-
染料废水由于具有高色度、高毒性、难降解性和易于在生物体内积累等特点引起人们高度重视[1],成为目前最难处理的工业废水之一。其中刚果红(congo red,CR)作为一种典型的联苯胺类直接偶氮染料,易溶于水,在生产和使用过程中流失率高,是染料废水中具有代表性的一种污染物[2],对人体和周围环境具有极大威胁,严重时可致癌、致畸、致突变,从而引起人们的广泛关注。近年来,常用的染料废水处理方法有化学氧化、生物降解、吸附、光催化等[3-6]。其中,吸附法由于效率高、设备简单、操作安全、可废物资源化等优点而受到普遍认可。常规吸附材料有活性炭、沸石、生物炭、有机膨润土和金属氧化物[7-11],虽然具有来源广泛、化学性能稳定等优点,但其成本高、产生二次污染等缺点也不容忽视。因此,选择价格低廉、吸附效果好、化学性能稳定的材料用于染料废水的处理备受关注。
市政污泥资源化问题是近期研究的热点,目前以污泥热解制取富氢燃气方向为主[12-13]。而污泥热解气化过程中的剩余半焦也是一种极具应用价值的吸附材料。相关研究[14-15]表明,其主要成分为固定碳,具有发达的孔隙结构和良好的表面特性,在吸附工业染料方面具有较好的应用前景[16-18]。李晋[19]的研究表明,以污泥焦炭为添加剂进行污泥热解制取剩余半焦,更有助于提高剩余半焦的比表面积。本研究以污泥焦炭为添加剂经机械化学预处理快速热解制备的半焦为吸附材料,以CR溶液模拟染料废水进行吸附实验。在制取高品质富氢燃气的同时,污泥热解剩余半焦可代替活性炭等传统吸附材料用于CR类染料废水的去除,可最大限度实现市政污泥资源化,具有“以废治废”的实践意义。
响应曲面法(response surface methodology, RSM)是一种能够优化工艺参数、减少实验次数以及评价各影响因素之间水平及交互作用的有效方法[20]。本研究采用响应曲面法中的Box-Behnken实验设计原理,探究了吸附剂投加量、CR初始浓度、温度及pH共4个影响因素对剩余半焦吸附CR的水平的影响及其之间的交互作用;同时通过比表面积及孔径分析、傅里叶变换红外光谱、X射线衍射等分析方法对剩余半焦进行了表征,分析了其吸附CR的机理,为污泥热解剩余半焦的利用探索新途径,为染料废水处理研究提供参考。
-
实验试剂:实验材料取自西安市第三污水处理厂机械脱水污泥,刚果红(分析纯)购于天津市科密欧化学试剂有限公司;氢氧化钠、盐酸均为分析纯。
实验仪器:分析天平(AL204-IC);恒温水浴振荡器(SHA-C);紫外-可见分光光度计(UV-1800)。
-
1)将一定量晾干的污泥置于反应器外陶瓷罐中,待固定床反应器热解炉内温度升至900 ℃时,迅速将陶瓷罐推入炉膛,在N2环境中快速热解20 min至不再产生气体,所得固体即为焦炭添加剂。
2)分别称取10 g干污泥和焦炭添加剂,放入行星式球磨机进行机械化学预处理,设置转速为425 r·min−1、小球质量为180 g(直径6 mm),球磨3 h得到混合物料。将混合物料再次进行快速热解(热解条件同1)),制备实验所需剩余半焦。
-
以ZEN 3700型Zeta电位仪,测定剩余半焦的等电点(isoelectric point, pI);采用比表面积及孔径分析仪(Gemini VII)测定剩余半焦的比表面积、微孔容积和孔径;X射线衍射仪(XRD,D8 ADVANCE型)在扫描速率8 (°)·min−1、扫描范围10°~80°条件下对剩余半焦的物质组成及结晶状况进行表征分析;通过傅里叶漫反射红外光谱仪(FT-IR IS50)对吸附CR前、后的剩余半焦进行扫描,分析其表面所含官能团的种类。
-
取不同浓度(20~150 mg·L−1)的CR溶液25 mL,加入50 mL锥形瓶,并投加一定量(0.8~4.0 g L−1)的剩余半焦,调节pH(2~12)后,将锥形瓶置于恒温水浴振荡器。在温度为20~40 ℃、150 r·min−1的条件下,振荡4 h后取样,以0.45 μm滤膜过滤,使用分光光度计测定波长为497 nm处的吸光度,计算吸附后CR的浓度。由式(1)计算可得剩余半焦对CR染料溶液的去除率η。
式中:η为去除率;C0为溶液的初始浓度,mg·L−1;Ce为溶液平衡浓度,mg·L−1。
-
为了更好地利用响应曲面法优化实验条件,研究剩余半焦对CR的吸附行为,本实验选取半焦投加量、CR初始浓度、温度和pH 4个变量,分别进行了单因素实验,以获取后续响应面吸附平衡时间和实验设计的参数范围。根据前期实验结果,剩余半焦振荡吸附CR 4 h后可达到吸附平衡状态,因此,选取4 h作为后续实验吸附时间,并以吸附剂用量2.0 g·L−1、CR初始浓度100 mg·L−1、温度30 ℃和溶液pH=4为响应面实验设计的中心点。同时,根据Box-Behnken实验原理,采用4因素3水平响应曲面法探究上述4种因素影响剩余半焦吸附CR的主效应和交互作用。实验设计的编码值和实验值见表1。
-
在刚果红类染料废水处理过程中,多采用去除率和出水水质评价处理效果。其中出水水质受污染物初始浓度影响较大,而去除率是二者进行处理后的客观指标,更为直观地表现了废水的处理效果。另外,还有一些报道[20-24]响应曲面法的研究过程和唐方云等[25]对污水处理厂评价指标的研究内容及结果中亦多采用去除率评价污水处理厂的处理效果。因此,本研究选择去除率为响应值,来呈现Box-Behnken实验设计和实验结果,结果如表2所示。由表2可知,以CR去除率为响应值,以吸附剂用量、CR初始浓度、温度、溶液pH为自变量建立响应面编码形式的二次多项式,如式(2)所示。
式中:A为吸附剂投加量;B为CR初始浓度;C为温度;D为pH。
式(2)表明,4个实验因素对响应值的影响是交互的,而不是简单的线性关系[21]。对响应曲面模型(式(2))进行方差分析评价和显著性检验,以检验该模型是否能够用来优化实验条件,其模型方程统计显著性以F值确定,每个回归系数的显著性以P值确定。模型的方差分析和二次回归方程的拟合结果如表3所示。
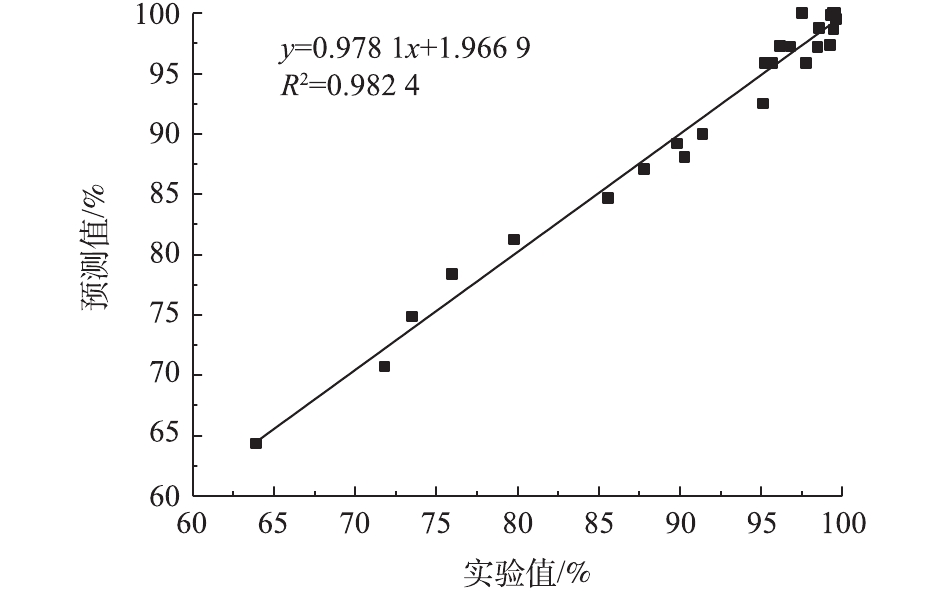
预测模型的拟合度R2=0.981 7,表明该模型与实验结果拟合良好,实验准确度高[22];校正判定系数
$R_{{\rm{Adj}}}^2 = 0.963\;3$ ,表明约96.33%响应值变化可以用该模型解释[23];CR去除率的实验值和模型预测值对比如图1所示,直线斜率接近于1,表明CR去除率的模型预测结果与实验结果高度吻合。残差正态概率可用于判断吸附模型是否服从正态分布,数据点越接近直线,回归模型拟合程度越高,图2中数据较好地拟合成一条直线,说明构建模型均符合正态分布。
由表3可以看出,响应曲面模型的F值为53.55,P<0.01,说明回归方程描述的4种因素与CR去除率之间的拟合曲线是显著的;同时,该响应模型的失拟项P值为0.098 7(P>0.05),表明预测模型的失拟性不显著,即回归模型显著[20]。综上所述,该模型可代替实验真实点对实验结果进行分析。
由表3可知,单因素条件下,吸附剂投加量、CR初始浓度、溶液pH对CR去除率的影响显著(P<0.01),同时,由F值可以得到各因素对CR去除率的影响,顺序为吸附剂投加量>CR初始浓度>溶液pH>温度,其中,温度对CR去除率的影响不显著,这是由于本研究所选的温度域较窄,染料分子运动不够剧烈,与吸附剂的碰撞概率较小,从而导致温度对CR的吸附行为影响较小。在交互作用中,吸附剂投加量与CR初始浓度、吸附剂投加量与溶液pH、CR初始浓度与溶液pH对CR的去除率呈显著效果。
-
利用Design Expert 8.0以两两自变量为坐标的3D图像,可以更加直观地说明吸附剂投加量、CR初始浓度和溶液pH对去除率的影响以及表征响应曲面函数的性状,结果如图3~图5所示。
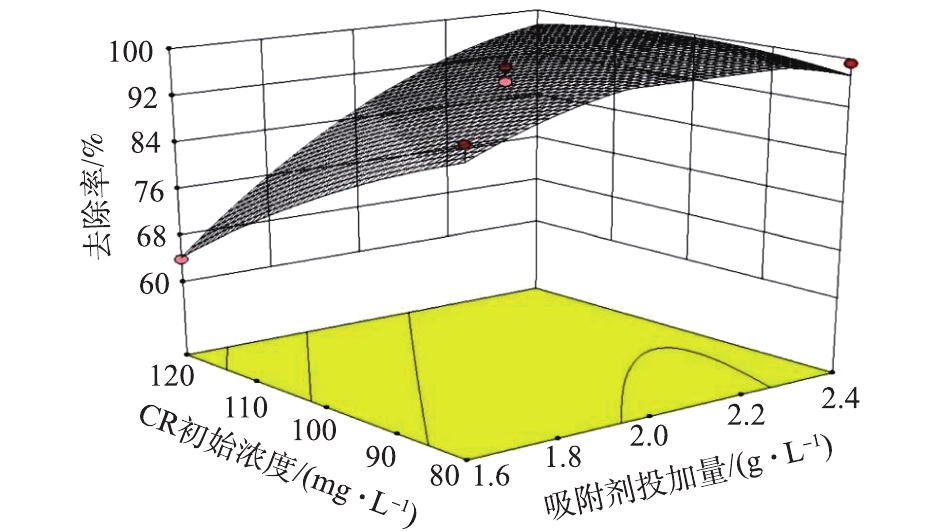
图3是以温度与溶液pH在中心条件下(温度30 ℃、pH=4),吸附剂投加量与CR初始浓度对CR去除率的影响。由图3可见,在CR浓度较高时,去除率随着投加量的增大而升高,这是因为随着吸附剂投加量的增大,可提供足够多的吸附位点吸附染料分子,去除率就越高;在CR浓度较低时,随着吸附剂投加量的增大,去除率先上升后趋于平缓,这是由于当吸附达到饱和时,继续增大投加量造成吸附剂颗粒间碰撞剧烈,使与染料分子接触的通道数目减少,从而影响去除率[24]。
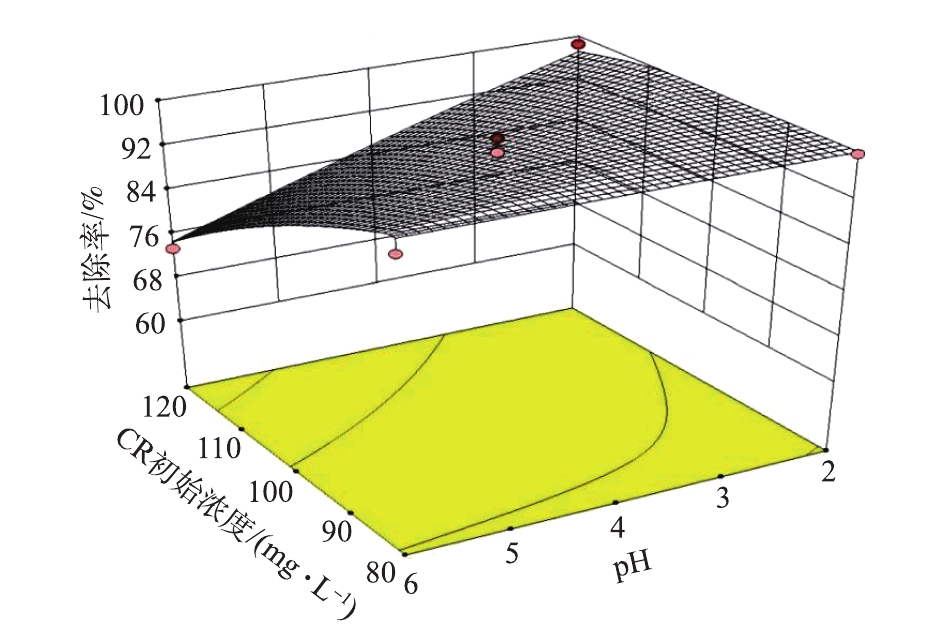
以吸附剂投加量、温度在中心条件下(投加量2.0 g·L−1、温度30 ℃),探究了CR初始浓度与溶液pH的交互作用对CR去除率的影响,结果如图4所示。可以看出,随着CR初始浓度和溶液pH的减小,CR去除率增大。这是因为CR浓度越小,吸附剂提供的吸附位点对于染料分子越充足,去除率就越高。经实验测得剩余半焦的等电点pI=2.6,当pH<2.6以后,随着pH的减小,剩余半焦吸附剂表面分布的正电荷数量增多,与CR的静电吸引作用越强,所以CR去除率升高。
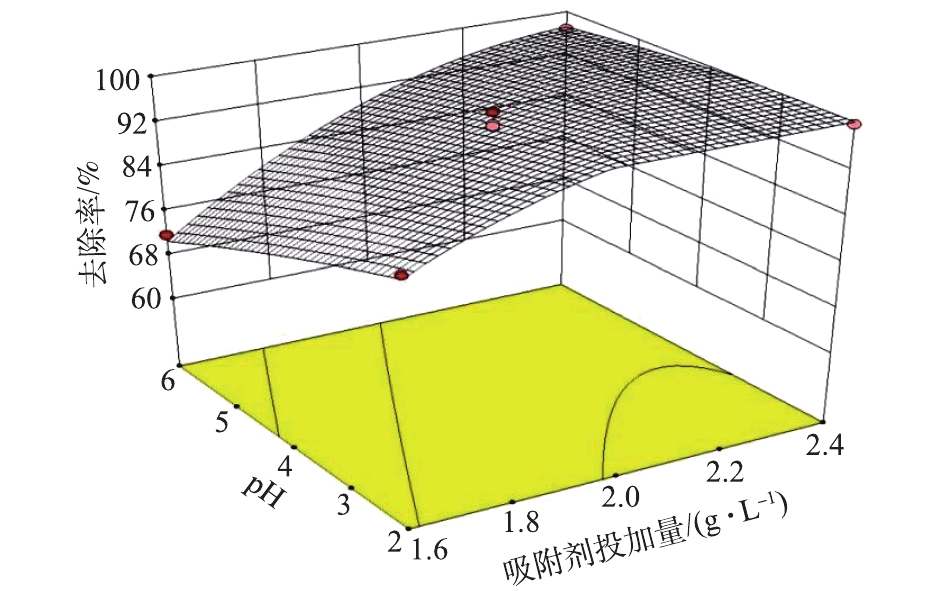
CR去除率随着吸附剂投加量与溶液pH而变化的3D响应曲面图如图5所示,此时CR初始浓度和温度处于中心条件下(初始浓度100 mg·L−1、温度30 ℃),可以看出,随着吸附剂投加量的增加和pH的降低,CR去除率增大。分析其原因是,随着吸附投加量的增大,吸附位点增多,同时,pH的减小使剩余半焦表面的正电荷增加,所以吸附剂带正电荷的吸附位点的比例升高,与CR产生的静电吸引作用变强,从而去除率就越高。
综上可知,适当增加吸附剂的投加量、降低CR初始浓度和溶液的pH有助于提高CR的去除率。
-
应用Design-Expert 8.0软件对最佳实验条件进行预测,得到剩余半焦吸附CR最佳实验条件:剩余半焦投加量为2.08 g·L−1、CR初始浓度为120 mg·L−1、温度32.67 ℃、溶液pH为2,此时CR去除率可达99.61%。为验证响应曲面模型的准确性,在预测的最佳条件下进行3组平行实验,测得去除率的平均值为98.75%,与预测值相差0.64%,说明该计算模型具有较好的预测效果,使用响应曲面法优化的工艺参数具有一定的指导意义。
-
通过BET、XRD方法对剩余半焦进行表征,再结合吸附CR前后剩余半焦的FT-IR图谱分析,初步探究了半焦对CR的吸附行为。
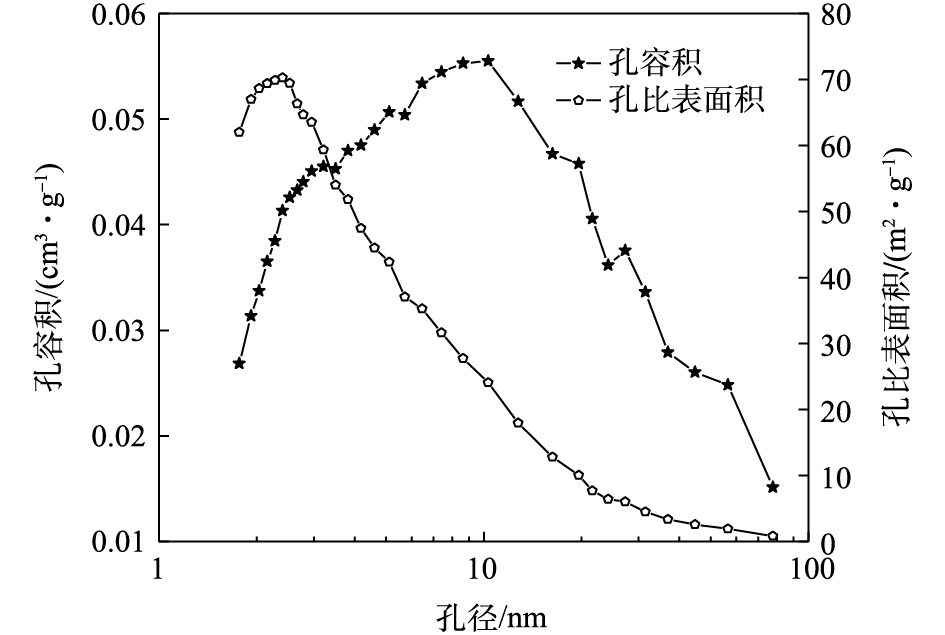
吸附材料的孔隙结构是影响吸附效果的重要因素。比表面积越大,可提供的吸附位点就越多[26]。本实验污泥热解剩余半焦与已报道的固体废弃物类吸附材料的孔隙参数对比如表4所示,可以看出污泥热解剩余半焦的孔隙比较发达,进一步证实了剩余半焦适合作为一种吸附材料去除水中CR;同时,由图6可以看出,剩余半焦的孔径主要是为2~20 nm的中孔,以及少量大于50 nm的大孔,表明剩余半焦是一种介孔材料,更有利于分子在孔隙内部扩散,从而促进对CR的吸附。
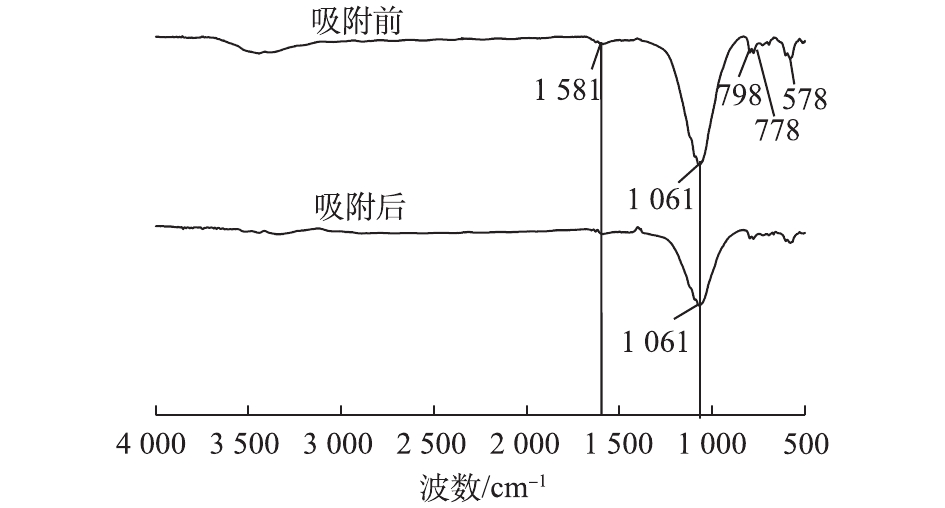
剩余半焦吸附CR前、后的红外光谱表征结果如图7所示,剩余半焦吸附CR前,波长1 061、778、798、578 cm−1处的吸收峰分别归属为由与不同桥氧原子相连的SiO4四面体的Si—O—Si和Si—O伸缩振动、石英族矿物产生的Si—O—Si对称伸缩振动、钙长石(CaAl2Si2O8)中O—Si(Al)—O的弯曲振动[31],说明污泥热解剩余半焦中主要成分为硅酸盐和碳酸盐化合物等无机物,这与PANEK等[32]对含油污泥热解所得剩余半焦的表征结果相似。剩余半焦吸附CR后主要吸收峰种类并未增加或减少,也未发生位移,即剩余半焦吸附后CR后没有新的官能团产生,说明污泥热解剩余半焦对CR主要是物理吸附过程,同时,吸附CR后剩余半焦的吸收峰强度减弱,推测是由于CR被吸附在剩余半焦表面从而削弱了剩余半焦的吸收峰强度。
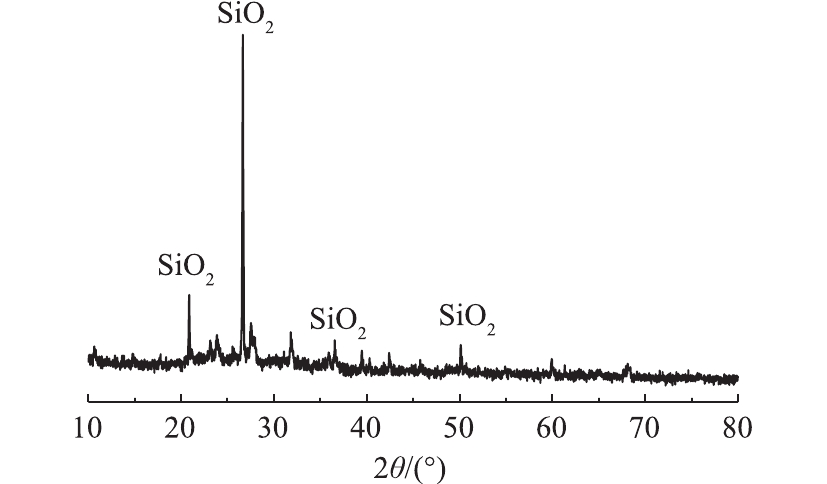
由图8中可以看出,剩余半焦在2θ=20.91°、26.68°、36.47°、50.20°处存在明显的特征衍射峰,对照标准卡片,确定为石英(SiO2),这与红外分析结果相似,这可能由于本研究所选取的市政污泥含砂量较大;常用的吸附材料如硅藻土[33]、黏土[34]、粉煤灰[35-36]的主要成分也为SiO2,它们都具有较大的比表面积和疏松多孔表面结构,因此,污泥热解所得的剩余半焦对CR具有一定的吸附效果。
综上可知,污泥热解剩余半焦主要成分为含硅无机物,具有较大的比表面积和丰富的孔隙结构,且其表面孔径以介孔为主,有助于促进其对染料分子的吸附。
-
1) Box-Behnken实验设计可以较好拟合剩余半焦对CR的吸附过程。单因素对吸附效果的影响顺序为吸附剂投加量>CR初始浓度>溶液pH>温度;预测模型的判定系数R2=0.981 7、校正判定系数
$R_{{\rm{Adj}}}^2 = 0.963\;3$ ,实验误差小;最佳吸附条件下,CR的去除率为98.97%,与预测值99.61%基本吻合。2)对剩余半焦吸附CR的交互作用的响应面分析可知,适当地增加吸附剂投加量、降低CR初始浓度以及减小溶液的pH,可以促进对CR的去除。
3)由XRD、BET分析结果可知,本研究所制备的剩余半焦是一种主要成分为含硅无机物且表面丰富的孔隙结构的介孔材料;由剩余半焦吸附CR前后的FT-IR图谱显示,剩余半焦吸附CR后未发生化学键的变化,其吸附过程主要为物理吸附。
4)本研究为市政脱水污泥的资源化利用探索了新思路,污泥热解剩余半焦可以有效吸附水体中的CR,并用响应曲面法得到最优吸附条件,研究成果具有一定的理论和实际意义。
响应曲面法对污泥热解剩余半焦吸附水中刚果红的优化
Optimization of congo red adsorption in water by semicoke residuals from sludge pyrolysis based on response surface methodology
-
摘要: 为了实现市政脱水污泥的资源化,以污泥快速热解制备富氢燃气剩余半焦作为吸附材料,对刚果红(CR)模拟染料废水进行了吸附研究。采用响应曲面法的Box-Behnken实验设计原理探究了吸附剂投加量、CR初始浓度、温度和溶液pH对CR去除率的影响,并分析了剩余半焦对CR的吸附机理。结果表明:各因素对CR去除率的影响顺序为吸附剂投加量>CR初始浓度>溶液pH>温度;最佳吸附条件下CR的去除率为98.97%,与预测值(99.61%)基本吻合;由交互作用响应曲面分析结果得出,适当的增加吸附剂投加量,降低CR初始浓度和溶液pH均有助于提高CR的去除率;污泥热解剩余半焦的XRD和FT-IR表征结果显示,其主要成分为含硅无机物,BET结果显示孔隙结构发达,可提供较多的吸附位点;吸附CR后,没有新的官能团产生,表明剩余半焦对CR的吸附主要为物理吸附。Abstract: As for the resource utilization of municipal sewage sludge, the semicoke residuals from sludge rapid pyrolysis for hydrogen-rich gas production was used to absorb the Congo red (CR) simulated dye wastewater. Box-Behnken design of response surface methodology was used to explore the effects of adsorbent dosage, initial concentration of CR, temperature and pH on CR removal rate. Furthermore, the CR adsorption mechanism by the semicoke residuals was analyzed. The results showed that the effect order of above factors on CR removal rate was following: the dosage of adsorbent > CR initial concentration > pH > temperature; the CR removal rate under the optimal adsorption conditions reached 98.97%, which was basically consistent with the predicted value of 99.61%. Through the analysis of the response surface interactions, increasing the amount of adsorbent to a certain extent, reducing the initial concentration of CR and the pH could improve the CR removal rate. The characterization results of XRD and FT-IR showed that the main components of semicoke residuals were silicon-containing inorganics, BET results demonstrated that the semicoke residuals had developed pore structure and provided many adsorption sites. No new functional groups were found after CR adsorption, which indicating that physical adsorption dominated CR adsorption on semicoke residuals.
-

-
表 1 刚果红吸附体系各实验因素水平取值
Table 1. Level of experimental factors in the congo red adsorption system
各水平
编码取值吸附剂
投加量/(g·L−1)CR初始
浓度/(mg·L−1)温度/℃ pH −1 1.6 80 25 2 0 2 100 30 4 1 2.4 120 35 6 表 2 实验设计与结果
Table 2. Experimental design and results
编号 各编码水平 去除率/% (A)吸附剂
投加量/(g·L−1)(B)CR初始
浓度/(mg·L−1)(C)温度/℃ (D) pH 实验值 预测值 1 1.6 80 30 4 95.12 92.51 2 2.4 80 30 4 99.26 97.36 3 1.6 120 30 4 63.90 64.34 4 2.4 120 30 4 96.15 97.29 5 2 100 25 2 99.61 99.53 6 2 100 35 2 99.52 100.00 7 2 100 25 6 90.29 88.06 8 2 100 35 6 91.39 90.00 9 1.6 100 30 2 89.80 89.21 10 2.4 100 30 2 99.57 100.00 11 1.6 100 30 6 71.82 70.73 12 2.4 100 30 6 96.81 97.24 13 2 80 25 4 99.29 99.84 14 2 120 25 4 85.56 84.66 15 2 80 35 4 99.40 100.00 16 2 120 35 4 87.79 87.07 17 1.6 100 25 4 75.95 78.39 18 2.4 100 25 4 98.57 98.78 19 1.6 100 35 4 79.80 81.23 20 2.4 100 35 4 99.45 98.64 21 2 80 30 2 99.55 99.85 22 2 120 30 2 98.48 97.19 23 2 80 30 6 97.50 100.00 24 2 120 30 6 73.51 74.86 25 2 100 30 4 97.75 95.87 26 2 100 30 4 95.24 95.87 27 2 100 30 4 95.35 95.87 28 2 100 30 4 95.67 95.87 29 2 100 30 4 95.33 95.87 表 3 CR去除率的方差分析结果
Table 3. ANOVA of CR removal efficiency
方差来源 平方和 自由度 均方 F P 显著性 模型 2 652.36 14 189.45 53.55 <0.000 1 显著 A 1 071.94 1 1 071.94 303.00 <0.000 1 显著 B 598.15 1 598.15 169.08 <0.000 1 显著 C 5.44 1 5.44 1.54 0.235 4 D 354.47 1 354.47 100.19 <0.000 1 显著 AB 197.47 1 197.47 55.82 <0.000 1 显著 AC 2.22 1 2.22 0.63 0.442 0 AD 57.93 1 57.93 16.38 0.001 2 显著 BC 1.12 1 1.12 0.32 0.582 5 BD 131.34 1 131.34 37.12 <0.000 1 显著 CD 0.36 1 0.36 0.10 0.755 6 A2 220.30 1 220.30 62.27 <0.000 1 显著 B2 30.37 1 30.37 8.58 0.011 0 显著 C2 3.93 1 3.93 1.11 0.309 6 D2 2.52 1 2.52 0.71 0.413 3 残差 49.53 14 3.54 失拟性 44.98 10 4.50 3.95 0.098 7 不显著 净误差 4.55 4 1.14 总离差 2 701.89 28 注:判定系数R2=0.981 7,校正判定系数 $R_{{\rm{Adj}}}^2 = 0.963\;3$ 。
-
[1] DU Q, SUN J, LI Y, et al. Highly enhanced adsorption of Congo red onto graphene oxide/chitosan fibers by wet-chemical etching off silica nanoparticles[J]. Chemical Engineering Journal, 2014, 245: 99-106. doi: 10.1016/j.cej.2014.02.006 [2] 张丽, 罗汉金, 方伟, 等. 改性氧化石墨烯/壳聚糖功能材料对刚果红的吸附研究[J]. 环境科学学报, 2016, 36(11): 3977-3985. [3] ZHENG L, WANG C, SHU Y, et al. Utilization of diatomite/chitosan Fe(III) composite for the removal of anionic azo dyes from wastewater: Equilibrium, kinetics and thermodynamics[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2015, 468: 129-139. [4] GAO H, ZHAO S, CHENG X, et al. Removal of anionic azo dyes from aqueous solution using magnetic polymer multi-wall carbon nanotube nanocomposite as adsorbent[J]. Chemical Engineering Journal, 2013, 223: 84-90. doi: 10.1016/j.cej.2013.03.004 [5] CHAKRABORTY S, BASAK B, DUTTA S, et al. Decolorization and biodegradation of congo red dye by a novel white rot fungus Alternariaalternata CMERI F6[J]. Bioresource Technology, 2013, 147: 662-666. doi: 10.1016/j.biortech.2013.08.117 [6] PENG H H, CHEN J, JIANG D Y, et al. Merging of memory effect and anion intercalation: MnOx-decorated MgAl-LDO as a high-performance nano-adsorbent for the removal of methyl orange[J]. Dalton Transactions, 2016, 45(26): 10530-10538. doi: 10.1039/C6DT00335D [7] CHENG Z, ZHANG L, GUO X, et al. Adsorption behavior of direct red 80 and congo red onto activated carbon/surfactant: process optimization, kinetics and equilibrium[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2014, 137: 1126-1143. doi: 10.1016/j.saa.2014.08.138 [8] RAFI M, SAMIEY B, CHENG C H. Study of adsorption mechanism of congo red on graphene oxide/PAMAM nanocomposite[J]. Materials, 2018, 11(4): 496-520. doi: 10.3390/ma11040496 [9] KIM K H, KIM J Y, CHO T S, et al. Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinusrigida)[J]. Bioresource Technology, 2012, 118: 158-162. doi: 10.1016/j.biortech.2012.04.094 [10] LIAN L, GUO L, GUO C. Adsorption of congo red from aqueous solutions onto Ca-bentonite[J]. Journal of Hazardous Materials, 2009, 161(1): 126-131. doi: 10.1016/j.jhazmat.2008.03.063 [11] ZHANG F, YIN X, ZHANG W. Development of magnetic Sr5(PO4)3(OH)/Fe3O4, nanorod for adsorption of congo red from solution[J]. Journal of Alloys & Compounds, 2016, 657: 809-817. [12] LUO S, FENG Y. The production of hydrogen-rich gas by wet sludge pyrolysis using waste heat from blast-furnace slag[J]. Energy, 2016, 113: 845-851. doi: 10.1016/j.energy.2016.07.130 [13] 韩融, 刘晋文, 赵晨曦, 等. 生物物理干化污泥快速热解半焦特性研究[J]. 中国给水排水, 2016, 32(15): 70-74. [14] LIU C, TANG Z, CHEN Y, et al. Characterization of mesoporous activated carbons prepared by pyrolysis of sewage sludge with pyrolusite[J]. Bioresource Technology, 2010, 101(3): 1097-1101. doi: 10.1016/j.biortech.2009.09.012 [15] 彭峰, 何丕文. 亚甲基蓝在污泥微波热解半焦上的吸附[J]. 水处理技术, 2011, 37(5): 51-54. [16] FAN S, JIE T, YI W, et al. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism[J]. Journal of Molecular Liquids, 2016, 220: 432-441. doi: 10.1016/j.molliq.2016.04.107 [17] MAHAPATRAK, RAMTEKE D S, PALIWAL L J. Production of activated carbon from sludge of food processing industry under controlled pyrolysis and its application for methylene blue removal[J]. Journal of Analytical & Applied Pyrolysis, 2012, 95: 79-86. [18] 王格格, 李刚, 陆江银, 等. 热解工艺对污泥制备生物炭物理结构的影响[J]. 环境工程学报, 2016, 10(12): 7289-7293. doi: 10.12030/j.cjee.201507124 [19] 李晋. 污泥经机械化预处理在不同条件下快速热解制氢研究[D]. 西安: 长安大学, 2018. [20] 王雅辉, 邹雪刚, 舒冉君, 等. 胡敏素对Pb2+吸附的响应面优化及机理[J]. 中国环境科学, 2017, 37(5): 1814-1822. doi: 10.3969/j.issn.1000-6923.2017.05.026 [21] 王利平, 刘静静, 沈肖龙, 等. 响应面法优化凹凸棒土吸附水中亚甲基蓝[J]. 环境工程学报, 2016, 10(9): 4912-4918. doi: 10.12030/j.cjee.201503263 [22] 李柳柳, 闫伯骏, 崔建升, 等. 基于响应曲面法优化H2O2/Fe3+脱除烧结烟气中的Hg0[J]. 环境工程学报, 2018, 12(4): 1083-1091. doi: 10.12030/j.cjee.201708060 [23] SEN K, MONDAL N K, CHATTORAJ S, et al. Statistical optimization study of adsorption parameters for the removal of glyphosate on forest soil using the response surface methodology[J]. Environmental Earth Sciences, 2017, 76(1): 22-37. doi: 10.1007/s12665-016-6333-7 [24] 姜春红, 赵研, 王鹏. 基于响应面法的改性玉米芯吸附水中Zn2+的工艺条件优化及机理研究[J]. 环境保护科学, 2017, 43(2): 76-80. [25] 唐方云, 银媛媛. 污水处理效果评价的一点思考[J]. 环境科学导刊, 2012, 31(2): 84-85. doi: 10.3969/j.issn.1673-9655.2012.02.022 [26] 安强, 蒋韵秋, 吴丹青, 等. 响应面法探究花生壳炭吸附水中镍离子的最优改性条件[J]. 重庆大学学报, 2018, 41(12): 46-54. [27] 王泽怿, 赵斌, 沈伯雄, 等. 热改性废茶叶吸附刚果红性能的研究[J]. 工业水处理, 2017, 37(4): 78-82. doi: 10.11894/1005-829x.2017.37(4).019 [28] NAUTIYAL P, SUBRAMANIAN K A, DASTIDAR M G. Adsorptive removal of dye using biochar derived from residual algae after in-situ transesterification: Alternate use of waste of biodiesel industry[J]. Journal of Environmental Management, 2016, 182: 187-197. doi: 10.1016/j.jenvman.2016.07.063 [29] 赵伟, 白云翔, 张春芳, 等. 水葫芦生物质炭的制备与染料吸附性能研究[J]. 应用化工, 2017, 46(10): 95-98. [30] MANE V S, VIJAY BABU P. Kinetic and equilibrium studies on the removal of congo red from aqueous solution using Eucalyptus wood (Eucalyptus globulus) saw dust[J]. Journal of the Taiwan Institute of Chemical Engineers, 2013, 44(1): 81-88. doi: 10.1016/j.jtice.2012.09.013 [31] 闻辂, 矿物红外光谱学[M]. 重庆: 重庆大学出版社, 1988. [32] PANEK P, KOSTURA B, CEPELAKOVA I, et al. Pyrolysis of oil sludge with calcium-containing additive[J]. Journal of Analytical and Applied Pyrolysis, 2014, 108: 274-283. doi: 10.1016/j.jaap.2014.04.005 [33] 徐阳. 硅藻土基吸附剂的制备及其吸附性能的研究[D]. 吉林: 吉林化工学院, 2018. [34] VIMONSES V, LEI S, JIN B, et al. Kinetic study and equilibrium isotherm analysis of congo red adsorption by clay materials[J]. Chemical Engineering Journal, 2009, 148(2/3): 354-364. [35] 段小月, 曹红丽, 刘伟, 等. 粉煤灰的改性及对刚果红的吸附[J]. 化工环保, 2013, 33(4): 294-298. doi: 10.3969/j.issn.1006-1878.2013.04.003 [36] 徐姗姗, 魏刚, 张晓丰, 等. 陶土-粉煤灰基吸附性陶瓷基体的制备及其吸附性能[J]. 北京化工大学学报(自然科学版), 2012, 39(4): 42-46. doi: 10.3969/j.issn.1671-4628.2012.04.009 -



 下载:
下载: