-
榨菜的工业生产过程中常伴随着大量高盐有机废水的排放,此类高盐有机废水对常规微生物的生长繁殖等活动有明显的抑制作用[1],因而常规的处理技术并不能完成高效处理,并且常规处理技术能耗和运行费用高、污泥产量大,污泥的后续治理费用也较高[2]。近年来,微生物燃料电池因具有工作条件温和、底物来源广泛等优点,且在降解污染物的同时能实现能量的资源化回收,故已成为一种真正绿色、可持续产能装置[3-4]并获得普遍关注,现已应用于生活废水、焦化废水、养猪废水、垃圾渗滤液等多种废水的资源化处理[5-10]。
榨菜废水高盐的环境可以提供良好的电导率和可生化性,这些优势使其具备了作为MFCs理想燃料的条件[11]。基于此,GUO等[12]首次运用MFCs技术处理榨菜废水,同步实现了榨菜废水微生物燃料电池的产电和化学需氧量(chemical oxygen demand,COD)去除。之后,研究人员在盐度、产电周期等方面开展了一些研究工作[11, 13-15]。
由于榨菜废水微生物燃料电池存在输出功率不高的问题,考虑到阴极电子受体对电池系统的产电性能影响较大[16],为了提高榨菜废水MFCs的输出功率,故选择合适的电子受体至关重要。为此,有研究人员[17-19]对微生物燃料电池阴极的电子受体和产电性能展开了研究,以期提高阴极性能和MFCs整体输出功率。JADHAVA等[17]以次氯酸盐和氧气为阴极电子受体研究黏土微生物燃料电池时发现,次氯酸钠的功率密度为氧气的9倍。GHADGE等[18]以次氯酸盐为微生物燃料电池电子受体以提高污泥消化速率。JUN等[19]以过硫酸钾、铁氰化钾为阴极电子受体作对比实验发现,过硫酸钾的功率密度要高于铁氰化钾,且过硫酸钾作为阴极电子受体时具有调节pH的能力。这些研究的进水水质大多为人工配置的废水,而非实际废水(如榨菜废水)。实际废水(如榨菜废水)的水质更为复杂,相关的研究不多。然而,从燃料电池实际应用出发,应对实际废水进行研究。且电子受体对高盐榨菜废水MFCs的产电性能和阳极微生物菌群分布方面的研究尚未见报道。为探究上述问题,本实验以高盐榨菜废水为双室微生物燃料电池底物,选用过硫酸钾、氧气和铁氰化钾等常见的氧化还原能力具有差异的氧化剂作为电子受体(三者的氧化还原电位如式(1)~式(3)所示),比较其产电性能和阳极微生物菌群,以期对后续高盐榨菜废水的研究提供参考。
-
榨菜废水取自重庆涪陵某榨菜厂,实验阳极底物为厌氧池出水(anaerobic reactor effluent,ARE),保存在4 ℃的冰箱内,其水质特征如下:pH为7.34±0.25,DO为(0.65±0.03) mg·L−1,COD为(1 600±80) mg·L−1,水温为(25±1.25) ℃,盐度(以NaCl计)为(14.7±0.74) g·L−1,电导率为(26.8±1.34) mS·cm−1。其中DO即溶解度(dissolved oxygen,DO),pH即酸碱度(purple heart,pH)。实验所用其他试剂均为分析纯,实验用水为超纯水(电阻率为18.2 MΩ·cm)。
-
方形反应器由有机玻璃制作而成。阳极室和阴极室尺寸相同,均为4 cm×5.5 cm×4 cm;有效容积均为80 mL。如图1所示,2个腔体通过质子交换膜从中间隔开,通过面积为22 cm2。阳极和阴极电极材料均为碳布,表面积均为44 cm2。电极由钛丝导线引出,并用铜线与变租箱(0~9 999.9 Ω)连接,形成闭合电路。在运行过程中,采用磁力搅拌器对阳极室底物进行搅拌混匀。实验所用的主要仪器和用途如下:变阻箱型号为ZX21旋转式直流电阻箱,作为负载电阻;磁力加热搅拌器型号为78-1,搅拌器预防出现短流;参比电极型号为RE-1C,测电极电势;数据采集模块型号为DAS,PISO-813,采集电压;便携式分光光度计型号为DRB200&DR5000,HACH,测COD;电导率仪型号为FE-30K,测电导率及盐度;便携式pH测试仪型号为Sension 1,HACH Co,测pH。
-
MFCs启动时外接500 Ω电阻,阳极室直接加入厌氧池废水进行启动,并加入厌氧池污泥进行接种。在运行过程中,保证阳极室的绝对厌氧状态。当电池电压低于50 mV时,认定完成一个产电周期;之后及时更换新鲜底物和阴极液,在至少连续3个周期内,电池均能达到相似电压(相差不超过5%)及持续时间,认为系统启动成功。所有实验采用续批式模式运行,平行检测,如无特殊说明,实验温度均在(25±1) ℃下进行。
实验分别以铁氰化钾、曝气阴极、过硫酸盐为电子受体,启动3组榨菜废水微生物燃料电池。曝气阴极的曝气强度为200 mL·min−1,这个曝气强度下阴极室内溶解氧浓度接近饱和,氧气浓度不是限制性因素。过硫酸钾和铁氰化钾的浓度皆为0.1 mol·L−1,阴极电子受体浓度不是实验限制因素。
不同阴极电子受体运行时,电池获得稳定的产电状态,然后再对电池性能进行测定评估。在运行期间,所有的底物都经过高温灭菌处理,除此之外不作其他任何改变。电池外部负载R=500 Ω,分别检测氧气、铁氰化钾、过硫酸钾等不同电子受体的微生物燃料电池的产电性能、COD的去除率及微生物群落的分布。
-
通过OMEGA Soil DNA试剂盒,提取阳极生物膜样品中微生物DNA。使用通用细菌引物16S rRNA的5′-ACTCCTACGGGAGGCAGCAG-3′和5′-GGACTACHVGGGTWTCTAAT-3′(338F和806R),对提取的DNA进行PCR扩增。将所有序列读数聚类到操作分类单位OTU(operational taxonomic units,OTU)(相似性阈值为97%)。高通量测序服务由上海美吉生物平台(上海,中国)提供。
-
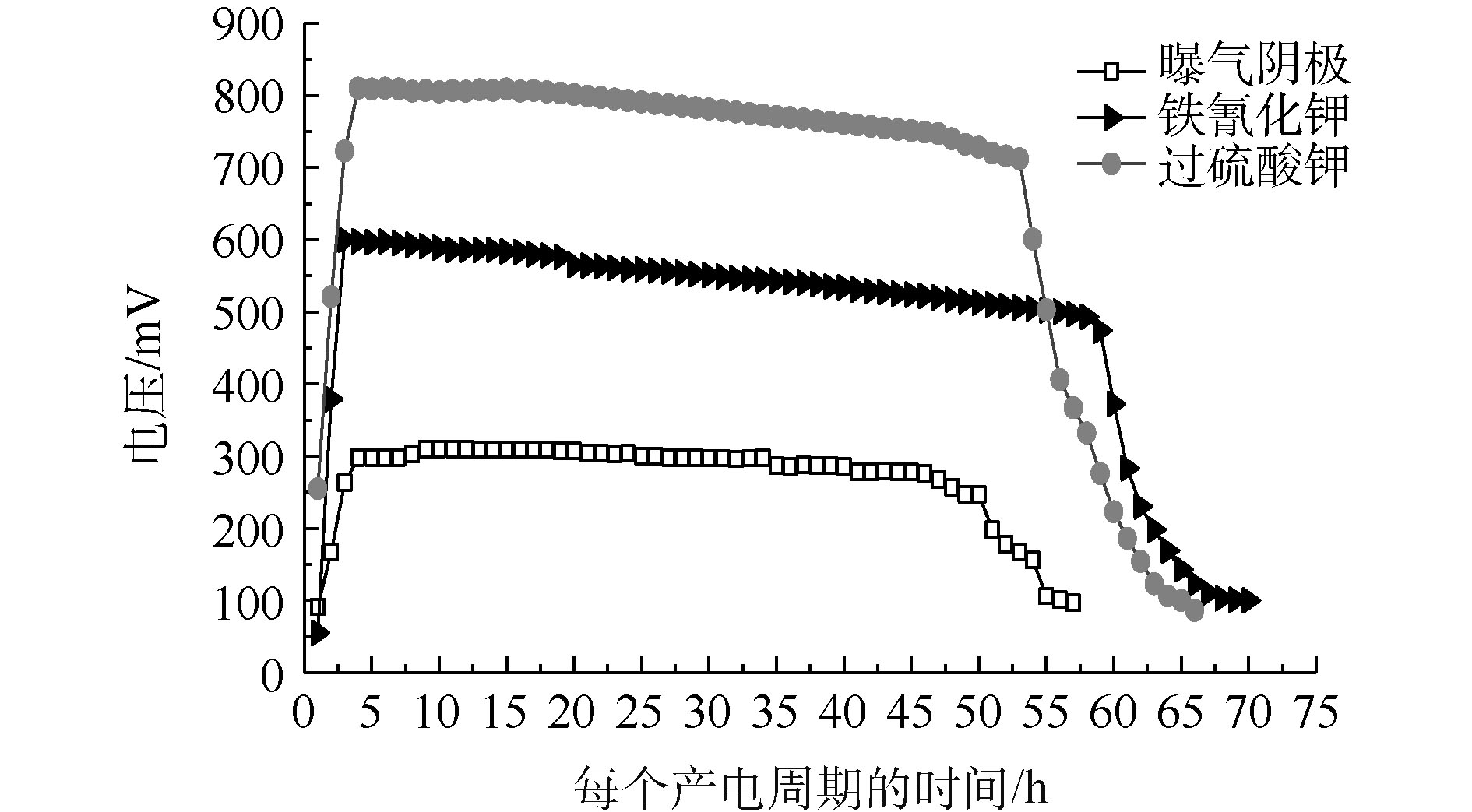
在外阻为500 Ω的负载下,不同阴极电子受体的输出电压如图2所示。过硫酸钾、铁氰化钾和曝气阴极的输出电压分别为802、588和300 mV,说明以榨菜废水为底物的MFCs不同电子受体的电压输出有较大的差别。在一个完整的产电周期内,铁氰化钾、过硫酸钾和曝气阴极的产电周期分别为70、66和57 h。造成输出电压和产电周期不同的主要原因可能是阴极电子受体的电势不同和氧化还原的能力不同[17]。曝气阴极的氧气输出电压较低,可能是因为活化损失较大,过电位较高。
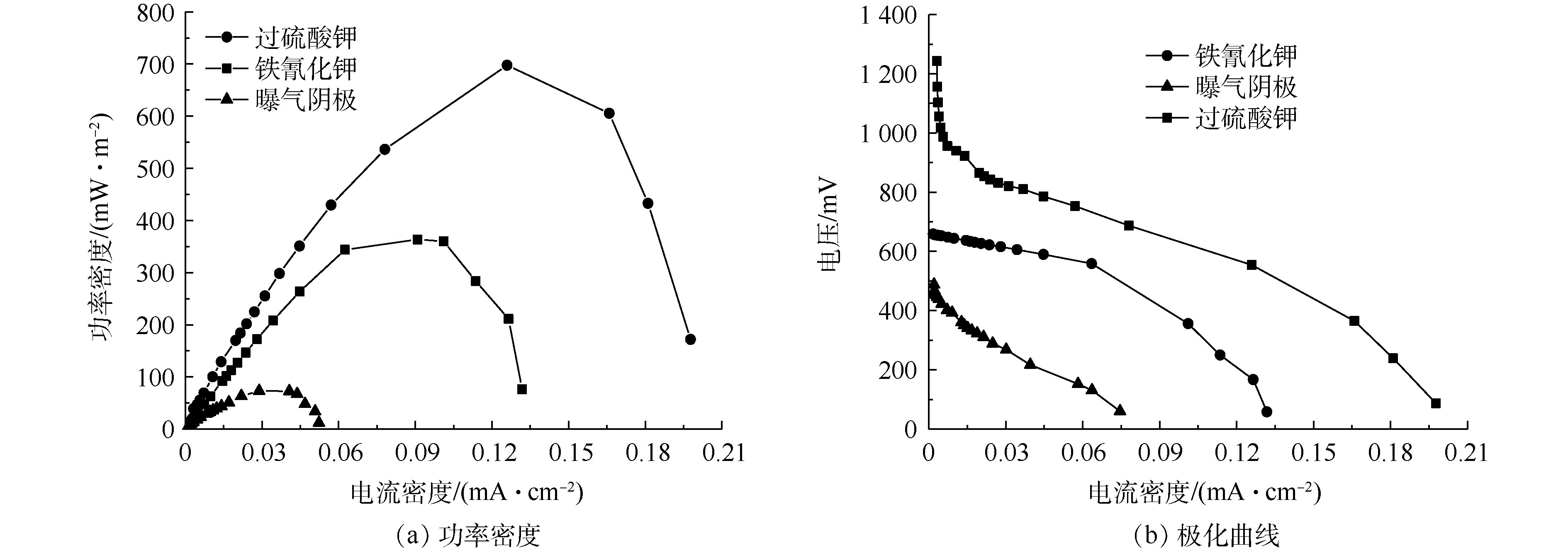
稳定运行3个周期以后,开始采用梯度改变外电阻的方法测量电池极化曲线及功率密度曲线。如图3(a)所示,不同电子受体的最大功率密度依次为697、363.64、73.3 mW·m−2。JUN等[19]利用人工废水为底物、过硫酸盐为阴极电子受体,构建的微生物燃料电池最大功率密度为317.5 mW·m−2,可能由于进水水质的差异导致其功率密度低于本研究。MIYAHARA等[20]发现,随着阳极液NaCl的添加,MFCs最大输出功率提高了近4倍。说明高盐高浓度榨菜废水MFCs是一种较为理想的电池燃料。
由图3(b)可知,系统的开路电压和内阻分别为688 mV和87 Ω(铁氰化钾)、1 303 mV和101 Ω(过硫酸钾)、612 mV和170 Ω(曝气阴极)。3种化学阴极MFCs(铁氰化钾、过硫酸钾和曝气阴极)开路电压的差别可能是因为阴极电子受体氧化还原能力和反应器的内阻不同。由图3(b)可知,铁氰化钾的内阻最小,过硫酸钾次之,曝气阴极的内阻最大。曝气阴极的内阻较大,可能是因为氧气作为阴极受体具有很高的Warburg扩散阻力[18]。微生物燃料电池系统内阻由欧姆内阻、活化内阻和传质内阻构成。过硫酸盐的电池内阻比铁氰化钾高,可能是因为碳化物表面上的铁氰化物溶液比过硫酸盐具有更快的电还原动力学[19],导致铁氰化钾的传质内阻和活化内阻比过硫酸钾小,并且过硫酸钾和铁氰化钾的欧姆内阻相差不大。因此,整个电池系统内阻铁氰化钾比过硫酸钾要小。
-
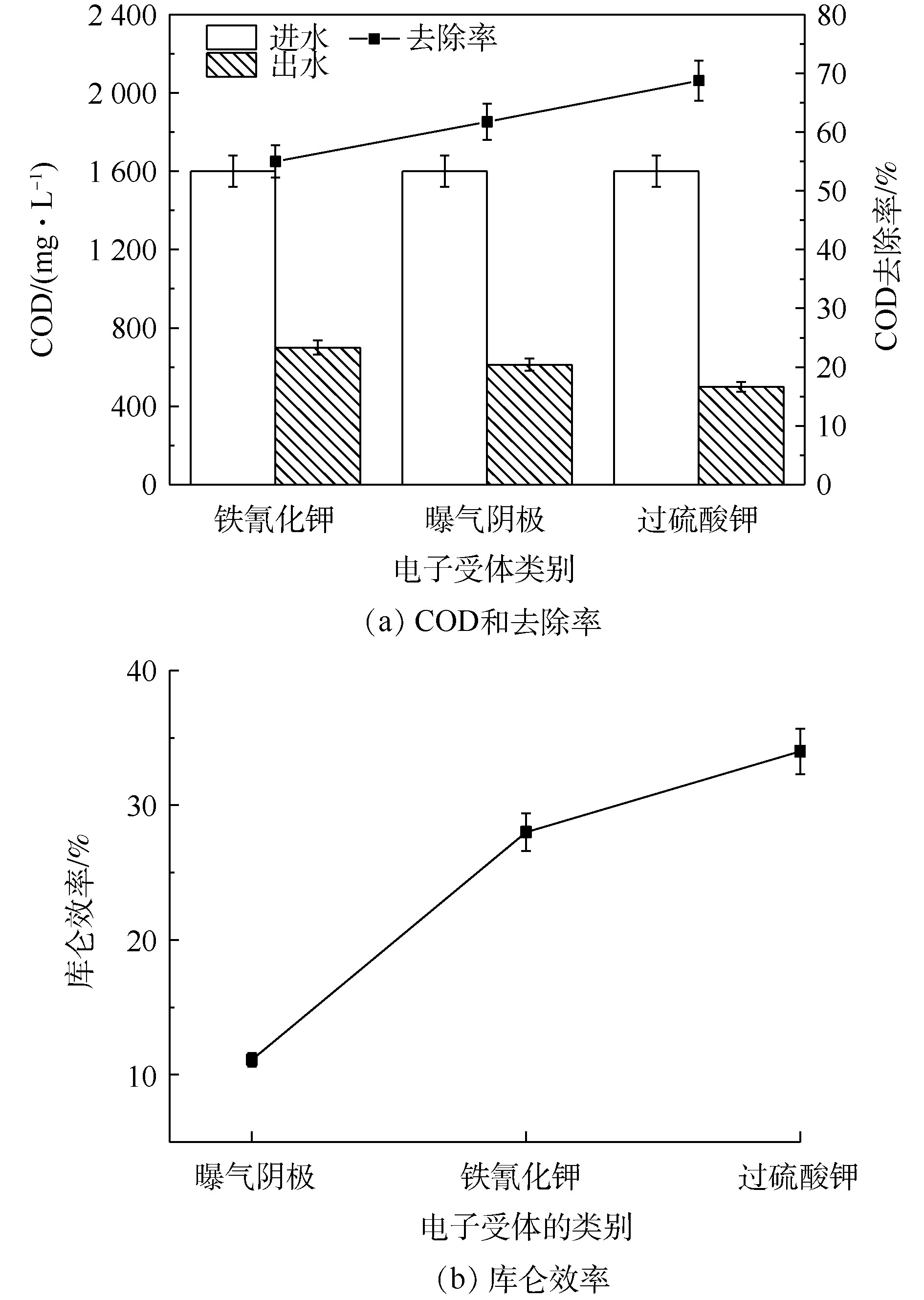
如图4(a)所示,铁氰化钾、曝气阴极和过硫酸钾的进水COD相同,COD去除率分别为(55±2.7)%、(61.7±3.1)%、(68.7±3.4)%。吴越[21]以榨菜废水为MFCs底物时,COD去除率在60%左右。由图4(a)可知,氧化还原能力强的阴极电子受体对COD的去除率要高一些。由图4(b)可知,不同电子受体的库仑效率不同。曝气阴极、铁氰化钾及过硫酸钾的库仑效率分别为(11.1±0.5)%、(28±1.4)%、(33±1.6)%。不同类型的高盐榨菜废水微生物燃料电池的库仑效率不同,其库仑效率为14%~44%[10-13]。库仑效率与多方面的因素有关,这些因素包括反应器的构形和腔室大小、阴极电子受体及受体浓度环境、运行的阶段不同。随着氧化还原能力的提高和内阻的降低,库仑效率会改变。
-
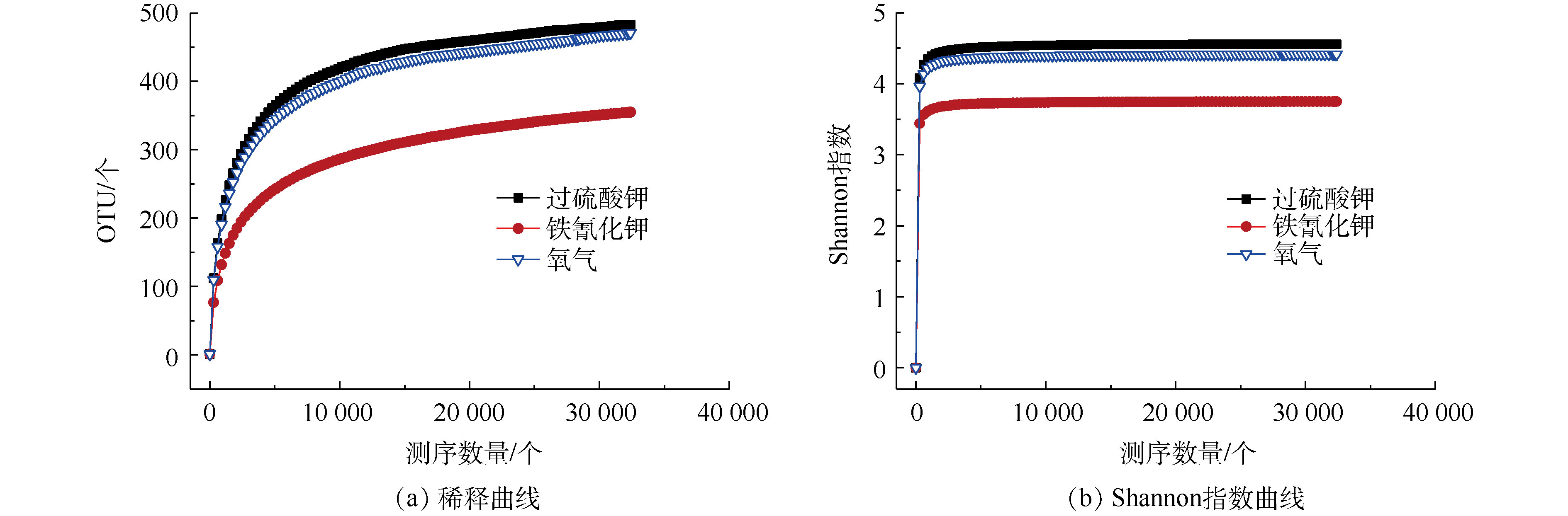
1)微生物多样性分析。为量化各组MFCs微生物的多样性,我们检测了几种常用微生物多样性指标。如表1所示,各组MFCs微生物测序中样本文库覆盖率(coverage)均大于0.98,说明测序结果能够很好地代表样本的真实情况。铁氰化钾、过硫酸钾和氧气的Chao指数和OTU数量随着氧化还原电位不同而不同,氧化还原电位高的Chao指数和OTU数量要高。这也表明在高盐度MFCs中,铁氰化钾和氧气、过硫酸钾的阳极生物膜显示出来微生物丰富度具有差异[22]。铁氰化钾、过硫酸钾和氧气的Shannon指数、Simpson指数和Ace指数也表明了过硫酸钾的阳极生物膜微生物多样性高于氧气和铁氰化钾。
如图5所示,在以过硫酸钾、氧气和铁氰化钾为阴极电子受体的MFCs中,发现了阳极微生物多样性具有差异。如图5(a)所示,读数范围从32 400个到最远处,表明大多数细菌群落被捕获,过硫酸钾的阳极富集群落比氧气和铁氰化钾中的阳极富集群落更丰富,这也可以通过Shannon多样性指数证明,如图5(b)所示。高盐度榨菜废水富含有机质,由于物种之间的协调,生物膜中较高的物种多样性可能对电力生产和污染物去除有益[23]。
2)微生物门水平组成分析。在门水平上,不同阴极电子受体的阳极生物膜群落分布如图6所示。可将微生物检测频率>1%的菌门做为主要的菌门[24]。在3种阴极电子受体的阳极生物膜中,共发现8个主要菌门,分别为拟杆菌门(Bacteroidetes)、厚壁菌门(Firmicutes)、变形菌门(Proteobacteria)、互养菌门(Synergistetes)、绿弯菌门(Chloroflexi)、螺旋菌门(Spirochaetae)、放线菌门(Actinobacteria)和Parcubacteria门。这进一步验证了在该电池系统中不同电子受体的菌群具有相似性,且拟杆菌门、厚壁菌门、互养菌门和变形菌门是主要的电化学活性菌[14-15],实现了电池的产电功能。在电池系统中,这4种主要产电菌的相对丰度不同,过硫酸钾(图6(c))相对丰度最多为82%、铁氰化钾相对丰度(图6(a))为80%、曝气阴极相对丰度(图6(b))为69%。这可能是MFCs输出电压不同的一个原因。厚壁菌门、拟杆菌门和变形菌门是厌氧发酵的优势群落[25],分别占铁氰化钾、氧气和过硫酸钾MFCs阳极微生物菌群的64.3%、63.6%和75.51%,这也说明了厌氧发酵菌群为优势菌群。从相关研究中可以推测,在电池运行过程中,厌氧发酵后的产物可能主要进行了产甲烷过程[26-29]。
从图6可以看出,不同电子受体的阳极微生物菌群种类类似,丰度有所不同。微生物群落丰度的不同可能由以下因素引起:微生物呼吸反应由电子供体和受体之间的氧化还原电位差驱动,不同的电子受体对微生物生长的影响不同[30-31];细菌电子传递途径的变化和生长速率与氧化还原电位相关[32-33],氧化还原电位是细胞外电子转移驱动力;电子供体和受体之间氧化还原电位的差异决定了通过呼吸可达到的最大微生物能量增益,较高电位下电极提取的生物膜蛋白质和细胞数量都较高[34-35]。
3)功能微生物群属分析。如图7所示,以铁氰化钾为电子受体的阳极生物膜优势菌属为Lentimicrobium(22.41%)、Synergistaceae(15.78%)、Thioclava(6.98%)、Sphaerochaeta(6.95%);以氧气为电子受体的阳极生物膜优势菌属为Lentimicrobium(21.39%)、Anaerolineaceae(10.95%)、Synergistaceae(4.96%);以过硫酸钾为电子受体的阳极生物膜优势菌属为Lentimicrobium(10.1%)、Marinobacterium(9.15%)、Desulfuromonas(7.89%)、Synergistaceae(5.43%)。Lentimicrobium属于Bacteroidetes门下的严格厌氧菌属革兰氏阴性细菌[36],其丰度会随着有机物的减少而降低[37]。Synergistaceae为严格厌氧细菌,主要代谢途径是发酵,为Synergistetes门下的厌氧发酵菌属[38]。Anaerolineaceae是Chloroflexi门下的厌氧消化池中的微生物菌属之一[39],长期培养后是可以为烷烃类的产甲烷提供有机酸的核心菌群[40]。Marinobacteriu为一种轻度嗜盐菌,为水解酸化的主要菌群。在以过硫酸盐作为阴极微生物燃料电池的微生物菌属中,发现了脱硫单胞菌属Desulfuromonas。在厌氧条件下,通过Desulfuromona等菌属把硫还原成H2S,说明在以过硫酸盐为阴极的微生物燃料电池中可能存在异化性硫还原作用。Thioclava为革兰氏阴性菌株,LIU等[41]发现,Thioclava能够氧化硫化合物(如硫代硫酸盐、硫)和硫化物,并以此作为能源。Draconibacteriace为兼性厌氧革兰氏阴性细菌,其生长环境必需有NaCl[42],主要代谢方式为发酵[43]。Sphaerochaeta为厌氧产甲烷菌属[44]。
从图5和图6可知,该电池系统中水解发酵菌为优势菌属,其厌氧水解产物可能主要为后续的产甲烷过程利用[29, 45-46]。另外,不同阴极电子受体MFCs的产电菌属的种类差别不大,丰度有所不同。
-
1)电子受体不同的微生物燃料电池的产电性能具有差异性。以铁氰化钾、过硫酸钾、曝气阴极为电子受体的微生物燃料电池,在外电阻500 Ω的负载下运行时,其在输出电压、产电周期、功率密度和内阻等方面均具有明显差异:铁氰化钾输出电压588 mV、产电周期70 h、功率密度363.64 mW·m−2、内阻87 Ω;过硫酸钾的输出电压802 mV、产电周期66 h、功率密度697 mW·m−2、内阻101 Ω;曝气阴极输出电压300 mV、产电周期57 h、功率密度73.3 mW·m−2、内阻170 Ω。
2)电子受体不同的微生物燃料电池的污染物去除及库仑效率具有差异性。铁氰化钾的COD去除率为(55±2.7)%、库仑效率为(28±1.4)%;过硫酸钾的COD去除率为(68.7±3.4)%、库仑效率为(33±1.6)%;曝气阴极的COD去除率为(61.7±3.1)%、库仑效率为(11±0.5)%。因此,从产电性能和污染物去除以及库仑效率等方面来考虑,过硫酸钾作为榨菜废水燃料电池的电子受体更优。
3)通过对微生物群落的分析,水解发酵菌属为电池系统阳极的核心微生物群落,在铁氰化钾、过硫酸钾和氧气MFCs阳极微生物菌群中,占比分别为64.3%、75.51%和63.6%。不同阴极电子受体的主要产电菌群种类相近,但丰度不同。铁氰化钾、过硫酸钾和氧气MFCs的核心产电菌群丰度分别为80%、82%和69%。微生物丰度的差异来源于不同电子受体的势差,势差较大时(本实验中即以过硫酸钾为电子受体时),微生物群落的多样性和丰富度较高,产电和污染物去除效果较好。
高盐废水MFCs不同阴极电子受体产电及微生物群落分析
Electricity generation and microbial community analysis of different cathodic electron acceptors in MFC treating mustard tuber wastewater
-
摘要: 为探查不同电子受体产电性能及对阳极微生物群落的影响,研究了3种电子受体(铁氰化钾、曝气阴极、过硫酸钾),构建了双室榨菜废水微生物燃料电池系统(microbial fuel cells,MFCs),实现了污水处理和能量回收的双重目的,探讨了不同电子受体(铁氰化钾、曝气阴极、过硫酸钾)对榨菜废水MFCs产电性能及阳极微生物群落的影响。结果表明:在产电性能方面,当过硫酸钾作为阴极电子受体时,电池输出电压、库仑效率、功率密度均优于另外2种常用阴极电子受体(铁氰化钾和氧气);在500 Ω的外接电阻间歇运行的条件下,其输出电压、库仑效率、功率密度分别为802 mV、(33±1.6)%、697 mW·m−2。阳极生物16S rRNA基因测序分析表明,水解发酵菌为榨菜废水微生物燃料电池阳极核心菌群,铁氰化钾、氧气和过硫酸钾MFCs阳极微生物菌群相对丰度分别为64.3%、63.6%和75.51%,包括Lentimicrobium、Synergistaceae、Sphaerochaeta、Anaerolineaceae、Draconibacteriacea菌属。阴极电子受体不同的MFCs的阳极微生物群落核心菌群类似,但是丰度有所不同。势差较大的电子受体(过硫酸钾)微生物群落多样性和丰富度较高,产电和污染物去除效果较好。
-
关键词:
- 微生物燃料电池 /
- 榨菜废水 /
- 电子受体 /
- 16S rRNA微生物分析
Abstract: In order to explore the relationship between the electrical properties of different electron acceptors and the anodic microbial community, three electron acceptors (potassium ferricyanide, aerated cathode, potassium persulfate) were experimentally studied. The microbial fuel cells (MFCs) of double-chamber mustard wastewater were constructed to achieve the dual purpose of sewage treatment and energy recovery. Effects of different electron acceptors (potassium ferricyanide, aerated cathode and potassium persulfate) on their electricity production performance and anode microbial community in mustard tuber wastewater were investigated. Results show that when potassium persulfate was used as the cathode electron acceptor, electricity production performance, such as the battery output voltage, coulombic efficiency and power density, was better than the other two common cathode electron acceptors (potassium ferricyanide and oxygen). Under the intermittent operation conditions with 500 Ω external resistors, the output voltage, coulombic efficiency, and power density were 802 mV, (33±1.6)% and 697 mW·m−2, respectively. The 16S rRNA gene sequencing analysis of the anode organism showed that the hydrolysis fermentation bacteria were the anode core bacteria of MFC treating mustard tuber wastewater, which accounted for 64.3%, 63.6%and 75.51% in the microbial flora of potassium ferricyanide, oxygen and potassium persulfate MFCs, respectively, and included Lentimicrobium, Synergistaceae, Sphaerochaeta, Anaerolineaceae, Draconibacteriacea. MFCs with different cathode electron acceptors had similar core bacteria of anode microflora, but had different abundance. The electron acceptor (potassium persulfate) with large potential difference caused higher diversity and richness of microbial community, and had better performance on electricity generation and pollutant removal than other electron acceptors. -

-
表 1 阳极生物膜的微生物多样性
Table 1. Microbial diversity of anode biofilm
电子受体类别 多样性指标 丰富度指标 覆盖率 Shannon指数 Simpson指数 OTU/个 Ace指数 Chao指数 铁氰化钾 3.75 0.072 0 355 396 406 0.988 过硫酸钾 4.53 0.003 1 483 525 535 0.998 氧气 4.41 0.005 4 471 484 483 0.990 -
[1] ZHAO Y, PARK H D, PARK J H, et al. Effect of different salinity adaptation on the performance and microbial community in a sequencing batch reactor[J]. Bioresource Technology, 2016, 216: 808-816. doi: 10.1016/j.biortech.2016.06.032 [2] CHAI H X, KANG W. Influence of biofilm density on anaerobic sequencing batch biofilm reactor treating mustard tuber wastewater[J]. Applied Biochemistry and Biotechnology, 2012, 168(6): 1664-1671. doi: 10.1007/s12010-012-9887-1 [3] 吴义诚, 王泽杰, 刘利丹, 等. 利用光微生物燃料电池实现养猪废水资源化利用研究[J]. 环境科学学报, 2015, 35(2): 456-460. [4] 刘斌, 尚均顶, 王许云. 微生物燃料电池构型研究进展[J]. 当代化工, 2018, 47(10): 2173-2177. doi: 10.3969/j.issn.1671-0460.2018.10.047 [5] 周亚, 彭新红, 阮国岭, 等. 微生物燃料电池阳极材料修饰研究进展[J]. 水处理技术, 2017, 43(3): 9-13. [6] 冯雅丽, 于莲, 李浩然, 等. 微生物燃料电池降解焦化废水过程研究[J]. 中国环境科学, 2018, 38(11): 4099-4105. doi: 10.3969/j.issn.1000-6923.2018.11.014 [7] 谢淼, 徐龙君, 程李钰. 处理过的老龄垃圾渗滤液为阴极液的微生物燃料电池性能研究[J]. 太阳能学报, 2018, 39(9): 2641-2647. [8] 程鹏, 袁浩然, 邓丽芳, 等. 基于广州市政垃圾渗滤液的MFC性能及阳极微生物分析[J]. 新能源进展, 2018, 6(5): 371-378. doi: 10.3969/j.issn.2095-560X.2018.05.006 [9] 倪红军, 卓露, 吕帅帅, 等. 运行因素对猪场废水微生物燃料电池产电性能的影响[J]. 现代化工, 2018, 38(11): 136-139. [10] 蒋沁芮, 李泽华, 杨暖, 等. 三维电极微生物燃料电池处理生活污水同步产电性能[J]. 应用与环境生物学报, 2018, 24(4): 873-878. [11] 付国楷, 吴越, 张林防, 等. 微生物燃料电池在高盐榨菜废水处理中的产电性能[J]. 环境工程学报, 2017, 11(4): 348-352. [12] GUO F, FU G K, ZHANG Z, et al. Mustard tuber wastewater treatment and simultaneous electricity generation using microbial fuel cells[J]. Bioresource Technology, 2013, 136: 425-430. doi: 10.1016/j.biortech.2013.02.116 [13] 付国楷, 张林防, 郭飞, 等. 榨菜废水MFC多周期运行产电性能及COD降解[J]. 中国环境科学, 2017, 37(4): 1401-1407. doi: 10.3969/j.issn.1000-6923.2017.04.026 [14] ZHANG L F, FU G K, ZHANG Z. Electricity generation and microbial community in long-running microbial fuel cell for high-salinity mustard tuber wastewater treatment[J]. Bioresource Electrochemistry, 2019, 126: 20-28. [15] ZHANG L F, FU G K, ZHANG Z. Simultaneous nutrient and carbon removal and electricity generation in self-buffered biocathode microbial fuel cell for high-salinity mustard tuber wastewater treatment[J]. Bioresource Technology, 2019, 272: 105-113. doi: 10.1016/j.biortech.2018.10.012 [16] 刘远峰, 孙伟, 宫磊. 电子受体对微生物燃料电池产电性能的影响[J]. 环境污染与防治, 2016, 38(11): 84-89. [17] JADHAVA D A, GHADGE A N, DEBIKA M, et al. Comparison of oxygen and hypochlorite as cathodic electron acceptor in microbial fuel cells[J]. Bioresource Technology, 2014, 154: 330-335. doi: 10.1016/j.biortech.2013.12.069 [18] GHADGE A N, JADHAV D A, PRADHAN H, et al. Enhancing waste activated sludge digestion and power production using hypochlorite as catholyte in clayware microbial fuel cell[J]. Bioresource Technology, 2015, 182: 225-231. doi: 10.1016/j.biortech.2015.02.004 [19] JUN L, QIAN F, QIANG L, et al. Persulfate a self-activated cathodic electron acceptor for microbial fuel cells[J]. Journal of Power Sources, 2009, 194: 269-274. doi: 10.1016/j.jpowsour.2009.04.055 [20] MIYAHARA M, KOUZUMA A, WATANABE K. Effects of NaCl concentration on anode microbes in microbial fuel cells[J]. AMB Express, 2015, 5(1): 1-9. doi: 10.1186/s13568-014-0092-1 [21] 吴越. 微生物燃料电池处理榨菜废水及甜菜碱影响研究[D]. 重庆: 重庆大学, 2016. [22] 杨瑞丽, 王晓君, 吴俊斌, 等. 厌氧氨氧化工艺快速启动策略及其微生物特性[J]. 环境工程学报, 2018, 12(12): 3341-3350. [23] DING A, ZHAN D, DING F, et al. Effect of inocula on performance of bio-cathode denitrification and its microbial mechanism[J]. Chemical Engineering, 2018, 343: 399-407. doi: 10.1016/j.cej.2018.02.119 [24] WU Y, HAN R, YANG X, et al. Correlating microbial community with physicochemical indices and structures of a full-scale integrated constructed wetland system[J]. Applied Microbiology & Biotechnology, 2016, 100(15): 6917-6926. [25] 陆玉, 钟慧, 丑三涛, 等. 乙酸驯化对厌氧污泥微生物群落结构及发酵特性的影响[J]. 环境科学学报, 2018, 38(5): 1835-1842. [26] RÓZSENBERSZKI T, KOÓK L, HUTVÁGNER D, et al. Comparison of anaerobic degradation processes for bioenergy generation from liquid fraction of pressed solid waste[J]. Waste and Biomass Valorization, 2015, 6(4): 465-473. doi: 10.1007/s12649-015-9379-y [27] DHIMAN S S, SHRESTHA N, DAVID A, et al. Producing methane, methanol and electricity from organic waste of fermentation reaction using novel microbes[J]. Bioresource Technology, 2018, 258: 270-278. doi: 10.1016/j.biortech.2018.02.128 [28] ZHEN G, KOBAYASHI T, LU X, et al. Biomethane recovery from egeria densa in a microbial electrolysis cell-assisted anaerobic system: Performance and stability assessment[J]. Chemosphere, 2016, 149: 121-129. doi: 10.1016/j.chemosphere.2016.01.101 [29] RÓZSENBERSZKI T, KOÓK L, BAKONYI P, et al. Municipal waste liquor treatment via bioelectrochemical and fermentation (H2+CH4) processes: Assessment of various technological sequences[J]. Chemosphere, 2017, 171: 692-701. doi: 10.1016/j.chemosphere.2016.12.114 [30] ROSENBAUM M A, BAR HY, BEG Q K, et al. Transcriptional analysis of shewanella oneidensis Mr-1 with an electrode compared to Fe (Ⅲ) citrate or oxygen as terminal electron acceptor[J]. Plos One, 2012, 7(2): 1-13. [31] PENG L, YOU S, WANG J. Electrode potential regulates cytochrome accumulation on Shewanella oneidensis cell surface and the consequence to bioelectrocatalytic current generation[J]. Biosensors and Bioelectronics, 2010, 25(11): 2530-2533. doi: 10.1016/j.bios.2010.03.039 [32] BUSALMEN J P, ESTEVE N A, FELIU J M. Whole cell electrochemistry of electricity-producing microorganisms evidence an adaptation for optimal exocellular electron transport[J]. Environmental Science & Technology, 2008, 42(7): 2445-2450. [33] TERAVEST M A, ANGENENT L T. Oxidizing electrode potentials decrease current production and coulombic efficiency through cytochrome c inactivation in Shewanella oneidensis Mr-1[J]. Chemelectrochem, 2014, 1(11): 2000-2006. doi: 10.1002/celc.v1.11 [34] GROBBLER C, VIRDIS B, NOUWENS A, et al. Effect of the anode potential on the physiology and proteome of Shewanella oneidensis Mr-1[J]. Bioelectrochemistry, 2018, 201: 172-179. [35] CARMONA M, HARNISCH F, KUHLICKE U, et al. Electron transfer and biofilm formation of Shewanella putrefaciens as function of anode potential[J]. Bioelectrochemistry, 2013, 93: 23-29. doi: 10.1016/j.bioelechem.2012.05.002 [36] SUN L, TOYONAGA M, OHASHI A, et al. Lentimicrobium saccharophilum gen. nov., sp. nov., a strictly anaerobic bacterium representing a new family in the phylum Bacteroidetes, and proposal of Lentimicrobiaceae fam. Nov[J]. International Journal of Systematic and Evolutionary Microbiology, 2016, 66: 2635-2642. doi: 10.1099/ijsem.0.001103 [37] RAGO L, ZECCHIN S, MARZORATI S, et al. A study of microbial communities on terracotta separator and on biocathode of air breathing microbial fuel cells[J]. Bioelectrochemistry, 2018, 120: 18-26. doi: 10.1016/j.bioelechem.2017.11.005 [38] 周蕾. 厌氧烃降解产甲烷菌系的组成及其代谢产物的特征[D]. 上海: 华东理工大学, 2012. [39] XIA Y, WANG Y, WANG Y, et al. Cellular adhesiveness and cellulolytic capacity in Anaerolineae revealed by omics-based genome interpretation[J]. Biotechnology for Biofuels, 2016, 9(1): 111. doi: 10.1186/s13068-016-0524-z [40] LIANG B, WANG L Y, MBADINGA S M, et al. Anaerolineaceae and Methanosaeta turne to be the dominant microorganisms in alkanes-dependent methanogenic culture after long-term of incubation[J]. AMB Express, 2015, 5: 37. doi: 10.1186/s13568-015-0117-4 [41] LIU Y, LAI Q, DU J, et al. Thioclava indica sp. nov., isolated from surface seawater of the Indian Ocean[J]. Antonie Van Leeuwenhoek, 2015, 107(1): 297-304. doi: 10.1007/s10482-014-0320-3 [42] DU Z J, WANG Y, DUNlAP C, et al. Draconibacterium orientale gen. nov., sp. nov., isolated from two distinct marine environments, and proposal of Draconibacteriaceae fam. nov[J]. International Journal of Systematic and Evolutionary Microbiology, 2014, 64: 1690-1696. doi: 10.1099/ijs.0.056812-0 [43] CHENG C, ZHOU Z, QIU Z, et al. Enhancement of sludge reduction by ultrasonic pretreatment and packing carriers in the anaerobic side-stream reactor: Performance, sludge characteristics and microbial community structure[J]. Bioresource Technology, 2018, 249: 298-306. doi: 10.1016/j.biortech.2017.10.043 [44] TROSHINA O, VIKTORIA O, NZTALIA S, et al. Sphaerochaeta associata sp. nov., a spherical spirochaete isolated from cultures of Methanosarcina mazei JL01[J]. International Journal of Systematic and Evolutionary Microbiology, 2015, 65: 4315-4322. doi: 10.1099/ijsem.0.000575 [45] LOGAN B E. Essential data and techniques for conducting microbial fuel cell and other types of bioelectrochemical system experiments[J]. Chemsuschem, 2012, 5(6): 988-994. doi: 10.1002/cssc.v5.6 [46] DAI X, HU C, ZHANG D, et al. Impact of a high ammonia-ammonium-pH system on methane-producing archaea and sulfate-reducing bacteria in mesophilic anaerobic digestion[J]. Bioresource Technology, 2017, 245: 598-605. doi: 10.1016/j.biortech.2017.08.208 -




 下载:
下载: