-
磷是一种生命必不可少的非金属元素,目前天然的磷矿正在不断枯竭,故磷的有效回收显得尤为重要[1]。由于城市生活污水中的磷酸盐含量较低,无法直接用磷酸铵镁结晶法(MAP)进行回收,因此,获取高浓度的磷酸盐浓缩液是高效回收磷的关键[2]。而在获取高浓度磷酸盐的过程中,聚磷微生物的生长环境从长期处于低浓度磷酸盐中转变为好氧低浓度磷酸盐与厌氧高浓度磷酸盐不断交替的状态,同时快速获取高浓度的磷酸盐浓缩液要求聚磷菌具有高效的释磷能力,因此,研究同步去除与富集磷酸盐工艺中聚磷微生物的种群结构变化显得十分必要。
SLATER等[3]研究发现,在相同条件下,运行的4个SBR中的Accumulibacter结构存在差别。而TIAN等[4]发现,在厌氧/好氧交替式生物滤池中随着磷吸收与磷富集的进行,占主导地位的菌群由β-变形菌纲转变为γ-变形菌纲(98%),且γ-变形菌纲中的假单胞菌为优势菌。孟璇等[5]发现,在厌氧/好氧交替式同步去除并富集磷酸盐尼龙生物膜反应器中,红环菌科(Rhodocyclaceae)含量最高,为58.32%。这些研究显示,在不同运行条件下,反应器中可能存在不同种类的聚磷菌。
目前,大部分研究把重点放在提高反应器的除磷效率上,而对于富集高浓度的磷酸盐以及在此过程中聚磷菌变化的研究较少。本研究以悬浮填料为载体,采用厌氧/好氧交替式运行的反应器,针对未来城市污水厂从污水中同时去除并回收利用磷的需求进行了实验;采用以人工配水模拟城市生活污水并结合扫描电子显微镜(SEM)和高通量测序技术,探讨了不同浓度的磷酸盐生长环境对同步去除与富集磷酸盐的悬浮填料生物膜工艺吸释磷性能的影响及其微生物群落的变化情况。
-
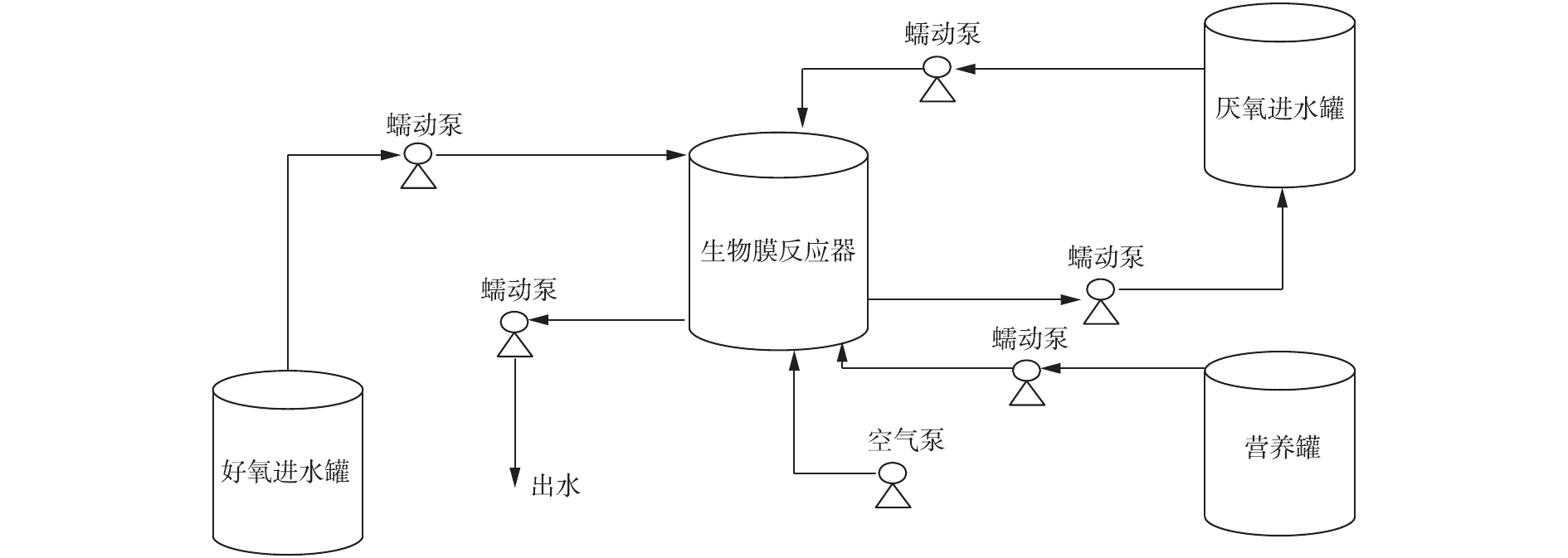
实验采用内径14 cm,高19 cm,有效容积为1.6 L的生物膜反应器。反应装置见图1。所用填料为k2型填料,材料为优质共聚材料,产品直径为15 mm。孔隙率高达95%以上,有效比表面积>500 m2·m−3。
实验用水采用人工配水来模拟城市生活污水水质,以乙酸钠作为厌氧碳源。好氧进水水质为5 mg·L−1
${\rm{PO}}_4^{3-} $ -P,40 mg·L−1${\rm{NH}}_4^{+} $ -N,少量微量元素[6],不添加碳源,加入适量NaHCO3,调节pH至7。控制厌氧进水水质为200 mg·L−1 COD,40 mg·L−1${\rm{NH}}_4^{+} $ -N,少量微量元素,不添加磷元素,pH为7。在第2阶段,使用投加浓缩液的方式在每个厌氧周期内补充200 mg·L−1 COD,40 mg·L−1${\rm{NH}}_4^{+} $ -N和少量微量元素[6]。 -
接种污泥来自苏州市某污水厂氧化沟。以人工配水模拟城市生活污水,采用厌氧/好氧交替式的运行模式在室温(20~25 ℃)下运行(好氧6 h,厌氧6 h),好氧阶段溶解氧控制在3 mg·L−1左右,蠕动泵的进出水流量约160 mL·min−1,进出水时间10 min,分2个阶段运行。
第1阶段(0~45 d),处于低浓度磷酸盐的生长环境(以下简称为低磷环境)。在好氧阶段,好氧进水通过蠕动泵进入生物膜反应器,曝气6 h,将溶液排出;在厌氧阶段,厌氧进水通过蠕动泵进入反应器,6 h后将溶液排出。
第2阶段(46~95 d),处于好氧低浓度磷酸盐与厌氧高浓度磷酸盐不断交替的生长环境(以下简称为低磷高磷交替环境)。在好氧阶段,好氧进水通过蠕动泵进入反应器,曝气6 h,之后将溶液排出;在厌氧阶段,厌氧进水通过蠕动泵进入反应器,同时营养罐中的碳源和氮源通过蠕动泵进入反应器,反应完成后,溶液回到厌氧进水罐中。以15 d为1个周期更新厌氧进水罐中的溶液。
-
COD采用重铬酸钾法[7]测定;
${\rm{PO}}_4^{3-} $ -P采用钼锑抗分光光度法[7]测定。采用扫描电子显微镜(SEM)观察第0、45和95天的微生物形态[4];采用高通量测序分析第0、45和95天反应器内的微生物群落组成[5]。 -
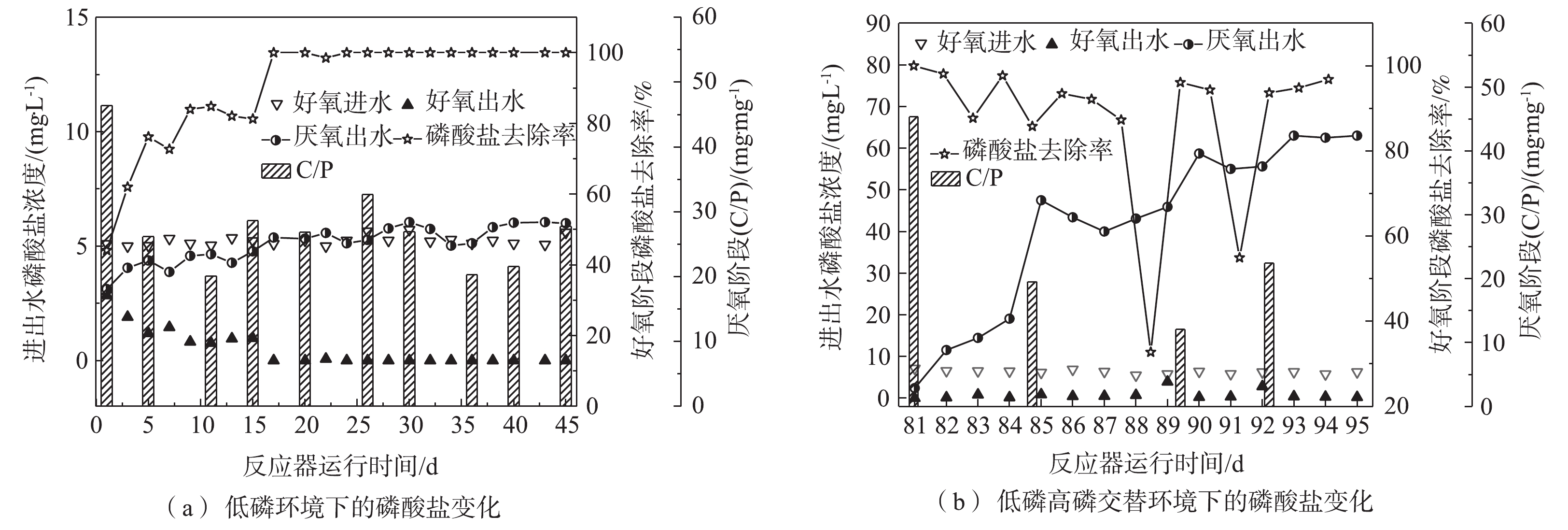
在同步去除与富集磷酸盐的工艺中,好氧进水COD为0 mg·L−1、
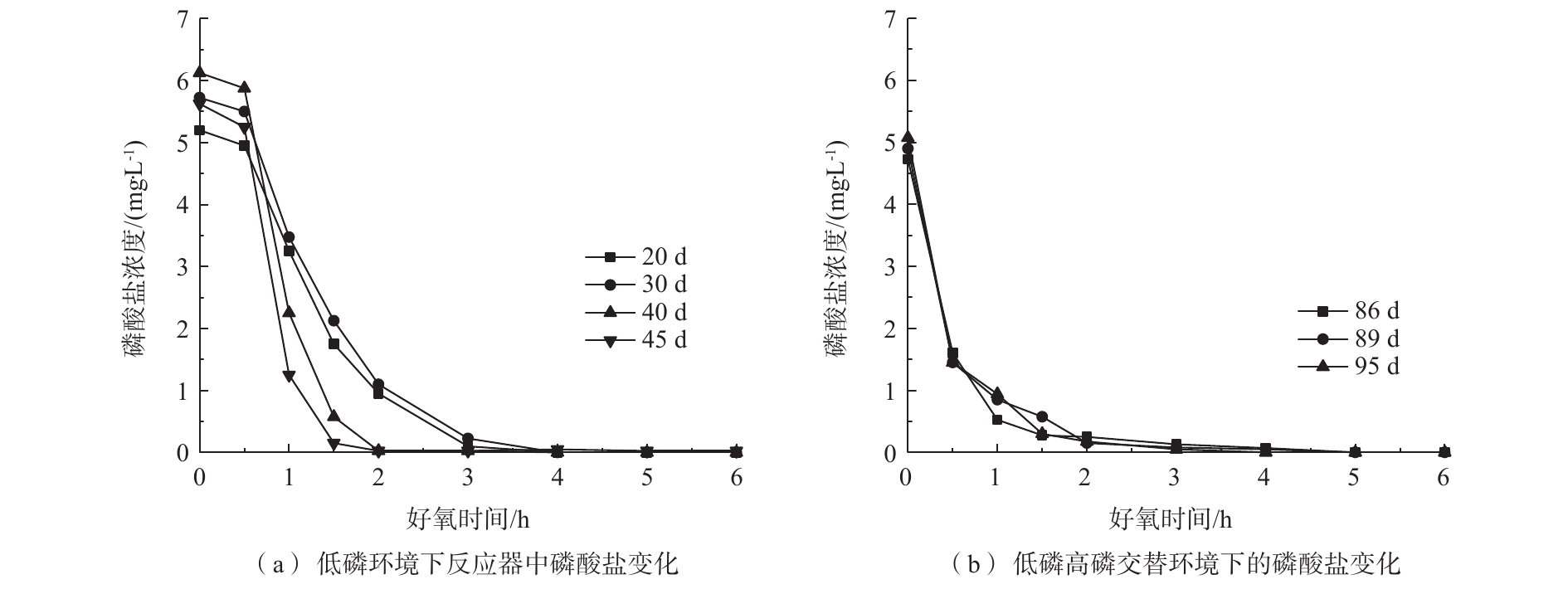
${\rm{PO}}_4^{3-} $ -P为5 mg·L−1。由图2(a)可知,在低磷环境下,随着反应器的运行,好氧阶段生物膜的吸磷速率逐渐变快,第45天时悬浮填料聚磷生物膜仅需1.5 h便可将磷酸盐浓度降低至0.15 mg·L−1;从图2(b)可以发现,低磷高磷交替环境下的悬浮填料聚磷生物膜也可在2 h内快速吸磷并使出水达标排放,具有良好的磷酸盐去除能力。在图3(a)中,在低磷环境下,运行稳定后,好氧出水磷酸盐浓度稳定在0.5 mg·L−1以下;由图3(b)可以看出,低磷高磷交替环境下的一个典型周期(以第81~95天为例)内,好氧出水磷酸盐浓度基本稳定在0.5 mg·L−1以下。图3的厌氧进水水质为200 mg·L−1 COD、0 mg·L−1
${\rm{PO}}_4^{3-} $ -P。由图3可知,低磷环境下厌氧阶段的释磷能力不断加强,最大释磷量为6.05 mg·L−1,在此期间,厌氧阶段C/P(消耗的COD量/释放的磷酸盐量)平均为24.84 mg·mg−1;低磷高磷交替环境下,厌氧进水在15 d内被重复利用(后简称富磷溶液),富磷溶液的磷酸盐浓度在15 d内逐渐增大,达到63 mg·L−1后趋于稳定,在此期间,C/P平均为27.74 mg·mg−1。在低磷环境中,同步去除与富集磷酸盐工艺的磷酸盐去除率基本稳定在98%以上,且其释磷能力不断加强;而在低磷高磷交替环境下,该工艺的磷酸盐去除能力受到了富磷溶液浓度的影响,该工艺前期对磷酸盐的去除率达到85%以上,厌氧阶段磷酸盐不断得到释放,富磷溶液达到了47 mg·L−1,之后富磷溶液的浓度出现了短期内变化幅度较小的现象。与此同时,好氧阶段磷酸盐的去除率下降至32%~54%。由此可见,聚磷生物膜的释磷能力与细胞外界的磷酸盐浓度密切相关。当富磷溶液中的磷酸盐浓度较高时,释磷能力会受到细胞内外磷酸盐浓度差太小的影响,导致富磷溶液磷酸盐浓度难以上升。有研究[8]表明,聚磷菌能够在好氧阶段吸收多于自己生长所需的磷,并以多聚磷酸盐颗粒的形式储存于体内。当聚磷生物膜进入蓄磷阶段,并且生物膜体内的磷酸盐量积累到一定程度时,聚磷菌细胞内外的磷酸盐浓度差增大,聚磷生物膜再次释磷,从而导致富磷溶液的磷酸盐浓度再次上升。
-
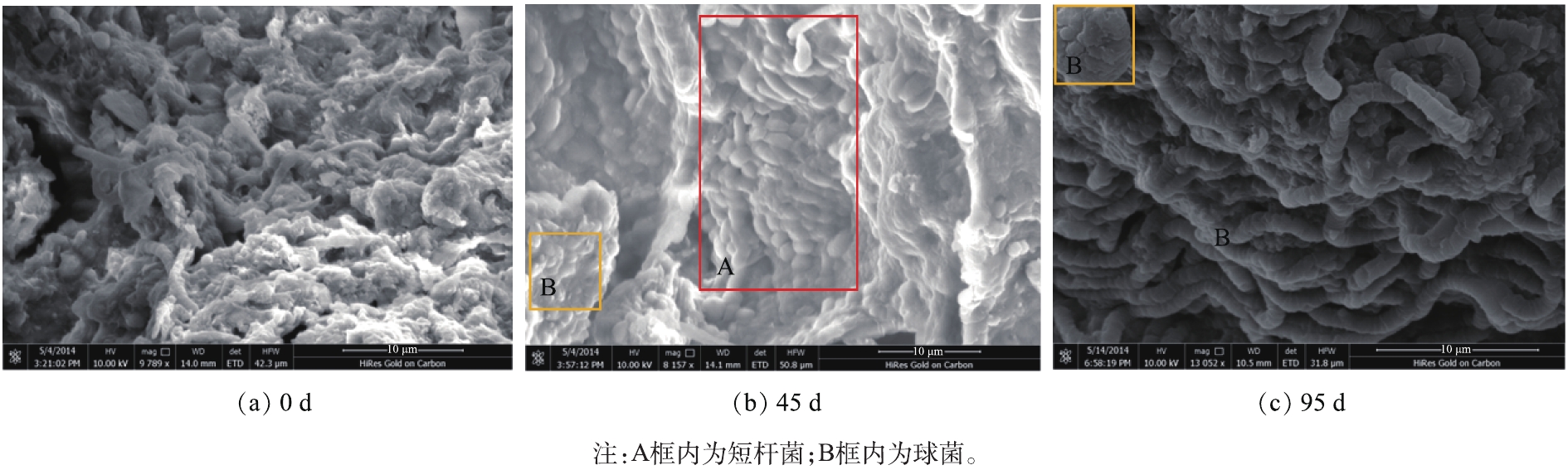
第0、45和95天的污泥扫描电子显微镜(SEM)表征结果如图4所示。在第0天的污泥中,可以观察到存在大量的胞外聚合物(EPS),通过相互作用与细胞结合在一起,此时有多种形态的微生物并存。第45天,聚磷生物膜的生长环境为低磷环境,此时反应器具有良好的除磷能力,好氧出水在0.5 mg·L−1以下,符合国家磷酸盐排放标准[9],污泥中存在较多的短杆菌和少量的球菌,污泥形态上逐渐均一化,此时杆菌正处于生长阶段,且优势菌种得到富集。第95天,生长环境为低磷高磷交替环境,在满足好氧出水达标排放的同时,富磷溶液的磷酸盐浓度为63 mg·L−1,污泥中长杆菌数量明显增加,仅存在少量的球菌,轮廓较为清晰,低磷高磷交替的生长环境对聚磷菌存在一定的筛选作用。CAO等[10]也指出聚磷菌主要为杆状和球状,与本研究结果一致。
-
1)聚磷生物膜的微生物多样性和丰富度。采用16S rDNA的测序方法,对聚磷生物膜运行过程中的微生物多样性和丰富度进行了评价。如表1所示,第0、45和95天的有效序列数分别为59 711、73 249和126 462。各样品的OTU数分别为52 736、64 715和92 618。第0、45和95天的覆盖率在0.995 0与0.996 4之间,所获得的结果可以说明样品中的微生物多样性。Shannon多样性指数可以说明样品中的物种丰富度,可见接种污泥中的物种丰富度最高(Shannon=7.781 0),第95天的物种丰富度最小(Shannon=5.630 7)。ACE、Chao和Simpson指数同样说明第95天的物种分布最为简单。第0、45和95天的种群变化表明,不同的磷酸盐生长环境会影响反应器中的微生物丰富度和多样性。
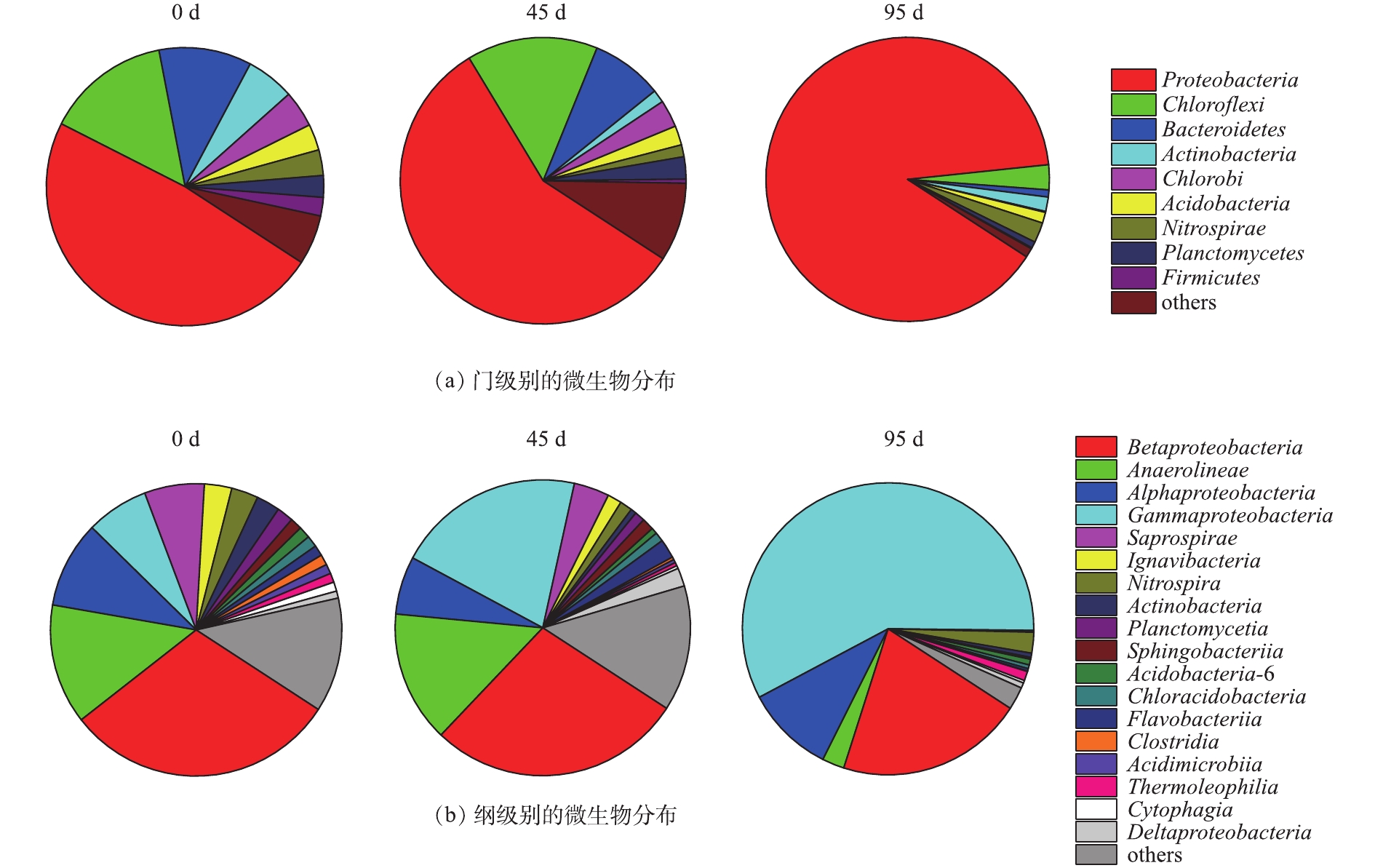
2)细菌种群特性分析。测序结果显示,在不同的生长环境下,聚磷生物膜中共同存在着一些微生物。第0、45和95天的样品在门级共有的OTUs主要有变形菌门(Proteobacteria)、绿弯菌门(Chloroflexi)、拟杆菌门(Bacteroidetes)、放线菌门(Actinobacteria)、绿菌门(Chlorobi)、酸杆菌门(Acidobacteria)、硝化螺旋菌门(Nitrospirae)、浮霉菌门(Planctomycetes)和厚壁菌门(Firmicutes)。
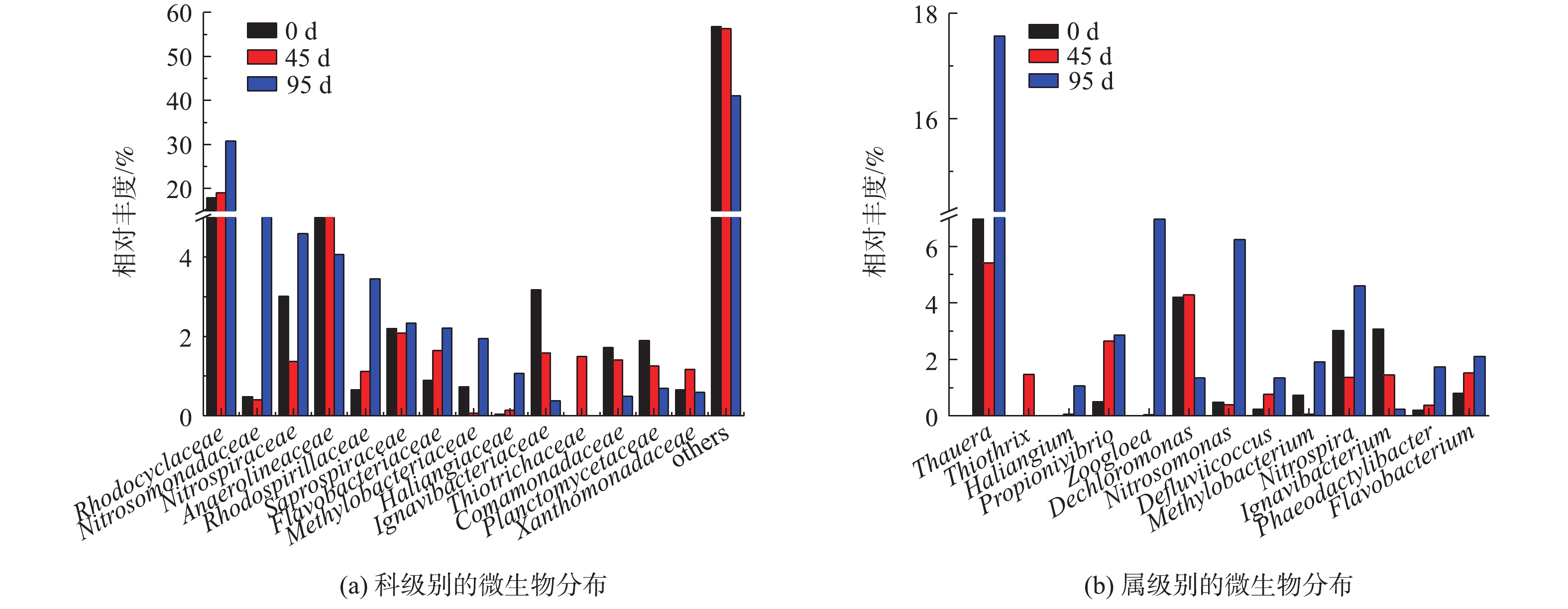
从门、纲2个水平对生物膜上的微生物群落进行分析,结果如图5所示。这3个污泥样品中相对丰度大于3%的门为变形菌门(48.3%、57.1%、89.1%)、绿弯菌门(14.5%、14.8%、2.8%)、拟杆菌门(10.8%、8.1%、0.8%)、绿菌门(4.2%、3.1%、0.1%),其中,变形菌门是3个污泥样品中最丰富的门。夏雪等[11]研究发现,使用不同碳源驯化得到的除磷污泥中变形菌门是最丰富的门。NGUYEN等[12]研究发现,变形菌门内的部分细菌具有在降解有机物的同时完成脱氮除磷的功能。在同步去除与富集磷酸盐的悬浮填料生物膜工艺中,变形菌门可能为潜在的聚磷菌。
在纲的水平上,第0、45和95天的γ-变形菌纲(Gammaproteobacteria)的相对丰度从6.9%上升至20.6%和57.9%,β-变形菌纲(Betaproteobacteria)的相对丰度从30.2%下降至27.9%和20.7%。在同步去除与富集磷酸盐的悬浮填料生物膜工艺中,主要的微生物属于γ-变形菌纲,这与强化生物除磷系统中主要微生物属于β-变形菌纲不同,可见生物膜系统与活性污泥系统中,存在不同种类的聚磷菌。
如图6所示,在科的水平上,相对丰度大于3%的有红环菌科(Rhodocyclaceae)、厌氧绳菌科(Anaerolineaceae)、亚硝化单胞菌科(Nitrosomonadaceae)、硝化螺旋菌科(Nitrospiraceae)、红螺菌科(Rhodospirillaceae)。红环菌科(Rhodocyclaceae)在运行过程中相对丰度逐渐增大(分别为18.1%、19.0%和30.8%),成为了反应器中相对丰度最大的科。据报道,目前被认可的聚磷菌大多属于红环菌科(Rhodocyclaceae)[13]。ZILLES等[14]的研究也发现,红环菌科(Rhodocyclaceae)是生物强化除磷系统中的优势聚磷菌,具有良好的除磷效果。测序结果证明,红环菌科(Rhodocyclaceae)是同步去除与富集磷酸盐的悬浮填料生物膜工艺中的优势菌群。
在属的水平上,反应器中的主要属类为陶厄氏菌属(Thauera)。在运行过程中,陶厄氏菌属(Thauera)、亚硝酸菌属(Nitrosomonas)、硝化螺旋菌属(Nitrospira)的相对丰度有所增加,这可能是由于微生物的生长导致生物膜逐渐变厚。在好氧阶段,氧气通过扩散作用能够进入到悬浮填料生物膜的一定深度处,生物膜外层处于好氧环境,亚硝酸菌属(Nitrosomonas)[15]、硝化螺旋菌属(Nitrospira)[16]等发生硝化作用产生硝酸盐,此时生物膜内层处于缺氧环境,陶厄氏菌属(Thauera)[17]等反硝化细菌吸收硝酸盐进行反硝化作用[18]。测序结果显示,动胶菌属(Zoogloea)是同步去除与富集磷酸盐的悬浮填料生物膜工艺中的主要功能菌。动胶菌属(Zoogloea)属于假单胞科(Pseudomonadaceae),是革兰氏阴性杆菌,属于兼性好氧细菌,能够将吸收的磷以多聚偏磷酸盐的形式贮存在细胞中[19]。动胶菌属(Zoogloea)也是活性污泥法强化除磷系统中的主要功能菌。在同步去除与富集磷酸盐的生物膜工艺中,动胶菌属的相对丰度不断增加,可见低磷高磷交替的生长环境比低磷的生长环境更适合聚磷菌的富集。
-
1)在低磷环境下,反应器好氧出水磷酸盐浓度稳定在0.5 mg·L−1以下,具有良好的磷酸盐去除能力,厌氧阶段的最大释磷量为6.05 mg·L−1,具有良好的磷酸盐释放能力;在低磷高磷交替的生长环境中,好氧出水磷酸盐浓度基本在0.5 mg·L−1以下,厌氧富磷溶液浓度最高可达63 mg·L−1,能够做到同步去除与富集磷酸盐。
2)杆菌是同步去除与富集磷酸盐的悬浮填料生物膜工艺中的优势菌群。反应器中的主要功能菌是动胶菌属(Zoogloea)。变形菌门是同步去除与富集磷酸盐的悬浮填料生物膜工艺中相对丰度最大的门,高达89.1%。γ-变形菌纲的相对丰度为57.9%,同步去除与富集磷酸盐的悬浮填料生物膜工艺中的优势菌种是红环菌科(Rhodocyclaceae),相对丰度为30.8%。
3)在悬浮填料生物膜工艺中,低磷高磷交替的生长环境下培养的聚磷生物膜能够使好氧出水磷酸盐达到国家排放标准,并在厌氧阶段得到高浓度的磷酸盐富集溶液,且这种生长环境更适合聚磷微生物的生长。
2种磷酸盐生长环境对同步去除与富集磷酸盐工艺的影响
Effects of two phosphate growth environments on simultaneous removal and enrichment of phosphate
-
摘要: 针对同步去除与富集磷酸盐溶液的问题,研究了在低磷环境和低磷高磷交替环境下悬浮填料生物膜反应器的除磷能力和释磷能力,采用扫描电子显微镜(SEM)和高通量测序对第0、45和95天的污泥进行了表征。结果表明:低磷环境下好氧出水磷酸盐浓度稳定在0.5 mg·L−1以下,厌氧阶段的最大释磷量为6.05 mg·L−1;在低磷高磷交替环境中,好氧出水磷酸盐浓度基本在0.5 mg·L−1以下,富磷溶液浓度最高可达63 mg·L−1。SEM结果表明,同步去除与富集磷酸盐的悬浮填料生物膜反应器中的主要微生物是杆状菌。高通量测序结果表明:第0、45和95天的变形菌门(Proteobacteria)的相对丰度分别为48.3%、57.1%和89.1%,占主导地位;而红环菌科(Rhodocyclaceae)的相对丰度分别为18.1%、19.0%和30.8%,是反应器中的优势菌科;动胶菌属(Zoogloea)是同步去除与富集磷酸盐的悬浮填料生物膜工艺中的主要功能菌。在悬浮填料生物膜工艺中,低磷高磷交替的生长环境下培养的聚磷生物膜能够使好氧出水的磷酸盐浓度达到国家排放标准,并在厌氧阶段得到高浓度的磷酸盐富集溶液,且这种生长环境更适合聚磷微生物的生长。
-
关键词:
- 同步去除与富集磷酸盐 /
- 微生物生长环境 /
- 生物膜 /
- 高通量测序
Abstract: The aim of this study was to simultaneously remove and enrich phosphate solutions. The phosphorus removal and release capacity of suspended packed biofilm reactor in low concentration phosphate environment or continuous alternation environment of aerobic low concentration phosphate and anaerobic high concentration phosphate were studied. Sludge samples taken from 0, 45 and 95 d were characterized by SEM and high-throughput sequencing. The results showed that the phosphate concentration of aerobic effluent was below 0.5 mg·L−1 and the maximum phosphorus release was 6.05 mg·L−1 in the low phosphorus environment. In the low phosphorus concentration and high phosphorus concentration alternating environment, most of the phosphate concentration of aerobic effluent was below 0.5 mg·L−1, and the concentration of anaerobic phosphate concentrate was up to 63 mg·L−1. The SEM images showed that the main microorganisms in the suspended packed biofilm reactor for simultaneous removal and enrichment of phosphoric acid were bacilli. The result of high-throughput sequencing showed that the relative abundance of Proteobacteria on 0, 45 and 95 d were 48.3%, 57.1% and 89.1%, respectively, and it was dominant bacteria phylum. The relative abundances of Rhodocyclaceae were 18.1%, 19.0% and 30.8%, respectively, which was the dominant bacteria family in the reactor. Zoogloea was the main functional bacterium. In the suspended packing biofilm process, phosphorus accumulating bacteria cultured in alternate growth environment of low phosphorus and high phosphorus could lead to phosphate content in aerobic effluent which meets the national discharge standard, and could obtain high concentration phosphate enrichment solution in anaerobic phase. This growth environment was more suitable for the growth of phosphorus-accumulating microorganisms. -

-
表 1 微生物种群丰度和多样性分析
Table 1. Species abundance and diversity ofmicrobial communities
时间 序列数 OTU数 ACE Chao Shannon Simpson 覆盖率 第0天 59 711 52 736 1 454.53 1 457.3 7.781 0 0.984 0.996 4 第45天 73 249 64 715 1 556.34 1 561.2 7.490 1 0.983 0.995 7 第95天 126 462 92 681 1 171.48 1 183.0 5.630 7 0.952 0.995 0 -
[1] HUTNIK N, KOZIK A, MAZIENCZUK A, et al. Phosphates (V) recovery from phosphorus mineral fertilizers industry wastewater by continuous struvite reaction crystallization process[J]. Water Research, 2013, 47(11): 3635-3643. doi: 10.1016/j.watres.2013.04.026 [2] 黄常青. MAP沉淀法回收化工废水中氮磷的研究[D]. 哈尔滨: 哈尔滨工程大学, 2017. [3] SLATER F R, JOHNSON C R, BLACKALL L L, et al. Monitoring associations between clade-level variation, overall community structure and ecosystem function in enhanced biological phosphorus removal (EBPR) systems using terminal-restriction fragment length polymorphism (T-RFLP)[J]. Water Research, 2010, 44(17): 4908-4923. doi: 10.1016/j.watres.2010.07.028 [4] TIAN Q, ONG S K, XIE X, et al. Enhanced phosphorus recovery and biofilm microbial community changes in an alternating anaerobic/aerobic biofilter[J]. Chemosphere, 2016, 144: 1797-1806. doi: 10.1016/j.chemosphere.2015.10.072 [5] 孟璇, 潘杨, 章豪, 等. 同步去除并富集磷酸盐生物膜驯化过程中微生物种群分析[J]. 环境科学, 2018, 39(6): 2802-2809. [6] KODERA H, HATAMOTO M, ABE K, et al. Phosphate recovery as concentrated solution from treated wastewater by a PAO-enriched biofilm reactor[J]. Water Research, 2013, 47(6): 2025-2032. doi: 10.1016/j.watres.2013.01.027 [7] 国家环境保护总局. 水和废水监测分析方法[M]. 4版. 北京: 中国环境科学出版社, 2016. [8] SEVIOUR R J, MINO T, ONUKI M. The microbiology of biological phosphorus removal in activated sludge systems[J]. FEMS Microbiology Reviews, 2003, 27(1): 99-127. doi: 10.1016/S0168-6445(03)00021-4 [9] 国家环境保护总局, 国家质量监督检验检疫总局.城镇污水处理厂污染物排放标准: GB 18918-2002[S]. 北京: 中国环境科学出版社, 2002. [10] CAO Y S, ALAERTS G J. Influence of reactor type and shear stress on aerobic biofilm morphology, population and kinetics[J]. Water Research, 1995, 29(1): 107-118. doi: 10.1016/0043-1354(94)00136-U [11] 夏雪, 邵明非, 吕小梅, 等. 不同碳源驯化除磷污泥的除磷效果及菌群结构分析[J]. 环境科学研究, 2014, 27(8): 936-942. [12] NGUYEN H T, LE V Q, HANSEN A A, et al. High diversity and abundance of putative polyphosphate-accumulating Tetrasphaera-related bacteria in activated sludge systems[J]. FEMS Microbiology Ecology, 2011, 76(2): 256-267. doi: 10.1111/fem.2011.76.issue-2 [13] OEHMEN A, SAUNDERS A M, VIVES M T, et al. Competition between polyphosphate and glycogen accumulating organisms in enhanced biological phosphorus removal systems with acetate and propionate as carbon source[J]. Journal of Biotechnology, 2005, 123(1): 22-32. [14] ZILLES J L, HUNG C H, NOGUERA D R. Presence of Rhodocyclus in a full-scale wastewater treatment plant and their participation in enhanced biological phosphorus removal[J]. Water Science & Technology, 2002, 46(1/2): 123-128. [15] PURKHOLD U, POMMERENINGRÖSER A, JURETSCHKO S, et al. Phylogeny of all recognized species of ammonia oxidizers based on comparative 16S rRNA and amoA sequence analysis: Implications for molecular diversity surveys[J]. Applied and Environmental Microbiology, 2000, 66(12): 5368-5382. doi: 10.1128/AEM.66.12.5368-5382.2000 [16] WOLGAST I, BARTOSCH S. Identification of nitrite-oxidizing bacteria with monoclonal antibodies recognizing the nitrite[J]. Applied & Environmental Microbiology, 1999, 65(9): 4126-4133. [17] YANG N, ZhAN G, WU T, et al. Effect of air-exposed biocathode on the performance of a Thauera-dominated membraneless single-chamber microbial fuel cell (SCMFC)[J]. Journal of Environmental Sciences, 2018, 66(4): 216-224. [18] 王健, 陈文兵, 黄传伟, 等. 序批式生物膜法除磷技术研究现状[J]. 水科学与工程技术, 2008, 32(S2): 22-24. [19] 安卫星, 高娜, 夏明, 等. 动胶菌属系统分类、生理特征及其在活性污泥中的作用[J]. 应用与环境生物学报, 2016, 22(6): 1167-1174. -



 下载:
下载: