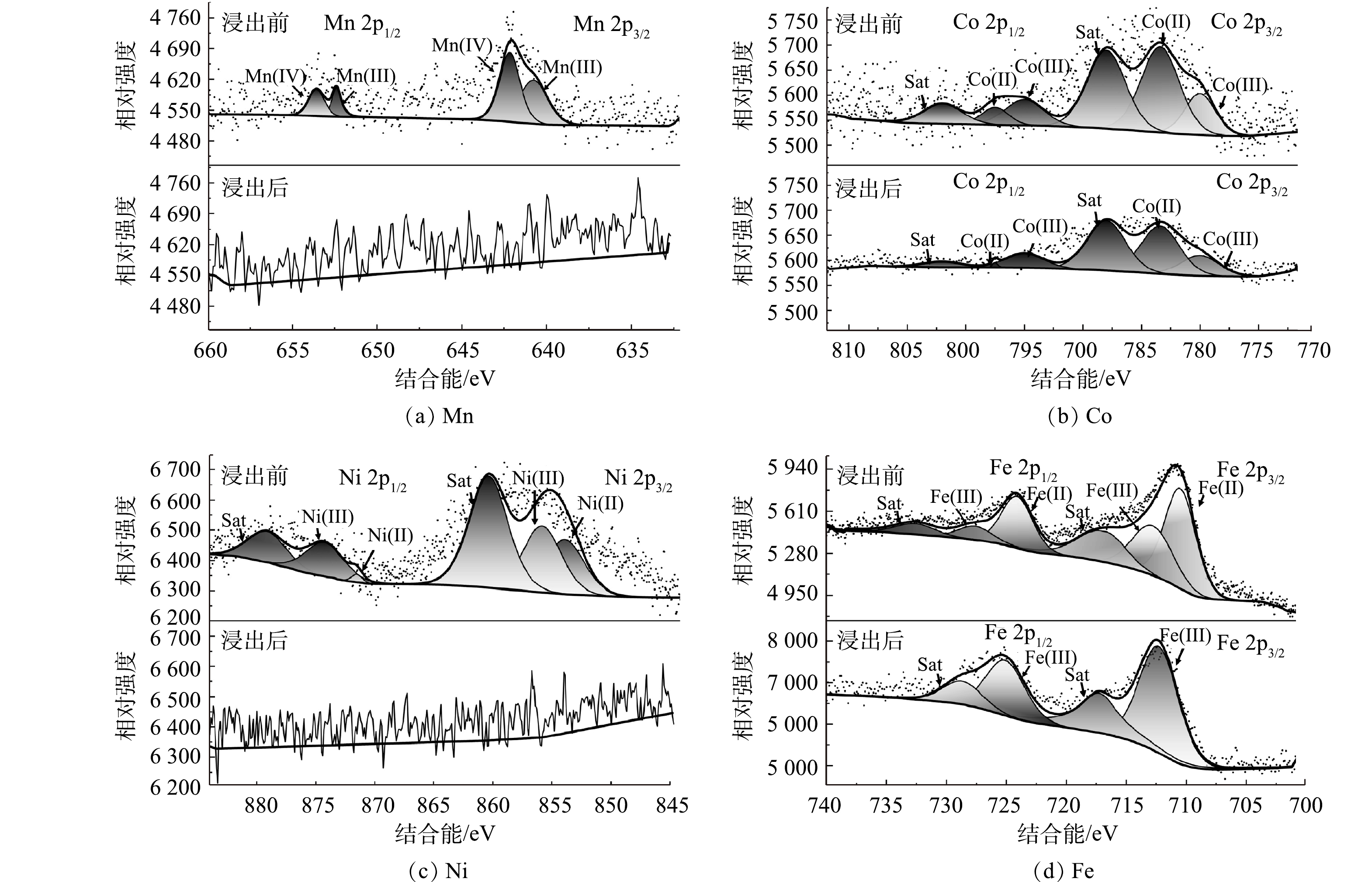
3) 金属价态分析。为了揭示A. ferrooxidans浸出废旧LIBs的机理,利用XPS分析C体系浸出前后固体金属价态变化,如图8所示。废旧LIBs中含有Mn(Ⅳ)、Mn(Ⅲ) [35]、Ni(Ⅲ)、Ni(Ⅱ) [36-37]、Co(III)、Co(II) [38]、Fe(Ⅱ) [39]和Fe(Ⅲ) [40],但是Mn(Ⅳ)、Ni(Ⅲ)和Co(III)是难以浸出的[33]。浸出完成后,Mn 2p的XPS谱图光谱嘈杂,浸出渣无明显谱峰,这表明浸出渣中Mn的表面浓度低,Mn基本浸出完成。这可能是因为,浸出体系中产生的Fe2+释放电子将Mn(Ⅳ)还原为Mn(Ⅲ),促进Mn容易被浸出。对于Ni 2p,浸出渣无明显谱峰,与AAS、SEM、XRD结果不一致,这可能是因为XPS是一种表面分析技术,分析深度约5~10 nm[41],而Ni表面覆盖率低导致谱峰不明显,这说明需要进一步破坏材料结构才能继续浸出Ni;对于Co 2p,与Ni 2p相比,有明显谱峰,这说明材料表面的Co未能完全浸出,Co相比于Ni更难浸出,同时,Co(Ⅱ)与Co(Ⅲ)面积比值增加,说明浸出过程中部分Co(Ⅲ)还原成Co(Ⅱ)[39];对于Fe 2p,浸出8 d后,浸出渣以Fe(Ⅲ)为主,表明大部分Fe(Ⅱ)氧化为Fe(Ⅲ),而该过程可以为Ni、Co和Mn的还原提供电子。因此,A. ferrooxidans能浸出废旧LIBs的LFP,释放的Fe(Ⅱ)可以还原高价态难溶解金属,从而有效提高金属浸出率。
-
1) 在A. ferrooxidans对数期的前、中和后期加入废旧LIBs,会影响金属浸出率。在对数期后期加入废旧LIBs,A. ferrooxidans有较强的产酸能力,较高的Fe2+产生量,从而获得最高的浸出率。对于不同金属,Mn和Li较容易浸出;Ni和Co较难浸出,且不同时期加入废旧LIBs对Ni和Co浸出率影响较大。
2) A. ferrooxidans均率先浸出废旧LIBs中的LMO,且可完全浸出LFP,而球状的NCM结构较为稳定,未能完全浸出;在对数增长期后期加入废旧LIBs,微生物对废旧LIBs的结构破坏更彻底,从而有利于金属的溶出。
3) A. ferrooxidans浸出废旧LIBs过程中产生的Fe2+可将LIBs中难溶解的Co(Ⅲ)、Ni(Ⅲ)、Mn(Ⅳ)还原为易溶的Co(Ⅱ)、Ni(Ⅱ)、Mn(Ⅱ),从而促进金属的浸出。
Acidithiobacillus ferrooxidans对数期的不同时期添加废旧锂电池对两步生物浸出工艺性能的影响
Effects of two-step bioleaching process on spent lithium ion batteries leaching during different stages of logarithmic growth of Acidithiobacillus ferrooxidans
-
摘要: 为了提高废旧锂电池 (LIBs) 的生物浸出效率,采用了氧化亚铁硫杆菌 (Acidithiobacillus ferrooxidans,简称A. ferrooxidans) 两步浸出废旧LIBs,考察在A. ferrooxidans对数期的前、中和后期向浸出体系中添加废旧LIBs对金属浸出效率的影响。结果表明,在对数期后期加入LIBs,A. ferrooxidans实现了100% Mn、76.82% Co、84.42% Ni和100% Li的浸出,比前和中期投加LIBs提高了4.51%~17.85% Co和16.38%~20.42% Ni。机理分析表明,A. ferrooxidans在对数期后期具有较强的产酸能力,比对数期前期和中期产生更多生物酸攻击废旧LIBs,导致金属释放。而释放的Fe2+可为A. ferrooxidans的生长提供能源物质,同时提供电子将LIBs中难溶解的Co(Ⅲ)、Ni(Ⅲ)和Mn(Ⅳ)还原为易被生物酸浸出的Co(Ⅱ)、Ni(Ⅱ)、Mn(Ⅱ),从而促进金属浸出。采用两步法生物浸出废旧LIBs时,为获得较高的生物浸出效率,需要在对数期的后期加入LIBs。本研究结果可为生物浸出废旧LIBs的工业化提供参考。Abstract: To improve the bioleaching efficiency of spent lithium batteries (LIBs), this study investigated Acidithiobacillus ferrooxidans (A. ferrooxidans) two-step leaching of spent LIBs during early, middle and late stages of logarithmic growth. The results showed that A. ferrooxidans could achieve a leaching efficiency of 100% Mn, 76.82% Co, 84.42% Ni and 100% Li by adding LIBs during late logarithmic phase and improve 4.51% to 17.85% Co and 16.38% to 20.42% Ni compared with early and mid-logarithmic growth. Mechanism analysis showed that A. ferrooxidans has a high capacity for acid production during the late logarithmic phase to attack spent LIBs, leading to an increased release of metal ions, as compared to early and mid-logarithmic growth. The released of Fe2+ could provide energy for the growth of A. ferrooxidans and release electrons to reduce the insoluble Co(Ⅲ), Ni(Ⅲ) and Mn(Ⅳ) in LIBs to Co(Ⅱ), Ni(Ⅱ) and Mn(Ⅱ), which were easily bioleached by this microorganism. In conclusion, to reach high leaching efficiencies, spent LIBs should be exposed to A. ferrooxidans during its the late logarithmic phase, when leaching was carried out by a two-step bioleaching method and the results of this study may provide a reference for the industrialisation of bioleaching of spent LIBs.
-

-
-
[1] ZHAO S Q, HE W Z, LI G M. Recycling technology and principle of spent Lithium-Ion Battery[M]. Cham: Springer International Publishing. 2019: 1-26. [2] ROY J J, CAO B, MADHAVI S. A review on the recycling of spent lithium-ion batteries (LIBs) by the bioleaching approach[J]. Chemosphere, 2021, 282: 130944. doi: 10.1016/j.chemosphere.2021.130944 [3] SETHURAJAN M, GAYDARDZHIEV S. Bioprocessing of spent lithium ion batteries for critical metals recovery – A review[J]. Resources, Conservation and Recycling, 2021, 165: 105225. doi: 10.1016/j.resconrec.2020.105225 [4] WANG X, GAUSTAD G, BABBITT C W, et al. Economic and environmental characterization of an evolving Li-ion battery waste stream[J]. Journal of Environmental Management, 2014, 135: 126-134. doi: 10.1016/j.jenvman.2014.01.021 [5] WANG M X, TIAN Y H, LIU W, et al. A moving urban mine: The spent batteries of electric passenger vehicles[J]. Journal of Cleaner Production, 2020, 265: 121769. doi: 10.1016/j.jclepro.2020.121769 [6] SRIVASTAVA N, SINGH S K, GUPTA H, et al. Electrochemical performance of Li-rich NMC cathode material using ionic liquid based blend polymer electrolyte for rechargeable Li-ion batteries[J]. Journal of Alloys and Compounds, 2020, 843: 155615. doi: 10.1016/j.jallcom.2020.155615 [7] LIAO X J, YE M Y, LIANG J L, et al. Feasibility of reduced iron species for promoting Li and Co recovery from spent LiCoO2 batteries using a mixed-culture bioleaching process[J]. Science of The Total Environment, 2022, 830: 154577. doi: 10.1016/j.scitotenv.2022.154577 [8] ROY J J, MADHAVI S, CAO B. Metal extraction from spent lithium-ion batteries (LIBs) at high pulp density by environmentally friendly bioleaching process[J]. Journal of Cleaner Production, 2021, 280: 124242. doi: 10.1016/j.jclepro.2020.124242 [9] XIN Y Y, GUO X M, CHEN S, et al. Bioleaching of valuable metals Li, Co, Ni and Mn from spent electric vehicle Li-ion batteries for the purpose of recovery[J]. Journal of Cleaner Production, 2016, 116: 249-58. doi: 10.1016/j.jclepro.2016.01.001 [10] BISWAL B K, JADHAV U U, MADHAIYAN M, et al. Biological Leaching and Chemical Precipitation Methods for Recovery of Co and Li from Spent Lithium-Ion Batteries[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 12343-12352. [11] MONBALLIU A, CARDON N, TRI NGUYEN M, et al. Tolerance of Chemoorganotrophic Bioleaching Microorganisms to Heavy Metal and Alkaline Stresses[J]. Bioinorganic Chemistry and Applications, 2015, 2015: 861874. [12] MISHRA D, KIM D-J, RALPH D E, et al. Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans[J]. Waste Management, 2008, 28(2): 333-338. doi: 10.1016/j.wasman.2007.01.010 [13] ZENG G S, DENG X R, LUO S L, et al. A copper-catalyzed bioleaching process for enhancement of cobalt dissolution from spent lithium-ion batteries[J]. Journal of Hazardous Materials, 2012, 199-200: 164-169. doi: 10.1016/j.jhazmat.2011.10.063 [14] ZENG G S, LUO S L, DENG X R, et al. Influence of silver ions on bioleaching of cobalt from spent lithium batteries[J]. Minerals Engineering, 2013, 49: 40-44. doi: 10.1016/j.mineng.2013.04.021 [15] BRYAN C G, WATKIN E L, MCCREDDEN T J, et al. The use of pyrite as a source of lixiviant in the bioleaching of electronic waste[J]. Hydrometallurgy, 2015, 152: 33-43. doi: 10.1016/j.hydromet.2014.12.004 [16] NATARAJAN G, TING Y-P. Gold biorecovery from e-waste: An improved strategy through spent medium leaching with pH modification[J]. Chemosphere, 2015, 136: 232-238. doi: 10.1016/j.chemosphere.2015.05.046 [17] ZHU N W, XIANG Y, ZHANG T, et al. Bioleaching of metal concentrates of waste printed circuit boards by mixed culture of acidophilic bacteria[J]. Journal of Hazardous Materials, 2011, 192(2): 614-9. doi: 10.1016/j.jhazmat.2011.05.062 [18] GHASSA S, FARZANEGAN A, GHARABAGHI M, et al. Novel bioleaching of waste lithium ion batteries by mixed moderate thermophilic microorganisms, using iron scrap as energy source and reducing agent[J]. Hydrometallurgy, 2020, 197: 105465. doi: 10.1016/j.hydromet.2020.105465 [19] JEGAN ROY J, SRINIVASAN M, CAO B. Bioleaching as an Eco-Friendly Approach for Metal Recovery from Spent NMC-Based Lithium-Ion Batteries at a High Pulp Density[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(8): 3060-3069. [20] MA L Y, WANG X J, FENG X, et al. Co-culture microorganisms with different initial proportions reveal the mechanism of chalcopyrite bioleaching coupling with microbial community succession[J]. Bioresource Technology, 2017, 223: 121-130. doi: 10.1016/j.biortech.2016.10.056 [21] NASERI T, BAHALOO-HOREH N, MOUSAVI S M. Bacterial leaching as a green approach for typical metals recovery from end-of-life coin cells batteries[J]. Journal of Cleaner Production, 2019, 220: 483-92. doi: 10.1016/j.jclepro.2019.02.177 [22] HOREH N B, MOUSAVI S M, SHOJAOSADATI S A. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger[J]. Journal of Power Sources, 2016, 320: 257-266. doi: 10.1016/j.jpowsour.2016.04.104 [23] 聂红燕, 朱能武, 杨婷婷, 等. 嗜酸性细菌对废旧线路板浸出的吸附行为及动力学[J]. 环境科学学报, 2015, 35(5): 1471-1476. doi: 10.13671/j.hjkxxb.2014.0913 [24] BOXALL N J, CHENG K Y, BRUCKARD W, et al. Application of indirect non-contact bioleaching for extracting metals from waste lithium-ion batteries[J]. Journal of Hazardous Materials, 2018, 360: 504-511. doi: 10.1016/j.jhazmat.2018.08.024 [25] LIU L Z, NIE Z Y, YANG Y, et al. In situ characterization of change in superficial organic components of thermoacidophilic archaeon Acidianus manzaensis YN-25[J]. Research in Microbiology, 2018, 169(10): 590-597. doi: 10.1016/j.resmic.2018.08.003 [26] 陈志, 王敏, 葛淑萍. 工科基础化学实验汇编[J]. 重庆:重庆大学出版社, 2018: 125. [27] SEONG M J, YIM T. Critical role of corrosion inhibitors modified by silyl ether functional groups on electrochemical performances of lithium manganese oxides[J]. Journal of Energy Chemistry, 2020, 51: 425-433. doi: 10.1016/j.jechem.2020.02.029 [28] MAHANDRA H, GHAHREMAN A. A sustainable process for selective recovery of lithium as lithium phosphate from spent LiFePO4 batteries[J]. Resources, Conservation and Recycling, 2021, 175: 105883. doi: 10.1016/j.resconrec.2021.105883 [29] LIU P, XIAO L, TANG Y, et al. Resynthesis and electrochemical performance of LiNi0.5Co0.2Mn0.3O2 from spent cathode material of lithium-ion batteries[J]. Vacuum, 2018, 156: 317-324. doi: 10.1016/j.vacuum.2018.08.002 [30] 王鹤茹, 杨琳琳, 王蕊, 等. 3种填料A. ferrooxidans挂膜效果及对模拟酸性矿山废水中Fe~(2+)生物矿化能力比较[J]. 环境科学学报, 2022, 42(5): 160-168. [31] 邱冠周, 柳建设, 王淀佐, 等. 氧化亚铁硫杆菌生长过程铁的行为[J]. 中南工业大学学报, 1998(3): 25-27. [32] JANG H-C, VALIX M. Overcoming the bacteriostatic effects of heavy metals on Acidithiobacillus thiooxidans for direct bioleaching of saprolitic Ni laterite ores[J]. Hydrometallurgy, 2017, 168: 21-25. doi: 10.1016/j.hydromet.2016.08.016 [33] HE L P, SUN S Y, SONG X F, et al. Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion batteries[J]. Waste Management, 2017, 64: 171-181. doi: 10.1016/j.wasman.2017.02.011 [34] WANG J, TIAN B Y, BAO Y H, et al. Functional exploration of extracellular polymeric substances (EPS) in the bioleaching of obsolete electric vehicle LiNixCoyMn1-x-yO2 Li-ion batteries[J]. Journal of Hazardous Materials, 2018, 354: 250-7. doi: 10.1016/j.jhazmat.2018.05.009 [35] GRISSA R, MARTINEZ H, COTTE S, et al. Thorough XPS analyses on overlithiated manganese spinel cycled around the 3V plateau[J]. Applied Surface Science, 2017, 411: 449-456. doi: 10.1016/j.apsusc.2017.03.205 [36] GAUTHIER N, COURRèGES C, DEMEAUX J, et al. Impact of the cycling temperature on electrode/electrolyte interfaces within Li4Ti5O12 vs LiMn2O4 cells[J]. Journal of Power Sources, 2020, 448: 227573. doi: 10.1016/j.jpowsour.2019.227573 [37] LIU J, GAO S, SI Z, et al. Lanthanum Oxyfluoride modifications boost the electrochemical performance of Nickel-rich cathode[J]. Applied Surface Science, 2022, 599: 153928. doi: 10.1016/j.apsusc.2022.153928 [38] HAN H, LEE H, LIM J, et al. Hopping conduction in (Ni, Co, Mn)O4 prepared by different synthetic routes: Conventional and spark plasma sintering[J]. Ceramics International, 2017, 43(18): 16070-16075. doi: 10.1016/j.ceramint.2017.08.105 [39] CHEN B P, LIU M, CAO S, et al. Regeneration and performance of LiFePO4 with Li2CO3 and FePO4 as raw materials recovered from spent LiFePO4 batteries[J]. Materials Chemistry and Physics, 2022, 279: 125750. doi: 10.1016/j.matchemphys.2022.125750 [40] XU Z D, DAI Y, HUA D, et al. Creative Method for Efficiently Leaching Ni, Co, Mn, and Li in a Mixture of LiFePO4 and LiMO2 Using Only Fe(III)[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(11): 3979-3984. [41] HU W Q, ZHANG C H, JIANG H, et al. Improving the electrochemistry performance of layer LiNi0.5Mn0.3Co0.2O2 material at 4.5V cutoff potential using lithium metaborate[J]. Electrochimica Acta, 2017, 243: 105-111. doi: 10.1016/j.electacta.2017.05.075 -



 下载:
下载: