-
甲醛(HCHO)是一种典型室内空气污染物,主要来源于油漆、家具及建筑装修材料等[1]。2004年,甲醛已被国际癌症研究机构(IARC)列为人类致癌物(Ⅰ类)[2]。一般来说,人类的大部分时间是在室内度过的,难免会接触甲醛,而甲醛会引起过敏、恶心、肿瘤等各种健康问题。因此,有效去除室内空气中的甲醛是保护人类健康的迫切需要。目前,催化氧化甲醛技术,因具有高效、节能、环境友好等优点而备受关注[3]。因此,开发一种能在室温条件下将甲醛完全转化为CO2和H2O的高效催化剂,已成为目前面临的最大挑战。
目前,用于消除甲醛的催化材料主要分为过渡金属氧化物和贵金属催化剂。现已证明,能有效氧化甲醛的过渡金属氧化物及其复合物有MnOx、Co3O4和CeO2。如3D-Co3O4在130 ℃时甲醛的转化率达到了100%[4]。不同晶型结构MnOx催化剂完全氧化甲醛的温度为80~150 ℃[5]。MnOx-CeO2催化剂完全氧化甲醛的温度为100 ℃[6]。但是,多数过渡金属氧化物及其复合物在低温(<100 ℃)条件下对甲醛去除率仍然不高。近年来,多项研究表明,负载型贵金属催化剂在室温下完全氧化甲醛的性能表现优异。ZHANG等[7]报道,催化剂Pt/TiO2中碱金属(Li+、Na+和K+)的加入可以促进和稳定Pt高度分散,改善了催化剂的性能,2% Na-1% Pt/TiO2在15 ℃将甲醛完全氧化。在Au/CeO2催化剂作用下,甲醛可以在28 ℃被完全氧化[8]。与Pt系和Au系催化剂相比,Pd系催化剂价格低廉,其在室温下氧化甲醛的性能受到广泛关注。ZHANG等[9]报道,催化剂2% Na-1% Pd/TiO2中Na与Pd的强相互作用,有利于带负电荷Pd物种的形成,进而促进O2的吸附,该催化剂在25 ℃条件下甲醛的转化率接近100%。1% Pd/CeO2催化剂在22 ℃时能将甲醛完全氧化[10]。尽管如此,仍然需要开发高效、低Pd含量的催化剂,以降低其成本。
鉴于碱金属Na+能够促进贵金属的高度分数、改变贵金属的电荷效应,本研究选择Na+作为贵金属Pd的助催化剂,以γ-Al2O3作为载体,制备了系列Pd质量分数为0.5%的Pd-x% Na/Al2O3(x%表示Na的质量分数,分别为0、1%、2%和4%)催化剂,对催化剂的物理化学性质进行了表征,对催化剂室温下催化氧化甲醛性能进行了评价,为开发室温下治理空气中甲醛污染技术提供参考。
全文HTML
-
以γ-Al2O3(购自国药集团化学试剂有限公司)为载体,采用共浸渍法制备了不同Na含量的负载型Pd催化剂。首先,将γ-Al2O3分散在20 mL蒸馏水中,在磁力搅拌下,将20 mL含有一定量Pd(NO3)2和NaNO3溶液缓慢加入γ-Al2O3悬浮液中;室温下连续搅拌24 h后,升温至80 ℃,在搅拌条件下蒸去多余水分;最后,样品在100 ℃干燥12 h,400 ℃焙烧4 h,升温速率为5 ℃·min−1。所得样品Pd质量分数均为0.5%,Na质量分数分别为0、1%、2%、4%,分别命名为Pd/Al2O3、Pd-1% Na/Al2O3、Pd-2% Na/Al2O3和Pd-4% Na/Al2O3。采用同样方法制备了Na质量分数为2%的2% Na/Al2O3催化剂。
-
采用ASAP2020HD88比表面积仪测定催化剂的比表面积。测试前,所有样品在250 ℃抽真空脱气处理4 h。用BET方法计算比表面积,用BJH方法计算催化剂的孔容和孔径。
X射线衍射(XRD)在Panalytical Empyrean X射线衍射仪上进行,衍射源为Cu-Kα辐射(λ= 0.154 056 nm),加速电压为40 kV,外加电流为30 mA,扫描速率为2(°)·min−1,扫描角度为10.0°~80.0°。
程序升温还原(H2-TPR)设备由热导检测器(TCD)和程序升温2个单元组成。测试前,在石英管反应器中装入50 mg催化剂,然后通入体积分数为10% H2/N2气(60 mL·min−1),以10 ℃·min−1的速率加热升温,从25 ℃加热至350 ℃。
氧程序升温脱附(O2-TPD)测试与H2-TPR在同一仪器上进行。60 mg样品先在体积分数为10% H2/N2(40 mL·min−1)的气氛下,350 ℃还原30 min,升温速率为10 ℃·min−1;再用He(40 mL·min−1)在200 ℃吹扫30 min后,He气氛条件下降温到50 ℃,切换为20 mL·min−1的体积分数为21% O2/N2,在50 ℃下进行O2吸附1 h;最后,在He(40 mL·min−1)气氛中以10 ℃·min−1的速率从50 ℃升温到450 ℃。
X射线光电子能谱(XPS)是在Thermo Scientific K-Alpha仪器上进行的,激发光源为MgKα(1 653.6 eV)。
-
甲醛的催化氧化反应在微型固定床反应器内进行,反应条件为常压和环境温度(25±1) ℃。首先,将50 mg催化剂(40~60目)填充在石英管(内径3 mm)中,用体积分数为10% H2/N2气(40 mL·min−1)在350 ℃下还原30 min。将多聚甲醛置于恒温水浴中,用体积分数为21% O2/N2气(30 mL·min−1)带出分解的甲醛,形成含甲醛的反应气体,反应气体中HCHO的体积分数为0.025%,反应气体流量为30 mL·min−1,质量空速(WHSV)为36 000 mL·(g·h)−1。反应1 h后,反应尾气通过配有甲烷转化炉和氢火焰离子化检测器(FID)的气相色谱仪进行在线检测。由于生成的含碳物种中仅有CO2,因此,甲醛转化率可由式(1)计算。
式中:η为HCHO转化率;
${C_{[{\rm{CO}}{_2}]}}_{{\rm{out}}}$ 为反应尾气中CO2的浓度;C[HCHO]in为入口气体中甲醛的浓度。
1.1. 催化剂制备
1.2. 催化剂表征
1.3. 催化性能测试
-
Pd-x% Na/Al2O3(x=0、1、2和4)催化剂和γ-Al2O3的SBET、孔容和孔径数据见表1。催化剂的SBET和孔容由大到小的顺序为:γ-Al2O3>Pd/Al2O3>Pd-1% Na/Al2O3>Pd-2% Na/Al2O3>Pd-4% Na/Al2O3。样品孔径由小到大顺序为:γ-Al2O3<Pd/Al2O3<Pd-1% Na/Al2O3<Pd-2% Na/Al2O3<Pd-4% Na/Al2O3。由此可知,Pd和Na粒子覆盖在γ-Al2O3表面上,并堵塞部分微孔,进而导致SBET和孔容减小[9]。
-
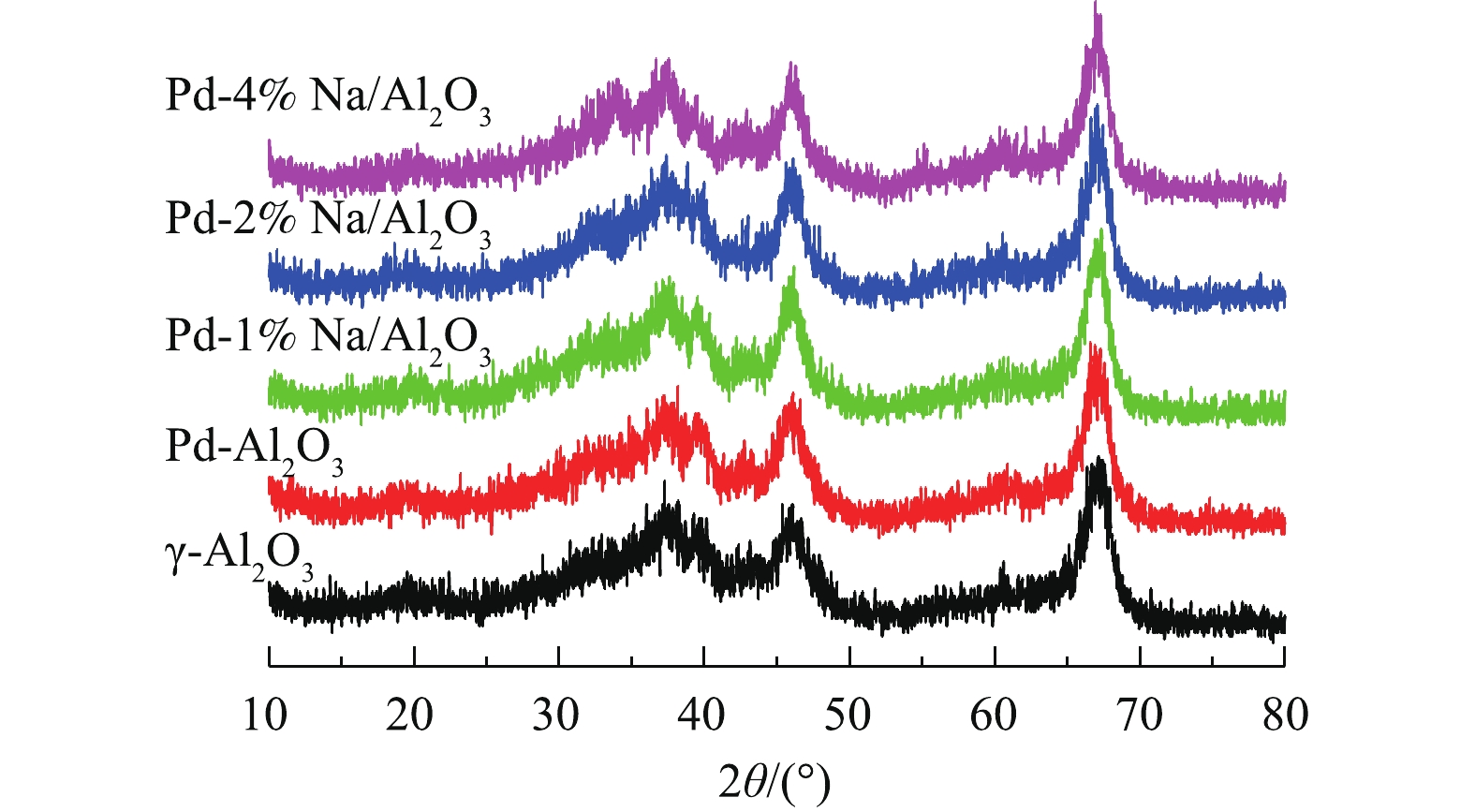
图1为Pd-x% Na/Al2O3(x=0、1、2和4)催化剂和γ-Al2O3的XRD图。所有催化剂都有4个特征衍射峰,分别在2θ=37.3°、42.6°、45.7°和67.1°处,归属于γ-Al2O3[11]。在Pd/Al2O3和Pd-Na/Al2O3的XRD图谱中没有Pd、Na或Pd-Na合金的衍射特征峰,说明这些物质粒径小、含量低、分散度高,难以检测到。
-
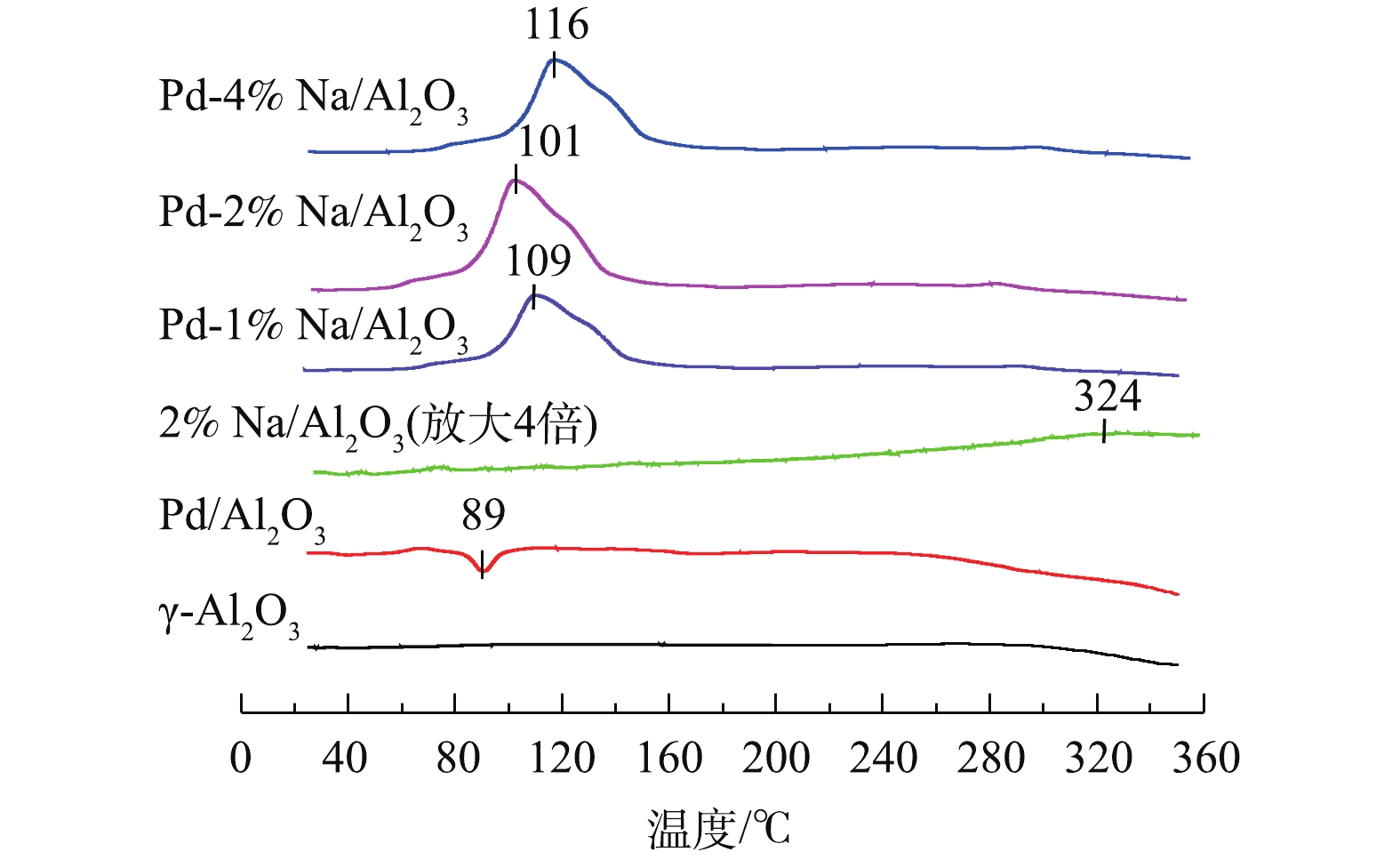
Pd-x% Na/Al2O3(x=0、1、2和4)催化剂和γ-Al2O3的H2-TPR结果见图2。可以看出,γ-Al2O3在25~350 ℃基本没有还原峰。Pd/Al2O3催化剂除了在89 ℃出现1个负峰外,没有PdO物种的还原峰。根据已有研究[12],在进行TPR测试之前,分散在载体Al2O3上的PdO物种可能在20 ℃以下已被H2还原。89 ℃的负峰归属于β-PdH分解产生H2脱附[13]。众所周知,金属Pd0大微晶在室温下可将H2解离成氢原子形成β-PdH,催化剂表面高度分散的Pd能够显著抑制β-PdH的形成[14]。由此说明,Pd/Al2O3催化剂表面上有较大的金属Pd0微晶生成。样品2% Na/Al2O3在324 ℃出现了一个弱的耗氢峰,可归属为Na物种的还原[9, 15]。
Pd-1% Na/Al2O3、Pd-2% Na/Al2O3和Pd-4% Na/Al2O3 3种催化剂H2脱附峰消失,在60~150 ℃出现了一个强的H2还原峰,归属于PdO和Na物种的还原。据报道[9],由于Pd与Na物种之间存在很强的相互作用,Na物种可以稳定Pd物种,Pd物种的存在可以促进Na物种的还原。同时,3种催化剂的还原性与Na含量密切相关,Pd-2% Na/Al2O3的低温还原性最好,其还原温度比Pd-1% Na/Al2O3和Pd-4% Na/Al2O3分别低了8 ℃和15 ℃。有研究[16-17]表明,催化剂的还原性与其氧空位有关,还原性较好的催化剂可能产生更多的氧空位。因此,Pd-2% Na/Al2O3可以产生更多的氧空位,从而在甲醛催化氧化过程中起到关键作用。这一结果与O2-TPD (图3)的分析结果一致。
-
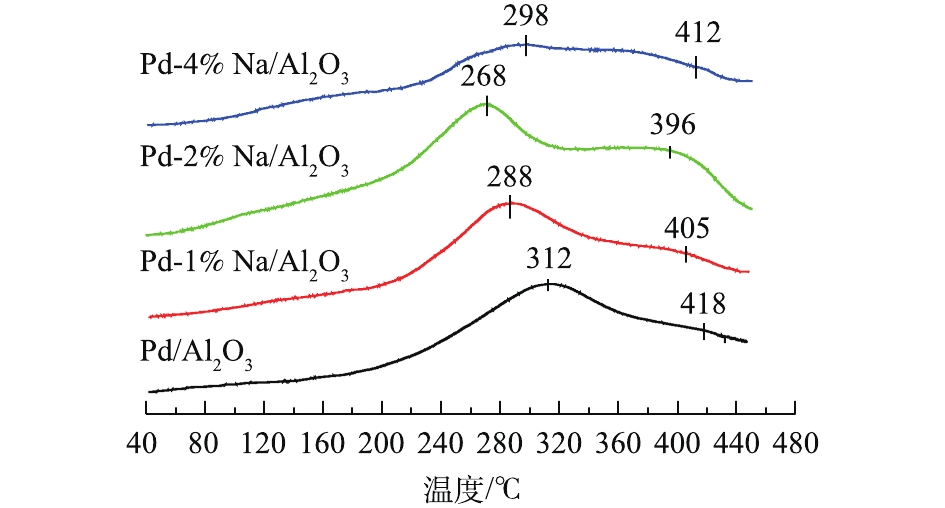
图3为Pd-x% Na/Al2O3(x=0、1、2和4)催化剂的O2-TPD图。催化剂表面活性氧如
$ {\rm{O}}_2^ - $ 和O−的脱附温度在350 ℃以下,而晶格氧(O2−)的脱附温度在350 ℃以上。一般来说,较低温度对应的O2脱附峰表明样品容易产生表面活性氧物种,有利于在氧化反应中提供较高的催化活性[18-19]。Pd/Al2O3催化剂在312 ℃和418 ℃的脱附峰分别归属于表面活性氧物种和晶格氧的脱附。添加Na后,Pd-1% Na/Al2O3、Pd-2% Na/Al2O3和Pd-4% Na/Al2O3催化剂的O2脱附峰较Pd/Al2O3的脱附峰向低温方向移动,特别是Pd-2% Na/Al2O3催化剂的O2脱附温度最低,为268 ℃,并且O2脱附峰的信号最强。由此可知,Pd-2% Na/Al2O3催化剂表面具有丰富的表面活性氧,这是催化剂具有优异催化活性不可缺少的。MA等[18]和BAI等[4]在研究中也得出了同样结论。MA等[18]发现,介孔催化剂Au/Co3O4-CeO2(7∶3)具有丰富的表面活性氧,其甲醛氧化活性较高。BAI等[4]研究发现,3D-Co3O4的催化剂因表面有丰富的活性氧,容易参与甲醛的催化氧化反应,其催化活性高。 -
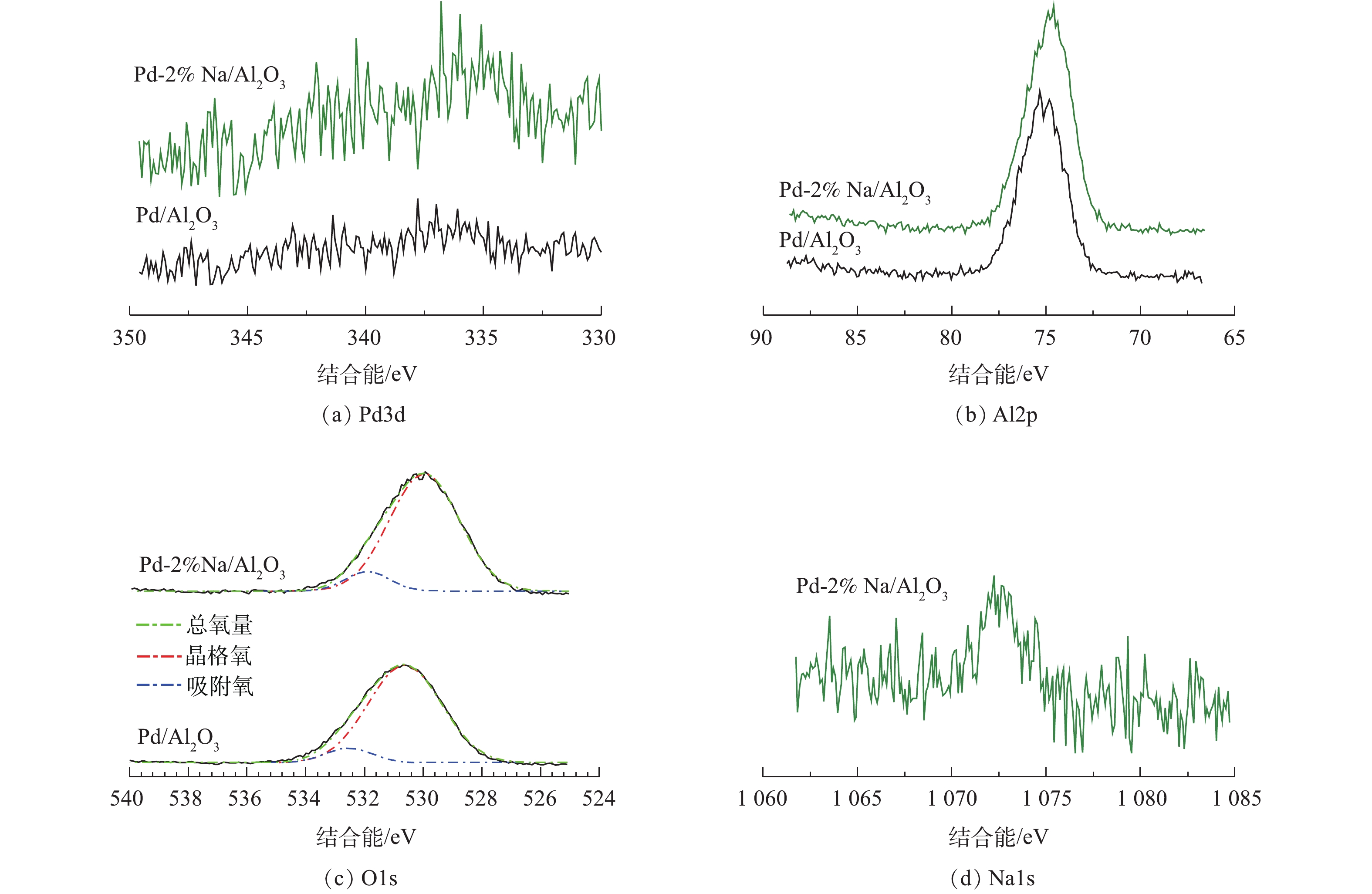
图4为Pd/Al2O3和Pd-2% Na/Al2O3催化剂的XPS谱图。如图4(a)所示,Pd/Al2O3在335.5 eV和336.5 eV时出现2个Pd3d5/2峰。BUERES等[20]发现,Pd0在Pd/AC、Pd/CNF和Pd/HSAG催化剂中的特征峰约在334.7~335.7 eV。NUTT等[21]报道了Pd0的特征峰在335.3 eV。HUANG等[22]将336.5 eV时的结合能归属于Pd氧化物。因此,本研究将335.5 eV和336.5 eV的特征峰分别归属于Pd0和Pd氧化物。Pd-2% Na/Al2O3的Pd3d5/2的峰向低结合能转移(334.6 eV和336.0 eV),表明Na作为给电子体,通过与Pd的强相互作用,导致部分带负电荷Pd物种的形成,而带负电荷的Pd又将负电荷转移给氧的反键π*轨道,进而促进了O2的吸附[23-24]。
Pd/Al2O3催化剂的Al2p谱图在74.9 eV处出现1个峰,Pd-2% Na/Al2O3的峰出现了0.4 eV的轻微负迁移,说明Na与Al2O3之间存在相互作用。此结论与ONISHI等[25]的研究结论一致。ONISHI等[25]报道了沉积在TiO2上的Na与表面氧原子间存在强烈的协同作用,导致电荷向TiO2转移。
O1s在529.0~531.0 eV的结合能归属于表面晶格氧,位于高结合能(531.5~533.0 eV)的肩峰归属于表面吸附氧或表面羟基[26]。样品Pd/Al2O3和Pd-2% Na/Al2O3中的表面吸附氧分别占8.70%和9.45%,表明Pd-2% Na/Al2O3催化剂中存在较多的表面活性氧物种,这与O2-TPD的结论一致(图3)。由图4(c)可知,Pd-2% Na/Al2O3的结合能比Pd/Al2O3低,较低的结合能是由于负电荷的增加造成的[27]。
催化剂的Na1s的XPS谱如图4(d)所示。在1 072.6 eV处的峰归属于Na+,表明Na物种已负载在Al2O3上。
-
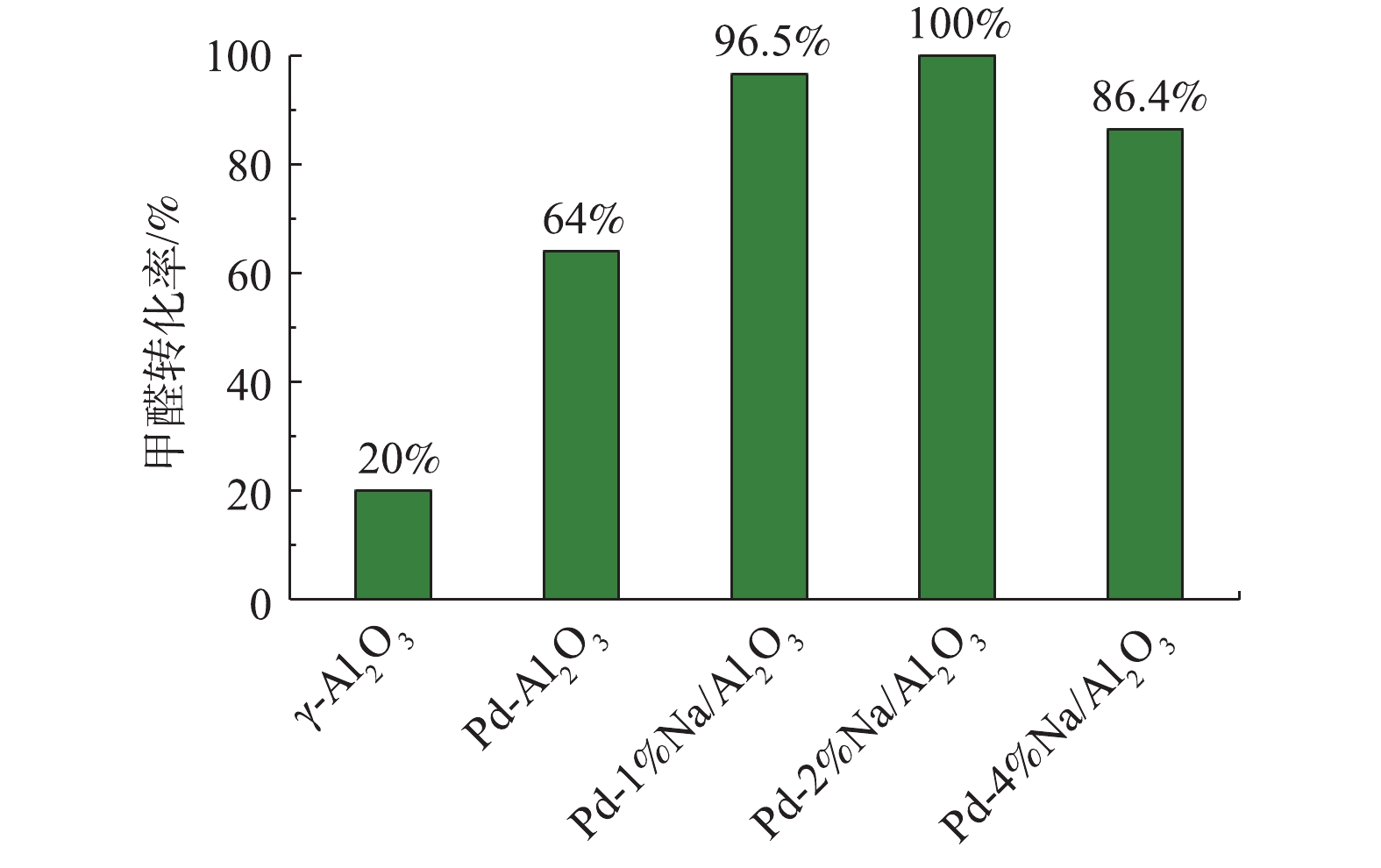
γ-Al2O3、Pd/Al2O3、Pd-1% Na/Al2O3、Pd-2% Na/Al2O3和Pd-4% Na/Al2O3催化剂在25 ℃催化氧化甲醛性能评价见图5,反应条件:反应气体中甲醛的体积分数为0.025%,WHSV=36 000 mL·(g·h)−1。可以看出,γ-Al2O3催化氧化甲醛的活性最差,25 ℃时甲醛转化率只有20.0%。负载Pd后,Pd/Al2O3催化剂催化活性提高至64.0%。同时负载Pd-Na后,Pd-1% Na/Al2O3的甲醛转化率提高至96.5%;Pd-2% Na/Al2O3的催化活性最好,甲醛转化率达到了100%,可以实现甲醛的完全氧化;而Pd-4% Na/Al2O3的甲醛转化率却降至86.4%,原因可能是Na粒子堵塞了部分微孔,覆盖了活性位Pd,使活性位减少。另外,随着Na含量增加到4%,由H2-TPR(图2)可知,催化剂Pd-4% Na/Al2O3耗氢峰的中心出现在116 ℃,相比催化剂Pd-2% Na/Al2O3,向高温偏移了15 ℃;O2-TPD(图3)中表面活性氧的脱附峰向高温方向偏移了。由此可见,Na含量增加到4%时,不利于改善催化剂的低温还原性能以及表面活性氧的脱附。甲醛转化率与Na的负载量密切相关,Na适宜负载量即质量分数为2%。
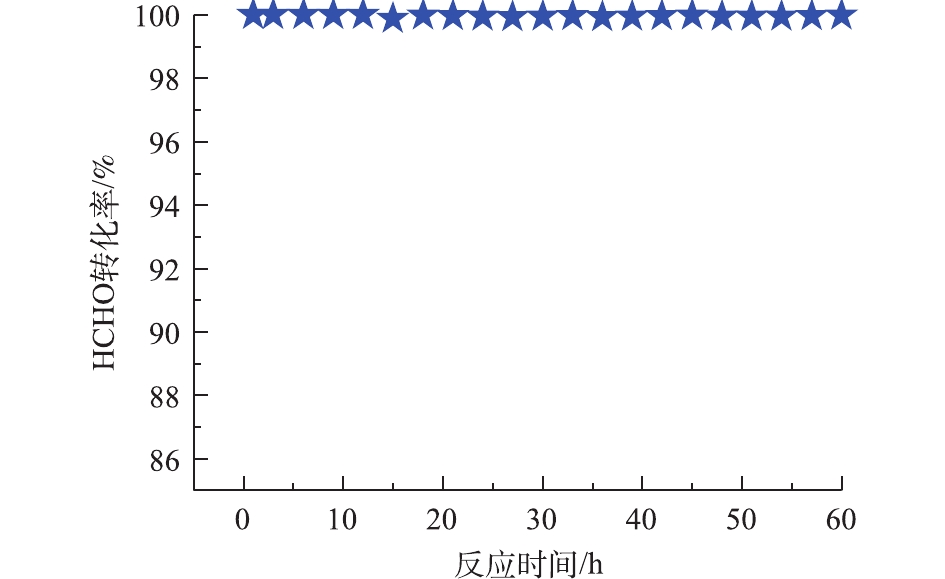
Pd-2% Na/Al2O3催化氧化甲醛的稳定性测试条件与催化氧化性能相同,间隔3 h采集数据计算HCHO转化率,结果如图6所示。连续使用60 h后,甲醛的转化率仍维持在99.0%以上,说明Pd-2% Na/Al2O3具有良好的催化稳定性。
众所周知,采用碱改性是提高催化剂的催化氧化活性的有效措施。ZHANG等[7]研究发现,碱金属物种通过促进表面OH−的形成,OH−与甲酸盐在室温下的反应,进而改变了催化氧化甲醛的途径,从而大大提高了催化剂的性能。何德东等[28]也报道了在Pt/MORn-H6催化剂中添加Na+可以提高氧化甲醛的催化活性。同时,ZHANG等[9]也发现,Na+对Pd/TiO2催化剂的催化活性有显著促进作用。除Na外,K对催化剂催化氧化甲醛的性能也能起到促进作用。引入K+离子后,催化剂表面OH−物种的存在明显提高了Ag/Co3O4催化剂的催化氧化甲醛的性能,Ag与Co的协同作用促使催化剂表面形成了更多的表面活性氧,进而Ag/Co3O4具有较高的催化氧化甲醛活性[29]。在本研究中,将Na引入到Pd/Al2O3催化体系中显著提高了催化氧化甲醛的催化活性,这是由于Na与Pd之间的强协同作用,使得催化剂具有良好的低温还原性和丰富的表面活性氧物种。因此,Pd-2% Na/Al2O3催化剂具有优异的催化活性和良好的稳定性。
2.1. BET分析
2.2. XRD分析
2.3. H2-TPR分析
2.4. O2-TPD分析
2.5. XPS分析
2.6. 催化剂的催化氧化性能评价
-
1)采用共浸渍法制备的一系列不同Na含量的Pd-1% Na/Al2O3、Pd-2% Na/Al2O3、Pd-4% Na/Al2O3催化剂中,Pd-2% Na/Al2O3催化剂性能最好,在25 ℃下可完全将甲醛氧化成CO2和H2O。
2) Pd与Na之间的强协同作用使得Pd-2% Na/Al2O3催化剂具有良好的低温还原性和丰富的表面活性氧,这对甲醛在Pd-2% Na/Al2O3催化剂上的完全氧化起着至关重要的作用。Pd-2% Na/Al2O3催化剂具有良好的稳定性,连续使用60 h后,甲醛的转化率仍维持在99.0%以上。



 下载:
下载: