-
紫外高级氧化工艺(ultraviolet-based advanced oxidation process, UV-AOPs)作为一种高效的深度处理技术,已被广泛研究用于去除水中的微量有机污染物。UV/H2O2工艺是一种传统的UV-AOPs,在波长254 nm下,氧化剂H2O2光解产生具有强氧化性的羟基自由基(hydroxyl radicals, HO·,氧化还原电位为1.8~2.7 V[1]),其可非选择性地将大分子有机物降解,从而达到高效去除污染物的目的。该工艺具有氧化效率高、有害副产物少等特点,因而得到了大量的研究关注并在实际工程中进行了应用[2]。
目前关于UV/H2O2工艺的研究主要关注污染物的去除效率、机理、水质条件的影响等方面,且大部分是在实验室完全混合序批式反应器中进行的[3-6]。为预测目标污染物在不同水质条件下的降解效率,从而更合理、高效地利用及调控UV-AOPs,研究人员开发了基于自由基浓度稳态假设(steady-state assumption, SSA)的动力学模型[7]。SSA模型的前提假设为反应器内自由基的浓度处于稳态且溶液完全混合,通过整合反应过程中的主要化学反应,可以计算出不同水质条件下反应器内自由基的平均稳态浓度,进而得到对应反应时间下微污染物的降解效率。因此,SSA模型在完全混合的序批式反应器中具有较高准确性[3,8-9]。TU等[10]通过SSA模型准确预测了丙烯氰在序批式UV/H2O2反应器中的降解速率。然而,UV-AOPs在工程应用中基本都采用过流式反应器,其中的水流远未达到完全混合状态。根据目标污染物的降解动力学和光化学反应原理可知,污染物的降解速率与自由基的浓度成正比,而自由基的浓度与紫外辐照强度以及氧化剂的浓度有关。已有研究[11-12]表明,UV光强呈从灯管向外逐渐降低,氧化剂浓度则呈现从进水口到出水口递减的趋势,因此,在溶液未完全混合时,部分自由基可能难以被目标污染物利用,从而影响污染物的降解效率。为了更好地指导UV/H2O2工艺在实际工程中的应用,开展过流式UV/H2O2工艺降解微量有机污染物的研究并评估SSA模型应用于过流式反应器的准确性,具有重要意义。但截至目前,相关研究仍比较缺乏,尤其对于SSA模型的适用性评估,尚未见报道。
基于此,本文选取阿特拉津(atrazine, ATZ)作为模型污染物,采用过流式UV/H2O2反应器对其进行降解,分别考察了H2O2浓度、反应器内径对污染物降解效率及经济性的影响。同时,建立UV/H2O2工艺的SSA模型,并与实验结果进行对比,评估分析SSA模型在过流式反应器中应用的准确性。
全文HTML
-
实验所用阿特拉津为分析纯,购于梯希爱(上海)化成工业发展有限公司;过氧化氢(质量分数为30%)购于国药集团化学试剂有限公司;所用甲酸、乙腈为色谱纯,硫酸氧钛溶液的质量分数15%,均购于Sigma-Aldrich公司。
-
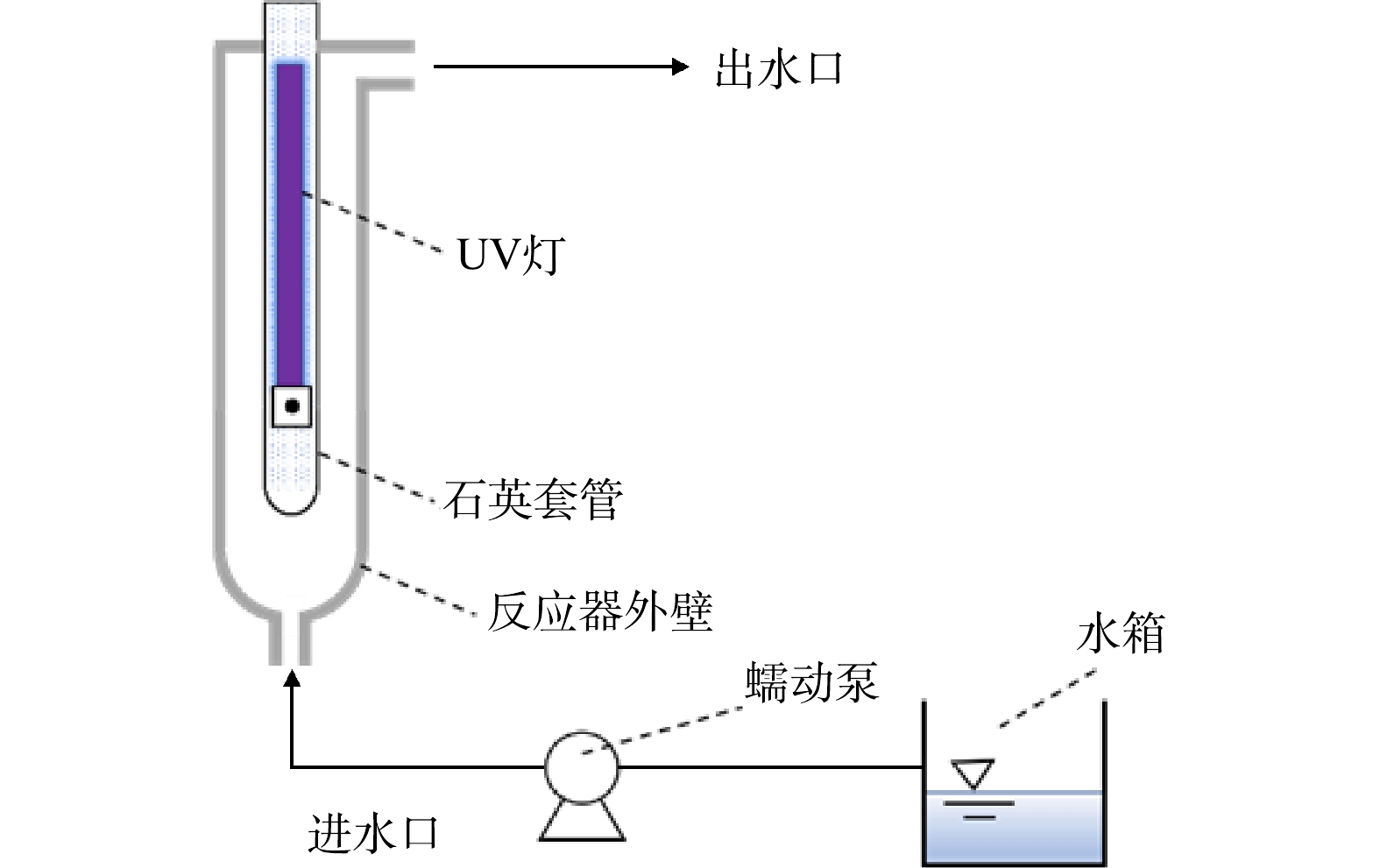
如图1所示,本研究在3种不同内径的过流式UV/H2O2反应器中进行,溶液从下方进入,一次性流过反应器后从侧上方的出水口流出。反应器长度均为500 mm,内径分别为35、50和80 mm,记作D35、D50和D80,其对应有效体积为418、950和2 500 mL。功率为21 W的一体化UV灯(GCL436T5L,美国莱劭思)置于反应器中间,灯管长度为436 mm,弧长为356 mm,其在254 nm处的输出功率为6.5 W。UV灯密封于石英套管内,套管外径为20.5 mm。反应器外壁为石英玻璃材质,用黑布包裹以避免外界光线的影响并防止紫外线泄露。以100 mmol·L−1 H2O2为感光剂,测得UV灯的光子流量(q0)为1.71×10−5 Einstein·s−1。以2 µmol·L−1ATZ为感光剂,测得D35、D50和D80反应器的有效路径(b)分别为0.67、1.33和2.29 cm[13]。
实验所用ATZ初始浓度为2.2 µmol·L−1,进水水温为21 ℃,根据实验条件,在水箱中加入一定量的H2O2溶液,搅拌混合均匀。UV灯在实验开始前预热15 min以保证输出功率稳定。打开蠕动泵,使ATZ和H2O2的混合溶液进入反应器,并在5次水力停留时间后进行取样以保证水质稳定。通过改变反应器进水流量,可得到不同辐照时间(紫外剂量)下ATZ的降解。对于处理高透光率溶液(UVT>95%)的UV反应器而言,反应器平均紫外强度和平均紫外剂量的近似计算如式(1)和式(2)所示。
式中:Eavg为平均紫外强度,mW·cm−2;V为反应器有效体积,L;U254为254 nm光子的摩尔能量,为471 528 J·Einstein−1;Favg为平均紫外剂量,mJ·cm−2;t为辐照时间,即反应器水力停留时间(hydraulic retention time, HRT),s。在本研究中,3个过流式UV反应器(D35、D50和D80)的平均紫外强度分别为12.9、11.3和7.4 mW·cm−2。基于获得的不同辐照时间(紫外剂量)下ATZ的去除率,可以分析过流式反应器中ATZ的降解动力学等特征规律。
-
H2O2浓度由紫外分光光度计(哈希DR6000)测得,显色剂为TiOSO4,测量波长为410 nm。ATZ浓度采用高效液相色谱(安捷伦1200)进行测量,采用紫外二极管阵列检测器,检测波长为234 nm,色谱柱为安捷伦C18柱(150 nm×2.1 nm,3 µm),流动相由0.2%的甲酸水和乙腈组成(10∶90,体积比),流速为0.8 mL·min−1,进样量为50 µL,柱温为30 ℃。
-
UV/H2O2降解ATZ过程涉及的主要化学反应及相关参数如表1所示。ATZ降解速率为直接光解速率与自由基氧化速率之和。根据稳态假设,反应器内自由基生成速率等于消耗速率,自由基浓度恒定不变。通过计算自由基的平均稳态浓度,可以得到对应反应时间下微污染物的自由基氧化降解效率。ATZ直接光解速率、HO·生成速率和消耗速率分别根据式(3)~(5)进行计算。
式中:rd为ATZ直接光解速率,mol·(L·s)−1;rHO·为HO·生成速率,mol·(L·s)−1;r'HO·为消耗速率,mol·(L·s)−1;Φ1和Φ2分别为ATZ和H2O2的量子产率,mol·Einstein−1;A为溶液在254 nm下的吸光度;f1和f2分别为ATZ和H2O2的吸光度占总吸光度的比例;k1和k2分别为HO·与ATZ和H2O2的二级反应速率常数,L·(mol·s)−1;CA、CH和CS分别为ATZ浓度、H2O2浓度和HO·的平均稳态浓度,mol·L−1。其中,A、f1和f2分别根据式(6)~式(8)进行计算。
式中:ɛ1和ɛ2分别为ATZ和H2O2的摩尔吸光系数,L·(mol·cm)−1。对高透光率溶液(A<0.02)而言,其中反应物的直接光解速率和HO·生成速率计算(式(3)和式(4))可进一步简化为式(9)和式(10)。
假定过流式UV/H2O2反应器内的相关反应符合稳态假设理论,即忽略其中非完全混合流态的影响,则根据式(5)和式(10)可计算得到CS,进而得到基于时间的ATZ降解速率和基于紫外剂量的ATZ降解速率,如式(11)~式(13)所示。
式中:r为基于时间的ATZ降解速率,mol·(L·s)−1;
$ r^{'} $ 为基于紫外剂量的ATZ降解速率,mol·cm2·(L·mJ)−1;kobs为ATZ基于时间的降解速率常数,s−1;$ k_{{\rm{obs}}}^{'} $ 为ATZ基于紫外剂量的降解速率常数,cm2·mJ−1。 -
根据定义,EEO为将单位体积中目标污染物浓度降低一个数量级(去除90%)所需的电能,kWh·(m3·order)−1,其计算过程如式(14)和式(15)所示。
式中:WL为灯的输出功率,kW;Q为反应器进水流量,L·h−1;Ci和Cf分别是进水和出水中ATZ的浓度,mol·L−1。kobs为基于时间的ATZ降解的拟一级反应速率常数,s−1;t为反应器的水力停留时间,s。综合式(14)和式(15)可以得到式(16)。
式中:V为反应器的有效体积,L。WL为灯的输出功率,kW;kobs为ATZ基于时间的拟一级速率常数,S−1。
1.1. 材料试剂
1.2. 降解实验
1.3. 分析方法
1.4. 稳态假设模型
1.5. 过流式UV/H2O2反应器降解ATZ的经济性计算
-
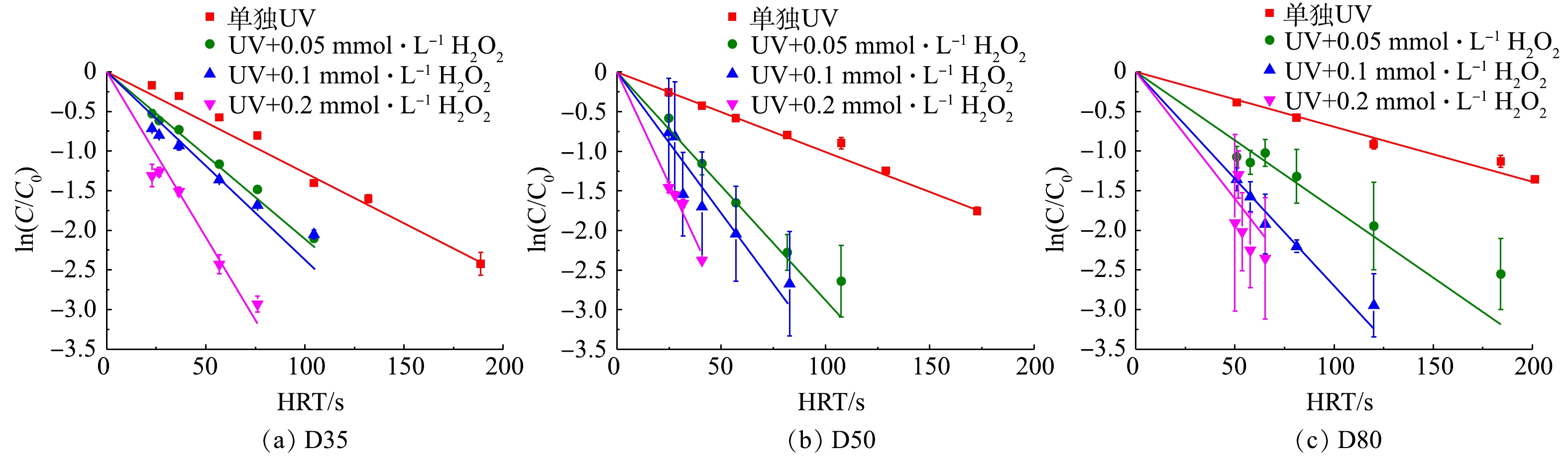
同一反应器条件下改变进水流量,得到目标污染物ATZ的降解效率如图2所示。由图2可以看到,随着辐照时间(HRT)的增加,ATZ的对数浓度接近于线性下降,即过流式反应器中ATZ的降解仍基本符合拟一级反应动力学规律。这表明对UV/H2O2工艺过程而言,反应器中流量及相应流态的变化对污染物降解反应规律影响较小。在单独UV照射下,ATZ能被部分降解。这是因为,虽然ATZ直接光解的量子产率较小(0.048 mol·Einstein−1),但其摩尔吸光系数较大(3 397 L·(mol·cm)−1),从而导致其直接光解速率较大。当在溶液中添加氧化剂H2O2时,H2O2光解产生强氧化性的HO·,其与ATZ的二级反应速率常数为2.3×109 L·(mol·s)−1,使得ATZ的降解速率显著加快,且投加的H2O2浓度越高,污染物降解速率越快。值得一提的是,由于过流式反应器固有的出水水质波动问题,2次平行实验得到的ATZ对数去除率误差相对序批式反应器中的更大,尤其是当反应器内径较大时(图2(b)和图2(c))。类似程度的实验结果偏差在采用过流式反应器的其他UV-AOPs工艺研究中也有过报道,故属正常现象[14]。
根据图2中的实验结果得到不同反应器内径和H2O2浓度下ATZ降解的拟一级降解速率常数,并与相同条件下SSA模型计算得到的降解速率常数进行对比,结果如表2所示。由表2可知,不同反应条件下ATZ拟一级降解速率常数的实验值和模拟值的偏差为−19.8%~12.9%(其中反应器D35的44%偏差可能为取样问题导致),且单独UV辐照时模型均出现一定程度的低估。对全部的实验值和模拟值进行线性拟合(排除44%异常值,n=11),可以得到一条经过原点且斜率为1.02的直线(R2=0.98)。因此,与序批式完全混合反应器中类似,SSA模型依然可以准确模拟过流式UV/H2O2反应器中ATZ的降解效率。
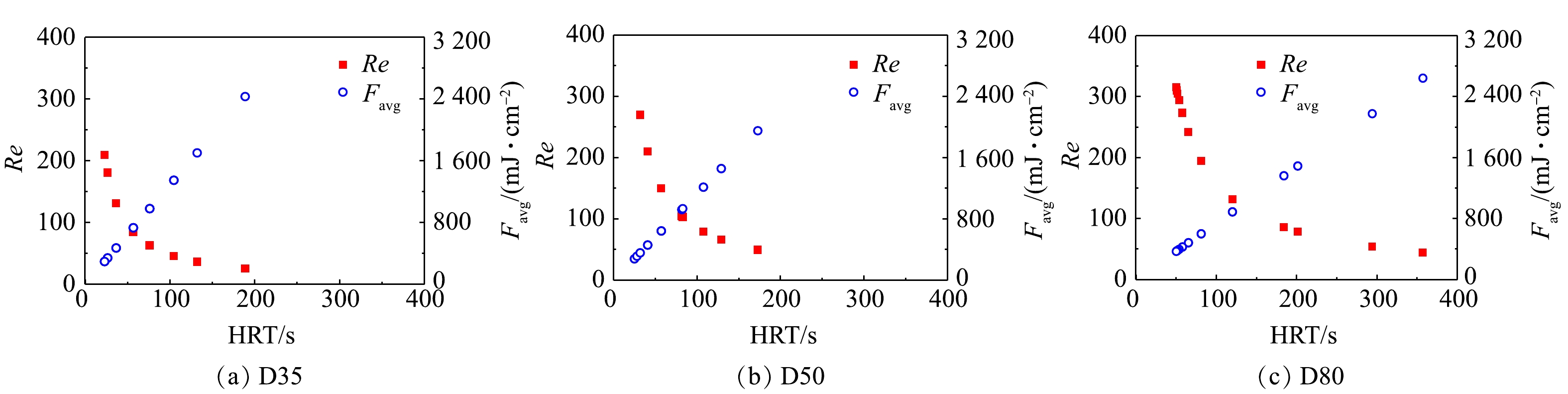
UV光解H2O2产生的HO·的不均匀分布是影响SSA模型在过流式反应器准确性的主要原因。不同于序批式反应器中通过搅拌实现反应物的近似完全混合,本研究中所用的过流式反应器中水流形态接近于推流,反应器内物质的径向混合程度有限,且具体混合效果与反应器内径和流量相关。在不同HRT下,3个反应器内水流的雷诺数(Reynolds, Re)结果如图3所示。由图3可知,在考察的流量条件下,反应器的雷诺数为25~344,远小于层流临界值2 300,这表明其中水流处于层流状态。同一紫外剂量下,反应器D80中的雷诺数最大,其次为D50、D35,这说明反应器内径越大,溶液的径向混合程度相对越高。这在一定程度上解释了SSA模型在D50和D80中的准确性更高的原因。此外,根据计算流体动力学模拟结果可知,当反应器流速较低时,未知中间产物的浓度将增大,从而导致模拟结果与实验值的偏差增大[15-16]。与其他2个反应器相比,相同紫外剂量下D35中的水流流速也更低,因而容易出现更大偏差。在UV/H2O2工艺的工程应用中,处理流量及水流雷诺数一般比本研究反应器D80中的要大,因此,SSA模型在过流式UV/H2O2反应器实际应用中仍具有较好的适用性。这为工程中UV/H2O2系统性能的快速评估提供了理论基础和技术方法。
-
如图2和表2所示,在反应器D35、D50和D80中,H2O2浓度增加均明显提高了ATZ的降解速率。当H2O2浓度增加至0.2 mmol·L−1时,ATZ的kobs分别增加至5.8×10−2、5.6×10−2和3.2×10−2 s−1,分别为单独UV辐照时的4.5、5.6和4.6倍。由式(10)可知,当H2O2浓度增加时,HO·的生成速率增加,从而促进了ATZ的降解。有研究[17]表明,当H2O2浓度为0.1 mmol·L−1和0.2 mmol·L−1时,序批式反应器中得到ATZ的
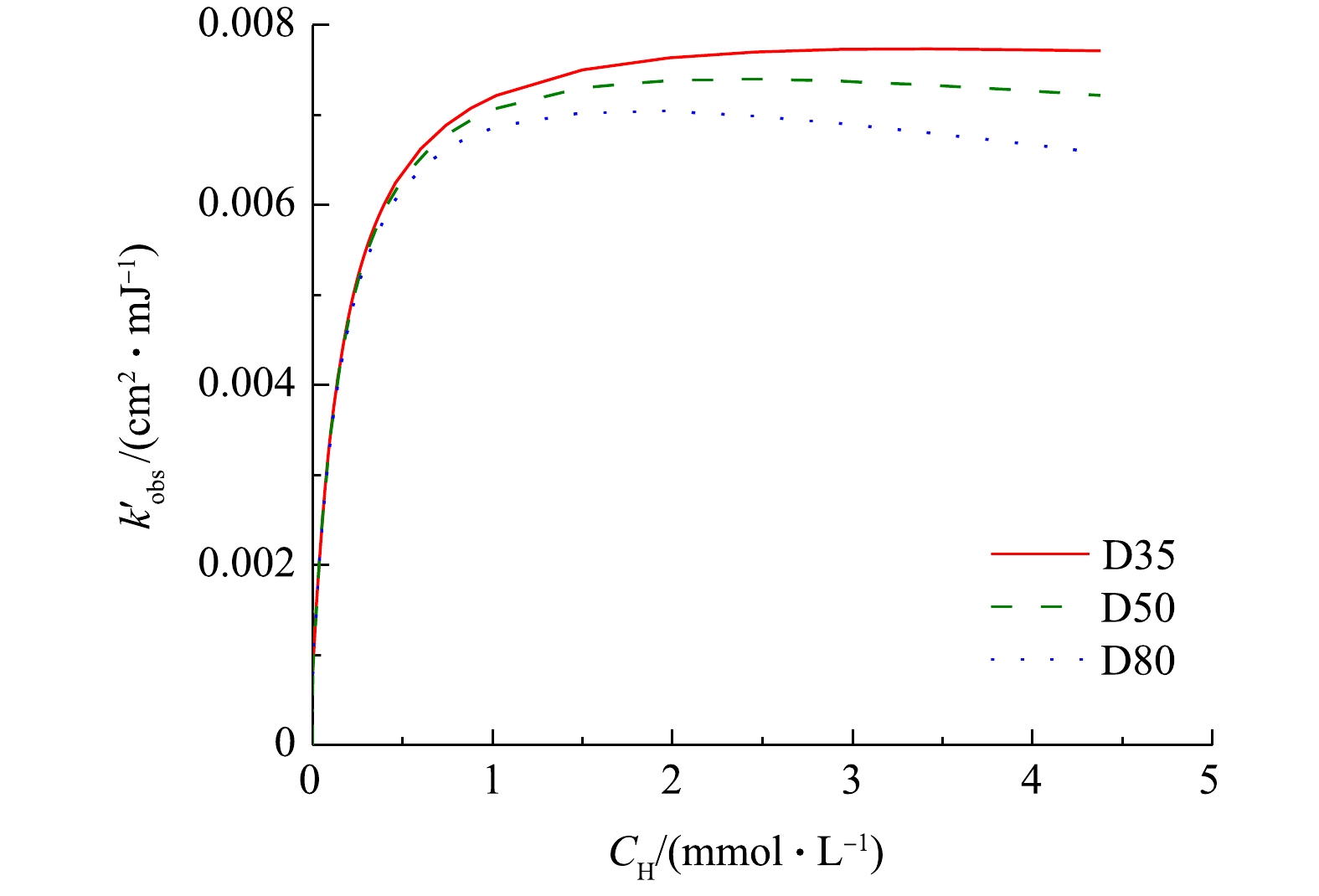
$ k_{{\rm{obs}}}^{'} $ 分别约为4.0×10−3 cm2·mJ−1和5.7×10−3 cm2·mJ−1,与本研究中所得结果(表2)较为接近。这表明在相同的紫外剂量下,UV/H2O2工艺降解目标污染物的效率可能不受反应器结构和水流流态的影响。事实上,MIKLOS等[2]在研究UV/H2O2工艺对污水厂出水中微量有机污染物的去除时也得到了类似结论,即目标污染物在序批式反应器和过流式反应器中的降解速率常数相差不大。进一步通过SSA模型计算不同反应器中H2O2浓度变化对ATZ降解速率的影响,结果如图4所示。与实验结果类似,在较低的浓度范围内,随着H2O2浓度的增加,3个反应器中ATZ的
$ k_{{\rm{obs}}}^{'} $ 均得到显著提升,其最大值出现在H2O2浓度约为3 mmol·L−1时,但当H2O2浓度继续增加时,ATZ降解速率缓慢降低。这是由于H2O2同时也是HO·的捕获剂,过量的H2O2会和HO·反应生成氧化性较弱的$ {\rm{HO}}_{\rm{2}}^{\cdot -}$ ,从而抑制ATZ降解速率的升高,甚至使反应速率降低[18]。在UV/H2O2工艺降解氧氟沙星[19]、美罗培南[20]、双酚A[21]等的研究中也有着类似的现象。高H2O2浓度下不同内径反应器中ATZ降解速率常数的下降幅度不一,这主要与有效路径不同导致的平均紫外强度下降程度差异有关。 -
反应器内径对ATZ降解的影响可以分别从kobs和
$ k_{{\rm{obs}}}^{'} $ 进行分析。如表2所示,在相同H2O2浓度下,ATZ的kobs随着反应器内径的增加而逐步减小。其原因在于:一方面,在同一光源辐射下,反应器内径越大,其中平均紫外强度Eavg越小,使得ATZ直接光降解速率及HO·产生速率、浓度降低,最终导致ATZ降解速率常数的减小;另一方面,相同H2O2浓度下,3个不同内径的反应器中ATZ的$ k_{{\rm{obs}}}^{'} $ 基本相同,如当H2O2浓度为0.2 mmol·L−1时,在反应器D35、D50和D80中分别为4.5×10−3、5.0×10−3和4.3×10−3 cm2·mJ−1。SSA模型模拟结果(表2)也进一步证实了相同紫外剂量下不同反应器中ATZ降解效率无显著差异。由式(1)可知,对高透光率反应体系而言,其中q0b/V与Eavg成正比。因此,不同反应器内的微量有机污染物的直接光解速率和自由基生成速率主要取决于反应器内的Eavg(式(9)和(10))。另一方面,反应器内自由基的消耗速率只与处理水质有关(式(5))而可认为是恒定值,则稳态自由基浓度也与Eavg成正比。综上所述,从稳态假设理论出发,3种内径的UV反应器中ATZ的kobs主要决定于相应的Eavg。将基于时间的降解速率除以Eavg可以得到
$ k_{{\rm{obs}}}^{'} $ (式(13)),因此,在相同的ATZ及H2O2浓度下,不同反应器内的ATZ的$ k_{{\rm{obs}}}^{'} $ 十分接近(表2)。随着H2O2浓度的增加,溶液在254 nm下的吸光度(A)大于0.02,导致式(3)和式(4)进行泰勒展开时误差较大,此时不能通过式(9)和式(10)预测反应器中ATZ的降解速率。结合SSA模型的模拟结果可知,当H2O2浓度大于0.5 mmol·L−1时,反应器内径将对ATZ的$ k_{{\rm{obs}}}^{'} $ 产生影响(图4)。在考察评估实际UV-AOPs反应器性能时,通常比较的是相同水质和流量条件下反应器出口处目标污染物的浓度(或去除率)大小。在本研究中,由于3个反应器内径不同,相应的水力停留时间及基于时间的ATZ降解速率常数都将不同,直接比较各反应器的kobs值并不能准确评估其效率。另一方面,同一条件下各反应器中得到的ATZ的
$ k_{{\rm{obs}}}^{'} $ 无明显差别,因此也不适合用作性能参数。根据拟一级反应原理,微量有机污染物在反应器出口处浓度的对数与速率常数和时间(或剂量)的乘积呈线性相关,因此,应综合kobs和HRT或$ k_{{\rm{obs}}}^{'} $ 和Favg结果来评估反应器性能或经济性。事实上,UV-AOPs工艺中常用的单位能耗(electrical energy per order, EEO)正是综合反应速率和处理流量后的性能评估参数。本研究将采用该参数分析上述3个反应器在不同H2O2浓度下的经济性。 -
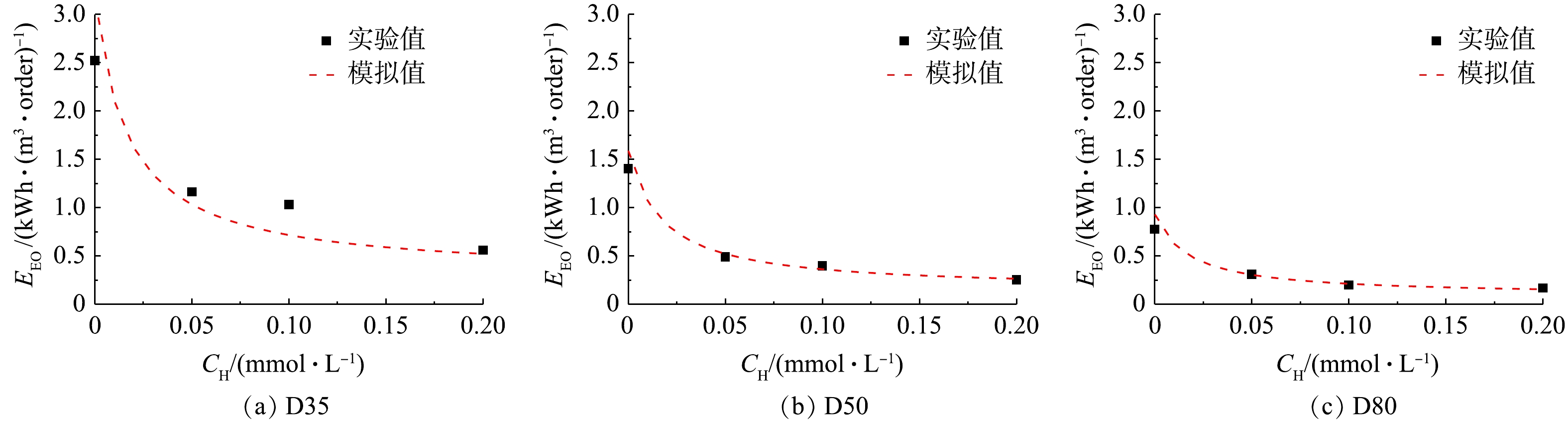
不同条件下过流式UV/H2O2反应器降解ATZ的EEO实验和模拟值结果如图5所示。与3.1部分的结果类似,在反应器D35中(图5(a)),SSA计算所得EEO值与实验值具有一定偏差,而在反应器D50(图5(b))和D80(图5(c))中,两者基本吻合。此外,在实验条件内,EEO值为0.17~2.52 kWh·(m3·order)−1。有研究[22]表明,UV-AOPs处理微量有机污染物的EEO低于2.5 kWh·(m3·order)−1时具有经济可行性,因此,UV/H2O2工艺在处理水中微量ATZ上具有良好的应用前景。在同一反应器内,随着H2O2浓度的增加,ATZ的降解速率常数逐渐增大,从而导致EEO值逐渐减小。在H2O2浓度相同时,虽然反应器内径的增加会降低ATZ基于时间的降解速率常数,但由于其有效体积的增大,UV/H2O2工艺处理ATZ的EEO值将降低。综上所述,增大H2O2浓度或反应器内径均能降低单位处理能耗,从而提高UV/H2O2工艺的经济效益。
2.1. 过流式UV/H2O2反应器中ATZ的降解及模拟
2.2. H2O2浓度对过流式UV/H2O2反应器中ATZ降解的影响
2.3. 反应器内径对过流式UV/H2O2反应器中ATZ降解的影响
2.4. 不同过流式UV/H2O2反应器降解ATZ的经济性
-
1)过流式UV/H2O2反应器可以有效降解去除水中微量有机污染物。在不同处理流量下,反应器中ATZ的降解基本符合拟一级反应动力学规律(R2>0.95),且其基于紫外剂量的速率常数与在序批式反应器中的接近。
2)虽然反应器内水流流态不同于序批式反应器中的完全混合,基于稳态假设的SSA模型仍能准确模拟过流式反应器中ATZ的降解速率,其偏差基本在20%以内。这为实际应用中UV/H2O2系统性能的快速评估提供了理论和技术可行性。
3)在考察的浓度范围内,H2O2浓度增加会导致HO·的生成速率增加,从而促进ATZ的降解,并提高反应器的经济性。当H2O2浓度过高时,会与HO·反应并抑制ATZ的降解。H2O2浓度大约为3 mmol·L−1时,ATZ的降解速率最快。
4)不同内径反应器中ATZ基于紫外剂量的降解速率常数无明显差异。反应器内径增大虽然降低了ATZ基于时间的降解速率常数,但由于有效体积的增大,反应器的EEO值将降低,从而使UV/H2O2工艺的效益得到提高。



 下载:
下载: