全氟和多氟化合物(per- and polyfluoroalkyl substances, PFASs),是一类长碳主链上的氢原子全部或部分被氟原子取代的有机化合物[1]。其分子结构中高能的C—F键使PFASs在实践应用中具备抗腐蚀、耐风化等优势,故此在日常生活和工业生产中应用广泛[2-5]。但是,研究发现传统的PFASs,尤其是其中最常见的全氟辛烷羧酸(perfluorooctanoic acid, PFOA)和全氟辛烷磺酸(perfluorooctane sulfonic acid, PFOS),具有生物持久性和长距离迁移性等特性,致使其在多种环境介质和生物体内广泛检出,并表现出生物积累性和毒性,对生态环境和生物体健康构成严重的威胁[4, 6-7]。因此此类物质逐渐受到学者和公众的关注,各个国家的主要制造商自愿逐步停止生产PFOA和PFOS及其相关产品,其在环境和生物中检出呈现出缓慢下降的趋势[3, 8-9]。然而,由于社会生产和经济发展的需要,此类物质的生产和使用无法完全避免,人们逐渐把重心转移到了它们的替代品上[3, 10]。
PFOA和PFOS替代品在传统的PFASs的结构基础上缩短碳链长度或增加功能官能团,以期在保证实用价值的同时降低其毒性[11-12]。PFOA和PFOS替代品大致可分为环状全氟烷酸、全氟聚醚及全氟醚基烷酸类化合物、氢代或氯代多氟化合物、短链(C4~C6)全氟化合物及其衍生物4类[13-14]。其中,近几年来较受学者关注的PFOA和PFOS替代品主要包括PFOA的替代品全氟丁烷羧酸(PFBA)、全氟己烷羧酸(PFHxA)、六氟环氧丙烷二聚酸(GenX)、4,8-二氧杂-3-氢-全氟壬酸(ADONA)、6:2氟调羧酸(6:2 FTCA)和PFOS的替代品全氟丁烷磺酸(PFBS)、全氟己烷磺酸(PFHxS)、6:2氯代多氟烷醚磺酸(F-53B)、6:2氟调磺酸(6:2 FTSA)、全氟壬烯氧基苯磺酸(OBS)(表1)[15-18]。由于全氟化碳链缩短,这些PFOA和PFOS替代品相比于PFOA和PFOS来说可能具有更强的亲水性,其生物积累性和毒性降低,但是生物过程中有机污染物对生物的毒性涉及多种复杂因素,关于“PFOA和PFOS氟化替代品到底是不是相对安全的替代品”这个问题还有待深入探究[19]。在一项对斑马鱼(Danio rerio)的暴露实验中明确指出,在毒性和生物积累方面GenX和被替代的PFOA相比并没有显著降低[20]。
表1 全氟辛烷羧酸(PFOA)和全氟辛烷磺酸(PFOS)及其替代品
Table 1 Perfluorooctanoic acid (PFOA) substitutes and perfluorooctane sulfonic acid (PFOS) substitutes

简称Abbreviations全称Full name分子式Molecular formula结构式Structural formulaPFOA全氟辛酸Perfluorooctanoic acidC7F15COOHPFBA全氟丁烷羧酸Perfluorobutanoic acidC3F7COOHPFHxA全氟己烷羧酸Perfluorohexanoic acidC5F11COOHGenX六氟环氧丙烷二聚酸2,3,3,3-tetrafluoro-2-(1,1,2,2,3,3,3-heptafluoropropoxy) propanoic acidC3F7OCF(CF3)COOHADONA4,8-二氧杂-3-氢-全氟壬酸4,8-dioxa-3H-perfluorononanoic acidCF3O(CF2)3OCHFCF2COOH6:2 FTCA6:2氟调羧酸6:2 fluorotelomer carboxylic acidC6F13CH2COOHPFOS全氟辛烷磺酸Perfluorooctane sulfonic acidC8F17SO3HPFBS全氟丁烷磺酸Perfluorobutane sulfonic acidC4F9SO3HPFHxS全氟己烷磺酸Perfluorohexane sulfonic acidC6F13SO3HF-53B6:2氯代多氟烷醚磺酸6:2 chlorinated polyfluoroalkylether sulfonic acidClC6F12OC2F4SO3H6:2 FTSA6:2氟调磺酸6:2 fluorotelomer sulfonic acidC6F13C2H4SO3HOBS全氟壬烯氧基苯磺酸Perfluorononenyloxy benzene sulfonateC9F17OC6H4SO3H
PFOA和PFOS替代品在生产和使用过程通过工业废水、生活污水或大气沉降等方式最终进入水环境中,对水生生物产生直接危害,且可通过食物链影响人类健康。因此,研究PFOA和PFOS替代品对水生生物的毒性效应,对控制水环境中的PFOA和PFOS替代品污染水平、保护水生生物和人类健康具有重要意义。本文通过查阅近期PFOA和PFOS替代品相关研究,对10种典型PFOA和PFOS替代品在水环境中的分布情况以及水生生物暴露情况进行总结归纳,重点总结了PFOA和PFOS替代品对水生生物的发育、免疫、神经行为等方面的毒性效应,以期为PFOA和PFOS替代品在水环境中的风险评估提供参考。
1 水环境中PFOA和PFOS替代品的分布(Distribution of PFOA and PFOS substitutes in aquatic environment)
PFOA和PFOS替代品在生产和使用过程中可通过多种途径释放到水体环境中,造成水环境污染[21]。多项研究表明,PFOA和PFOS替代品在水环境中广泛检出(表2)。
表2 部分水体中PFOA和PFOS替代品的浓度
Table 2 PFOA and PFOS substitutes concentrations in some water bodies

介质类型Media type采样点Sampling sitesPFBA/(ng·L-1) PFHxA/(ng·L-1)GenX/(ng·L-1)ADONA/(ng·L-1)6:2 FTCA/(ng·L-1)PFBS/(ng·L-1)PFHxS/(ng·L-1)F-53B/(ng·L-1)6:2 FTSA/(ng·L-1)OBS/(ng·L-1)参考文献References地表水Surface water中国天津 Tianjin, China2.77~66.90.34~25.0---0.09~4.17nd~0.18---[23]中国巢湖 Chaohu Lake, China19.20±19.704.03±0.66---8.44±1.492.60±0.67---[26]中国天津 Tianjin, China2.52~1 7524.04~3 224-nd-0.60~33.90.56~186nd~18.8ndnd~193[28]中国湟水河 Huangshui River, Chinand~1 942-----nd~3 207---[29]中国汾河/渭河Fen/Wei River, China---0.11~3.28--nd~44.19-1.34-[45]中国渤海附近河口Estuary near Bohai Sea, Chinand~5310.13~1 985nd~663nd~0.04nd~7.26nd~281nd~305nd~36.9nd~28.7-[47]中国鄱阳湖 Poyang Lake, China7.20~5300.33~6.900.29~5.70-nd~5.700.69~3200.039~250.28~3.200.91~7.601.70~28.0[50]中国辽宁 Liaoning, China24.7~4 6061.14~20.2nd~0.60--4.55~2 492nd~2.97nd~1.93--[52]中国观澜河Guanlan River, China- 续表2介质类型Media type采样点Sampling sitesPFBA/(ng·L-1) PFHxA/(ng·L-1)GenX/(ng·L-1)ADONA/(ng·L-1)6:2 FTCA/(ng·L-1)PFBS/(ng·L-1)PFHxS/(ng·L-1)F-53B/(ng·L-1)6:2 FTSA/(ng·L-1)OBS/(ng·L-1)参考文献References地下水Groundwater中国江西 Jiangxi, Chinand~129.3nd~27.4nd~8.93--nd~6.90-nd~13.4nd~118-[25]中国辽宁 Liaoning, China4.14~2 5010.47~936---17.1~51 818nd~539.60.01~0.40--[30]中国湖北 Hubei, China22.8~8 7640.05~2 214---2.98~2 744nd~307---[33]中国常熟 Changshu, China13.8~4980.50~4530.01~1530.01~5.94~0.14~16.30.01~8.810.15~5.570.08~7.21-[39]中国江苏 Jiangsu, Chinand~33.5nd~30.6---nd~143nd~4.010.17~1.830.32~8.54-[58]美国北卡罗来纳州North Carolina, United States of America--50.0~1 151-------[42]海水Seawater中国东海 East Sea, China1.66~71.2------nd~2.13--[24]中国黄海 Yellow Sea, China----1.75~13.81.86~15.5-0.04~0.17--[46]中国渤海 Bohai Sea, Chinand~8.690.89~33.4nd~6.87nd~0.05nd~1.38nd~6.59nd~3.62nd~0.32nd~20.7-[47]中国边缘海域The marginal seas of China--------0.07~0.15-[59]红海沙特阿拉伯沿岸Red Sea (Coastal area near Saudi Arabia)-nd to >198---nd~50.85nd~245-nd to >450-[31] 注:nd表示未检测到;MDL表示方法检出限;MQL表示方法定量限;LOD表示检出限。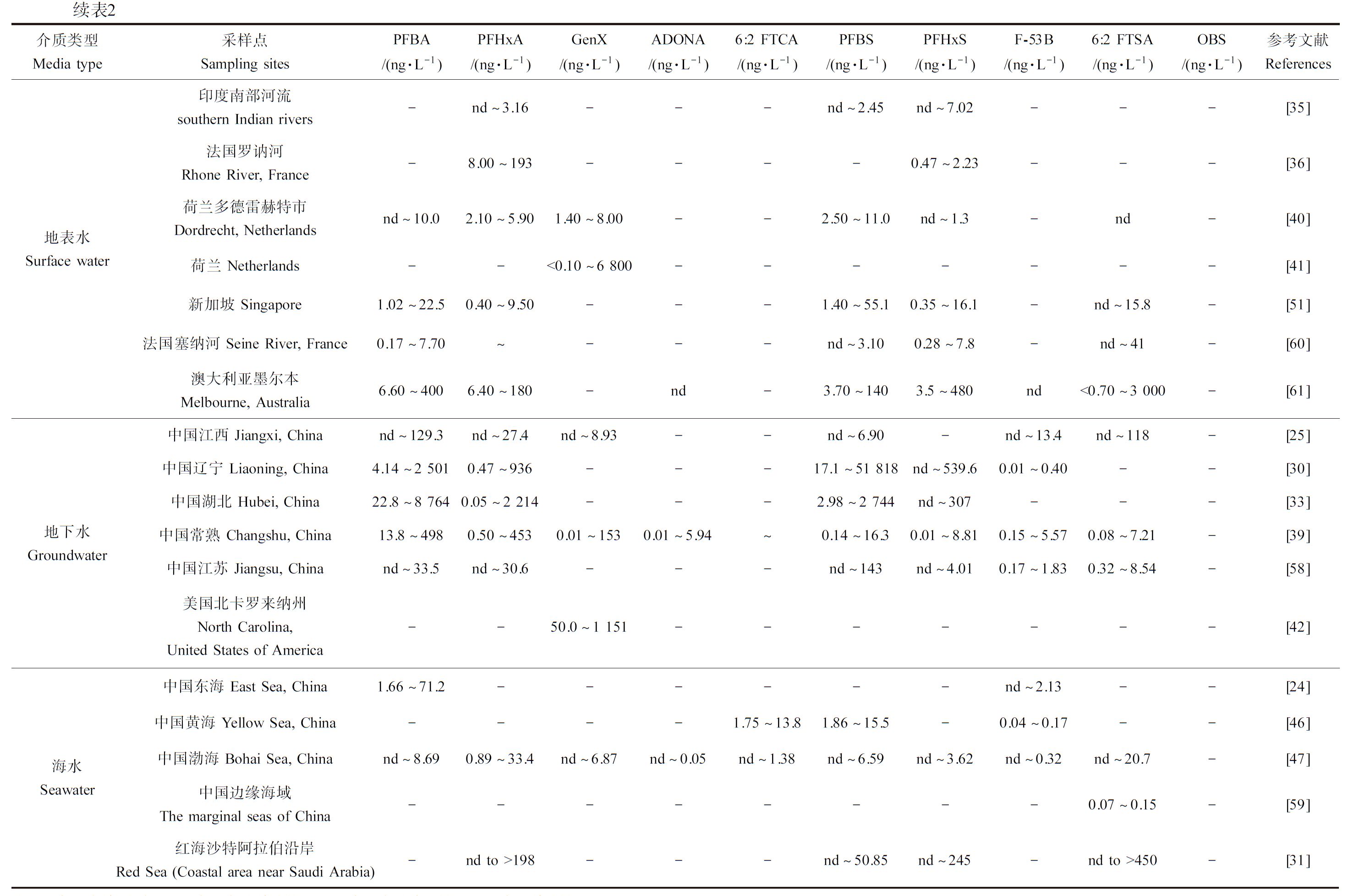
Note: nd stands for not detected; MDL stands for method detection limit; MQL stands for method quantification limit; LOD stands for limit of detection.
PFBA在水体中检出历史悠久,但检出浓度和检出频率均较低,直至近几年PFBA作为PFOA的替代品被广泛使用,其在水体中的浓度也随之增加。例如,Lalonde和Garron[22]对2013—2020年间加拿大多个地点的淡水进行了采样和PFASs分析,发现该地区PFOA呈现出下降的趋势,PFBA则呈现出上升的趋势。Cao等[23]分析了中国天津市于桥水库中的PFASs污染情况,发现水样中PFBA(2.77~66.93 ng·L-1)浓度高于PFOA(0.48~5.33 ng·L-1)。同样,在对中国东南部海水样品[24]、中国江西地下水样品[25]、中国巢湖地表水样品[26]、中国青藏高原雅鲁藏布江河水样品[27]等多地区水样进行PFASs检测分析后发现,PFBA为主要的检出PFASs,且其浓度均高于PFOA。PFBA在天津大港油田水样[28]、青藏高原湟水流域水样[29]和辽宁阜新氟化工厂附近地下水水样[30]中最高浓度分别达到1 752、1 942和2 501 ng·L-1。与PFBA类似,近几年PFHxA在水体中的浓度也呈上升的趋势[31]。Khan等[32]首次报道了PFASs在巴基斯坦印度河表层水水样中的检出情况,其中PFHxA浓度为0.11~46.41 ng·L-1,占所有检出PFASs的18%,仅次于PFBA,高于PFOA浓度(
随着近年来PFBS作为PFOS替代品大量应用于工业和生活中,水体中PFBS的浓度不断升高,在许多地区PFBS已成为主要的PFASs污染物[48]。例如,Wang等[25]对中国江西地下水PFASs进行调查,发现PFBS是主要污染物。Li等[49]调查了中国石家庄市地表水中的PFASs分布,发现PFBS为水样中的优势污染物,平均检出浓度为14.3 ng·L-1。除在中国鄱阳湖[50]、巢湖[26]、黄海[46]、渤海[47]、青藏高原[27]等地外,在新加坡[51]、印度[35]和沙特阿拉伯[31]等地区采集的水样中均发现较高浓度的PFBS(最高浓度范围为4.01~710 ng·L-1),其浓度均高于同水样中检出的PFOS,表明PFBS的广泛使用。除此以外,Gao等[52]在中国辽宁氟化工厂附近水体中检测到PFBS的浓度高达2 492 ng·L-1,而Tang等[30]在同一地区附近水体中检测到PFBS的最高浓度达到51 818 ng·L-1。Zhou等[45]通过对中国汾河、渭河的PFASs调查发现作为PFOS替代品之一的PFHxS已成为两地主要的PFASs。Ali等[31]在红海东部沙特阿拉伯沿岸水体中也发现PFHxS较高的检出频率和检出浓度。近期研究表明在中国小清河[47]、中国深圳观澜河[53]、中国辽宁阜新氟工业园[54]、印度南部河流[35]等多地采集的水样中检出的PFHxS浓度均高于PFOS,说明PFHxS在这些地区的广泛应用。Guo等[55]在中国河北白洋淀水体中检测到PFHxS浓度高达1 688 ng·L-1,与之类似,PFHxS在中国巢湖周边河流水样中的最高浓度达到1 866 ng·L-1[56]。F-53B仅在中国作为PFOS的替代品,因此有关其在水体中污染的报道范围多集中在中国。Feng等[46]对中国南黄海水域进行了PFASs检测,发现F-53B在所有水样中的检出率为100%。Wang等[25]报道了中国江西地下水水样中F-53B的检出情况,结果显示F-53B在水样中的浓度范围为0.04~13.4 ng·L-1,检出率为100%,表明F-53B在该区域的广泛使用。Wang等[57]对中国19条河流入海口处PFASs进行了评估,在多条河流中均检出F-53B的存在,其最高浓度达78.5 ng·L-1。除此以外,F-53B在中国非工业区地下水[58],美国、英国、韩国、德国、瑞典和荷兰地区地表水[43]中均有检出。近期研究表明6:2 FTSA在中国黄土高原[44]、中国汾河和渭河[45]、中国边缘海域[59]、新加坡[51]和法国塞纳河[60]等地区普遍存在。不仅如此,近年来某些水体中的6:2 FTSA浓度已超过PFOS。例如,对中国江西地下水[25]、渤海海水[47]等水体水样进行PFASs检测,发现水样中6:2 FTSA浓度范围分别为nd~117.5 ng·L-1、nd~20.7 ng·L-1,均高于同水样中PFOS的含量。此外,Ali等[31]在红海东部沙特阿拉伯沿岸水体中检测到6:2 FTSA浓度高达450 ng·L-1,Marchiandi等[61]在澳大利亚墨尔本某失火场所附近水域进行了长达3年的固定取样监测,发现6:2 FTSA在水样中广泛存在,其浓度最高可达3 000 ng·L-1。因OBS在石油行业的应用,OBS在中国天津大港油田[28]、中国大庆油田[62]附近水样中均有检出,其中OBS在大庆油田附近水样中浓度最高可达3 200 ng·L-1。Hou等[63]在中国宿迁氟化工厂附近地表水中也检测到较高浓度的OBS(7.78~10 358 ng·L-1),比其他PFASs高出1~4个数量级。此外,在中国翻阳湖中也检测到OBS的存在,其浓度高于水样中的PFOS浓度[50]。
综上,PFOA和PFOS替代品在水环境中分布广泛,短链替代品(如PFBA、PFBS等)已成为多个地区的主要检出PFASs,检出浓度远高于PFOA和PFOS,醚类或氯(氢)代类替代品(如6:2 FTCA、OBS等)已在多种水环境介质中检出且浓度和频率呈上升趋势,其检出浓度与PFOA和PFOS相当甚至更高。水环境中广泛存在的PFOA和PFOS替代品可能会造成其在水生生物中积累,继而引起对水生生物的毒性效应,存在潜在生物安全风险。
2 PFOA和PFOS替代品在水生生物中的积累(Bioaccumulation of PFOA and PFOS substitutes in aquatic organisms)
在PFOA和PFOS替代品的生产和使用过程中,部分PFOA和PFOS替代品随之释放到环境中,经过降解、迁移、分配和转化等过程继而被生物体所吸收[64]。基于此类物质固有的持久性、半挥发性和长距离迁移性,目前PFOA和PFOS替代品除在水环境中普遍检出外,还可在水生生物体内检出,且其浓度和频率均呈现出一定的增长趋势[65]。
先前研究表明PFBA在水生生物中的生物富集系数(BCF)值通常低于PFOA,因此相对于PFOA,PFBA不易在水生生物体内积累,然而近期研究发现PFBA在多地水生生物体内的浓度超过了PFOA[66-69]。例如,在美国卡罗莱纳州皮迪河地区采集水生生物样本进行PFASs监测时发现,PFBA在鱼体内平均浓度为118.25 ng·g-1,远大于PFOA(33.43 ng·g-1)。推测这种情况的出现与PFBA作为PFOA替代品大量使用相关[66]。Zhang等[70]分析了PFASs在中国东海海豚(Neophocaena asiaeorientalis sunameri)体内的积累情况,并进行了不同时间(2009—2010、2018—2019)尺度上的对比,发现PFBA表现出上升趋势。在对中国北京密云水库鱼类[71]、意大利加尔达湖鳗鱼(Anguilla anguilla)[72]进行采样分析,结果显示PFBA已成为样品中主要的PFASs污染物。在中国黄海海鲜产品中PFBA的浓度最高可达605 ng·g-1[73]。与PFBA类似,在多个地区采集的水生生物样品中均以PFHxA为优势污染物[68, 74-75]。
Zhang等[73]收集了中国黄海海鲜产品并对其进行PFASs分析,在梭子蟹(Portunus trituberculatus)中测得PFBS平均浓度为38.9 ng·g-1,高于PFOS的平均浓度(5.7 ng·g-1)。在一项有关PFASs在中国东海领域海豚的研究中,将2008—2009、2018—2019年时间尺度对比后发现,作为PFOA和PFOS替代品之一的PFHxS在海豚体内的浓度呈现上升趋势[70]。Xu等[76]采集了海南岛12种人造礁珊瑚样本,结果表明PFHxS为优势污染物(贡献率为43%)。Shi等[77]首次在小清河和汤逊湖野生鲫鱼(Carassius carassius)样本中检测到了F-53B的存在,其在鲫鱼全鱼中的生物积累系数(logBAF=4.12)高于PFOS(logBAF=3.43)。类似的,Wu等[78]在实验室模拟实验中也发现F-53B在斑马鱼中的生物富集系数(BCF=3.56)与PFOS(BCF=2.84~3.70)相似或更高,说明与PFOS相比,F-53B具有相似或更高的生物积累能力。在综合评估北半球生物的PFASs暴露情况的筛查中发现海洋哺乳动物体内存在F-53B[79-80]。同样,有研究在东格陵兰岛的野生动物(环斑海豹(Pusa hispida)、北极熊(Ursus maritimus)、虎鲸(Orcinus orca))肝脏中检测到了F-53B[81]。现有资料指出F-53B仅在中国境内被用作电镀行业中的抑雾剂,北极地区生物体内检出F-53B从侧面反映出该物质具有持久性和长距离迁移性。Munoz等[82]对加拿大梅金蒂克湖和乔迪埃河中白亚口鱼(Catostomus commersonii)体内的PFASs进行检测,首次在取样地的鱼肌肉样本中检测到了n:2-FTSAs的存在。此外,在对加拿大东部不同淡水区鱼类样品的调查中,也检测了6:2 FTSA的存在[83]。在对一项针对中国江苏省氟化工厂附近河流中鲫鱼体内的PFASs监测中发现了高检出率、高浓度的OBS(检出率:100%;血液:25.3~641 ng·mL-1;肌肉:5.27~66.6 ng·g-1)[63]。类似的,Shi等[84]对中国河北省地区野生鲫鱼中PFASs做了分析,发现OBS的检出浓度高于PFOS,计算两者的生物积累系数发现OBS在鲫鱼肌肉中的生物积累系数(logBAF=3.06)与PFOS(logBAF=3.09)相似。
鉴于PFOA和PFOS替代品在水环境中的广泛存在,其在水生生物体内的积累浓度呈上升趋势,甚至在部分水生生物体内超过PFOA和PFOS成为生物体内主要的PFASs。外源化合物对生物的毒性效应与其在生物体内的积累浓度密切相关,PFOA和PFOS替代品对水生生物的毒性效应可能与PFOA和PFOS相当,甚至PFOA和PFOS替代品可能表现出更强的毒性效应。因此,有必要了解PFOA和PFOS替代品对水生生物的毒性效应研究现状,为今后PFOA和PFOS替代品对水生生物的毒性效应研究提供参考依据。
3 PFOA和PFOS替代品对水生生物的毒性效应(Toxic effects of PFOA and PFOS substitutes on aquatic organisms)
3.1 急性毒性(Acute toxicity)
研究PFOA和PFOS替代品对水生生物的急性毒性有助于理解其接触极限,并可为后续亚慢性、慢性试验提供理论依据。目前关于PFOA和PFOS替代品对水生生物的急性毒性研究主要包括其对水生生物致死率、形态学变化等影响,以半数致死浓度(LC50)和半数效应浓度(EC50)等毒理学参数表征。
对比表3中急性毒性参数可以发现,大部分PFOA和PFOS替代品对水生生物的急性毒性较低,环境浓度的PFOA和PFOS替代品可能不会对水生生物造成急性致死效应。Godfrey等[85]研究发现,PFBA对斑马鱼的半数致死浓度(96 h-LC50=13 795 mg·L-1)远高于被替代的PFOA(96 h-LC50=473 mg·L-1),说明PFBA对斑马鱼的毒性较低且远低于PFOA。该结论在Vogs等[69]的实验中得到证明,即实验得到PFOA对斑马鱼的120 h-EC50值为211 mg·L-1,而将斑马鱼暴露于2 568 mg·L-1的PFBA 120 h后,未观察到PFBA对斑马鱼形态变化的影响。同样,PFBA对大型溞(Daphnia magna)的48 h-EC50值(5 251 mg·L-1)也大于PFOA的48 h-EC50值(239 mg·L-1)[86]。该研究还发现PFHxA对大型溞的48 h-EC50值为1 048 mg·L-1[86],同样大于PFOA,说明PFHxA对大型溞的急性毒性小于PFOA。Kim等[87]测定PFHxA在非洲爪蟾(Xenopus)胚胎中的LC50值为478 mg·L-1,EC50值为329 mg·L-1。Gebreab等[20]发现GenX对斑马鱼的LC50为(383±30) mg·L-1,也高于PFOA的LC50 (232±29) mg·L-1。然而,Shi等[88]对斑马鱼胚胎急性暴露的研究表明,6:2 FTCA对斑马鱼的120 h-LC50值仅为7.33 mg·L-1,对斑马鱼的毒性为高毒,且其LC50值远小于PFOA,即该替代品表现出了更高的毒性。Tornabene等[89]研究了PFASs对两栖动物的急性毒性,结果表明在测试的所有物种中,存活率均随着PFHxS暴露浓度的增加而降低,其中绿蛙(Rana clamitans)对PFHxS的敏感性要强于美国牛蛙(Rana catesbaeiana)(后者的平均致死浓度值比前者高1.4倍~1.6倍)。
表3 PFOA和PFOS替代品对水生生物的急性毒性
Table 3 Acute toxicity of PFOA and PFOS substitutes on aquatic organisms
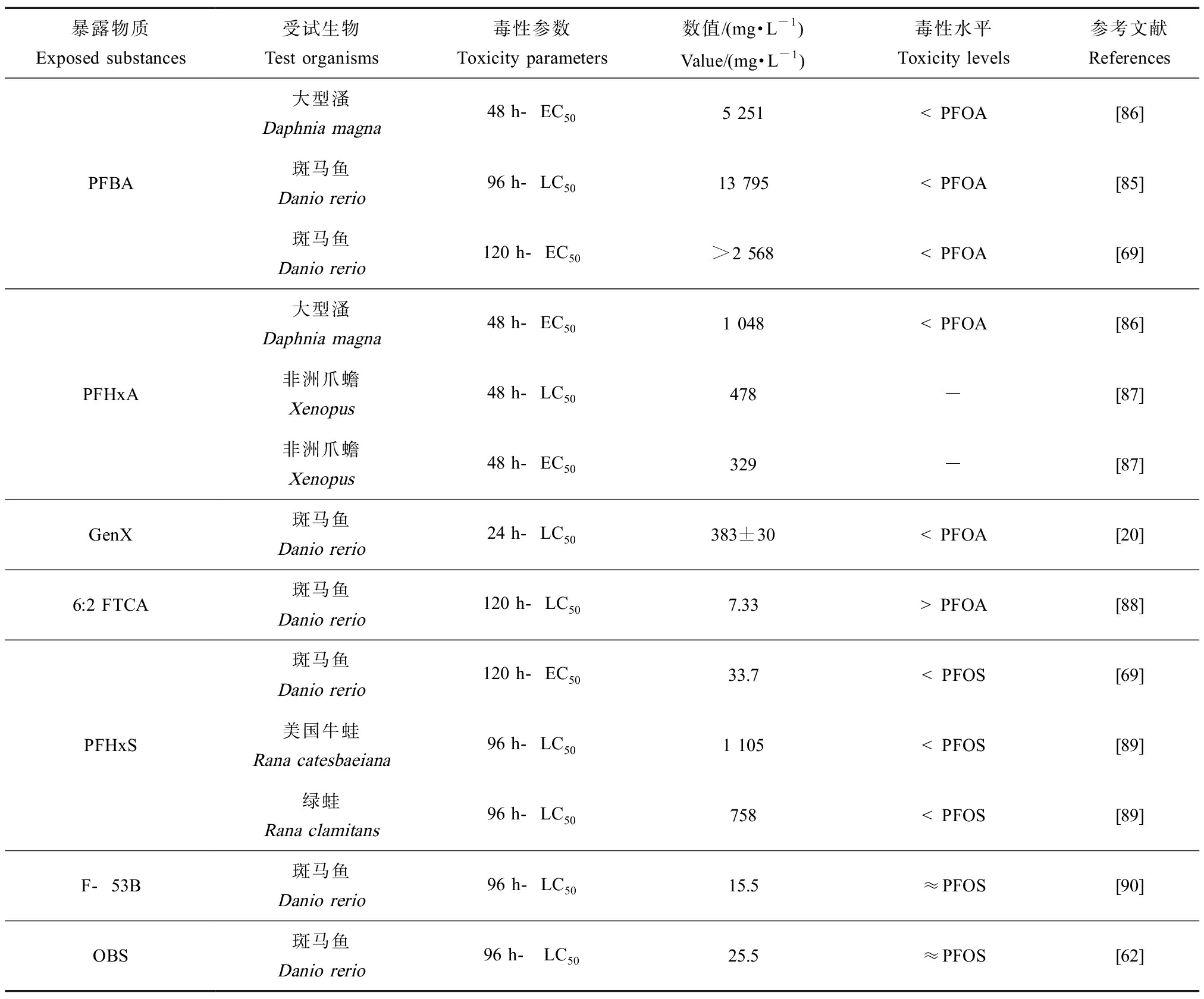
暴露物质Exposed substances受试生物Test organisms毒性参数Toxicity parameters数值/(mg·L-1)Value/(mg·L-1)毒性水平Toxicity levels参考文献ReferencesPFBA大型溞Daphnia magna48 h-EC505 251 注:EC50表示半数效应浓度;LC50表示半致死浓度。
Note: EC50 stands for median effect concentration; LC50 stands for median lethal concentration.
研究发现将斑马鱼胚胎分别暴露在PFHxS和PFOS中120 h,发生胚胎畸变的EC50值分别为33.7 mg·L-1和1.90 mg·L-1,即PFHxS对斑马鱼的急性毒性小于被替代的PFOS[69]。然而,当斑马鱼在F-53B中暴露96 h后,测得其LC50值为15.5 mg·L-1,与PFOS(96 h-LC50 =17 mg·L-1)的毒性相当[90]。此外,研究还发现斑马鱼在OBS中暴露96 h,测得LC50值为25.5 mg·L-1,根据危险化学品分类(GHS)其急性毒性与PFOS相似[62]。
可以看出,大部分PFOA和PFOS替代品的毒性较低并明显低于PFOA或PFOS,但仍有部分PFOA和PFOS替代品,如F-53B、OBS等物质急性毒性与PFOA或PFOS相当,甚至存在毒性更高的情况(如6:2 FTCA)。
3.2 发育毒性(Developmental toxicity)
研究表明斑马鱼早期发育阶段(胚胎期和仔鱼期)易受到环境污染物的影响,诱导发育毒性,因此以往的研究主要考察了处于胚胎期和仔鱼期的斑马鱼暴露于PFOA和PFOS替代品可能发生的形态学变化,如畸形、心率、孵化率、体长改变等。
研究发现PFBA暴露会导致斑马鱼幼鱼鱼鳔充气障碍,可能与体内甲状腺激素受到干扰有关[91-92]。Kim等[87]研究发现PFHxA(0.1、0.5、1和1.5 mmol L-1)可以导致非洲爪蟾胚胎发育异常,在发育过程中出现多发性水肿、小头畸形、骨骼扭曲、体长变短等畸形现象。此外,PFHxA还会损害非洲爪蟾肝脏和心脏发育,导致其肝脏肿大、心房扩大、房间隔缺失等形态损伤,在研究中可观察到相关标志物xPTB(肝脏)和NKX2.5(心脏)的mRNA表达量显著降低。Annunziato等[93]将斑马鱼胚胎暴露在0.01 mg·L-1的PFHxA时,未观察到明显胚胎畸形和生长延迟等变化。然而,当斑马鱼胚胎暴露在较高浓度(0.48、2.4和12 mg·L-1)的PFHxA时,下丘脑-垂体-甲状腺轴(HPT)上部分基因和激素(如促进甲状腺激素)发生变化,其正常的生长进程受到扰乱[94-95]。上述研究表明,PFHxA对斑马鱼发育毒性强弱受到暴露剂量的影响。此外,物质状态也会影响其发育毒性强弱,例如当PFHxA与其他污染物共存时,可能会引起受试生物体内基因表达模式的改变,进而增强或减弱生物毒性[96]。
L-1)可以导致非洲爪蟾胚胎发育异常,在发育过程中出现多发性水肿、小头畸形、骨骼扭曲、体长变短等畸形现象。此外,PFHxA还会损害非洲爪蟾肝脏和心脏发育,导致其肝脏肿大、心房扩大、房间隔缺失等形态损伤,在研究中可观察到相关标志物xPTB(肝脏)和NKX2.5(心脏)的mRNA表达量显著降低。Annunziato等[93]将斑马鱼胚胎暴露在0.01 mg·L-1的PFHxA时,未观察到明显胚胎畸形和生长延迟等变化。然而,当斑马鱼胚胎暴露在较高浓度(0.48、2.4和12 mg·L-1)的PFHxA时,下丘脑-垂体-甲状腺轴(HPT)上部分基因和激素(如促进甲状腺激素)发生变化,其正常的生长进程受到扰乱[94-95]。上述研究表明,PFHxA对斑马鱼发育毒性强弱受到暴露剂量的影响。此外,物质状态也会影响其发育毒性强弱,例如当PFHxA与其他污染物共存时,可能会引起受试生物体内基因表达模式的改变,进而增强或减弱生物毒性[96]。
Tang等[97]将海洋青鳉鱼(O. melastigma)胚胎暴露在PFBS中,发现PFBS可诱导胚胎孵化时间提前。当斑马鱼胚胎暴露在PFBS中,可观察到明显的胰腺和尾鳍畸形、鱼鳔膨胀延迟和卵黄利用受损等发育畸形[98]。同样,Sun等[99]将斑马鱼幼鱼暴露于PFBS(3.3 mg·L-1和10 mg·L-1)中发现幼鱼体质量和生长率降低。他们利用转录组分析技术进一步探究PFBS对斑马鱼发育的影响,发现下丘脑-垂体-肾间质轴(HPI)相关基因,如促肾上腺皮质激素释放激素结合蛋白(CRHBP)和前阿片黑素细胞皮质激素(POMC)对应基因显著上调,糖皮质激素受体(GR)基因下调,这些基因的改变会导致斑马鱼体内皮质醇浓度升高,与之相关的应激活动消耗了维持早期发育的能量,从而导致斑马鱼幼鱼生长迟缓,此外生长激素/胰岛素样生长因子(GH/IGF)和HPT上相关基因的改变也会影响斑马鱼正常的生长发育[100]。Flynn等[101]发现PFHxS(1 mg·L-1)暴露会导致豹蛙(Rana pipiens)发育延迟,蝾螈(Ambystoma tigrinum)体长变短,还可以导致蟾蜍(Anaxyrus americanus)的体长和体质量受到影响。然而,Annunziato等[93]对斑马鱼胚胎进行PFHxS暴露,发现0.8 mg·L-1的PFHxS会导致斑马鱼体长和卵黄囊面积增加,这一结果与同浓度PFOS对斑马鱼造成的影响相反。已有研究指出F-53B可以在斑马鱼体内快速积累,在发育毒性方面与PFOS相当甚至更高,作为PFOS替代品的安全性有待评估[102]。Shi等[103]通过在实验室条件下设置不同的浓度梯度(1.5、3.0、6.0和12.0 mg·L-1)对斑马鱼胚胎(6-132 hpf)进行F-53B暴露,发现F-53B可以引起与PFOS类似的发育毒性(如胚胎畸形、孵化延迟、死亡率升高等)。研究发现F-53B对斑马鱼的发育毒性强弱与暴露浓度呈正相关关系。例如,Liu等[104]对比了F-53B暴露对斑马鱼胚胎期和仔鱼期的影响,发现在0~300 μg·L-1的低暴露浓度处理下除仔鱼期斑马鱼体长明显变短之外,其余各暴露组斑马鱼的存活率和体长均未发生明显改变,而在0~30 mg·L-1的高暴露浓度处理下处于仔鱼期的斑马鱼比胚胎期的斑马鱼更敏感,发育受到明显抑制。此外,研究还发现F-53B对斑马鱼发育的影响具有持续性。例如,Deng等[102]发现斑马鱼胚胎经F-53B暴露后,即使将斑马鱼转移到不含F-53B的环境中,其体长仍然短于对照组斑马鱼,说明F-53B持续阻碍斑马鱼胚胎的正常发育进程。研究发现1 mg·L-1的6:2 FTSA暴露会导致蝾螈的体长,蟾蜍的体长和体质量均受到影响[101]。当斑马鱼暴露于OBS后,会发现心包水肿、尾部弯曲、脊柱弯曲、体长变短等发育畸形,推测与孵化过程中的纤毛功能障碍有关[105]。
水生生物的发育毒性评价往往涉及多个测量终点,毒性强弱受到暴露剂量大小、物质结构和物质共存状态等的影响,毒性作用机理可能与干扰HPI轴、HPT轴和GH/IGF轴的调节能力或者影响孵化过程中正常的细胞活动有关。然而,PFOA和PFOS替代品对HPI轴、HPT轴和GH/IGF轴上相关基因表达量的影响是否与其结构性质相关有待进一步研究。
3.3 免疫毒性(Immunotoxicity)
生物体的免疫系统主要防御病原微生物和外源化合物的侵害,当机体受到环境污染物干扰时,免疫系统可灵敏地作出响应。研究表明PFOA和PFOS替代品暴露会影响水生生物体内免疫活性物质的表达,并引发一系列生理反应。
Ishibashi等[106]通过体外模拟实验的方式评估PFBA和PFHxA(1.68~78.8 mg·L-1)对贝加尔湖海豹过氧化物酶体增殖物激活受体α(BS PPARα)的活化作用,结果发现PFBA和PFHxA可以增加PPARα的转录活性。类似的,Søderstrøm等[107]也发现PFHxA也可激活大西洋鳕鱼(Gadus morhua)的PPARs。在生态毒理学研究中,常将氧化应激作为一种敏感的生物标志物,作为机体免疫毒性预警信号[108]。生物体内存在氧化和抗氧化系统的平衡,其中由多种酶(如超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和谷胱甘肽转移酶(GST))组成的抗氧化防御系统,可保护细胞免受氧化损伤,避免氧化应激的产生[109]。Liu等[110]将小球藻(C. pyrenoidosa)暴露于GenX(12.5、25、50和100 mg·L-1)中96 h,发现GenX可以激活小球藻体内的氧化应激,导致体内活性氧(ROS)含量、SOD活性、谷胱甘肽过氧化物酶(GPX)活性和总抗氧化能力(T-AOC)水平发生变化。通过转录组分析发现,谷胱甘肽-抗坏血酸循环和光合作用相关基因上调,推测这是小球藻应对氧化应激损伤的内部调节机制。
研究表明,皮肤黏液中存在的微生物是抵制病原体入侵的免疫屏障重要组成部分,对鱼类免疫发育和维持至关重要[111]。Hu等[112]的研究指出,PFBS暴露会造成成年斑马鱼皮肤黏液中的微生物菌群失调,并导致雄性斑马鱼皮肤黏液蛋白浓度升高,对其免疫功能产生影响。与PFHxA类似,研究发现PFHxS也可以对海豹、鳕鱼等水生生物的PPAR起活化作用,引起氧化应激的产生[106-107]。Xu等[113]通过脂质组学技术发现,PFHxS暴露会引起斑马鱼胚胎体内与氧化应激等免疫相关脂质的种类和表达量发生改变,并激发机体防御系统,推测与PPAR信号通路有关。Wu等[114]的研究表明F-53B可以引起斑马鱼体内氧化应激相关标志物(如SOD、CAT、GST、丙二醛(MDA)等)的变化,诱导氧化应激反应,激活其抗氧化防御系统,此现象可能是F-53B对P13k/Akt信号通路和Nrf2-ARE信号通路的影响有关[78, 108]。Liu等[104]根据特定指标、基因和蛋白水平的研究发现,F-53B暴露对不同发育期的斑马鱼均有一定的免疫毒性,且结合基准计量水平(BMD)值可以看出F-53B对仔鱼期斑马鱼毒性大于胚胎期。毒性研究常以单一暴露为主,而环境中的污染物往往以混合形式存在,因此污染物联合暴露研究更具有现实意义。Yang等[115]发现聚苯乙烯塑料微粒(PS-MPs)的存在可以显著降低F-53B在斑马鱼仔鱼体内的积累能力,却诱导了更为严重的氧化应激和炎症反应,并指出该免疫过程与NF-κB信号通路有关。Zhang和Liang[116]通过测定氧化应激相关指标(如SOD、CAT、过氧化物酶(POD)等)确定6:2 FTSA暴露(10 μg·L-1和200 μg·L-1)对浮萍(common duckweed)的影响,发现暴露至第7天和第14天时浮萍中CAT酶活性受到了显著抑制,并呈现出剂量依赖性,说明6:2 FTSA暴露抑制了浮萍体内的抗氧化防御系统。当斑马鱼胚胎暴露于30 mg·L-1的OBS 96 h后,斑马鱼体内的GSH、SOD、CAT等抗氧化酶含量下降,ROS含量显著增加,表明OBS暴露促进了其氧化应激反应,进一步研究发现,OBS暴露后体内的免疫相关基因(如il-1β和mmp9等)表达上调,白细胞介素1β(IL-1β)、白细胞介素-8(CXCL8)、基质金属蛋白酶(MMP9)和胱天蛋白酶8(Casp8)的蛋白表达水平也显著升高[117]。此外有研究指出,OBS对斑马鱼氧化应激的产生与Nrf2-ARE信号通路有一定的关系[118]。除氧化应激外,炎症反应也可作为生物体免疫毒性的预警信号,且炎症反应与氧化应激之间存在着错综复杂的联系[119]。Huang等[120]研究了低浓度(0.6 mg·L-1)OBS暴露21 d对斑马鱼的影响,发现OBS可以通过影响促炎细胞因子的表达诱导抗炎作用,并引起与PFOS类似的免疫毒性。
PFOA和PFOS替代品暴露会快速激活生物体内的抗氧化和免疫防御系统,实现机体自我保护[121]。在PFASs对水生生物的免疫毒性研究中,除直接表现为免疫相关基因或蛋白的差异表达外,还可表现为机体氧化应激、炎症反应的出现,其毒性效应机制可能与PPAR激活,NF-κB、Nrf2-ARE等信号通路受到影响,或机体皮肤菌群改变、体内代谢紊乱有关。
3.4 神经毒性(Neurotoxicity)
外源化合物可以通过影响生物体神经系统结构或者神经系统功能(如影响神经信号传导等),进而引起神经毒性。研究表明PFASs可以穿过血脑屏障并在生物体脑组织中积累,其对水生生物的神经毒性不可忽略[122]。目前关于PFOA和PFOS替代品对水生生物的神经毒性研究主要包括PFOA和PFOS替代品对发育期水生生物的发育神经毒性的研究和对成年水生生物神经毒性的研究。
当斑马鱼暴露于PFBA 120 h后,斑马鱼在水中的移动距离和移动速度发生明显变化[123]。Guo等[94]发现将斑马鱼胚胎暴露于PFHxA(0.48、2.4和12 mg·L-1)120 h后,虽然低浓度PFHxA暴露没有引起斑马鱼胚胎明显异常,然而高浓度PFHxA暴露下的斑马鱼体内与神经发育相关的多个基因(如mbp、syn2a、a1-tubulin、elavl3等)和蛋白(如α1-tubulin、elavl3和gap43等)表达水平改变,神经递质浓度受到显著影响,斑马鱼幼鱼游泳行为发生变化,说明PFHxA在较高暴露水平下会对斑马鱼产生发育神经毒性,其机理可能与神经元发育受到抑制、神经信号传导受到影响有关。Rericha等[124]对斑马鱼进行PFHxA膳食暴露研究,结果发现PFHxA引起了斑马鱼异常焦虑,出现回避行为和惊恐反应。Gaballah等[125]也发现PFHxA暴露会引起斑马鱼异常行为,然而当斑马鱼暴露在GenX和ADONA时,没有观察到明显的异常行为。
视觉系统在感知外界信息和启动运动反应中起着重要作用。Chen等[126]将海洋青鳉鱼胚胎暴露于环境浓度的PFBS(1.0、2.9和9.5 μg·L-1)至性成熟,解剖成年青鳉鱼的眼睛,在眼组织中检测到较高浓度的PFBS。该研究发现PFBS的暴露会造成雌性青鳉鱼眼睛质量减少,其内含水量增加,并可能导致其眼压升高。此外,PFBS暴露还会干扰多个神经信号传导过程,并造成与视觉感知和运动活动显著相关的视觉蛋白(例如,β和γ晶体蛋白、arrestin和lumican等)发生改变,表明低浓度的PFBS暴露可诱发神经毒性。Tang等[127]将海洋青鳉鱼胚胎暴露于3.3 mg·L-1 PFBS 15 d,结合高通量测序技术筛选差异基因,发现PFBS暴露会导致青鳉鱼嗅觉相关基因表达下调,影响G蛋白偶联受体活性(如嗅觉受体)和嗅觉传导。多项研究表明将成年斑马鱼暴露于10 mg·L-1和100 mg·L-1的PFBS 28 d后,斑马鱼肠道和脑组织中的胆碱能、谷氨酸能、血清素能、γ-氨基丁酸(GABA)能和单胺能等神经信号传导受到干扰[126, 128-129]。研究发现PFBS暴露除对斑马鱼神经信号传导造成干扰,还会影响斑马鱼体内视黄醇代谢,导致其接收到错误的视觉信号[130]。此外,这些研究发现当益生菌与PFBS联合暴露时,可以拮抗PFBS对斑马鱼神经传导信号以及视觉信号的干扰[128]。Annunziato等[93]研究发现,PFHxS(8.76 mg·L-1)暴露会对斑马鱼的行为产生影响,主要表现为移动距离的减少。当斑马鱼暴露于一定梯度浓度(0.15、1.5和5 μg·L-1)的F-53B 120 h后,斑马鱼视觉功能受损,其眼睛体积、晶状体面积、视网膜神经节细胞层面积等随着暴露浓度的增加而减小,对相关基因进行分析发现,斑马鱼体内与细胞凋亡相关基因(如p53等)上调,与视黄酸代谢、晶状体发育、视网膜发育相关基因(如aldhla2、crybb等)下调,对斑马鱼幼鱼的反应行为进行评估发现,斑马鱼游泳速度增加,推测F-53B引发的视觉功能紊乱导致斑马鱼过度活跃[131]。在一项探究6:2 FTSA暴露对斑马鱼胚胎影响的研究中,通过将斑马鱼幼鱼的游泳活动进行量化记录,发现斑马鱼在黑暗条件下的游泳距离显著增加,推测可能是通过影响感觉器官或者多巴胺能系统继而导致其行为异常[132]。将斑马鱼胚胎暴露于30 mg·L-1的OBS 4 d后,斑马鱼幼鱼的运动距离显著减少,体内多巴胺含量明显降低,相关神经元控制基因(如bdnf)表达量下调,表明OBS对斑马鱼具有明显的神经毒性作用[105]。
PFOA和PFOS替代品对水生生物的神经毒性强弱与物质结构、剂量大小密切相关,其对水生生物神经毒性可以用行为变化进行评估,推测其毒性效应机制可能是通过影响神经元和感觉器官发育,干扰神经信号和视觉信号传导。
3.5 生殖毒性(Reproductive toxicity)
与PFASs类似,PFOA和PFOS替代品对水生生物具有生殖毒性。多项研究表明暴露于PFOA和PFOS替代品会影响水生生物的生殖能力并对其子代的发育、代谢等多方面产生影响。
Barmentlo等[86]研究发现PFHxA在低浓度下会促进大型溞的繁殖,而在高浓度下会抑制其繁殖,表现出低促高抑的现象。当非洲爪蟾暴露在环境浓度的PFBS 2个月后,其大脑中雌激素受体(ER)和雄激素受体(AR)的mRNA水平显著提高,预示着PFBS可能会对非洲爪蟾性别发育产生影响[133]。Chen等[134]将海洋青鳉鱼暴露于低浓度的PFBS中,发现PFBS暴露会改变海洋青鳉鱼的性别比、导致雄性比例提高,还会造成雌性青鳉鱼卵巢体积变小,卵母细胞发育受阻,产卵量降低等现象,使得其生殖功能受到严重损害。此外,他们还发现PFBS对雌性青鳉鱼表现出抗雌激素活性,对雄性青鳉鱼表现出雌激素活性,此不利影响主要是由于下丘脑-垂体-性腺(HPG)轴上关键激素(如促性腺激素释放激素(GnRH)、黄体生成素(LH)、促卵泡激素(FSH)等)水平发生变化,引起性激素紊乱。除此以外,将海洋青鳉鱼暴露于PFBS后,其后代也会受到PFBS的影响。例如,Chen等[135]对暴露于PFBS的青鳉鱼的子代(F1)、子代的子代(F2)进行跟踪分析,发现F1、F2和亲代产生相同的毒性效应,即证明传代毒性和跨代毒性的产生。Chen等[136]通过进一步研究证明PFBS的传代毒性可能与父代甲基化标记的异常遗传有关。与海洋青鳉鱼类似,PFBS暴露也会导致雌性斑马鱼卵巢体积变小,卵母细胞的生长和成熟受到干扰,正常产卵周期受到影响[137]。此外,亲代暴露还会对母体转录本的转移过程产生干扰,引发基因层次上的表达差异[138]。研究发现将成年斑马鱼暴露在F-53B中180 d后,成年斑马鱼体内性腺组织结构发生改变,成熟精母/卵母细胞数量减少,血清睾酮(T)水平升高、睾酮(T)与雌二醇(E2)比例显著提高,HPG轴上相关基因转录水平发生改变,此外,雄性斑马鱼血清中的E2和卵黄蛋白原(VTG)含量显著升高,不仅如此,F-53B还可以通过产卵转移到子代体内,引起子代发育异常[139]。在其他研究中也发现了F-53B暴露会对子代产生毒性作用,例如,Shi等[140]发现将斑马鱼暴露在F-53B中会引起脂质代谢异常,对其子代进行基因检测分析发现子代斑马鱼体内部分脂质代谢相关的基因也发生异常表达。另一项研究也发现亲代斑马鱼暴露在F-53B中会导致子代HPT轴相关基因发生改变,影响其甲状腺正常发育[141]。
较低浓度的PFOA和PFOS替代品暴露就会造成水生生物生殖器官受损,生殖功能受到影响,还会导致体内性激素紊乱,其作用机理可能是通过影响HPG轴内分泌功能。此外,PFOA和PFOS替代品还会影响子代正常发育,产生传代毒性和跨代毒性,其作用机理可能是PFOA和PFOS替代品通过母体转移到子代体内或者通过亲代遗传信息错误传递到子代体内引起毒性效应。
3.6 其他毒性和内分泌干扰作用(Other toxic and endocrine disrupting effect)
PFOA和PFOS替代品对水生生物的毒性作用除了上述几种典型毒性效应外,还存在多种其他毒性效应,如肝毒性、心脏毒性等,此外,PFOA和PFOS替代品还会干扰生物内分泌系统。肝脏被认为是脊椎动物PFASs污染的主要靶器官,呈现出空泡化、代谢紊乱、功能受损等肝毒性。Mahapatra等[142]调查了PFOA和其替代物PFHxA暴露对斑马鱼肝细胞的影响,研究结果表明所有实验组的细胞代谢均受到明显影响(如还原型辅酶Ⅰ(NADH)的寿命降低)。Liu等[143]研究发现,暴露于0.01 mg·L-1的PFBS会导致成年斑马鱼肝脏糖脂代谢紊乱,出现肝脏空泡化的现象。体内实验证明,PFOS、OBS和F-53B暴露后最早是在肝脏进行蓄积[144]。Shi等[140]调查了F-53B长期暴露对斑马鱼的影响,研究发现F-53B诱导了斑马鱼肝损伤,推测可能与体内PPAR信号通路受到影响有关。Wang等[145]发现F-53B暴露会造成斑马鱼肝脏空泡化以及细胞核固缩,并导致肝功异常,其对斑马鱼的肝毒性大于PFOS。该研究还发现斑马鱼暴露于OBS中也会导致肝脏空泡化、肝功异常等现象,通过KEGG分析发现OBS与F-53B、PFOS相似,均可通过引发脂质紊乱导致肝细胞损伤[145]。同样,在另一项针对雄性成年斑马鱼的OBS长期暴露研究中,也发现了类似的肝脏代谢失衡现象[146]。Gong等[147]在一项研究中揭示了PFBS对斑马鱼的心脏毒性。PFBS暴露导致斑马鱼胚胎心房和心室组织缺陷、心脏瓣膜边界不清,通过转录组分析发现PFBS可导致斑马鱼体内与心脏发育相关的基因转录水平发生变化。此外,F-53B暴露也会影响斑马鱼心脏的正常功能(主要表现为心率降低),其毒性机理可能是F-53B暴露后导致斑马鱼胚胎体内的Wnt信号通路受到了影响[103]。Li等[148]将小球藻暴露于GenX溶液中12 d,以此探究GenX对小球藻的毒性效应。研究结果表明,GenX通过影响光合碳循环酶导致CO2同化减少,从而影响小球藻的光合活性,此外,基因分析结果显示小球藻中大部分与光合蛋白相关的基因表达下调。Labine等[149]发现GenX暴露会诱导大型溞甘油磷脂、半胱氨酸和蛋氨酸代谢异常。Liu等[150]探讨了F-53B对中华稀有鮈鲫(Gobiocypris rarus)幼鱼的甲状腺功能干扰效应,研究发现鱼体内甲状腺激素和HPT轴相关基因(如CRH、TSHβ、TTR、Dio1、Dio2、TRα、TRβ等)受到影响,T3(总T3)和fT3(游离T3)含量均显著增加,该研究表明,F-53B暴露对中华稀有鮈鲫的甲状腺功能具有干扰作用。
4 总结与展望(Conclusion and prospect)
研究表明PFOA和PFOS替代品在水环境中广泛存在,其对水环境及水生生物健康的影响不可忽视。目前PFOA和PFOS替代品对水生生物的毒性效应研究已证明PFOA和PFOS替代品对水生生物的发育、免疫、神经等方面均具有一定的毒性。尽管环境浓度下PFOA和PFOS替代品几乎不会导致水生生物急性致死,但可通过诱发神经毒性、生殖毒性等毒性效应,影响水生生物的健康安全。研究PFOA和PFOS替代品对水生生物的毒性效应具有重要的环境意义,然而,有关PFOA和PFOS替代品对水生生物的毒性效应的研究尚不全面,其毒性效应机制仍不明确。今后有关PFOA和PFOS替代品对水生生物的毒性效应研究应关注以下几个方面:(1)拓展PFOA和PFOS替代品水生生物毒性效应研究的广度。目前部分PFOA和PFOS替代品对水生生物的毒性效应研究较少,例如ADONA的内分泌毒性、GenX的免疫毒性均在大鼠中得到证实,而其对水生生物类似毒性的研究几乎没有。不同PFOA和PFOS替代品的分子结构、理化性质存在较大差异,因此今后需要扩展实验室研究中PFOA和PFOS替代品的种类,探究其对水生生物的毒性效应及存在的构效关系。此外,目前用于PFOA和PFOS替代品毒性效应研究的水生生物种类有限,特别是对水生植物、底栖生物等水生生物的研究较少。不同生物对同种污染物的敏感度存在差异,因此今后需加强PFOA和PFOS替代品对多种水生生物以及不同营养级生物的毒性效应研究,以期全面评价PFOA和PFOS替代品对水生生态系统的安全性。(2)加强PFOA和PFOS替代品毒性效应研究的深度。尽管环境浓度下部分PFOA和PFOS替代品可能不会引起水生生物形态学改变,但水生生物的基因表达和代谢过程已受到显著影响,因此基于分子水平探究环境浓度下PFOA和PFOS替代品对水生生物的毒性效应有助于为早期预测提供参考,后续应加强分子水平的毒性效应研究。此外,目前大部分PFOA和PFOS替代品对水生生物的毒性效应研究仅局限于单一水平和有限的技术手段,后续研究应结合代谢组学、蛋白组学等技术手段,综合基因、细胞、个体等不同水平深入探究其毒性效应机制。(3)开展PFOA和PFOS替代品复合污染毒性效应研究。目前的研究主要是针对单一污染物对水生生物的毒性效应,关于不同污染物复合毒性的研究较少。真实水环境中通常同时存在多种PFOA和PFOS替代品,为更好地评估真实环境下PFOA和PFOS替代品对水生生物的毒性效应,应重视多种PFOA和PFOS替代品对水生生物的复合毒性。(4)针对PFOA和PFOS替代品,加强其环境风险和健康风险研究。研究发现部分PFOA和PFOS替代品对水生生物的毒性超过被替代品,其安全性存在争议。因此应综合评估PFOA和PFOS替代品对水生生物的毒性效应,开展环境和健康风险评价,在此基础上提出应对之策,开发更加安全、绿色、经济的新型PFOA和PFOS替代品。
[1] Jantzen C E, Annunziato K M, Cooper K R. Behavioral, morphometric, and gene expression effects in adult zebrafish (Danio rerio) embryonically exposed to PFOA, PFOS, and PFNA [J]. Aquatic Toxicology, 2016, 180: 123-130
[2] Hoover G M, Chislock M F, Tornabene B J, et al. Uptake and depuration of four per/polyfluoroalkyl substances (PFASS) in northern leopard frog Rana pipiens tadpoles [J]. Environmental Science &Technology Letters, 2017, 4(10): 399-403
[3] Zhang S H, Chen K, Li W M, et al. Varied thyroid disrupting effects of perfluorooctanoic acid (PFOA) and its novel alternatives hexafluoropropylene-oxide-dimer-acid (GenX) and ammonium 4,8-dioxa-3H-perfluorononanoate (ADONA) in vitro [J]. Environment International, 2021, 156: 106745
[4] Duan Y S, Sun H W, Yao Y M, et al. Serum concentrations of per-/polyfluoroalkyl substances and risk of type 2 diabetes: A case-control study [J]. The Science of the Total Environment, 2021, 787: 147476
[5] Dong G Z, Zhang R, Huang H Y, et al. Exploration of the developmental toxicity of TCS and PFOS to zebrafish embryos by whole-genome gene expression analyses [J]. Environmental Science and Pollution Research International, 2021, 28(40): 56032-56042
[6] Ojo A F, Xia Q, Peng C, et al. Evaluation of the individual and combined toxicity of perfluoroalkyl substances to human liver cells using biomarkers of oxidative stress [J]. Chemosphere, 2021, 281: 130808
[7] Jo A, Ji K, Choi K. Endocrine disruption effects of long-term exposure to perfluorodecanoic acid (PFDA) and perfluorotridecanoic acid (PFTrDA) in zebrafish (Danio rerio) and related mechanisms [J]. Chemosphere, 2014, 108: 360-366
[8] Khan E A, Zhang X K, Hanna E M, et al. Application of quantitative transcriptomics in evaluating the ex vivo effects of per- and polyfluoroalkyl substances on Atlantic cod (Gadus morhua) ovarian physiology [J]. The Science of the Total Environment, 2021, 755(Pt 1): 142904
[9] Schröter-Kermani C, Müller J, Jürling H, et al. Retrospective monitoring of perfluorocarboxylates and perfluorosulfonates in human plasma archived by the German Environmental Specimen Bank [J]. International Journal of Hygiene and Environmental Health, 2013, 216(6): 633-640
[10] Coperchini F, Croce L, Denegri M, et al. Adverse effects of in vitro GenX exposure on rat thyroid cell viability, DNA integrity and thyroid-related genes expression [J]. Environmental Pollution, 2020, 264: 114778
[11] Chappell G A, Thompson C M, Wolf J C, et al. Assessment of the mode of action underlying the effects of GenX in mouse liver and implications for assessing human health risks [J]. Toxicologic Pathology, 2020, 48(3): 494-508
[12] 周秀鹃, 盛南, 王建设, 等. 全氟和多氟化合物替代品的研究进展[J]. 生态毒理学报, 2017, 12(3): 3-12
Zhou X J, Sheng N, Wang J S, et al. The Current research status of several kinds of fluorinated alternatives [J]. Asian Journal of Ecotoxicology, 2017, 12(3): 3-12 (in Chinese)
[13] Xu C, Song X, Liu Z Y, et al. Occurrence, source apportionment, plant bioaccumulation and human exposure of legacy and emerging per- and polyfluoroalkyl substances in soil and plant leaves near a landfill in China [J]. The Science of the Total Environment, 2021, 776: 145731
[14] Pasecnaja E, Bartkevics V, Zacs D. Occurrence of selected per- and polyfluorinated alkyl substances (PFASs) in food available on the European market - A review on levels and human exposure assessment [J]. Chemosphere, 2022, 287(Pt 4): 132378
[15] Thompson C M, Fitch S E, Ring C, et al. Development of an oral reference dose for the perfluorinated compoundGenX [J]. Journal of Applied Toxicology, 2019, 39(9): 1267-1282
[16] Sheng N, Zhou X J, Zheng F, et al. Comparative hepatotoxicity of 6:2 fluorotelomer carboxylic acid and 6:2 fluorotelomer sulfonic acid, two fluorinated alternatives to long-chain perfluoroalkyl acids, on adult male mice [J]. Archives of Toxicology, 2017, 91(8): 2909-2919
[17] Zhou X J, Wang J S, Sheng N, et al.Subchronic reproductive effects of 6:2 chlorinated polyfluorinated ether sulfonate (6:2 Cl-PFAES), an alternative to PFOS, on adult male mice [J]. Journal of Hazardous Materials, 2018, 358: 256-264
[18] Cai Y P, Wang Q Y, Zhou B H, et al. A review of responses of terrestrial organisms to perfluorinated compounds [J]. The Science of the Total Environment, 2021, 793: 148565
[19] Brase R A, Mullin E J, Spink D C. Legacy and emerging per- and polyfluoroalkyl substances: Analytical techniques, environmental fate, and health effects [J]. International Journal of Molecular Sciences, 2021, 22(3): 995
[20] Gebreab K Y, Eeza M N H, Bai T Y, et al. Comparative toxicometabolomics of perfluorooctanoic acid (PFOA) and next-generation perfluoroalkyl substances [J]. Environmental Pollution, 2020, 265: 114928
[21] 陈家苗, 王建设. 新型全氟和多氟烷醚类化合物的环境分布与毒性研究进展[J]. 生态毒理学报, 2020, 15(5): 28-34
Chen J M, Wang J S. Research progress in environmental distribution and toxicity of per-and polyfluoroalkyl ether substances [J]. Asian Journal of Ecotoxicology, 2020, 15(5): 28-34 (in Chinese)
[22] Lalonde B, Garron C. Perfluoroalkyl substances (PFASs) in the Canadian freshwater environment [J]. Archives of Environmental Contamination and Toxicology, 2022, 82(4): 581-591
[23] Cao X H, Wang C C, Lu Y L, et al. Occurrence, sources and health risk of polyfluoroalkyl substances (PFASs) in soil, water and sediment from a drinking water source area [J]. Ecotoxicology and Environmental Safety, 2019, 174: 208-217
[24] Sun Q P, Bi R, Wang T Y, et al. Are there risks induced by novel and legacy poly- and perfluoroalkyl substances in coastal aquaculture base in South China? [J]. The Science of the Total Environment, 2021, 779: 146539
[25] Wang Q, Song X, Wei C L, et al. Distribution, source identification and health risk assessment of PFASs in groundwater from Jiangxi Province, China [J]. Chemosphere, 2022, 291(Pt 2): 132946
[26] Chen S Q, Yan M, Chen Y, et al. Perfluoroalkyl substances in the surface water and fishes in Chaohu Lake, China [J]. Environmental Science and Pollution Research International, 2022, 29(50): 75907-75920
[27] Ren J, Yu M J, Chen F, et al. Occurrence, spatial heterogeneity, and risk assessment of perfluoroalkyl acids (PFAAs) in the major rivers of the Tibetan Plateau [J]. The Science of the Total Environment, 2023, 856(Pt 1): 159026
[28] Meng Y, Yao Y M, Chen H, et al. Legacy and emerging per- and polyfluoroalkyl substances (PFASs) in Dagang Oilfield: Multimedia distribution and contributions of unknown precursors [J]. Journal of Hazardous Materials, 2021, 412: 125177
[29] Zhang F S, Wang Y L, Wei Z, et al. Perfluorinated compounds in a river basin from QingHai-Tibet Plateau: Occurrence, sources and key factors [J]. Ecotoxicology and Environmental Safety, 2021, 228: 113043
[30] Tang J X, Zhu Y L, Xiang B, et al. Multiple pollutants in groundwater near an abandoned Chinese fluorine chemical park: Concentrations, correlations and health risk assessments [J]. Scientific Reports, 2022, 12: 3370
[31] Ali A M, Higgins C P, Alarif W M, et al. Per- and polyfluoroalkyl substances (PFASs) in contaminated coastal marine waters of the Saudi Arabian Red Sea: A baseline study [J]. Environmental Science and Pollution Research International, 2021, 28(3): 2791-2803
[32] Khan K, Younas M, Zhou Y Q, et al. First report of perfluoroalkyl acids (PFAAs) in the Indus Drainage System: Occurrence, source and environmental risk [J]. Environmental Research, 2022, 211: 113113
[33] Gao Y, Liang Y, Gao K, et al. Levels, spatial distribution and isomer profiles of perfluoroalkyl acids in soil, groundwater and tap water around a manufactory in China [J]. Chemosphere, 2019, 227: 305-314
[34] Bai X L, Son Y. Perfluoroalkyl substances (PFAS) in surface water and sediments from two urban watersheds in Nevada, USA [J]. The Science of the Total Environment, 2021, 751: 141622
[35] Selvaraj K K, Murugasamy M, Nikhil N P, et al. Investigation of distribution, sources and flux of perfluorinated compounds in major southern Indian Rivers and their risk assessment [J]. Chemosphere, 2021, 277: 130228
[36] Schmidt N, Fauvelle V, Castro-Jiménez J, et al. Occurrence of perfluoroalkyl substances in the Bay of Marseille (NW Mediterranean Sea) and the Rh ne River [J]. Marine Pollution Bulletin, 2019, 149: 110491
ne River [J]. Marine Pollution Bulletin, 2019, 149: 110491
[37] Gebbink W A, van Asseldonk L, van Leeuwen S P J. Presence of emerging per- and polyfluoroalkyl substances (PFASs) in river and drinking water near a fluorochemical production plant in the Netherlands [J]. Environmental Science &Technology, 2017, 51(19): 11057-11065
[38] Xu B T, Liu S, Zhou J L, et al. PFAS and their substitutes in groundwater: Occurrence, transformation and remediation [J]. Journal of Hazardous Materials, 2021, 412: 125159
[39] Liu Z Y, Xu C, Johnson A C, et al. Exploring the source, migration and environmental risk of perfluoroalkyl acids and novel alternatives in groundwater beneath fluorochemical industries along the Yangtze River, China [J]. Science of the Total Environment, 2022, 827: 154413
[40] Brandsma S H, Koekkoek J C, van Velzen M J M, et al. The PFOA substitute GenX detected in the environment near a fluoropolymer manufacturing plant in the Netherlands [J]. Chemosphere, 2019, 220: 493-500
[41] Gebbink W A, van Leeuwen S P J. Environmental contamination and human exposure to PFASs near a fluorochemical production plant: Review of historic and current PFOA and GenX contamination in the Netherlands [J]. Environment International, 2020, 137: 105583
[42] Pétré M A, Genereux D P, Koropeckyj-Cox L, et al. Per- and polyfluoroalkyl substance (PFAS) transport from groundwater to streams near a PFAS manufacturing facility in North Carolina, USA [J]. Environmental Science &Technology, 2021, 55(9): 5848-5856
[43] Pan Y T, Zhang H X, Cui Q Q, et al. Worldwide distribution of novel perfluoroether carboxylic and sulfonic acids in surface water [J]. Environmental Science &Technology, 2018, 52(14): 7621-7629
[44] Zhou J, Li S J, Liang X X, et al. First report on the sources, vertical distribution and human health risks of legacy and novel per- and polyfluoroalkyl substances in groundwater from the Loess Plateau, China [J]. Journal of Hazardous Materials, 2021, 404(Pt A): 124134
[45] Zhou J, Li Z, Guo X T, et al. Evidences for replacing legacy per- and polyfluoroalkyl substances with emerging ones in Fen and Wei River Basins in Central and Western China [J]. Journal of Hazardous Materials, 2019, 377: 78-87
[46] Feng X M, Ye M Q, Li Y, et al. Potential sources and sediment-pore water partitioning behaviors of emerging per/polyfluoroalkyl substances in the South Yellow Sea [J]. Journal of Hazardous Materials, 2020, 389: 122124
[47] Zhao Z, Cheng X H, Hua X, et al. Emerging and legacy per- and polyfluoroalkyl substances in water, sediment, and air of the Bohai Sea and its surrounding rivers [J]. Environmental Pollution, 2020, 263: 114391
[48] Du D, Lu Y L, Zhou Y Q, et al. Perfluoroalkyl acids (PFAAs) in water along the entire coastal line of China: Spatial distribution, mass loadings, and worldwide comparisons [J]. Environment International, 2022, 169: 107506
[49] Li X T, Wang Y, Qian C J, et al. Perfluoroalkyl acids (PFAAs) in urban surface water of Shijiazhuang, China: Occurrence, distribution, sources and ecological risks [J]. Journal of Environmental Sciences (China), 2023, 125: 185-193
[50] Tang A P, Zhang X H, Li R F, et al. Spatiotemporal distribution, partitioning behavior and flux of per- and polyfluoroalkyl substances in surface water and sediment from Poyang Lake, China [J]. Chemosphere, 2022, 295: 133855
[51] Chen H T, Reinhard M, Nguyen T V, et al. Characterization of occurrence, sources and sinks of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in a tropical urban catchment [J]. Environmental Pollution, 2017, 227: 397-405
[52] Gao L J, Liu J L, Bao K, et al. Multicompartment occurrence and partitioning of alternative and legacy per- and polyfluoroalkyl substances in an impacted river in China [J]. The Science of the Total Environment, 2020, 729: 138753
[53] Wu J, Junaid M, Wang Z F, et al. Spatiotemporal distribution, sources and ecological risks of perfluorinated compounds (PFCs) in the Guanlan River from the rapidly urbanizing areas of Shenzhen, China [J]. Chemosphere, 2020, 245: 125637
[54] Tang J X, Zhu Y L, Li Y, et al. Occurrence characteristics and health risk assessment of per- and polyfluoroalkyl substances from water in residential areas around fluorine chemical industrial areas, China [J]. Environmental Science and Pollution Research International, 2022, 29(40): 60733-60743
[55] Guo R, Liu X L, Liu J, et al. Occurrence, partition and environmental risk assessment of per- and polyfluoroalkyl substances in water and sediment from the Baiyangdian Lake, China [J]. Scientific Reports, 2020, 10(1): 4691
[56] Pan X, Ye J, Zhang H, et al. Occurrence, removal and bioaccumulation of perfluoroalkyl substances in Lake Chaohu, China [J]. International Journal of Environmental Research and Public Health, 2019, 16(10): 1692
[57] Wang T, Vestergren R,Herzke D, et al. Levels, isomer profiles, and estimated riverine mass discharges of perfluoroalkyl acids and fluorinated alternatives at the mouths of Chinese Rivers [J]. Environmental Science &Technology, 2016, 50(21): 11584-11592
[58] Wei C L, Wang Q, Song X, et al. Distribution, source identification and health risk assessment of PFASs and two PFOS alternatives in groundwater from non-industrial areas [J]. Ecotoxicology and Environmental Safety, 2018, 152: 141-150
[59] Wang S Q, Ding G H, Liu Y H, et al. Legacy and emerging persistent organic pollutants in the marginal seas of China: Occurrence and phase partitioning [J]. Science of the Total Environment, 2022, 827: 154274
[60] Munoz G, Fechner L C, Geneste E, et al. Spatio-temporal dynamics of per and polyfluoroalkyl substances (PFASs) and transfer to periphytic biofilm in an urban river: Case-study on the River Seine [J]. Environmental Science and Pollution Research, 2018, 25(24): 23574-23582
[61] Marchiandi J, Szabo D, Dagnino S, et al. Occurrence and fate of legacy and novel per- and polyfluoroalkyl substances (PFASs) in freshwater after an industrial fire of unknown chemical stockpiles [J]. Environmental Pollution, 2021, 278: 116839
[62] Xu L, Shi Y L, Li C X, et al. Discovery of a novel polyfluoroalkyl benzenesulfonic acid around oilfields in Northern China [J]. Environmental Science &Technology, 2017, 51(24): 14173-14181
[63] Hou M M, Jin Q, Na G S, et al. Emissions, isomer-specific environmental behavior, and transformation of OBS from one major fluorochemical manufacturing facility in China [J]. Environmental Science &Technology, 2022, 56(12): 8103-8113
[64] Vongphachan V, Cassone C G, Wu D M, et al. Effects of perfluoroalkyl compounds on mRNA expression levels of thyroid hormone-responsive genes in primary cultures of avian neuronal cells [J]. Toxicological Sciences: An Official Journal of the Society of Toxicology, 2011, 120(2): 392-402
[65] Arinaitwe K, Koch A, Taabu-Munyaho A, et al. Spatial profiles of perfluoroalkyl substances and mercury in fish from northern Lake Victoria, East Africa [J]. Chemosphere, 2020, 260: 127536
[66] Penland T N, Cope W G, Kwak T J, et al. Trophodynamics of per- and polyfluoroalkyl substances in the food web of a large Atlantic slope river [J]. Environmental Science &Technology, 2020, 54(11): 6800-6811
[67] Diao J Y, Chen Z W, Wang T Y, et al. Perfluoroalkyl substances in marine food webs from South China Sea: Trophic transfer and human exposure implication [J]. Journal of Hazardous Materials, 2022, 431: 128602
[68] Abafe O A, Macheka L R, Abafe O T, et al. Concentrations and human exposure assessment of per and polyfluoroalkyl substances in farmed marine shellfish in South Africa [J]. Chemosphere, 2021, 281: 130985
[69] Vogs C, Johanson G, Näslund M, et al. Toxicokinetics of perfluorinated alkyl acids influences their toxic potency in the zebrafish embryo (Danio rerio) [J]. Environmental Science &Technology, 2019, 53(7): 3898-3907
[70] Zhang B, He Y, Yang G, et al. Legacy and emerging poly- and perfluoroalkyl substances in finless porpoises from East China Sea: Temporal trends and tissue-specific accumulation [J]. Environmental Science &Technology, 2022, 56(10): 6113-6122
[71] Meng J, Liu S F, Zhou Y Q, et al. Are perfluoroalkyl substances in water and fish from drinking water source the major pathways towards human health risk? [J]. Ecotoxicology and Environmental Safety, 2019, 181: 194-201
[72] Chiesa L M, Nobile M, Pasquale E, et al. Detection of perfluoroalkyl acids and sulphonates in Italian eel samples by HPLC-HRMS Orbitrap [J]. Chemosphere, 2018, 193: 358-364
[73] Zhang A Q, Wang P, Lu Y L, et al. Occurrence and health risk of perfluoroalkyl acids (PFAAs) in seafood from Yellow Sea, China [J]. The Science of the Total Environment, 2019, 665: 1026-1034
[74] Wu J Y, Liu W X, He W, et al. Comparisons of tissue distributions and health risks of perfluoroalkyl acids (PFAAs) in two fish species with different trophic levels from Lake Chaohu, China [J]. Ecotoxicology and Environmental Safety, 2019, 185: 109666
[75] Wang Q, Ruan Y F, Jin L J, et al. Oysters for legacy and emerging per- and polyfluoroalkyl substances (PFASs) monitoring in estuarine and coastal waters: Phase distribution and bioconcentration profile [J]. The Science of the Total Environment, 2022, 846: 157453
[76] Xu L J, Chen H, Han X, et al. First report on per- and polyfluoroalkyl substances (PFASs) in coral communities from the Northern South China Sea: Occurrence, seasonal variation, and interspecies differences [J]. Environmental Pollution, 2022, 314: 120214
[77] Shi Y L, Vestergren R, Zhou Z, et al. Tissue distribution and whole body burden of the chlorinated polyfluoroalkyl ether sulfonic acid F-53B in crucian carp (Carassius carassius): Evidence for a highly bioaccumulative contaminant of emerging concern [J]. Environmental Science &Technology, 2015, 49(24): 14156-14165
[78] Wu Y M, Deng M, Jin Y X, et al. Uptake and elimination of emerging polyfluoroalkyl substance F-53B in zebrafish larvae: Response of oxidative stress biomarkers [J]. Chemosphere, 2019, 215: 182-188
[79] Spaan K M, van Noordenburg C, Plassmann M M, et al. Fluorine mass balance and suspect screening in marine mammals from the Northern Hemisphere [J]. Environmental Science &Technology, 2020, 54(7): 4046-4058
[80] He Y X, Lv D, Li C H, et al. Human exposure to F-53B in China and the evaluation of its potential toxicity: An overview [J]. Environment International, 2022, 161: 107108
[81] Gebbink W A, Bossi R, Rigét F F, et al. Observation of emerging per- and polyfluoroalkyl substances (PFASs) in Greenland marine mammals [J]. Chemosphere, 2016, 144: 2384-2391
[82] Munoz G, Desrosiers M, Duy S V, et al. Environmental occurrence of perfluoroalkyl acids and novel fluorotelomer surfactants in the freshwater fish Catostomus commersonii and sediments following firefighting foam deployment at the lac-mégantic railway accident [J]. Environmental Science &Technology, 2017, 51(3): 1231-1240
[83] Kaboré H A, Goeury K, Desrosiers M, et al. Novel and legacy per- and polyfluoroalkyl substances (PFAS) in freshwater sporting fish from background and firefighting foam impacted ecosystems in Eastern Canada [J]. The Science of the Total Environment, 2022, 816: 151563
[84] Shi Y L, Song X W, Jin Q, et al. Tissue distribution and bioaccumulation of a novel polyfluoroalkyl benzenesulfonate in crucian carp [J]. Environment International, 2020, 135: 105418
[85] Godfrey A, Abdel-moneim A, Sepúlveda M S. Acute mixture toxicity of halogenated chemicals and their next generation counterparts on zebrafish embryos [J]. Chemosphere, 2017, 181: 710-712
[86] Barmentlo S H, Stel J M, van Doorn M, et al. Acute and chronic toxicity of short chained perfluoroalkyl substances to Daphnia magna [J]. Environmental Pollution, 2015, 198: 47-53
[87] Kim M, Park M S, Son J, et al. Perfluoroheptanoic acid affects amphibian embryogenesis by inducing the phosphorylation of ERK and JNK [J]. International Journal of Molecular Medicine, 2015, 36(6): 1693-1700
[88] Shi G H, Cui Q Q, Pan Y T, et al. 6:2 fluorotelomer carboxylic acid (6:2 FTCA) exposure induces developmental toxicity and inhibits the formation of erythrocytes during zebrafish embryogenesis [J]. Aquatic Toxicology, 2017, 190: 53-61
[89] Tornabene B J, Chislock M F, Gannon M E, et al. Relative acute toxicity of three per- and polyfluoroalkyl substances on nine species of larval amphibians [J]. Integrated Environmental Assessment and Management, 2021, 17(4): 684-690
[90] Wang S W, Huang J, Yang Y, et al. First report of a Chinese PFOS alternative overlooked for 30 years: Its toxicity, persistence, and presence in the environment [J]. Environmental Science &Technology, 2013, 47(18): 10163-10170
[91] Horie Y, Nomura M, Okamoto K, et al. Effect of thyroid hormone-disrupting chemicals on swim bladder inflation and thyroid hormone-related gene expression in Japanese medaka and zebrafish [J]. Journal of Applied Toxicology, 2022, 42(8): 1385-1395
[92] Godfrey A, Hooser B, Abdelmoneim A, et al. Thyroid disrupting effects of halogenated and next generation chemicals on the swim bladder development of zebrafish [J]. Aquatic Toxicology, 2017, 193: 228-235
[93] Annunziato K M, Jantzen C E, Gronske M C, et al. Subtle morphometric, behavioral and gene expression effects in larval zebrafish exposed to PFHxA, PFHxS and 6:2 FTOH [J]. Aquatic Toxicology, 2019, 208: 126-137
[94] Guo X C, Zhang S N, Liu X H, et al. Evaluation of the acute toxicity and neurodevelopmental inhibition of perfluorohexanoic acid (PFHxA) in zebrafish embryos [J]. Ecotoxicology and Environmental Safety, 2021, 225: 112733
[95] Zhang S N, Guo X C, Lu S Y, et al. Perfluorohexanoic acid caused disruption of the hypothalamus-pituitary-thyroid axis in zebrafish larvae [J]. Ecotoxicology and Environmental Safety, 2022, 232: 113283
[96] Blanc M, Kärrman A, Kukucka P, et al. Mixture-specific gene expression in zebrafish (Danio rerio) embryos exposed to perfluorooctane sulfonic acid (PFOS), perfluorohexanoic acid (PFHxA) and 3,3’,4,4’,5-pentachlorobiphenyl (PCB126) [J]. The Science of the Total Environment, 2017, 590-591: 249-257
[97] Tang L Z, Liu M Y, Song S W, et al. Interaction between hypoxia and perfluorobutane sulfonate on developmental toxicity and endocrine disruption in marine medaka embryos [J]. Aquatic Toxicology, 2020, 222: 105466
[98] Sant K E, Venezia O L, Sinno P P, et al. Perfluorobutanesulfonic acid disrupts pancreatic organogenesis and regulation of lipid metabolism in the zebrafish, Danio rerio [J]. Toxicological Sciences: An Official Journal of the Society of Toxicology, 2019, 167(1): 258-268
[99] Sun B L, Liu M Y, Tang L Z, et al. Probiotic supplementation mitigates the developmental toxicity of perfluorobutanesulfonate in zebrafish larvae [J]. The Science of the Total Environment, 2021, 799: 149458
[100] Sun B L, Liu M Y, Tang L Z, et al. Probiotics inhibit the stunted growth defect of perfluorobutanesulfonate via stress and thyroid axes in zebrafish larvae [J]. Environmental Pollution, 2021, 290: 118013
[101] Flynn R W, Hoover G, Iacchetta M, et al. Comparative toxicity of aquatic per- and polyfluoroalkyl substance exposure in three species of amphibians [J]. Environmental Toxicology and Chemistry, 2022, 41(6): 1407-1415
[102] Deng M, Wu Y M, Xu C, et al. Multiple approaches to assess the effects of F-53B, a Chinese PFOS alternative, on thyroid endocrine disruption at environmentally relevant concentrations [J]. The Science of the Total Environment, 2018, 624: 215-224
[103] Shi G H, Cui Q Q, Pan Y T, et al. 6:2 chlorinated polyfluorinated ether sulfonate, a PFOS alternative, induces embryotoxicity and disrupts cardiac development in zebrafish embryos [J]. Aquatic Toxicology, 2017, 185: 67-75
[104] Liu S, Lai H, Wang Q Y, et al. Immunotoxicity of F53B, an alternative to PFOS, on zebrafish (Danio rerio) at different early life stages [J]. Science of the Total Environment, 2021, 790: 148165
[105] Huang J, Sun L W, Mennigen J A, et al. Developmental toxicity of the novel PFOS alternative OBS in developing zebrafish: An emphasis on cilia disruption [J]. Journal of Hazardous Materials, 2021, 409: 124491
[106] Ishibashi H, Kim E Y, Iwata H. Transactivation potencies of the Baikal seal (Pusa sibirica) peroxisome proliferator-activated receptor α by perfluoroalkyl carboxylates and sulfonates: Estimation of PFOA induction equivalency factors [J]. Environmental Science &Technology, 2011, 45(7): 3123-3130
[107] Søderstrøm S, Lille-Langøy R, Yadetie F, et al. Agonistic and potentiating effects of perfluoroalkyl substances (PFAS) on the Atlantic cod (Gadus morhua) peroxisome proliferator-activated receptors (PPARs) [J]. Environment International, 2022, 163: 107203
[108] Wu Y M, Deng M, Jin Y X, et al. Toxicokinetics and toxic effects of a Chinese PFOS alternative F-53B in adult zebrafish [J]. Ecotoxicology and Environmental Safety, 2019, 171: 460-466
[109] Dasgupta S, Choyke S, Ferguson P L, et al. Antioxidant responses and oxidative stress in sheepshead minnow larvae exposed to Corexit 9500® or its component surfactant, DOSS [J]. Aquatic Toxicology, 2018, 194: 10-17
[110] Liu X L, Li Y Y, Zheng X W, et al. Anti-oxidant mechanisms of Chlorella pyrenoidosa under acute GenX exposure [J]. The Science of the Total Environment, 2021, 797: 149005
[111] Hoseinifar S H, Shakouri M, Yousefi S, et al. Humoral and skin mucosal immune parameters, intestinal immune related genes expression and antioxidant defense in rainbow trout (Oncorhynchus mykiss) fed olive (Olea europea L.) waste [J]. Fish &Shellfish Immunology, 2020, 100: 171-178
[112] Hu C Y, Huang Z L, Liu M Y, et al. Shift in skin microbiota and immune functions of zebrafish after combined exposure to perfluorobutanesulfonate and probiotic Lactobacillus rhamnosus [J]. Ecotoxicology and Environmental Safety, 2021, 218: 112310
[113] Xu M M, Legradi J, Leonards P. Using comprehensive lipid profiling to study effects of PFHxS during different stages of early zebrafish development [J]. The Science of the Total Environment, 2022, 808: 151739
[114] Wu Y M, Huang J, Deng M, et al. Acute exposure to environmentally relevant concentrations of Chinese PFOS alternative F-53B induces oxidative stress in early developing zebrafish [J]. Chemosphere, 2019, 235: 945-951
[115] Yang H L, Lai H, Huang J, et al. Polystyrene microplastics decrease F-53B bioaccumulation but induce inflammatory stress in larval zebrafish [J]. Chemosphere, 2020, 255: 127040
[116] Zhang W L, Liang Y N. Interactions between Lemna minor (common duckweed) and PFAS intermediates: Perfluorooctane sulfonamide (PFOSA) and 6:2 fluorotelomer sulfonate (6:2 FTSA) [J]. Chemosphere, 2021, 276: 130165
[117] Huang J, Wang Q Y, Liu S, et al. Crosstalk between histological alterations, oxidative stress and immune aberrations of the emerging PFOS alternative OBS in developing zebrafish [J]. Science of the Total Environment, 2021, 774: 145443
[118] Zou Y L, Wu Y M, Wang Q Y, et al. Comparison of toxicokinetics and toxic effects of PFOS and its novel alternative OBS in zebrafish larvae [J]. Chemosphere, 2021, 265: 129116
[119] Park S, Moon N R, Kang S N, et al. Ferulic acid and vinpocetine intake improves memory function by enhancing insulin sensitivity and reducing neuroinflammation and oxidative stress in type 2 diabetic animals with induced Alzheimer’s disease [J]. Journal of Functional Foods, 2022, 95: 105180
[120] Huang J, Wang Q Y, Liu S, et al. Comparative chronic toxicities of PFOS and its novel alternatives on the immune system associated with intestinal microbiota dysbiosis in adult zebrafish [J]. Journal of Hazardous Materials, 2022, 425: 127950
[121] Bonato M, Corrà F, Bellio M, et al. PFAS environmental pollution and antioxidant responses: An overview of the impact on human field [J]. International Journal of Environmental Research and Public Health, 2020, 17(21): 8020
[122] Shi Y L, Wang J M, Pan Y Y, et al. Tissue distribution of perfluorinated compounds in farmed freshwater fish and human exposure by consumption [J]. Environmental Toxicology and Chemistry, 2012, 31(4): 717-723
[123] Wasel O, Thompson K M, Freeman J L. Assessment of unique behavioral, morphological, and molecular alterations in the comparative developmental toxicity profiles of PFOA, PFHxA, and PFBA using the zebrafish model system [J]. Environment International, 2022, 170: 107642
[124] Rericha Y, Truong L, Leong C, et al. Dietary perfluorohexanoic acid (PFHxA) exposures in juvenile zebrafish produce subtle behavioral effects across generations [J]. Toxics, 2022, 10(7): 372
[125] Gaballah S, Swank A, Sobus J R, et al. Evaluation of developmental toxicity, developmental neurotoxicity, and tissue dose in zebrafish exposed to GenX and other PFAS [J]. Environmental Health Perspectives, 2020, 128(4): 47005
[126] Chen L G, Tsui M M P, Shi Q P, et al. Accumulation of perfluorobutane sulfonate (PFBS) and impairment of visual function in the eyes of marine medaka after a life-cycle exposure [J]. Aquatic Toxicology, 2018, 201: 1-10
[127] Tang L Z, Liu M Y, Hu C Y, et al. Binary exposure to hypoxia and perfluorobutane sulfonate disturbs sensory perception and chromatin topography in marine medaka embryos [J]. Environmental Pollution, 2020, 266(Pt 3): 115284
[128] Liu M Y, Song S W, Hu C Y, et al. Dietary administration of probiotic Lactobacillus rhamnosus modulates the neurological toxicities of perfluorobutane sulfonate in zebrafish [J]. Environmental Pollution, 2020, 265(Pt B): 114832
[129] Slotkin T A, MacKillop E A, Melnick R L, et al. Developmental neurotoxicity of perfluorinated chemicals modeled in vitro [J]. Environmental Health Perspectives, 2008, 116(6): 716-722
[130] Hu C Y, Tang L Z, Liu M Y, et al. Probiotic modulation of perfluorobutane sulfonate toxicity in zebrafish: Disturbances in retinoid metabolism and visual physiology [J]. Chemosphere, 2020, 258: 127409
[131] Wu L Y, Zeeshan M, Dang Y, et al. Environmentally relevant concentrations of F-53B induce eye development disorders-mediated locomotor behavior in zebrafish larvae [J]. Chemosphere, 2022, 308(Pt 1): 136130
[132] Menger F, Pohl J, Ahrens L, et al. Behavioural effects and bioconcentration of per- and polyfluoroalkyl substances (PFASs) in zebrafish (Danio rerio) embryos [J]. Chemosphere, 2020, 245: 125573
[133] Lou Q Q, Zhang Y F, Zhou Z, et al. Effects of perfluorooctane sulfonate and perfluorobutane sulfonate on the growth and sexual development of Xenopus laevis [J]. Ecotoxicology, 2013, 22(7): 1133-1144
[134] Chen L G, Lam J C W, Hu C Y, et al. Perfluorobutane sulfonate exposure skews sex ratio in fish and transgenerationally impairs reproduction [J]. Environmental Science &Technology, 2019, 53(14): 8389-8397
[135] Chen L G, Hu C Y, Tsui M M P, et al. Multigenerational disruption of the thyroid endocrine system in marine medaka after a life-cycle exposure to perfluorobutane sulfonate [J]. Environmental Science &Technology, 2018, 52(7): 4432-4439
[136] Chen L G, Tsui M M P, Hu C Y, et al. Parental exposure to perfluorobutane sulfonate impairs offspring development through inheritance of paternal methylome [J]. Environmental Science &Technology, 2019, 53(20): 12018-12025
[137] Hu C Y, Liu M Y, Tang L Z, et al. Probiotic Lactobacillus rhamnosus modulates the impacts of perfluorobutane sulfonate on oocyte developmental rhythm of zebrafish [J]. The Science of the Total Environment, 2021, 776: 145975
[138] Tang L Z, Song S W, Hu C Y, et al. Parental exposure to perfluorobutane sulfonate disturbs the transfer of maternal transcripts and offspring embryonic development in zebrafish [J]. Chemosphere, 2020, 256: 127169
[139] Shi G H, Guo H, Sheng N, et al. Two-generational reproductive toxicity assessment of 6:2 chlorinated polyfluorinated ether sulfonate (F-53B, a novel alternative to perfluorooctane sulfonate) in zebrafish [J]. Environmental Pollution, 2018, 243(Pt B): 1517-1527
[140] Shi G H, Cui Q Q, Wang J X, et al. Chronic exposure to 6:2 chlorinated polyfluorinated ether sulfonate acid (F-53B) induced hepatotoxic effects in adult zebrafish and disrupted the PPAR signaling pathway in their offspring [J]. Environmental Pollution, 2019, 249: 550-559
[141] Shi G H, Wang J X, Guo H, et al. Parental exposure to 6:2 chlorinated polyfluorinated ether sulfonate (F-53B) induced transgenerational thyroid hormone disruption in zebrafish [J]. The Science of the Total Environment, 2019, 665: 855-863
[142] Mahapatra C T, Damayanti N P, Guffey S C, et al. Comparative in vitro toxicity assessment of perfluorinated carboxylic acids [J]. Journal of Applied Toxicology, 2017, 37(6): 699-708
[143] Liu M Y, Tang L Z, Hu C Y, et al. Antagonistic interaction between perfluorobutane sulfonate and probiotic on lipid and glucose metabolisms in the liver of zebrafish [J]. Aquatic Toxicology, 2021, 237: 105897
[144] Cao H M, Zhou Z, Wang L, et al. Screening of potential PFOS alternatives to decrease liver bioaccumulation: Experimental and computational approaches [J]. Environmental Science &Technology, 2019, 53(5): 2811-2819
[145] Wang Q Y, Huang J, Liu S, et al. Aberrant hepatic lipid metabolism associated with gut microbiota dysbiosis triggers hepatotoxicity of novel PFOS alternatives in adult zebrafish [J]. Environment International, 2022, 166: 107351
[146] Wang C Y, Zhao Y, Jin Y X. The emerging PFOS alternative OBS exposure induced gut microbiota dysbiosis and hepatic metabolism disorder in adult zebrafish [J]. Comparative Biochemistry and Physiology Part C: Toxicology &Pharmacology, 2020, 230: 108703
[147] Gong H J, Du J, Xu J, et al. Perfluorononanoate and perfluorobutane sulfonate induce cardiotoxic effects in zebrafish [J]. Environmental Toxicology and Chemistry, 2022, 41(10): 2527-2536
[148] Li Y Y, Liu X L, Zheng X W, et al. Toxic effects and mechanisms of PFOA and its substitute GenX on the photosynthesis of Chlorella pyrenoidosa [J]. The Science of the Total Environment, 2021, 765: 144431
[149] Labine L M, Oliveira Pereira E A,Kleywegt S, et al. Comparison of sub-lethal metabolic perturbations of select legacy and novel perfluorinated alkyl substances (PFAS) in Daphnia magna [J]. Environmental Research, 2022, 212(Pt D): 113582
[150] Liu W, Yang J, Li J W, et al. Toxicokinetics and persistent thyroid hormone disrupting effects of chronic developmental exposure to chlorinated polyfluorinated ether sulfonate in Chinese rare minnow [J]. Environmental Pollution, 2020, 263(Pt B): 114491