-
随着我国高校实验室种类和规模不断扩大,实验产生的污水量逐渐增加[1]。实验室污水具有成分复杂、危害大、间歇性排放、排水量无明显规律的特点,对环境的污染问题也日益严重[2]。目前,实验室污水常规的处理方法主要包括物理处理、化学处理、生物处理[3]。物理处理存在能耗高、废水处理量小等缺点,化学处理过程中会产生二次污染和处理成本较高的问题[4-5]。传统生物处理是利用活性污泥法、生物转盘法和生物膜法等处理污水,该方法存在污泥膨胀、处理效果不稳定等缺点[6]。悬浮填料生物膜反应器(MBBR)是集活性污泥法和生物转盘法优点于一体的新型生物膜反应器,可以高效、稳定地处理成分复杂的污水[7]。
MBBR具有抗冲击负荷能力较强,处理效果稳定,微生物量大等优点[8]。贾方明[9]利用MBBR在14~18 ℃水温处理北方地区生活污水,当DO=4.5 mg·L−1,出水氨氮去除率最高达到65%;当DO=2.0 mg·L−1时,出水总氮和总磷去除率分别为45.2%和58.2%,对于低温环境处理北方生活污水具有较大帮助。庄海峰等[10]采用缺氧/好氧MBBR处理煤化工废水,硝态氮/亚硝态氮混合液回流比200%,最佳HRT=12 h,出水COD、氨氮、总氮去除率分别达到68.1%、84.0%、74.7%,出水达到国家一级A排放标准,出水酚类化合物的数量和种类分别减少了84.4%和54.5%。李月等[11]采用MBBR处理低浓度氨氮(2 mg·L−1)养殖废水,MBBR在水力停留时间为6~8 min和曝气量为180 L·h−1的条件下氨氮去除率可达到70%~75%,氨氮去除负荷为560~700 g·(m3·d−1),能高效地处理低浓度氨氮养殖废水。
MBBR作为污水处理的新技术,目前研究主要集中于MBBR处理生活污水和工业废水,而对实验室污水的处理效果,尤其是环境因子影响下生物膜细菌群落变化的研究则较少[12]。本研究构建了2个相同的MBBR,在秋冬季节处理实验室污水,并监测系统的Tw、DO和pH,探究MBBR水质COD、
${\rm{NH}}_4^ + $ -N处理效果以及生物膜细菌群落的动态变化,分析出水水质波动和细菌群落变化之间的相关性。
全文HTML
-
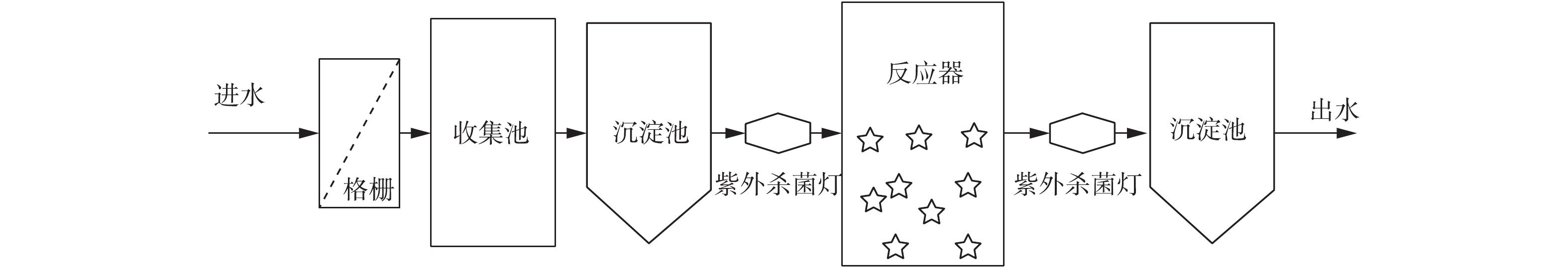
本研究构建2套相同的1号MBBR和2号MBBR,实验装置如图1所示。MBBR反应桶有效容积为800 L,MBBR载体填充率为30%。反应器底部曝气采用多管砂芯曝气,曝气设置好氧 6 h,间隔2 h。1号和2号MBBR分别对应实验室A、B座污水,反应器进水方式采用间歇式进水,实验来水经格栅除杂、紫外杀菌后进入反应器,MBBR连续运行的水力停留时间(HRT)为8~12 h。出水经过紫外杀菌后进入沉淀池,检测出水水质后排放。
-
MBBR填料挂膜采用闷曝自然挂膜法,活性污泥取自武汉沌口污水处理厂脱水污泥,通过添加0.02%葡萄糖间隔曝气活化24 h,取SV30沉降在5 min左右的污泥,按照活性污泥和污水1∶2的比例投放,曝气时间为2 h,间隔时间为1 h[13]。经过约20 d,填料挂膜成功,MBBR运行处理实验室污水。MBBR处理实验室污水期间为自然环境温度,实验持续时间为106 d。每3 d,利用便携式多参数分析仪(HQ30D,哈希公司,美国)现场测定系统的Tw、DO、pH。同时,采集进出水水样,分别采用重铬酸盐法(GB 11914-1989)、纳氏试剂比色法(GB 7479-1987)测定样品的COD、
${\rm{NH}}_4^ + $ -N。 -
在反应器运行7、37、64、97 d(每月下旬)时,取填料生物膜提取总DNA,生物膜总DNA的提取按照土壤细菌DNA提取试剂盒(fast DNA SPIN kit for soil,MP biomedicals)的操作方法。总DNA利用微量紫外分光光度计(ND-1 000,Fisher Scientific,USA)检测其浓度和纯度,通过0.8%的琼脂糖凝胶电泳检测总DNA的完整性,并对其进行高通量测序。
-
采用Illumina MiSeq平台对细菌群落DNA片段进行双端(Paired-end)测序,运用QIIME软件检查并剔除嵌合体等疑问序列,对获得的高质量序列按97%的序列相似度进行归并和OTU划分,并选取每个OTU中丰度最高的序列作为该OTU的代表序列进行统计。利用R软件统计样本细菌群落多样性((即Alpha多样性)以及在各分类水平的组成并进行分析。用Canoco for Windows 4.5软件对细菌优势菌属的相对丰度和环境因子进行冗余分析(redundancy analysis, RDA)。
1.1. 实验装置
1.2. 实验条件和检测方法
1.3. DNA提取和高通量测序
1.4. 数据分析
-
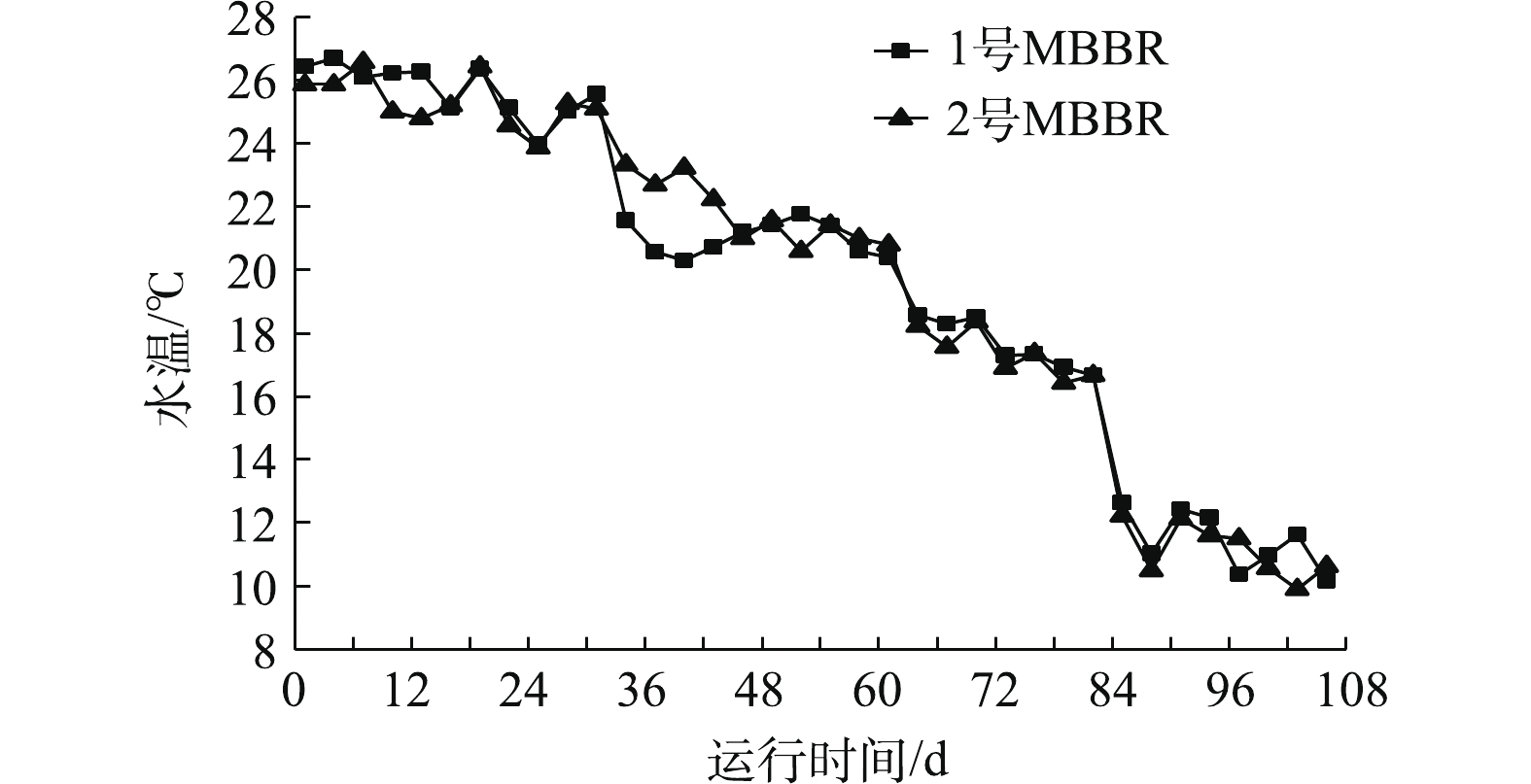
图2是MBBR处理污水期间Tw的变化。由图2看出,Tw下降的趋势基本相同,由前期的26 ℃下降到中期的16 ℃,再到后期的10 ℃。水温是MBBR处理污水过程中重要的环境因子之一,对MBBR出水水质和生物膜微生物群落的演变具有显著的影响[14]。
-
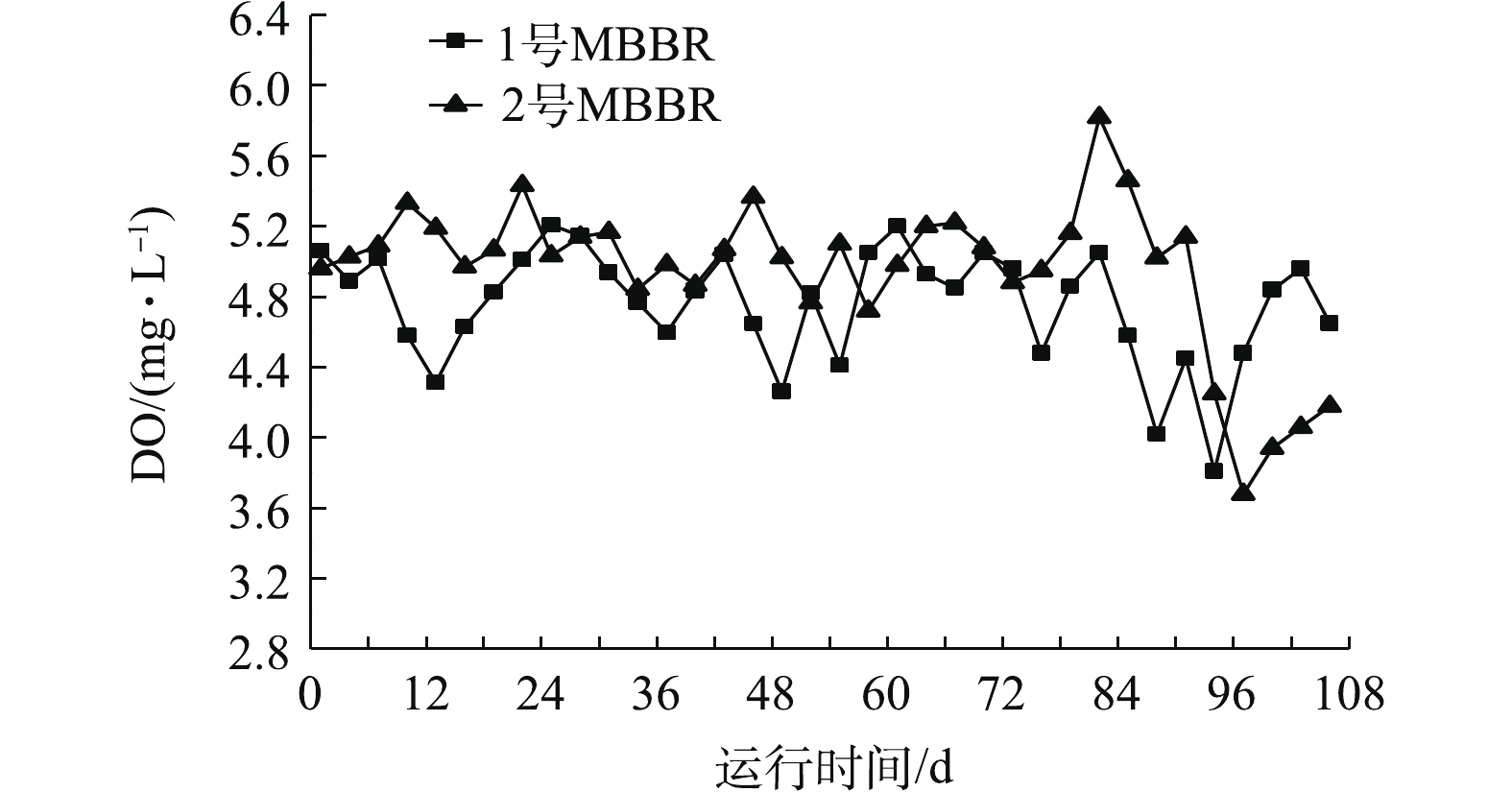
图3是MBBR处理污水期间DO的变化。1号和2号MBBR的DO含量整体保持在(4.8±0.3) mg·L−1和(5.0±0.2) mg·L−1。1号反应器在第94天时,DO含量降到最低3.8 mg·L−1,2号MBBR在94~106 d时,DO含量降低到3.7~4.3 mg·L−1。有研究[15]表明,微生物在分解利用污水中有机物等物质时,会大量消耗DO,从而引起污水中的DO含量波动。反应器DO含量增高有利于有机质的分解和硝化反应的进行,较低的DO含量有利于脱氮微生物进行反硝化作用。
-
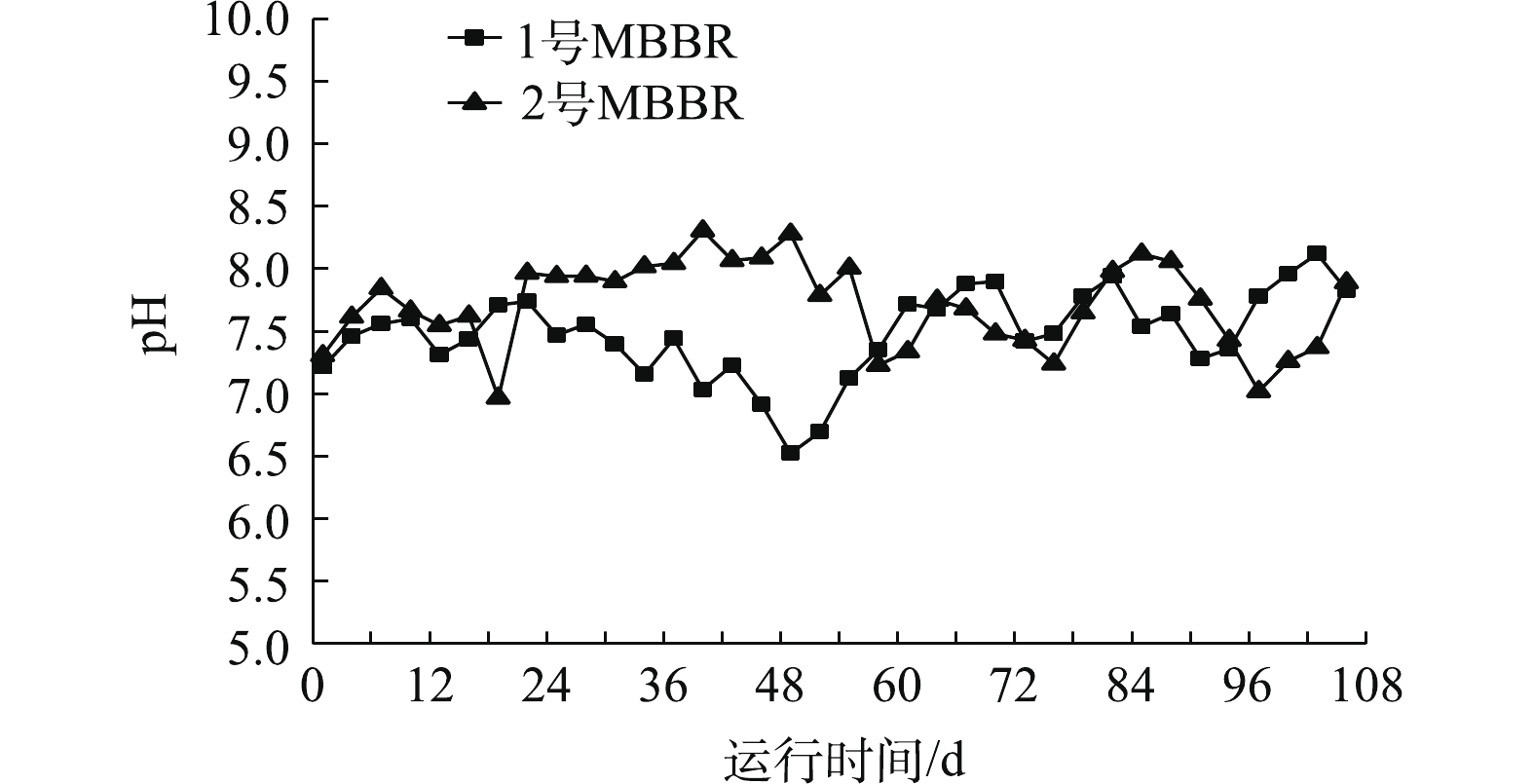
图4是MBBR处理污水期间pH的变化。1号、2号MBBR的pH稳定在7.5±0.3、7.8±0.3左右,整体偏弱碱性。1号反应器在第103天时,pH最高达到8.1,2号反应器在第19天时,pH最低下降到6.9。pH对微生物代谢活动影响显著,硝化过程产酸积累会导致反应受抑制,碱性环境有利于
${\rm{NH}}_4^ + $ -N的去除[16]。反硝化过程产生的碱度会消耗环境酸度,较低的 pH有利于污水TN的去除。MBBR处理过程中pH处于弱碱性,有利于硝化反应的进行。 -
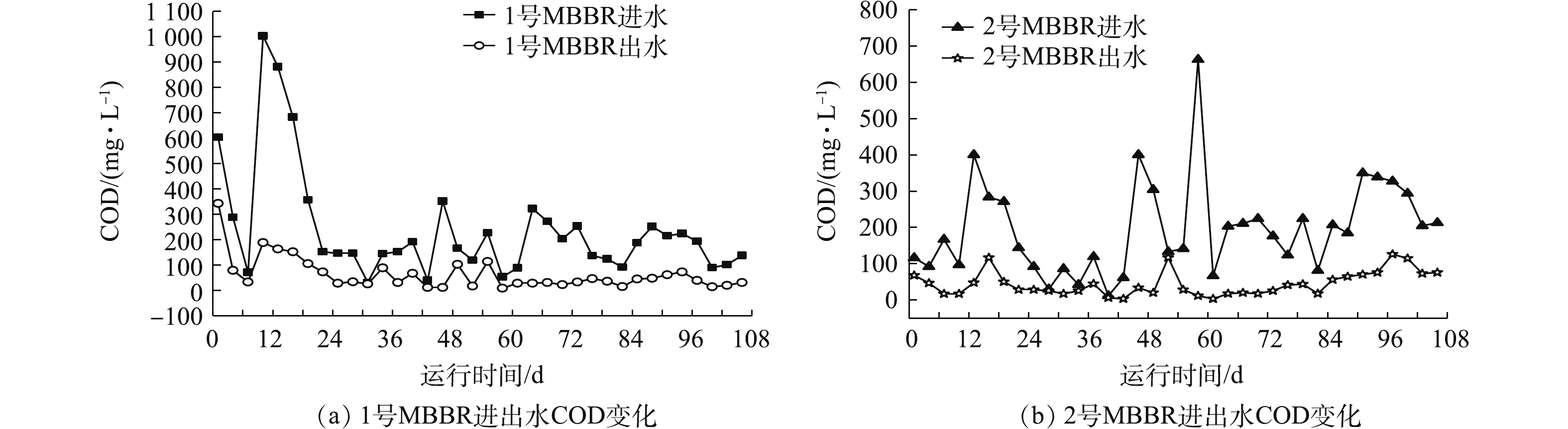
图5(a)和图5(b)是MBBR进出水水质COD的变化。实验期间,1号和2号MBBR进水COD分别为31.02~1 003.16 mg·L−1和11.71~662.69 mg·L−1。1号MBBR整体出水COD低于50 mg·L−1,但在前22 d,出水COD为33.28~342.75 mg·L−1。监测数据表明,进水COD最高时(1 003.16 mg·L−1),现有微生物种类和数量不能使耗氧有机污染物(以COD计)完全降解,随着微生物快速繁殖适应环境,出水COD逐渐下降且恢复正常值。2号MBBR平均出水COD低于50 mg·L−1,而在后期第97 天,出水COD达到126 mg·L−1,后期较低水温(10 ℃)和高有机负荷的进水(349 mg·L−1)是造成出水COD增加的原因。本研究中MBBR进水具有滞后性,高有机负荷的污水会对出水造成影响,而现有微生物种类、数量不足和低Tw是引起MBBR出水COD增高的主要因素[17]。
-
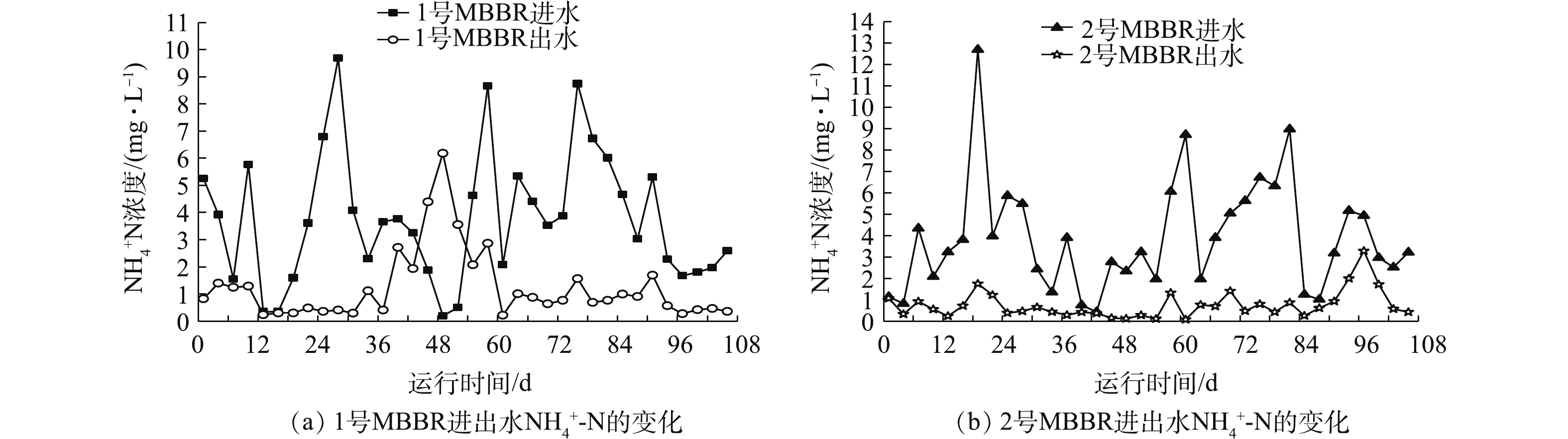
图6(a)和图6(b)为MBBR进出水
${\rm{NH}}_4^ + $ -N的变化结果。1号和2号MBBR进水${\rm{NH}}_4^ + $ -N浓度分别为0.20~9.69 mg·L−1和0.44~12.71 mg·L−1,进水${\rm{NH}}_4^ + $ -N浓度低但波动较大。1号和2号MBBR平均出水${\rm{NH}}_4^ + $ -N浓度保持在0.76~1.25 mg·L−1左右。1号MBBR在第46~52天,出水${\rm{NH}}_4^ + $ -N浓度均高于进水,最高可达到6.19 mg·L−1。环境因子数据表明,在49 d时,DO含量只有4.3 mg·L−1,pH降低到6.5,下降的DO和弱酸性的环境不利于硝化反应的进行,硝化细菌不能快速将${\rm{NH}}_4^ + $ -N转化为$ {\rm{NO}}_3^{-}$ -N、${\rm{NO}}_2^{-} $ -N,导致${\rm{NH}}_4^ + $ -N浓度积累,造成出水的${\rm{NH}}_4^ + $ -N浓度较高[18]。2号MBBR在后期97 d时(Tw=11.5 ℃,DO=3.7 mg·L−1),出水${\rm{NH}}_4^ + $ -N浓度升高到3.28 mg·L−1,随后又快速恢复到正常水平,反应器较低的Tw和DO影响出水${\rm{NH}}_4^ + $ -N。结果表明,随着季节Tw下降,MBBR仍能稳定去除污水中的${\rm{NH}}_4^ + $ -N,出水${\rm{NH}}_4^ + $ -N浓度保持在达标值,而Tw、DO、pH等环境因子是引起出水${\rm{NH}}_4^ + $ -N波动的重要因素。 -
MBBR不同月份样品OTU数和Alpha多样性指数变化见表1。从表1可以看出,2个MBBR生物膜样品的高通量测序总共获得410 649个高质量的序列,覆盖率达到97.98%~99.97%。1号和2号MBBR样品OTU总数分别达到4 512、5 533(97%的序列相似度)。细菌群落多样性可以用侧重体现群落丰富度的Chao1和群落均匀度的Shannon指数来反映,1号和2号MBBR在12月份的Chao1指数均最大(1 689、2 050),Shannon指数没有明显降低。2个MBBR在冬季12月份细菌群落多样性指数仍然保持在较高值,这说明填料微生物能很好地适应冬季低温环境,群落微生物多样性较高,从而能在低温环境下稳定地处理生物实验室污水。
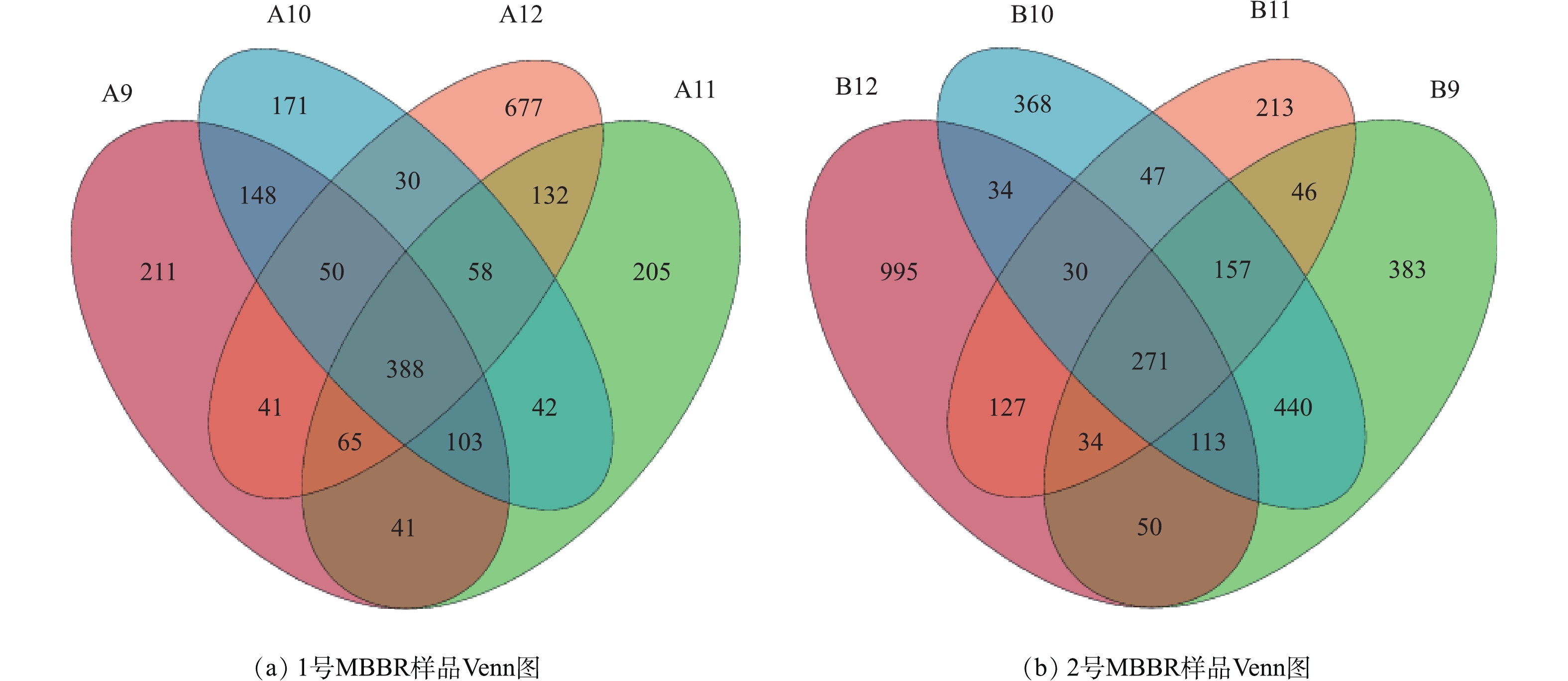
图7反映2个MBBR样品的Venn图。1号MBBR(A)在9、10、11、12月共有OTU数388个,独有的OTU数分别有211、171、205和677个;2号MBBR(B)在9、10、11、12月共有OTU数271个,独有的OTU数分别有383、368、213和995个。2个MBBR在不同月份都具有丰富的物种,2号MBBR的物种数量高于1号,在12月,2个MBBR独有的物种均达最高,说明MBBR在不同月份物种组成具有相似性,但每月都有其独有的物种,随着不同月份Tw的变化,物种数量也有相应的动态变化。
-
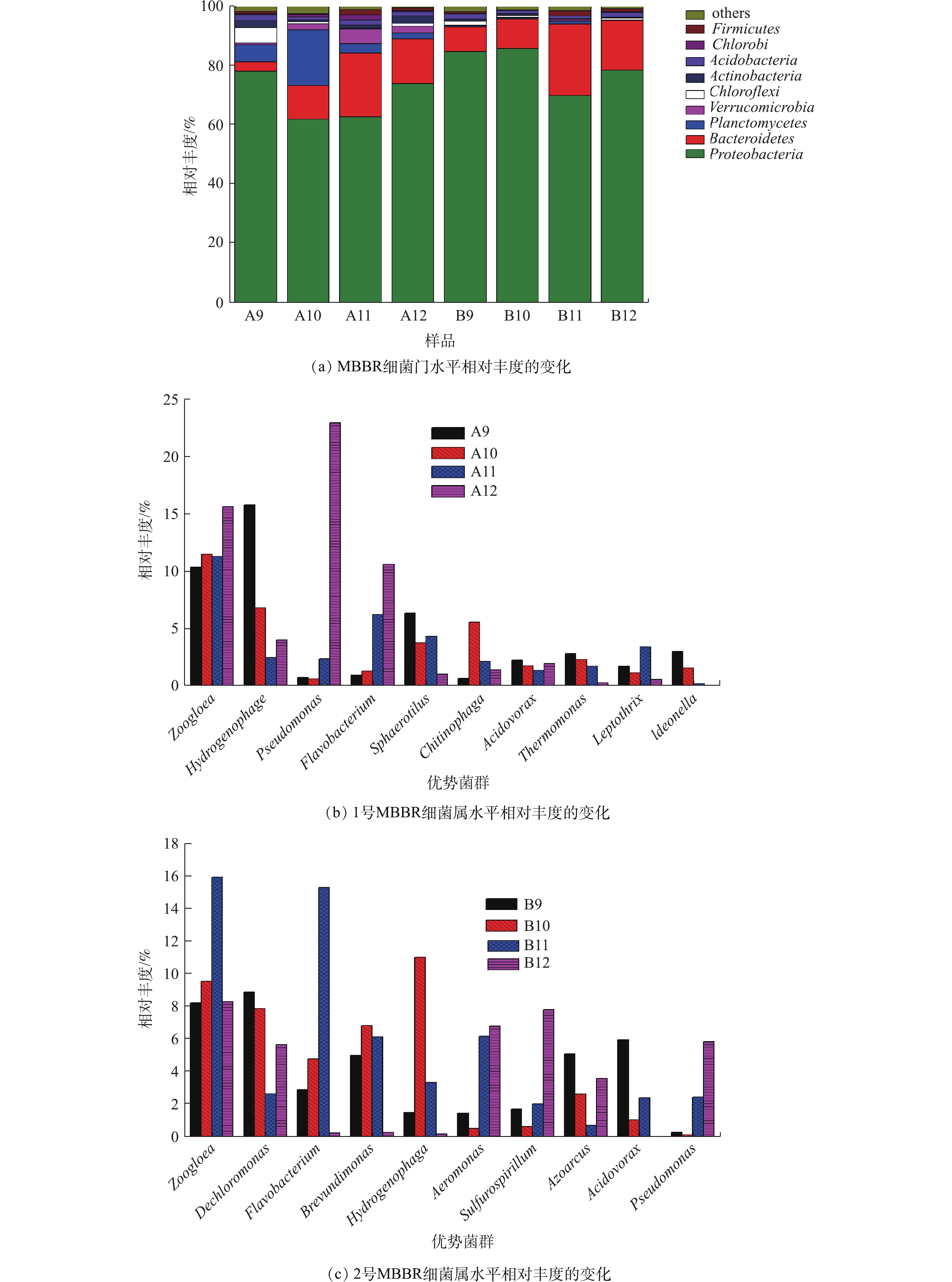
图8为 MBBR细菌群落相对丰度的变化。由图8(a)看出,MBBR处理实验室污水过程中变形菌门(Proteobacteria)、拟杆菌门(Bacteroidetes)和浮霉菌门(Planctomycetes)是主要优势菌门。变形菌门是所有MBBR样品最大的优势菌门,占据61.87% ~ 85.78%的相对丰度。变形菌门包含多种代谢类型,广泛分布在填料生物膜好氧和厌氧环境中,在MBBR除碳脱氮中发挥关键性作用[19]。拟杆菌门是主要优势菌门之一,1号MBBR样品拟杆菌门在9月和10月的相对丰度分别是3.12%和11.41%,在11月和12月的相对丰度升高到21.6%和15.05%。同样,2号MBBR样品相对丰度在11月和12月也升高到24.1%和16.73%。拟杆菌门的相对丰度在水温较低的环境显著增加,说明拟杆菌门细菌可以很好地适应低温环境,从而在MBBR低温处理污水中发挥显著作用[20]。
图8(b)和图8(c)为MBBR样品细菌属水平相对丰度的变化。可以看出,在MBBR细菌群落属水平上,动胶菌属(Zoogloea)占据8.27%~15.96%的相对丰度,是明显的优势菌属之一。有研究[21]表明,动胶菌属是化能异养的专性好氧菌,通过群聚形成菌胶团,具有吸附、氧化分解、凝聚沉降等能力,高丰度的动胶菌属可以稳定生物絮团,保证充足的生物量分解污水中有机物,从而降低污水中COD值。氢噬胞菌属(Hydrogenophaga)在1号、2号MBBR样品中相对丰度最高分别达到15.78%(9月)和11.06%(10月);而在12月,分别下降到4.02%和0.21%,氢噬胞菌属更适合Tw较高的季节。氢噬胞菌属的细菌具有降解多环芳烃化合物(PAHs)的能力,通过产酶作用于苯环,经过氧化反应,进一步代谢为邻苯二甲酸等中间产物[22]。氢噬胞菌属可能在降解实验室污水的多环芳烃物质中发挥重要作用。假单胞菌属(Pseudomonas)的相对丰度随Tw变化较显著,1号的假单胞菌属从9月的0.77%升高到12月的22.95%,同样2号的假单胞菌属从9月的0.32%上升到12月的5.86%。这表明假单胞菌属能很好地适应低温环境,成为生物膜的优势菌种,是低温环境重要的脱氮微生物之一。
在11月和12月,MBBR出水水质能稳定保持在较低浓度,这是因为适应低温环境的微生物(如假单胞菌属)逐渐增加成为优势菌属,而不适应低温的微生物(如氢噬胞菌属)逐渐减少,通过生物膜菌群的演替,保证了MBBR在秋冬季节仍然能稳定达标的处理实验室污水。
-
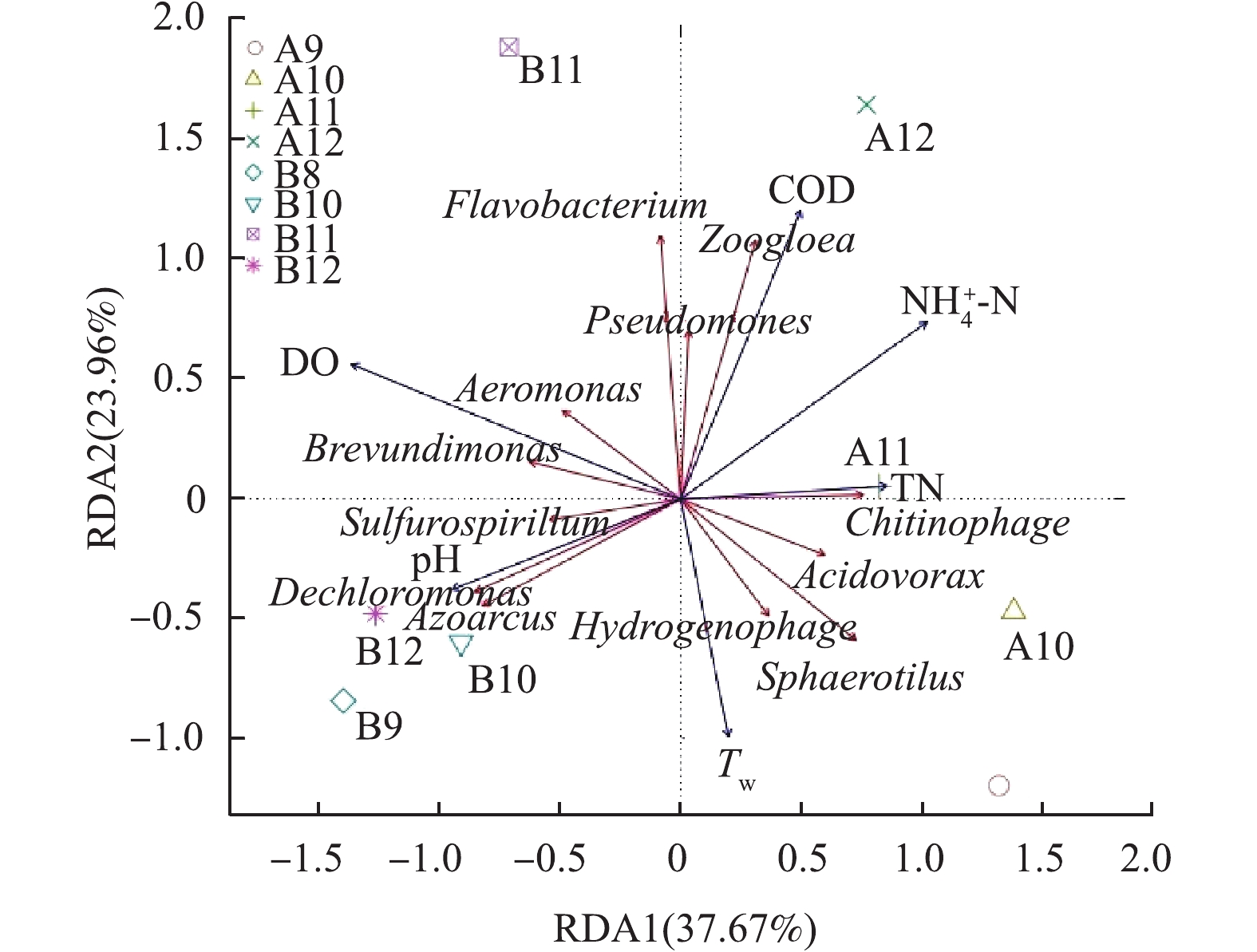
图9是环境因子与细菌群落的冗余分析(RDA)结果。MBBR的RDA排序图第1和第2主轴分别解释了优势菌群相对丰度变化的37.67%和23.96%,第1轴上,相关性较大的环境因子是DO(r=− 0.918)、
${\rm{NH}}_4^ + $ -N(r=0.687)和pH(r=−0.634);第2轴上,相关性较大的环境因子是COD(r=0.811)和Tw(r=−0.668)。可以看出,DO与短波单胞菌属(Brevundimonas)、气单胞菌属(Aeromonas)具有显著正相关性,较高的DO有利于短波单胞菌属和气单胞菌属的生长。短波单胞菌属具有广谱的烷烃类有机物降解能力,还能降解芳香族有毒物质[23]。pH与脱氯单胞菌(Dechloromonas)、固氮弧菌属(Azoarcus)具有显著正相关性,这说明脱氯单胞菌和固氮弧菌属更适合碱性的环境。脱氯单胞菌和固氮弧菌属是常见的脱氮微生物,脱氯单胞菌具有很强的硝酸盐还原能力,固氮弧菌属具有脱硫脱氮的重要作用[24-25]。Tw明显与黄杆菌属(Flavobacterium)、假单胞菌属(Pseudomonas)具有负相关性,黄杆菌属和假单胞菌属在较低Tw环境成为优势菌种,从而在低温环境发挥分解有机物和脱氮的重要作用。综上所述,Tw、DO、pH是影响MBBR细菌群落更替的重要环境因子,对MBBR处理生物实验室污水产生较大影响。
2.1. MBBR环境因子的变化
2.2. MBBR进出水质的变化
2.3. MBBR细菌群落的OTU和多样性
2.4. MBBR细菌群落结构的变化
2.5. 环境因子对MBBR细菌群落结构的影响
-
1)随着秋冬季节Tw从26 ℃下降到10 ℃,MBBR能稳定达标地处理实验室污水。当进水COD、
${\rm{NH}}_4^ + $ -N分别为200 mg·L−1和6 mg·L−1时,MBBR出水COD、${\rm{NH}}_4^ + $ -N去除率仍然保持在75%和80%左右。2)通过RDA分析发现,DO与短波单胞菌属、pH与脱氯单胞菌、固氮弧菌属具有显著正相关性,Tw与假单胞菌属、黄杆菌属具有显著负相关性,环境因子Tw、pH、DO显著影响生物膜细菌群落的变化。
3)高通量测序表明,MBBR样品中变形菌门、拟杆菌门和浮霉菌门是生物膜主要优势菌门,Tw的下降引起拟杆菌门的相对丰度显著增加,假单胞菌属和黄杆菌属的相对丰度随Tw的降低而极大地升高,动胶菌属相对丰度保持稳定,说明细菌群落动态更替是MBBR出水水质保持稳定达标的重要原因。





 下载:
下载: