-
科技的高速发展在提高人类生活水平的同时对环境也会造成极大影响,近年来,国内外水体污染问题日益严峻[1-3]。2015年,中国废水排放总量为7.353×1010 t。其中,城镇生活污水排放量达到了5.352×1010 t[4]。城镇生活污水的处理问题备受关注,因此,AO、A2O、OAO、氧化沟等优良工艺应运而生[5-6]。
缺氧好氧工艺即AO工艺是我国目前广泛应用的一种生物脱氮工艺,由于其脱氮效果较好、原理简单被国内外学者和工程师持续研究和改进[7-9]。OAO工艺即是AO工艺的一种改良工艺,其在传统AO工艺的缺氧池前端增设一级曝气池,具有良好的废水处理效果[10-11]。
微生物是污水生物处理的核心部分,利用分子生物学技术对污水处理过程中活性污泥的微生物菌群进行动态跟踪和功能群种鉴定,有助于筛选并培养出利于污水处理的优势菌种,构建更加合理的活性污泥生态系统,提高污水处理效率[12]。MATSUDA等[13]对日本大阪8个城市污水厂的活性污泥微生物组成进行了分析,发现不同工艺在不同时期微生物群落差异很大。DING等[14]和YE等[15]分别应用AO工艺和A2O工艺处理混合废水和酱油废水,发现Proteobacteria是污水处理中的第一优势菌门。HAN等[16]、曾涛涛等[17]研究氧化沟工艺处理生活污水时发现,大部分细菌属于Proteobacteria和Bacteroidetes这2大门类,其优势菌属有Pseudomonas、Phycisphaera、Methylocystis等。但是,关于OAO工艺处理城镇生活污水过程中活性污泥的微生物特性,目前还少有研究。本研究拟采用16S rDNA高通量测序技术对OAO和AO工艺处理城镇生活污水过程中各反应池的活性污泥中的微生物进行测序分析,为OAO工艺处理城镇生活污水中优势菌种的筛选及培养提供参考。
-
实验装置如图1所示,AO和OAO工艺所用一体化装置均为有机玻璃制作。AO工艺装置长、宽、高分别为0.78、0.4、0.6 m,其中,缺氧池有效容积为30 L,好氧池有效容积为90 L。OAO工艺则在AO工艺基础上,前置1个容积为46 L的曝气池及1个一级沉淀池,装置尺寸为1.16 m×0.4 m×0.6 m。
-
本实验采用实验室配置的模拟城镇生活污水,水质指标如下:COD为340~360 mg·L−1、NH3-N为50.2~72.3 mg·L−1、TN为54.2~75.5 mg·L−1、TP为3.3~8.0 mg·L−1、pH为7.3~8.0。
-
本研究从郑州市某生活污水处理厂的污泥脱水机房获取污泥,其含水率约为80%,将反应器平均污泥浓度设为3 500 mg·L−1,以此计算出OAO工艺需投加的污泥量为2 905 g,AO工艺为2 100 g。同时,从该污水厂硝化液回流管道中取部分混合液,将混合液与污泥一并加入反应器内。而后,开始进水并搅拌混合液,使泥水混合均匀。同时,开启风机进行闷曝,闷曝2 d后,污泥基本恢复生物活性。
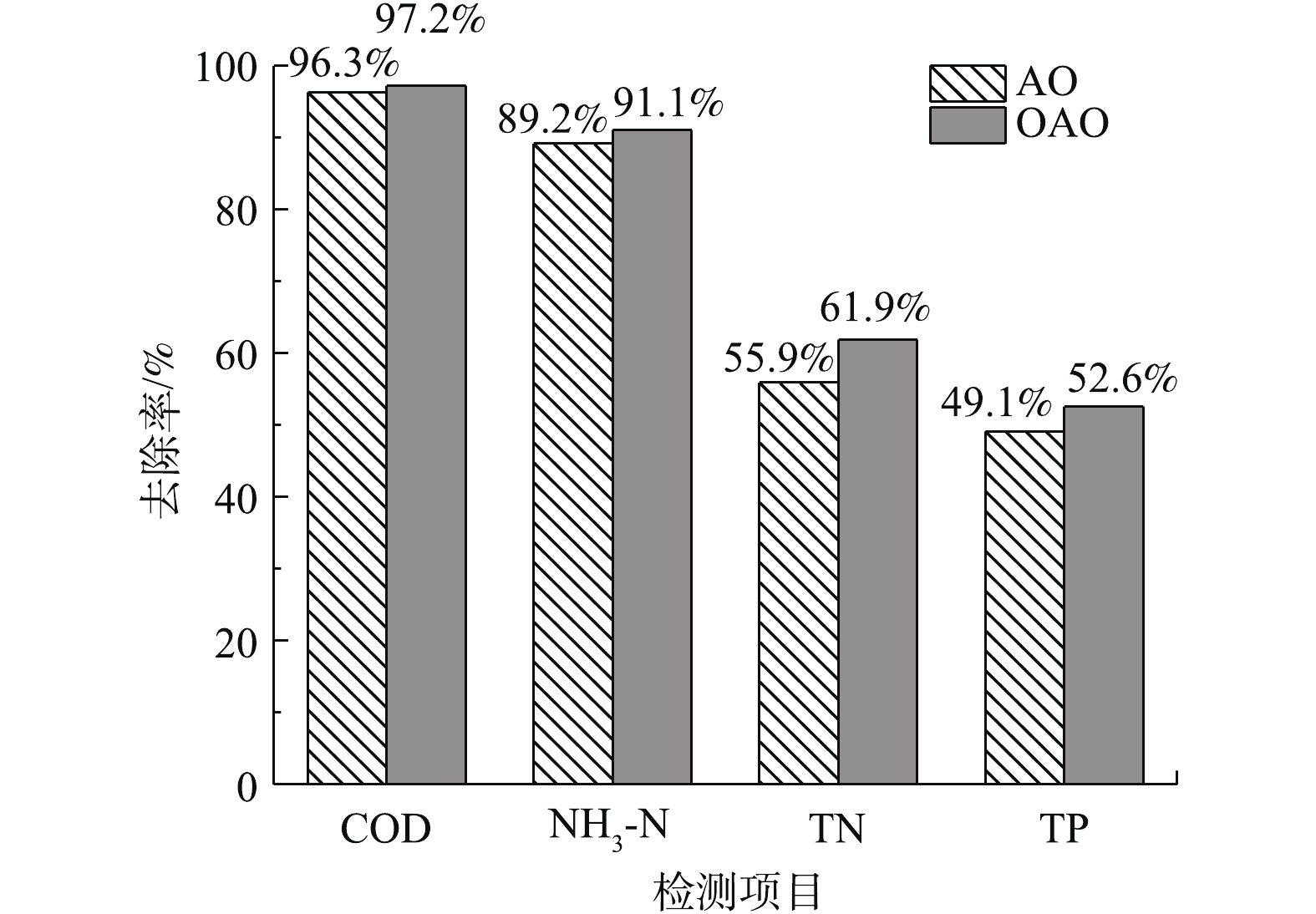
闷曝工作完成后,开始连续不间断地向反应器内进水以驯化污泥,AO工艺进水流量约为12 L·h−1,OAO工艺约为16.6 L·h−1。AO工艺污泥回流比为100%,混合液回流比为200%,OAO工艺2段污泥和混合液回流比均为100%。AO工艺为单点进水,新鲜污水均进入缺氧池中。考虑到缺氧池碳源问题,OAO工艺采用两点进水,1/3的污水进入缺氧池,2/3进入曝气池。驯化期间,每天对进出水水质进行检测,COD采用重铬酸钾法(HJ 828-2017)、NH3-N采用纳氏试剂分光光度法(HJ 535-2009)、TN采用碱性过硫酸钾消解紫外分光光度法(HJ 636-2012)、TP采用钼酸铵分光光度法(GB 11893-1989)、溶解氧采用电化学探头法(HJ 506-2009)、pH采用玻璃电极法(GB 6920-1986)、温度使用水温计法(GB 13195-1991)进行测量[18-24]。进水检测大约持续30 d,AO工艺在第9天达到稳定状态,OAO工艺在第12天时达到稳定,稳定后,2种工艺对COD、NH3-N、TN、TP的平均去除率见图2。2种工艺稳定后的出水COD、NH3-N指标基本符合《城镇污水处理厂污染物排放标准》中的一级A标准[25]。
-
在连续进水第30天时进行取样,此时2种工艺各反应池均已稳定运行。污泥样品取自AO工艺的好氧池、AO工艺的缺氧池、OAO工艺的好氧池、OAO工艺的缺氧池、OAO工艺的曝气池,分别记为AO.H、AO.Q、OAO.H、OAO.Q、OAO.B。每个反应池取100 mL泥水混合液,静置30 min,除去上清液,所得沉淀混合均匀后,分装于瓶中,保存于−30 ℃环境中,以便后续DNA提取。污泥样品中的DNA采用PowerSoil®DNA提取试剂盒,按照试剂盒使用方法提取。
-
采用NanoDrop 1000分光光度法测定样品中提取的DNA的浓度和纯度[26],将符合要求的DNA样品使用无菌水稀释至1 ng·μL−1后,用作模板DNA。以上述基因组DNA为模板,在16S rDNA基因的保守区V4区域,通过融合引物515F(5′-GTGCCAGCMGCCGCGG-3′)和806R(5′-GGACTACHVGGGTWTCTAAT-3′)进行PCR扩增,扩增反应体系体积为30 μL,扩增流程为:95 ℃预变性3 min;95 ℃变性30 s,55 ℃退温30 s,72 ℃延伸40 s,进行30个循环;72 ℃延伸10 min;最后将样品保持在10 ℃恒温环境中。扩增产物采用1%琼脂糖凝胶电泳检测,选择明亮主条带的样品用于进一步的实验。使用AxyPrep DNA凝胶抽提试剂盒(AXYGEN)对PCR产物切胶纯化。之后,通过Illumina建库试剂盒和相应的质检方案完成文库的构建和质检。将合格的文库送往Illumina平台(Miseq)进行测序,用下机得到的数据进行相应的生物信息分析。
-
为了反映样品中微生物菌群的丰富度及多样性,对每个样本分别进行微生物菌群的Alpha多样性分析[27],结果见表1。覆盖率均在99%以上,证明本次测序数据可靠度极高。在97%相似度下,将样本中的优质Tags进行聚类,共得到1 237个OTUs,其中OAO工艺的OTUs为1 135个,AO工艺为1 063个。OAO工艺中的3个反应器都比AO工艺中的OTU数目高,反映了本实验中OAO工艺的细菌数目要大于AO工艺。从Chao指数和Ace指数来看,2种工艺各反应器的物种丰富度为OAO曝气池<AO好氧池<AO缺氧池<OAO缺氧池<OAO好氧池。OAO工艺独有的曝气池丰富度较低,初步分析其可能的原因是,该曝气池的相对进水流量为AO工艺和OAO工艺后续反应池的2/3,污水水量较少,营养物质浓度较低,且没有混合液回流,水源单一,进水水质的差异造成了其丰富度的差异[28]。同时,曝气池后的初沉池污泥直接内回流于曝气池,处于一种相对封闭的状态,故OAO工艺的一级曝气池丰度要略低于其他反应器。从Shannon和Simpson指数来看,OAO工艺中的微生物群落多样性显著高于AO工艺。2种工艺好氧池物种多样性均大于缺氧池,其原因可能是,在缺氧和好氧2种不同运行条件下,微生物的生态系统体系有所差异[29]。总体来看,OAO工艺的微生物物种丰度和多样性均优于AO工艺。
-
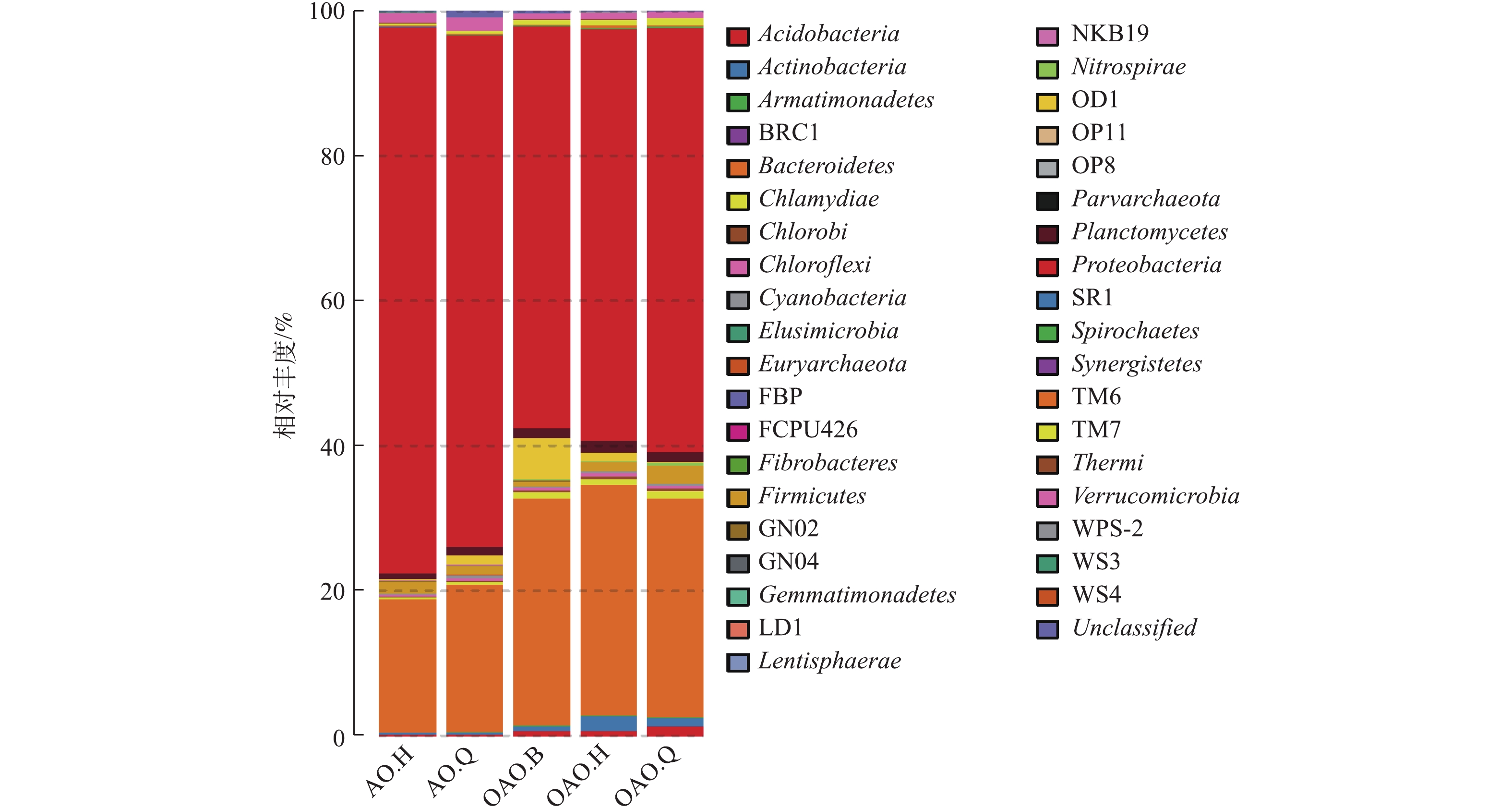
本次测序共检测出已知门38个,结果见图3。OAO工艺中的细菌门类较AO多。Proteobacteria和Bacteroidetes为2种工艺的第1和第2优势菌门,这与HAN等[16]、曾涛涛等[17]、郑向阳等[30]在氧化沟和AO工艺研究中的研究结果一致。Proteobacteria和Bacteroidetes是自然界细菌中2个较大的门类,一些反硝化细菌和大多数固氮细菌也属于这2种门类[31]。康晓荣[32]研究发现总氮和总磷的去除率与Proteobacteria和Bacteroidetes的丰度成正相关。本实验中,AO工艺中的Proteobacteria丰度要大于OAO工艺,但OAO工艺中的Bacteroidetes丰度要远大于AO工艺。
在纲水平下,对Proteobacteria门和Bacteroidetes门的细菌在各反应器中的分布进行统计分析,结果如表2所示。γ-Proteobacteria除在OAO的曝气池为第2优势菌纲外,在其他各反应器均为第1优势菌纲。在Proteobacteria门里,除γ-Proteobacteria外,β-Proteobacteria、α-Proteobacteria、δ-Proteobacteria也为2种工艺的优势菌纲。β-Proteobacteria和γ-Proteobacteria的富集可以大大提高污水中有机物和氮、磷去除率[33];α-Proteobacteria作为一种成员变异性极大、共通点极少的菌纲[34],在OAO的各反应器内的占比均明显高于AO工艺,其成员有根瘤菌、立克次氏体、光合细菌等,在某些光生物反应器实验中表现为优势菌种[33, 35]。在Bacteroidetes门中,Saprospirae、Sphingobacteria、Flavobacteriia、Cytophagia在2种工艺里占比较高。Cytophagia在AO工艺中的占比几乎是OAO工艺的2倍,Flavobacteriia在OAO缺氧池占比较小,其他该门菌纲在OAO工艺中的各反应器占比明显高于AO工艺。OGONOWSKI等[36]研究发现,Cytophagia在天然纤维素基材上富集较多,该菌纲在OAO工艺较少且曝气池>缺氧池>好氧池,这可能是因为部分纤维素作为部分微生物的碳源在前置曝气池中被转化。Flavobacteriia在许多海洋环境中都很丰富,可以在极端环境中降解水中不稳定的有机碳[37]。Saprospirae、Sphingobacteria在一些其他AO改良工艺和厌氧、好氧工艺中也表现为优势菌纲[38-40]。
除上述2种菌门外,在2种工艺中均存在且丰度较高的菌门还有Nitrospirae、Planctomycetes、Firmicutes、Verrucomicrobia、OD1等,Nitrospirae、Planctomycetes、Firmicutes中的某些细菌具有较好的碳氮去除能力,短程硝化反硝化中的厌氧氨氧化菌就来自Planctomycetes。TM7、Actinobacteria、Chlamydiae、Acidobacteria等菌门在OAO工艺中的丰度较高,但在AO工艺中则相反。Actinobacteria是OAO好氧池中的第3优势菌门,具有多样化的次级代谢,在生态环境中有许多潜在用途[41];Acidobacteria是OAO缺氧池中的第4优势菌门,在土壤生态中占细菌总量的30%~50%,具有纤维素降解的功能,并且参与土壤中的铁循环和光合作用[42]。除Verrucomicrobia外,AO工艺中相对丰度大于0.5%的菌门在OAO中的相对丰度也均在0.5%以上。Verrucomicrobia作为一种在氮、磷、钾含量较低的情况下依然能利用碳源生长的菌门[43-44],在AO工艺中的平均占比几乎是OAO工艺中的2倍,这与OAO工艺前置曝气池消耗掉部分有机物不无关系。
-
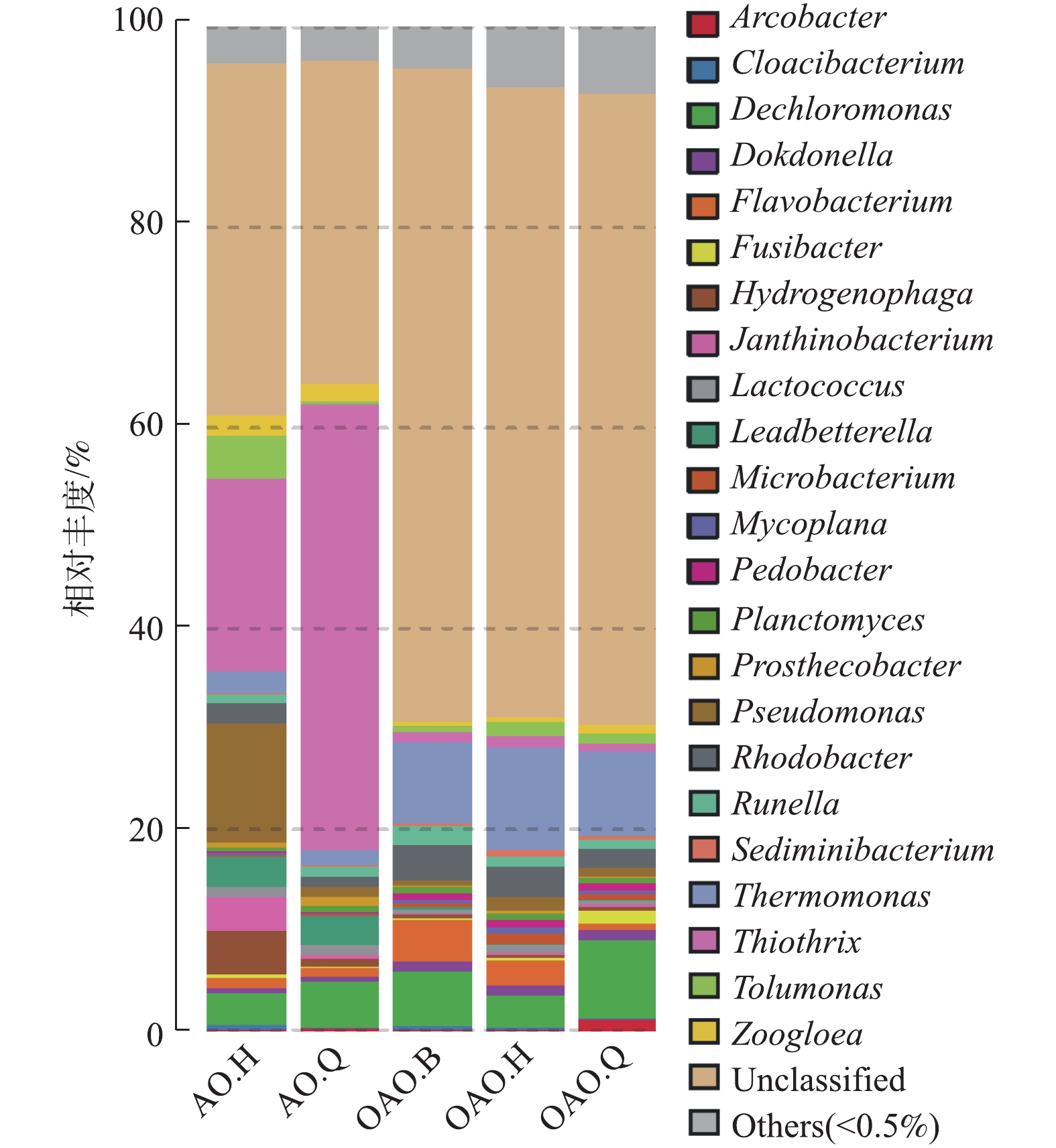
在本次测序的5个样本中,共检出菌属347个,丰度大于0.5%的已知菌属为23个,各池微生物群落相对丰度见图4。未知菌属(Unclassified)在各池的占比为35.07%~65.13%,在OAO工艺内的相对丰度要远大于AO工艺。已知属中来自Proteobacteria门的Thiothrix、Thermomonas、Dechloromonas相对丰度较高,三者总丰度占比为24%。与何嘉鹏[45]的实验结果不同,作为硝化细菌的重要菌属Nitrosomonas、Nitrospira虽均在2种工艺中检出,但含量皆小于0.5%,在本实验中,被归类为其他菌属(others)。Thiothrix、Pseudomonas、Hydrogenophaga是AO工艺好氧池中的优势菌属;Thiothrix、Dechloromonas、Leadbetterella是AO工艺缺氧池中的优势均属;Thermomonas、Flavobacterium、Dechloromonas是OAO工艺曝气池中的优势菌属;Thermomonas、Dechloromonas是OAO工艺好氧池中的优势菌属;Thermomonas、Dechloromonas、Rhodobacter是OAO工艺缺氧池中的优势菌属。
WANG等[46]研究发现,Thermomonas与硝酸盐还原酶基因呈正相关,对同时厌氧氨氧化-反硝化系统中的硝酸盐还原起重要作用;WANG等[47]研究发现,Dechloromonas在反硝化过程时可以进行磷的去除。在本实验中,上述2种菌属在OAO工艺各部分的相对丰度均大于AO工艺,这可能是OAO工艺对TN和TP去除效果显著优于AO工艺的原因之一。OAO工艺里其他优势菌属中,Flavobacterium为好氧反硝化菌[48],Rhodobacter具有固氮的功能,也属于好氧反硝化菌[49]。AO工艺里,Pseudomonas为第1优势菌属,其主要功能为异养硝化-好氧反硝化菌[50]和反硝化聚磷菌[48],其在AO工艺和OAO工艺好氧池中的大量存在可能是2种工艺高效脱氮的重要原因之一。Thiothrix作为一种能以硫化物为唯一能源生长的菌属,被认为是造成活性污泥膨胀的主要原因之一[51]。本次实验虽未见明显的污泥膨胀现象,但2种工艺在HRT、温度、水质等条件相同的情况下,Thiothrix在AO工艺中的相对丰度要远大于OAO工艺,这使得AO工艺发生污泥膨胀的可能性要远大于OAO工艺。Nitrosomonas在2种工艺的好氧池中的相对丰度分别为AO.H 0.05%、OAO.H 0.47%;Nitrospira则为AO.H 0.02%、OAO.H 0.45%。MA等[52]研究发现,污水厂活性污泥中的AOB和NOB相对丰度在0.1%~1%时,脱氮效率依旧很高,这与本次实验水质检测结果基本相符。2种硝化菌属在2种工艺中好氧池的丰度均为OAO好氧池>AO好氧池,这可能是因为OAO工艺中的第1段曝气池的作用使得工艺整体的污泥BOD负荷较AO工艺小,从而减轻硝化细菌与其他异养型细菌的竞争压力,为硝化细菌提供了更佳的生长环境。
-
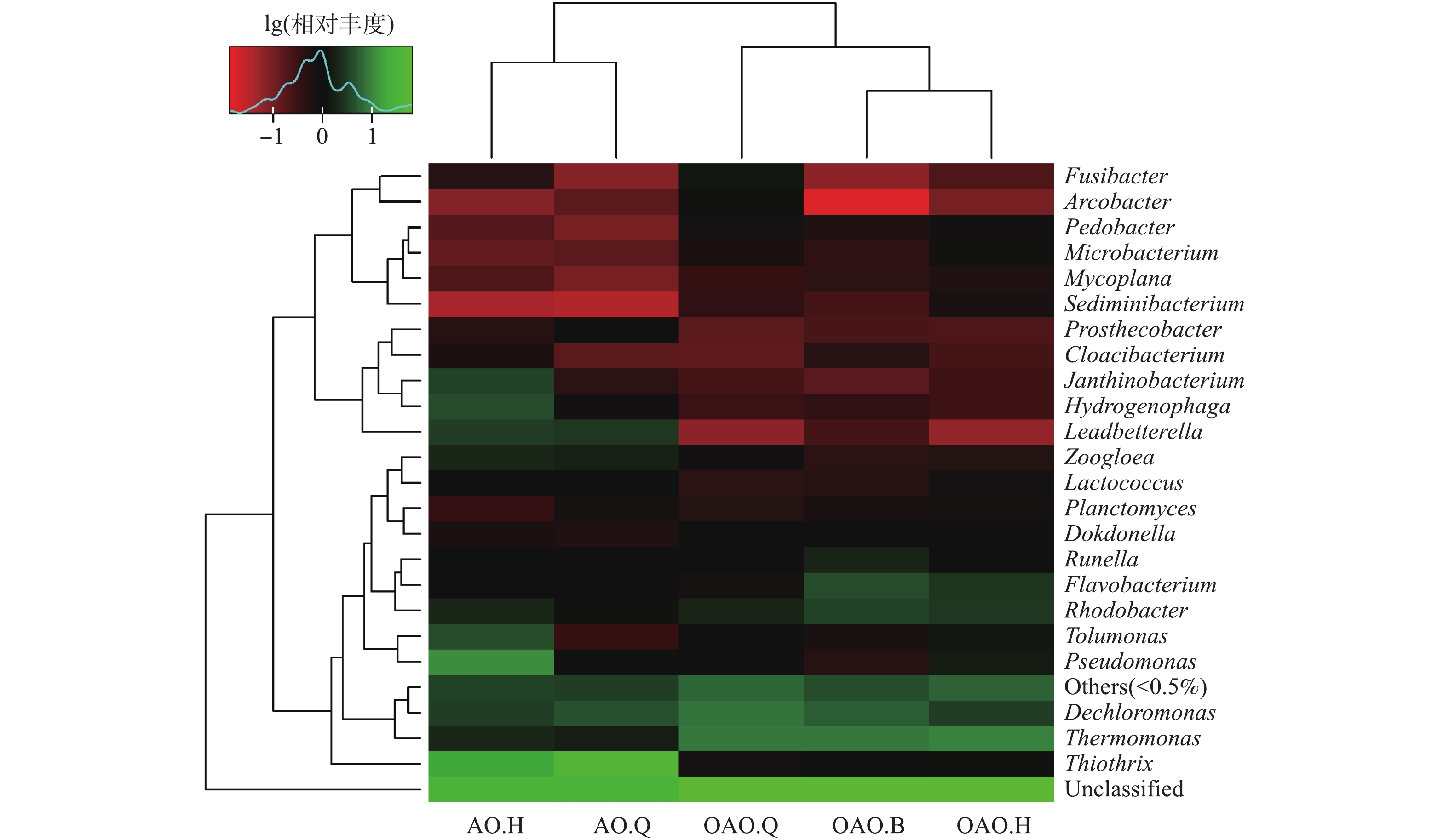
图5为2种工艺各反应池中微生物在属水平下的物种丰度热图,该图将微生物属水平的聚类结果按照其在该样品中所占相对丰度数值用不同梯度的颜色表示,用颜色矩阵来直观地表示不同样品中物种的丰度。微生物群落组成在不同反应池间的相似性和差异性由纵向聚类表示,距离越近,枝长越短,说明样品的物种组成及丰度越相似。由于本次测序结果显示不同物种间相对丰度差异较大,颜色梯度难以满足要求,因此,将原始相对丰度以10为底进行对数转化,若某物种相对丰度为0,则取样品中物种相对丰度最小(大于0)的值除以2然后进行对数转化。
由图5可知,OAO和AO工艺间微生物群落具有较大的差异性,OAO工艺内部的缺氧池、曝气池和好氧池3个反应池间的差异性要略大于AO工艺内部的2个反应池间的差异性。这进一步说明OAO工艺中活性污泥微生物生态体系更为复杂,多样性更高。Planctomyces、Dokdonella、Mycoplana等在2种工艺中的相对丰度相近,初步推测其受2种工艺间差异的影响较小,采用OAO工艺并不能使该类菌属在原AO工艺中发挥的作用发生改变。Thiothrix、Pseudomonas、Tolumonas、Zoogloea、Leadbetterella、Hydrogenophaga、Janthinobacterium在AO工艺的丰度明显大于OAO工艺,同时,Thermomonas、Runella、Flavobacterium、Rhodobacter在OAO工艺中的丰度又显著大于AO工艺。采用OAO工艺会增加或减少上述菌属在原AO工艺反应器内的丰度,某些具有脱氮除磷功能的菌属的增减使污水处理效果发生变化。
5个反应池内的优势菌属中部分功能菌属的相对丰度统计分析结果见表3。Hydrogenophaga、Pseudomonas作为异养硝化-好氧反硝化菌[53],在BOD负荷较高有机碳源充足的AO工艺好氧池中大量存在,其可在同一个空间内进行硝化和反硝化,在本次AO工艺生物脱氮过程中起到了重要作用。Dechloromonas、Flavobacterium、Rhodobacter、Thermomonas均为具有反硝化功能的菌属,在OAO工艺中的相对丰度大于AO工艺,尤其是Thermomonas作为一种自养反硝化菌[54],在BOD负荷较小的OAO工艺中的相对丰度远高于AO工艺。这解释了OAO工艺在对TN的去除效果上优于AO工艺的原因。
-
1) OAO和AO工艺在稳定运行时对COD、NH3-N、TN、TP的平均去除率分别为97.23%和96.31%、91.07%和89.16%、61.91%和55.90%、52.59%和49.15%,OAO工艺对TN和TP的去除效果要显著优于AO工艺。
2)微生物群落的Alpha多样性分析表明,AO工艺和OAO工艺二者间的丰度和多样性有显著差异,OAO工艺的丰度和多样性皆优于AO工艺。
3)微生物物种结构分析结果表明,2种工艺的优势菌门均为Proteobacteria和Bacteroidetes,且Proteobacteria门中的β-Proteobacteria和γ-Proteobacteria均为2种工艺的优势菌纲。2种工艺在优势菌属上差异较大,OAO工艺的优势菌属为Thermomonas、Dechloromonas、Rhodobacter,AO工艺的优势菌属为Pseudomonas、Thiothrix、Dechloromonas。
4)属水平下物种丰度热图表明,OAO和AO工艺间微生物群落具有较大的差异性,Thiothrix、Pseudomonas、Thermomonas等受2工艺间差异的影响,相对丰度差异性较大。
5)由于OAO工艺前置曝气池的设置,OAO工艺污泥BOD负荷较AO工艺小,导致Verrucomicrobia门、Cytophagia纲等对碳源需求较高的微生物在OAO中丰度较小,Thermomonas属、Dechloromonas属、Nitrospira属、Nitrosomonas属等具有脱氮除磷功能的细菌有了合适的生长环境,其在OAO的丰度明显高于AO工艺。据此推测,若将OAO工艺用于处理中高浓度的污水,其脱氮效率要远大于AO工艺。
OAO和AO工艺处理城镇生活污水的微生物群落特征分析
Analysis of microbial community characteristics of OAO and AO processes for domestic wastewater treatment
-
摘要: 以模拟城镇生活污水为对象,采用AO和OAO 2种工艺进行处理,通过常规水质检测和高通量测序技术来分析和探究导致2种工艺运行效果不同的微生物层面原因。结果表明:OAO工艺对COD和NH3-N的平均去除率略高于AO工艺,但OAO工艺对TN和TP的去除效果较为显著,平均去除率分别比AO工艺高6.01%和3.44%;2种工艺的优势菌门均为Proteobacteria和Bacteroidetes,2种工艺的优势菌纲也均为β-Proteobacteria和γ-Proteobacteria;2种工艺间微生物群落差异性较大,OAO工艺的微生物种群丰度和多样性均大于AO工艺;AO工艺优势菌属为Pseudomonas、Thiothrix、Dechloromonas,OAO工艺为Thermomonas、Dechloromonas、Rhodobacter。此外,Nitrosomonas、Nitrospira作为亚硝化和硝化阶段的重要菌属在AO工艺好氧池的相对丰度为0.05%和0.02%,在OAO工艺中则显著提高,为0.47%和0.45%。其原因是OAO工艺的好氧池污泥BOD负荷较AO小,更适合硝化细菌生长。硝化细菌及其他功能菌在OAO工艺中的大量存在是OAO工艺的能够高效脱氮的主要原因。以上结果可为OAO工艺处理城镇生活污水中优势菌种的筛选及培养提供参考。Abstract: To reveal the reason for the different domestic wastewater treatment effects of OAO(oxic-anoxic-oxic) and AO(anoxic-oxic) process at the perspective of microorganism, the ordinary wastewater quality test and high-throughput sequencing technique were used to analyze the different bacterial community in the activated sludge. The results showed that the average removal rates of COD and NH3-N in OAO process were slightly higher than those in AO process, while TN and TP removal effect by OAO process were significant, and its average removal rates were 6.01% and 3.44% higher than in AO process, respectively. Proteobacteria and Bacteroidetes were the dominant phyla in the two processes, and β-Proteobacteria and γ-Proteobacteria were the dominant classes. A significant difference in the bacteria community occurred in these two systems, the abundance and diversity of species in OAO process were greater than AO process. Pseudomonas, Thiothrix, Dechloromonas were the dominant genus in AO process. Thermomonas, Dechloromonas, Rhodobacter were the dominant genus in OAO process. Nitrosomonas and Nitrospira, as important genus of nitrosation and nitrification stages, had a relative abundance of 0.05% and 0.02% in AO process and 0.47% and 0.45% in OAO process, respectively. The higher abundance of these two bacteria in OAO process might correspond to its lower BOD loading of the activated sludge, which was suitable for the growth of nitrifying bacteria. The high abundances of nitrifying bacteria and other functional bacteria in the OAO process ultimately resulted in high nitrogen removal efficiency. The above results can be used as the theoretical basis for the screening and culture of the dominant bacterial in domestic wastewater treatment using OAO process.
-

-
表 1 2种工艺各反应器中微生物的Alpha多样性指数
Table 1. Alpha diversity index of microorganisms in each tank of the two processes
样本 Chao Ace Shannon Simpson 覆盖率/% AO.H 928.01 954.09 3.96 0.060 99.79 AO.Q 938.10 967.41 3.42 0.202 99.81 OAO.B 913.39 927.97 4.28 0.030 99.81 OAO.H 959.64 980.46 4.44 0.029 99.85 OAO.Q 943.01 980.01 4.37 0.034 99.82 表 2 Proteobacteria和Bacteroidetes中的各纲在各反应池中的分布
Table 2. Distribution of bacteria classes belonging to Proteobacteria and Bacteroidetes in each tank %
菌门 菌纲 AO.H AO.Q OAO.B OAO.H OAO.Q Proteobacteria α-Proteobacteria 3.75 3.04 6.52 7.16 5.24 β-Proteobacteria 22.30 14.14 26.98 19.24 24.67 δ-Proteobacteria 2.99 2.58 2.58 2.83 1.86 γ-Proteobacteria 46.22 50.51 19.28 27.45 25.48 Other 0.19 0.27 0.16 0.05 1.16 Bacteroidetes Sphingobacteria 2.95 4.29 6.65 13.36 11.02 Cytophagia 4.36 4.15 2.81 1.69 1.71 Flavobacteriia 1.74 1.18 4.85 3.13 1.10 Saprospirae 8.83 10.20 16.67 13.42 15.62 Other 0.53 0.54 0.20 0.33 0.64 表 3 功能菌在各反应池中的分布
Table 3. Distribution of functional bacteria in each tank
% 菌属 主要功能 AO.H AO.Q OAO.B OAO.H OAO.Q Dechloromonas 好氧反硝化、氨氧化 3.08 4.57 5.44 3.18 7.78 Flavobacterium 好氧反硝化 0.95 0.85 4.17 2.59 0.67 Hydrogenophaga 异养硝化-好氧反硝化 4.30 0.82 0.38 0.29 0.30 Pseudomonas 异养硝化-好氧反硝化 11.90 0.92 0.46 1.43 0.85 Rhodobacter 好氧反硝化 2.01 1.10 3.53 3.03 1.91 Thermomonas 自养反硝化 2.12 1.60 7.97 10.20 8.48 -
[1] 中华人民共和国生态环境部. 2018年中国生态环境状况公报[R]. 北京, 2019. [2] BARLETTA M, LIMA A R A, COSTA M F. Distribution, sources and consequences of nutrients, persistent organic pollutants, metals and microplastics in South American estuaries[J]. Science of the Total Environment, 2019, 651: 1199-1218. doi: 10.1016/j.scitotenv.2018.09.276 [3] WEN Y, SCHOUPS G, VAN DE GIESEN N. Organic pollution of rivers: Combined threats of urbanization, livestock farming and global climate change[J]. Scientific Reports, 2017, 7: 43289. doi: 10.1038/srep43289 [4] 中华人民共和国环境保护部. 中国环境统计年报·(2015)[M]. 北京: 中国环境科学出版社, 2016. [5] JIN L, ZHANG G, TIAN H. Current state of sewage treatment in China[J]. Water Research, 2014, 66: 85-98. doi: 10.1016/j.watres.2014.08.014 [6] ZHANG Q H, YANG W N, NGO H H, et al. Current status of urban wastewater treatment plants in China[J]. Environment International, 2016, 92-93: 11-22. doi: 10.1016/j.envint.2016.03.024 [7] 张健. A/O-MBR+O3溶胞组合工艺污泥减量及强化脱氮效能与机制研究[D]. 哈尔滨: 哈尔滨工业大学, 2018. [8] XU Y, FANG Y, WANG Z, et al. In-situ sludge reduction and carbon reuse in an anoxic/oxic process coupled with hydrocyclone breakage[J]. Water Research, 2018, 141: 135-144. doi: 10.1016/j.watres.2018.05.010 [9] 邹胜萍, 洪平. 一种四内循环改良A/O一体化污水处理装置: CN207645902U[P]. 2018-07-24. [10] 汤清泉, 魏宏斌, 陈良才. AAO与OAO工艺处理焦化废水的对比研究[J]. 工业用水与废水, 2016, 47(3): 31-35. doi: 10.3969/j.issn.1009-2455.2016.03.007 [11] 岳培恒, 王宝强, 夏辉, 等. OAO工艺在非熔渣-熔渣气化废水处理中的应用[J]. 中国给水排水, 2016, 32(10): 116-119. [12] 彭永臻, 高守有. 分子生物学技术在污水处理微生物检测中的应用[J]. 环境科学学报, 2005, 25(9): 1143-1147. doi: 10.3321/j.issn:0253-2468.2005.09.001 [13] MATSUDA M, INOUE D, ANAMI Y, et al. Comparative analysis of DNA-based microbial community composition and substrate utilisation patterns of activated sludge microorganisms from wastewater treatment plants operated under different conditions[J]. Water Science & Technology, 2010, 61(11): 2843-2851. [14] DING L, ZHOU Q, WANG L, et al. Dynamics of bacterial community structure in a full-scale wastewater treatment plant with anoxic-oxic configuration using 16S rDNA PCR-DGGE fingerprints[J]. African Journal of Biotechnology, 2011, 10(4): 589-600. [15] YE D, LIANG H, ZHOU W, et al. Total and active microbial communities in a full-scale system treating wastewater from soy sauce production[J]. International Biodeterioration & Biodegradation, 2017, 123: 206-215. [16] HAN Y P, LI L, LIU J X. Characterization of the airborne bacteria community at different distances from the rotating brushes in a wastewater treatment plant by 16S rRNA gene clone libraries[J]. Journal of Environmental Sciences, 2013, 25(1): 5-15. doi: 10.1016/S1001-0742(12)60018-7 [17] 曾涛涛, 蒋小梅, 韩科昌, 等. 生活污水处理厂微生物群落结构解析[J]. 安全与环境学报, 2018, 18(2): 697-703. [18] 国家环境保护局. 水质pH值的测定玻璃电极法: GB 6920-1986[S]. 北京: 中国标准出版社, 1986. [19] 中华人民共和国环境保护部. 水质氨氮的测定纳氏试剂分光光度法: HJ 535-2009[S]. 北京: 中国环境科学出版社, 2009. [20] 中华人民共和国环境保护部. 水质化学需氧量的测定重铬酸盐法: HJ 828-2017[S]. 北京: 中国环境科学出版社, 2017. [21] 中华人民共和国环境保护部. 水质溶解氧的测定电化学探头法: HJ 506-2009[S]. 北京: 中国环境科学出版社, 2009. [22] 国家环境保护局. 水质水温的测定温度计或颠倒温度计测定法: GB 13195-1991[S]. 北京: 中国标准出版社, 1991. [23] 中华人民共和国环境保护部. 水质总氮的测定碱性过硫酸钾消解紫外分光光度法: HJ 636-2012[S]. 北京: 中国环境科学出版社, 2012. [24] 国家环境保护总局. 水质总磷的测定钼酸铵分光光度法: GB 11893-1989[S]. 北京: 中国标准出版社, 1989. [25] 国家环境保护总局, 国家质量监督检验检疫总局. 城镇污水处理厂污染物排放标准: GB 18918-2002[S]. 北京: 中国环境科学出版社, 2003. [26] LI Y, HE J, HE Z, et al. Phylogenetic and functional gene structure shifts of the oral microbiomes in periodontitis patients[J]. ISME Journal, 2014, 8(9): 1879-1891. doi: 10.1038/ismej.2014.28 [27] SCHLOSS P D, WESTCOTT S L, RYABIN T, et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities[J]. Applied and Environmental Microbiology, 2009, 75(23): 7537-7541. doi: 10.1128/AEM.01541-09 [28] ZHANG T, SHAO M F, YE L. 454 pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants[J]. ISME Journal, 2012, 6(6): 1137-1147. doi: 10.1038/ismej.2011.188 [29] WELLS G F, PARK H, EGGLESTON B, et al. Fine-scale bacterial community dynamics and the taxa-time relationship within a full-scale activated sludge bioreactor[J]. Water Research, 2011, 45(17): 5476-5488. doi: 10.1016/j.watres.2011.08.006 [30] 郑向阳, 罗晓, 袁立霞, 等. AO工艺处理淀粉污水效能及微生物群落解析[J]. 环境工程学报, 2018, 12(3): 804-814. doi: 10.12030/j.cjee.201708047 [31] MADIGAN M A J M. Brock Biology of Microorganisms[M]. 11th Ed. Upper Saddle River: Prentice Hall, 2005. [32] 康晓荣. 超声联合碱促进剩余污泥水解酸化及产物研究[D]. 哈尔滨: 哈尔滨工业大学, 2013. [33] ZHANG B, LENS P N L, SHI W, et al. Enhancement of aerobic granulation and nutrient removal by an algal-bacterial consortium in a lab-scale photobioreactor[J]. Chemical Engineering Journal, 2018, 334: 2373-2382. doi: 10.1016/j.cej.2017.11.151 [34] WILLIAMS K P, SOBRAL B W, DICKERMAN A W. A robust species tree for the Alphaproteobacteria[J]. Journal of Bacteriology, 2007, 189(13): 4578-4586. doi: 10.1128/JB.00269-07 [35] BEROUAL W, BIONDI E G. A new factor controlling cell envelope integrity in Alphaproteobacteria in the context of cell cycle, stress response and infection[J]. Molecular Microbiology, 2019, 111(3): 553-555. doi: 10.1111/mmi.14201 [36] OGONOWSKI M, MOTIEI A, ININBERGS K, et al. Evidence for selective bacterial community structuring on microplastics[J]. Environmental Microbiology, 2018, 20(8): 2796-2808. doi: 10.1111/1462-2920.14120 [37] LIU J, XUE C, SUN H, et al. Carbohydrate catabolic capability of a Flavobacteriia bacterium isolated from hadal water[J]. Systematic and Applied Microbiology, 2019, 42(3): 263-274. doi: 10.1016/j.syapm.2019.01.002 [38] 宁欣强. A+OSA污泥减量工艺微生物群落结构及代谢特征研究[D]. 重庆: 重庆大学, 2015. [39] 戴昕. 好氧颗粒污泥工艺运行过程重要功能菌群研究[D]. 杭州: 浙江大学, 2014. [40] 胡健. 混凝-两级A/O组合工艺处理高浓度聚酯树脂废水技术研究[D]. 杭州: 浙江大学, 2015. [41] VAN DER MEIJ A, WORSLEY S F, HUTCHINGS M I, et al. Chemical ecology of antibiotic production by actinomycetes[J]. FEMS Microbiology Reviews, 2017, 41(3): 392-416. doi: 10.1093/femsre/fux005 [42] 刘彩霞, 董玉红, 焦如珍. 森林土壤中酸杆菌门多样性研究进展[J]. 世界林业研究, 2016, 29(6): 17-22. [43] AGUIRRE-VON-WOBESER E, ROCHA-ESTRADA J, SHAPIRO L R, et al. Enrichment of Verrucomicrobia, Actinobacteria and Burkholderiales drives selection of bacterial community from soil by maize roots in a traditional milpa agroecosystem[J]. Plos One, 2018, 13(12): e0208852. [44] NAVARRETE A A, SOARES T, ROSSETTO R, et al. Verrucomicrobial community structure and abundance as indicators for changes in chemical factors linked to soil fertility[J]. Antonie van Leeuwenhoek, 2015, 108(3): 741-752. doi: 10.1007/s10482-015-0530-3 [45] 何嘉鹏. 多点进水OAO工艺模拟城镇生活污水处理的生物特性研究[D]. 郑州: 郑州大学, 2018. [46] WANG D, LI T, HUANG K, et al. Roles and correlations of functional bacteria and genes in the start-up of simultaneous anammox and denitrification system for enhanced nitrogen removal[J]. Science of the Total Environment, 2019, 655: 1355-1363. doi: 10.1016/j.scitotenv.2018.11.321 [47] WANG X, ZHAO J, YU D, et al. Evaluating the potential for sustaining mainstream anammox by endogenous partial denitrification and phosphorus removal for energy-efficient wastewater treatment[J]. Bioresource Technology, 2019, 284: 302-314. doi: 10.1016/j.biortech.2019.03.127 [48] 肖晶晶, 郭萍, 霍炜洁, 等. 反硝化微生物在污水脱氮中的研究及应用进展[J]. 环境科学与技术, 2009, 32(12): 97-102. doi: 10.3969/j.issn.1003-6504.2009.12.022 [49] 李小义, 王丽萍, 杜雅萍, 等. 好氧反硝化微生物多样性及其反硝化功能初步研究[J]. 氨基酸和生物资源, 2016, 38(2): 37-45. [50] 孙庆花, 于德爽, 张培玉, 等. 1株海洋异养硝化-好氧反硝化菌的分离鉴定及其脱氮特性[J]. 环境科学, 2016, 37(2): 647-654. [51] MAESTRE J P, ROVIRA R, ÁLVAREZ-HORNOS F J, et al. Bacterial community analysis of a gas-phase biotrickling filter for biogas mimics desulfurization through the rRNA approach[J]. Chemosphere, 2010, 80(8): 872-880. doi: 10.1016/j.chemosphere.2010.05.019 [52] MA Q, QU Y, SHEN W, et al. Bacterial community compositions of coking wastewater treatment plants in steel industry revealed by Illumina high-throughput sequencing[J]. Bioresource Technology, 2015, 179: 436-443. doi: 10.1016/j.biortech.2014.12.041 [53] 杨浩, 张国珍, 杨晓妮, 等. 16S rRNA高通量测序研究集雨窖水中微生物群落结构及多样性[J]. 环境科学, 2017, 38(4): 1704-1716. [54] XING W, LI J, LI D, et al. Stable-isotope probing reveals the activity and function of autotrophic and heterotrophic denitrifiers in nitrate removal from organic-limited wastewater[J]. Environmental Science & Technology, 2018, 52(14): 7867-7875. -




 下载:
下载: