-
随着我国畜禽养殖业规模化、集约化的快速发展,养殖粪污环境污染问题日益严重,养殖废弃物处理与资源化利用受到业界关注。为此,厌氧发酵作为养殖废弃物处理技术具有运行成本低、管理方便、能源回收等优点,得到广泛的应用。然而,厌氧发酵的效果受多种因素影响。其中,总氨氮(total ammonia nitrogen, TAN)既能为微生物提供必须的氮源营养,又能提高体系的缓冲能力,但高浓度氨氮也是微生物抑制剂[1],一般可使产气量降低30%[2]。对于猪粪等富氮原料的沼气工程,高浓度氨氮是发酵低效甚至失败的最重要因素。为此,国内外学者对厌氧发酵氨氮抑制进行了大量研究。RAJAGOPAL等[3]系统总结了TAN质量浓度对厌氧发酵系统的影响;AKINDELE等[4]采用连续实验研究了不同pH和外源氨氮添加对城镇垃圾厌氧发酵的影响;乔玮等[5]对比了中高温条件下,氨氮质量浓度对鸡粪厌氧发酵产甲烷性能和挥发性脂肪酸产生特点。对于猪粪厌氧发酵,由于猪粪、猪尿中含有较高浓度的尿素、氨基酸等有机氮,发酵过程中有机氮分解,从而提高了氨抑制的风险[6]。HENSEN等[7]观察到,在不同温度条件下,约6 000 mg·L−1质量浓度的TAN就会对猪粪底物的全混合式厌氧发酵反应器(continuous stirred tank reactor, CSTR)产生抑制。孟晓山等[8]以猪粪为底物,以外源氯化铵为氮源开展序批次实验,发现氨氮添加量在2 000 mg·L−1以上时会产生显著抑制,添加量大于4 000 mg·L−1时会加剧氨氮抑制。由于厌氧发酵氨抑制受原料、温度、pH和接种物等诸多因素影响,抑制浓度、发生过程及机理机制差异较大。其中,发酵温度影响游离氨(free ammonia nitrogen, FAN)和铵氮间的转化及产甲烷的生化过程[9],是重要参数之一,提高发酵温度,有利于提升产气效率,但同时会增加氨抑制的风险。以往的研究一般关注中温(30~40 ℃)和高温(50~60 ℃)下的厌氧发酵,但部分研究表明[10-12],在40~50 ℃这一温度区间内,存在发酵的最适温度。有研究者[13-14]指出,与中温条件相比,高氮原料在42~45 ℃下具有更好的产气性能,且伴随着微生物群落和甲烷代谢方式的转变。目前的研究多以序批次实验为实验对象,缺乏以温度为变量的连续发酵实验。然而,厌氧发酵氨抑制研究的重点在于针对不同温度条件,探究发酵体系在不同梯度质量浓度氨氮下的响应,以明确温度对发酵体系氨氮耐受性的影响效应及机制。
本研究以猪粪为底物,分别在35、44、55 ℃下开展氨氮胁迫下厌氧发酵半连续实验,研究pH、甲烷产率、挥发性脂肪酸(volatile fatty acids, VFAs)等对氨氮质量浓度和温度变化的响应,并开展相关性分析,旨在进一步明确不同温度下氨氮胁迫对猪粪厌氧发酵产气性能的影响,为猪粪厌氧发酵工艺优化和效率提升提供数据支持。
-
实验用猪粪取自天津市西青区某养猪场,鲜猪粪取回后于(4±1) ℃下保存备用;接种物取自实验室正常运行的CSTR,猪粪和接种物的理化性质见表1。
-
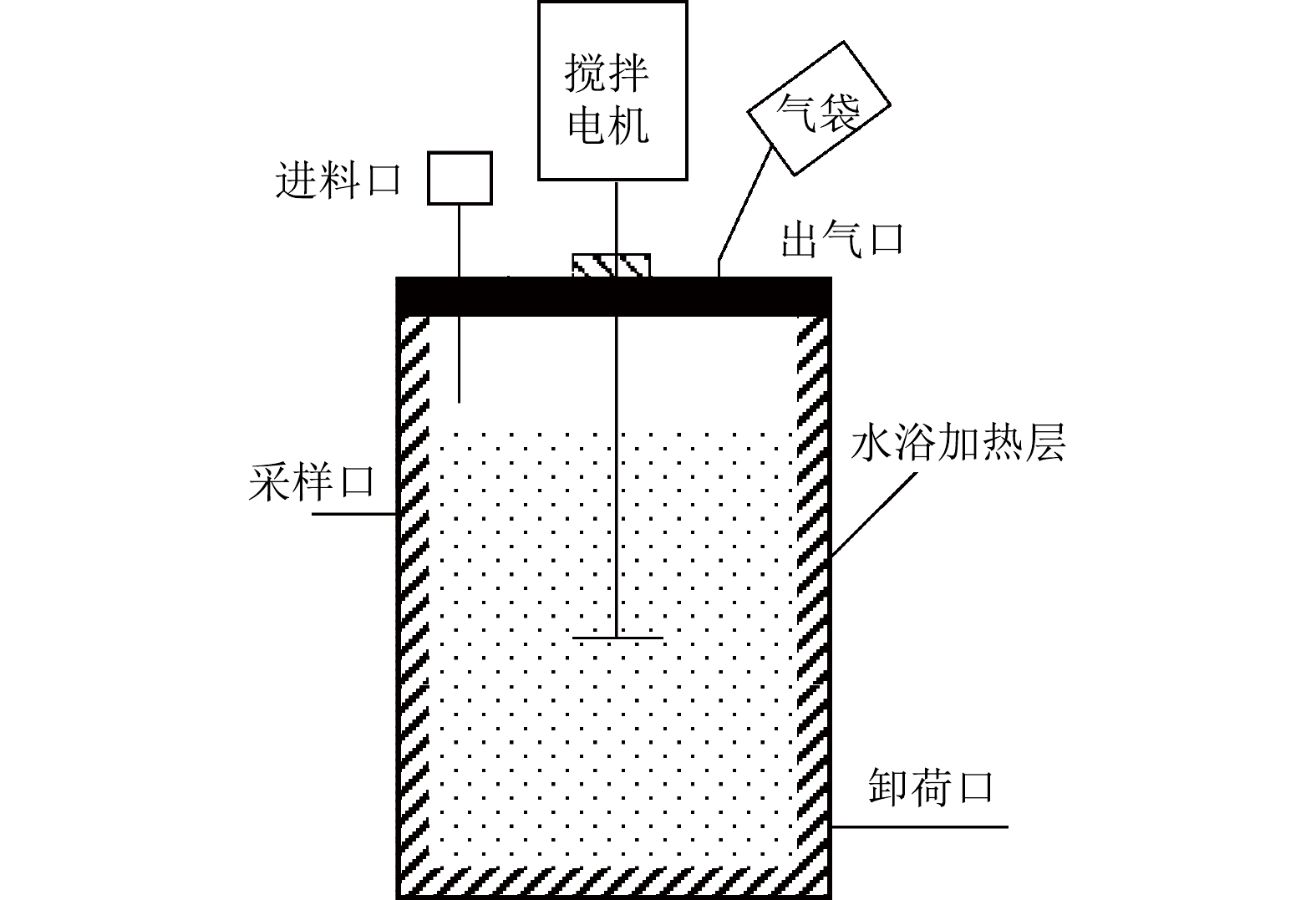
实验装置采用半连续搅拌厌氧反应器系统(CSTR-10S,瑞典Bioprocess Control公司),有效容积10 L、双层水浴加热、机械搅拌、转速为70 r·min−1,反应器连接10 L铝箔集气袋(图1)。
-
本实验以猪粪为原料,反应物料体积为10 L,采用半连续进料,有机负荷率(organic loading rate, OLR)为3 g·(L·d)−1,水力停留时间(hydraulic retention time, HRT)为30 d。通过在进料中添加氯化铵调整发酵体系氨氮质量浓度,发酵过程分为5个阶段,对应发酵时长及进料中外源氨氮质量浓度分别为:P1(0~42 d、0 mg·L−1),P2(43~95 d、2 000 mg·L−1),P3(96~132 d、3 000 mg·L−1),P4(133~159 d、4 000 mg·L−1),P5(160~181 d、5 000 mg·L−1)。每个阶段进料中添加氯化铵的浓度分别为0、7.63、11.45、15.27、19.08 g·L−1。每天进出料1次,进料时将猪粪与蒸馏水混合,加入氯化铵固体,用蒸馏水调至333.33 mL,搅拌均匀后加入反应器。在进料前出料,出料量约333.33 mL。实验设3个反应器(R1、R2和R),分别在35、44和55 ℃下进行发酵,通过外接铝箔气袋收集气体,每天计量产生气体体积,取样分析CH4和CO2体积分数。取发酵后鲜样品,分析pH、氨氮质量浓度、VFA质量浓度等指标。取鲜样时,用取样勺通过采样口快速舀取发酵罐内样品,取样完成后,关闭采样口,避免空气进入。
-
氨氮质量浓度采用纳氏试剂分光光度法(DR 6000, 美国HACH公司)测量;pH采用pH计(WTW 7110, 德国WTW公司)测定;总固体(total solid, TS)和挥发性固体(volatile solid, VS)采用重量法测定;沼气体积用防腐湿式气体流量计进行测量;沼气组分采用气相色谱仪(Thermo trace 1300, 美国Thermo Fisher公司)测定,PP-Q色谱柱(2 m×φ2 mm),He为载气(75 kPa恒压),炉温40 ℃,进样口和检测器温度均为200 ℃;VFAs采用气相色谱仪测定(Thermo trace 1300, 美国Thermo Fisher公司),气相色谱配M12毛细管柱(30 m×0.53 mm×1 μm),载气为He(恒流:7.00 mL·min−1),进样口温度200 ℃,FID温度220 ℃。总挥发性脂肪酸(total volatile fatty acids, TVFAs)的质量浓度为换算成乙酸后的各种VFA浓度之和。碱度(alkalinity, ALK)采用电位滴定法(WTW 7110, 德国WTW公司)测定。FAN质量浓度计算方法参照式(1)[7]。
式中:CFAN为FAN质量浓度,mg·L−1;CTAN为TAN质量浓度,mg·L−1;T为厌氧发酵反应温度,K;pH为样品实测pH值。
采用SPSS 25.0统计软件对实验数据进行Pearson相关性分析,采用Origin 9.1绘图软件进行绘图。
-
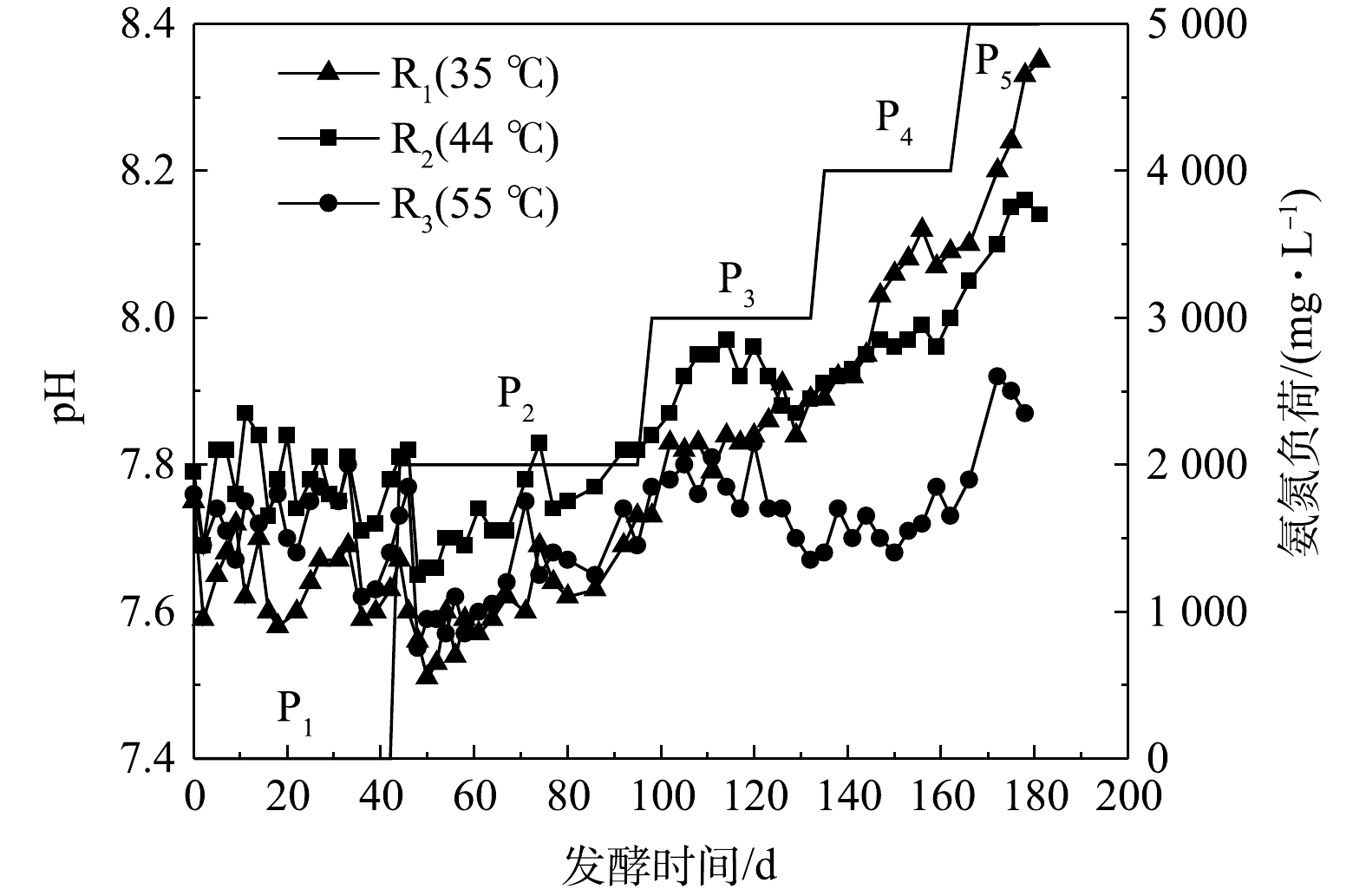
厌氧发酵过程中,pH反映了发酵体系中大分子有机物水解酸化成VFAs和VFAs被产甲烷菌利用的动态过程,一般认为产甲烷菌的最适pH值为6.80~8.00。由图2可知,无外源氨氮添加时(P1),3组反应器pH为7.60~7.90,其中R2最高、R1最低。当外源氨氮质量浓度为2 000 mg·L−1时,各反应器中的pH开始下降,可能是因为加入的NH4+与OH−结合生成NH3·H2O所致。当外源氨氮质量浓度达到4 000 mg·L−1后,各反应器中pH大小为R1>R2>R3。该现象反映了高氨氮浓度下,较高的温度不利于产甲烷菌对于有机酸的利用,导致其产生积累。
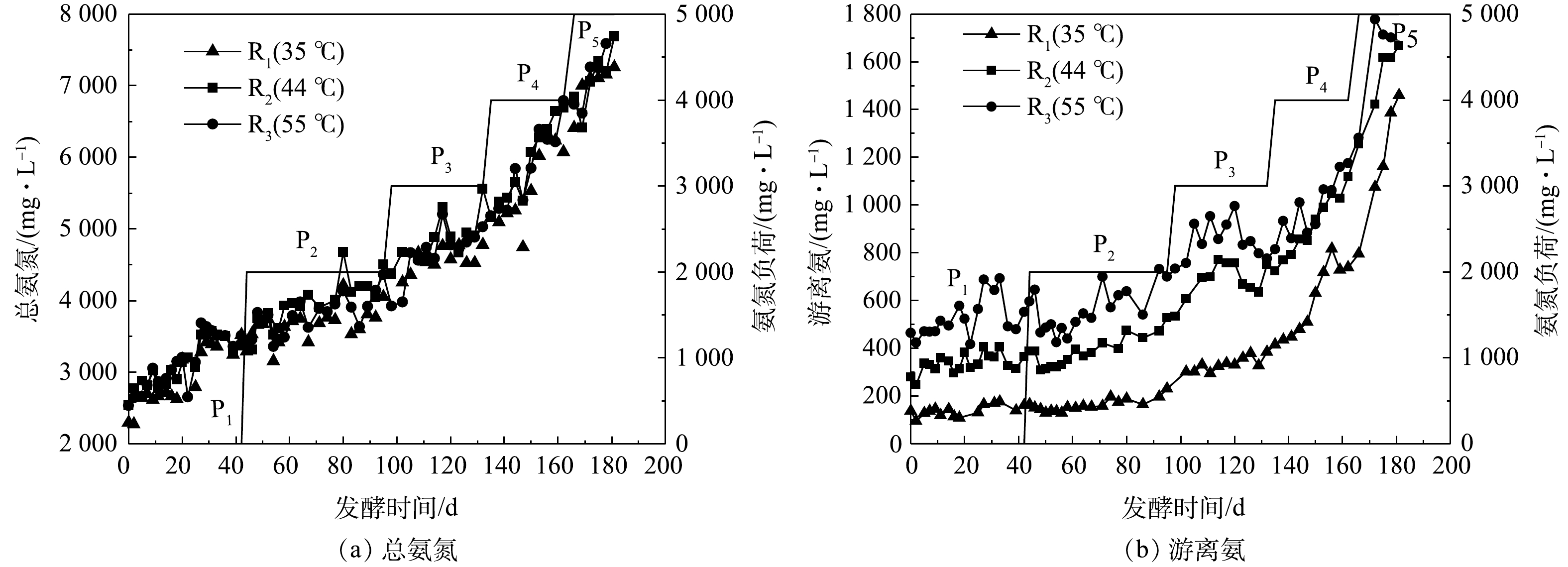
图3为发酵过程中不同反应器内TAN和FAN质量浓度的变化。由图3(a)可知,TAN质量浓度从初始的2 500 mg·L−1左右逐渐升高至7 000 mg·L−1以上,且不同温度下无明显差异;与TAN相似,FAN的质量浓度随着氨氮负荷的增加逐渐升高(图3(b))。整个发酵过程中,不同反应器中的FAN质量浓度高低依次为R3>R2>R1。结合式(1)和图2可以推断,该实验中FAN质量浓度的差异应主要归因于反应温度的不同。
-
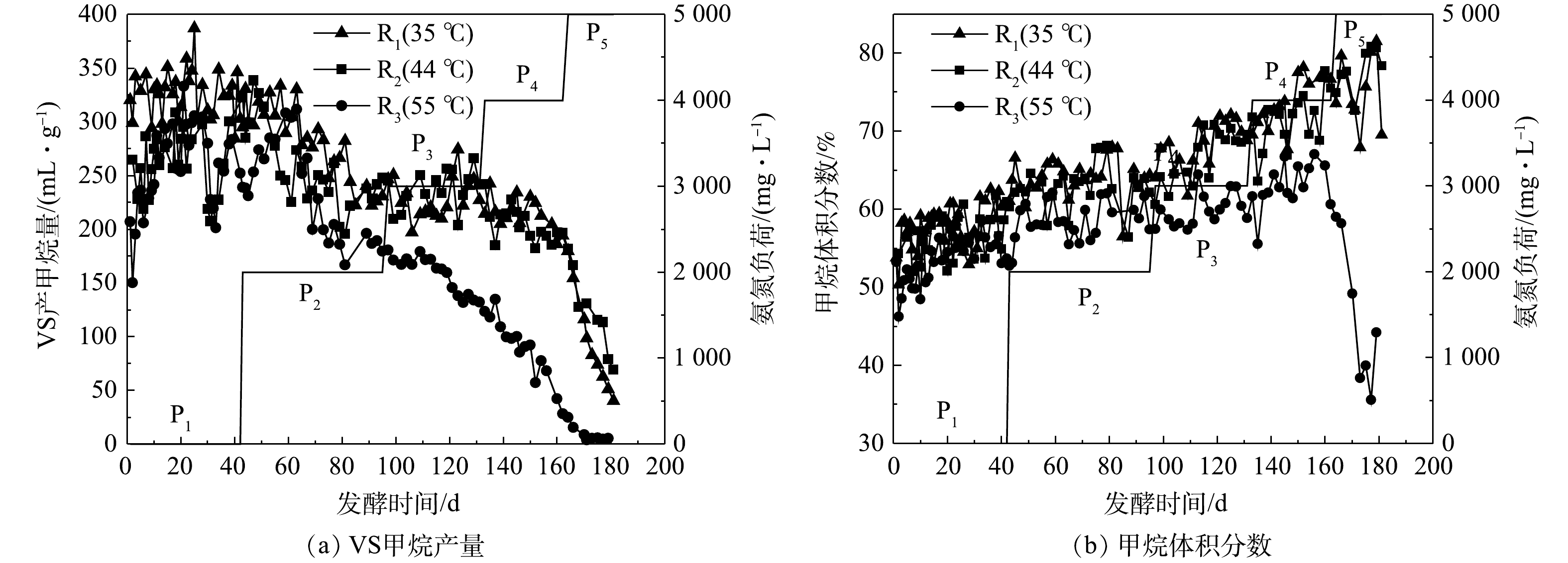
图4(a)为发酵过程中各处理猪粪VS产甲烷量(specific methane yield, SMY)的变化情况。P1时,R1的SMY较R2和R3稳定,这可能是因为发酵接种物取自35 ℃运行的CSTR反应器,虽然经过中温和高温的驯化,但仍未完全适应,导致其稳定性略差,随着发酵的进行趋于稳定。以P1阶段平均SMY为对照,各反应器SMY下降50%时对应的氨氮质量浓度为半抑制阈值。R1、R2和R3的半抑制点分别出现在P5、P5和P4,对应的TAN质量浓度分别为6 416、6 845和5 034 mg·L−1,这说明高温厌氧发酵体系对TAN更敏感,更容易被抑制[15]。该阈值高于乔玮等[5]在鸡粪中温(37 ℃、5 500 mg·L−1)和高温(55 ℃、2 500 mg·L−1)厌氧发酵的半抑制阈值,特别是55 ℃下的半抑制阈值增加了1倍以上。R1(35 ℃)、R3(55 ℃)中与TAN半抑制阈值相对应的FAN质量浓度分别为795和776 mg·L−1,与高文萱等[16]、SASAKI等[17]的研究结果接近。而44 ℃下相关研究鲜见报道,本实验中该温度下(R2)FAN的半抑制阈值约为1 257 mg·L−1。
表2为不同外源氨氮添加量下各反应器的平均SMY。在P2~P4阶段,R1和R2的甲烷产量相近,均明显高于R3,且随着外源氨氮添加量的增加,该差距相应增大。一般认为,与铵态氮相比,FAN对产甲烷微生物的毒性更强,是导致厌氧发酵氨氮抑制的主要原因[18]。但是,各反应器SMY(表2和图4)和FAN变化(图3)显示,在P4和P5阶段,虽然R2和R3的FAN质量浓度接近,但R3的SMY远低于R2。这可能的原因是,在55 ℃下,FAN具有更强的细胞穿透性,从而导致产甲烷菌活性降低。另外,产甲烷微生物的种群结构可能发挥重要的作用。中温发酵(20~45 ℃、通常35 ℃)较高温发酵(通常55 ℃)具有更高的生物多样性,对环境变化具有更强的抵御能力,因此R1和R2的甲烷产量高于R3。P5阶段R2的甲烷产量高于R1可能与甲烷代谢途径有关。有研究表明,与氢营养型产甲烷菌相比,乙酸营养型产甲烷菌对FAN更敏感,耐受阈值为700 mg·L−1,而氢营养型菌达到1 200 mg·L−1[19]。该结论与本实验R1和R2中FAN的半抑制阈值接近,故推测44 ℃下的产甲烷途径可能存在由乙酸营养型向氢营养型的转变。
图4(b)为沼气中的甲烷体积分数。随着发酵体系氨氮质量浓度的提高,R1和R2中甲烷体积分数逐渐升高,R3中平均甲烷体积分数从P1的53.31%增加到P4的62.62%,但在P5阶段急剧下降至47.56%,与SMY的结果一致。在本实验中,P4、P5阶段的R1和R2中不断升高的甲烷体积分数和逐渐降低的SMY表明,在猪粪等高含氮原料厌氧发酵体系中,不能单纯以甲烷体积分数作为衡量厌氧发酵系统是否良好的参考指标。
-
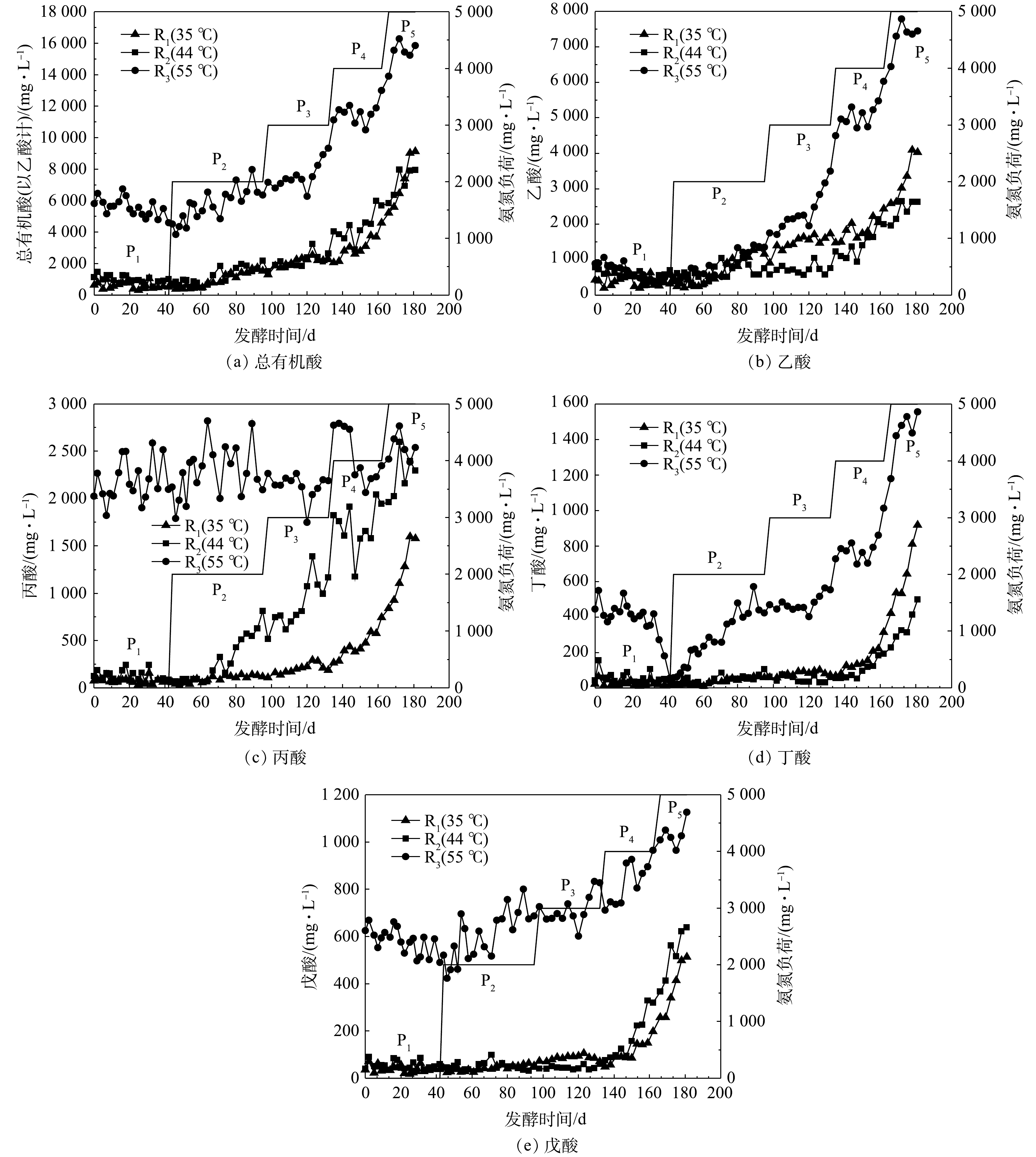
VFAs是有机物厌氧发酵的中间产物,其浓度可在一定程度上反映有机物水解酸化和产甲烷之间的动态平衡。图5为各处理实验过程中发酵液总挥发性脂肪酸(total volatile fatty acids, TVFAs)、乙酸、丙酸、丁酸(包含异丁酸)和戊酸(包含异戊酸)质量浓度的变化。P1阶段,R1、R2的TVFAs质量浓度接近且较稳定,这表明体系中产酸和消耗酸的速率达到动态平衡[8];R3的TVFAs质量浓度虽然达到4 500 mg·L−1左右,但仍低于产甲烷菌的耐受浓度10 000 mg·L−1[20]。P1和P3阶段,35和44 ℃发酵体系中TVFAs的质量浓度略微升高,但55 ℃发酵体系中表现出更明显的升高趋势,这可能是因为55 ℃下加速了猪粪的水解、产酸反应所致。当TAN质量浓度高于5 000 mg·L−1时(P4和P5),各反应器内TVFAs出现积累。
图5(b)、图5(c)分别为乙酸、丙酸质量浓度变化情况。可见,33和44 ℃下,P4后期(FAN约730 mg·L−1),乙酸出现明显积累,而55 ℃下P3后期(FAN约800 mg·L−1)即发生明显积累,而且积累更为严重。结合产甲烷情况可以推断,该FAN质量浓度下已经发生了明显的氨氮抑制,导致TVFAs积累。P3之后,R2中的乙酸质量浓度低于R1,这说明R2中乙酸产甲烷过程受到的抑制小于R1,该现象与SMY趋势相吻合。从发酵开始至P2中期,丙酸在R1和R2中质量浓度接近,均在200 mg·L−1以下。丙酸质量浓度在R1的P4阶段逐渐升高,在R2的P2中期后逐渐升高,而在R3中基本不受氨氮质量浓度影响(平均值2 273 mg·L−1上下波动),明显高于R1和R2,这与李红丽等[21]的研究结果相似。这可能是高温环境发酵体系微生物种群多样性低,影响了丙酸降解微生物的互营代谢[22]所致。
图5(d)、图5(e)分别为发酵体系中丁酸(包含异丁酸)、戊酸(包含异戊酸)质量浓度变化曲线。可以看出,R1与R2中,丁酸与戊酸质量浓度具有相同的变化过程,6 000 mg·L−1左右的TAN对丁酸和戊酸的降解产生一定的抑制作用,即氨胁迫导致了35 ℃和44 ℃下丁酸和戊酸的积累。R3中,丁酸和戊酸质量浓度在P1阶段呈现下降趋势,添加外源氨氮后不断升高。其中,丁酸的积累现象更加明显,至发酵末期丁酸和戊酸质量浓度分别达到1 500和1 100 mg·L−1左右。
-
碱度(ALK)是衡量厌氧发酵过程稳定性的重要指标,通常以TVFAs质量浓度和ALK的比值(TVFAs/ALK=α)衡量体系的缓冲能力。一般认为,α<0.35时发酵系统具有较好的稳定性[23]。由该实验α变化曲线(图6)可知,对于R1、R2,P1至P4阶段的α值均小于0.4;出现明显抑制的P5中,α值仅为0.25左右。R3的α值明显高于R1和R2,在P1~P3期间于0.4上下波动,P4和P5的α值在0.55~0.80变化,对应的SMY明显降低。结合图5(a)发现,在35、44 ℃发酵体系中,α值在0.2~0.3的范围即表现出酸积累的趋势。55 ℃发酵中,α<0.5时体系具有较好的稳定性。因此,在猪粪厌氧发酵中,应根据不同发酵温度对α值进行更为具体的划分,从而准确判断体系稳定性。
-
表3为各反应器的SMY、TAN质量浓度、FAN质量浓度、乙酸质量浓度和丙酸质量浓度5个指标之间Pearson相关性分析结果。在35和44 ℃发酵条件下,SMY与其余4个指标均呈极显著负相关关系(P<0.01),且较于TAN,FAN对于SMY抑制作用更大。TAN、FAN、乙酸和丙酸质量浓度4个指标之间的相关系数均大于0.8,存在极显著的正相关(P<0.01),这说明TAN和FAN质量浓度对乙酸和丙酸质量浓度的增加具有直接的作用。
在55 ℃厌氧发酵条件下,SMY与FAN质量浓度、TAN质量浓度、乙酸质量浓度均呈极显著负相关关系(P<0.01)。该温度下的产生氨氮抑制主要因素为总氨氮,其次为游离氨,这与BI等[24]在鸡粪厌氧发酵中的研究结果相似。此外,该温度下丙酸质量浓度与其它各个指标之间相关系数均小于0.5,相关性不显著,说明该温度下的丙酸代谢生成和分解的过程和速率与35和44 ℃存在差异。
-
1) 35、44、55 ℃下,厌氧发酵体系中半抑制TAN质量浓度分别为6 416、6 845和5 034 mg·L−1,对应的FAN质量浓度分别为795、1 257和776 mg·L−1。55 ℃发酵中氨氮抑制的影响最大。7 000 mg·L−1左右的高TAN质量浓度条件下,44 ℃发酵体系的耐受性较好。
2) 35、44 ℃下,体系中丙酸、丁酸和戊酸质量浓度随氨氮负荷增加逐渐增大,氨胁迫导致了35、44 ℃下3种脂肪酸的积累。55 ℃下丙酸、丁酸和戊酸等脂肪酸生成和分解的过程和速率与35和44 ℃存在明显差异,55 ℃下发酵全过程中丙酸和戊酸质量浓度稳定且维持在较高范围内,这2种脂肪酸质量浓度水平基本不受氨胁迫影响,该温度下丁酸存在受氨胁迫而积累的趋势。
3) Pearson相关性分析结果表明,35、44 ℃下通过氨氮抑制影响甲烷产率的主要因素为FAN,而55 °C下的主要因素为TAN。因此,在不同温度条件下,应根据不同形态氨氮,制定相应的氨氮抑制缓解策略。
不同温度下猪粪厌氧发酵的氨胁迫效应
Ammonia inhibition effect of anaerobic digestion of swine manure at different temperatures
-
摘要: 为了明确不同温度下氨胁迫对猪粪厌氧发酵性能的影响,以猪粪为底物,通过添加外源氯化铵逐步提高氨氮质量浓度,在3种温度下开展了半连续厌氧发酵实验。结果表明,在3种温度下,当总氨氮质量浓度达到4 000 mg·L−1时,VS甲烷产率明显降低。VS甲烷产率半抑制时,44 ℃发酵体系中的总氨氮质量浓度达到6 845 mg·L−1,对应游离氨质量浓度为1 257 mg·L−1;在7 000 mg·L−1左右的总氨氮质量浓度下,44 ℃发酵体系表现出更好的耐受性。氨胁迫导致了35、44 ℃下丙酸、丁酸和戊酸等3种脂肪酸的积累;在55 ℃下,该3种脂肪酸生成和分解的过程和速率与35、44 ℃存在明显差异。Pearson相关性分析结果表明,35、44 ℃下通过氨抑制影响甲烷产率的主要因素为游离氨(NH3),而55 ℃下的主要因素为总氨氮。该研究结果可为养殖粪污厌氧发酵的工艺优化提供参考。Abstract: In order to understand the effect of ammonia stress on the performance of anaerobic digestion (AD) of swine manure at different temperatures, the semi-continuous anaerobic digestion experiments with swine manure as substrate were carried out at three temperatures. During the experiments, the concentration of ammonia nitrogen was increased by adding exogenous ammonium chloride. The results showed that the specific methane yield (SMY) decreased significantly when the total ammonia nitrogen (TAN) mass concentration reached 4 000 mg·L−1 under different temperatures, and half maximal inhibitory concentration (IC50) of TAN on SMY at 44 ℃ was 6 845 mg·L−1, corresponding to free ammonia nitrogen (FAN) mass concentrations 1 257 mg·L−1. At a high TAN mass concentrations of approximately 7 000 mg·L−1, the 44 ℃ digestion system showed better tolerance. Ammonia stress caused the accumulation of three fatty acids at 35 ℃ and 44 ℃, including propionic acid, butyric acid, and valeric acid. The processes and rates of the formation and decomposition of the three fatty acids at 55 ℃ were significantly different from those at 35 ℃ and 44 ℃. Pearson correlation analysis results showed that the main factor affecting methane yield through ammonia inhibition at 35 ℃ and 44 ℃ was FAN (NH3), and the main factor at 55 ℃ was TAN. The results of this study can provide a reference for the optimization of the anaerobic fermentation process of aquaculture manure.
-

-
表 1 原料和接种物理化性质
Table 1. Characteristics of feedstock and inoculum
供试原料 总固体/% 挥发性固体/% 总氮 氨氮 C/N pH 猪粪 26.01 82.83 3.96%±0.32% 0.61%±0.08% 11.56±0.37 7.94 接种物 13.69 58.46 1 976 mg·L−1 1 562 mg·L−1 — 8.23 注:猪粪与接种物中总氮和氨氮单位不同,猪粪总氮和氨氮为干重下质量分数。 表 2 各反应器不同阶段的平均VS产甲烷量
Table 2. Average SMY of each reactor at different stages
反应器 平均VS产甲烷量/(mL·g−1) P1 P2 P3 P4 P5 R1 326.0 281.5 229.8 214.3 95.7 R2 261.3 257.9 236.0 204.3 122.9 R3 256.3 234.7 158.1 88.3 9.2 表 3 各指标之间Pearson相关性分析
Table 3. Correlation analysis among variables
反应器 检测指标 VS甲烷产量 总氨氮 游离氨 乙酸 丙酸 R1(35 ℃) VS甲烷产量 1 总氨氮 −0.732** 1 游离氨 −0.784** 0.924** 1 乙酸 −0.820** 0.931** 0.948** 1 丙酸 −0.752** 0.871** 0.979** 0.925** 1 R2(44 ℃) VS甲烷产量 1 总氨氮 −0.609** 1 游离氨 −0.658** 0.954** 1 乙酸 −0.653** 0.849** 0.907** 1 丙酸 −0.628** 0.941** 0.934** 0.886** 1 R3(55 ℃) VS甲烷产量 1 总氨氮 −0.812** 1 游离氨 −0.795** 0.934** 1 乙酸 −0.870** 0.942** 0.906** 1 丙酸 −0.281* 0.334** 0.285* 0.426** 1 注:* 代表P<0.05,** 代表P<0.01。 -
[1] YENIGUN O, DEMIREL B. Ammonia inhibition in anaerobic digestion: A review[J]. Process Biochemistry, 2013, 48(5/6): 901-911. [2] YANG Z Y, WANG W, HE Y F, et al. Effect of ammonia on methane production, methanogenesis pathway, microbial community and reactor performance under mesophilic and thermophilic conditions[J]. Renewable Energy, 2018, 125: 915-925. doi: 10.1016/j.renene.2018.03.032 [3] RAJAGOPAL R, MASSE D I, SINGH G. A critical review on inhibition of anaerobic digestion process by excess ammonia[J]. Bioresource Technology, 2013, 143: 632-641. doi: 10.1016/j.biortech.2013.06.030 [4] AKINDELE A A, SARTAJ M. The toxicity effects of ammonia on anaerobic digestion of organic fraction of municipal solid waste[J]. Waste Management, 2018, 71: 757-766. doi: 10.1016/j.wasman.2017.07.026 [5] 乔玮, 姜萌萌, 赵婧, 等. 中温和高温环境下乙酸和丙酸厌氧发酵产甲烷动力学特征[J]. 农业工程学报, 2018, 34(21): 234-238. doi: 10.11975/j.issn.1002-6819.2018.21.029 [6] ZHENG H Y, LI D W, STANISLAUS M S, et al. Development of a bio-zeolite fixed-bed bioreactor for mitigating ammonia inhibition of anaerobic digestion with extremely high ammonium concentration livestock waste[J]. Chemical Engineering Journal, 2015, 280: 106-114. doi: 10.1016/j.cej.2015.06.024 [7] HANSEN K H, ANGELIDAKI I, AHRING B K. Anaerobic digestion of swine manure: Inhibition by ammonia[J]. Water Research, 1998, 32(1): 5-12. doi: 10.1016/S0043-1354(97)00201-7 [8] 孟晓山, 张玉秀, 隋倩雯, 等. 氨氮浓度对猪粪厌氧消化及产甲烷菌群结构的影响[J]. 环境工程学报, 2018, 12(8): 2346-2356. doi: 10.12030/j.cjee.201802064 [9] POIRIER S, DESMOND-LE QUEMENER E, MADIGOU C, et al. Anaerobic digestion of biowaste under extreme ammonia concentration: Identification of key microbial phylotypes[J]. Bioresource Technology, 2016, 207: 92-101. doi: 10.1016/j.biortech.2016.01.124 [10] HUPFAUF S, PLATTNER P, WAGNER A O, et al. Temperature shapes the microbiota in anaerobic digestion and drives efficiency to a maximum at 45 ℃[J]. Bioresource Technology, 2018, 269: 309-318. doi: 10.1016/j.biortech.2018.08.106 [11] LIU R, GONG H, XU Y, et al. The transition temperature (42 ℃) from mesophilic to thermophilic micro-organisms enhances biomethane potential of corn stover[J]. Science of the Total Environment, 2021, 759: 143549. [12] TIAN H L, FOTIDIS I A, MANCINI E, et al. Acclimation to extremely high ammonia levels in continuous biomethanation process and the associated microbial community dynamics[J]. Bioresource Technology, 2018, 247: 616-623. doi: 10.1016/j.biortech.2017.09.148 [13] WESTERHOLM M, MULLER B, ISAKSSON S, et al. Trace element and temperature effects on microbial communities and links to biogas digester performance at high ammonia levels[J]. Biotechnology for Biofuels, 2015, 8(1): 1-19. doi: 10.1186/s13068-014-0179-6 [14] MOESTEDT J, NORDELL E, SCHNURER A. Comparison of operating strategies for increased biogas production from thin stillage[J]. Journal of Biotechnology, 2014, 175: 22-30. doi: 10.1016/j.jbiotec.2014.01.030 [15] DE VRIEZE J, SMET D, KLOK J, et al. Thermophilic sludge digestion improves energy balance and nutrient recovery potential in full-scale municipal wastewater treatment plants[J]. Bioresource Technology, 2016, 218: 1237-1245. doi: 10.1016/j.biortech.2016.06.119 [16] 高文萱, 张克强, 梁军锋, 等. 氨胁迫对猪粪厌氧消化性能的影响[J]. 农业环境科学学报, 2015, 34(10): 1997-2003. doi: 10.11654/jaes.2015.10.023 [17] SASAKI K, MORITA M, HIRANO S I, et al. Decreasing ammonia inhibition in thermophilic methanogenic bioreactors using carbon fiber textiles[J]. Applied Microbiology and Biotechnology, 2011, 90(4): 1555-1561. doi: 10.1007/s00253-011-3215-5 [18] HO L, HO G. Mitigating ammonia inhibition of thermophilic anaerobic treatment of digested piggery wastewater: Use of pH reduction, zeolite, biomass and humic acid[J]. Water Research, 2012, 46(14): 4339-4350. doi: 10.1016/j.watres.2012.05.016 [19] CALLI B, MERTOGLU B, INANC B, et al. Methanogenic diversity in anaerobic bioreactors under extremely high ammonia levels[J]. Enzyme and Microbial Technology, 2005, 37(4): 448-455. doi: 10.1016/j.enzmictec.2005.03.013 [20] 周晓臣. 城镇有机垃圾厌氧发酵中有机酸及氨氮抑制效应研究[D]. 重庆: 重庆大学, 2006. [21] 李红丽, 曹霏霏, 王岩. 挥发性脂肪酸对厌氧干式发酵产甲烷的影响[J]. 环境工程学报, 2014, 8(6): 2572-2578. [22] LI Y, ZHANG Y, SUN Y, et al. The performance efficiency of bioaugmentation to prevent anaerobic digestion failure from ammonia and propionate inhibition[J]. Bioresource Technology, 2017, 231: 94-100. doi: 10.1016/j.biortech.2017.01.068 [23] LI L, PENG X Y, WANG X M, et al. Anaerobic digestion of food waste: A review focusing on process stability[J]. Bioresource Technology, 2018, 248: 20-28. doi: 10.1016/j.biortech.2017.07.012 [24] BI S J, QIAO W, XIONG L P, et al. Effects of organic loading rate on anaerobic digestion of chicken manure under mesophilic and thermophilic conditions[J]. Renewable Energy, 2019, 139: 242-250. doi: 10.1016/j.renene.2019.02.083 -



 下载:
下载: