双酚S(bisphenol S, BPS)作为双酚A(bisphenol A, BPA)的结构类似物已被广泛使用,在地表水、湖泊[1]、沉积物[2]、洗发水等日用品[3]以及尿液[4]和血液等多种介质中被检出,其检出浓度和检出率呈逐年上升趋势[5]。调查发现BPS在我国流溪河中含量高达65 μg·L-1[6],且辽宁地区孕妇血清中BPS平均浓度已达1.24 μg·L-1[7-8]。视网膜是胚胎-发生期中枢神经系统的延伸,极易受环境因素的影响[9-10]。已有研究表明,BPA、四溴双酚A和四溴双酚S具有潜在的视网膜发育毒性,对人类早期视网膜发育表现出类似的不利影响[11-12],因而我们推测BPS极有可能存在与其相似的视网膜发育毒性。
实验室前期研究表明BPS长期暴露斑马鱼会使成年斑马鱼的眼动反应(optokinetic response, OKR)受损,并降低其视觉追踪能力[13];同时BPS还会导致斑马鱼幼鱼趋光反应减弱[14]。正常的OKR依赖于发育成熟的完整视网膜,而BPS很有可能通过影响斑马鱼幼鱼的视网膜发育导致其OKR受损。Lobo等[15]的研究指出视觉功能OKR的损伤与光感受器视锥和视杆细胞变性及眼内视黄酸(retinoic acid, RA)类物质含量降低有关。感光细胞是高度特化的感觉神经元,课题组前期研究表明BPS能够造成感光细胞视锥细胞DNA损伤和结构损伤,但其是否干扰了视网膜发育期感光细胞的发育尚未见报道。在眼中,眼中发色团与感光细胞中的视蛋白共价结合,在光激发过程中形成视觉色素,从而启动视觉转导级联反应,即视觉的起始。RA除了与视觉功能密切相关外,也是脊椎动物视觉系统尤其是光感受器发育过程中的重要信号分子[16-17],RA代谢通路扰乱会显著影响生物的视觉功能[18],如将视黄醛代谢为RA的视黄醛脱氢酶(retinal dehydrogenase, RALDH)突变后会导致严重的小眼症[19]。已有研究表明BPA暴露母体后会增加雄性小鼠肝脏中全反式视黄酸含量并上调了RA合成相关基因的表达,导致维甲酸信号通路异常[20]。因而,BPS是否会通过影响视黄酸信号通路并干扰感光细胞发育导致幼年斑马鱼视觉行为出现障碍需进一步探究。
综上,本文首先检测了1、10、100和1 000 μg·L-1 BPS暴露15 d对不同发育阶段斑马鱼幼鱼眼睛大小的影响。随后,采用组织切片方法观察幼鱼视网膜及其各层结构变化,并在分子水平进一步检测了斑马鱼幼鱼RA代谢、视蛋白基因表达量变化,旨在探究BPS对早期视网膜发育的影响。
1 材料与方法(Materials and methods)
1.1 材料
斑马鱼品系为Tuebingen (Tu)系,成年斑马鱼已在实验室内繁殖饲养一年以上。饲养条件如下: DO (7.0±0.1) mg·L-1,pH (7.8±0.2),水温(27±1) ℃,光暗比为14 h(L)∶10 h(D),每天早晚2次喂食适量新孵化的丰年虾。雌雄斑马鱼以1∶2的比例混合,次日开灯刺激其追逐产卵,收集健康的受精卵,清洗后用于暴露实验。
BPS纯度≥98%,购自Sigma-Aldrich (上海,中国)。暴露液配制使用二甲基亚砜(DMSO,分析纯,购自北京索莱宝公司,中国)作为助溶剂,储备液浓度为0.01 mg·L-1和1 mg·L-1。麻醉剂MS-222(纯度≥98%)、甲基纤维素均购自Sigma-Aldrich (上海,中国)。
1.2 双酚S暴露实验
实验采用半静态暴露方法,设置溶剂对照组(DMSO,体积比0.002%)和浓度为1、10、100和1 000 μg·L-1的BPS暴露组,每个暴露组设置3个烧杯。暴露至受精后12 h(12 hpf)挑取死卵,每天更换2/3的暴露溶液。待受精卵孵化24 h后开始喂食草履虫,自受精后10 d(10 dpf)起开始喂食新孵化的丰年虾(Artemia),暴露至15 dpf后取样。
1.3 斑马鱼幼鱼体长、体质量、头宽和眼睛直径测量
不同浓度BPS暴露斑马鱼胚胎至5、7和15 dpf后,每组取20条于10%中性福尔马林固定液中固定24 h后光学显微镜下观察,CCD拍照后采用Image J软件测量横轴方向眼睛长度、身体长度(沿体轴测量从头宽最前端到尾巴最后端的体长)和纵轴方向眼睛长度、头宽(图1),每组取30条幼鱼置于1.5 mL离心管中后称重,每组取6个平行样品。
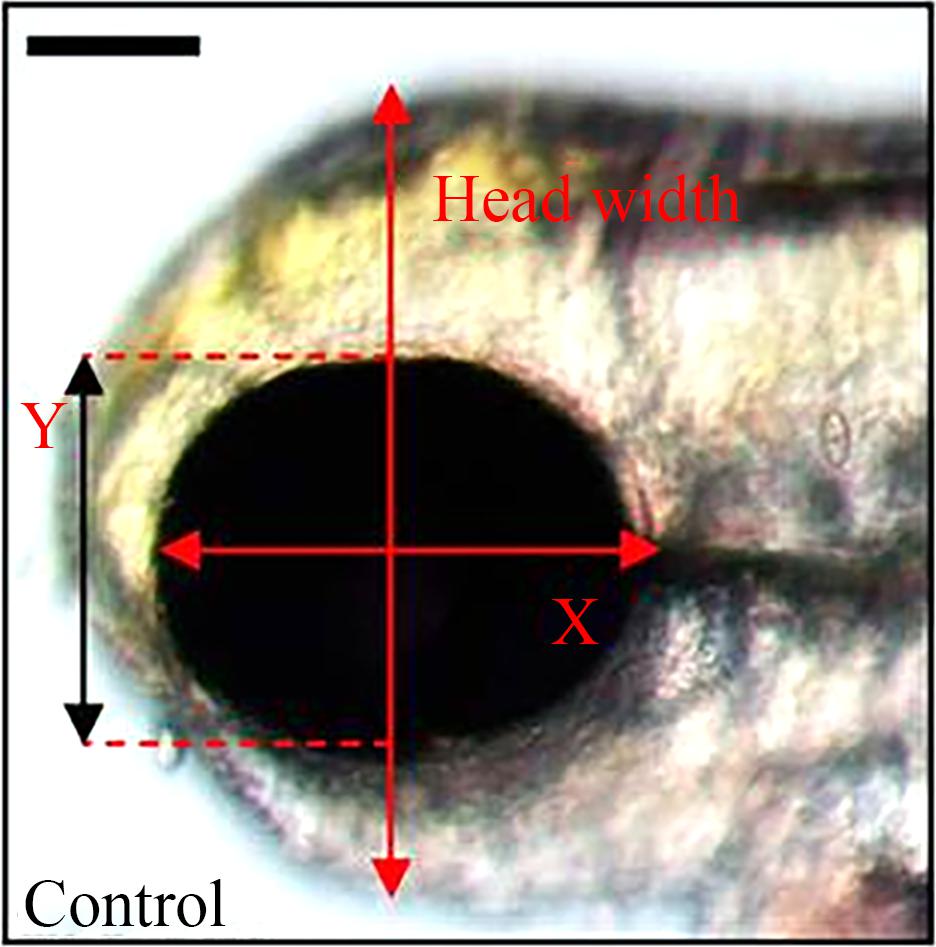
图1 斑马鱼幼鱼头宽、眼睛直径测量示意图[21]
Fig. 1 Arrows indicate locations of the measurements of the diameter of eye and the width of head[21]
1.4 石蜡切片的制备
随机取各组斑马鱼幼鱼若干,对其固定后乙醇梯度洗脱,石蜡包埋常规切片制片,使用苏木精-伊红染色,用中性树胶封片。光学显微镜下观察并拍照,选择有代表性的显微照片分析各暴露组斑马鱼幼鱼视网膜组织学变化,并用Image J软件测量各组眼睛各层结构:视网膜色素上皮(retinal pigmented epithelium, RPE)、外核层(outer nuclear layer, ONL)、内核层(inner nuclear layer, INL)、内丛状层(inner plexiform layer, IPL)、神经节细胞层(ganglion cell layer, GCL)、视网膜(包括RPE、ONL、INL、IPL和GCL)的厚度,每组选取7个鱼的眼睛,每个眼睛选取4个切片,每个眼睛选取视网膜中央部位测5处。
1.5 Real-time RT-PCR
Trizol(美国Invitrogen公司)法提取斑马鱼幼鱼总RNA后,使用微量分光光度计(美国Thermo公司,NanoDrop 2000c)检测RNA样品的OD230、OD260、OD280和RNA浓度,并使用琼脂糖凝胶电泳检测提取RNA的质量。按PrimeScriptTM RT Reagent Kit with gDNA Eraser试剂盒(日本TaKaRa公司)说明对等量的质量合格的RNA进行反转录。琼脂糖为Biowest公司产品;焦碳酸二乙酯(diethylpyrocarbonate, DEPC)、溴化乙啶(ethidium bromide, EB)为Sigma公司产品。
根据Genebank已发表的基因序列,利用引物设计软件Primer Premier 5.0 software (PREMIER Biosoft Int. Palo Alto, USA)按照实时定量PCR的引物设计原则设计目的基因的特异性引物。引物序列见表1。RT-PCR按照SYBR Premix Ex TaqTM Ⅱ Kit试剂盒(日本TaKaRa公司)说明,使用荧光定量PCR仪(德国Eppendorf公司,Eppendorf MasterCycler® ep RealPlex4)进行。反应程序设置为:95 ℃ 30 s;95 ℃5 s,60 ℃ 30 s,40 cycles;扩增后使用2%的琼脂糖凝胶对产物进行电泳检测,从而进一步确定扩增片段的特异性和长度。以β-actin和elf-α为内参基因计算目的基因的相对表达量。
表1 荧光定量引物序列
Table 1 Primer sequences for the genes

基因GeneGenBank号GenBank No.引物序列(5’~3’)Primer sequence (5’~3’)elfαNM_200009F: TGCCAGTGTTGCCTTCGTCR: AATCTTCCATCCCTTGAACCAGβ-actinBC045846F: GTTTTCCCCTCCATTGTTGR: GTAGAAGGTGTGATGCCAGATrbp4NM-130920F: CAGCAACTTCGCCGTCCAACAR: AACAGCCCAACAGGGTCTTTCTTGcrbp1aNM-199528F: CGATGTGAACACTGGCAGGATGAAR: AGTCCACCGTAGTTTGGCACTTTCrdh1NM-198069F: CTCATCGCTGCTGTCTGGTTCTTCR: GCCGCTGTCACATCCCGTTATCraldh2AF-339837F: TGAACTGCCAGGAGAGGTGAAGAAR: GCCGCTCACAGAATCATGCCATcrabp1aNM-182858F: TCAAGACCTCCACCACTGTCCGR: TTCTGCCATCCACCGTCTCCTCcrabp2aNM-182859F: GGACCACCAACGTCACCTTCACR: GTCTGTTACCCAGCGAGGAAAGCrarαaNM-131406F: AGGCTTCGGTCCGCTAACAGAR: CCGTCTCAGCATCGTCCATCTCTAcrxNM_152940F: CCCGTACCTTTCTCCCATGACCAR: CCAACGACGCAGTGCTGTATCCotx2NM_131251.1F: CCTCTATCTCGCCGCTGTCAGAR: TCCAGACGCTTGAGTGTAGGTCATrx2NM_131226.2F: CACAGACGCAACAGAACCACCTTR: AACTCGAACTTCAGGCAGGTTGACnrlNM_001040331.1F: TCCACCACCCACACCATCCAATR: ACGCTCTTCCACACCGCACTzfrhoL11014F: GTACGTCACCATCGAGCACAAGAAR: GAACACCATGAAGAGGTCGGCAATzfblueAF109372F: TTCGGTTCCTCGGTAGCGTTCTR: CACCACAGCAAGAGACCACAAACTzfredEH435011F: CCCAGCACAAGAAACTCCGACAGR: TGTAGCCCACAAGTGAGCAGTAGAzfgrBC060896F: GCCGAGAAGGAAGTGTCCAGAATGR: AAGCAGGCGAAGAACGTGTAAGGzfuvAF109373F: TGGTTGTTGTGATGGTTGGCTCTTR: GGCGGTAATCCTTGTTTGGCTCAT
1.6 统计学分析
应用SPSS(version 19.0; SPSS Inc., Chicago, IL, USA)软件对数据进行统计学分析。数据均以平均数±标准差形式表示,采用One-Way ANOVA和Tukey’s multiple range test检验各暴露组与对照组之间的统计学差异,P<0.05、P<0.01表示差异显著(用*、**标记)。
2 结果(Results)
2.1 双酚S短期暴露对斑马鱼体长、体质量和眼睛大小的影响
不同浓度的BPS暴露斑马鱼胚胎至5 dpf和7 dpf后,与溶剂对照组相比,各暴露组幼鱼的体长均没有显著性的变化,但暴露15 dpf后,100 μg·L-1暴露组斑马鱼的体长出现显著下降(图2(a))。经称量后发现,在各个暴露时间点,各暴露组幼鱼的体质量均没有显著性变化(图2(b))。空白对照组与溶剂对照组相比,均无显著性的变化。暴露15 dpf后,与溶剂对照组相比,10、100和1 000 μg·L-1暴露组X和Y的长度均显著减少(图2(d)和(e)),且100 μg·L-1暴露组的头宽也显著减少(图2(c))。相关性分析发现,15 dpf斑马鱼幼鱼眼睛的大小与头宽、体长均显著相关(表2),对体长、头宽与眼睛直径数据的比较表明10 μg·L-1和1 000 μg·L-1 BPS诱导15 dpf的斑马鱼幼鱼产生了小眼。

图2 不同浓度的双酚S(BPS)暴露斑马鱼胚胎至5、7和15 dpf后对斑马鱼幼鱼形态的影响
注:(a)对斑马鱼幼鱼体长的影响;(b)对斑马鱼幼鱼体质量的影响;(c)对斑马鱼幼鱼头宽的影响;(d)对斑马鱼幼鱼眼睛横向直径X的影响; (e)对斑马鱼幼鱼眼睛纵向直径Y的影响;SC表示二甲基亚砜(DMSO)溶剂对照;n=20;*表示P<0.05,**表示P<0.01。
Fig. 2 Effects on morphology in zebrafish larvae exposed to different concentrations of bisphenol S (BPS) from embryos to 5, 7 and 15 dpf
Note: (a) Length, (b) Weight, (c) Head width of larvae, (d), (e) The diameter of eye; SC stands for solution control of dimethyl sulfoxide (DMSO); n=20, *P<0.05, **P<0.01.
2.2 双酚S暴露对斑马鱼幼鱼视网膜结构的影响
如图3(a)~(e)所示,溶剂对照组和各暴露组斑马鱼幼鱼视网膜结构完整,各核层排布规则紧密。对视网膜及各核层定量结果表明(图3(f)),与溶剂对照组相比10 μg·L-1组GCL厚度显著减少了7.24%;100 μg·L-1和1 000 μg·L-1组INL厚度分别显著降低7.92%和14.86%;1 000 μg·L-1组ONL厚度显著降低了13.95%,且该组视网膜总厚度显著降低了6.03%。
2.3 双酚S暴露对斑马鱼幼鱼视黄酸信号通路的影响
与溶剂对照组相比,不同浓度的BPS暴露斑马鱼胚胎至15 dpf后,10、100和1 000 μg·L-1暴露组视黄醇结合蛋白基因(rbp4)的表达量显著上调;10 μg·L-1组中Ⅰ型细胞视黄醇结合蛋白基因(crbp1a)被显著上调;视黄醛脱氢酶基因(raldh2)的表达量在100 μg·L-1暴露组出现显著上调,且该组视黄醇脱氢酶基因(rdh1)的表达量出现显著下调;1 μg·L-1暴露组视黄酸结合蛋白基因Ⅰ(crabp1a)的表达显著上调,其余暴露组中视黄酸结合蛋白基因Ⅱ(crabp2a)均出现了显著性的上调;而视黄酸受体α(rarαa)的表达量在各暴露组均无显著性的变化(图4)。
2.4 双酚S暴露对斑马鱼幼鱼视觉发育相关基因的影响
使用不同浓度的BPS暴露斑马鱼受精卵至15 d后,与溶剂对照组相比,10 μg·L-1和100 μg·L-1组神经视网膜亮氨酸拉链(nrl)的表达被显著下调,其他与感光细胞发育相关基因表达无显著变化。除最低浓度1 μg·L-1暴露组外,其他各暴露组紫外敏感视蛋白(zfuv)的表达量均显著上调(P<0.01),10、100和1 000 μg·L-1暴露组蓝光敏感视蛋白(zfblue)的表达量也出现显著上调;10 μg·L-1和1 000 μg·L-1暴露组绿光敏感视蛋白(zfgr)的表达量显著上调(P<0.01),但是1 μg·L-1和100 μg·L-1暴露组无显著性变化;各暴露组视紫红质视蛋白(zfrho)和红光敏感视蛋白(zfred)的表达量均无显著性变化(图5)。
3 讨论(Discussion)
课题组前期研究表明,100 μg·L-1 BPS能够降低斑马鱼幼鱼的趋光性和视觉追踪能力,损伤其视觉功能[22]。视网膜发育障碍可能直接影响视觉功能,组织病理学分析表明,BPS于发育早期暴露15 d对斑马鱼幼鱼视网膜结构并无明显损伤,但低浓度组(10 μg·L-1)GCL、高浓度组ONL及INL厚度均显著降低。GCL主要由视网膜神经节细胞组成,该细胞是连接视网膜和大脑视觉中心的重要视网膜神经元[23],可整合来自光感受器的信息。GCL厚度的减小会导致眼睛或视觉处理中心功能障碍。
大量研究表明,视黄酸类物质对维持脊椎动物眼睛正常发育至关重要[24]。眼睛是动物体内维生素A含量最高的部位,也是其活性调控中心,缺乏维生素A的大鼠子代表现出包括小眼在内的多种眼部缺陷[25]。本研究结果发现,低浓度(10 μg·L-1)及高浓度的BPS暴露导致15 dpf斑马鱼幼鱼眼睛横向直径X和纵向直径Y均显著减小,出现小眼。在斑马鱼胚胎发育过程中,视黄醇与视黄醇结合蛋白结合,通过血液运输至眼部等靶器官。眼中的视黄醇由Ⅰ型细胞视黄醇结合蛋白运输至靶细胞,在视黄醇脱氢酶的催化下转化为视黄醛,该反应为RA生成中的限速步骤,而过量视黄醛则在RALDH催化下不可逆地氧化为RA。本研究中100 μg·L-1 BPS暴露组rbp4和crbp1a表达显著上调、rdh1表达显著下调,表明BPS导致幼鱼眼中视黄醛生成过程受损,可能导致视黄醇累积、RA生成减少。虽然100 μg·L-1 BPS暴露导致斑马鱼幼鱼raldh2基因表达量显著上调,但课题组相关研究结果发现该浓度BPS导致幼鱼眼中RA含量显著降低(未发表)。Begemann等[26]研究发现,RA可通过作用于raldh2启动子进而负反馈调节该基因的表达[27-28]。本研究高浓度BPS暴露组raldh2表达量显著上调,可能是由于RA不足的负反馈作用所致。
表2 BPS暴露后斑马鱼的头宽、体长和眼睛直径之间的皮尔逊相关性分析
Table 2 Pearson’s correlation analysis of head width, body length and eye diameter in zebrafish exposed to BPS
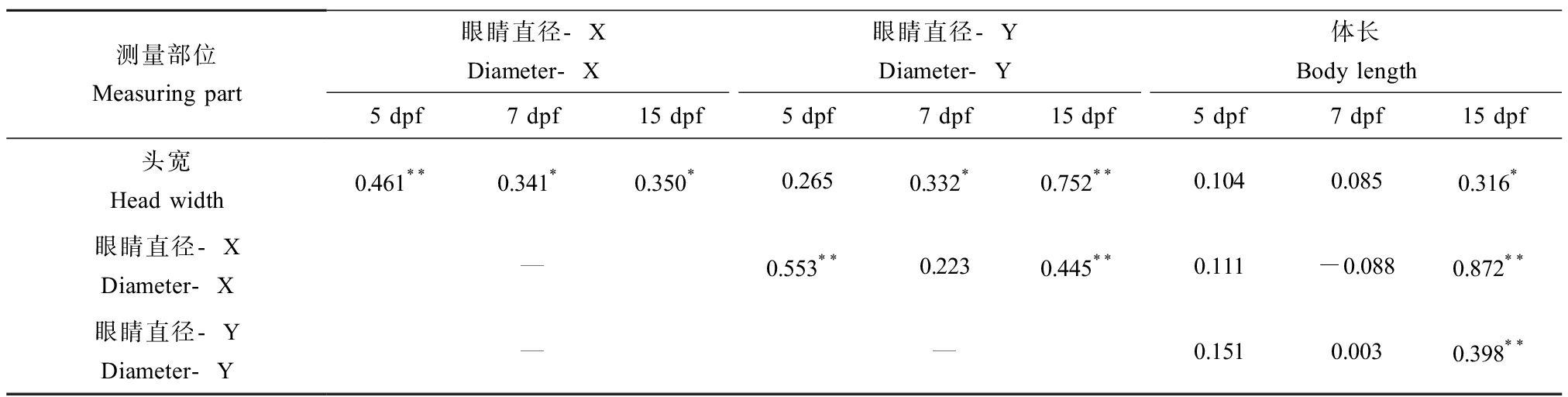
测量部位Measuring part眼睛直径-XDiameter-X眼睛直径-YDiameter-Y体长Body length5 dpf7 dpf15 dpf5 dpf7 dpf15 dpf5 dpf7 dpf15 dpf头宽Head width0.461**0.341*0.350*0.2650.332*0.752**0.1040.0850.316*眼睛直径-XDiameter-X—0.553**0.2230.445**0.111-0.0880.872**眼睛直径-YDiameter-Y——0.1510.0030.398**
注:数据为皮尔逊相关系数;n=50;*表示P<0.05,**表示P<0.01。
Note: Data in the figure are Pearson’s correlation coefficients; n=50; *P<0.05, **P<0.01.

图3 不同浓度BPS暴露斑马鱼胚胎至15 dpf对幼鱼视网膜结构及厚度的影响
注:(a) 溶剂对照组;(b) 1 μg·L-1 BPS暴露组;(c) 10 μg·L-1 BPS暴露组;(d) 100 μg·L-1 BPS暴露组;(e) 1 000 μg·L-1 BPS暴露组; (f)不同浓度BPS对15 dpf幼鱼视网膜及各核层厚度的影响;GCL表示神经节细胞层,IPL表示内丛状层,INL表示内核层, ONL表示外核层,RPE表示视网膜色素上皮层;标尺为50 μm;n=10;*表示P<0.05,**表示P<0.01。
Fig. 3 Histopathological analysis of larval zebrafish retina structure in the solvent control and different concentrations of BPS groups
Note: (a) The solvent control group; (b) 1 μg·L-1 BPS treated group; (c) 10 μg·L-1 BPS treated group; (d) 100 μg·L-1 BPS treated group; (e) 1 000 μg·L-1 BPS treated group; (f) Thickness of retina and nuclear layers of zebrafish larvae; GCL stands for ganglion cell layer, IPL stands for inner plexiform layer, INL stands for inner nuclear layer, ONL stands for outer nuclear layer, and RPE stands for retinal pigmented epithelium; scale bar is 50 μm; n=10; *P<0.05, **P<0.01.

图4 不同浓度(1、10、100和1 000 μg·L-1)BPS暴露斑马鱼胚胎至15 dpf对幼鱼视黄酸信号通路相关基因表达的影响
注:n=6;*表示P<0.05,**表示P<0.01。
Fig. 4 Effects on the expression of retinoid signaling-related genes in zebrafish larvae exposed to 1, 10, 100, and 1 000 μg·L-1 BPS from embryos to 15 dpf
Note: n=6; *P<0.05, **P<0.01.
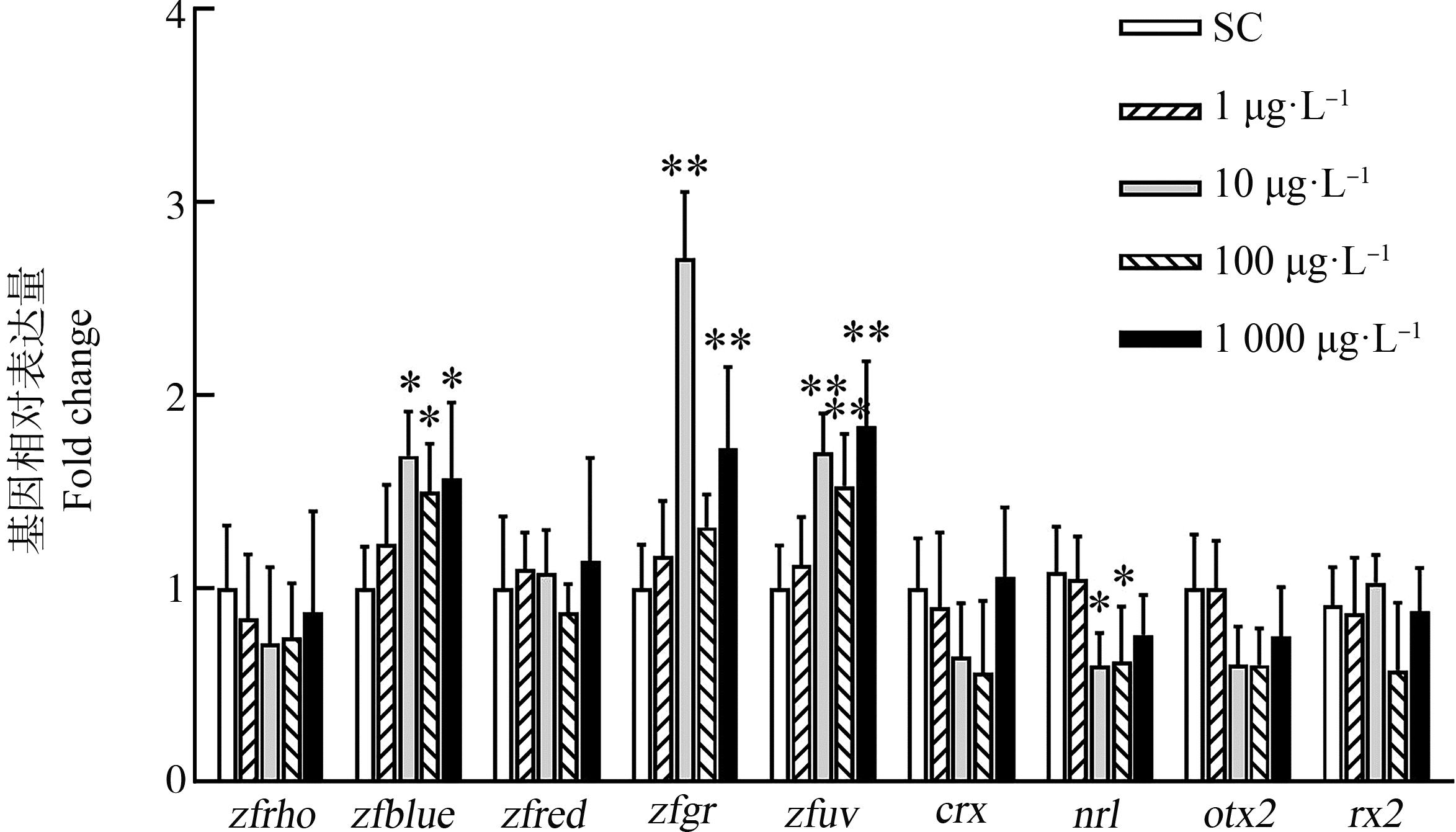
图5 不同浓度(1、10、100和1 000 μg·L-1)BPS暴露斑马鱼胚胎至15 dpf对幼鱼眼睛 发育(crx、otx2、rx2和nrl)和视蛋白相关基因(zfred、zfgr、zfrho、zfblue和zfuv)表达的影响
注:n=6;*表示P<0.05,**表示P<0.01。
Fig. 5 Effects on the expression of eye development-related genes (crx, otx2, rx2 and nrl) and opsin genes (zfred, zfgr, zfrho, zfblue, and zfuv) in zebrafish larvae exposed to 1, 10, 100 and 1 000 μg·L-1 BPS from embryos to 15 dpf
Note: n=6; *P<0.05, **P<0.01.
胞内的RA被视黄酸结合蛋白(cellular retinoic acid binding protein, CRABP)运输至细胞核后,将与视黄酸受体(retinoic acid receptors/retinoid X receptors, RARs/RXRs)结合,可启动与眼睛发育相关的特定基因的转录。Sharma等[29]发现CRABPs可以调节RA与其核受体RAR结合,从而在靶基因的转录启动中发挥重要作用。斑马鱼具有2种CRABP异构体(CRABP Ⅰ和CRABP Ⅱ),CRABP Ⅰ主要负责运送细胞色素P450家族酶解的RA;当RA与CRABPⅡ结合后,将被转运至细胞核,以驱动RARs/RXRs介导的RA靶向基因表达[30]。在大多数动物中,维甲酸类化合物无法从头合成,需从外界摄取并在需要时输送至靶器官[25]。本研究中,BPS暴露显著上调crabp2a表达量,可能是由于RA生成不足产生的补偿性反应,需要更多的RA转运到细胞核中,从而与RARs结合来调节目标基因转录[31]。RA是脊椎动物视觉系统中光感受器发育的重要信号分子[32],RA缺失会导致包括视网膜发育障碍[33-34]、OKR减弱等视觉损伤,是本研究中高浓度组视网膜核层变薄的可能原因。
与视黄醛共价结合的视蛋白是由ONL处的光感受器合成的光敏蛋白质,是脊椎动物光感受器中视觉色素的重要组成部分[35],不同的视蛋白决定视色素的光谱敏感性。本研究中,低浓度(10 μg·L-1)及高浓度BPS显著上调了zfblue、zfuv和zfgr基因表达量,增强了对蓝光、紫外和绿光的敏感性。接触多溴联苯醚[36]和BPS[13]等持久性有毒物质会改变光感受器视蛋白基因的转录,从而导致视觉行为改变。且本研究与Qiu等[22]的研究结果一致,即BPS会上调绿锥和紫外锥等视锥视蛋白基因的表达。这些结果表明,为了提高对光的敏锐度,斑马鱼对眼部光感受器中视黄醛供应减少和RA信号传导的干扰可能存在补偿性反应。本研究中otx2、crx和rx2在15 dpf的基因转录水平无显著性变化,推测是由于它们主要在光感受器的早期发育中发挥其主要作用[37-38]。本研究还发现,低浓度(10 μg·L-1)及高浓度(100 μg·L-1)BPS处理会下调nrl的转录水平。敲除该基因的斑马鱼在幼年时缺乏视杆细胞,并且有过量的紫外锥[39]。因此nrl下调表明BPS暴露致使视杆细胞发育受阻,推测是ONL厚度降低,视蛋白zfuv显著上调的原因之一。
已有研究报道了BPS对鱼类的视觉毒性,但其对视网膜早期发育的影响研究还较为缺乏。本研究中,我们发现10、100和1 000 μg·L-1 BPS抑制了RA的合成并下调了感光细胞发育相关基因的表达,减小了视网膜核层中GCL、INL和ONL的厚度,导致斑马鱼视网膜早期发育障碍,诱导15 dpf斑马鱼幼鱼出现小眼现象。100 μg·L-1 BPS暴露组中斑马鱼幼鱼可能通过上调相应视蛋白基因表达,增强对蓝光、紫外和绿光的敏感性而对上述发育障碍进行补偿调节。综上,本研究表明BPS短期暴露即会对早期视网膜发育产生毒性,完善的视觉功能对鱼类的觅食[40-41]、避敌[42]和求偶[43]等行为至关重要,因而BPS的早期发育视觉毒性可能对鱼类的生存繁衍构成严重威胁。且随着BPS的广泛使用,婴幼儿的暴露风险不断增加,所造成的潜在健康问题如视网膜发育不良等的风险也不容忽视。
[1] Yan Z Y, Liu Y H, Yan K, et al. Bisphenol analogues in surface water and sediment from the shallow Chinese freshwater lakes: Occurrence, distribution, source apportionment, and ecological and human health risk [J]. Chemosphere, 2017, 184: 318-328
[2] Jin H B, Zhu L Y. Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China [J]. Water Research, 2016, 103: 343-351
[3] Wu L H, Zhang X M, Wang F, et al. Occurrence of bisphenol S in the environment and implications for human exposure: A short review [J]. The Science of the Total Environment, 2018, 615: 87-98
[4] Liao C Y, Liu F, Alomirah H, et al. Bisphenol S in urine from the United States and seven Asian countries: Occurrence and human exposures [J]. Environmental Science &Technology, 2012, 46(12): 6860-6866
[5] Rochester J R, Bolden A L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes [J]. Environmental Health Perspectives, 2015, 123(7): 643-650
[6] Huang C, Wu L H, Liu G Q, et al. Occurrence and ecological risk assessment of eight endocrine-disrupting chemicals in urban river water and sediments of South China [J]. Archives of Environmental Contamination and Toxicology, 2018, 75(2): 224-235
[7] Li A J, Zhuang T F, Shi W, et al. Serum concentration of bisphenol analogues in pregnant women in China [J]. The Science of the Total Environment, 2020, 707: 136100
[8] Liu Y H, Yan Z Y, Zhang Q, et al. Urinary levels, composition profile and cumulative risk of bisphenols in preschool-aged children from Nanjing suburb, China [J]. Ecotoxicology and Environmental Safety, 2019, 172: 444-450
[9] Fox D A. Retinal and visual system: Occupational and environmental toxicology [J]. Handbook of Clinical Neurology, 2015, 131: 325-340
[10] Maurya M, Bora K, Blomfield A K, et al. Oxidative stress in retinal pigment epithelium degeneration: From pathogenesis to therapeutic targets in dry age-related macular degeneration [J]. Neural Regeneration Research, 2023, 18(10): 2173-2181
[11] Li M H, Yang T, Gao L X, et al. An inadvertent issue of human retina exposure to endocrine disrupting chemicals: A safety assessment [J]. Chemosphere, 2021, 264(Pt 1): 128484
[12] Pannetier P, Poulsen R, Gölz L, et al. Reversibility of thyroid hormone system-disrupting effects on eye and thyroid follicle development in zebrafish (Danio rerio) embryos [J]. Environmental Toxicology and Chemistry, 2023, 42(6): 1276-1292
[13] Liu W M, Zhang X N, Wei P H, et al. Long-term exposure to bisphenol S damages the visual system and reduces the tracking capability of male zebrafish (Danio rerio) [J]. Journal of Applied Toxicology, 2018, 38(2): 248-258
[14] Wei S H, Qiu L G, Ru S G, et al. Bisphenol S disrupts opsins gene expression and impairs the light-sensing function via antagonizing TH-TRβ signaling pathway in zebrafish larvae [J]. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 2023, 172: 113588
[15] Lobo G P, Pauer G, Lipschutz J H, et al. The retinol-binding protein receptor 2 (Rbpr2) is required for photoreceptor survival and visual function in the zebrafish [J]. Advances in Experimental Medicine and Biology, 2018, 1074: 569-576
[16] Prabhudesai S N, Cameron D A, Stenkamp D L. Targeted effects of retinoic acid signaling upon photoreceptor development in zebrafish [J]. Developmental Biology, 2005, 287(1): 157-167
[17] Sanjurjo-Soriano C, Erkilic N, Damodar K, et al. Retinoic acid delays initial photoreceptor differentiation and results in a highly structured mature retinal organoid [J]. Stem Cell Research &Therapy, 2022, 13(1): 478
[18] Cho K, Lee S M, Heo J, et al. Retinaldehyde dehydrogenase inhibition-related adverse outcome pathway: Potential risk of retinoic acid synthesis inhibition during embryogenesis [J]. Toxins, 2021, 13(11): 739
[19] Fares-Taie L, Gerber S, Chassaing N, et al. ALDH1A3 mutations cause recessive anophthalmia and microphthalmia [J]. American Journal of Human Genetics, 2013, 92(2): 265-270
[20] Esteban J, Serrano-Maciá M, Sánchez-Pérez I, et al. In utero exposure to bisphenol-A disrupts key elements of retinoid system in male mice offspring [J]. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 2019, 126: 142-151
[21] Huang L X, Wang C G, Zhang Y Y, et al. Phenanthrene causes ocular developmental toxicity in zebrafish embryos and the possible mechanisms involved [J]. Journal of Hazardous Materials, 2013, 261: 172-180
[22] Qiu L G, Wei S H, Yang Y X, et al. Mechanism of bisphenol S exposure on color sensitivity of zebrafish larvae [J]. Environmental Pollution, 2023, 316(Pt 2): 120670
[23] Bilotta J, Saszik S. The zebrafish as a model visual system [J]. International Journal of Developmental Neuroscience, 2001, 19(7): 621-629
[24] Amann P M, Eichmüller S B, Schmidt J, et al. Regulation of gene expression by retinoids [J]. Current Medicinal Chemistry, 2011, 18(9): 1405-1412
[25] Thompson B, Katsanis N, Apostolopoulos N, et al. Genetics and functions of the retinoic acid pathway, with special emphasis on the eye [J]. Human Genomics, 2019, 13(1): 61
[26] Begemann G, Schilling T F, Rauch G J, et al. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain [J]. Development, 2001, 128(16): 3081-3094
[27] Dobbs-McAuliffe B, Zhao Q S, Linney E. Feedback mechanisms regulate retinoic acid production and degradation in the zebrafish embryo [J]. Mechanisms of Development, 2004, 121(4): 339-350
[28] Wang C, Kane M A, Napoli J L. Multipleretinol and retinal dehydrogenases catalyze all-trans-retinoic acid biosynthesis in astrocytes [J]. Journal of Biological Chemistry, 2011, 286(8): 6542-6553
[29] Sharma M K, Saxena V, Liu R Z, et al. Differential expression of the duplicated cellular retinoic acid-binding protein 2 genes (crabp2a and crabp2b) during zebrafish embryonic development [J]. Gene Expression Patterns, 2005, 5(3): 371-379
[30] Nagpal I, Wei L N. All-trans retinoic acid as a versatile cytosolic signal modulator mediated by CRABP1 [J]. International Journal of Molecular Sciences, 2019, 20(15): 3610
[31] Novák J, Beníšek M, Hilscherová K. Disruption of retinoid transport, metabolism and signaling by environmental pollutants [J]. Environment International, 2008, 34(6): 898-913
[32] Isla-Magrané H, Zufiaurre-Seijo M, García-Arumí J, et al. All-trans retinoic acid modulates pigmentation, neuroretinal maturation, and corneal transparency in human multiocular organoids [J]. Stem Cell Research &Therapy, 2022, 13(1): 376
[33] Chen X F, Lin Z C, Qi Z H, et al. Effects of pollutant toxicity on the eyes of aquatic life monitored by visual dysfunction in zebrafish: A review [J]. Environmental Chemistry Letters, 2023, 21(2): 1177-1201
[34] Zhang S Y, Gan X F, Shen B G, et al. 6PPD and its metabolite 6PPDQ induce different developmental toxicities and phenotypes in embryonic zebrafish [J]. Journal of Hazardous Materials, 2023, 455: 131601
[35] Chinen A, Hamaoka T, Yamada Y, et al. Gene duplication and spectral diversification of cone visual pigments of zebrafish [J]. Genetics, 2003, 163(2): 663-675
[36] Chen L G, Huang Y B, Huang C J, et al. Acute exposure to DE-71 causes alterations in visual behavior in zebrafish larvae [J]. Environmental Toxicology and Chemistry, 2013, 32(6): 1370-1375
[37] Garelli A, Rotstein N P, Politi L E. Docosahexaenoic acid promotes photoreceptor differentiation without altering Crx expression [J]. Investigative Ophthalmology &Visual Science, 2006, 47(7): 3017-3027
[38] An M J, Lee H M, Kim C H, et al. c-Jun N-terminal kinase 1 (JNK1) phosphorylates OTX2 transcription factor that regulates early retinal development [J]. Genes &Genomics, 2023, 45(4): 429-435
[39] Oel A P, Neil G J, Dong E M, et al. Nrl is dispensable for specification of rod photoreceptors in adult zebrafish despite its deeply conserved requirement earlier in ontogeny [J]. iScience, 2020, 23(12): 101805
[40] 张瑞祺, 郝月月, 宋银都, 等. 鳜视觉和侧线感觉调控捕食行为的动态观察[J]. 中国水产科学, 2020, 27(10): 1136-1144
Zhang R Q, Hao Y Y, Song Y D, et al. Predation behavior of mandarin fish (Siniperca chuatsi) regulated by visual and lateral line sensory [J]. Journal of Fishery Sciences of China, 2020, 27(10): 1136-1144 (in Chinese)
[41] Kamermans M, Hawryshyn C. Teleost polarization vision: How it might work and what it might be good for [J]. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 2011, 366(1565): 742-756
[42] Mussen T D, Cech J J Jr. The roles of vision and the lateral-line system in Sacramento splittail’s fish-screen avoidance behaviors: Evaluating vibrating screens as potential fish deterrents [J]. Environmental Biology of Fishes, 2013, 96(8): 971-980
[43] Camargo-dos-Santos B, Gonçalves B B, Bellot M S, et al. Water turbidity-induced alterations in coloration and courtship behavior of male guppies (Poecilia reticulata) [J]. Acta Ethologica, 2021, 24(2): 127-136