自1907年塑料发明以来,塑料制品已被应用在人们生产生活的各个领域[1]。在过去的几十年里,全球塑料产量激增,预计到2050年,将达到12亿t[2]。自然界中的塑料可通过机械磨损、紫外辐射以及生物降解等作用下分解成<5 mm的塑料碎片[3],也被称为微塑料。已有的研究证据表明在自然界的各个环境中如大气[4]、水体[5]、土壤[6]和海洋沉积物[7]等中发现了微塑料,甚至在瓶装水[8]、啤酒[9]、茶叶[9]和鱼罐头[10]中也发现了微塑料的存在。自然环境和生活环境中的微塑料主要通过摄入、吸入和皮肤暴露等途径进入人体。当其他纳米颗粒经不同暴露方式进入人体后,一旦到达肺部就会通过穿越肺泡上皮细胞进入间质组织或经由淋巴循环后进入血液[11],而微塑料主要通过穿越胃肠道黏膜接触等途径进入到血液中[12],经由血液循环分布至全身,对人体产生潜在的健康风险。由于微塑料最早在海洋环境中被发现,因此早期的微塑料健康风险研究通常集中在水生鱼类[13]和贝类[14],但由于身体结构以及生活环境上的差异,不能很好地反映人体微塑料暴露的健康风险。但近年来随着啮齿动物微塑料健康风险模型的研究逐渐增多,对评估微塑料暴露对人体的健康风险提供了更好的参考。为此,本文系统的综述了微塑料暴露在啮齿动物中的蓄积部位和蓄积量及其影响因素,毒性效应影响因素、单一微塑料暴露和与其他污染物联合暴露下的毒性效应及机制,微塑料的浸出液及添加剂的毒性效应,为评估微塑料暴露对人体的健康风险提供参考。
1 组织积累(Organizational accumulation)
啮齿动物摄入微塑料后可在体内多器官蓄积,可以通过荧光标记法、拉曼光谱以及全动物成像等方法直观观察到其在体内蓄积的部位。微塑料在体内蓄积的部位以及蓄积量又受到暴露方式以及微塑料粒径大小的影响,口服和吸入微塑料后会分别在其胃肠道和肺部产生大量蓄积,又可以经循环系统蓄积在体内各组织中;同时小粒径微塑料可穿越体内各屏障,更容易在体内产生较大的蓄积量。
1.1 蓄积部位
将啮齿动物暴露于荧光微塑料或荧光标记的微塑料后,可以通过多种方式检测到组织中的微塑料蓄积。Liu等[15]通过激光共聚焦显微镜在小鼠冷冻切片后的卵巢组织中发现了荧光微塑料的存在,通过荧光光谱法在小鼠新鲜组织如心脏、肝脏、脾脏等器官中均检测到了微塑料的荧光信号。同样的,Fan等[16]将小鼠暴露于罗丹明B标记的微塑料后发现,通过荧光光度计可在小鼠的肺部、心脏和脑中发现荧光信号。除此之外,拉曼光谱分析通常作为物质理化检验的常用方法之一,Schwarzfischer等[17]通过拉曼光谱相对应的拉曼峰检测到微塑料可在小鼠的不同组织中蓄积,如小肠、脾脏和肝脏等。由于局部组织观察区域的有限以及组织大小的限制,为了进一步说明微塑料摄入后的生物组织积累,Amereh等[18]将大鼠荧光微塑料暴露后,采用全动物荧光成像系统观察到摄入的微塑料在全身各处都有分布,但主要在睾丸区域聚集,且聚集程度最高。有关微塑料在啮齿动物体内蓄积部位如表1所示[14-17]。
1.2 影响因素
1.2.1 暴露方式
自然界中的微塑料可通过口服、吸入和皮肤接触等途径进入生物体内,并通过循环系统逐渐分布至全身[19]。在啮齿动物染毒模型中,灌胃(饮水)染毒以及气管滴注染毒是最常用的2种方式。由于灌胃(饮水)染毒是模拟人体经口暴露微塑料,因此经灌胃(饮水)染毒后微塑料通常会大量蓄积在胃肠道组织[20,21]。但目前有证据表明,微塑料经口暴露后会进入血液[22],经循环系统分布至全身,因此在其他组织如肝脏[15]和肾脏[23]中也发现了少量微塑料存在。气管滴注染毒是模拟人体吸入暴露微塑料,这也导致了经气管滴注染毒后微塑料主要蓄积部位为肺组织[24]。此外,微塑料经吸入暴露后同样会进入血液循环中,并蓄积在心脏和脾脏中[25]。皮肤接触暴露微塑料在啮齿动物染毒模型中还未见应用,但啮齿动物皮肤接触其他微纳米颗粒已经被证实能够渗透皮肤并引发皮肤损伤[26],因此开展相关领域的研究尤为必要。
1.2.2 粒径
不同的粒径大小会影响微塑料在生物体内不同部位的蓄积程度。总体来看,小粒径微塑料更容易在生物体内蓄积,Yang等[27]将小鼠暴露于100 nm、3 μm和10 μm 3种粒径的微塑料后,在皮下、肾脏和睾丸周围脂肪中发现了100 nm的微塑料蓄积,而3 μm和10 μm的微塑料未发现;在小脑和睾丸组织中发现了100 nm和3 μm的微塑料蓄积,而10 μm的微塑料未发现。Deng等[19]发现小鼠暴露于5 μm和20 μm粒径的微塑料后,5 μm的微塑料在肾脏和肠道中的蓄积量显著高于20 μm的微塑料。除此之外,小粒径微塑料在体内会穿过血睾屏障和血脑屏障进而在睾丸和脑组织组织中蓄积,Jin等[28]发现0.4 μm和4 μm大小的微塑料均可以穿过小鼠血睾屏障在睾丸组织中蓄积;Shan等[29]发现50 nm的微塑料会诱导小鼠脑内血脑屏障的通透性增加,在脑部蓄积。
2 毒性效应影响因素(Factors influencing toxic effects)
微塑料所致啮齿动物毒性效应主要受粒径大小及聚合物类型的不同所影响。微塑料在其体内的吸收和分布具有粒径依赖性,小粒径微塑料更容易在体内蓄积,因而往往会诱发更大的毒性效应。不同类型的塑料制品均会产生微塑料,使得微塑料的理化性质会因材质不同而有所区别,影响其对啮齿动物的毒性效应。
2.1 粒径
微塑料在小鼠体内的吸收和分布具有粒径依赖性,粒径越小的微塑料越容易在其体内蓄积[27],且蓄积得更多[30]。Meng等[31]发现小鼠暴露50 nm大小的微塑料会诱发明显的肾小管扩张,而300 nm、600 nm和4 μm暴露下肾小管偶见扩张。Liu等[32]发现小鼠暴露于100 nm的微塑料相比于5 μm的微塑料会诱发更严重的肝毒性,具体表现在出现了更明显的肝脏内脂滴的积聚以及更明显的氧化损伤。Wang等[33]研究了1~10 μm和50~100 μm 2种粒径的微塑料对小鼠泌尿系统的损害,发现前者能够引起小鼠膀胱更严重的坏死和氧化损伤,后者则能够引起更高程度的炎性损伤。尽管小粒径微塑料被证实能够产生更大的毒性效应,但 Liang等[21]发现当小鼠同时暴露50 nm和500 nm粒径的微塑料后,能显著提高微塑料的生物利用度和生物分布,所产生的毒性效应远远超过单独暴露一种粒径的微塑料。
2.2 聚合物类型
不同类型的塑料均可以产生微塑料,但由于其材质不同导致各微塑料的理化性质如表面官能团修饰、电位以及结晶度等特征也不尽相同,影响其毒性效应。Xie等[34]研究发现小鼠暴露于聚苯乙烯、聚乙烯、聚氯乙烯、聚对苯二甲酸乙二醇酯和聚丙烯微塑料7 d后,各微塑料暴露组中小鼠均出现了结肠炎症损伤,按聚合物所致损伤大小依次排序为:聚苯乙烯>聚氯乙烯>聚对苯二甲酸乙二醇酯>聚丙烯。Danso等[35]对比了灌胃和气管滴注2种染毒方式下聚苯乙烯、聚丙烯和聚氯乙烯3种微塑料对小鼠的肺损伤作用,发现在灌胃染毒方式下聚苯乙烯会导致小鼠肺泡灌洗液(BALF)中细胞总数和中性粒细胞数显著上升,而聚丙烯和聚氯乙烯处理下无显著变化;在气管滴注染毒方式下聚苯乙烯会导致小鼠肺组织出现炎性细胞浸润,而其他2种微塑料处理下则未出现。
表1 微塑料在啮齿动物体内蓄积部位
Table 1 Accumulated site of microplastics in rodents
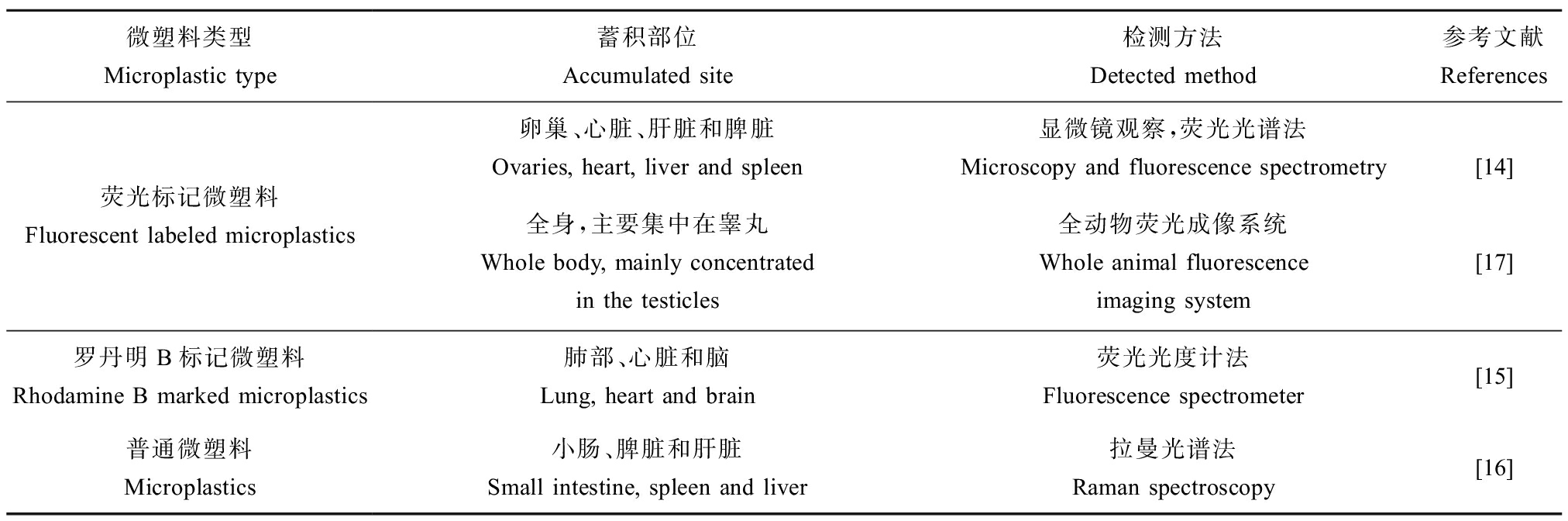
微塑料类型Microplastic type蓄积部位Accumulated site检测方法Detected method参考文献References荧光标记微塑料Fluorescent labeled microplastics卵巢、心脏、肝脏和脾脏Ovaries, heart, liver and spleen显微镜观察,荧光光谱法Microscopy and fluorescence spectrometry[14]全身,主要集中在睾丸Whole body, mainly concentrated in the testicles全动物荧光成像系统Whole animal fluorescence imaging system[17]罗丹明B标记微塑料Rhodamine B marked microplastics肺部、心脏和脑Lung, heart and brain荧光光度计法Fluorescence spectrometer[15]普通微塑料Microplastics小肠、脾脏和肝脏Small intestine, spleen and liver拉曼光谱法Raman spectroscopy[16]
3 单一暴露毒性效应及其机制(Toxic effects and mechanisms of single exposure)
自然环境中的塑料制品在风力以及机械降解等各种外力作用下被分解成微塑料,通过口服、吸入以及皮肤接触等途径进入到生物体内,对生殖、胃肠道、神经、肺脏、肝脏、肾脏、心血管及子代等多系统产生损伤,具体表现为组织病理变化、炎症作用、细胞凋亡、氧化损伤、微生物群落失调、代谢紊乱、相关激素水平异常以及子代发育障碍等毒性效应,如图1所示。
3.1 生殖毒性
生殖系统是生物体最敏感的系统之一,微塑料进入小鼠体内后,可破坏[36]并穿过血睾屏障,在睾丸组织中蓄积[27]。Jin等[28]发现,4 μm和10 μm大小的微塑料均可以通过血睾屏障进入小鼠睾丸组织中,诱发小鼠精子质量下降、睾丸生精细胞排列紊乱和睾酮(T)水平下降等生殖毒性效应。Xu等[37]发现25、50和100 nm 3种粒径的微塑料可穿透小鼠睾丸及附睾微结构,并在睾丸细胞和精子中蓄积,导致睾丸组织中正常生精小管百分比显著低于对照组,异常生精小管的百分比显著提高,诱发睾丸细胞凋亡。Xie等[38]将小鼠连续暴露于(0.01、0.1和1 mg·d-1)微塑料42 d后,发现微塑料可通过激活p38 MAPK信号通路诱发小鼠睾丸出现炎症损伤和细胞凋亡,通过显著提高睾丸组织中活性氧(ROS)和丙二醛(MDA)的含量诱发氧化损伤。此外,Jin等[39]研究发现小鼠慢性暴露低浓度微塑料后,微塑料可通过下调促黄体生成素(LH)介导的LHR/cAMP/PKA/StAR信号通路诱导睾酮水平的降低。微塑料暴露对于雌性个体同样存在损伤作用,主要体现在对卵巢组织的影响。Hou等[40]将雌性Wistar大鼠通过连续饮水暴露于(0.015、0.15和1.5 mg·kg-1)微塑料90 d后,次级卵泡颗粒层厚度变薄,卵泡生长数量变少;在同样的染毒浓度下,An等[41]发现微塑料可通过Wnt/β-Catenin信号通路激活导致大鼠卵巢纤维化,并通过氧化应激导致卵巢颗粒细胞凋亡。作为典型的多胎动物,大鼠和小鼠的胚胎吸收率可作为评价雌性生殖毒性的重要指标,Hu等[42]研究发现,母鼠在妊娠期暴露微塑料会使其体内蜕膜NK细胞减少,进而干扰体内免疫微环境导致胎儿流失,使母体胚胎吸收率升高。
3.2 胃肠道毒性
胃肠道是生物体内最重要的消化吸收器官,生物体摄入体内的微塑料更容易在胃肠道中产生蓄积。胃肠道组织病理、炎症因子表达以及相关氧化酶的变化均可以反映胃肠道的损伤情况,He等[43]发现小鼠暴露于5 μg·g-1浓度的微塑料2周后,十二指肠绒毛长度和隐窝深度明显下降,并且能通过驱动ROS驱动的NF-κB/NLRP3途径加重脂多糖(LPS)诱导的小鼠十二指肠炎症反应。同样的,Djouina等[44]发现小鼠暴露于36 μm和116 μm 2种粒径的微塑料6周后,小鼠近端小肠和远端小肠隐窝深度增加并出现绒毛萎缩。Liang等[21]通过氧化应激的激动剂和抑制剂来探究氧化应激损伤与肠道损伤的关系,发现小鼠肠道中ROS的增长能显著诱导肠上皮细胞凋亡,增加肠道通透性。肠道微生物群在调节生物体新陈代谢和宿主肥胖的发展中起着至关重要的作用,Lu等[45]将小鼠暴露于100 μg·L-1和1 000 μg·L-1微塑料 5周后,肠道微生物群失调,血清中肝甘油三酯(TG)和总胆固醇(TCH)水平下降,诱发肝脏脂质代谢紊乱;Li等[46]同样发现微塑料暴露后显著提高了小鼠肠道中葡萄球菌的丰度,引发肠道微生物群的失调。此外,微塑料暴露还可以加重患有胃肠道疾病小鼠的损伤,Liu等[47]发现,与未暴露微塑料的急性结肠炎小鼠相比,暴露微塑料的急性结肠炎小鼠会诱发更严重的肠黏膜组织病理损伤,对结肠微生物群落及代谢产生更大的干扰。

图1 微塑料暴露所致啮齿动物毒性作用
注:本图由Figdraw绘制。
Fig. 1 Toxic effects of microplastic exposure on rodents
Note: By Figdraw.
3.3 神经毒性
有研究发现微塑料暴露可通过小胶质细胞的吞噬作用蓄积在小鼠脑中[48],使小鼠脑部静止的小胶质细胞激活,表现为细胞核增大、分支短粗、端点体素减少[29],进而破坏血脑屏障[49],增加血脑屏障的通透性,产生神经毒性效应。Liang等[50]将小鼠暴露于0.25、2.5、25和250 mg·kg-1微塑料28 d后,发现微塑料可穿过小鼠的血脑屏障进入到小鼠大脑各处,蓄积在皮质和海马体中;引起小鼠神经行为活动减弱,发生PD样神经变性。学习和记忆能力通常作为神经毒性的一个重要指标,Wang等[51]将小鼠暴露于0.01、0.1和1 mg·d-1的微塑料4周后,发现微塑料可通过降低乙酰胆碱(ACh)水平以及诱导氧化应激来影响小鼠的学习和记忆能力。Lee等[52]发现,小鼠暴露在浓度为0.008 mg·g-1和0.016 mg·g-1的微塑料8周后,神经元活动依赖基因和突出蛋白的表达发生变化,诱发了神经炎症反应和行为学改变。Jin等[49]将小鼠通过饮水暴露于3种粒径(0.5、4和10 μm)2种浓度(100 μg·L-1和1 000 μg·L-1)微塑料180 d后,树突棘密度提高,海马体中肿瘤坏死因子α(TNF-α)、白细胞介素6(IL-6)和白细胞介素1b(IL-1b)等炎症相关基因的mRNA表达显著升高,诱发海马体出现炎症反应。
3.4 肺脏毒性
已经有证据表明微塑料广泛的存在于大气环境中[53],生物体很有可能从空气中吸入微塑料,并在肺部蓄积[54]。Cao等[55]研究发现小鼠连续鼻内滴注40 mg·kg-1微塑料3周后,可引起小鼠肺组织的炎症、凋亡和纤维化,具体表现为:肺组织基本被实变病灶所覆盖;细胞凋亡率增加和肺组织纤维化生物标志物α-SMA的表达显著增加。Xu等[56]发现小鼠暴露10 mg·mL-1微塑料28 d后,肺组织结构发生显著性变化,出现肺泡壁增厚和肺间质纤维化的现象。除此之外,Li等[57]研究发现氧化应激同样能诱导肺纤维化,将小鼠暴露微塑料3周后,引起小鼠肺组织中超氧化物歧化酶(SOD)的含量显著上升而引起氧化应激反应,进而导致肺部纤维化。Lee等[54]发现小鼠暴露500、1 000和2 000 mg·kg-1·d-1微塑料28 d后,小鼠肺部存在明显的微塑料蓄积,肺泡间隙出现肉芽肿性炎症,并伴有混合炎症细胞的出现。同样的,Wu等[58]通过转录组学分析发现微塑料可能通过激活NLRP3/capase-1/IL-1β信号通路来引起小鼠肺组织早期的炎症反应;Fan等[24]采用全基因组测序以及PCR验证等方式发现非编码RNA如:lncRNA XLOC _ 031479、circRNA 014924以及circRNA 006603可能在微塑料所致肺部炎症中发挥着重要作用。
3.5 肝脏毒性
肝脏作为机体最大的代谢器官之一,在许多代谢性疾病中发挥着重要的作用。微塑料暴露后可引起机体内肝脏代谢紊乱,诱发炎症损伤。胰岛素抵抗(IR)作为一种代谢性疾病,有研究表明小鼠暴露微塑料后可通过抑制胰岛素信号通路、调节肠道微生物群以及刺激炎症反应诱发小鼠出现胰岛素抵抗[59]。Wang等[60]将小鼠通过饮水暴露于100 μg·L-1和1 000 μg·L-1的微塑料8周后,发现小鼠的糖耐量显著降低,引发肝脏脂质沉积和脂质代谢紊乱。Fan等[16]研究发现,小鼠暴露于5 mg·kg-1和15 mg·kg-1浓度的微塑料均可以导致转氨酶水平升高,肝脏脂肪变性和肝细胞内脂滴的沉积。Choi等[61]发现小鼠连续暴露于100 μg·mL-1浓度的微塑料2周后,小鼠肝脏中炎症反应蛋白一氧化氮合酶(iNOS)和环氧化酶-2(COX-2)以及炎症细胞因子的mRNA的表达水平显著升高,诱发炎症损伤。Park等[20]发现小鼠暴露于0.5 mg·d-1微塑料60 d后,小鼠肝细胞微结构有明显的损伤作用,表现为肝细胞膨胀、空泡化和细胞间隙扩张;血清中甘油三酯(TG)、总胆汁酸(TBA)和总胆固醇(TCH)水平显著下降。此外,微塑料暴露也会使小鼠肝组织出现肝索结构[62]。
3.6 肾脏毒性
肾脏是研究微塑料对机体损害的重要靶器官之一,其对于生物体内超细颗粒的排泄是不可或缺的[31]。Meng等[31]发现小鼠暴露于5 mg·d-1微塑料28 d后,小鼠肾小管上皮明显脱落,肾小管出现扩张,肾囊腔偶见扩张,全肾出现严重的炎性细胞浸润;同样的,Choi等[61]发现小鼠暴露于微塑料后肾脏组织中炎症反应蛋白iNOS和COX-2以及炎症细胞因子的mRNA的表达水平显著升高,诱导肾脏出现炎症损伤。Shi等[63]将小鼠连续饮水暴露于1 000 μg·L-1浓度的微塑料180 d后,发现小鼠肾脏中肾管状细胞质空泡化和脂质下降,显著提高了肾损伤发生的概率。除病理损伤外,微塑料暴露还会通过相关生化指标的改变来诱导肾脏损伤,Xiong等[64]发现小鼠暴露于微塑料后可促进肾脏出现纤维化,并在转录组学上发现长期暴露微塑料可以改变与免疫应答和昼夜节律有关的多个mRNA的表达。Wang等[23]发现微塑料暴露会导致小鼠血清中肌酐(Cre)水平显著下降,尿蛋白含量显著提高。有证据表明微塑料暴露后同样会造成小鼠肾脏出现氧化损伤,李欢等[65]发现小鼠暴露于100 nm微塑料28 d后,肾脏中炎症因子TNF-α和IL-6表达显著提高,抗氧化酶的表达显著提高,提示肾脏出现炎症和氧化损伤。
3.7 心血管毒性
心脏作为微塑料暴露的靶组织之一,具有较大的研究价值。Lin等[66]研究发现,小鼠暴露于5 μg·g-1浓度的微塑料2周后,破坏了小鼠的心肌结构,纤维化蛋白和胶原水平升高,诱发心肌发生纤维化。Li等[67]发现微塑料可通过激活Wnt/β-catenin信号通路诱导小鼠出现心肌纤维化,并通过氧化应激和上调血清肌钙蛋白I(TnI)和肌酸激酶同工酶(CK-MB)水平触发心肌细胞凋亡,从而导致心血管毒性。心血管疾病(CVD)是全球主要的死亡原因之一,其危险因素主要包括代谢紊乱、糖尿病和肥胖等。Zhao等[68]将小鼠暴露于浓度为(0.1 μg·mL-1和1 μg·mL-1)微塑料12周后,小鼠肠道微生物的组成发生改变,空腹血糖升高以及体脂和体质量增加,这表明微塑料暴露很有可能诱发这些危险因素,诱发心血管疾病(CVD)的发生。以往的研究中在人类血液中发现了微塑料的存在[12],血管通常被认为是微塑料在体内循环的重要通道,微塑料暴露后可通过血管进入到各靶器官中,但微塑料对血管本身的损伤也是应该值得注意的,Wang等[69]研究发现,小鼠暴露于(5、10和20 mg·kg-1)3种浓度的微塑料后,所有暴露组小鼠血液中白细胞数量显著增加,动脉血管壁增厚并出现炎性细胞浸润,并通过激活JAK1/STAT3/TF信号通路诱导凝血功能障碍和血管栓塞的形成。除此之外,Wang等[70]研究发现微塑料暴露能够通过引起巨噬细胞受体(MARCO)上调、巨噬细胞活化以及脂质代谢紊乱诱发小鼠出现血管动脉粥样硬化损伤。
3.8 跨代毒性
微塑料不仅会直接对机体产生损害,而且会通过母体-子代的代际传递效应对子代产生跨代毒性。Yang等[71]在母鼠妊娠的第1天到第17天连续使其暴露微塑料,发现微塑料进入母体后,可通过胎盘屏障进入到子鼠大脑中,并在子鼠丘脑中蓄积。此外,Zhang等[72]研究发现母代在妊娠期暴露微塑料会导致后代小鼠出生体质量和出生后体质量下降,显著降低其雌性后代的卵母细胞成熟、受精率和胚胎发育。除了在妊娠期外,母代在哺乳期暴露微塑料仍可以对子代产生影响,Jeong等[73]发现母代在哺乳期暴露于50 nm和500 nm大小的微塑料,可通过母乳传递至子鼠体内,引起子鼠脑部发育异常,导致大脑功能障碍和认知障碍。除跨代神经毒性外,Huang等[74]还发现母代微塑料暴露会对子代产生跨代生殖毒性,具体表现为雄性子代小鼠的精子数量减少和睾丸氧化损伤。此外,Han等[75]发现母代在妊娠期和哺乳期暴露于微塑料后均会导致F1代小鼠体质量增加,肾脏、肺、心、肝和脾脏等器官的绝对质量显著提高。
4 与其他污染物的复合毒性(Combined toxicity with other pollutants)
微塑料具有粒径小、比表面积大及表面带有负电荷等特性,使得其能够作为一个有效的载体来吸附环境中的重金属、有机污染物及其他微生物,这些吸附了其他污染物的微塑料被生物体摄入后,使生物体同时暴露微塑料和有毒物质,增强了生物体对这些有毒物质的吸收率,相比于单一暴露微塑料显著提高了其毒性作用,如表2所示[76-85]。
4.1 重金属
环境中的微塑料可与重金属发生静电相互作用,使重金属吸附在微塑料表面,被生物吸收,导致重金属在生物体内蓄积。Liu等[76]研究发现微塑料与铁离子具有良好的结合能力,联合暴露后可增加铁离子在小鼠脑部的蓄积,干扰小鼠脑内铁稳态,诱发小鼠认知功能障碍。Feng等[77]研究发现雌性小鼠同时暴露于微塑料和铅离子时,子宫和卵巢脏器系数显著降低,卵泡数量减少,子宫内膜变薄,微塑料加重了铅离子对雌性小鼠的生殖损伤。自然环境中的镉(Cd)是一种剧毒的重金属,肾脏是其最重要的靶器官之一,Zou等[78]发现小鼠联合暴露于微塑料和镉离子90 d后,相比于单独暴露于镉离子加重了对肾小管、肾脏线粒体和细胞核的损伤,会诱发更显著的全身和肾脏氧化损伤的程度。
表2 微塑料与其他污染物的复合毒性
Table 2 Compound toxicity of microplastics and other pollutants
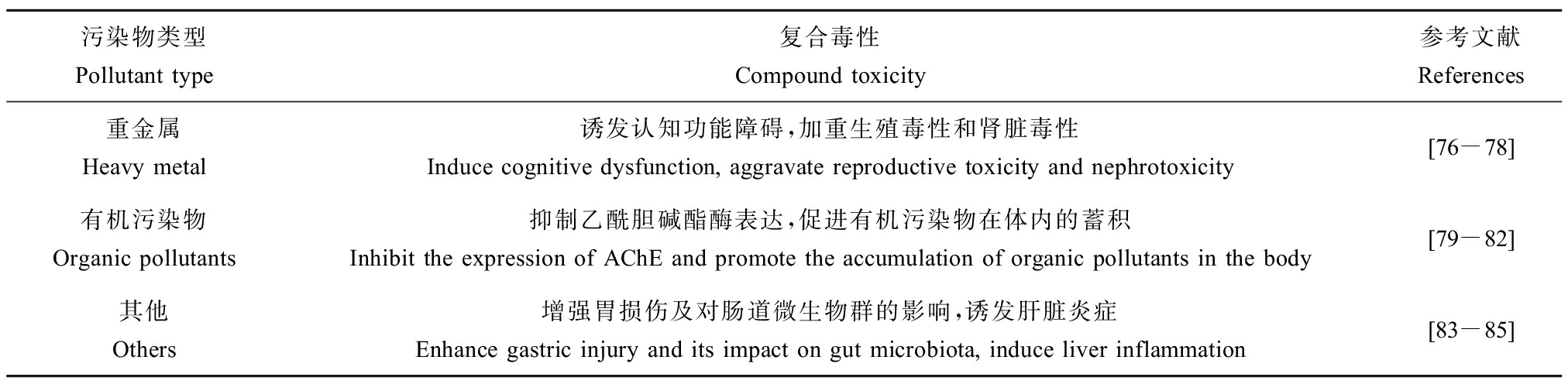
污染物类型Pollutant type复合毒性Compound toxicity参考文献References重金属Heavy metal诱发认知功能障碍,加重生殖毒性和肾脏毒性Induce cognitive dysfunction, aggravate reproductive toxicity and nephrotoxicity[76-78]有机污染物Organic pollutants抑制乙酰胆碱酯酶表达,促进有机污染物在体内的蓄积Inhibit the expression of AChE and promote the accumulation of organic pollutants in the body[79-82]其他Others增强胃损伤及对肠道微生物群的影响,诱发肝脏炎症Enhance gastric injury and its impact on gut microbiota, induce liver inflammation[83-85]
4.2 有机污染物
微塑料在自然界中可作为添加剂和环境污染物的载体,吸附环境中的有机污染物。Deng等[79]发现相比于单独暴露有机磷阻燃剂(OPFRs),微塑料与其联合暴露会干扰小鼠的氨基酸代谢和能量代谢,抑制小鼠脑中乙酰胆碱酯酶(AChE)的表达。同样的,Zhang等[80]发现微塑料与邻苯二甲酸二(2-乙基己基)酯(DEHP)联合暴露会使小鼠大脑线粒体发生肿胀和破裂,诱发氧化损伤,导致大脑神经元和细胞发生凋亡。除此之外,微塑料暴露还会促进有机污染物在生物体内蓄积,Deng等[81]将小鼠联合暴露于微塑料和邻苯二甲酸酯(PAEs),发现微塑料能显著促进邻苯二甲酸酯(PAEs)在小鼠肠道和肝脏中的蓄积,并诱发生殖毒性如精子质量下降和睾丸氧化损伤。Jiang等[82]发现微塑料单独或与三丁基锡(TBT)联合暴露均能降低小鼠血清中甘油三酯(TG)的表达水平,诱发肝脏炎症,提高肝脏中总胆汁酸(TBA)的含量;此外,联合暴露也会通过降低锡的生物利用度上来削弱锡单独暴露的毒性,如肝组织中谷草转氨酶(AST)和γ干扰素(IFN-γ)相比于对照组未显著升高以及肠道菌群未发生明显改变等。
4.3 其他
微塑料颗粒由于其比表面积较大,更容易吸附环境中的细菌和真菌等微生物,产生联合毒性。Tong等[83]研究发现,微塑料可作为载体吸附幽门螺旋杆菌,微塑料与幽门螺旋杆菌联合暴露后,会加重小鼠胃组织炎性细胞浸润,诱导更多的炎症因子,引起更严重的胃黏膜损伤。抗生素如今广泛应用于人类和动物体当中,与微塑料的共存是不可避免的,Liu等[84]发现磺胺甲噁唑(SMX)与微塑料的联合暴露会增强其对小鼠肠道微生物群和小鼠抗生素耐药性的影响。农药作为农业中广泛使用的化学品,与微塑料一起残留在环境和食物中并被人体摄入的风险很高,很可能对人体产生潜在的健康风险。Sun等[85]发现微塑料与环氧康唑联合暴露会抑制小鼠体质量增加,减少肝脏质量,引起肝内炎性细胞浸润和肾小管上皮空泡变形。
5 微塑料浸出液及添加剂毒性效应(Toxic effects of microplastics leachate and additives)
塑料制品是由高分子聚合物及各种添加剂构成,自然环境和生活环境中的塑料制品在各种复杂的环境下不断老化,经由各种外力作用,不但改变了其表面性质,而且加速了其聚合物和添加剂释放到环境中[86]。这些释放到环境中的聚合物和添加剂很容易浸出到周围环境中,对生物体产生潜在的健康风险,因此也被称为塑料浸出液[87]。而微塑料具有粒径小、比表面积大等特点,使得微塑料更容易向周围环境释放这些潜在的有害物质。一些生活中常见的塑料聚合物如聚苯乙烯(PS)、聚氯乙烯(PVC)以及丙烯腈(ABS)等在特定条件下都可浸出一些致癌和剧毒物质,如苯、氯化氢及丙烯腈单体等。当这些有毒物质暴露在啮齿动物染毒模型中时,会诱发其出现血液毒性[88]、胃肠道毒性[89]、免疫毒性[90]、肺部毒性[91]及生殖毒性[92]等。在塑料制品加工过程中,为了使塑料具备可塑性、延展性、稳定性以及阻燃性等特性,往往会添加一些添加剂,常见的塑料添加剂包括增塑剂、阻燃剂、抗氧化剂和稳定剂等。Deng等[93]发现微塑料可以吸附邻苯二甲酸脂类增塑剂(PAEs),将其转运到小鼠肠道中,增强肠道通透性和诱发炎症反应。Wang[94]将小鼠暴露在十溴二苯醚(BDE-209)、四溴双酚A(TBBPA)和六溴环十二烷(HBCD)3种溴化阻燃剂(BFRs)28 d后,发现小鼠的空间记忆能力受到损害,凋亡基因Caspase-3和bax的mRNA表达显著提高,诱发神经毒性。除增塑剂和阻燃剂外,Kazemi等[95]发现大鼠连续暴露于抗氧化剂壬基酚(NP)35 d后,显著增强了血清中碱性磷酸酶的表达,诱导肝损伤。塑料在制造过程中往往会添加一些重金属作为稳定剂,如铅、锌、锡等,这些重金属离子通常会对生物体产生毒性作用,Gąssowska等[96]发现新生大鼠短期暴露于铅(Pb)会促进脑内突触结构的病变,包括神经末梢肿胀、突触间隙结构模糊和增厚以及突触前区突触小泡密度增加。
6 总结与展望(Summary and prospect)
微塑料作为一种新型环境污染物,近年来受到国内外学者的广泛关注。自然界中的微塑料可被生物体摄入,并在体内蓄积,通过食物链的富集和传递作用逐步传递至人体内,对人体产生潜在的健康风险。随着微塑料生物毒性效应相关的研究不断增多,越来越多的学者关注到微塑料对啮齿动物的损害。正如文中所述,微塑料暴露会在啮齿动物体内多器官蓄积,不同粒径及暴露方式都会影响微塑料在其体内的蓄积部位及蓄积量;单一微塑料暴露会对啮齿动物多器官产生毒性效应,不同粒径和聚合物类型都会影响其毒性损伤,母代暴露微塑料也会对子代产生多种跨代毒性。除此之外,微塑料还会联合其他污染物如有机物、重金属和细菌等产生复合毒性或加重原有污染物的毒性作用,以及塑料浸出液和添加剂同样对啮齿动物存在着一定的毒性效应。
虽然目前已有大量相关的研究,但由于微塑料在自然环境中存在的多样性以及特殊的理化性质,仍有较多的问题亟待解决。首先,口服、吸入和皮肤接触等途径都能使微塑料进入到人体中,对人体产生潜在的健康风险。但目前啮齿动物染毒模型中常用的染毒方式为灌胃(饮水)染毒及气管滴注染毒,还未见皮肤暴露微塑料颗粒时对其毒性效应的报道,因此开展相关领域的研究是非常必要的。其次,已知不同尺寸和聚合物类型的微塑料都会影响微塑料对啮齿动物的毒性效应,但有关不同形状的微塑料是否会影响其生物毒性效应的研究尚存在不足,有必要开展相关研究为微塑料生物毒性效应的影响因素提供更多证据。最后,目前有较多的研究发现微塑料吸附其他环境污染物可能诱发更大的毒性效应,但其中具体吸附原理的研究尚存在不足,后续应多关注微塑料表面与污染物的吸附原理及微塑料的团聚效应与污染物相互作用。上述领域的深入研究有助于完善啮齿动物微塑料健康风险模型,使之更好地应用到人类健康风险评估中。
[1] Zhu X P, Ran W, Teng J, et al. Microplastic pollution in nearshore sediment from the Bohai Sea coastline [J]. Bulletin of Environmental Contamination and Toxicology, 2021, 107(4): 665-670
[2] Suaria G, Avio C G, Mineo A, et al. The Mediterranean Plastic Soup: Synthetic polymers in Mediterranean surface waters [J]. Scientific Reports, 2016, 6: 37551
[3] Qiao J Y, Chen R, Wang M J, et al. Perturbation of gut microbiota plays an important role in micro/nanoplastics-induced gut barrier dysfunction [J]. Nanoscale, 2021, 13(19): 8806-8816
[4] Fang M Z, Liao Z L, Ji X L, et al. Microplastic ingestion from atmospheric deposition during dining/drinking activities [J]. Journal of Hazardous Materials, 2022, 432: 128674
[5] Chen F J, Lao Q B, Liu M Y, et al. Impact of intensive mariculture activities on microplastic pollution in a typical semi-enclosed bay: Zhanjiang Bay [J]. Marine Pollution Bulletin, 2022, 176: 113402
[6] Wang K, Chen W, Tian J Y, et al. Accumulation of microplastics in greenhouse soil after long-term plastic film mulching in Beijing, China [J]. The Science of the Total Environment, 2022, 828: 154544
[7] Peng B, Hossain K B, Lin Y, et al. Assessment and sources identification of microplastics, PAHs and OCPs in the Luoyuan Bay, China: Based on multi-statistical analysis [J]. Marine Pollution Bulletin, 2022, 175: 113351
[8] Rist S, Carney Almroth B, Hartmann N B, et al. A critical perspective on early communications concerning human health aspects of microplastics [J]. The Science of the Total Environment, 2018, 626: 720-726
[9] Li Y N, Peng L, Fu J X, et al. A microscopic survey on microplastics in beverages: The case of beer, mineral water and tea [J]. The Analyst, 2022, 147(6): 1099-1105
[10] Diaz-Basantes M F, Nacimba-Aguirre D, Conesa J A, et al. Presence of microplastics in commercial canned tuna [J]. Food Chemistry, 2022, 385: 132721
[11] Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles [J]. Environmental Health Perspectives, 2005, 113(7): 823-839
[12] Leslie H A, van Velzen M J M, Brandsma S H, et al. Discovery and quantification of plastic particle pollution in human blood [J]. Environment International, 2022, 163: 107199
[13] Cheng H D, Duan Z H, Wu Y H, et al. Immunotoxicity responses to polystyrene nanoplastics and their related mechanisms in the liver of zebrafish (Danio rerio) larvae [J]. Environment International, 2022, 161: 107128
[14] Alnajar N, Jha A N, Turner A. Impacts of microplastic fibres on the marine mussel, Mytilus galloprovinciallis [J]. Chemosphere, 2021, 262: 128290
[15] Liu Z Q, Zhuan Q R, Zhang L Y, et al. Polystyrene microplastics induced female reproductive toxicity in mice [J]. Journal of Hazardous Materials, 2022, 424(Pt C): 127629
[16] Fan X P, Wei X J, Hu H L, et al. Effects of oral administration of polystyrene nanoplastics on plasma glucose metabolism in mice [J]. Chemosphere, 2022, 288(Pt 3): 132607
[17] Schwarzfischer M, Niechcial A, Lee S S, et al. Ingested nano- and microsized polystyrene particles surpass the intestinal barrier and accumulate in the body [J]. NanoImpact, 2022, 25: 100374
[18] Amereh F, Babaei M, Eslami A, et al. The emerging risk of exposure to nano(micro)plastics on endocrine disturbance and reproductive toxicity: From a hypothetical scenario to a global public health challenge [J]. Environmental Pollution, 2020, 261: 114158
[19] Deng Y F, Zhang Y, Lemos B, et al. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure [J]. Scientific Reports, 2017, 7: 46687
[20] Park E J, Han J S, Park E J, et al. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation [J]. Toxicology Letters, 2020, 324: 75-85
[21] Liang B X, Zhong Y Z, Huang Y J, et al. Underestimated health risks: Polystyrene micro- and nanoplastics jointly induce intestinal barrier dysfunction by ROS-mediated epithelial cell apoptosis [J]. Particle and Fibre Toxicology, 2021, 18(1): 20
[22] Sun W, Jin C H, Bai Y L, et al. Blood uptake and urine excretion of nano- and micro-plastics after a single exposure [J]. The Science of the Total Environment, 2022, 848: 157639
[23] Wang Y L, Lee Y H, Hsu Y H, et al. The kidney-related effects of polystyrene microplastics on human kidney proximal tubular epithelial cells HK-2 and male C57BL/6 mice [J]. Environmental Health Perspectives, 2021, 129(5): 57003
[24] Fan Z, Xiao T, Luo H J, et al. A study on the roles of long non-coding RNA and circular RNA in the pulmonary injuries induced by polystyrene microplastics [J]. Environment International, 2022, 163: 107223
[25] Fournier S B, D’Errico J N, Adler D S, et al. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy [J]. Particle and Fibre Toxicology, 2020, 17(1): 55
[26] Mortensen L J, Oberdörster G, Pentland A P, et al. In vivo skin penetration of quantum dot nanoparticles in the murine model: The effect of UVR [J]. Nano Letters, 2008, 8(9): 2779-2787
[27] Yang Z S, Bai Y L, Jin C H, et al. Evidence on invasion of blood, adipose tissues, nervous system and reproductive system of mice after a single oral exposure: Nanoplastics versus microplastics [J]. Biomedical and Environmental Sciences, 2022, 35(11): 1025-1037
[28] Jin H B, Ma T, Sha X X, et al. Polystyrene microplastics induced male reproductive toxicity in mice [J]. Journal of Hazardous Materials, 2021, 401: 123430
[29] Shan S, Zhang Y F, Zhao H W, et al. Polystyrene nanoplastics penetrate across the blood-brain barrier and induce activation of microglia in the brain of mice [J]. Chemosphere, 2022, 298: 134261
[30] Tsou T Y, Lee S H, Kuo T H, et al. Distribution and toxicity of submicron plastic particles in mice [J]. Environmental Toxicology and Pharmacology, 2023, 97: 104038
[31] Meng X M, Zhang J W, Wang W J, et al. Effects of nano- and microplastics on kidney: Physicochemical properties, bioaccumulation, oxidative stress and immunoreaction [J]. Chemosphere, 2022, 288(Pt 3): 132631
[32] Liu S, Wang Z Z, Xiang Q, et al. A comparative study in healthy and diabetic mice followed the exposure of polystyrene microplastics: Differential lipid metabolism and inflammation reaction [J]. Ecotoxicology and Environmental Safety, 2022, 244: 114031
[33] Wang Y, Wang S C, Xu T, et al. A new discovery of polystyrene microplastics toxicity: The injury difference on bladder epithelium of mice is correlated with the size of exposed particles [J]. Science of the Total Environment, 2022, 821: 153413
[34] Xie L L, Chen T L, Liu J Y, et al. Intestinal flora variation reflects the short-term damage of microplastic to the intestinal tract in mice [J]. Ecotoxicology and Environmental Safety, 2022, 246: 114194
[35] Danso I K, Woo J H, Lee K. Pulmonary toxicity of polystyrene, polypropylene, and polyvinyl chloride microplastics in mice [J]. Molecules, 2022, 27(22): 7926
[36] Wei Y X, Zhou Y, Long C L, et al. Polystyrene microplastics disrupt the blood-testis barrier integrity through ROS-mediated imbalance of mTORC1 and mTORC2 [J]. Environmental Pollution, 2021, 289: 117904
[37] Xu W Q, Yuan Y Y, Tian Y, et al. Oral exposure to polystyrene nanoplastics reduced male fertility and even caused male infertility by inducing testicular and sperm toxicities in mice [J]. Journal of Hazardous Materials, 2023, 454: 131470
[38] Xie X M, Deng T, Duan J F, et al. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway [J]. Ecotoxicology and Environmental Safety, 2020, 190: 110133
[39] Jin H B, Yan M H, Pan C, et al. Chronic exposure to polystyrene microplastics induced male reproductive toxicity and decreased testosterone levels via the LH-mediated LHR/cAMP/PKA/StAR pathway [J]. Particle and Fibre Toxicology, 2022, 19(1): 13
[40] Hou J Y, Lei Z M, Cui L L, et al. Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats [J]. Ecotoxicology and Environmental Safety, 2021, 212: 112012
[41] An R, Wang X F, Yang L, et al. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats [J]. Toxicology, 2021, 449: 152665
[42] Hu J N, Qin X L, Zhang J W, et al. Polystyrene microplastics disturb maternal-fetal immune balance and cause reproductive toxicity in pregnant mice [J]. Reproductive Toxicology, 2021, 106: 42-50
[43] He Y J, Li Z, Xu T, et al. Polystyrene nanoplastics deteriorate LPS-modulated duodenal permeability and inflammation in mice via ROS drived-NF-κB/NLRP3 pathway [J]. Chemosphere, 2022, 307(Pt 1): 135662
[44] Djouina M, Vignal C, Dehaut A, et al. Oral exposure to polyethylene microplastics alters gut morphology, immune response, and microbiota composition in mice [J]. Environmental Research, 2022, 212(Pt B): 113230
[45] Lu L, Wan Z Q, Luo T, et al. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice [J]. The Science of the Total Environment, 2018, 631-632: 449-458
[46] Li B Q, Ding Y F, Cheng X, et al. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice [J]. Chemosphere, 2020, 244: 125492
[47] Liu S, Li H, Wang J, et al. Polystyrene microplastics aggravate inflammatory damage in mice with intestinal immune imbalance [J]. The Science of the Total Environment, 2022, 833: 155198
[48] Kwon W, Kim D, Kim H Y, et al. Microglial phagocytosis of polystyrene microplastics results in immune alteration and apoptosis in vitro and in vivo [J]. The Science of the Total Environment, 2022, 807(Pt 2): 150817
[49] Jin H B, Yang C, Jiang C Y, et al. Evaluation of neurotoxicity in BALB/c mice following chronic exposure to polystyrene microplastics [J]. Environmental Health Perspectives, 2022, 130(12): 107002
[50] Liang B X, Huang Y J, Zhong Y Z, et al. Brain single-nucleus transcriptomics highlights that polystyrene nanoplastics potentially induce Parkinson’s disease-like neurodegeneration by causing energy metabolism disorders in mice [J]. Journal of Hazardous Materials, 2022, 430: 128459
[51] Wang S W, Han Q, Wei Z L, et al. Polystyrene microplastics affect learning and memory in mice by inducing oxidative stress and decreasing the level of acetylcholine [J]. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 2022, 162: 112904
[52] Lee C W, Hsu L F, Wu I L, et al. Exposure to polystyrene microplastics impairs hippocampus-dependent learning and memory in mice [J]. Journal of Hazardous Materials, 2022, 430: 128431
[53] Liu Z, Bai Y, Ma T T, et al. Distribution and possible sources of atmospheric microplastic deposition in a valley basin city (Lanzhou, China) [J]. Ecotoxicology and Environmental Safety, 2022, 233: 113353
[54] Lee S, Kang K K, Sung S E, et al. Toxicity study and quantitative evaluation of polyethylene microplastics in ICR mice [J]. Polymers, 2022, 14(3): 402
[55] Cao J W, Xu R, Geng Y, et al. Exposure to polystyrene microplastics triggers lung injury via targeting toll-like receptor 2 and activation of the NF-κB signal in mice [J]. Environmental Pollution, 2023, 320: 121068
[56] Xu D H, Ma Y H, Han X D, et al. Systematic toxicity evaluation of polystyrene nanoplastics on mice and molecular mechanism investigation about their internalization into Caco-2 cells [J]. Journal of Hazardous Materials, 2021, 417: 126092
[57] Li X R, Zhang T T, Lv W T, et al. Intratracheal administration of polystyrene microplastics induces pulmonary fibrosis by activating oxidative stress and Wnt/β-catenin signaling pathway in mice [J]. Ecotoxicology and Environmental Safety, 2022, 232: 113238
[58] Wu Y L, Yao Y R, Bai H J, et al. Investigation of pulmonary toxicity evaluation on mice exposed to polystyrene nanoplastics: The potential protective role of the antioxidant N-acetylcysteine [J]. The Science of the Total Environment, 2023, 855: 158851
[59] Huang D J, Zhang Y, Long J L, et al. Polystyrene microplastic exposure induces insulin resistance in mice via dysbacteriosis and pro-inflammation [J]. The Science of the Total Environment, 2022, 838(Pt 1): 155937
[60] Wang Q, Wu Y L, Zhang W J, et al. Lipidomics and transcriptomics insight into impacts of microplastics exposure on hepatic lipid metabolism in mice [J]. Chemosphere, 2022, 308(Pt 3): 136591
[61] Choi Y J, Park J W, Lim Y, et al. In vivo impact assessment of orally administered polystyrene nanoplastics: Biodistribution, toxicity, and inflammatory response in mice [J]. Nanotoxicology, 2021, 15(9): 1180-1198
[62] Mu Y W, Sun J Y, Li Z Y, et al. Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice [J]. Chemosphere, 2022, 291(Pt 2): 132944
[63] Shi J, Deng H P, Zhang M. Whole transcriptome sequencing analysis revealed key RNA profiles and toxicity in mice after chronic exposure to microplastics [J]. Chemosphere, 2022, 304: 135321
[64] Xiong X, Gao L K, Chen C, et al. The microplastics exposure induce the kidney injury in mice revealed by RNA-seq [J]. Ecotoxicology and Environmental Safety, 2023, 256: 114821
[65] 李欢, 刘苏, 张静丽, 等. 聚苯乙烯微塑料对糖尿病小鼠肾脏的影响[J]. 中国环境科学, 2022, 42(3): 1369-1378
Li H, Liu S, Zhang J L, et al. Effects of polystyrene microplastics on kidney of diabetic mice [J]. China Environmental Science, 2022, 42(3): 1369-1378 (in Chinese)
[66] Lin P, Tong X, Xue F, et al. Polystyrene nanoplastics exacerbate lipopolysaccharide-induced myocardial fibrosis and autophagy in mice via ROS/TGF-β1/Smad [J]. Toxicology, 2022, 480: 153338
[67] Li Z K, Zhu S X, Liu Q, et al. Polystyrene microplastics cause cardiac fibrosis by activating Wnt/β-catenin signaling pathway and promoting cardiomyocyte apoptosis in rats [J]. Environmental Pollution, 2020, 265(Pt A): 115025
[68] Zhao J J, Gomes D, Jin L X, et al. Polystyrene bead ingestion promotes adiposity and cardiometabolic disease in mice [J]. Ecotoxicology and Environmental Safety, 2022, 232: 113239
[69] Wang X X, Jia Z Z, Zhou X R, et al. Nanoplastic-induced vascular endothelial injury and coagulation dysfunction in mice [J]. The Science of the Total Environment, 2023, 865: 161271
[70] Wang B, Liang B X, Huang Y J, et al. Long-chain acyl carnitines aggravate polystyrene nanoplastics-induced atherosclerosis by upregulating MARCO [J]. Advanced Science, 2023, 10(19): e2205876
[71] Yang D Q, Zhu J D, Zhou X S, et al. Polystyrene micro- and nano-particle coexposure injures fetal thalamus by inducing ROS-mediated cell apoptosis [J]. Environment International, 2022, 166: 107362
[72] Zhang Y B, Wang X Y, Zhao Y F, et al. Reproductive toxicity of microplastics in female mice and their offspring from induction of oxidative stress [J]. Environmental Pollution, 2023, 327: 121482
[73] Jeong B, Baek J Y, Koo J, et al. Maternal exposure to polystyrene nanoplastics causes brain abnormalities in progeny [J]. Journal of Hazardous Materials, 2022, 426: 127815
[74] Huang T, Zhang W J, Lin T T, et al. Maternal exposure to polystyrene nanoplastics during gestation and lactation induces hepatic and testicular toxicity in male mouse offspring [J]. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 2022, 160: 112803
[75] Han Y, Song Y, Kim G W, et al. No prominent toxicity of polyethylene microplastics observed in neonatal mice following intratracheal instillation to dams during gestational and neonatal period [J]. Toxicological Research, 2021, 37(4): 443-450
[76] Liu X, Yang H K, Yan X Z, et al. Co-exposure of polystyrene microplastics and iron aggravates cognitive decline in aging mice via ferroptosis induction [J]. Ecotoxicology and Environmental Safety, 2022, 233: 113342
[77] Feng Y Y, Yuan H B, Wang W Z, et al. Co-exposure to polystyrene microplastics and lead aggravated ovarian toxicity in female mice via the PERK/eIF2α signaling pathway [J]. Ecotoxicology and Environmental Safety, 2022, 243: 113966
[78] Zou H, Chen Y, Qu H Y, et al. Microplastics exacerbate cadmium-induced kidney injury by enhancing oxidative stress, autophagy, apoptosis, and fibrosis [J]. International Journal of Molecular Sciences, 2022, 23(22): 14411
[79] Deng Y F, Zhang Y, Qiao R X, et al. Evidence that microplastics aggravate the toxicity of organophosphorus flame retardants in mice (Mus musculus) [J]. Journal of Hazardous Materials, 2018, 357: 348-354
[80] Zhang W Y, Sun X Y, Qi X, et al. Di-(2-ethylhexyl) phthalate and microplastics induced neuronal apoptosis through the PI3K/AKT pathway and mitochondrial dysfunction [J]. Journal of Agricultural and Food Chemistry, 2022, 70(35): 10771-10781
[81] Deng Y F, Yan Z H, Shen R Q, et al. Enhanced reproductive toxicities induced by phthalates contaminated microplastics in male mice (Mus musculus) [J]. Journal of Hazardous Materials, 2021, 406: 124644
[82] Jiang P, Yuan G H, Jiang B R, et al. Effects of microplastics (MPs) and tributyltin (TBT) alone and in combination on bile acids and gut microbiota crosstalk in mice [J]. Ecotoxicology and Environmental Safety, 2021, 220: 112345
[83] Tong X H, Li B Q, Li J, et al. Polyethylene microplastics cooperate with Helicobacter pylori to promote gastric injury and inflammation in mice [J]. Chemosphere, 2022, 288(Pt 2): 132579
[84] Liu J, Lv M, Sun A Q, et al. Exposure to microplastics reduces the bioaccumulation of sulfamethoxazole but enhances its effects on gut microbiota and the antibiotic resistome of mice [J]. Chemosphere, 2022, 294: 133810
[85] Sun W, Yan S, Meng Z Y, et al. Combined ingestion of polystyrene microplastics and epoxiconazole increases health risk to mice: Based on their synergistic bioaccumulation in vivo [J]. Environment International, 2022, 166: 107391
[86] Bejgarn S, MacLeod M, Bogdal C, et al. Toxicity of leachate from weathering plastics: An exploratory screening study with Nitocra spinipes [J]. Chemosphere, 2015, 132: 114-119
[87] Lithner D, Damberg J, Dave G, et al. Leachates from plastic consumer products: Screening for toxicity with Daphnia magna [J]. Chemosphere, 2009, 74(9): 1195-1200
[88] Zhang W, Wang J Y, Liu Z Y, et al. Iron-dependent ferroptosis participated in benzene-induced anemia of inflammation through IRP1-DHODH-ALOX12 axis [J]. Free Radical Biology and Medicine, 2022, 193: 122-133
[89] Sun R L, Xu K, Ji S B, et al. Benzene exposure induces gut microbiota dysbiosis and metabolic disorder in mice [J]. The Science of the Total Environment, 2020, 705: 135879
[90] Karaulov A V, Smolyagin A I, Mikhailova I V, et al. Assessment of the combined effects of chromium and benzene on the rat neuroendocrine and immune systems [J]. Environmental Research, 2022, 207: 112096
[91] Bourgois A, Saurat D, de Araujo S, et al. Nose-only inhalations of high-dose alumina nanoparticles/hydrogen chloride gas mixtures induce strong pulmonary pro-inflammatory response: A pilot study [J]. Inhalation Toxicology, 2021, 33(9-14): 308-324
[92] Luo Y S, He Q K, Sun M X, et al. Acrylonitrile exposure triggers ovarian inflammation and decreases oocyte quality probably via mitochondrial dysfunction induced apoptosis in mice [J]. Chemico-Biological Interactions, 2022, 360: 109934
[93] Deng Y F, Yan Z H, Shen R Q, et al. Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut [J]. Environment International, 2020, 143: 105916
[94] Wang J, Dai G D. Comparative effects of brominated flame retardants BDE-209, TBBPA, and HBCD on neurotoxicity in mice [J]. Chemical Research in Toxicology, 2022, 35(9): 1512-1518
[95] Kazemi S, Mousavi Kani S N, Ghasemi-Kasman M, et al. Nonylphenol induces liver toxicity and oxidative stress in rat [J]. Biochemical and Biophysical Research Communications, 2016, 479(1): 17-21
[96] Gąssowska M, Baranowska-Bosiacka I, Moczyd owska J, et al. Perinatal exposure to lead (Pb) induces ultrastructural and molecular alterations in synapses of rat offspring [J]. Toxicology, 2016, 373: 13-29
owska J, et al. Perinatal exposure to lead (Pb) induces ultrastructural and molecular alterations in synapses of rat offspring [J]. Toxicology, 2016, 373: 13-29