-
我国污泥产量日渐增多,污泥的处理处置问题日益突出。对污泥进行脱水是最终处置前必不可少的环节,也是实现污泥减量的方式之一[1]。过硫酸盐高级氧化调理技术是近年来发展起来的新兴氧化技术,主要活性物质为SO4−·,具有氧化性强、稳定性高、适用范围广等优点[2],可以有效破坏污泥絮体和污泥微生物细胞,释放胞内结合水,改善污泥脱水性能[3],因此其在污泥调理中具有很高的研究价值。活化过硫酸盐的方法有热活化、微波活化、超声波活化、碱活化、过渡金属活化等[4]。其中,过渡金属Fe2+因为价格低廉、无污染而得到广泛关注[5]。如ZHEN等[6]采用Fe2+活化PDS调理污泥脱水,在1.2 mmol∙g−1 VSS PDS、1.5 mmol∙g−1 VSS Fe2+、pH 3.00~8.50条件下,1 min内CST可降低88.80%。这是因为,Fe2+活化过硫酸盐产生的强氧化自由基可以破坏微生物细胞、EPS结构和其中的某些特定的荧光官能团遭到显著破坏,使得絮体中包含的结合水被释放出来,污泥的脱水性能得到改善[7]。然而,Fe2+在溶液中不稳定,且过量存在时容易淬灭SO4−·,导致其活化效率较低[8-9]。为此,研究人员将铁负载在活性炭等材料表面来提高其催化效率[10]。例如,丁敬林[11]采用生物质和高铁酸钾共热解制备负载纳米零价铁的生物炭活化过硫酸盐去除雌二醇 (E2) ,发现其对E2的去除效果明显优于单独生物炭和纳米零价铁;WANG等[12]采用氧化铁改性废咖啡渣生物炭联合过硫酸盐对氯四环素的去除率显著提高,达到83.48%。由此可知,采用生物质炭负载铁元素活化过硫酸盐可在一定程度上提高催化氧化效率,解决Fe2+活化存在的问题,但目前该方法在调理污泥脱水方面研究的较少,有关机理需要深入探讨。
污水厂污泥中有机质含量较高,有文献[13-14]证明,可以通过热解将其制备成具有丰富孔隙结构和表面官能团的污泥生物炭。而给水厂含铁污泥中铁含量较多,可以作为铁源。如果将给水厂铁污泥和污水厂污泥共热解制备铁基污泥炭材料,不仅可以解决Fe2+催化效率较低的问题,还可实现2种污泥的资源化利用,有利于水厂的碳减排。此外,还研究表明,在污泥调理过程中,炭材料还可以作为骨架颗粒,降低污泥的可压缩性,构建过水通道,有利于污泥的脱水性能的提高[15-16]。
本研究拟以给水厂含铁污泥为铁源,污水厂污泥为碳源,将2者混合后热解制备铁基污泥炭 (Iron-SBC) ,探究Iron-SBC的最佳制备条件并对其进行了表征,对比Iron-SBC/PDS体系与其他体系对污泥的调理效果,并探究其活化机制,以期为污泥调理中过硫酸盐的活化提供参考。
-
污水厂脱水污泥和给水厂含铁污泥分别取自天津市某污水处理厂和某给水厂。分别将取回的污水厂污泥和给水厂含铁污泥去除杂质,在自然条件下风干,研磨粉碎,过100目筛,置于干燥器中备用。用X射线衍射法分析污水厂污泥和给水厂含铁污泥的元素组成,见表1。
剩余污泥取自同一污水处理厂的二次沉淀池。每次取足量实验用泥,通过4 mm的筛网去除污泥中的杂质,经自由沉淀后,去除上清液,在4 ℃条件下存放。实验用泥基本性质见表2。
-
采用热解法制备铁基污泥炭。即将粉碎、研磨、过100目筛的给水厂含铁污泥与污水厂污泥以一定的比例混合,取一定量混合后的污泥投入去离子水中,搅拌2 h使其混合均匀,离心过滤后将固体物质放入烘箱在105 ℃下烘2 h,得到混合污泥 (mixed sludge,MS) 。将MS粉碎、研磨,过100目筛后放入磁舟,将磁舟放至管式炉,封闭后通15 min氮气 (气体流速200 mL·min−1) 以排出炉腔内的空气,将气体流速调至100 mL·min−1,开启管式炉加热程序,在5 ℃·min−1的速率下升温至一定温度,热解一定时间后,冷却至室温,得到铁基污泥炭 (iron-sludge biochar,Iron-SBC) ,并对材料进行了XRD、FT-IR和BET表征分析。
-
取200 mL供试污泥置于300 mL锥形瓶中,将锥形瓶置于恒温水浴锅中调至65 ℃,投加350 mg·g−1 (以污泥干固体计,以下同) Iron-SBC和150 mg·g−1 PDS,将数显增力电动搅拌器转速调整为250 r·min−1,反应20 min。反应结束后,测定污泥毛细吸水时间 (capillary suction time,CST) 、污泥比阻 (specific resistance to filtration,SRF) 和泥饼含水率 (moisture content,Wc) ;将调理后的污泥滤液经0.45 μm滤膜过滤,测定滤液中SCOD、TN和TP质量浓度;对比Iron-SBC/PDS、Fe0/ SBC/PDS、Fe0/PDS和SBC/PDS 4种体系调理污泥脱水的效果,其中SBC为采用污水厂污泥按照1.2所述方法制备的污泥生物质炭。以上4种体系中Iron-SBC和SBC投加量均为350 mg·g−1,PDS投加量均为150 mg·g−1,Fe0与PDS摩尔比选用文献[17]中的最优值 (5∶1) ,操作条件同本节所述实验操作,反应结束后测定CST、SRF和Wc。以上实验均重复3次。采用自由基猝灭实验对反应过程中产生的自由基种类进行分析,采用XPS分析反应前后Iron-SBC表面元素种类和官能团变化,综合探讨Iron-SBC活化PDS的机理。
-
样品的组成成分和晶相结构采用X射线粉末衍射仪 (Smartlab 9KW,日本株式会社理学) 测定;样品的特征官能团采用傅里叶红外光谱仪 (V80,德国布鲁克公司) 分析;材料的比表面积和孔容采用全自动比表面积与孔隙度分析仪 (ASAP 2 460,美国麦克仪器公司) 分析;样品材料中的元素组成、化学价态和电子态以及相对含量采用X 射线光电子能谱仪 (ESCALAB 250Xi,美国赛默飞世尔科技有限公司) 分析;CST采用CST测定仪 (DFC-10A,杭州典范科技有限公司) 测定;SRF采用布氏漏斗法[18]测定;Wc采用重量法[19]测定。
-
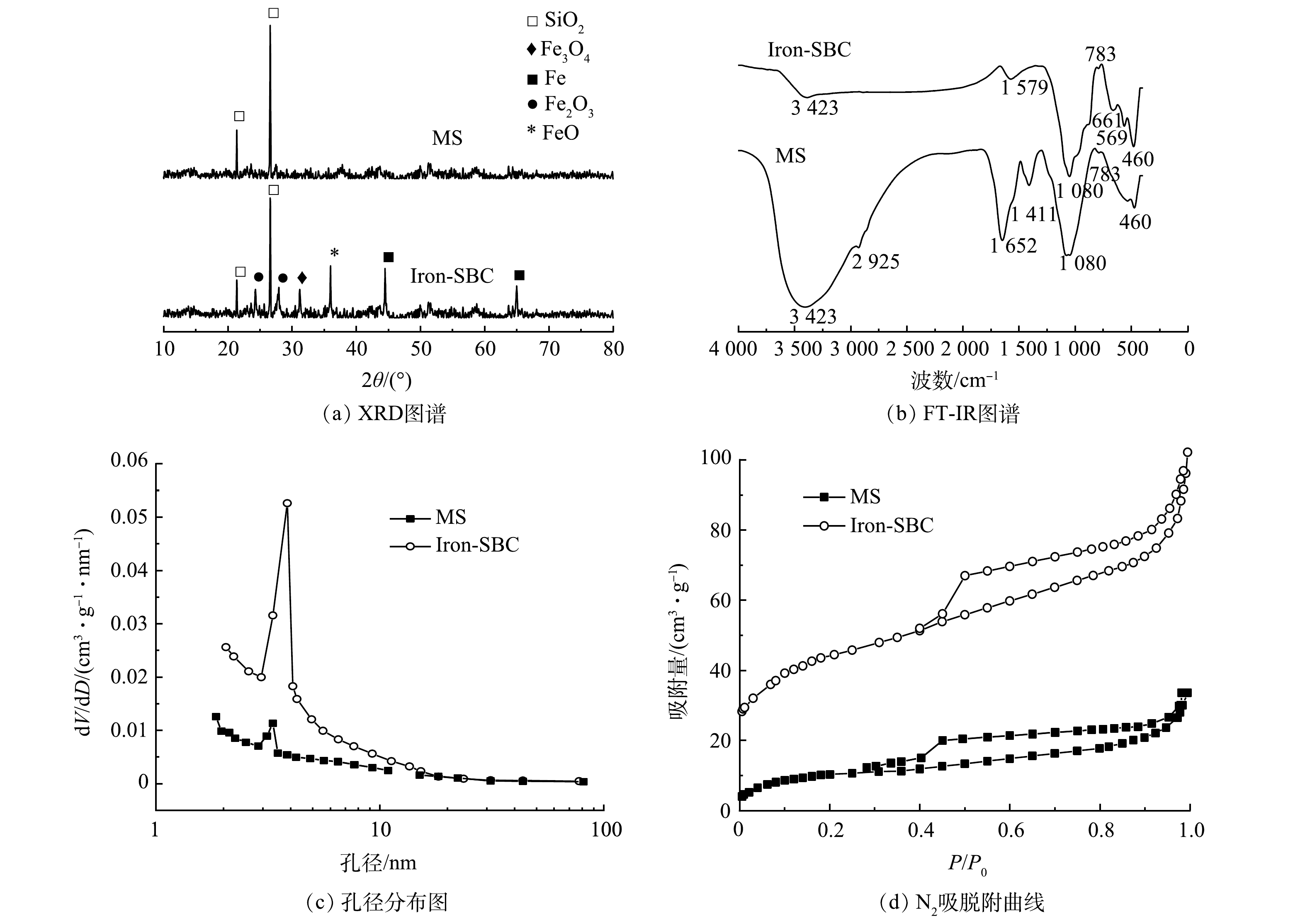
为探究Iron-SBC的组成成分,对Iron-SBC进行了XRD测试,并以MS为对照 (图1(a)) 。MS在2θ为 21.4°、26.6°处存在较大的特征峰,这说明存在SiO2[20]。与MS相比,高温热解后形成的Iron-SBC的XRD图谱上出现很多新的衍射峰。其中,31.2°处的衍射峰代表存在Fe3O4[21];44.5°和65.0°处的衍射峰与Fe0的标准PDF卡片一致[22];24.3°和27.8°处存在衍射峰,这说明存在Fe2O3[23];36.0°处的衍射峰代表存在FeO[24]。XRD分析表明Iron-SBC的表面形成了Fe0及多种形式的铁氧化物。
图1(b)为Iron-SBC和MS的傅里叶变换红外光谱图。MS分别在3 423、1 652、1 080 cm−1处存在较大的吸收峰,分别说明存在醇、酚类 (-OH) [25]、烯烃类-C=C-和Si-O。另外,在2 925 cm−1处和1 411 cm−1处也出现吸收峰,分别对应-CH2-和-CH3的振动特征峰;783 cm−1附近为C-H苯环间二取代[26-27];460 cm−1附近为Fe-O的特征峰[28]。与MS相比,Iron-SBC在3 423 cm−1处的特征峰明显减小,说明经过高温热解,-OH减少;在1 579 cm−1处出现特征峰,表明存在烯烃类-C=C-,与混合污泥中1 652 cm−1处的特征峰相比较小,说明经过热解-C=C-部分发生分解;661、569 cm−1处也出现新的特征峰,说明铁与周围氧离子结合形成新的物质,分别归因于Fe3O4和FeO[29];460 cm−1附近的峰也略微增强,表明FeO含量增多。
表3列出了Iron-SBC和MS的比表面积和孔容大小。MS的比表面积和孔容分别为38.22 m2·g−1和0.052 cm3·g−1,与MS相比,Iron-SBC的比表面积和孔容都增大了很多,分别达到89.51 m2·g−1和0.158 cm3·g−1。这是因为,在高温热解过程中,污泥中的有机物挥发,增大了污泥炭的比表面积与孔容。图1(c)、图1(d)为MS和Iron-SBC的孔径分布和N2吸脱附曲线图。由图1(c)可知,MS和Iron-SBC的峰值孔径主要集中在3~5 nm,说明这2种材料的孔径在这个范围内出现的频率最大。孔径分析表明,2种材料的孔径大多处于介孔 (2~50 nm) 范围内,大孔 (>50 nm) 占比非常少。氮气吸附脱附曲线的形状能够反映固体表面结构、孔结构和固体-吸附质的相互作用[30]。由图1(d)可知,MS和Iron-SBC的N2吸附-脱附曲线均具有IUPAC分类中的Ⅳ等温线特征,低压时发生的为单分子层吸附;随着压力增加,等温线近似线性,样品表面发生的是多分子层吸附;相对压力接近饱和蒸汽压时,发生毛细凝聚现象,吸附等温线表现为突跃。介孔回滞环属于H4类,回滞环等温线没有明显的饱和吸附平台,表明孔结构很不规整[31]。Iron-SBC的N2吸脱附曲线的滞后环较宽,表示比表面积较大[30]。
-
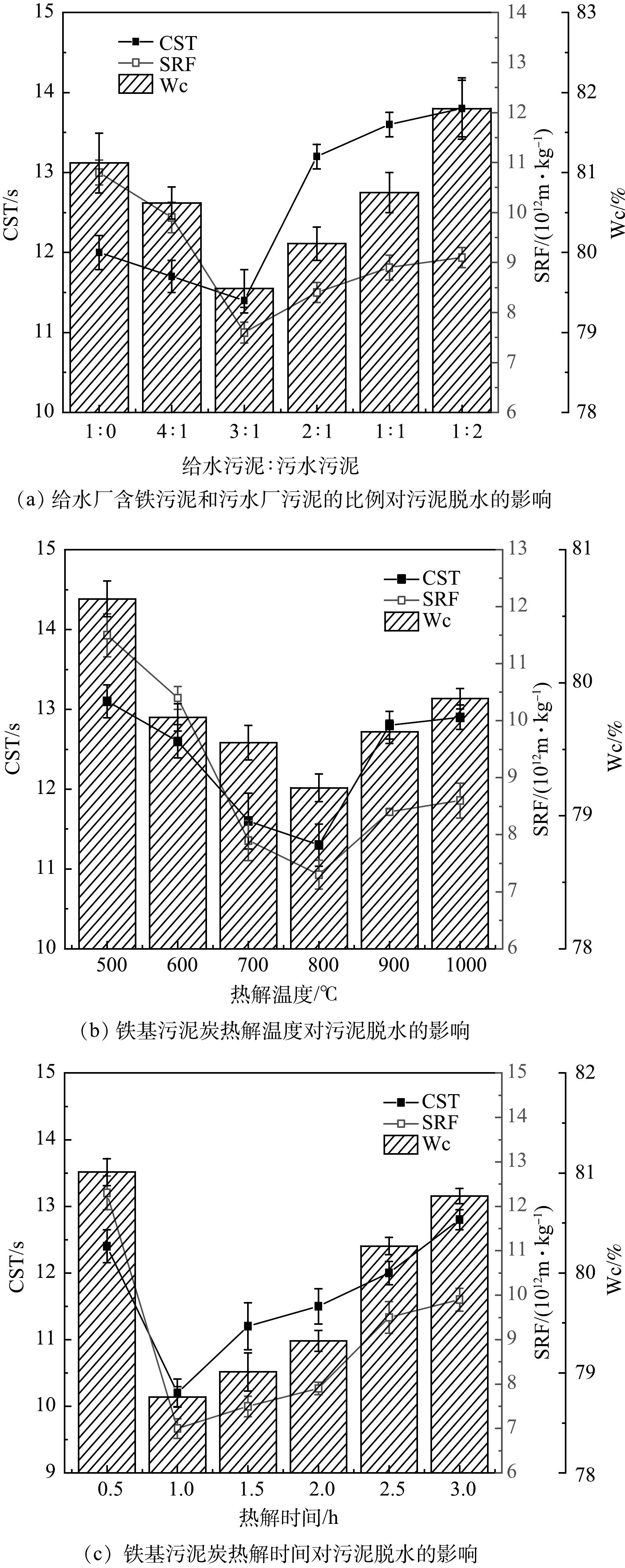
给水厂含铁污泥和污水厂污泥质量的比例、热解温度、热解时间对制备的Iron-SBC活化PDS调理污泥脱水性能的影响见图2。由图2(a)可知,当给水厂含铁污泥和污水厂污泥比例取1∶0,即只使用给水厂含铁污泥时,CST、SRF和Wc分别为12 s、10.8×1012 m·kg−1和81.12%。随着污水厂污泥比例的增多,CST、SRF和Wc都逐渐下降,在给水厂含铁污泥和污水厂污泥比例为3∶1时均达到最小。此后再增加污水厂污泥的比例,CST、SRF和Wc都逐渐变大。这可能是因为,污水厂污泥中有机质含量较高,高温热解过程中可作为碳源将高价态铁还原为低价态铁[32],当不添加或添加污水厂污泥比例过小时,不能有效还原给水厂污泥中高价态的铁,从而使得活化过程中可提供电子的还原态铁较少,从而产生的自由基较少,污泥脱水效果较差;而污水厂污泥添加比例过高时,混合污泥中的铁含量相对变少,部分活性位点-铁被包裹在热解后的生物炭中,无法有效活化PDS。
图2(b)为热解温度对Iron-SBC活化PDS调理污泥脱水性能的影响。图2(b)结果显示,热解温度为800 ℃时,CST、SRF和Wc均达到最低值,污泥脱水效果最好。喻江维[22]的研究表明,600 ℃条件下,热解的温度未达到将污泥中固化的铁还原改性的条件。当热解温度大于800 ℃时,在热解过程中,泥饼中含有有机物转化得来的还原性的炭,在高温热解条件下可将污泥中固化的铁改性还原[22]。有研究[33]表明,当热解温度低于800 ℃时,Fe3O4是碳热还原的主要铁产物;在热解温度为800和1 000 ℃时,产物中均存在Fe0。而这些铁的氧化物及Fe0都可以有效活化PDS,从而氧化破解污泥,提高污泥的脱水性能。
图2(c)为热解时间对Iron-SBC活化过硫酸盐调理污泥脱水的影响。由图2(c)可知,热解时间由0.5 h增加到1 h时,CST、SRF和Wc都降低,这说明所制备的Iron-SBC活化PDS调理污泥脱水的效果增强。这是因为,热解时间过短时,热解过程中碳热还原不足以进行完全;1 h之后,随着热解时间的增加,CST、SRF和Wc都逐渐升高,活化过硫酸盐调理污泥的效果逐渐下降。有研究提出,随着热解时间增长,污泥中有机物逐渐分解,灰分物质增加,孔隙被大量无机化合物充填或堵塞[34],造成污泥炭中孔的大小和数量降低,减少污泥滤水通道,从而降低污泥的脱水性能。
-
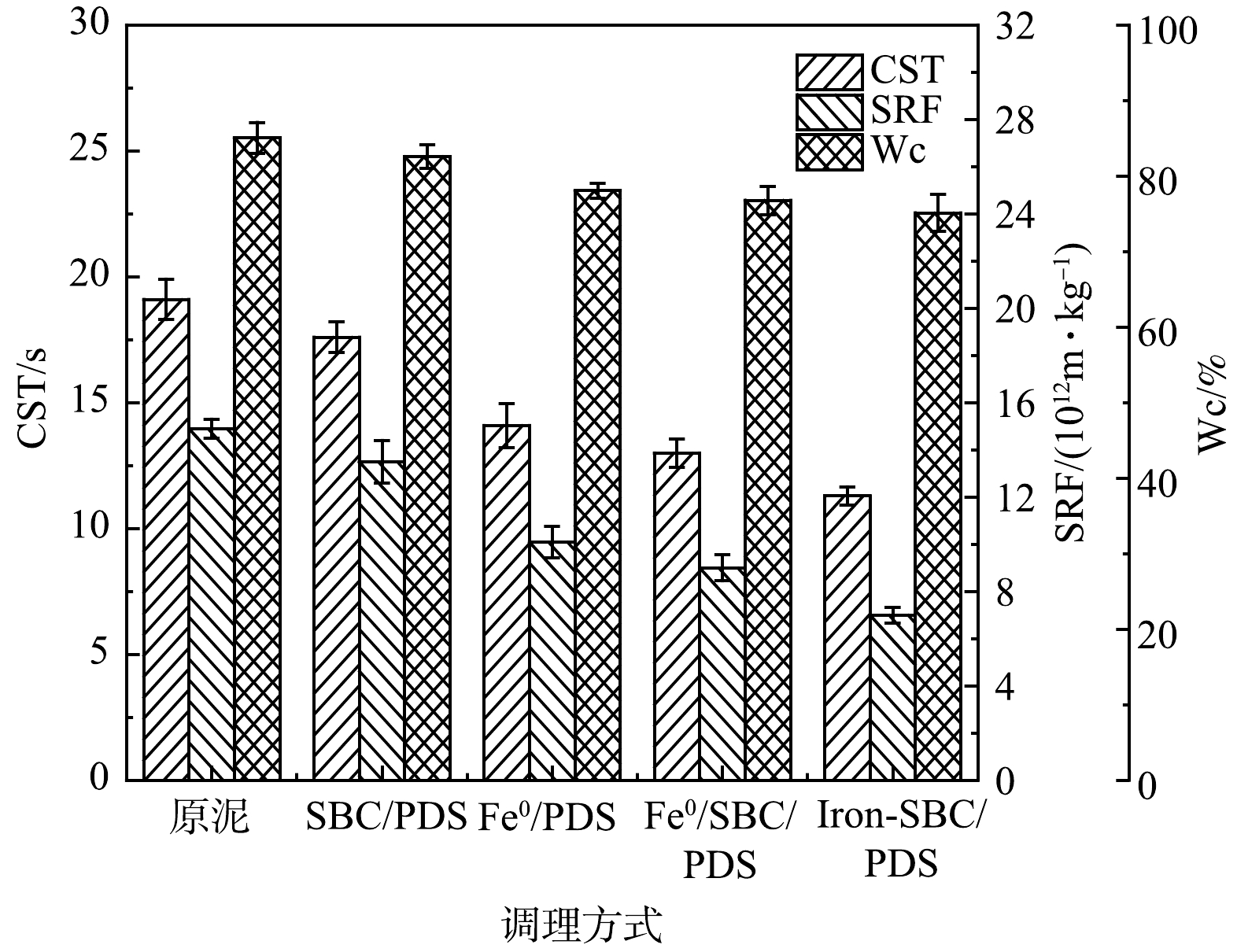
对比Iron-SBC/PDS、Fe0/ SBC/PDS、Fe0/PDS、SBC/PDS 4种体系调理污泥后,污泥脱水性能的变化如图3所示。由图3可看出,Iron-SBC/PDS联合调理污泥脱水的效果更为明显,反应20 min后,CST、SRF和Wc分别由原泥的19.1 s、14.9×1012 m·kg−1和85.06%下降到8.4 s、5.4×1012 m·kg−1和73.48%,是4种污泥调理体系中效果最佳的1种。这是因为,Iron-SBC/PDS体系中存在的PDS同时由Iron-SBC表面的Fe0、铁的氧化物以及官能团活化,产生了SO4−·,SO4−·的强氧化作用破坏了污泥絮体,释放出内部结合水,从而大幅降低了污泥含水率;且体系中的Iron-SBC可以作为骨架支撑,为水分脱出提供通道,同样有利于污泥脱水。SBC/PDS和Fe0/PDS 2种体系对污泥脱水的调理作用不如Iron-SBC/PDS体系。前者是由于可以活化PDS的物质不足,只有污泥炭表面官能团;后者虽然PDS可以被Fe0充分活化,但无法构建过水通道,污泥可压缩性较高,不利于水分脱出。SBC/Fe0/PDS体系对污泥脱水的调理效果与Iron-SBC/PDS相差不大,但从经济角度分析,额外加入Fe0会使经济耗费增加,而采用给水厂含铁污泥与污水厂污泥混合热解制备Iron-SBC,可以回收利用给水厂污泥中的铁,达到废物利用的目的。
-
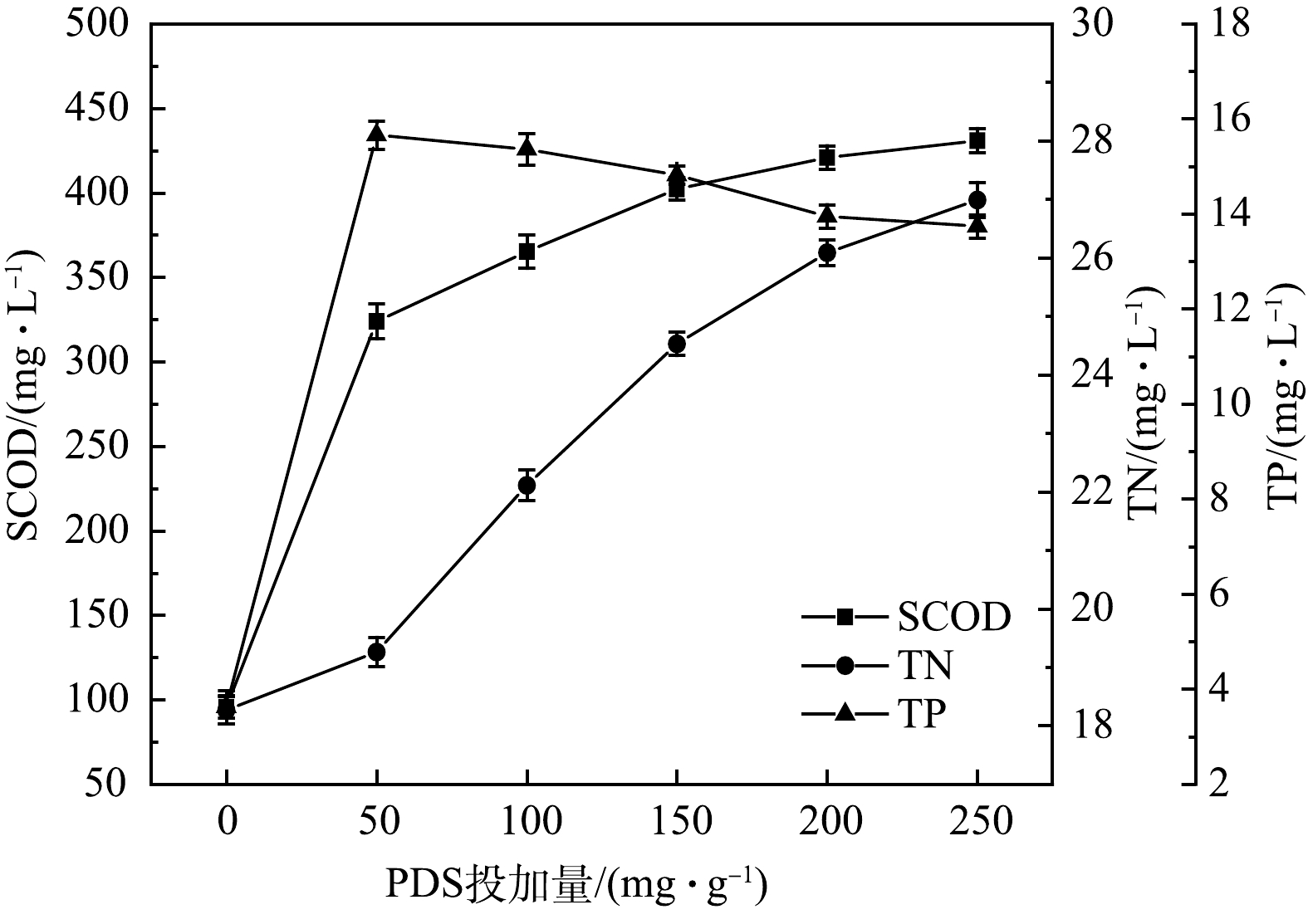
污泥上清液中溶解性化学需氧量 (SCOD) 、总氮 (TN) 和总磷 (TP) 的质量浓度随PDS投加量的变化如图4所示。由图4可知,上清液中SCOD随PDS投加量的增多而增大。当PDS投加量为150 mg·g−1 时,SCOD由原泥的95.54 mg·L−1上升为402.30 mg·L−1。这是因为,PDS的投加量是影响污泥破解效果的关键因素,随着PDS投加量的增加,体系中产生的SO4−·就越多,对污泥的破解效果越显著,污泥中有机物质溶出,SCOD随之增大,该结果与万甜等[35]的研究结论一致。经Iron-SBC/PDS体系调理后,污泥滤液中SCOD增加,滤液回流至生物处理系统后,能够增加系统的碳源,有利于生物处理系统中反硝化的进行。
原污泥中TN和TP的质量浓度分别为18.27和3.63 mg·L−1。投加PDS后,TN和TP的质量浓度都较原污泥增多,PDS投加量为150 mg·g−1时,TN和TP的质量浓度分别达到24.53和14.82 mg·L−1。这说明,在SO4−·的氧化作用下,污泥细胞被破解,释放出了细胞内部的氮和磷。PDS投加量越多,TN质量浓度越高,这是因为,体系产生的SO4−·随PDS的增多而增多,污泥细胞的破解程度随之增强,释放出的氮也变多。TP的质量浓度随PDS投加量的增多而逐渐减小。这是因为,Iron-SBC活化PDS后,活化剂表面的铁离子溶出,产生了Fe2+与Fe3+,体系中的铁盐与磷发生反应形成沉淀物,在污泥脱水过程中进入到了泥饼中。
-
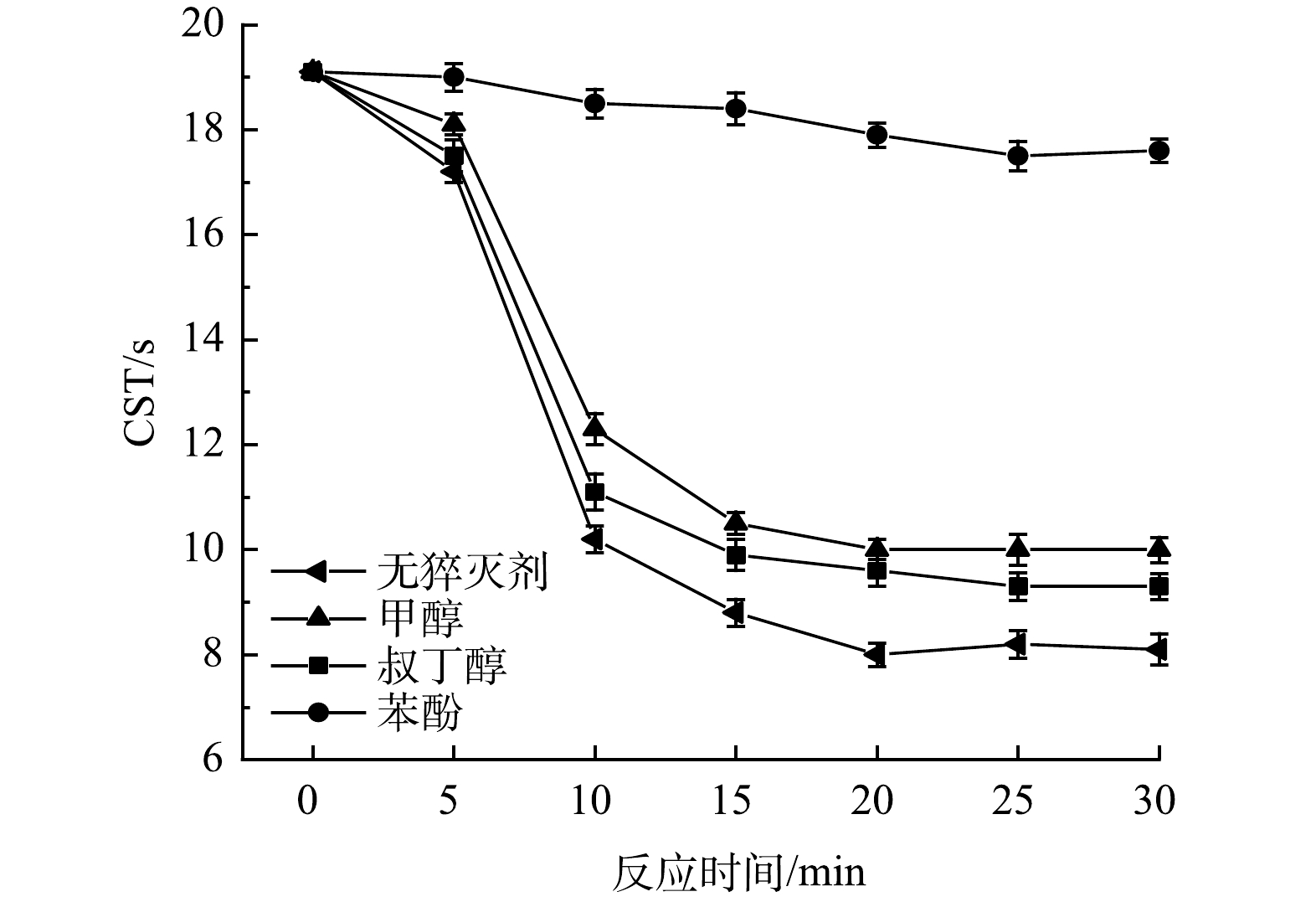
为了探究Iron-SBC/PDS体系调理污泥过程中发挥作用的主要自由基种类,分别采用甲醇、叔丁醇和苯酚3类自由基抑制剂进行猝灭反应。甲醇和苯酚都对SO4−·和·OH自由基均有较高的反应活性,可以作为SO4−·和·OH的淬灭剂[25, 36]。其中,苯酚作为强猝灭剂,更容易接近催化剂表面。叔丁醇对·OH的反应速率常数高 (K·OH=3.8~7.6×108 M−1S−1),而对SO4−·的反应速率常数较低 (KSO4-·=4.0~9.1×108 M−1S−1),是良好、高效的·OH淬灭剂[37]。
取猝灭剂与PDS的初始浓度比为100∶1 (mol·L−1)[25],自由基猝灭反应实验结果见图5。不添加猝灭剂的情况下,随着反应时间的进行,CST快速大幅下降,20 min时达到8 s,此后趋于平稳。分别添加甲醇或叔丁醇时,20 min时CST分别下降到10和9.6 s,此后下降不明显。实验结果表明,加入甲醇或叔丁醇均对CST的降低有一定的抑制作用,这主要是因为,在反应过程中加入猝灭剂能够抑制SO4−·和·OH的生成,对污泥的氧化破解作用减弱,从而使CST值上升。而且,甲醇比叔丁醇抑制作用更明显,这说明体系中既存在·OH,又存在SO4−·。反应体系中加入苯酚时,CST在20 min时仅由最初的19.1 s下降到17.9 s。这说明,苯酚对体系中自由基的猝灭作用远大于甲醇和叔丁醇,该结果与张倩等[25]的研究结果相似。其原因可能是,苯酚是一种强猝灭剂,不仅可以猝灭溶液中的·OH和SO4−·,也可以接近到Iron-SBC的表面,猝灭催化剂表面的自由基。这说明,活化过程主要发生在非均质催化剂的表面,即除了Iron-SBC上的Fe0及铁氧化物产生的溶解性Fe2+活化过硫酸盐外,Iron-SBC表面的含氧官能团,如羟基、羧基等,也对过硫酸盐具有一定的活化作用[38]。
-
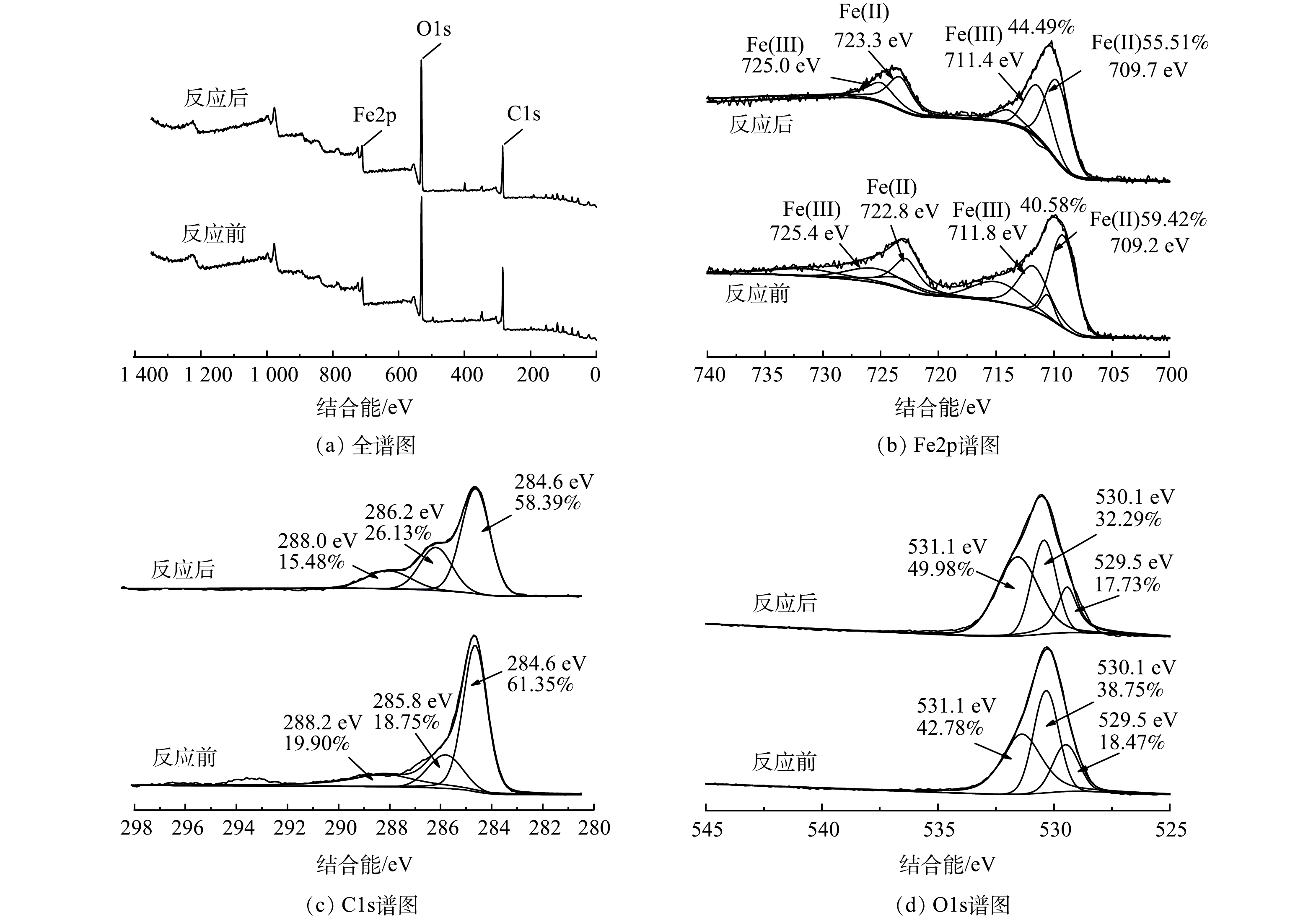
为分析Iron-SBC活化PDS调理污泥脱水的反应机理,对反应前后Iron-SBC进行XPS表征,分析了材料表面Fe、C、O元素的变化,结果见图6。图6(a)为 Iron-SBC的XPS全谱扫描图,724.1 eV附近为Fe2p的峰[39],说明Iron-SBC中含有铁元素;532.1 eV附近为O1s的峰,306.1 eV附近为C1s的峰[39],且2峰较高,说明Iron-SBC中含有较多的氧和碳元素。图6(b)为Fe2p分峰后的图谱,反应前铁元素在结合能709.2和722.8 eV处的峰分别属于Fe2+的Fe2p3/2和Fe2p1/2;在711.8和725.4 eV处的特征峰相对较弱,分别属于Fe3+的Fe2p3/2和Fe2p1/2。其中,Fe2+的质量分数占59.42%,Fe3+的质量分数占40.58%。经过调理污泥的反应后,Fe2+的质量分数下降到55.51%,Fe3+的相对含量增加到44.49%,这说明反应过程中有部分Fe2+被氧化成Fe3+。XPS检测中并没有分析出Fe0,这可能是材料表面被比较厚的物质包覆形成了核壳结构,而XPS只能探测材料表面<10 nm范围的元素分布情况[40],因此,此处XPS的特征峰不明显。图6(c)为C1s分峰后对应的谱图,反应前在284.6、285.8和288.2 eV处对应的官能团分别为C-C官能团[37]、C-OH官能团和C=O官能团[41],3种官能团分别占比为61.53%、18.75%和19.9%。反应后,在284.6、286.2和288.0 eV处对应的官能团分别为C-C官能团、C-O官能团和O-CO官能团,占比分别为58.39%、26.13%和15.48%,这种变化可能是因为Iron-SBC表面发生了氧化反应。图6(d)为O1s的谱图,在结合能为530.1 eV处对应的为C-O键[42],结合能531.1 eV处存在的应该是Fe-O键[42]。
-
1) Iron-SBC的最佳制备条件为给水厂含铁污泥和污水厂污泥比例为3∶1、热解温度800 ℃、热解时间1 h。最佳条件下制备的Iron-SBC上负载了Fe0和多种形式的铁氧化物,包括FeO、Fe2O3和Fe3O4;与混合污泥相比,Iron-SBC上-OH和烯烃类-C=C-含量减少;比表面积和孔容增大,孔径主要集中在3~5 nm。
2) Iron-SBC/PDS联合调理污泥脱水的效果优于Fe0/SBC/PDS、Fe0/PDS和SBC/PDS 3种体系。Iron-SBC/PDS反应体系中强氧化自由基作用,导致污泥破解后有机物质和细胞内氮和磷的溶出,污泥滤液中SCOD、TN和TP的质量浓度升高。
3) Iron-SBC/PDS体系中主要有SO4−·和·OH 2种自由基,且活化过程主要发生在Iron-SBC的表面。XPS分析表明Iron-SBC主要含有Fe、C、O元素,活化PDS过程中Iron-SBC表面发生官能团的氧化反应,且部分Fe2+转化为Fe3+。
铁基污泥炭活化过硫酸盐调理污泥脱水
Preparation of iron-based sludge biochar and its application in sludge dewatering as a activator for persulfate
-
摘要: 利用给水厂含铁污泥和污水厂污泥混合热解制备铁基污泥炭 (Iron-SBC) ,作为过硫酸盐活化剂,用于调理污泥脱水。研究了Iron-SBC的最佳制备条件及其活化PDS调理污泥脱水的效果,并探究其活化机制。结果表明,Iron-SBC最佳制备条件为给水厂含铁污泥和污水厂污泥比例3∶1、热解温度800 ℃、热解时间1 h。XRD、FT-IR及BET分析结果表明,与原混合污泥相比,Iron-SBC比表面积和孔容增大,表面负载了Fe0和FeO、Fe2O3、Fe3O4等铁的氧化物,并含有大量官能团。在活化PDS过程中,Iron-SBC表面的Fe0、铁的氧化物及官能团等均能有效活化PDS,产生SO4−·和·OH自由基。XPS分析结果表明,Iron-SBC表面部分Fe2+被氧化为Fe3+,官能团C-C、C-OH和C=O等被氧化,并有Fe-O键生成。经Iron-SBC/PDS调理污泥后,CST、SRF和Wc分别由原泥的19.1 s、14.9×1012 m·kg−1和85.06%下降到8.4 s、5.4×1012 m·kg−1和73.48%。本研究结果可为含铁污泥和剩余污泥资源化及污泥深度脱水提供参考。Abstract: Iron-based sludge biochar (Iron-SBC) was prepared by pyrolysis using mixed iron-contained sludge from water supply plant and waste activated sludge from sewage treatment plant. It was used as a activator for persulfate to condition sludge for dewatering. The optimal preparation conditions of Iron-SBC, and the effect and mechanism of PDS/Iron-SBC on sludge dewatering were studied. The results showed that the optimum preparation conditions of Iron-SBC were as follows: the ratio of iron-contained sludge to waste activated sludge was 3:1, pyrolysis temperature was 800 ℃, and the pyrolysis time was 1 hour. XRD, FT-IR and BET analysis showed that compared with the original mixed sludge, the specific surface area and pore volume of Iron-SBC increased, and its surface was loaded with iron oxides, as Fe0, FeO, Fe2O3 and Fe3O4, and contained a large number of functional groups. During the process of activating PDS, Fe0, iron oxides and functional groups on the surface of Iron-SBC all could effectively activate PDS and generate SO4−· and ·OH free radicals. XPS analysis showed that Fe2+ on the surface of Iron-SBC was oxidized to Fe3+, and functional groups as C-C, C-OH and C=O were also oxidized, and Fe-O bonds were formed. After the sludge treatment by Iron-SBC/PDS, CST, SRF and Wc were reduced from 19.1 s, 14.9×1012 m∙kg−1 and 85.06% of the raw sludge to 8.4 s, 5.4×1012 m∙kg−1 and 73.48%, respectively. The results of this study can provide a technical reference for the utilization of iron-contained sludge and waste activated sludge, and also for the sludge deep dewatering.
-
Key words:
- iron-based sludge biochar /
- persulfate /
- sludge dewatering /
- advanced oxidation
-

-
表 1 给水厂脱水污泥与污水厂脱水污泥的化学组成
Table 1. Chemical composition of water supply plant dewatered sludge and sewage dewatered sludge %
供试污泥 C O Si Al Fe 其他 给水厂含铁污泥 40.09 34.76 4.97 5.92 8.14 6.12 污水厂污泥 48.49 34.62 3.45 2.96 0.94 9.54 表 2 剩余污泥的基本参数
Table 2. Basic parameters of waste activated sludge
TSS/
(g·L−1)含水
率/%滤饼
含水率/%CST/s SRF/
(×1012 m·kg−1)pH 16.0±0.3 98.5±0.2 85.12±0.53 19.1±0.4 14.9±0.2 6.68±0.1 表 3 MS和Iron-SBC的比表面积及孔容
Table 3. Specific surface area and pore volume of MS and Iron-SBC
样品名称 比表面积/(m2·g−1) 孔容/(cm3·g−1) MS 38.22 0.052 Iron-SBC 89.51 0.158 -
[1] 陈彦秀, 李刚. 市政污泥脱水技术研究进展[J]. 环境科学与技术, 2021, 44(S1): 308-311. doi: 10.19672/j.cnki.1003-6504.2021.S1.049 [2] LI Y F, PAN L Y, ZHU Y Q, et al. How does zero valent iron activating peroxydisulfate improve the dewatering of anaerobically digested sludge?[J]. Water Research, 2019, 163: 114912. doi: 10.1016/j.watres.2019.114912 [3] 张彦平, 裴佳华, 郑松超, 等. 污泥炭负载Fe2+活化过硫酸盐联合PAM调理污泥[J]. 环境科学与技术, 2021, 44(12): 113-119. [4] LIU C G, WU B, CHEN X E. Sulfate radical-based oxidation for sludge treatment: a review[J]. Chemical Engineering Journal, 2018, 335: 865-875. doi: 10.1016/j.cej.2017.10.162 [5] GE D D, DONG Y T, ZHANG W R, et al. A novel Fe2+/persulfate/tannic acid process with strengthened efficacy on enhancing waste activated sludge dewaterability and mechanism insight[J]. Science of the Total Environment, 2020, 733: 139146. doi: 10.1016/j.scitotenv.2020.139146 [6] ZHEN G Y, LU X Q, ZHAO Y C, et al. Enhanced dewaterability of sewage sludge in the presence of Fe(II)-activated persulfate oxidation[J]. Bioresource Technology, 2012, 116: 259-265. doi: 10.1016/j.biortech.2012.01.170 [7] ZHEN G Y, LU X Q, LI Y Y, et al. Novel insights into enhanced dewaterability of waste activated sludge by Fe(II)-activated persulfate oxidation[J]. Bioresource Technology, 2012, 119: 7-14. doi: 10.1016/j.biortech.2012.05.115 [8] XIONG Q, ZHOU M, YANG H, et al. Improving the dewaterability of sewage sludge using rice husk and Fe2+-sodium persulfate oxidation[J]. ACS Sustainable Chemistry & Engineering, 2017, 6(1): 872-881. [9] FENG Y, ZHONG J, ZHANG L Y, et al. Activation of peroxymo nosulfate by Fe0@Fe3O4 core-shell nanowires for sulfate radical generation: electron transfer and transformation products[J]. Separation and Purification Technology, 2020, 247: 116942. doi: 10.1016/j.seppur.2020.116942 [10] 简铃, 严丽丽, 鞠梦灿, 等. 铁/炭复合材料的制备及其类Fenton反应的研究进展[J]. 应用化工, 2023,52(2): 625-632. [11] 丁敬林. 负载纳米零价铁的生物炭活化过硫酸盐去除雌二醇的研究[D]. 长沙: 湖南大学, 2021. [12] WANG Y, TIAN Q B, YANG G Y, et al. Enhanced chlortetracycline removal by iron oxide modified spent coffee grounds biochar and persulfate system[J]. Chemosphere, 2022, 301: 134654. doi: 10.1016/j.chemosphere.2022.134654 [13] 李玉双, 杨嘉鑫, 魏建兵, 等. 城市污泥资源化利用技术研究进展[J]. 工业水处理, 2022, 42(12): 41-46. [14] 郝晓地,李佳勇,郝丽婷,等.剩余污泥制取生物炭可行性分析与评价[J/OL].中国给水排水(2023-03-02). http://kns.cnki.net/kcms/detail/12.1073.tu.20230109.1728.001.html. [15] WEI H, GAO B Q, REN J, et al. Coagulation/flocculation in dewatering of sludge: a review[J]. Water Research, 2018, 143: 608-631. doi: 10.1016/j.watres.2018.07.029 [16] 张彦平, 裴佳华, 高珊珊, 等. 生物质材料用于污泥深度脱水的研究进展[J]. 工业水处理, 2022, 42(7): 24-32. [17] HU L L, LIAO Y, HE C, et al. Enhanced dewaterability of sewage sludge with zero-valent iron-activated persulfate oxidation system[J]. Water Science & Technology, 2015, 72(2): 245-251. [18] 王澜. 基于Fe2+/HSO5-体系的污泥氧化调理工艺研究[D]. 哈尔滨: 哈尔滨工业大学, 2019. [19] 曾婧, 荀久玉. 过硫酸钠与PAM联合改善污泥脱水效果的研究[J]. 江西化工, 2020, 150(4): 45-49. doi: 10.3969/j.issn.1008-3103.2020.04.015 [20] WU H L, CHE X D, DING Z H, et al. Release of soluble elements from biochars derived from various biomass feedstocks[J]. Environmental Science and Pollution Research International, 2016, 23(2): 1905-1915. doi: 10.1007/s11356-015-5451-1 [21] LI R N, WANG Z W, ZHAO X T, et al. Magnetic biochar-based manganese oxide composite for enhanced fluoroquinolone antibiotic removal from water[J]. Environmental Science and Pollution Research International, 2018, 25(31): 31136-31148. doi: 10.1007/s11356-018-3064-1 [22] 喻江维. 臭氧与零价铁/铁基生物炭协同调理市政污泥促进污泥深度脱水的研究[D]. 武汉: 华中科技大学, 2018. [23] 邹意义, 袁怡, 沈涛, 等. FeCl3改性污泥生物炭对水中吡虫啉的吸附性能研究[J]. 环境科学学报, 2021, 41(9): 3478-3486. [24] ALIREZA N E, AREZOO S. Enhancement of the photocatalytic activity of ferrous oxide by doping onto the nano-clinoptilolite particles towards photodegradation of tetracycline[J]. Chemosphere, 2014, 107: 136-144. doi: 10.1016/j.chemosphere.2014.02.015 [25] 张倩, 谢陈飞洋, 仇玥, 等. Fe/污泥基生物炭持久活化过硫酸盐降解酸性橙G[J]. 中国环境科学, 2019, 39(9): 3879-3886. doi: 10.3969/j.issn.1000-6923.2019.09.034 [26] 胡益, 李培生, 余亮英. 污泥与煤混烧中含碳官能团的演化过程[J]. 武汉大学学报(工学版), 2013, 46(5): 649-653. [27] 胡艳军, 王琳洁, 卢艳军, 等. 污泥含碳有机官能团分布及其模型化合物构建[J]. 中国环境科学, 2019, 39(9): 3872-3878. doi: 10.3969/j.issn.1000-6923.2019.09.033 [28] WU W, ZHU S S, HUANG X C, et al. Mechanisms of persulfate activation on biochar derived from two different sludges: Dominance of their intrinsic compositions[J]. Journal of Hazardous Materials, 2021, 408: 124454. doi: 10.1016/j.jhazmat.2020.124454 [29] OU Y D, YAN J, QIAN L, et al. Degradation of 1, 4-dioxane by biochar supported nano magnetite particles activating persulfate[J]. Chemosphere, 2017, 184: 609-617. doi: 10.1016/j.chemosphere.2017.05.156 [30] 方帅. 地下水厂铁泥制备磁性吸附剂的研究[D]. 长春: 东北师范大学, 2015. [31] 高少敏. 磁性氧化铈材料的制备及其在染料废水处理中的应用[D]. 上海: 上海应用技术大学, 2019. [32] XU Z H, ZHOU Y W, SUN Z H, et al. Understanding reactions and pore-forming mechanisms between waste cotton woven and FeCl3 during the synthesis of magnetic activated carbon[J]. Chemosphere, 2020, 241: 125120. doi: 10.1016/j.chemosphere.2019.125120 [33] LIU X Y, YANG L, ZHAO H T, et al. Pyrolytic production of zerovalent iron nanoparticles supported on rice husk-derived biochar: simple, in situ synthesis and use for remediation of Cr(VI)-polluted soils[J]. Science of the Total Environment, 2020, 708(C): 134479. [34] 郑松超. 污泥炭负载Fe(Ⅱ)活化过硫酸盐对污泥脱水及重金属去除的研究[D]. 天津: 河北工业大学, 2021. [35] 万甜, 闫幸幸, 任杰辉, 等. Fe(Ⅱ)活化过硫酸盐改善污泥脱水性能[J]. 环境工程学报, 2020, 14(1): 189-196. doi: 10.12030/j.cjee.201902067 [36] 彭小明, 吴健群, 戴红玲, 等. Ni-N-C单原子催化剂活化过硫酸盐降解苯酚[J]. 高等学校化学学报, 2021, 42(8): 2581-2591. doi: 10.7503/cjcu20210009 [37] WANG D, SUN Y, TANG D C, et al. Synergistic utilization of inherent halides and alcohols in hydraulic fracturing wastewater for radical-based treatment: a case study of di-(2-ethylhexyl) phthalate removal[J]. Journal of Hazardous Materials, 2020, 384: 121321. doi: 10.1016/j.jhazmat.2019.121321 [38] 肖鹏飞, 安璐, 韩爽. 炭质材料在活化过硫酸盐高级氧化技术中的应用进展[J]. 化工进展, 2020, 39(8): 3293-3306. doi: 10.16085/j.issn.1000-6613.2019-1833 [39] 梁宇坤. 生物炭负载纳米零价铁镍激活过硫酸盐降解诺氟沙星废水[D]. 太原: 太原理工大学, 2019. [40] LI J X, ZHANG X Y, LIU M C, et al. Enhanced reactivity and electron selectivity of sulfidated zerovalent iron toward chromate under aerobic conditions[J]. Environmental Science & Technology, 2018, 52(5): 2988-2997. [41] HAN Q, WANG Z H, XIA J F, et al. Facile and tunable fabrication of Fe3O4 /graphene oxide nanocomposites and their application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples[J]. Talanta, 2012, 101: 388-395. doi: 10.1016/j.talanta.2012.09.046 [42] 曹华莉. 基于过硫酸盐的铁基复合材料制备及去除活性黑5的效能研究[D]. 南昌: 南昌大学, 2020. -



 下载:
下载: