-
伴随着我国工业化进程的加快和社会经济的迅速发展,产生了大量含有难降解和具生物毒性的典型污染物的工业废水,其中包括:印染、造纸、海水利用等众多工业领域产生的浓度越来越高的含盐废水[1];制革工业产生的重金属污染种类排名第2的含铬废水[2];生产四环类抗生素的制药厂所产生的含四环素废水[3]。污水处理厂的设计本身不具备处理这类污染物的能力,此类工业废水进入城镇污水处理系统后,势必会对生物脱氮系统带来负面影响[4-5]。
目前,工业废水中一些典型污染物对反硝化过程的影响的相关研究已经取得了一定的进展。YU等[6]在研究苯酚胁迫对高硝酸盐厌氧反硝化的影响中提到,一定浓度的苯酚会减少硝酸盐的去除率,积累亚硝酸盐,增加微生物群落的多样性和丰富度;FENG等[7]研究了锌、铜、铅、镉等重金属对膜生物反应器硝化反硝化性能的影响,发现微生物在低浓度或高浓度重金属处理后反硝化率降低显著,可减小污泥颗粒粒径并增加胞外聚合物;GUI等[8-9]研究发现,盐度和重金属均会抑制好氧反硝化细菌去除硝酸盐氮,盐度和重金属会影响反硝化功能基因的表达。据报道,某些从天然环境如河床[10]、海域[11]、活性污泥[12]中筛选得到的好氧反硝化细菌不仅具有高效好氧反硝化能力,还具备良好的耐盐耐重金属或耐低温特性,反硝化过程基本不受污染物的影响。
好氧反硝化技术为实现同步硝化反硝化提供了良好的基础,部分好氧反硝化细菌甚至可以耐受污染物的生物毒性,在接纳工业废水的城镇污水厂的生物脱氮系统中具有极大的应用潜力。但目前污染物抑制好氧反硝化作用的研究多集中于反硝化率和相关基因表达的影响方面,对污染物长期胁迫下细胞胞外多聚物,尤其是微生物群落结构的变化等方面的研究相对较少。本研究以活性污泥作为接种源,富集筛选好氧反硝化菌群,持续投加氯化钠、六价铬、盐酸四环素(tetracycline hydrochloride,TC),对富集菌群进行驯化,最后比较了驯化前后菌群微生物的反硝化速率、EPS含量、群落多样性以及napA基因丰度,以揭示典型工业污染物对好氧反硝化菌群的影响。
-
本研究所用样品取自南昌市某城市污水处理厂二沉池的活性污泥。
富集培养基/富集测试培养基:无水乙酸钠3 g·L−1,NH4Cl 0.306 g·L−1,KNO3 1 g·L−1,KH2PO4 0.15 g·L−1,K2HPO4 0.252 g·L−1,MgSO4·7H2O 0.1 g·L−1,FeSO4·7H2O 0.006 g·L−1,CaCl2 0.01 g·L−1。去离子水溶解,pH 7.0~7.3。
驯化测试培养基:无水乙酸钠6 g·L−1,NH4Cl 0.612 g·L−1,KNO3 2 g·L−1,KH2PO4 0.15 g·L−1,K2HPO4 0.252 g·L−1,MgSO4·7H2O 0.1 g·L−1,FeSO4·7H2O 0.006 g·L−1,CaCl2 0.01 g·L−1。去离子水溶解,pH 7.0~7.3。
-
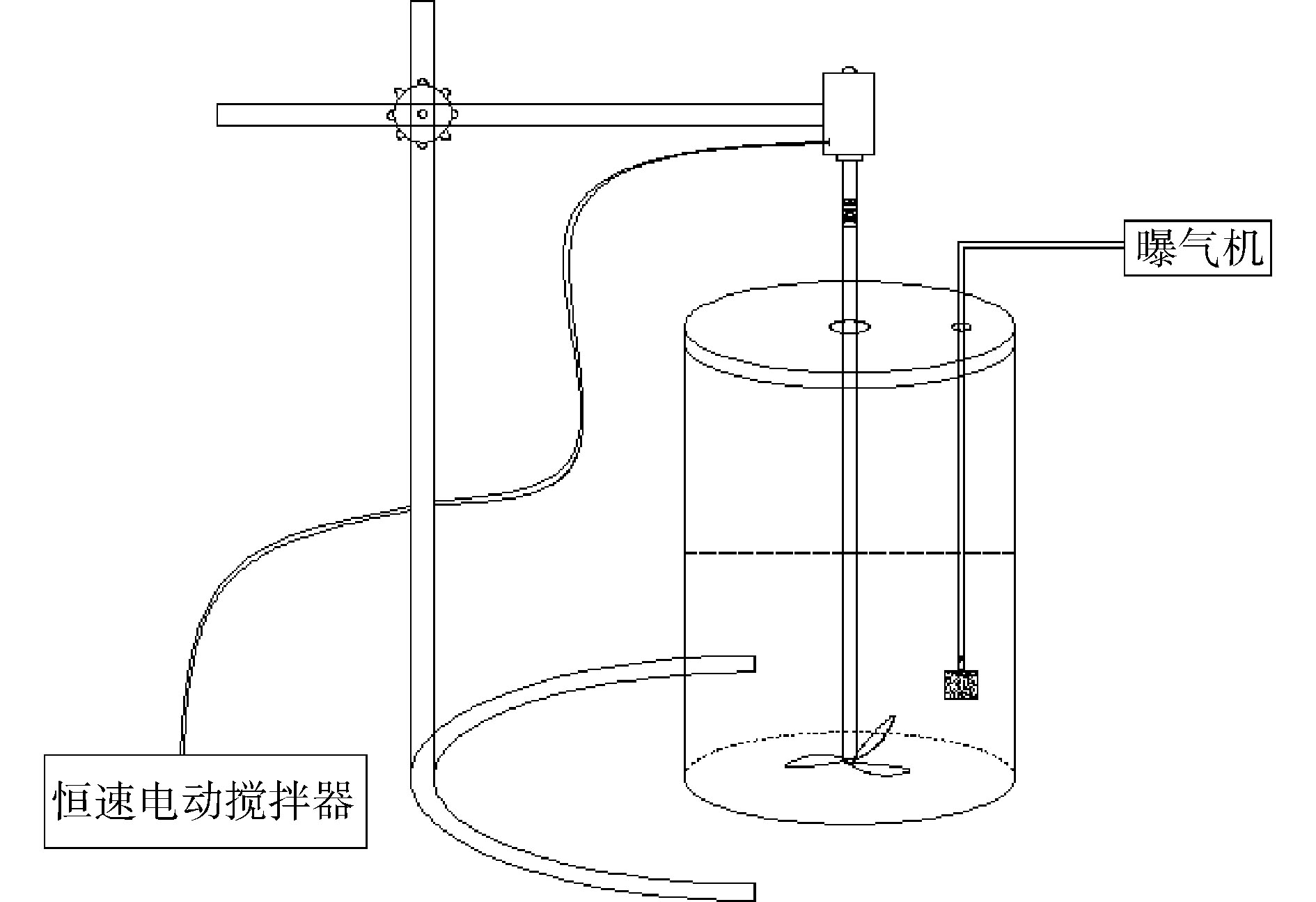
富集过程在自制的SBR中进行,反应器内部容积为3 L,反应器中部接有恒速电动搅拌器,内部接有可调式空气泵的气石。驯化过程和反硝化测试实验在250 mL的蓝盖瓶中进行,瓶盖不密封,用透气膜封口。反应装置如图1所示。
-
取200 g污泥于SBR中,倒入2 L富集培养基,设置60 r·min−1的电动搅拌,打开气泵开始运行。运行周期约为24 h,好氧反应时间22~23 h,每天监测三氮(氨氮、硝氮、亚硝氮)浓度,至出水检测不到硝氮且无亚硝氮积累后,以富集测试培养基对富集前后的活性污泥进行反硝化性能测试。
反硝化测试方法:取20 mL的污泥或菌群至蓝盖瓶中,各加入200 mL测试培养基,混匀后测定MLVSS,将蓝盖瓶放置在30 ℃、150 r·min−1的恒温振荡箱中,来回振荡24 h,定期取样测定三氮浓度。
-
以富集得到的好氧反硝化菌群为样品,分别取20 mL混匀后的样品于4个蓝盖瓶中,各加入200 mL富集培养基。向其中3个蓝盖瓶分别加入氯化钠、重铬酸钾和盐酸四环素,置于恒温振荡箱中以30 ℃、150 r·min−1的条件振荡,周期与富集过程相同。根据硝氮去除效果,梯度浓度提高硝酸盐氮和抑制物(氯化钠、重铬酸钾和盐酸四环素)的浓度负荷共分为4个阶段。其中,A阶段中NaCl、Cr(Ⅵ)和TC浓度分别为10 g·L−1、1 mg·L−1和5 mg·L−1;B阶段为30 g·L−1、2 mg·L−1和10 mg·L−1;C阶段为50 g·L−1、5 mg·L−1和25 mg·L−1;D阶段为70 g·L−1、10 mg·L−1和50 mg·L−1。
驯化结束后,以驯化测试培养基对驯化前后的菌群进行反硝化性能测试,测试方法同1.3节。不同的是,投加NaCl、K2Cr2O4和TC驯化后的菌群中测试培养基分别含70 g·L−1的NaCl、10 mg·L−1的Cr(Ⅵ)和50 mg·L−1的TC,并以驯化前的菌群作为对照,同时进行反硝化测试。
-
对接种源、富集过程和驯化过程结束后取出的菌群样品进行EPS的提取和测定。提取采用超声波提取法[13]。取4 mL的菌群样品,在5 500 r·min−1下离心20 min,倾去上清液,补充0.9%生理盐水至原体积,振荡均匀,重复2次。将清洗后的污泥样品,冰浴下40 W超声2 min。再将样品在8 000 r·min−1下离心30 min,上清液经0.22 μm纤维素滤膜过滤得到EPS样品。蛋白质(PN)采用考马斯亮蓝法[14]测定;多糖(PS)采用苯酚-硫酸法[15]测定。
-
取1 mL污泥或菌群样品,采用细菌DNA提取试剂盒(申能博彩,上海)提取细菌的基因组DNA,最后用100 μL TAE洗脱。琼脂糖凝胶电泳检测合格后,采用高通量测序技术对提取的DNA样品进行微生物群落结构及多样性分析。
-
对提取的DNA样品进行q-PCR分析,目的基因为napA,是在有氧条件下转化硝酸盐所必需的周质硝酸盐还原酶Nap的编码基因[16]。napA基因丰度的qPCR采用Stepone plus型荧光定量PCR仪(ABI)进行分析,使用的引物[17]和扩增条件见表1。反应体系20 μL,包括2.0 μL的DNA模板、10 μL的SybrGreen qPCR Master Mix(2X)、0.4 μL的上游引物和下游引物(10 μmol·L−1)和7.2 μL的双蒸水。
-
硝酸盐氮:紫外分光光度法[18];氨氮:纳氏试剂光度法[19];亚硝酸盐氮:N-(1-萘)-乙二胺光度法[20];六价铬:二苯碳酰二肼分光光度法[21];pH、DO:玻璃电极法。MLVSS:重量法。
-
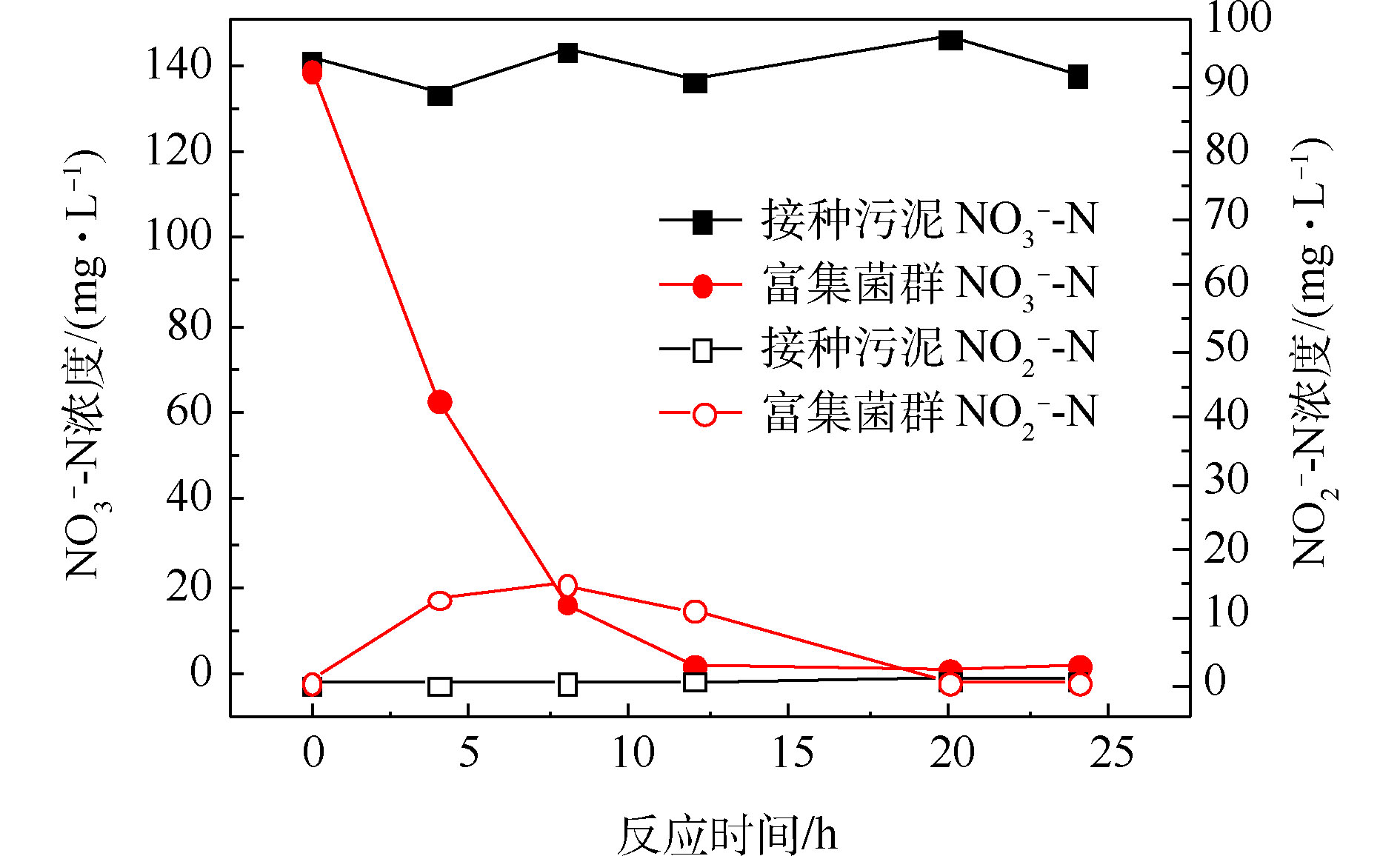
经过一段时间的间歇曝气,反应器出水基本检测不到硝酸盐氮,且能在7 d内保持稳定,视为富集得到好氧反硝化菌群,此时菌群中参与反硝化作用的主要是好氧反硝化细菌。由图2可知,好氧条件下,接种污泥基本无法去除硝酸盐氮,而富集得到的菌群在12 h内能将140 mg·L−1左右的硝酸盐氮完全去除,硝酸盐氮的比去除速率达到11.67 mg·(L·h)−1。在去除硝酸盐氮的过程中,存在亚硝酸盐氮的积累,在8 h时达到峰值15.16 mg·L−1,但随后逐渐被降解。
-
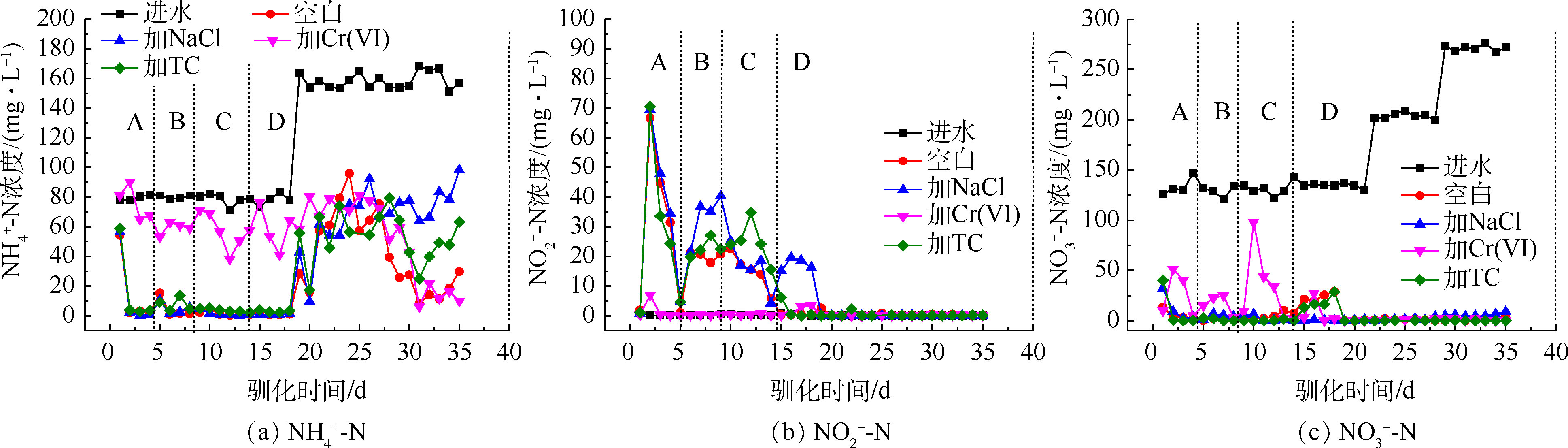
1)驯化过程中的三氮变化。从图3(a)和图3 (b)可以看出,NaCl和TC的胁迫基本不影响氨氮的去除和亚硝酸盐氮的积累量,Cr(Ⅵ)的胁迫显著抑制了氨氮的去除,氨氮去除率仅有为对照组的25%~50%,并减少了亚硝酸盐氮的积累,这可能是因为Cr(Ⅵ)影响了菌群中硝化细菌的活性。汪敏刚等[22]在研究低浓度铬对SBR中微生物作用的影响中提到,Cr(Ⅵ)的毒性作用使菌群中的硝化细菌无法维持正常的生长和工作,减少了氨氮的去除效果,影响程度与铬浓度具有相关性。
从图3(c)可以看出,梯度投加NaCl和TC对硝酸盐氮的去除没有产生抑制作用;而梯度投加Cr(Ⅵ)的过程中减少了硝酸盐氮的去除,当Cr(Ⅵ)浓度提升到5 mg·L−1的时候,硝酸盐氮的去除被抑制得最明显,对硝酸盐氮的去除率降为32%,但在持续投加Cr(Ⅵ)后,抑制作用在5 d内逐渐降低,直至消失。HE等[11]研究发现,铜绿假单胞菌PCN-2在有氧条件可同时去除硝酸盐氮和Cr(Ⅵ),且两者能同时作为电子受体而相互竞争。菌群中可能存在兼具铬还原和反硝化作用的好氧反硝化细菌,这类细菌在驯化过程中逐渐成为优势菌种,削弱了Cr(Ⅵ)对反硝化过程的抑制作用。
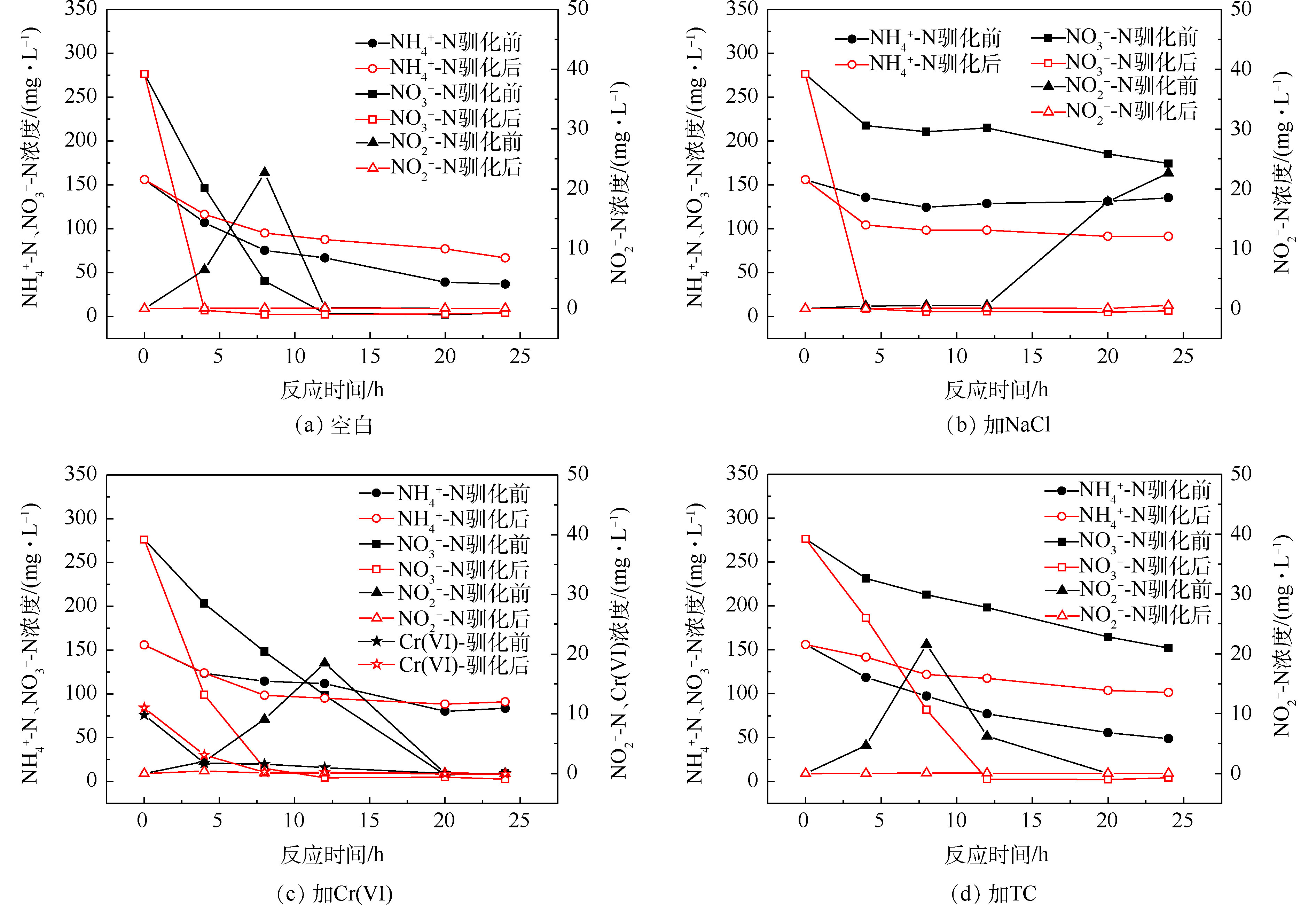
2)污染物对驯化前后菌群脱氮性能的影响。驯化前后反硝化测试结果见图4和表2。结果表明,NaCl、Cr(Ⅵ)和TC均会影响驯化前的菌群去除硝酸盐氮的效果,反硝化速率仅为空白组的19%、62%和23%,可见NaCl和TC对好氧反硝化过程的抑制效果更为突出,高盐和含高浓度四环素的工业废水对好氧反硝化细菌的生物脱氮有很大负面影响;驯化后的菌群在NaCl、Cr(Ⅵ)和TC的胁迫下,24 h内可快速去除280 mg·L−1的
NO−3 -N,反硝化速率分别从0.44、1.43和0.52 mg·(g·h)−1增加到7.95、4.53和6.49 mg·(g·h)−1,表明污染物的长期胁迫提高了菌群中好氧反硝化细菌抵抗生物毒性的能力。另外,测试过程中对加铬的菌群进行了Cr(Ⅵ)的检测,发现10 mg·L−1的Cr(Ⅵ)在8 h内快速去除,这证实了菌群中很可能存在具有还原六价铬能力的好氧反硝化细菌。 -
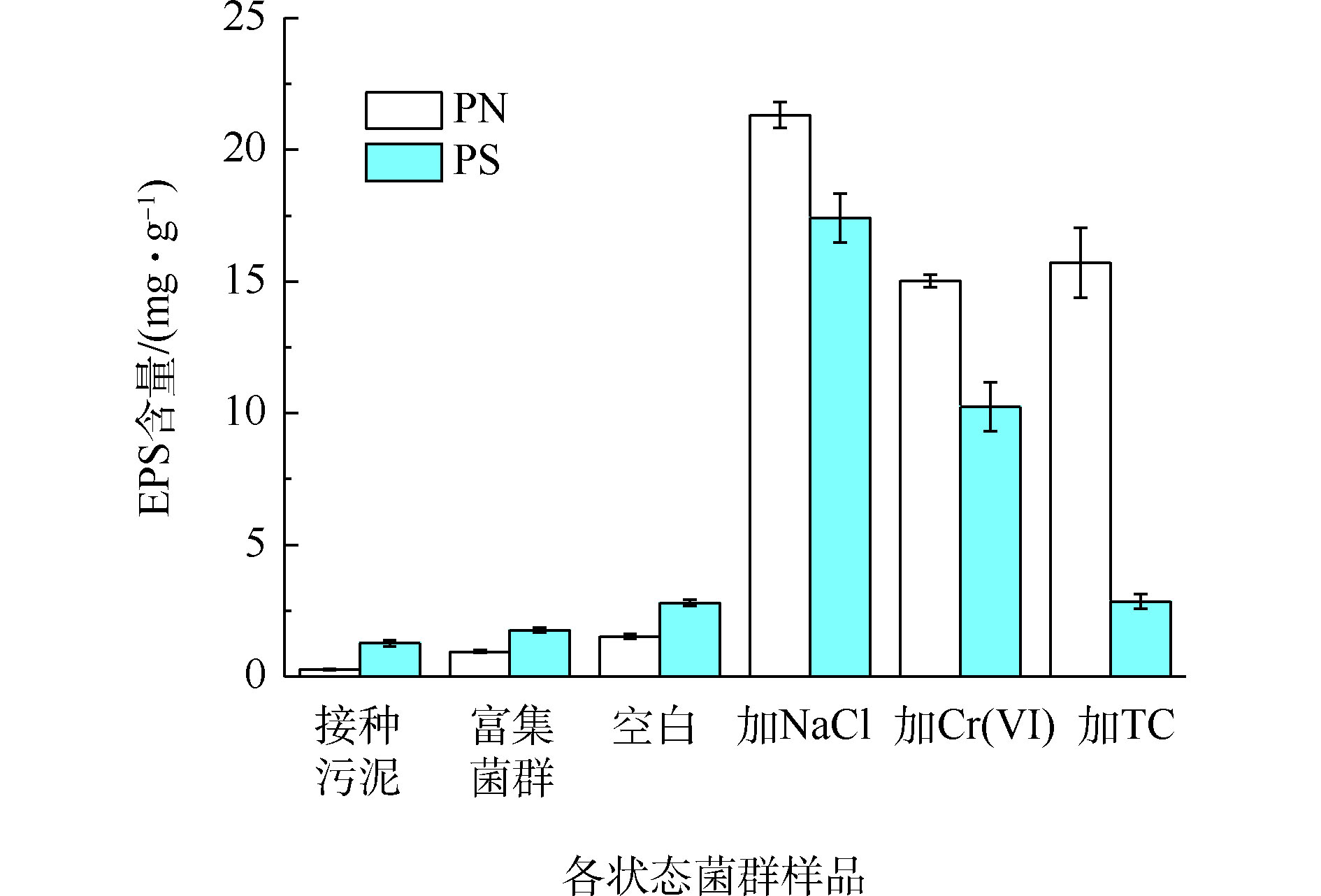
EPS作为细菌与环境介质间的缓冲层,有助于降低环境因素对菌体的损害[23],污染物的胁迫可能影响菌群EPS的产量和组分构成。从图5可以看出,污染物的长期胁迫大幅提高了菌群EPS的产量,相比之下,培养基中的氮素和其他无机盐对菌群EPS产生量影响甚微。
未加污染物的菌群的PN和PS含量较低,随着氮负荷的提升呈现缓慢增加的趋势。这可能是因为在颗粒污泥解体为絮状污泥的过程中,由于有机负荷低使微生物内源代谢水平升高,造成细胞的自溶,从而导致EPS含量的增加[24]。与空白组相比,NaCl、Cr(Ⅵ)和TC胁迫下的菌群PN含量增长8.7~12.8倍,NaCl、Cr(Ⅵ)胁迫下的菌群PS含量增长2.6~5.2倍,PS含量未受TC影响。盐度的增加会导致细胞内外离子浓度差异增大,迫使细胞分泌出大量的蛋白酶,增强扩散和主动运输的能力,同时产生多糖分子来限制细胞中水分的流动以适应渗透压的变化[25]。许睿骁等[26]发现,逐渐提高盐度至3.5%,污泥EPS含量较稳定,当盐度提高到5%,EPS含量增长明显。为了降低重金属毒性,细胞可以分泌出更多的蛋白物质和糖类物质,通过这些物质的官能团来络合重金属离子[27],因此,四环素的胁迫致使系统产生过量的EPS与水中的四环素结合,这主要是EPS中产生的一些蛋白类物质的参与导致的[28]。
-
以16S rRNA基因序列97%相似度水平归类OUT统计菌群群落的α-多样性结果见表3。从表3可知,原污泥群落多样性最丰富,好氧反硝化细菌的富集显著减少了菌群群落多样性,加Cr(Ⅵ)和TC的菌群多样性低于相应空白组。这说明Cr(Ⅵ)和TC的胁迫进一步造成了群落多样性的降低,相反,NaCl的胁迫提高了菌群的群落多样性。由此可知,菌群中对铬污染和四环素污染耐受能力强的好氧反硝化细菌较少,耐高盐的好氧反硝化细菌较多;部分嗜盐菌在高盐条件下甚至繁殖更快[29],如果该菌群应用到高盐含氮废水的治理中,菌群多样性的增加可能使生物系统的稳定性更高。
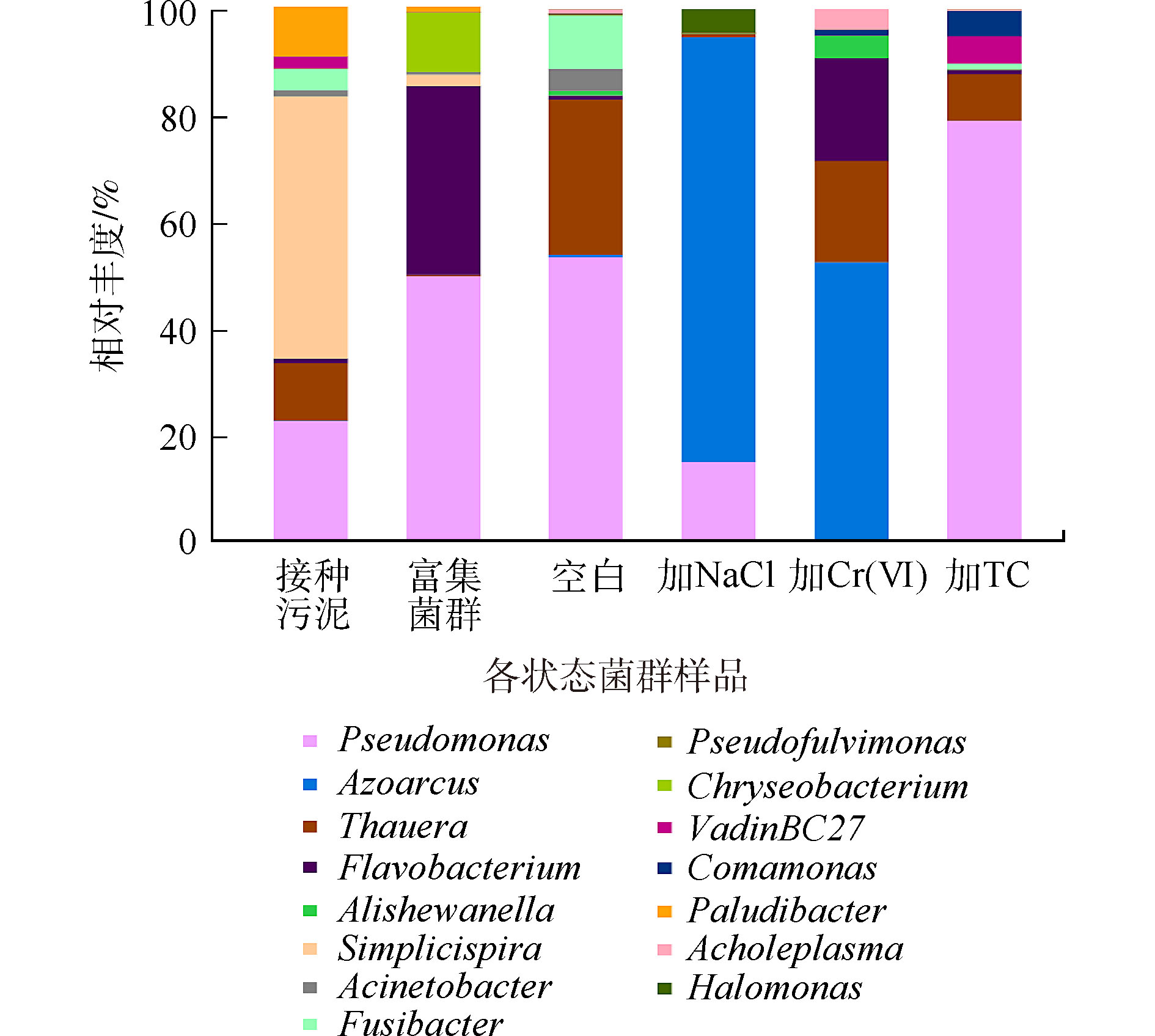
图6是各菌群群落结构在属水平上的相对丰度。接种污泥中优势菌种主要为专性厌氧的简单螺旋菌属(Simplicispira),富集后,假单胞菌属(Pseudomonas)成为优势菌种,这可能是导致富集前后菌群好氧反硝化能力差异的主要原因。空白组的优势菌种为假单胞菌属(Pseudomonas)和陶厄氏菌属(Thauera),相对丰度为55%和28%,陶厄氏菌(Thauera)具有较高的反硝化活性[30],高浓度的硝酸盐氮环境可能促进了陶厄氏菌的繁殖。加NaCl驯化后的菌群优势菌种为固氮弧菌属(Azoarcus)和假单胞菌属(Pseudomonas),相对丰度为79%和15%,其中还有约4%的盐单胞菌属(Halomonas),它们都具有一定的耐盐特性[31],其中固氮弧菌属(Azoarcus)有高效生物脱氮的功能[32],是驯化菌群在高盐环境实现高效反硝化的主要功能菌。加铬驯化后优势菌种主要为固氮弧菌属(Azoarcus)和陶厄氏菌属(Thauera),相对丰度分别为52%和18%。固氮弧菌(Azoarcus)能够在苯酚、甲苯等有机碳源下还原Cr(Ⅵ)[33],驯化菌群能够同时还原硝酸盐氮和Cr(Ⅵ),很可能是固氮弧菌(Azoarcus)丰度增加造成的结果;陶厄氏菌(Thauera)在低浓度Cr(Ⅵ)下活性不高,但在高浓度Cr(Ⅵ)环境的反硝化过程中发挥主要作用[34]。加TC驯化后的菌群的优势菌属与空白组相同,假单胞菌属(Pseudomonas)相对丰度增长至79%。这与ZOU等[35]的研究结果一致,在四环素的胁迫下,假单胞菌属(Pseudomonas)为反硝化过程的主要功能菌。TC对菌群脱氮性能的抑制可能与反硝化活性高的陶厄氏菌丰度下降有关。
-
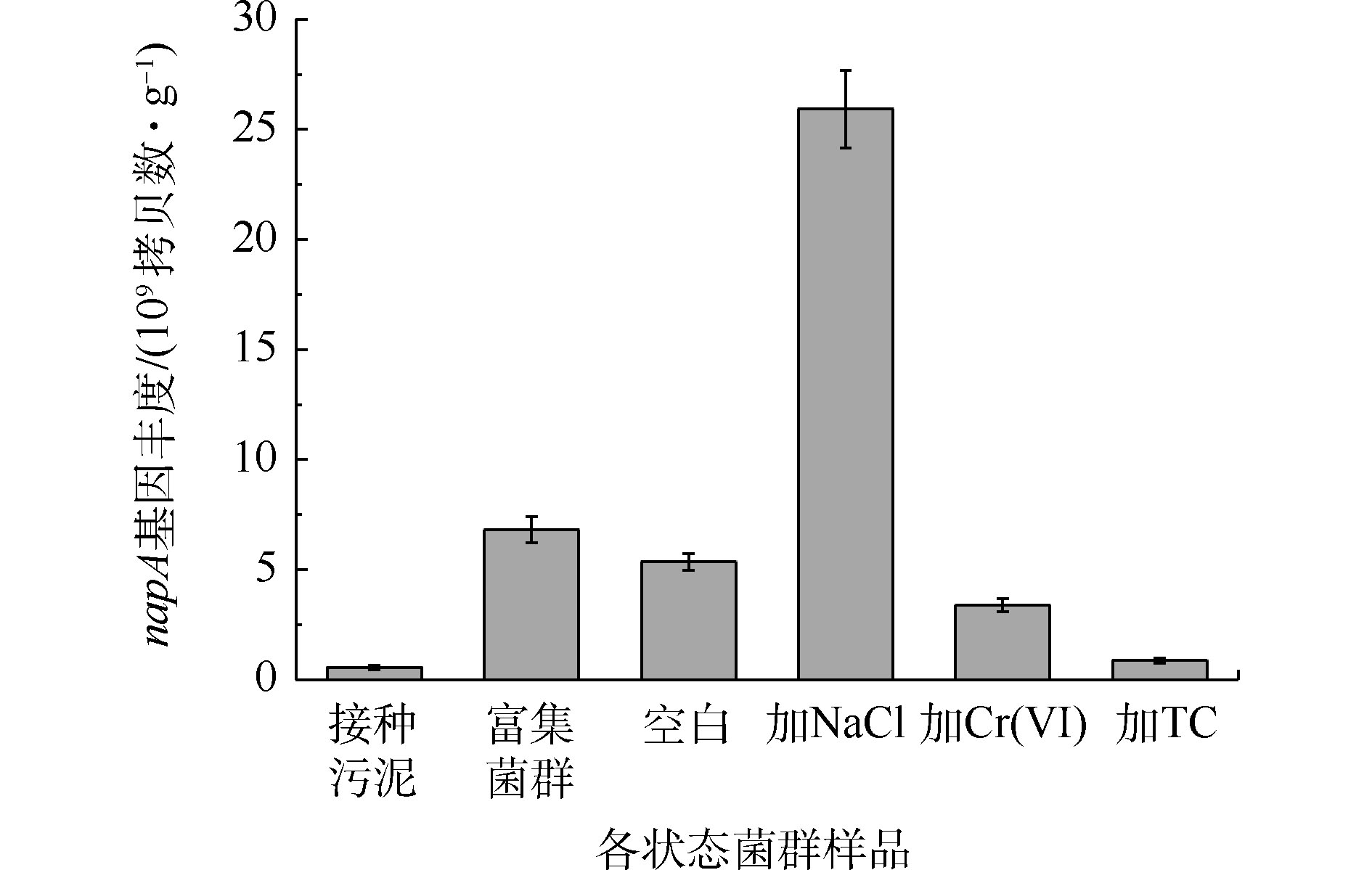
由图7可知,富集过程使菌群napA基因丰度从5.54×108拷贝数·g−1提高到6.82×109 拷贝数·g−1,提高了1个数量级。Cr(Ⅵ)和TC的长期胁迫减少了菌群napA基因丰度,而NaCl的胁迫增加了菌群napA基因丰度,达到 2.59×1010 拷贝数·g−1。结果表明,Cr(Ⅵ)和TC会导致菌群中具有好氧反硝化能力的细菌数量的减少,这与Cr(Ⅵ)和TC能够抑制好氧反硝化性能的结果一致。其中,TC对napA基因丰度的影响更大,其原因可能是和四环素相关的耐药基因与napA基因相互作用所致[36]。然而,高盐环境虽然促进了嗜盐好氧反硝化细菌的繁殖,但有氧条件下的硝酸盐氮的去除效果仍低于空白组,其原因可能是细菌在NaCl的胁迫下改变了Na+/H+反向转运蛋白的活性,从而提高了napA基因的丰度,但这些基因未能正常表达[37-38]。
-
1) NaCl、Cr(Ⅵ)和TC均能抑制好氧反硝化菌群的反硝化性能,梯度浓度投加NaCl、Cr(Ⅵ)和TC可以提高好氧反硝化菌群对这些抑制物的耐受能力,并实现在较广浓度范围的NaCl、Cr(Ⅵ)和TC废水中快速去除硝酸盐氮的目标。
2)好氧反硝化菌群在污染物环境中会提高胞外蛋白和胞外多糖的产量,其目的可能是降解或者隔离污染物,减少污染物对细菌细胞的毒害作用。对于胞外多聚物如何保护细胞抵御生物毒性作用是后续工作需要完善的内容。
3)典型污染物会显著影响好氧反硝化菌群群落多样性和种群结构。Cr(Ⅵ)和TC的长期胁迫会降低好氧反硝化菌群的群落多样性并减少napA基因丰度,NaCl胁迫的结果与其相反;NaCl和Cr(Ⅵ)的胁迫使固氮弧菌属(Azoarcus)替代假单胞菌属(Pseudomonas)和陶厄氏菌属(Thauera)成为好氧反硝化菌群中的优势菌种,TC不影响假单胞菌属(Pseudomonas)和陶厄氏菌属(Thauera)的优势地位,但会改变两者在群落中的相对丰度。
典型工业污染物对好氧反硝化菌群脱氮性能及群落结构的影响
Effects of typical industrial pollutants on the denitrification performance and the community structure of aerobic denitrifying bacteria
-
摘要: 针对工业废水中的典型污染物影响城镇污水生物脱氮系统正常运行的问题,以富集筛选到的好氧反硝化菌群为研究对象,深入研究了工业废水中的典型污染物(包括NaCl、Cr(Ⅵ)和TC等)对好氧反硝化菌群的影响机理。结果表明:NaCl、Cr(Ⅵ)和TC均会抑制好氧反硝化菌群的脱氮性能,提高胞外多聚物(EPS)的含量;与此同时,Cr(Ⅵ)和TC的胁迫导致微生物群落多样性减少,降低了napA基因的丰度,然而,NaCl的胁迫呈现相反的变化趋势;NaCl和Cr(Ⅵ)使得菌群中优势菌属由Pseudomonas向Azoarcus转变,反之,TC能够显著增加Pseudomonas的相对丰度,降低Thauera的相对丰度。工业废水中的典型污染物对好氧反硝化菌群的脱氮性能具有较显著的抑制作用,长期胁迫下菌群通过产生胞外多聚物和改变菌群结构来抵御污染物。研究结果对于接纳工业废水的城镇污水处理厂应用好氧反硝化技术进行生物脱氮具有指导和借鉴意义。
-
关键词:
- 工业污染物 /
- 好氧反硝化 /
- 脱氮性能 /
- 群落结构 /
- 胞外多聚物(EPS)
Abstract: The normal operation of biological nitrogen removal systems for urban sewage is affected by typical contaminants in industrial wastewater, while the biological nitrogen removal based on pollutant-tolerated aerobic denitrifying bacteria provides a solution to this toxic wastewater treatment. In this study, the effects of typical contaminants in industrial wastewater, i.e., sodium chloride, hexavalent chromium, and tetracycline hydrochloride on the denitrifying performance of the enriched aerobic denitrifying bacteria were investigated. The results showed that these contaminants apparently inhibited the denitrifying performance of aerobic denitrifying bacteria, and enhanced the EPS production. Meanwhile, the long-term stress from hexavalent chromium and tetracycline hydrochloride reduced the diversity of the microbial community and the abundance of napA gene, while the long-term stress from sodium chloride resulted in an opposite trends. The stress of sodium chloride and hexavalent chromium led to the transformation of the dominant genus from Pseudomonas to Azoarcus, but the stress of tetracycline hydrochloride could significantly improve the relative abundance of Pseudomonas, and reduce that of Thauera. Although the initial significant inhibitory effect by typical industrial contaminants on the performance of aerobic denitrifying bacteria occurred, these bacteria could produce extracellular polymers and change their microbial community structure to relieve this inhibitory effect under long-term stress conditions. The study can provide a reference for the practical application of aerobic denitrification technology for bio-denitrification in urban sewage treatment plants with admission of industrial wastewater. -
伴随着我国工业化进程的加快和社会经济的迅速发展,产生了大量含有难降解和具生物毒性的典型污染物的工业废水,其中包括:印染、造纸、海水利用等众多工业领域产生的浓度越来越高的含盐废水[1];制革工业产生的重金属污染种类排名第2的含铬废水[2];生产四环类抗生素的制药厂所产生的含四环素废水[3]。污水处理厂的设计本身不具备处理这类污染物的能力,此类工业废水进入城镇污水处理系统后,势必会对生物脱氮系统带来负面影响[4-5]。
目前,工业废水中一些典型污染物对反硝化过程的影响的相关研究已经取得了一定的进展。YU等[6]在研究苯酚胁迫对高硝酸盐厌氧反硝化的影响中提到,一定浓度的苯酚会减少硝酸盐的去除率,积累亚硝酸盐,增加微生物群落的多样性和丰富度;FENG等[7]研究了锌、铜、铅、镉等重金属对膜生物反应器硝化反硝化性能的影响,发现微生物在低浓度或高浓度重金属处理后反硝化率降低显著,可减小污泥颗粒粒径并增加胞外聚合物;GUI等[8-9]研究发现,盐度和重金属均会抑制好氧反硝化细菌去除硝酸盐氮,盐度和重金属会影响反硝化功能基因的表达。据报道,某些从天然环境如河床[10]、海域[11]、活性污泥[12]中筛选得到的好氧反硝化细菌不仅具有高效好氧反硝化能力,还具备良好的耐盐耐重金属或耐低温特性,反硝化过程基本不受污染物的影响。
好氧反硝化技术为实现同步硝化反硝化提供了良好的基础,部分好氧反硝化细菌甚至可以耐受污染物的生物毒性,在接纳工业废水的城镇污水厂的生物脱氮系统中具有极大的应用潜力。但目前污染物抑制好氧反硝化作用的研究多集中于反硝化率和相关基因表达的影响方面,对污染物长期胁迫下细胞胞外多聚物,尤其是微生物群落结构的变化等方面的研究相对较少。本研究以活性污泥作为接种源,富集筛选好氧反硝化菌群,持续投加氯化钠、六价铬、盐酸四环素(tetracycline hydrochloride,TC),对富集菌群进行驯化,最后比较了驯化前后菌群微生物的反硝化速率、EPS含量、群落多样性以及napA基因丰度,以揭示典型工业污染物对好氧反硝化菌群的影响。
1. 材料与方法
1.1 菌群来源和培养基
本研究所用样品取自南昌市某城市污水处理厂二沉池的活性污泥。
富集培养基/富集测试培养基:无水乙酸钠3 g·L−1,NH4Cl 0.306 g·L−1,KNO3 1 g·L−1,KH2PO4 0.15 g·L−1,K2HPO4 0.252 g·L−1,MgSO4·7H2O 0.1 g·L−1,FeSO4·7H2O 0.006 g·L−1,CaCl2 0.01 g·L−1。去离子水溶解,pH 7.0~7.3。
驯化测试培养基:无水乙酸钠6 g·L−1,NH4Cl 0.612 g·L−1,KNO3 2 g·L−1,KH2PO4 0.15 g·L−1,K2HPO4 0.252 g·L−1,MgSO4·7H2O 0.1 g·L−1,FeSO4·7H2O 0.006 g·L−1,CaCl2 0.01 g·L−1。去离子水溶解,pH 7.0~7.3。
1.2 实验装置
富集过程在自制的SBR中进行,反应器内部容积为3 L,反应器中部接有恒速电动搅拌器,内部接有可调式空气泵的气石。驯化过程和反硝化测试实验在250 mL的蓝盖瓶中进行,瓶盖不密封,用透气膜封口。反应装置如图1所示。
1.3 菌群的富集
取200 g污泥于SBR中,倒入2 L富集培养基,设置60 r·min−1的电动搅拌,打开气泵开始运行。运行周期约为24 h,好氧反应时间22~23 h,每天监测三氮(氨氮、硝氮、亚硝氮)浓度,至出水检测不到硝氮且无亚硝氮积累后,以富集测试培养基对富集前后的活性污泥进行反硝化性能测试。
反硝化测试方法:取20 mL的污泥或菌群至蓝盖瓶中,各加入200 mL测试培养基,混匀后测定MLVSS,将蓝盖瓶放置在30 ℃、150 r·min−1的恒温振荡箱中,来回振荡24 h,定期取样测定三氮浓度。
1.4 菌群的驯化
以富集得到的好氧反硝化菌群为样品,分别取20 mL混匀后的样品于4个蓝盖瓶中,各加入200 mL富集培养基。向其中3个蓝盖瓶分别加入氯化钠、重铬酸钾和盐酸四环素,置于恒温振荡箱中以30 ℃、150 r·min−1的条件振荡,周期与富集过程相同。根据硝氮去除效果,梯度浓度提高硝酸盐氮和抑制物(氯化钠、重铬酸钾和盐酸四环素)的浓度负荷共分为4个阶段。其中,A阶段中NaCl、Cr(Ⅵ)和TC浓度分别为10 g·L−1、1 mg·L−1和5 mg·L−1;B阶段为30 g·L−1、2 mg·L−1和10 mg·L−1;C阶段为50 g·L−1、5 mg·L−1和25 mg·L−1;D阶段为70 g·L−1、10 mg·L−1和50 mg·L−1。
驯化结束后,以驯化测试培养基对驯化前后的菌群进行反硝化性能测试,测试方法同1.3节。不同的是,投加NaCl、K2Cr2O4和TC驯化后的菌群中测试培养基分别含70 g·L−1的NaCl、10 mg·L−1的Cr(Ⅵ)和50 mg·L−1的TC,并以驯化前的菌群作为对照,同时进行反硝化测试。
1.5 EPS分析
对接种源、富集过程和驯化过程结束后取出的菌群样品进行EPS的提取和测定。提取采用超声波提取法[13]。取4 mL的菌群样品,在5 500 r·min−1下离心20 min,倾去上清液,补充0.9%生理盐水至原体积,振荡均匀,重复2次。将清洗后的污泥样品,冰浴下40 W超声2 min。再将样品在8 000 r·min−1下离心30 min,上清液经0.22 μm纤维素滤膜过滤得到EPS样品。蛋白质(PN)采用考马斯亮蓝法[14]测定;多糖(PS)采用苯酚-硫酸法[15]测定。
1.6 群落结构分析
取1 mL污泥或菌群样品,采用细菌DNA提取试剂盒(申能博彩,上海)提取细菌的基因组DNA,最后用100 μL TAE洗脱。琼脂糖凝胶电泳检测合格后,采用高通量测序技术对提取的DNA样品进行微生物群落结构及多样性分析。
1.7 q-PCR分析
对提取的DNA样品进行q-PCR分析,目的基因为napA,是在有氧条件下转化硝酸盐所必需的周质硝酸盐还原酶Nap的编码基因[16]。napA基因丰度的qPCR采用Stepone plus型荧光定量PCR仪(ABI)进行分析,使用的引物[17]和扩增条件见表1。反应体系20 μL,包括2.0 μL的DNA模板、10 μL的SybrGreen qPCR Master Mix(2X)、0.4 μL的上游引物和下游引物(10 μmol·L−1)和7.2 μL的双蒸水。
表 1 荧光定量PCR引物、序列及反应条件Table 1. Primers, sequences and reaction conditions for fluorescent q-PCR引物 序列 反应条件 napA Z3F CGCGAACAAGCTGATGAAGG 95 ℃预变性3 min; 95 ℃变性15 s, 57 ℃退火20 s, 72 ℃延伸30 s, 45个循环 napA Z3R AAGATCATCGGGATGTCGGC 95 ℃预变性3 min; 95 ℃变性15 s, 57 ℃退火20 s, 72 ℃延伸30 s, 45个循环 1.8 分析方法
硝酸盐氮:紫外分光光度法[18];氨氮:纳氏试剂光度法[19];亚硝酸盐氮:N-(1-萘)-乙二胺光度法[20];六价铬:二苯碳酰二肼分光光度法[21];pH、DO:玻璃电极法。MLVSS:重量法。
2. 结果与讨论
2.1 富集前后好氧反硝化性能
经过一段时间的间歇曝气,反应器出水基本检测不到硝酸盐氮,且能在7 d内保持稳定,视为富集得到好氧反硝化菌群,此时菌群中参与反硝化作用的主要是好氧反硝化细菌。由图2可知,好氧条件下,接种污泥基本无法去除硝酸盐氮,而富集得到的菌群在12 h内能将140 mg·L−1左右的硝酸盐氮完全去除,硝酸盐氮的比去除速率达到11.67 mg·(L·h)−1。在去除硝酸盐氮的过程中,存在亚硝酸盐氮的积累,在8 h时达到峰值15.16 mg·L−1,但随后逐渐被降解。
2.2 菌群的驯化
1)驯化过程中的三氮变化。从图3(a)和图3 (b)可以看出,NaCl和TC的胁迫基本不影响氨氮的去除和亚硝酸盐氮的积累量,Cr(Ⅵ)的胁迫显著抑制了氨氮的去除,氨氮去除率仅有为对照组的25%~50%,并减少了亚硝酸盐氮的积累,这可能是因为Cr(Ⅵ)影响了菌群中硝化细菌的活性。汪敏刚等[22]在研究低浓度铬对SBR中微生物作用的影响中提到,Cr(Ⅵ)的毒性作用使菌群中的硝化细菌无法维持正常的生长和工作,减少了氨氮的去除效果,影响程度与铬浓度具有相关性。
从图3(c)可以看出,梯度投加NaCl和TC对硝酸盐氮的去除没有产生抑制作用;而梯度投加Cr(Ⅵ)的过程中减少了硝酸盐氮的去除,当Cr(Ⅵ)浓度提升到5 mg·L−1的时候,硝酸盐氮的去除被抑制得最明显,对硝酸盐氮的去除率降为32%,但在持续投加Cr(Ⅵ)后,抑制作用在5 d内逐渐降低,直至消失。HE等[11]研究发现,铜绿假单胞菌PCN-2在有氧条件可同时去除硝酸盐氮和Cr(Ⅵ),且两者能同时作为电子受体而相互竞争。菌群中可能存在兼具铬还原和反硝化作用的好氧反硝化细菌,这类细菌在驯化过程中逐渐成为优势菌种,削弱了Cr(Ⅵ)对反硝化过程的抑制作用。
2)污染物对驯化前后菌群脱氮性能的影响。驯化前后反硝化测试结果见图4和表2。结果表明,NaCl、Cr(Ⅵ)和TC均会影响驯化前的菌群去除硝酸盐氮的效果,反硝化速率仅为空白组的19%、62%和23%,可见NaCl和TC对好氧反硝化过程的抑制效果更为突出,高盐和含高浓度四环素的工业废水对好氧反硝化细菌的生物脱氮有很大负面影响;驯化后的菌群在NaCl、Cr(Ⅵ)和TC的胁迫下,24 h内可快速去除280 mg·L−1的
NO−3 -N,反硝化速率分别从0.44、1.43和0.52 mg·(g·h)−1增加到7.95、4.53和6.49 mg·(g·h)−1,表明污染物的长期胁迫提高了菌群中好氧反硝化细菌抵抗生物毒性的能力。另外,测试过程中对加铬的菌群进行了Cr(Ⅵ)的检测,发现10 mg·L−1的Cr(Ⅵ)在8 h内快速去除,这证实了菌群中很可能存在具有还原六价铬能力的好氧反硝化细菌。表 2 驯化前后反硝化速率Table 2. Denitrification rates before and after acclimation驯化状态 投加抑制物 MLVSS/(g·L−1) 反硝化速率(以VSS计)/(mg·(g·h)−1) 驯化前 空白 9.84 2.31 加NaCl 9.76 0.44 加Cr(Ⅵ) 9.41 1.43 加TC 9.93 0.52 驯化后 空白 7.18 9.37 加NaCl 8.4 7.95 加Cr(Ⅵ) 7.21 4.53 加TC 3.51 6.49 2.3 菌群EPS分析
EPS作为细菌与环境介质间的缓冲层,有助于降低环境因素对菌体的损害[23],污染物的胁迫可能影响菌群EPS的产量和组分构成。从图5可以看出,污染物的长期胁迫大幅提高了菌群EPS的产量,相比之下,培养基中的氮素和其他无机盐对菌群EPS产生量影响甚微。
未加污染物的菌群的PN和PS含量较低,随着氮负荷的提升呈现缓慢增加的趋势。这可能是因为在颗粒污泥解体为絮状污泥的过程中,由于有机负荷低使微生物内源代谢水平升高,造成细胞的自溶,从而导致EPS含量的增加[24]。与空白组相比,NaCl、Cr(Ⅵ)和TC胁迫下的菌群PN含量增长8.7~12.8倍,NaCl、Cr(Ⅵ)胁迫下的菌群PS含量增长2.6~5.2倍,PS含量未受TC影响。盐度的增加会导致细胞内外离子浓度差异增大,迫使细胞分泌出大量的蛋白酶,增强扩散和主动运输的能力,同时产生多糖分子来限制细胞中水分的流动以适应渗透压的变化[25]。许睿骁等[26]发现,逐渐提高盐度至3.5%,污泥EPS含量较稳定,当盐度提高到5%,EPS含量增长明显。为了降低重金属毒性,细胞可以分泌出更多的蛋白物质和糖类物质,通过这些物质的官能团来络合重金属离子[27],因此,四环素的胁迫致使系统产生过量的EPS与水中的四环素结合,这主要是EPS中产生的一些蛋白类物质的参与导致的[28]。
2.4 群落多样性及群落结构分析
以16S rRNA基因序列97%相似度水平归类OUT统计菌群群落的α-多样性结果见表3。从表3可知,原污泥群落多样性最丰富,好氧反硝化细菌的富集显著减少了菌群群落多样性,加Cr(Ⅵ)和TC的菌群多样性低于相应空白组。这说明Cr(Ⅵ)和TC的胁迫进一步造成了群落多样性的降低,相反,NaCl的胁迫提高了菌群的群落多样性。由此可知,菌群中对铬污染和四环素污染耐受能力强的好氧反硝化细菌较少,耐高盐的好氧反硝化细菌较多;部分嗜盐菌在高盐条件下甚至繁殖更快[29],如果该菌群应用到高盐含氮废水的治理中,菌群多样性的增加可能使生物系统的稳定性更高。
表 3 细菌群落α-多样性Table 3. α-diversity of microbial community处理组 OTU 深度指数 Chao1指数 Shannon多样性指数 Simpson多样性指数 接种污泥 413 0.999 1 419 6.31 0.965 富集菌群 142 0.998 7 170 3.76 0.849 空白 120 0.999 0 141 2.95 0.756 加NaCl 245 0.998 4 255 3.12 0.806 加Cr(Ⅵ) 76 0.999 4 88 2.20 0.531 加TC 68 0.999 6 75 2.21 0.591 图6是各菌群群落结构在属水平上的相对丰度。接种污泥中优势菌种主要为专性厌氧的简单螺旋菌属(Simplicispira),富集后,假单胞菌属(Pseudomonas)成为优势菌种,这可能是导致富集前后菌群好氧反硝化能力差异的主要原因。空白组的优势菌种为假单胞菌属(Pseudomonas)和陶厄氏菌属(Thauera),相对丰度为55%和28%,陶厄氏菌(Thauera)具有较高的反硝化活性[30],高浓度的硝酸盐氮环境可能促进了陶厄氏菌的繁殖。加NaCl驯化后的菌群优势菌种为固氮弧菌属(Azoarcus)和假单胞菌属(Pseudomonas),相对丰度为79%和15%,其中还有约4%的盐单胞菌属(Halomonas),它们都具有一定的耐盐特性[31],其中固氮弧菌属(Azoarcus)有高效生物脱氮的功能[32],是驯化菌群在高盐环境实现高效反硝化的主要功能菌。加铬驯化后优势菌种主要为固氮弧菌属(Azoarcus)和陶厄氏菌属(Thauera),相对丰度分别为52%和18%。固氮弧菌(Azoarcus)能够在苯酚、甲苯等有机碳源下还原Cr(Ⅵ)[33],驯化菌群能够同时还原硝酸盐氮和Cr(Ⅵ),很可能是固氮弧菌(Azoarcus)丰度增加造成的结果;陶厄氏菌(Thauera)在低浓度Cr(Ⅵ)下活性不高,但在高浓度Cr(Ⅵ)环境的反硝化过程中发挥主要作用[34]。加TC驯化后的菌群的优势菌属与空白组相同,假单胞菌属(Pseudomonas)相对丰度增长至79%。这与ZOU等[35]的研究结果一致,在四环素的胁迫下,假单胞菌属(Pseudomonas)为反硝化过程的主要功能菌。TC对菌群脱氮性能的抑制可能与反硝化活性高的陶厄氏菌丰度下降有关。
2.5 功能基因napA丰度
由图7可知,富集过程使菌群napA基因丰度从5.54×108拷贝数·g−1提高到6.82×109 拷贝数·g−1,提高了1个数量级。Cr(Ⅵ)和TC的长期胁迫减少了菌群napA基因丰度,而NaCl的胁迫增加了菌群napA基因丰度,达到 2.59×1010 拷贝数·g−1。结果表明,Cr(Ⅵ)和TC会导致菌群中具有好氧反硝化能力的细菌数量的减少,这与Cr(Ⅵ)和TC能够抑制好氧反硝化性能的结果一致。其中,TC对napA基因丰度的影响更大,其原因可能是和四环素相关的耐药基因与napA基因相互作用所致[36]。然而,高盐环境虽然促进了嗜盐好氧反硝化细菌的繁殖,但有氧条件下的硝酸盐氮的去除效果仍低于空白组,其原因可能是细菌在NaCl的胁迫下改变了Na+/H+反向转运蛋白的活性,从而提高了napA基因的丰度,但这些基因未能正常表达[37-38]。
3. 结论
1) NaCl、Cr(Ⅵ)和TC均能抑制好氧反硝化菌群的反硝化性能,梯度浓度投加NaCl、Cr(Ⅵ)和TC可以提高好氧反硝化菌群对这些抑制物的耐受能力,并实现在较广浓度范围的NaCl、Cr(Ⅵ)和TC废水中快速去除硝酸盐氮的目标。
2)好氧反硝化菌群在污染物环境中会提高胞外蛋白和胞外多糖的产量,其目的可能是降解或者隔离污染物,减少污染物对细菌细胞的毒害作用。对于胞外多聚物如何保护细胞抵御生物毒性作用是后续工作需要完善的内容。
3)典型污染物会显著影响好氧反硝化菌群群落多样性和种群结构。Cr(Ⅵ)和TC的长期胁迫会降低好氧反硝化菌群的群落多样性并减少napA基因丰度,NaCl胁迫的结果与其相反;NaCl和Cr(Ⅵ)的胁迫使固氮弧菌属(Azoarcus)替代假单胞菌属(Pseudomonas)和陶厄氏菌属(Thauera)成为好氧反硝化菌群中的优势菌种,TC不影响假单胞菌属(Pseudomonas)和陶厄氏菌属(Thauera)的优势地位,但会改变两者在群落中的相对丰度。
-
表 1 荧光定量PCR引物、序列及反应条件
Table 1. Primers, sequences and reaction conditions for fluorescent q-PCR
引物 序列 反应条件 napA Z3F CGCGAACAAGCTGATGAAGG 95 ℃预变性3 min; 95 ℃变性15 s, 57 ℃退火20 s, 72 ℃延伸30 s, 45个循环 napA Z3R AAGATCATCGGGATGTCGGC 95 ℃预变性3 min; 95 ℃变性15 s, 57 ℃退火20 s, 72 ℃延伸30 s, 45个循环 表 2 驯化前后反硝化速率
Table 2. Denitrification rates before and after acclimation
驯化状态 投加抑制物 MLVSS/(g·L−1) 反硝化速率(以VSS计)/(mg·(g·h)−1) 驯化前 空白 9.84 2.31 加NaCl 9.76 0.44 加Cr(Ⅵ) 9.41 1.43 加TC 9.93 0.52 驯化后 空白 7.18 9.37 加NaCl 8.4 7.95 加Cr(Ⅵ) 7.21 4.53 加TC 3.51 6.49 表 3 细菌群落α-多样性
Table 3. α-diversity of microbial community
处理组 OTU 深度指数 Chao1指数 Shannon多样性指数 Simpson多样性指数 接种污泥 413 0.999 1 419 6.31 0.965 富集菌群 142 0.998 7 170 3.76 0.849 空白 120 0.999 0 141 2.95 0.756 加NaCl 245 0.998 4 255 3.12 0.806 加Cr(Ⅵ) 76 0.999 4 88 2.20 0.531 加TC 68 0.999 6 75 2.21 0.591 -
[1] 陈浩, 张枫, 王中正, 等. 高盐废水处理技术研究进展[J]. 广州化工, 2017, 45(22): 17-18. doi: 10.3969/j.issn.1001-9677.2017.22.008 [2] 耿淑英, 付伟章, 郑书联, 等. 皮革厂含铬污泥铬回收及资源化利用[J]. 环境工程学报, 2017, 11(6): 3767-3772. [3] 张昱, 唐妹, 田哲, 等. 制药废水中抗生素的去除技术研究进展[J]. 环境工程学报, 2018, 12(1): 1-14. [4] 高欣, 马鹏飞. 工业废水对城市污水处理厂设计及运行的影响[J]. 环境科学与管理, 2006, 31(3): 72-73. doi: 10.3969/j.issn.1673-1212.2006.03.025 [5] 赵凯峰, 王淑莹, 叶柳, 等. NaCl盐度对耐盐活性污泥沉降性能及脱氮的影响[J]. 环境工程学报, 2010, 4(3): 570-574. [6] YU M, ZHU W, LIAO R H, et al. Assessment of phenol effect on microbial community structure and function in an anaerobic denitrifying process treating high concentration nitrate wastewater[J]. Chemical Engineering Journal, 2017, 330: 757-763. doi: 10.1016/j.cej.2017.08.011 [7] FENG B, FANG Z, HOU J, et al. Effects of heavy metal wastewater on the anoxic/aerobic-membrane bioreactor bioprocess and membrane fouling[J]. Bioresource Technology, 2013, 142(8): 32-38. [8] GUI M Y, CHEN Q, MA T, et al. Effects of heavy metals on aerobic denitrification by strain Pseudomonas stutzeri PCN-1[J]. Applied Microbiology and Biotechnology, 2017, 101(4): 1717-1727. doi: 10.1007/s00253-016-7984-8 [9] GUI M, CHEN Q, NI J. Effect of NaCl on aerobic denitrification by strain Achromobacter sp. GAD-3[J]. Applied Microbiology & Biotechnology, 2017, 101(12): 1-9. [10] 白洁, 陈琳, 黄潇, 等. 1株耐盐异养硝化-好氧反硝化菌Zobellella sp. B307的分离及脱氮特性[J]. 环境科学, 2018, 39(10): 4793-4801. [11] HE D, ZHENG M, MA T, et al. Interaction of Cr(VI) reduction and denitrification by strain Pseudomonas aeruginosa PCN-2 under aerobic conditions[J]. Bioresource Technology, 2015, 185(1): 346-352. [12] 魏荷芬, 王田野, 张宏才, 等. 一株多重耐受性高效反硝化细菌的分离鉴定及特性[J]. 环境工程学报, 2016, 10(12): 7367-7374. doi: 10.12030/j.cjee.201507080 [13] 龙腾锐, 李金印, 龙向宇, 等. 超声波提取活性污泥胞外聚合物的研究[J]. 环境化学, 2008, 27(3): 310-313. doi: 10.3321/j.issn:0254-6108.2008.03.007 [14] 王孝平, 邢树礼. 考马斯亮蓝法测定蛋白含量的研究[J]. 天津化工, 2009, 23(3): 40-42. doi: 10.3969/j.issn.1008-1267.2009.03.016 [15] 姜琼, 谢妤. 苯酚-硫酸法测定多糖方法的改进[J]. 江苏农业科学, 2013, 41(12): 316-318. doi: 10.3969/j.issn.1002-1302.2013.12.114 [16] ZHU L, DING W, FENG L J, et al. Isolation of aerobic denitrifiers and characterization for their potential application in the bioremediation of oligotrophic ecosystem[J]. Bioresource Technology, 2012, 108: 1-7. doi: 10.1016/j.biortech.2011.12.033 [17] THROBACK I N, ENWALL K, JARVIS A, et al. Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE[J]. FEMS Microbiology Ecology, 2004, 49(3): 401-417. doi: 10.1016/j.femsec.2004.04.011 [18] 国家环境保护总局. 水质 硝酸盐氮的测定 紫外分光光度法(试行): HJ/T 346-2007[S]. 北京: 中国环境科学出版社, 2007. [19] 国家环境保护部. 水质 氨氮的测定 纳氏试剂分光光度法: HJ 535-2009[S]. 北京: 中国环境科学出版社, 2010. [20] 国家环境保护局. 水质 亚硝酸盐氮的测定 分光光度法: GB 7493-1987[S]. 北京: 中国标准出版社, 1987. [21] 国家环境保护局. 水质 六价铬的测定 二苯碳酰二肼分光光度法: GB/T 7467-1987[S]. 北京: 中国标准出版社, 1987. [22] 汪敏刚, 孙培德, 罗涛, 等. 低浓度铬对SBR中微生物抑制影响研究[J]. 环境科学学报, 2016, 36(6): 1979-1985. [23] 康福星, 龙健, 王倩, 等. 微生物胞外聚合物对水体重金属和富营养元素的环境生化效应研究展望[J]. 应用与环境生物学报, 2010, 16(1): 129-134. [24] 金淑芳, 张燚, 刘敏. 不同种类污泥中胞外多聚物的研究[J]. 四川化工, 2015, 18(4): 7-9. doi: 10.3969/j.issn.1672-4887.2015.04.003 [25] 王子超, 高孟春, 魏俊峰, 等. 盐度变化对厌氧污泥胞外聚合物的影响[J]. 环境科学学报, 2016, 36(9): 3273-3281. [26] 许睿骁, 李冰, 张勇, 等. 压力环境下活性污泥的耐盐驯化过程[J]. 环境工程学报, 2017, 11(7): 3952-3956. [27] 张恩华, 戴幼芬, 肖勇, 等. 还原Cr(Ⅵ)的混菌胞外聚合物和细菌群落结构分析[J]. 中国环境科学, 2017, 37(1): 352-357. [28] LIU C, XU J, LEE D J, et al. Denitrifying sulfide removal process on high-tetracycline wastewater[J]. Bioresource Technology, 2016, 205: 254-257. doi: 10.1016/j.biortech.2016.01.026 [29] HAMODA M F. Effects of high sodium chloride concentrations on activated sludge treatment[J]. Water Science & Technology, 1995, 31(9): 61-72. [30] FERNANDO M S, NIELSEN J L, NIELSEN P H. Substrate-dependent denitrification of abundant probe-defined denitrifying bacteria in activated sludge[J]. FEMS Microbiology Ecology, 2008, 66(2): 447-461. doi: 10.1111/fem.2008.66.issue-2 [31] ZHU I X, LIU J R. Nitrification and Denitrification: Introductory Chapter: Effects of Salinity on Biological Nitrate Removal from Industrial Wastewater[M/OL]. [2018-12-01]. https://www.intechopen.com/books/nitrification-and-denitrification/introductory-chapter-effects-of-salinity-on-biological-nitrate-removal-from-industrial-wastewater. [32] 马晓丹, 高灵芳, 谭文博, 等. 一株异养脱硫反硝化菌株的筛选及其生物脱硫脱氮特性研究[J]. 微生物学通报, 2015, 42(5): 853-857. [33] FINGER K A, RHINE E D, YOUNG L. Bacterial chromate reduction and degradation of aromatic compounds[C]//American Society for Microbiology. 102nd General Meeting of the American Society for Microbiology. Salt Lake City, UT, USA, 2002: 411. [34] YU M, LIAO R, ZHANG X X, et al. Metagenomic insights into Cr(VI) effect on microbial communities and functional genes of an expanded granular sludge bed reactor treating high-nitrate wastewater[J]. Water Research, 2015, 76: 43-52. doi: 10.1016/j.watres.2015.02.042 [35] ZOU Y, LIN M, XIONG W, et al. Metagenomic insights into the effect of oxytetracycline on microbial structures, functions and functional genes in sediment denitrification[J]. Ecotoxicology & Environmental Safety, 2018, 161: 85-91. [36] SUN M, YE M, LIU K, et al. Dynamic interplay between microbial denitrification and antibiotic resistance under enhanced anoxic denitrification condition in soil[J]. Environmental Pollution, 2017, 222: 583-591. doi: 10.1016/j.envpol.2016.10.015 [37] CHRISTINE M, FLORIAN M, RICHARD V, et al. Comparative analysis of denitrifying activities of hyphomicrobium nitrativorans, hyphomicrobium denitrificans, and hyphomicrobium zavarzinii[J]. Applied and Environmental Microbiology, 2015, 81(15): 5003-5014. doi: 10.1128/AEM.00848-15 [38] WASER M, HESS-BIENZ D, DAVIES K, et al. Cloning and disruption of a putative NaH-antiporter gene of Enterococcus hirae[J]. Journal of Biological Chemistry, 1992, 267(8): 5396-5400. 期刊类型引用(3)
1. 张紫薇,陈召莹,张甜娜,周石磊,崔建升,罗晓. 岗南水库沉积物好氧反硝化菌群落时空分布特征. 环境科学. 2022(01): 314-328 .  百度学术
百度学术
2. 蒋宇豪,李敏,唐明哲,滕泽栋. 砾间接触氧化/水平潜流人工湿地净化微污染河道水. 中国给水排水. 2021(05): 57-65 .  百度学术
百度学术
3. 史文超,桂梦瑶,杜俊逸,马志飞,吴代赦. 典型微塑料对好氧反硝化菌群脱氮特性及反硝化相关基因的影响. 环境工程学报. 2021(04): 1333-1343 .  本站查看
本站查看
其他类型引用(4)
-






 下载:
下载: