-
高浓度硝态氮废水来源广泛,其中大部分源于工业废水。例如,化肥加工厂产生的废水中硝态氮浓度可达950 mg·L−1[1];在核工业处理放射性金属制品过程中,通常也会产生高浓度硝态氮废水[2]。此外,采用物化法处理低浓度硝态氮废水时产生的浓缩液,也是其来源之一。例如,离子交换法产生的脱附液[3]、NF浓缩液与RO浓缩液[4]等。在利用能够彻底去除氮素的生物法处理此类废水时,反硝化过程会消耗大量碳源,甲醇、乙酸和葡萄糖等传统外加碳源会带来高昂的成本问题[5],且容易出现 pH升高过快和NO2−-N积累现象[6]。因此,可采用新型外加碳源来代替传统碳源。垃圾渗滤液原液就是其中之一,但却不可避免地引入了大量难降解有机物和重金属离子等。然而,针对浓度比原液较低但碳源依旧可观的前处理垃圾渗滤液作为反硝化外加碳源的研究则鲜有报道。
国内外在垃圾渗滤液处理方面的技术还处在不断发展和研究的阶段。随着人民生活水平的改善,城市生活垃圾的产量迅速增加[7],现有的NF、反渗透、活性炭吸附等深度处理工艺,由于结构复杂、成本高昂,导致其处理能力跟不上垃圾渗滤液的产生速度,不能及时处理经过前序工艺所带来的初级处理液。因此,寻求合理处置前处理垃圾渗滤液(pretreated landfill leachate,PLL)的途径意义重大[8]。相对于垃圾渗滤液原液,前处理垃圾渗滤液的成分复杂度降低,大分子有机物、重金属离子等有毒物质含量大大减少,TN和COD也得到一定的去除[9-10];同时,PLL中所含的短链脂肪酸 (SCFA)、挥发性脂肪酸(VFAs)等快速可生物降解有机物(属于第一类基质)[11-12],可为反硝化过程提供更易于微生物同化的碳源物质。
本研究以无水乙酸钠和PLL为混合碳源,对比在不同PLL添加比例条件下,活性污泥处理高浓度硝态氮时的反硝化特性,结合实时荧光定量PCR(qPCR)技术及16S rDNA测序分析,揭示了活性污泥系统内部微生物结构特征和功能基因与系统反硝化效能之间的关系,以期为处理高浓度硝态氮废水选择经济高效的外加碳源提供技术参考,并深入探究前处理垃圾渗滤液的高效利用模式。
全文HTML
-
模拟废水水质。实验以模拟高浓度硝态氮废水为考察对象,驯化阶段用无水乙酸钠为COD唯一来源,启动成功后添加PLL作为混合碳源;利用KNO3作为唯一氮源;以KH2PO4配制磷酸盐质量浓度;以MgSO4和CaCl2满足反硝化菌对Mg2+和Ca2+的需求;微量元素添加量为1 mL·L−1,1 L微量元素溶液中含有10.00 g EDTA、1.50 g FeCl3·6H2O、0.03 g CuSO4·5H2O、0.12 g ZnSO4·7H2O、0.15 g CoCl2·6H2O、0.12 g MnCl2·4H2O、0.06 g Na2MoO4·2H2O、0.18 g KI、0.15 g H3BO3;实验全过程各反应器内DO ≤ 0.4 mg·L−1,保持缺氧状态;进水pH控制在7.5 ± 0.1;水温为室温,即21.5~25.6 ℃。
前处理垃圾渗滤液水质及来源。深圳市下坪垃圾填埋场采用氨吹脱+厌氧生物滤池+A/A/O+MBR+NF的新型组合工艺处理垃圾渗滤液,本研究所用前处理垃圾渗滤液取自该垃圾填埋场厌氧生物滤池出水,此系统前2步流程已经有效降解垃圾渗滤液原液中的氨氮、SS等主要污染物,前处理垃圾渗滤液中的COD主要来源于VFAs和腐殖酸。具体水质成分如下:COD (1 989±50) mg·L−1,
${\rm{NH}}_4^{+} $ -N (8.5±3.5) mg·L−1,DO (0.2±0.1) mg·L−1,Ca2+ (335±14) mg·L−1,Mg2+ (13.2±3.8) mg·L−1,As (0.253±0.096) mg·L−1,Cd (0.04±0.01) mg·L−1。 -
实验装置由2个相同的有机玻璃材质柱形SBR (R0和R1)组成,有效柱高为65 cm,内径为10 cm,有效容积10 L,在反应器底部设有排泥口,直径为10 mm。反应器侧面15、25、35、45、55 cm处分别设有取样口,反应器顶部设置搅拌器,使泥水保持混合状态,反应器进水方式为上进下出,反应装置如图1所示。
-
实验分为3个阶段,即驯化阶段、反硝化速率对比阶段、最佳投加比例研究阶段。驯化阶段将活性污泥接种在R0及R1反应器中,无水乙酸钠作为唯一碳源培养,控制进水碳氮比(COD/
${\rm{NO}}_3^{-} $ -N,下称C/N)为5.0,硝态氮浓度根据驯化程度采取阶梯式上升的方式在50~500 mg·L−1内逐渐增加。微生物驯化完成后,R0作为对照组按原驯化方式运行,而R1反应器则以无水乙酸钠 + PLL为碳源进行培养,此时PLL添加体积分数为10%,提供足量电子供体以排除碳源不足的影响,从而对2种不同碳源的反硝化速率进行比较。为了探究前处理垃圾渗滤液的最佳掺入比例,设置C/N分别为2.5、3.2、4.5、4.9、5.6、6.1的批次实验,每批6组反应器(在六连搅拌器内进行)中,前处理垃圾渗滤液的投加体积分数分别为0%、5%、10%、15%、20%、25%,无水乙酸钠的添加量则依次减少,具体分配情况见表1。测定每组每批次的反硝化速率和COD去除率,重复3个周期;批次实验活性污泥均取自R1反应器,接种300 mL纯水洗涤3次后的污泥,最终体积为1.0 L。 -
在固定时间点于采样口取10 mL泥水混合液,静沉5 min,取适量上清液并稀释一定倍数后,用0.22 μm微滤膜过滤,再经消解器(DRB200,美国哈希)消解2 h后用分光光度计(DR3900,美国哈希)对COD进行测定,硝态氮和亚硝态氮浓度采用离子色谱法[13]测定,MLVSS采用马弗炉灼烧重量法[13]测定,以单位重量MLVSS在单位时间内对硝态氮的去除量表征反硝化速率(VDN, mg·(g·h)−1)。在系统运行的第65天,分别从R0、R1反应器底部取进水后15 min泥水混合液50 mL,3 500 r·min−1离心15 min后,去除上清液取其沉淀物,将样品标记为R0-0、R1-1;16S rDNA测序送至测序公司检测。
1.1. 实验材料
1.2. 实验装置
1.3. 实验方法
1.4. 分析方法
-
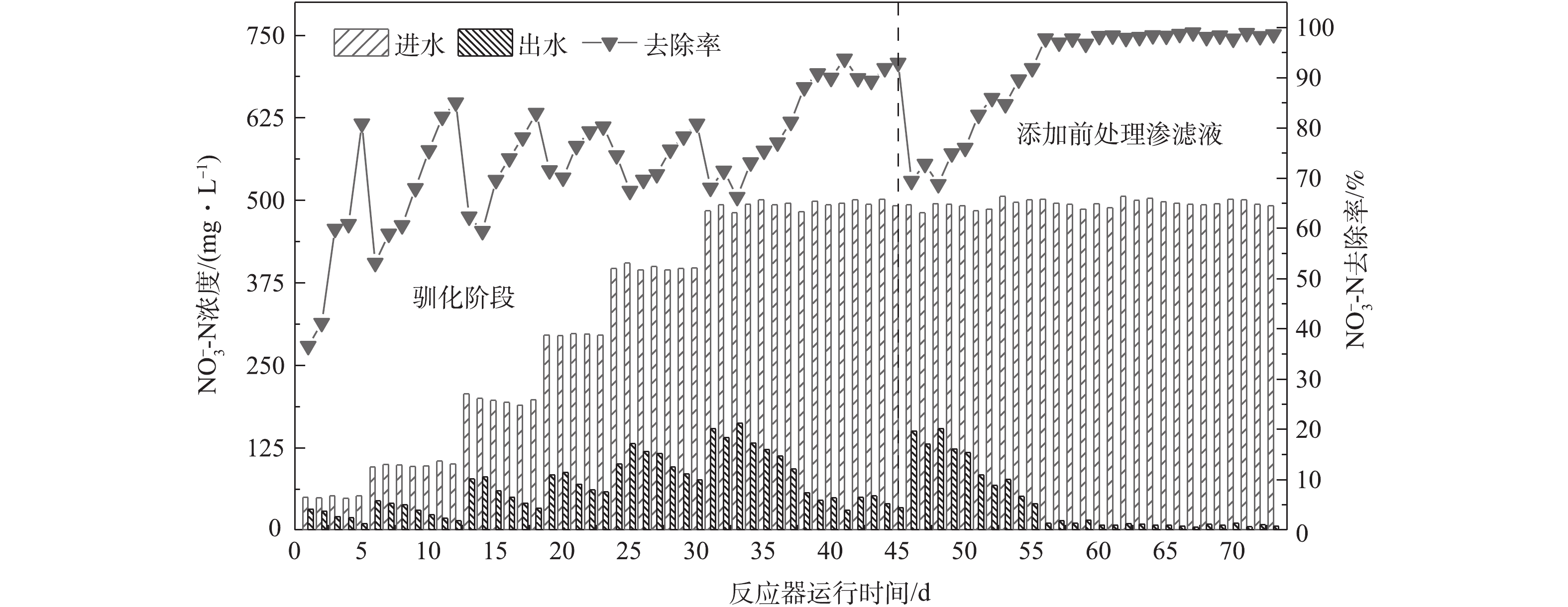
R1系统运行结果如图2所示。0~45 d为驯化阶段,以无水乙酸钠作为碳源培养接种污泥以提高反硝化效能,每天测定1个周期的出水
${\rm{NO}}_3^{-} $ -N浓度。初始进水${\rm{NO}}_3^{-} $ -N质量浓度设置为50 mg·L−1,启动阶段共经过5级逐次提高,具体为50 mg·L−1→100 mg·L−1→200 mg·L−1→300 mg·L−1→400→500 mg·L−1。每级去除率达到80%时,提高硝酸钾投加量,使${\rm{NO}}_3^{-} $ -N质量浓度最终维持在约500 mg·L−1,当去除率保持在 90%以上,并连续1周效果保持稳定时,视为反应器启动成功;第46天开始以10%的体积分数添加前处理垃圾渗滤液,此后出水硝态氮浓度出现较大波动,系统经过11 d的适应,硝态氮去除率慢慢提高到98.3% ± 1.0%,第56天之后达到稳定。整个运行过程出水未检测出氨氮,亚硝氮质量浓度均小于10 mg·L−1。经驯化的反硝化菌已经很好地适应高浓度的${\rm{NO}}_3^{-} $ -N,能够满足后续批次实验在高负荷条件下研究添加前处理垃圾渗滤液作为碳源时脱氮效能的要求。 -
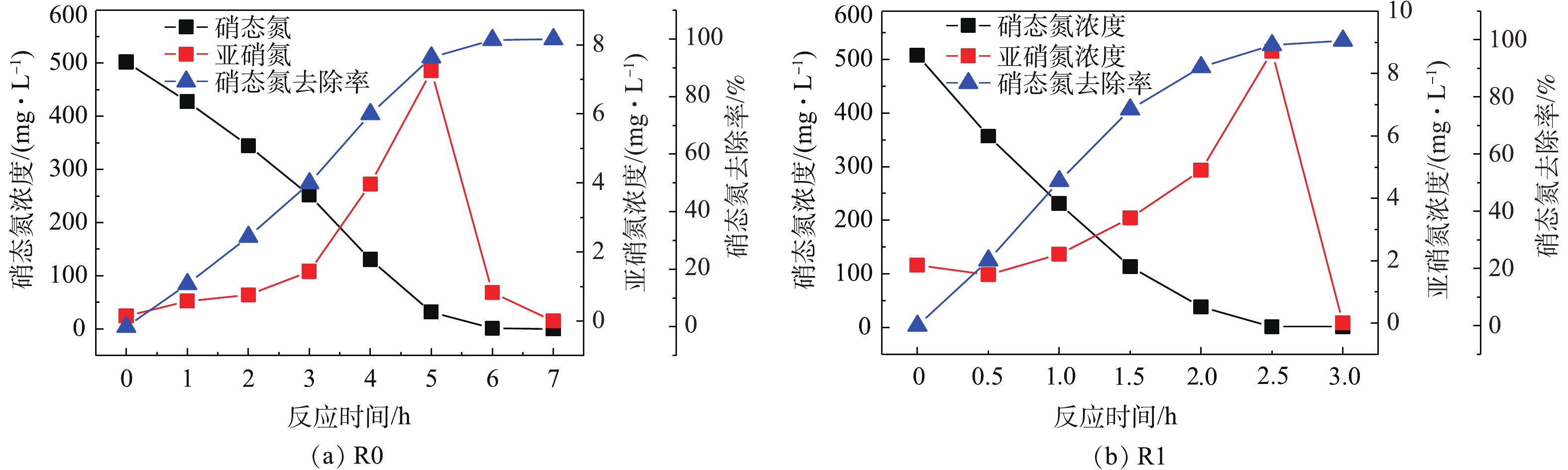
在R0、R1反应器中分别以无水乙酸钠、PLL+无水乙酸钠为碳源,并使PLL添加的体积分数为10%,控制各反应器C/N=5.0,在电子供体足量情况下对比其反硝化速率,结果如图3所示。由图3可以看出,R0系统将硝态氮几乎完全去除(出水浓度低于检出下限)需要约6.0 h,反硝化速率为32.32 mg·(g·h)−1,而R1系统在约需2.5 h即可去除相同浓度的硝态氮,平均反硝化速率可高达58.05 mg·(g·h)−1,是R0的1.79倍。这主要是因为PLL中COD来源较为多样,相对于R0的单一碳源,R1的混合碳源更有利于加速SBR的反硝化过程,从而提高脱氮速率。ELEFSINIOTIS等[14-15]研究认为,当系统内存在多种碳源时,微生物可以同时利用多种电子供体。由此可推测,R1系统内发生的不是单一的零级反应,而是结合多种碳源参与的一种耦合反应,故相比只有单一碳源的R0系统而言,R1系统有着更快的反硝化速率。此外,PLL中富含的Ca2+和Mg2+也是促进微生物快速生长的一个因素。刘沛然[16]用ASBR法处理垃圾渗滤液时,发现25 mmol·L−1的Ca2+和Mg2+能够有效促进反硝化菌的代谢活性。
-
为进一步探究不同PLL添加比例时碳、氮的去除情况,批次实验结果如图4所示。由图4(a)和图4(b)可以看出,C/N为2.5、3.2时,反硝化速率与PLL添加比例呈反比例关系。这是由于在C/N过低条件下,随着PLL添加量的增多,乙酸钠添加量减少,大分子有机物的相对含量会超过专性大分子有机物的降解需求量,导致电子供体在周期内不能把高浓度的
${\rm{NO}}_3^{-} $ -N彻底还原,从而降低单位时间硝态氮的还原量。在C/N为4.5和5.0条件下,PLL添加体积分数 ≤ 10%时,VDN随PLL添加量增加而升高,最大值分别为61.03 mg·(g·h)−1和62.28 mg·(g·h)−1;当PLL添加体积分数大于10%时,反硝化速率随添加量的增加而降低,VDN在PLL添加的体积分数为25%时达到最低值,分别为45.82 mg·(g·h)−1和32.71 mg·(g·h)−1。这是由于此条件下小分子碳源和复杂碳源在系统内的相对含量与不同菌属对各种碳源的需求之间达到一种平衡,微生物间的协同作用加快了硝态氮的还原速率。相反,可以看到C/N为5.6和6.1时的反硝化速率随PLL体积分数的增加而降低。这可能是因为R1系统内发生了共代谢作用,在共代谢机制下,生长基质和非生长基质的质量浓度比值过高会对关键酶的生成产生竞争性抑制作用[17],在R1系统中乙酸钠和硝态氮充当生长基质的一部分,PLL中的复杂有机物(腐殖酸等)则充当非生长基质,当C/N为5.6和6.1时乙酸钠的投加量很高,故可以合理推测,此时生长基质和非生长基质的质量浓度比值超过了共代谢的需求范围,添加PLL反而会降低共代谢作用,进而降低硝态氮的去除速率[18]。将PLL添加量换算为COD占比,碳氮比4.5~5.0且PLL添加的体积分数为5%~15%时所对应的COD占比为4.10%~13.5%。由此可见,投加PLL所带来的COD占比在4.10%~13.5%范围内能够促进硝态氮的还原速率。另外,由图4(c)~(f)还可以看出,在C/N为4.5~6.1时,COD去除率随PLL添加比例的增加逐渐升高。这说明添加PLL作为混合碳源可以提高乙酸钠利用率,减少乙酸钠投加量。但是,在C/N为5.6和6.1时,COD去除率均小于64.96%;而在C/N 为3.2、4.5、5.0时的COD去除率最大分别可达到96.77%、84.38%、82.67%。因此,综合考虑COD利用率和反硝化速率2方面因素,建议在C/N为3.2~5.0条件下,将PLL的投加体积分数控制在5%~15%,能够在兼顾COD去除率的同时,也可以得到高效的反硝化速率。
-
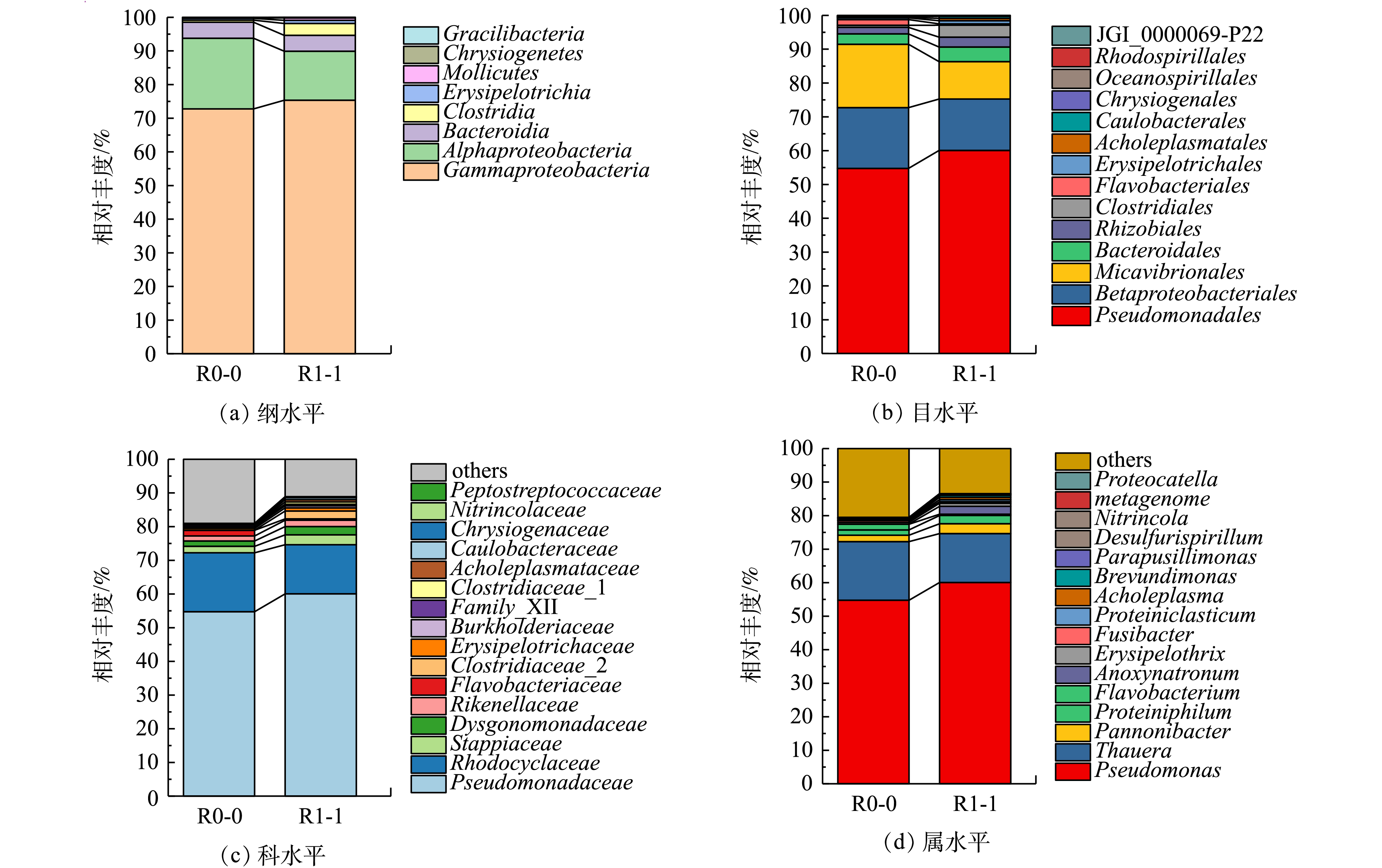
1)添加前处理垃圾渗滤液对菌群结构及相对丰度的影响。经高通量测序,2个样本去除嵌合体后的有效序列数分别为36 591和39 831,Shannon和Simpson指数分别为2.366、2.511和0.738、0.686,文库覆盖率均为100%,表明测序结果真实可靠。图5为R0和R1系统运行第65天时不同分类水平上的微生物群落结构。由图5(a)可知,2个反应器均被检出8个纲,对照组R0反应器泥样中相对丰度大于1%的优势菌有3种,分别为Gammaproteobacteria(γ-变形菌纲,72.78%)、Alphaproteobacteria(α-变形菌纲,20.98%)、Bacteroidia(拟杆菌纲,4.77%),占比之和为98.53%。这与周梦娟等[19]用乙酸钠为碳源培养富集的微生物群落具有较高的相似度。实验组R1反应器泥样中相对丰度大于1%的优势菌有4种,分别为γ-变形菌纲(77.66%)、α-变形菌纲(13.34%)、拟杆菌纲(4.17%)和Clostridia(梭菌纲,3.21%),占比之和为98.38%。添加PLL可以提高纲水平上Gammaproteobacteria和Clostridia的相对丰度。
由图5(d)可知,在属水平,R0和R1都被检出有17种菌属,其中Pseudomonas(假单胞菌属)、Thauera(陶厄氏菌属)、Pannonibacter和Proteiniphilum均为2个反应器内的优势菌属,相对丰度分别为54.75%、13.26%、1.49%、1.79%和59.39%、17.7%、1.95%、1.97%。从丰度变化能够看出,这4种反硝化功能菌在R1中都得到强化和富集,而Pseudomonas[20]、Thauera、Pannonibacter均具有良好的反硝化特性。QIAO等[21]在石化废水中分离出1株革兰氏阴性杆状菌Thauera sp.K11,发现其能利用10多种酚类衍生物作为电子供体进行反硝化;王艳青[22]利用MBR处理芳香族磺酸类有机废水时,发现Pannonibacter sp.W1能够有效降解对氨基苯磺酸。这进一步证明了添加前处理垃圾渗滤液可提高反硝化效率;此外,与科层面相似,属层面R0的Flavobacterium丰度高于R1,但R1中的Anoxynatronum丰度为1.85%,属于优势菌属,为R0的46.25倍(其在R0中仅占0.04%)。这说明添加前处理垃圾渗滤液有利于Anoxynatronum生长。ELENA等[23]在贝加尔湖分离出了一种嗜碱厌氧型菌属Anoxynatronum,并发现它可以利用纤维素等大分子有机物为碳源实现反硝化,能够分解结构较为复杂的化合物。这解释了批次实验中COD去除率随前处理渗滤液添加量增加而升高的现象。
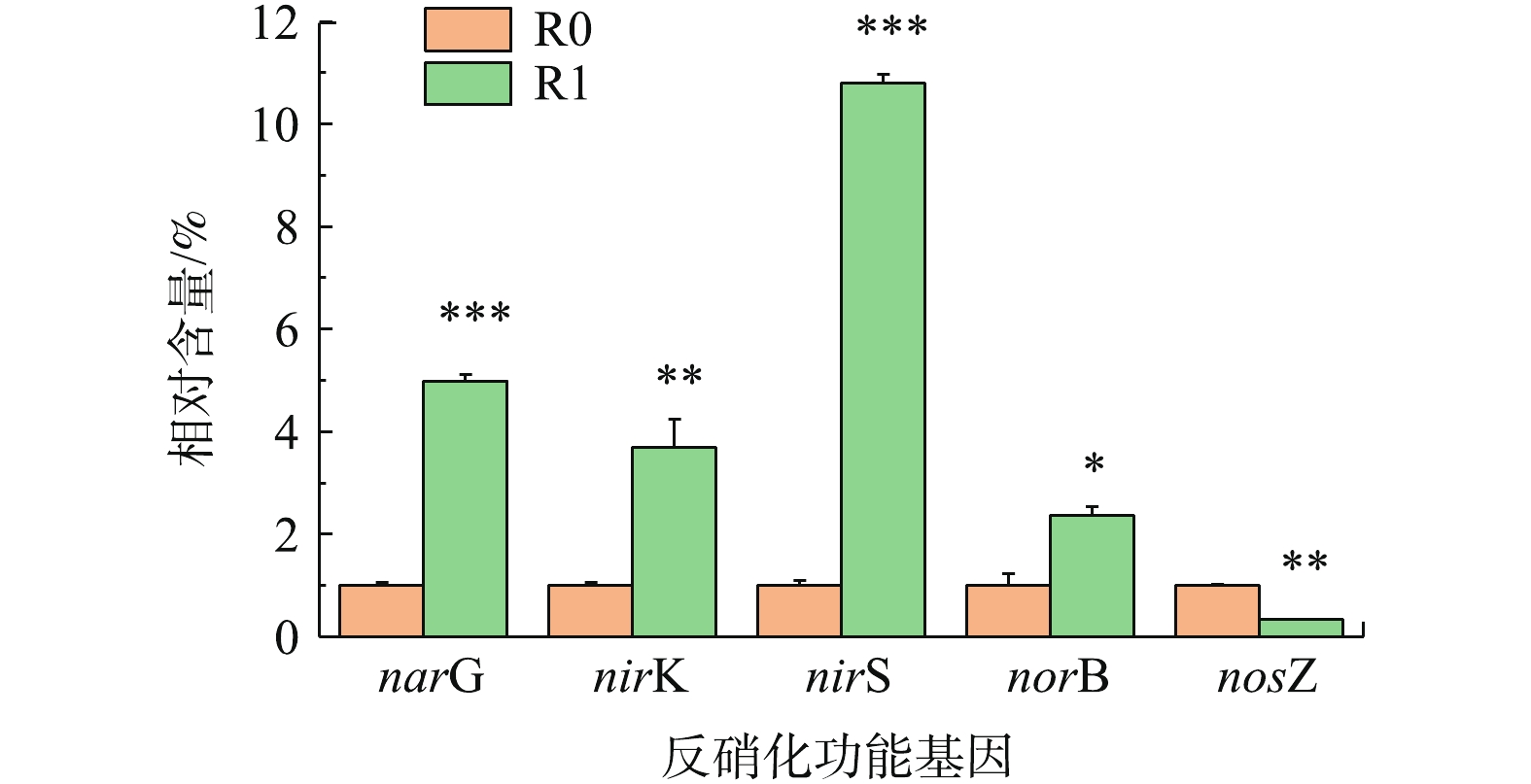
2)添加前处理垃圾渗滤液对反硝化功能基因相对丰度的影响。反硝化过程包括4步反应(
${\rm{NO}}_3^{-} $ -N→${\rm{NO}}_2^{-} $ -N→NO→N2O→N2),分别由硝酸还原酶(NAR)、亚硝酸还原酶(NIR)、一氧化氮还原酶(NOR)和一氧化二氮还原酶(NOS)进行催化,这4种功能蛋白的编码基因分别是nar、nir、nor和nos。由图6可知,除了NOS编码基因nosZ外,基因narG、nirS、nirK和norB的相对含量在R1中分别提高了4.98、3.69、10.80和2.38倍。NOS的作用是将N2O催化还原为N2,但这并非是反硝化的限速步骤,决定整个反硝化速率的是NIR将${\rm{NO}}_2^{-} $ N转化为NO的过程[24]。因此,R1中的nosZ相对含量低于R0并不会抵消其他功能基因的高表达活性对反硝化速率的促进作用。nir基因分为编码铜型亚硝酸还原酶(Cu-NiRs)的nirK和编码血红素cd1型亚硝酸还原酶(cd1-NiRs)的nirS,PLL所含的铜离子为Cu-NiRs单体的Ⅱ型Cu提供了足量的底物结合位点,因此,增强了nirK的表达;cd1-NiRs为一种同二聚体双功能酶,发挥催化作用时以天青蛋白、假天青蛋白或细胞色素c551作为电子供体,由此可以推测,PLL为其提供了所需电子供体,从而提高了nirK的相对表达量。PINTATHONG等[25]的研究显示,添加Fe(III)和Mo(Ⅵ)可以提高NAR活性,这可能与PLL含有的Fe3+、Mo6+可以增强narG的相对表达量有关。qPCR对功能基因的相对定量结果很好地解释了2个反应器所表现出的反硝化效能差异。
2.1. 反应器运行实验结果
2.2. 添加前处理渗滤液对反硝化速率的影响
2.3. 前处理垃圾渗滤液最佳掺入比的确定
2.4. 分子生物学分析
-
1)以无水乙酸钠为唯一碳源处理高浓度硝态氮废水,获得了快速高效反硝化活性污泥,最大反硝化速率为32.32 mg·(g·h)−1;当PLL添加体积分数为10%时,最大反硝化速率升至58.05 mg·(g·h)−1。
2) PLL充当碳源时能提高乙酸钠利用率。在C/N为3.2~5.0条件下,PLL投加比例为10%~15%,最大COD去除率为96.77%,是添加PLL前的1.23~1.41倍。
3)添加PLL对微生物种群类别没有明显影响,但改变了优势菌种群的相对丰度,Pseudomonas、Thauera、Pannonibacter和Proteiniphilum相对丰度有所提高,Alphaproteobacteria和Bacteroidia相对丰度则有所降低。
4) PLL的加入使反硝化功能基因 narG、nirK、nirS和norB的相对含量分别提高了4.98、3.69、10.80和2.38倍,但一氧化二氮还原酶编码基因nosZ的相对表达量却有所降低。




 下载:
下载: