-
微生物燃料电池(microbial fuel cell,MFC)是污水处理领域的一个研究热点。MFC阳极表面生长的产电微生物的生长、活性和产电能力对MFC的性能、污水处理的效果均有重要影响。地杆菌(Geobacter)因其出色的产电能力成为这类微生物中广泛被学术界关注的电活性细菌[1]。然而,污水中重金属等具有生物毒性的组分会影响Geobacter的生长生存和产电能力,进而制约MFC的产电性能和运行效果[2]。因此,研究Geobacter对重金属的耐受机制对MFC在污水处理领域的应用和发展具有重要意义和现实价值。
银及其相关产品广泛应用于电子、电镀、感光材料、化工工业和科研领域。其中,照相业和电镀业是可溶性含银废液的2大主要来源,主要涉及到定影废液和电镀废液[3]。以定影废液为例,世界每年生产感光材料所消耗的白银在3 000 t以上,我国每年的消耗量也超过了100 t。在洗印过程中,这些感光材料中70%以上的Ag会溶解进入定影废液[4]。除了工业含银废水,Ag在环境中的迁移还来自于金属银纳米颗粒(nAg)。银纳米颗粒是一种广泛应用的抗菌剂。自2010年以来,我国纳米银抗菌剂的市场需求量已超过5×106 t[5]。然而,人类活动释放的银纳米颗粒进入自然生态环境中会形成银胶体。银胶体主要以银单质颗粒和银离子(Ag+)形式存在[6]。同时,银胶体的毒性主要来源于Ag+[7],应妥善监测和处理。
细菌对重金属的耐受能力即其细胞结构具有的将有毒金属离子外排或泵出细胞的能力。目前,已发现3种将有毒重金属转运排出细胞的机制,分别依赖于抗瘤细胞分裂蛋白(resistance-nodulation-cell division super family,RND)家族、助阳离子扩散体(cation diffusion failitator,CDF)家族和P型ATP酶(P-type ATPase)家族。其中,由P型ATP酶驱动的金属外排被认为是细菌抵抗重金属毒性的主要模式[8]。在基于P型ATP酶的重金属抵抗模式中,有毒重金属离子(如Ag+、Cu2+)进入细胞后会诱导细胞合成CopZ和CopA蛋白。其中,CopZ蛋白负责结合细胞质中的金属离子,并将其转移至位于细胞膜的转运蛋白CopA上,并由CopA将其转运出细胞[8]。因此,由copZ基因编码的CopZ蛋白对细菌抵抗重金属毒性具有重要作用。然而,不同细菌利用P型ATP酶家族系统所能抵抗的重金属种类存在较大差别,需要分别探究。
截至目前,对于Geobacter的P型ATP酶系统耐受重金属的研究尚未见报道。本研究以Geobacter的模式菌种硫还原地杆菌(Geobacter sulfurreducens)为代表,研究其对Ag+的耐受能力,并对其P型ATP酶系统中copZ基因对Ag+耐受能力的内在影响机制进行深入探究,以期在MFC系统内揭示较高浓度Ag+对G. sulfurreducens产能性能的影响。
全文HTML
-
本研究中所用菌株为实验室收集保存的野生型Geobacter sulfurreducens DL1(ATCC 51573),在含有10 mmol·L−1的乙酸作为唯一电子供体和50 mmol·L−1的富马酸作为唯一电子受体的厌氧培养基中恒温(30 °C)培养。
培养基的制备:每升去离子水中加入0.42 g KH2PO4、0.22 g K2HPO4、0.2 g NH4Cl、0.38 g KCl、0.36 g NaCl、0.04 g CaCl2·2H2O、0.10 g MgSO4·7H2O、1.80 g NaHCO3,0.50 g Na2CO3,2.04 g NaC2H3O2·3H2O、6.40 g Na2C4H4O4,再加入0.50 mL质量分数为0.1%刃天青,1 mL浓度为100 mmol·L−1的Na2SeO4溶液,10 mL 维生素溶液以及10 mL 微量矿物质溶液。维生素溶液成分参考文献[9]。微量矿物质溶液的制备:每升去离子水中添加0.10 g MnCl2·4H2O、0.3 g FeSO4·7H2O、0.17 g CoCl2·6H2O、0.20 g ZnSO4·7H2O、0.30 g CuCl2·2H2O、0.005 g AlK(SO4)2·12H2O、0.005 g H3BO3、0.09 g Na2MoO4、0.11 g NiSO4·6H2O及0.2 g Na2WO4·2H2O。
Ag+以硝酸银(AgNO3)形式添加,浓度为0.000 1~1 mmol·L−1。在厌氧条件下,用一次性无菌注射器从接种培养后的培养基中取出1 mL菌液至一次性比色皿中,在可见分光光度计600 nm波长下检测菌液的OD值,再根据培养时间换算出生长速率[10]。
-
用上述培养基培养野生型G. sulurreducens。培养条件分别为菌液中不加Ag+和添加0.05 mmol·L−1 的Ag+。培养至指数增长期后,使用RNEasy Plus minikit(Qiagen)提取RNA,并用不含DNA的DNase(Ambion)处理。RNA样品纯度通过琼脂糖凝胶电泳进行检查,A260/A280的比率为1.8~2.0,并用相关基因引物以提取的RNA样品为模版进行PCR扩增,确保RNA样品不含DNA污染。最后,用Superscript III first-strand synthesis SuperMix(Invitrogen)试剂盒将纯化后的RNA样品反转录为cDNA。
从美国能源部联合基因组研究所网站(www.jgi.doe.gov)获得基因组序列数据用于设计定量反转录PCR(qRT-PCR)引物,主要关注的基因包括助阳离子扩散体(CDF)家族的cdf1(GSU0487)、cdf2(GSU2613),抗瘤细胞分裂蛋白(RND)家族的czcA1(GSU3400)、czcA2(GSU0830)和P型ATP酶(P-type ATPase)家族的copZ(GSU1338)、copA(GSU2452)(见表1)。然后,以上述反转录的cDNA为模版,用7500 qPCR系统(Applied Biosystems)进行qRT-PCR扩增和检测,并用已知浓度的纯化cDNA做系列稀释,构建覆盖6个数量级的标准曲线。
-
构建copZ基因缺失型G. sulfurreducens菌株采用基因重组方法[11],对G. sulfurreducens DL1的copZ基因的上游和下游各约500 bp的区域设计PCR引物(见表2),并在上游后引物的3′端和下游前引物的5′端添加AvrII(CCTAGG,NEB)限制性酶切位点。对copZ基因的上游和下游区间进行高保真的JumpStart AccuTaq LA DNA聚合酶(Sigma-Aldrich)的PCR扩增,产物用AvrII限制性核酸内切酶(NEB,Beverly,MA)消化,乙醇沉淀,并与T4 DNA连接酶(NEB)连接。然后将连接反应混合物的约1 kb长度的产物条带进行琼脂糖凝胶回收Qiaquick凝胶提取试剂盒Qiagen),并连接到pCR2.1 TOPO克隆载体中,形成PCR2.1上游5′+3′下游质粒,通过Sanger测序以验证克隆产物的序列。然后提取测序结果完全准确的克隆子的质粒载体,再用AvrII限制性核酸内切酶消化,用T4 DNA连接酶将消化产物与同样用AvrII酶切处理过的庆大霉素抗性基因片段连接形成PCR2.1上游5′+庆大霉素抗性基因+3′下游质粒,测序验证质粒序列的准确性。接着用限制性内切酶KpnI (GGTACC,NEB)对上述构建的质粒进行酶切使质粒线性化。线性化的质粒通过电转进入G. sulfurreducens菌株的感受态细胞,方法见文献[9]。最后,利用厌氧手套箱中制备的已添加了20 μg·mL−1庆大霉素的NBAF固体培养基筛选copZ基因缺失型G. sulfurreducens菌株,并通过PCR检验copZ基因是否被替换成功。
-
反应体系由2组双极室MFC组成,阴阳两极室的容积均为250 mL,并由质子膜(CMI;CMI7000,Membranes International Inc.,美国)隔开。每升阴阳极溶液含0.42 g KH2PO4、0.22 g K2HPO4、0.20 g NH4Cl2、0.38 g KCl、0.36 g NaCl、0.04 g CaCl2·2H2O、0.10 g MgSO4·7H2O、1.80 g NaHCO3、0.50 g Na2CO3,以及1 mmol·L−1的Na2SeO4溶液10 mL、10 mL 微量矿物质溶液和15 mL 维生素溶液[9]。阳极室培养液中还加入10 mmol·L−1乙酸作为唯一电子供体。配制时将以上组分溶解于800 mL去离子水中,溶解后定容至1 L,各取200 mL溶液并用N2∶CO2=80∶20的混合气曝气30 min。阳极室用丁腈橡胶塞密封以保证厌氧环境,并利用磁力搅拌器进行搅拌。阴阳极均为石墨棒电极(Φ 6 mm×80 mm),参比电极为饱和甘汞电极(相对标准氢电势为+199 mV)。分别在2组MFC的阳极室接种野生型G. sulfurreducens和copZ基因缺失型G. sulurreducens。通过电化学工作站(ChI1030C,上海辰华仪器有限公司)控制阳极电势为+300 mV,同时监测2组MFC的输出电流。
1.1. 实验菌株培养条件及生长速率的测定方法
1.2. 定量反转录PCR分析相关基因转录量的方法
1.3. copZ基因缺失型Geobacter sulfurreducens菌株的构建
1.4. MFC体系装置及运行条件
-
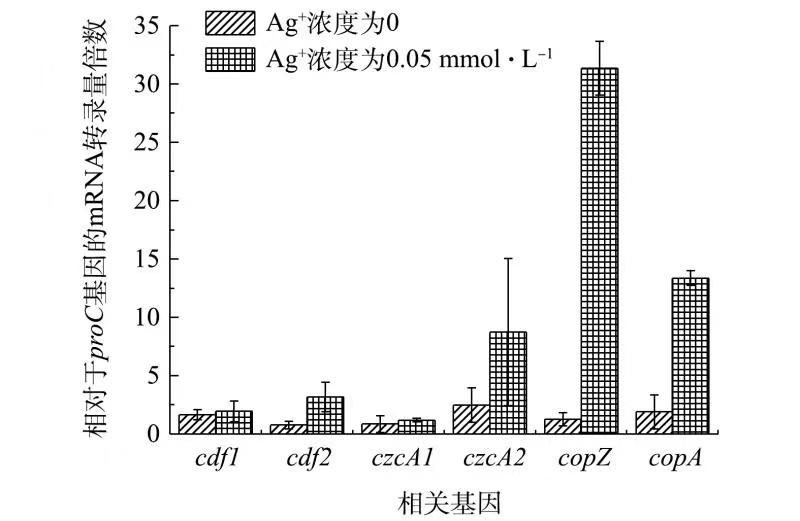
根据助阳离子扩散体(CDF)家族、抗瘤细胞分裂蛋白(RND)家族、P型ATP酶家族这3种已知金属转运机制,选取了G. sulfurreducens对Ag+耐受性可能相关的助阳离子扩散体(CDF)家族的cdf1/cdf2基因、抗瘤细胞分裂蛋白(RND)家族的czcA1/czcA2基因和P型ATP酶家族的copA/copZ基因进行研究。分别在不加Ag+和已添加0.05 mmol·L−1 Ag+条件下,对野生型G. sulfurreducens的上述基因的转录量进行比较分析,结果如图1所示。
由图1可见,Ag+的存在对cdf1和czcA1的表达量影响较小,可推测这2种基因与G. sulfurreducens对Ag+解毒特性的相关性并不大。在Ag+浓度为0.05 mmol·L−1 的条件下,Ag+的存在对cdf2与czcA2的表达量有一定影响,cdf2与czcA2的表达量分别增加了3.5倍与4.2倍。而对于与P-ATPase家族相关的copZ和copA基因,在Ag+的浓度为0.05 mmol·L−1的培养条件下,其表达量分别提升了24.8倍与8.1倍。copZ基因的表达量变化受Ag+的影响更为明显。
由copZ基因所编码的CopZ蛋白是一种金属伴侣蛋白,其在多种细菌如海氏肠球菌[12](Enterococcus hirae)、枯草芽孢杆菌[13](Bacillus subtilis)等耐受某种特定重金属(如Cu2+)的过程中发挥了重要作用。由此可推测,copZ对Ag+的响应相比于其他重金属转运相关基因更为显著,可能调控了G. sulfurreducens对Ag+的耐受能力。
-
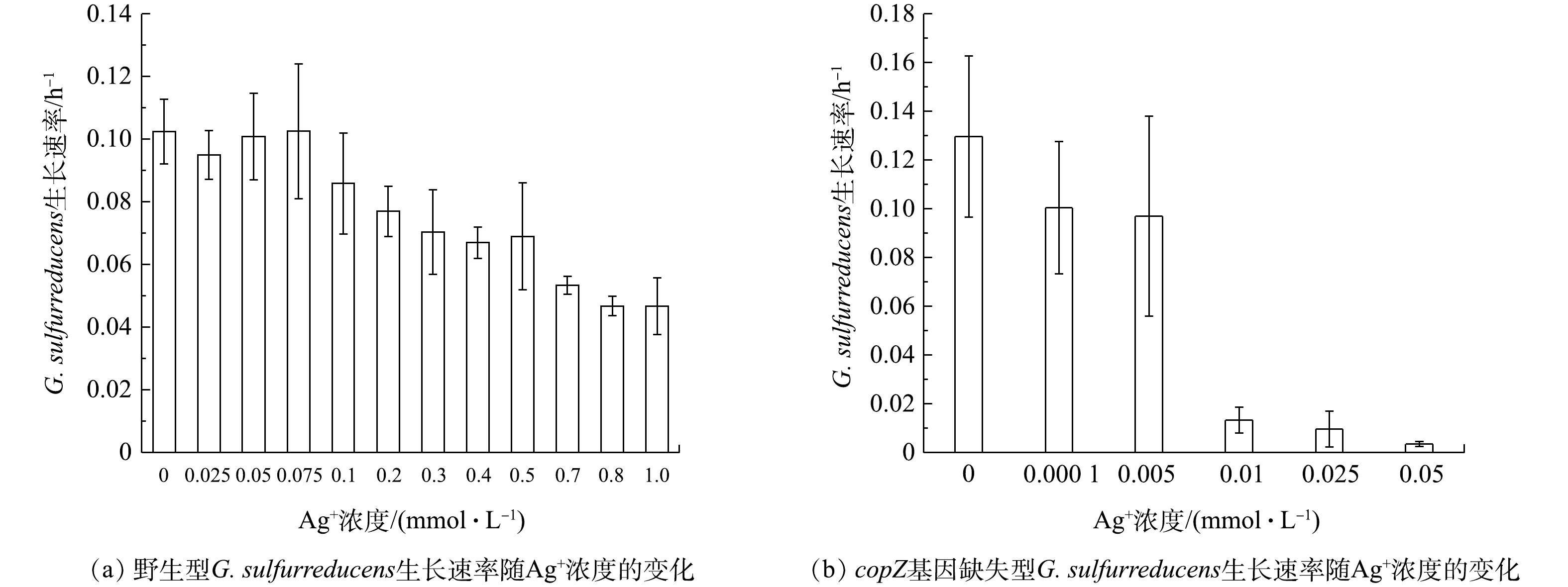
为进一步证实copZ基因在G. sulfurreducens耐受Ag+过程中的关键作用,本研究通过同源重组的方式构建了copZ基因缺失型G. sulfurreducens菌株,且在不同Ag+浓度的培养条件下,分别研究了野生型与copZ基因缺失型的G. sulfurreducens菌株对Ag+耐受浓度阈值,结果如图2所示。
由图2(a)可知,野生型G. sulfurreducens菌株对Ag+浓度耐受能力较强,当Ag+浓度为0~0.075 mmol·L−1时,G. sulfurreducens菌株生长速率可保持在0.10 h−1左右。随着Ag+浓度的增大,G. sulfurreducens菌株生长速率虽有下降,但在Ag+浓度增大至1.00 mmol·L−1时,菌株生长速率仍能达到0.046 h−1。其他细菌,如埃希大肠杆菌(Escherichia coli)、嗜肺军团菌(Legionella pneumophila)和铜绿假单胞菌(Pseudomonas aeruginosa)对Ag+的耐受浓度仅为 7×10−4~9×10−4 mmol·L−1[14],由此说明,野生型G. sulfurreducens对Ag+有很强的耐受能力。
然而,从图2(b)可知,当copZ从G. sulfurreducens的基因组中敲除后,G. sulfurreducens对Ag+的耐受能力显著下降。在Ag+浓度高于0.01 mmol·L−1时,copZ基因缺失型菌株的生长受到明显抑制,生长速率低于0.013 h−1,仅为野生型G. sulfurreducens菌株在Ag+浓度为1.00 mmol·L−1培养条件下的33.3%。由此可见,copZ的缺失使G. sulfurreducens对Ag+的耐受浓度阈值降低了约100倍,也证实了copZ参与了G. sulfurreducens对Ag+耐受能力的调控。由于copZ的高表达协助了G. sulfurreducens菌株对Ag+的解毒,从而保证了其生长速率,而缺失了copZ的G. sulfurreducens菌株的生长则受到了Ag+毒性的抑制。
-
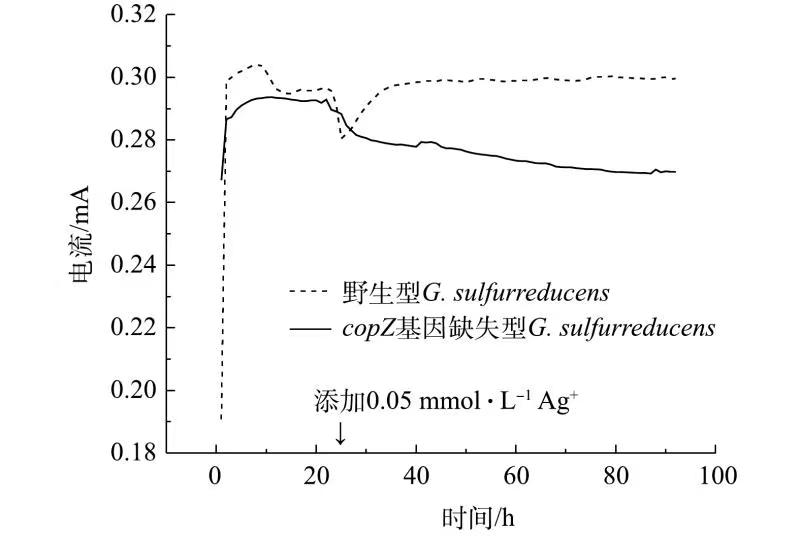
G. sulfurreducens具有出色的产电能力。分别构建接种野生型与copZ基因缺失型G. sulfurreducens菌株的MFC体系,通过对比2组MFC的电化学性能,探究copZ的缺失对于G. sulfurreducens产电能力的影响,同时在稳定运行24 h后,向2组MFC阳极室加入0.05 mmol·L−1的Ag+,研究Ag+的存在对2种G. sulfurreducens菌株产电能力的影响,结果如图3所示。
由图3可见,当体系中不存在Ag+时,2种菌株产电性能差别不大,体系输出电流均为0.29 mA左右。当体系运行24 h时加入0.05 mmol·L−1Ag+后,接种野生型G. sulfurreducens菌株的MFC体系电流出现了短暂下降,后又回升至原来的电流水平。这可能是由于Ag+的出现对于MFC阳极中的G. sulfurreducens细胞产生了一定毒害作用,但由于copZ基因的存在,野生型G. sulfurreducens可以实现Ag+的解毒,适应该Ag+浓度下的生存环境,使MFC体系电流又得以恢复。然而,加入0.05 mmol·L−1Ag+后,接种了copZ基因缺失型G. sulfurreducens菌株的MFC系统的电流持续下降,表明基因缺失型G. sulfurreducens菌株的产电能力受到了外加Ag+的影响,且无法自我恢复。当体系运行到90 h后,体系电压由加入Ag+前的0.29 mA下降到加入Ag+后的0.27 mA,下降了6.99%。
copZ基因编码的金属伴侣CopZ蛋白与P-ATPase金属转运机制密切相关,当CopZ蛋白与金属结合后能够促进其外排[15]。根据前文叙述的实验结果,当环境中Ag+浓度超过G. sulfurreducens细胞的适应范围时,野生型菌株可通过细胞质中的CopZ蛋白将Ag+运送到跨膜金属结合位点上,激活基于P-ATPase的Ag+转运机制,从而保持细胞内Ag+浓度的稳定,继而维持自身的生长与生命活动。在此过程中,copZ基因通过调控CopZ蛋白的表达影响了G. sulfurreducens对Ag+的耐受。
低浓度Ag+对copZ基因缺失型G. sulfurreducens菌株的产电能力会产生影响,进而改变MFC的输出电压。因此,copZ基因对G. sulfurreducens的Ag+毒性的解毒起到了关键作用,也展现了Geobacter型MFC在实际含Ag+废水中潜在的应用可行性。另一方面,通过多基因敲除构建出对痕量Ag+极其敏感的G. sulfurreducens工程菌株,可以利用接种该菌株的MFC系统构建生物电化学传感器,对自然水环境中的Ag+污染通过电信号进行实时监测。该设想的可行性还有待后续研究证实。
2.1. G. sulfurreducens对Ag+耐受的相关基因表达分析
2.2. copZ基因对G. sulfurreducens菌株耐受Ag+的浓度阈值的影响
2.3. Ag+对G. sulfurreducens产电性能的影响
-
1)野生型G. sulfurreducens对Ag+有较强的耐受能力,在Ag+浓度高达1 mmol·L−1时仍能保持一定的生长速率,在含Ag+废水的处理领域具有潜在的应用潜力。
2) cdf2和czcA2基因与G. sulfurreducens对Ag+耐受作用的相关性较弱,而与P-ATPase家族相关的copZ和copA基因对Ag+的响应较强,表现出与G. sulfurreducens对Ag+耐受作用更强的相关性。copZ基因在G. sulfurreducens对Ag+的耐受过程中起到关键作用,该基因通过调控CopZ蛋白的表达影响了G. sulfurreducens对Ag+的耐受。
3) copZ基因的缺失不影响野生型G. sulfurreducens MFC的产电能力,但Ag+会对接种了copZ基因缺失型G. sulfurreducens菌株的MFC的产电能力造成较大影响,且短时间内无法自我恢复。



 下载:
下载: