授粉昆虫在保障农业粮食生产和维护生态系统稳定等方面发挥重要作用[1]。研究表明,2015年授粉昆虫对中国主要农作物授粉功能量达1.8亿t,授粉服务价值达8 860.5亿元[2];其中蜜蜂及熊蜂等膜翅目昆虫作为众多农作物及野生植物的优势授粉类群,其授粉价值得到广泛认可[3-4]。熊蜂(Bombus spp.)隶属于膜翅目(Hymenoptera)蜜蜂科(Apidae)熊蜂属(Bombus),因其个体大、绒毛多、趋光性弱、耐低温及声震大等独特形态学与生物学特性,成为众多农作物及濒危植物的理想传粉昆虫,尤其适宜设施作物及深花冠植物,显著提升农林果蔬产量及品质[5]。研究显示,与传统人工授粉或蜜蜂授粉相比,合理采用地熊蜂(Bombus terrestris)授粉可使番茄、蓝莓、茄子、黄瓜、油茶、杏仁等植物坐果率提高15.5%~35.94%,增产7.1%~68.28%,目前熊蜂在全球被广泛应用于作物授粉,带来显著经济效益[6-11]。
然而,近年来全球熊蜂多样性呈减少趋势,部分地区熊蜂蜂群出现衰败现象,甚至少数物种濒临灭绝[12]。农药施用、耕地扩张、气候变化、夜间人造光以及病原体侵害等因素对熊蜂生存造成严重威胁(图1)[13-14],其中农药是严重威胁因素之一。全球年均投入200万t农药用于提高作物产量,只有不到0.1%的农药作用于目标害虫,其余农药进入环境危害蜜蜂及熊蜂等非靶标生物及生态系统[15-16]。研究表明,农药不仅会直接造成熊蜂死亡,还会对熊蜂生长发育、采食、授粉、归巢等行为及学习记忆能力等方面带来危害,导致熊蜂丰度降低[17-20]。因此,农药对熊蜂危害及毒理研究受到国内外学者的广泛关注。有关熊蜂毒理研究主要包括急性致死效应(lethal effects)和慢性亚致死效应(sub-lethal effects)2个方面,毒理研究作为农药的生态评估重要基础,其结果反映农药对试验动物的危害程度,可为农药田间施用进行指导与建议。
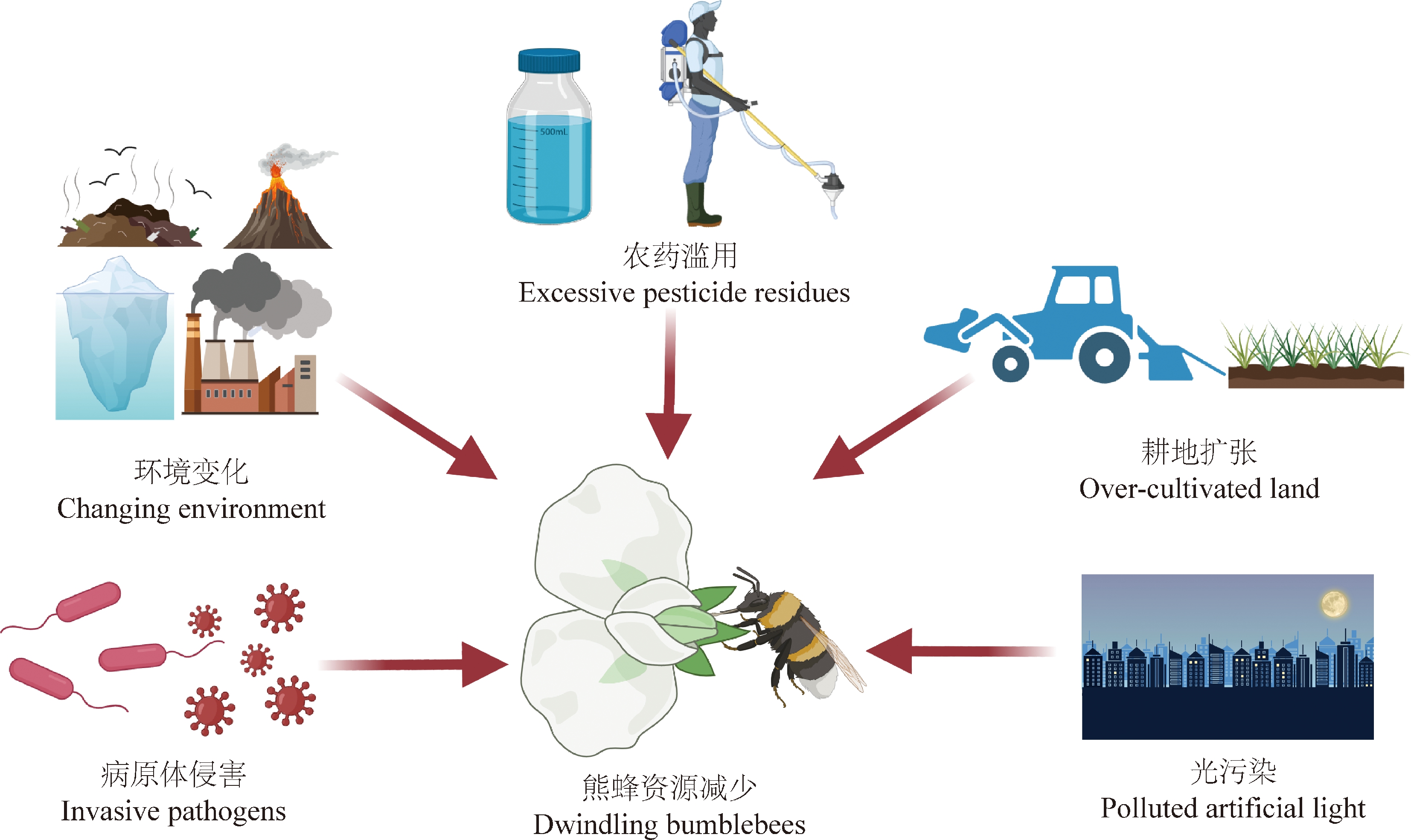
图1 熊蜂生存威胁的诸多因素
Fig. 1 The factors of threatening the survival of bumblebees
本文结合已有研究结果,整理了熊蜂急性致死和亚致死毒理研究方法,综述了农药对熊蜂急性致死效应研究进展及农药对熊蜂发育及行为的亚致死效应研究,其中着重介绍了农药对熊蜂肠道微生物及解毒因子细胞色素P450的不利影响,进而对存在的问题和未来研究进行了展望,旨在为农药科学使用、生物环境安全评估及生物多样性维护提供参考。
1 农药对熊蜂毒理研究方法(Methods of pesticide toxicology research on bumblebees)
1.1 急性致死效应的研究方法
急性致死效应(lethal effects)是指供试药物直接造成试验生物的死亡,根据不同浓度的供试药剂在短时间内对试验生物的致死效应来评估药物的生态风险等级。蜜蜂常作为全球生态风险评估和决策农药登记的模式生物[21],其毒理研究方法较为成熟,可作为农药对熊蜂急性毒理评估的参考。但研究表明熊蜂在形态及生活习性等方面与蜜蜂存在明显差异,部分农药对熊蜂与蜜蜂的毒性评估结果无明确相关性[22-23]。蜜蜂作为风险评估对象的普适性受到质疑,其评估结果不适用于其他野生物种,农药对熊蜂毒性评估不能完全参考蜜蜂评估。2017年经济合作与发展组织(Organization for Economic Co-Operation and Development, OECD)出版了熊蜂急性毒理试验手册(OECD Guidelines for the Testing of Chemicals, Section 2, Test No. 247: Bumblebee, Acute Oral/Contact Toxicity Test),并将熊蜂正式列为明确的保护目标[24]。另外,欧洲食品安全局(European Food Safety Authority, EFSA)对已有的蜜蜂及熊蜂的生态毒理学研究结果进行了分析,提出将熊蜂作为生态评估对象并阐述其合理性[25]。
1.1.1 熊蜂急性效应试验方法
熊蜂急性效应试验方法具体应依照OECD熊蜂急性毒理试验手册,其详细描述了试验方法以及试验单元所用装置要求(图2),熊蜂毒理试验应采用单独饲喂方式,但不能阻碍蜂个体间的交流,允许个体之间进行嗅觉和视觉接触;将4周内未接触药物的成年工蜂用不同浓度梯度农药进行饲喂,建议每只饲喂40 μL处理液,记录48 h的死亡数,并使用Probit等数学模型计算出该农药的半致死量(median lethal dose, LD50)或半致死浓度(median lethal concentration, LC50)[24]。
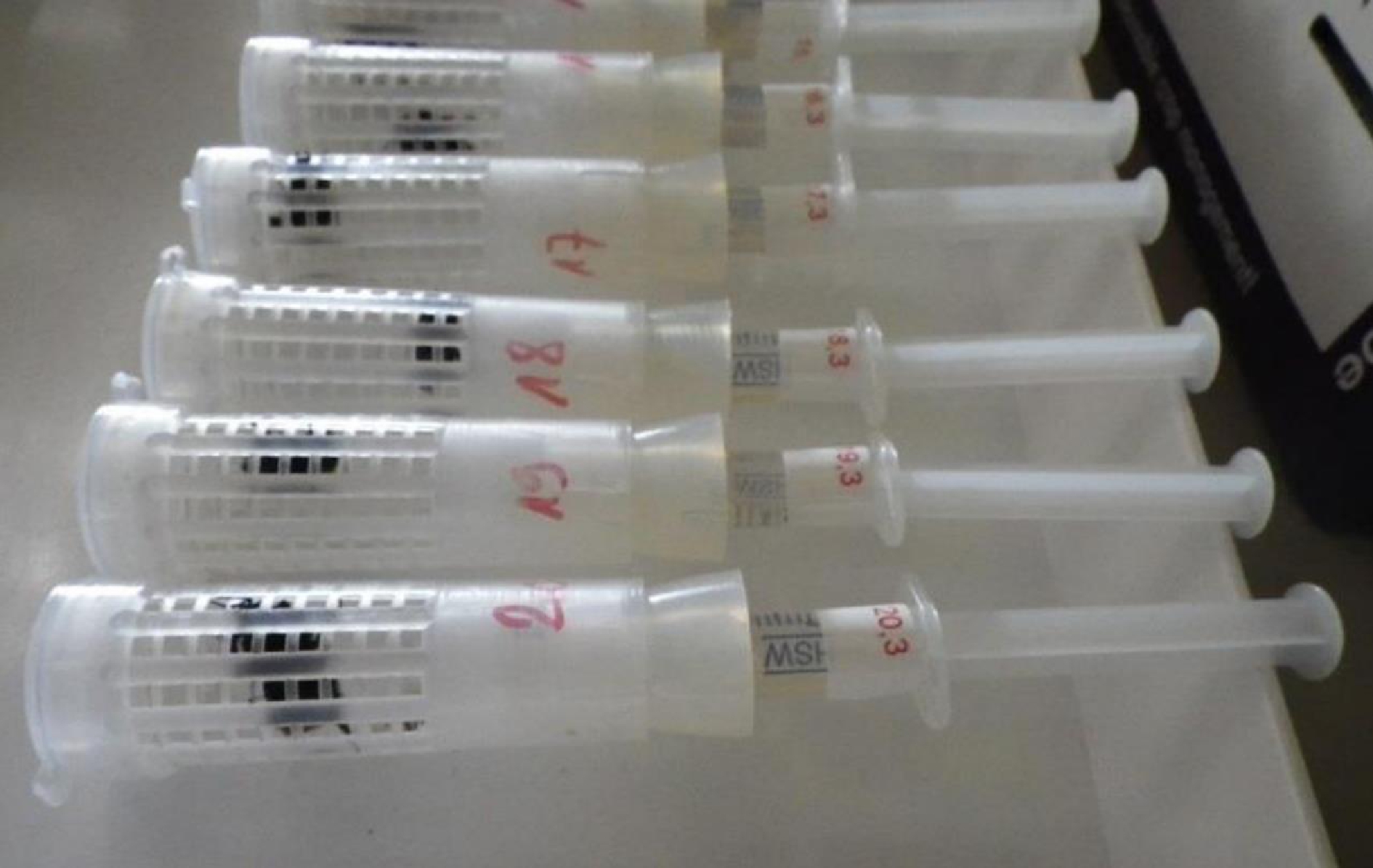
图2 熊蜂急性毒理试验装置图[24]
Fig. 2 Experimental set-up of bumblebee acute toxicology test[24]
1.1.2 死亡率校正
若试验对照组死亡率<5%,可直接计算LD50或LC50;若对照组数据异常,死亡率>5%且<20%时须进行死亡率校正后再计算。通常采用Abbott、Henderson-Tilton、Sun-Shepard及Schneider-Orelli等公式对死亡率进行校正,还可借助LDP line等软件进行。
1.1.3 数据分析方法
LD50及LC50可运用DSP、SPASS、SAS、PoloPlus、R语言等数据分析软件(包)采用Probit或Logit回归模型计算;其中PoloPlus软件还可比较不同药剂间的毒力、抗药性水平以及分析农药增效剂试验的数据等[26]。
1.1.4 生态评估方法
农药的生态评估是进行毒理研究中的重要内容,根据急性毒理试验所得农药对熊蜂的LD50或LC50数据评估植物保护产品的生态风险,以确定该产品对试验动物的威胁程度。生态风险评估通常采用风险系数(hazard quotient, HQ)与毒性值(exposure toxicity ratio, ETR)2个指标评价。HQ用来判定化学农药的毒性风险级别,公式为HQ = A(有效用量)/LD50,其中:A表示该药物田间参考有效使用量,毒性风险等级划分依据如表1所示[27]。EFSA于2013年提出利用ETR来评价农药风险等级,公式为ETR = 实际使用量(mg a.i.·株-1)×SV值(shortcut value, SV)/急性经口LD50 (μg a.i.·蜂-1),该评估方法将农药实际施用量及喷淋方式考虑其中,且提出一个新的系数SV值,SV值因向下喷淋和侧面喷淋2种喷淋情况不同,该风险评估反映结果更为准确(表2)[28]。HQ和ETR是分别用来评价不同农药对蜂群安全性的安全值(trigger value, TV),如果计算值小于或等于安全值,则说明该农药对熊蜂的威胁较小。通常采用LD50或LC50的最低值来保守评估农药安全性,或结合试验尽可能模拟野外真实生存环境,以提高农药对熊蜂安全性评估的准确性与可靠性。
表1 风险系数HQ值的风险等级划分依据[27]
Table 1 Risk factor HQ value risk classification basis[27]

风险级别Risk assessmentHQ<50低毒 Low50 注:HQ表示风险系数(商)。
Note: HQ represents hazard quotient.
表2 毒性值(ETR)风险评估计算表[28]
Table 2 The calculation table of toxicity value (ETR) for risk assessment [28]
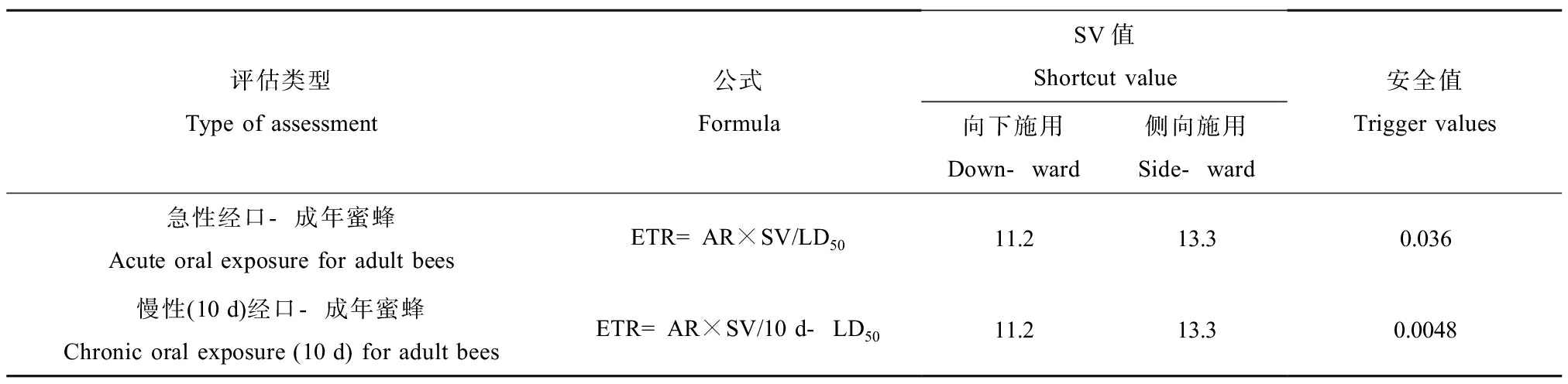
评估类型Type of assessment公式FormulaSV值Shortcut value向下施用Down-ward侧向施用Side-ward安全值Trigger values急性经口-成年蜜蜂Acute oral exposure for adult beesETR=AR×SV/LD5011.213.30.036慢性(10 d)经口-成年蜜蜂Chronic oral exposure (10 d) for adult beesETR=AR×SV/10 d-LD5011.213.30.0048
注:ETR表示暴露毒性比;AR表示实际施用量,单位kg a.s.·ha-1。
Note: ETR represents exposure toxicity ratio; AR represents the actual application rate, with the unit being expressed as kg a.s.·ha-1
1.2 亚致死效应研究方法
亚致死效应(sub-lethal effects)是指一般不引起试验对象直接死亡,但对其正常的行为或生理活动产生不利影响,并把农药产生亚致死效应的剂量称为亚致死剂量[29]。低剂量或低毒性的农药可引起熊蜂发生亚致死效应,如影响熊蜂个体和蜂群的发育、降低熊蜂繁殖性能及学习行为等,其对熊蜂的危害性与急性致死效应同样严重[30-31]。
1.2.1 熊蜂亚致死效应的试验方法
熊蜂亚致死效应研究中农药亚致死剂量/浓度设置及处理方式为关键因素。
(1)农药亚致死剂量/浓度设置:熊蜂亚致死效应研究中,农药试验浓度设置通常有以下几种方式,如根据田间推荐最大施用量的10倍、2倍、0.5倍、0.2倍、0.1倍设置[32-33];采用大田农药残留浓度极值;根据田间施用农药的药代动力学数据或根据48 h-LC50的1×10-1~1×10-2取值范围等方式确定蜂群亚致死暴露浓度[30,34]。
(2)农药亚致死效应处理方式:亚致死效应试验常分为室内、野外田间以及两者相结合的形式。其中室内试验环境因素可控,试验数据更精准[30,35-36],一般建立1~20只不等的无王工蜂群,使之暴露在亚致死剂量的农药下,记录相关生理指标及行为学的变化,评估低剂量农药对熊蜂的危害[37];而田间试验常将采集的优质新蜂王人工饲养,形成稳定蜂群后进行亚致死浓度农药饲喂后再进行野外定植,试验更接近实际情况[30,32,38]。
1.2.2 亚致死效应的评估方法
熊蜂亚致死效应的评估主要集中在发育和行为等2个方面[30,38]。
(1)发育。通过对比受试熊蜂咽下腺小囊大小[39],雄性蜂的精子活率[38-39],肠道微生物结构变化[40-41],细胞色素P450酶、谷胱甘肽-S-转移酶和羧酸酯酶等解毒酶活性[42]及飞行肌线粒体功能[43]等指标来评估农药对熊蜂个体发育的影响。此外,相关研究还以长期给药后受试蜂群死亡率[36]、子代熊蜂的数量及体质量[31,36,39]、产蛹数量、蜂巢质量及体积变化、蜂群温度调节的能力[44]、对供试糖液及花粉等人工饲料的消耗量[41]、携带花粉归巢的工蜂数量、蜂巢中花粉和花蜜储罐的数量[45]来评估整个蜂群的发育状况。
(2)行为。目前相关研究学者常采用给药后蜂群翅拍频率、不同颜色辨别能力、气味辨识能力[46]等方面评估农药对熊蜂行为的影响。其中不同颜色辨别能力是指熊蜂通过采食含有农药的不同颜色的花朵,并在间隔2 d后评估其成功回到原来花色采食的能力;气味辨识能力指将农药处理和空白对照组的熊蜂分别采食特定气味的糖溶液,持续2 d后对比飞行及着陆等行为学差异[46]。
2 农药对熊蜂的危害(The harm of pesticides to bumblebees)
2.1 农药对熊蜂的急性致死效应
研究表明,不同种类的农药对同一蜂种的急性毒性存在较大的差异;如杀虫剂噻虫嗪(thiamethoxam)对地熊蜂(Bombus terrestris)的LD50值为0.061 μg·蜂-1,具有高毒性;天然植物源农药藜芦碱(veratrum alkaloids)对其LD50值为69.936 μg·蜂-1,为低毒作用;杀菌剂苯醚甲环唑(difenoconazole)对地熊蜂的LD50为336.094 μg·蜂-1,杀螨剂唑螨酯(feupyroximate)对地熊蜂的LD50为4.938 μg·蜂-1(表3)[47-50]。Reid等[51]开展杀虫剂生态评估时发现,同类型中不同品种的杀虫剂对地熊蜂的毒性存在一定差异,同为新烟碱类杀虫剂,吡虫啉(imidacloprid)对地熊蜂表现为剧毒,而噻虫啉(thiacloprid)却表现无毒;拟除虫菊酯类溴氰菊酯(deltamethrin)为剧毒性,氟胺氰菊酯(tau-fluvalinate)却表现轻微毒性;有机磷酸盐类毒死蜱(chlorpyrifos)对熊蜂有严重急性致死作用,而香豆磷(coumaphos)却表现无毒。另外,研究表明,同一农药对不同熊蜂物种亦存在差异。如毒死蜱对小峰熊蜂(Bombus hypocrita)和红光熊蜂(Bombus ignitus)的毒性存在较大差异,2种熊蜂的LC50相差近百倍[50](表4)。
表3 不同农药对地熊蜂(Bombus terrestris)的急性经口毒性[47-50]
Table 3 Acute oral toxicity of different pesticides against Bombus terrestris [47-50]
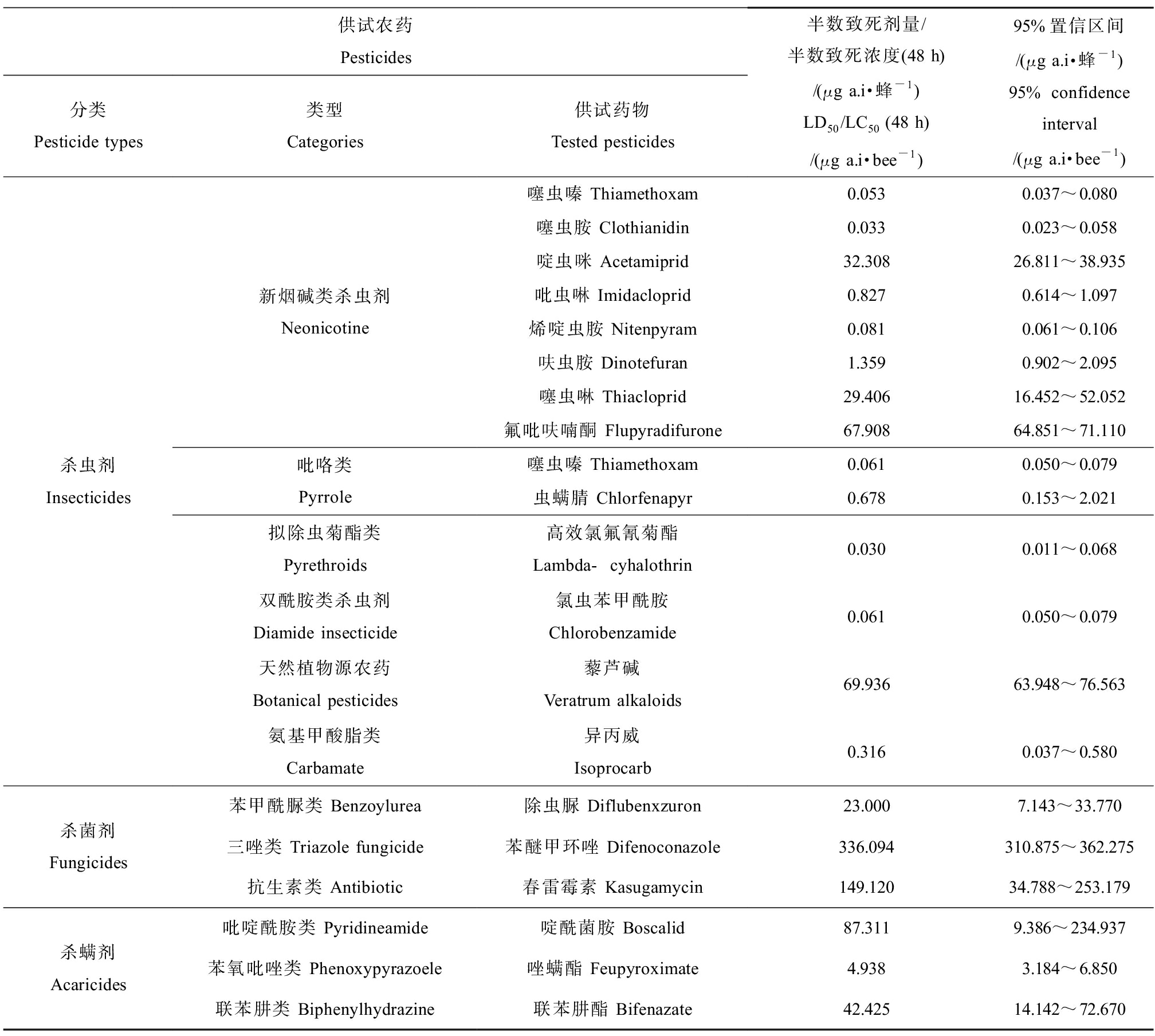
供试农药 Pesticides分类Pesticide types类型Categories供试药物Tested pesticides半数致死剂量/半数致死浓度(48 h)/(μg a.i·蜂-1)LD50/LC50(48 h)/(μg a.i·bee-1)95%置信区间/(μg a.i·蜂-1)95% confidence interval/(μg a.i·bee-1)杀虫剂Insecticides新烟碱类杀虫剂Neonicotine吡咯类Pyrrole拟除虫菊酯类Pyrethroids双酰胺类杀虫剂Diamide insecticide天然植物源农药Botanical pesticides氨基甲酸脂类Carbamate噻虫嗪 Thiamethoxam0.0530.037~0.080噻虫胺 Clothianidin0.0330.023~0.058啶虫咪 Acetamiprid32.30826.811~38.935吡虫啉 Imidacloprid0.8270.614~1.097烯啶虫胺 Nitenpyram0.0810.061~0.106呋虫胺 Dinotefuran1.3590.902~2.095噻虫啉 Thiacloprid29.40616.452~52.052氟吡呋喃酮 Flupyradifurone67.90864.851~71.110噻虫嗪 Thiamethoxam0.0610.050~0.079虫螨腈 Chlorfenapyr0.6780.153~2.021高效氯氟氰菊酯Lambda-cyhalothrin0.0300.011~0.068氯虫苯甲酰胺Chlorobenzamide0.0610.050~0.079藜芦碱Veratrum alkaloids69.93663.948~76.563异丙威Isoprocarb0.3160.037~0.580杀菌剂Fungicides苯甲酰脲类 Benzoylurea除虫脲 Diflubenxzuron23.0007.143~33.770三唑类 Triazole fungicide苯醚甲环唑 Difenoconazole336.094310.875~362.275抗生素类 Antibiotic春雷霉素 Kasugamycin149.12034.788~253.179杀螨剂Acaricides吡啶酰胺类 Pyridineamide啶酰菌胺 Boscalid87.3119.386~234.937苯氧吡唑类 Phenoxypyrazoele唑螨酯 Feupyroximate4.9383.184~6.850联苯肼类 Biphenylhydrazine联苯肼酯 Bifenazate42.42514.142~72.670
表4 不同农药对小峰熊蜂(Bombus hypocrita)和红光熊蜂(Bombus ignitus)的急性经口毒性[50]
Table 4 Acute oral toxicity of different pesticides against Bombus hypocorita and Bombus ignites[50]
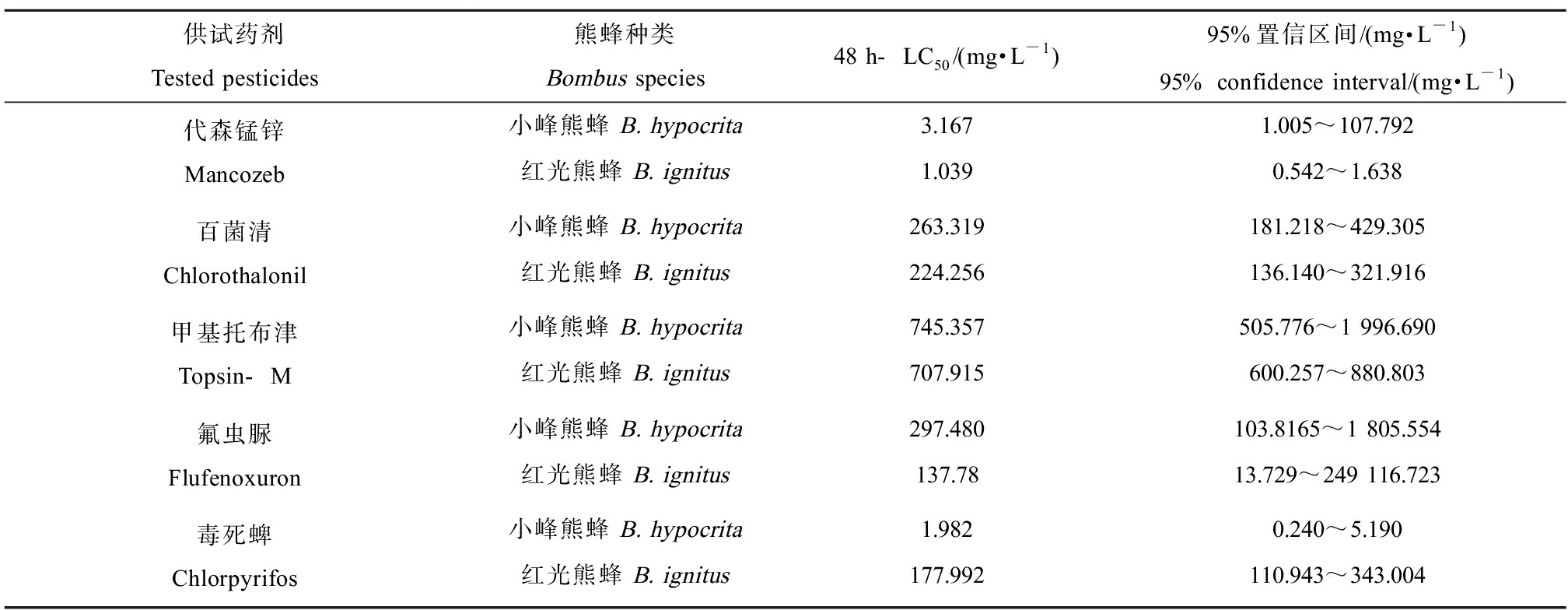
供试药剂Tested pesticides熊蜂种类Bombus species48 h-LC50/(mg·L-1)95%置信区间/(mg·L-1)95% confidence interval/(mg·L-1)代森锰锌Mancozeb小峰熊蜂 B. hypocrita3.1671.005~107.792红光熊蜂 B. ignitus1.0390.542~1.638百菌清Chlorothalonil小峰熊蜂 B. hypocrita263.319181.218~429.305红光熊蜂 B. ignitus224.256136.140~321.916甲基托布津Topsin-M小峰熊蜂 B. hypocrita745.357505.776~1 996.690红光熊蜂 B. ignitus707.915600.257~880.803氟虫脲Flufenoxuron小峰熊蜂 B. hypocrita297.480103.8165~1 805.554红光熊蜂 B. ignitus137.7813.729~249 116.723毒死蜱Chlorpyrifos小峰熊蜂 B. hypocrita1.9820.240~5.190红光熊蜂 B. ignitus177.992110.943~343.004
比较农药对熊蜂的急性致死研究发现,不同类型的农药或相同类型不同种类的农药对熊蜂的急性毒性亦存在较大差异;不同熊蜂种类对同种农药的敏感性表现出较大差异;剧毒和高毒的农药产品以杀虫剂为主,杀菌剂和杀螨剂对熊蜂的毒性相对较低。
2.2 农药对熊蜂的慢性亚致死效应
农业生产中残留的低剂量农药对熊蜂的影响不容忽视,亚致死剂量的农药给熊蜂生长发育、采食、授粉、归巢等行为及学习记忆能力等方面带来危害[17-20,52];研究表明肠道微生物及相关解毒因子在维系宿主健康方面发挥重要作用,农药对熊蜂肠道微生物及解毒因子的影响成为研究热点;鉴于这些因素相关学者针对杀虫剂、杀菌剂及除草剂等不同农药对熊蜂发育、行为、肠道微生物及解毒因子等方面的影响开展研究。
2.2.1 农药对熊蜂发育的危害
新烟碱类杀虫剂在全球范围内使用广泛,在土壤、水、花粉及蜂蜜中大量残留,给熊蜂的生存能力带来严重的损害[53-54]。新烟碱类杀虫剂使熊蜂蜂群发育放缓,生殖成功率降低,子代新蜂王数量大大减少,这些伤害对一年一世代的熊蜂打击巨大[38,55]。Minnameyer等[39]研究发现,噻虫嗪(thiamethoxam)造成地熊蜂咽下腺小囊发育受损,进而可能抑制蛋白质、糖类及脂类物质的合成代谢,导致蜂群生长发育缓慢;且噻虫嗪显著降低雄性蜂的精子活率,抑制熊蜂生殖能力。研究证实,熊蜂更喜采食含有噻虫嗪的食物,这无疑加重了新烟碱类杀虫剂对熊蜂的伤害[56]。另外,熊蜂外出采食花蜜及花粉的过程中会接触到除草剂,同样给熊蜂造成亚致死效应[57];Weidenmüller等[44]发现草甘膦(glyphosate)会造成地熊蜂蜂群体温调节受损,草甘膦处理过的蜂群维持巢内温度稳定的时间比对照组减少26%,且巢内温度失衡导致巢中幼虫及蛹的发育放缓。除此之外,杀菌剂给地熊蜂带来伤害,影响其生长发育、授粉和归巢能力,Syromyatnikov等[58]发现低剂量的杀菌剂苯醚甲环唑(difenoconazole)和二噻农(dithianon),均会引起熊蜂飞行肌线粒体发生功能障碍以及新陈代谢的速率降低,最终可能抑制熊蜂飞行能力,使其无法正常采集和归巢,从而影响整个蜂群发育;另外,杀菌剂吡唑醚菌酯(pyraclostrobin)会损害地熊蜂体内相关解毒酶和脂肪体,造成熊蜂排泄和解毒等生理功能严重受损,导致熊蜂生长发育迟缓[36,59]。生物农药对生态环境的友好表现使其在新时期的农药研发中受到持续关注[60],但研究表明,部分生物农药也会造成对非靶标生物的危害[33,61]。其中从印楝树提取开发的印楝素相关的植物农药,对熊蜂生存能力亦会产生影响,其中印楝杀虫剂(nimbecidine)显著降低地熊蜂工蜂的存活率,影响整个蜂群的发育[31];印楝素(azadirachtin)严重抑制地熊蜂工蜂卵巢的发育,卵巢长度随着印楝素浓度的增加而减少,当浓度达到64 mg·L-1时,地熊蜂工蜂的卵巢平展长度仅为空白对照组的1/3[33]。
2.2.2 农药对熊蜂行为的影响
熊蜂超频震动是其高效授粉的基础,Switzer和Combes[62]的试验证明低剂量的吡虫啉会影响熊蜂的翅拍频率,这可能抑制熊蜂的授粉能力。Stanley和Raine[63]发现经过噻虫胺处理的蜂群,外出采集的工蜂数量显著增加,并且采集花粉的频率也增加,但是其学习记忆能力减弱,采食偏好也有所不同,在给定2种花卉百脉根(Lotus corniculatus)和白三叶(Trifolium repens)为采集对象时,农药处理后的熊蜂更爱采食百脉根,而未经处理的熊蜂偏爱白三叶;Straub等[64]利用电生理技术(electroantennographic, EAG)发现噻虫胺会影响熊蜂的嗅觉系统,降低其对不同挥发物质的敏感性。自然生存条件下,熊蜂面对复杂的采食环境,需要具备一些精准定位的能力,研究发现,草甘膦还会降低熊蜂对细微差异颜色的辨别能力,损伤熊蜂的学习记忆功能,这将大大降低熊蜂的环境适应能力,对其造成不可逆的伤害[46]。
2.2.3 农药对熊蜂肠道菌群的影响
熊蜂肠道内栖息着多样性相对较少但特异性较高的微生物,且微生物群落主要分布于后肠[65-66]。熊蜂多为一年一世代,当年蜂群产生的新蜂王交配后冬眠,次年重新繁殖建立新蜂群,因此蜂群中熊蜂个体肠道菌群具有高度统一性,并且母本蜂王为子代工蜂肠道微生物的主要来源[67]。熊蜂属于完全变态昆虫,具有卵、幼虫、蛹及成虫4个发育阶段,已有研究证明其菌群结构会随着不同的发育阶段变化,卵发育到幼虫的过程中相关群落的多样性降低,蛹期的肠道内含有更少且不同与其他阶段的菌群,成虫阶段熊蜂肠道菌群逐渐形成由斯诺德格拉斯氏菌属(Snodgrassella)、吉列姆氏菌属(Gilliamella)、乳杆菌属(Lactobacillus)和双歧杆菌属(Bifidobacterium)等组成的稳定菌群结构[67-70]。这些肠道菌群可分为益生菌、共生菌及致病菌,肠道菌群对于宿主健康的影响主要取决于这3类菌之间的互作模式;研究证明,这些菌群参与熊蜂体内营养物质的消化吸收和能量供给,并在防御病原体侵害及毒素代谢等维系熊蜂健康方面发挥重要作用[71-73]。熊蜂肠道核心菌可促进熊蜂对短膜虫(Crithidia bombi)抵御作用,且肠道益生菌所建立起的优势可降低病原体的丰度,而非核心菌相对丰度增高会增加短膜虫的侵染概率[74-75];同时,熊蜂肠道核心细菌Gilliamela apicola还可参与甘露糖、木糖和鼠李糖等对危害熊蜂的化学物质的代谢[71]。
近年来,相关学者开展农药对熊蜂肠道微生物影响的研究,已证明农药暴露会损害熊蜂肠道微生物群落结构。除草剂显著影响熊蜂肠道菌群结构,地熊蜂进食2.5 mg·L-1的草甘膦后,虽对其肠道菌群的总体丰度影响较小,菌群多样性却显著增加,其中真菌Zygosaccharomyces和Cladosporium的丰度显著增加[42];Motta和Moran[41]将连续进食1 mg·L-1草甘膦5 d后的美洲熊蜂(Bombus impatiens)工蜂放归原有王群,并对比当天、第3天及第7天的肠道菌群结构,发现益生菌斯诺德格拉斯氏菌属(Snodgrassella)的相对丰度随之增加,说明草甘膦显著影响熊蜂肠道菌群,并且停药后回归原有王群可使其肠道菌群结构逐渐恢复。研究表明,杀虫剂对熊蜂肠道微生态结构的影响不均,10 μg·L-1吡虫啉显著降低地熊蜂(Bombus terrestris)肠道内乳杆菌属(Lactobacillus Firm-5)和蜜蜂杆菌属(Apibacter)的相对丰度,改变肠道微生物结构[76];但同剂量下的吡虫啉对美洲熊蜂(Bombus impatiens)的肠道菌群影响较少[77],造成该现象的原因可能为不同物种肠道微生物结构存在差异,在应对同一农药侵害时的抵御能力可能不同[78];另外多杀菌素(spinetoram)对地熊蜂肠道菌群无显著影响[42]。目前,未见杀菌剂影响肠道菌群结构的研究。
2.2.4 农药对熊蜂解毒因子的影响
经过数百万年的进化历程,昆虫体内形成了部分克服自然环境中有毒化合物的机制[79]。昆虫体内解毒反应是降低其有害化合物伤害的重要途径[80],其中羧酸酯酶、谷胱甘肽-S-转移酶及细胞色素P450等解毒酶是参与昆虫体内解毒反应的重要解毒酶[81],但少有关于农药对熊蜂解毒酶的研究。Tang等[42]测定了采食草甘膦10 d后地熊蜂羧酸酯酶和谷胱甘肽-S-转移酶的活性,并未发现显著影响。细胞色素P450家族蛋白(简称P450)为近年来昆虫解毒因子研究热点,P450参与多种农药的解毒过程[82],是一个可自身氧化的血红蛋白家族,催化超过200 000种化学物质的生物转化,可将疏水性药物催化成易代谢的亲水性化合物,其主要活性成分为血红素(Heme),血红素内含有的原卟啉铁促进外源化合物代谢[83-86],其代谢原理见图3。P450可介导昆虫产生抗药性,其机制表现在基因的差异性表达及其酶活性的变化,一般当昆虫体内该家族基因表达量增加或酶活性上升时,抗药性增强[87];P450家族共有4个分支,包括CYP2、CYP3、CYP4及线粒体家族Mito CYP,其中CYP3分支中的CYP6和CYP9这2个家族广泛参与昆虫对外源化合物的代谢过程[82,84];研究表明,CYP9家族蛋白酶参与了噻虫啉在地熊蜂体内代谢过程,在试验药液中添加P450抑制剂后,发现噻虫啉对其毒性增加4.2 倍[88-89],另外CYP9Q3已被证实可有效代谢噻虫啉和啶虫脒[90];因此,P450在熊蜂体内解毒反应中具有重要作用。研究表明,农药影响P450家族基因在熊蜂体内的表达,Bebane等[91]以10 μg·L-1的吡虫啉饲喂6日龄的地熊蜂工蜂,并对其进行脑部的转录组测序,结果显示4个P450家族基因出现差异性表达,其中CYP6k1和CYP4c3上调表达,CYP28d1和CYP9e2下调表达,推测这些变化介导熊蜂对吡虫啉产生抗药性。
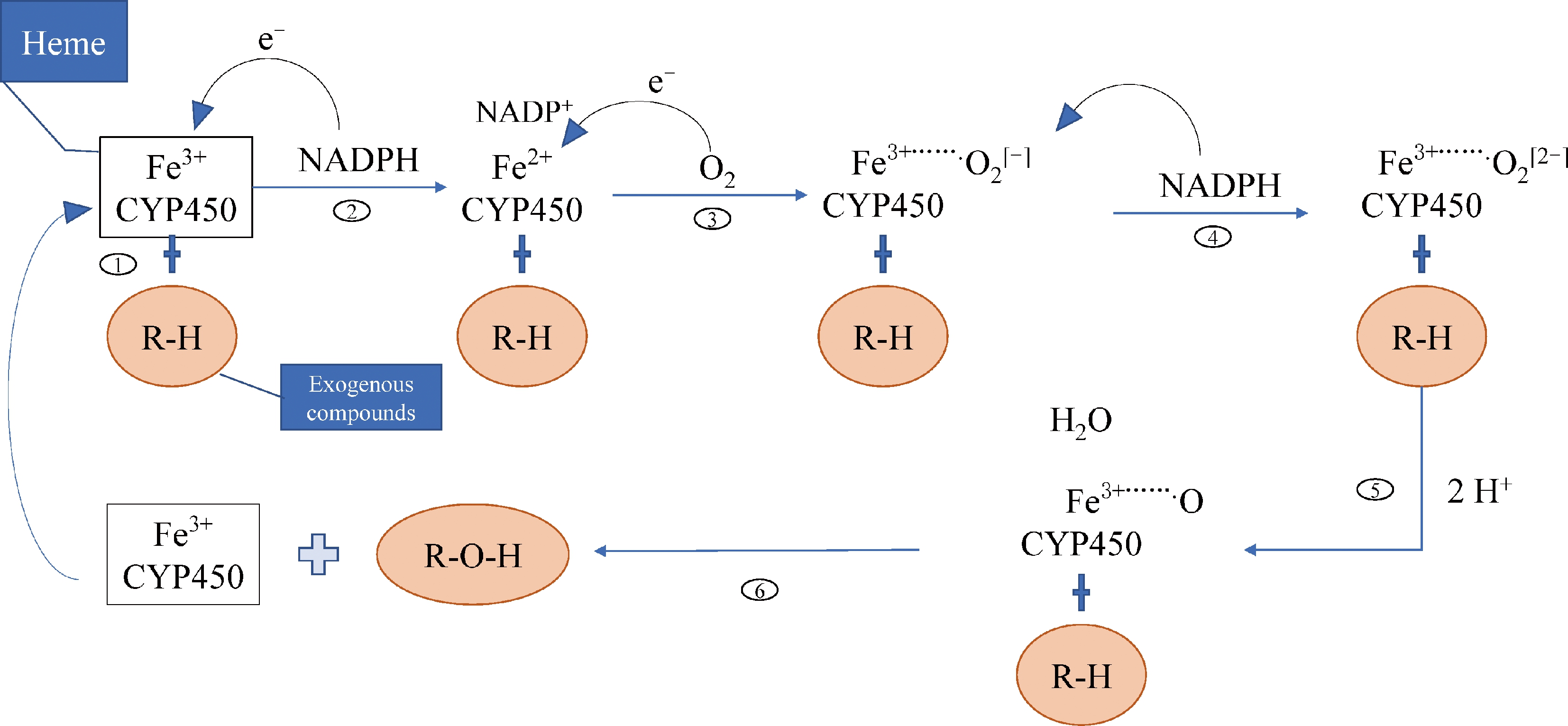
图3 P450促进化合物代谢的途径
注:Heme为血红素;NADPH表示还原性辅酶Ⅱ;CYP450表示细胞色素P450家族蛋白;R-H表示外源化合物。
Fig. 3 Pathway of P450 promoting the compound metabolism
Notes: NADPH represents nicotinamide adenine dinucleotide phosphate, CYP450 represents cytochrome P450 family proteins and R-H represents an exogenous compound.
3 总结与展望(Summary and prospect)
农药不合理的施用对熊蜂的影响复杂多变,熊蜂外出活动接触喷洒农药的叶片,采食含有农药的花粉及花蜜,或通过其他途径接触到农药,均可能产生急性致死或慢性亚致死效应,直接导致其死亡或对其生长发育、繁殖性能及传粉、归巢等行为产生不利影响。目前有关熊蜂毒理学研究较滞后,其毒理研究方法主要参考蜜蜂,但同一农药对不同蜜蜂属昆虫的作用效果存在较大差异[55,92],如东方蜜蜂(Apis cerana)进食R-二硝基呋喃(R-dinotefuran)的24 h-LD50是西方蜜蜂(Apis mellifera)的3倍[93],联苯肼酯(bifenazate)、弥拜菌素(milbemectin)、溴虫腈(chlorfenapyr)和喹螨醚(fenazaquin)等农药对地熊蜂无毒(LD50>100 μg a.i.·蜂-1),但对西方蜜蜂有毒(LD50<1 μg a.i.·蜂-1)[22-23];因此农药评估需考虑模式生物的差异[94]。
综上,熊蜂作为新的模式生物,开展农药及其他有害物质对其毒理研究具有合理性,开展熊蜂毒理研究应遵循熊蜂急性毒理试验手册,充分考虑不同农药对不同物种及危害的差异性。现有熊蜂急性毒性研究大多在室内条件下开展,其数据结果并不能切实反映大田自然环境下农药对熊蜂影响的真实水平,建议在开展毒理试验时尽可能模拟自然环境或田间条件。农药的急性毒理及生态风险数据常作为大田中农药的安全使用指南,科学开展农药对熊蜂的急性毒理及生态风险评估具有重要意义。
为避免农药对熊蜂的急性致死作用,可调整放蜂和农药施用时间以达到保护熊蜂的目的,但作物最佳授粉时期亦为病虫害高发期,加之部分高毒且半衰期长的农药残留,依然可能导致熊蜂亚致死效应,目前尚未报道有效抑制熊蜂亚致死效应的方法。因此,一方面研发针对熊蜂及蜜蜂等非靶标生物低毒高效的农药或针对靶标性强的农药具有重要意义;另一方面,深入开展熊蜂等有益生物的亚致死效应机制研究,加强相关益生菌及解毒代谢基因的挖掘;探究农药代谢的关键菌种或菌种群,进而形成熊蜂解毒代谢的微生物制剂,或利用营养措施或分子手段有效调控解毒基因表达,改变有益生物对特定毒性物质的耐受性[95-96],对未来环境安全及生物多样性保护具有重要价值。
[1] van der Sluijs J P, Vaage N S. Pollinators and global food security: The need for holistic global stewardship [J]. Food Ethics, 2016, 1(1): 75-91
[2] 欧阳芳, 王丽娜, 闫卓, 等. 中国农业生态系统昆虫授粉功能量与服务价值评估[J]. 生态学报, 2019, 39(1): 131-145
Ouyang F, Wang L N, Yan Z, et al. Evaluation of insect pollination and service value in China’s agricultural ecosystems [J]. Acta Ecologica Sinica, 2019, 39(1): 131-145 (in Chinese)
[3] 秦加敏, 苏睿, 梁铖, 等. 熊蜂生物学及种群影响因素研究进展[J]. 环境昆虫学报, 2020, 42(6): 1383-1393
Qin J M, Su R, Liang C, et al. Research progress of biology and population influencing factors in bumblebee [J]. Journal of Environmental Entomology, 2020, 42(6): 1383-1393 (in Chinese)
[4] Lemanski N J, Williams N M, Winfree R. Greater bee diversity is needed to maintain crop pollination over time [J]. Nature Ecology &Evolution, 2022, 6(10): 1516-1523
[5] 黄家兴, 安建东. 中国熊蜂多样性、人工利用与保护策略[J]. 生物多样性, 2018, 26(5): 486-497
Huang J X, An J D. Species diversity, pollination application and strategy for conservation of the bumblebees of China [J]. Biodiversity Science, 2018, 26(5): 486-497 (in Chinese)
[6] 钟培星, 谢再成, 吴延旭, 等. 熊蜂对油茶授粉结实的影响初探[J]. 中国蜂业, 2019, 70(7): 69-72
Zhong P X, Xie Z C, Wu Y X, et al. Study on the effects of pollination by Bombus terrestris on Camellia oleifera [J]. Apiculture of China, 2019, 70(7): 69-72 (in Chinese)
[7] 安建东, 童越敏, 国占宝, 等. 熊蜂为温室茄子授粉试验[J]. 中国养蜂, 2004, 55(3): 7-8
An J D, Tong Y M, Guo Z B, et al. A study on Bombus terrestris pollination to greenhouse eggplant [J]. Apiculture of China, 2004, 55(3): 7-8 (in Chinese)
[8] 国占宝, 安建东, 彭文君, 等. 熊蜂和蜜蜂为日光温室甜椒授粉的研究[J]. 中国养蜂, 2005, 56(10): 8-9
Guo Z B, An J D, Peng W J, et al. Research which the bumblebee and honeybee pollinate for the sweet pepper of sunlight greenhouse [J]. Apiculture of China, 2005, 56(10): 8-9 (in Chinese)
[9] 程尚, 周容, 罗文华, 等. 熊蜂为温室番茄授粉的效果研究[J]. 四川畜牧兽医, 2011, 38(2): 23-24
Cheng S, Zhou R, Luo W H, et al. Effect of Bombus terrestris pollination on greenhouse tomato [J]. Sichuan Animal &Veterinary Sciences, 2011, 38(2): 23-24 (in Chinese)
[10] 吴光安, 尹园园, 陈浩, 等. 地熊蜂和意大利蜜蜂为设施蓝莓授粉效果比较研究 [J]. 中国蜂业, 2019, 70(9): 68-70
Wu G A, Yin Y Y, Chen H, et al. Comparative study on pollination by Bombus terrestris and Apis mellifera for blueberry in greenhouse [J]. Apiculture of China, 2019, 70(9): 68-70 (in Chinese)
[11] 孙永深, 安建东, 童越敏, 等. 熊蜂(Bombus terrestris)为温室黄瓜授粉的效果研究[J]. 蜜蜂杂志, 2003, 23(8): 3-5
Sun Y S, An J D, Tong Y M, et al. A study on the effect of Bombus terrestris pollination to greenhouse cucumber [J]. Journal of Bee, 2003, 23(8): 3-5 (in Chinese)
[12] Brown M J F. The trouble with bumblebees [J]. Nature, 2011, 469(7329): 169-170
[13] Knop E, Zoller L, Ryser R, et al. Artificial light at night as a new threat to pollination [J]. Nature, 2017, 548(7666): 206-209
[14] Potts S G, Imperatriz-Fonseca V, Ngo H T, et al. Safeguarding pollinators and their values to human well-being [J]. Nature, 2016, 540(7632): 220-229
[15] Pimentel D. Amounts of pesticides reaching target pests: Environmental impacts and ethics [J]. Journal of Agricultural and Environmental Ethics, 1995, 8(1): 17-29
[16] Yatoo A M, Ali M N, Zaheen Z, et al. Assessment of pesticide toxicity on earthworms using multiple biomarkers: A review [J]. Environmental Chemistry Letters, 2022, 20(4): 2573-2596
[17] Feltham H, Park K, Goulson D. Field realistic doses of pesticide imidacloprid reduce bumblebee pollen foraging efficiency [J]. Ecotoxicology, 2014, 23(3): 317-323
[18] Stanley D A, Garratt M P, Wickens J B, et al. Neonicotinoid pesticide exposure impairs crop pollination services provided by bumblebees [J]. Nature, 2015, 528(7583): 548-550
[19] Stanley D A, Russell A L, Morrison S J, et al. Investigating the impacts of field-realistic exposure to a neonicotinoid pesticide on bumblebee foraging, homing ability and colony growth [J]. The Journal of Applied Ecology, 2016, 53(5): 1440-1449
[20] Phelps J D, Strang C G, Sherry D F. Imidacloprid impairs performance on a model flower handling task in bumblebees (Bombus impatiens) [J]. Ecotoxicology, 2020, 29(3): 359-374
[21] Organisation for Economic Co-operation and Development (OECD). Honeybees, acute oral toxicity test [R]. Paris: OECD, 1998
[22] Besard L, Mommaerts V, Vandeven J, et al. Compatibility of traditional and novel acaricides with bumblebees (Bombus terrestris): A first laboratory assessment of toxicity and sublethal effects [J]. Pest Management Science, 2010, 66(7): 786-793
[23] Thompson H M, Hunt L V. Extrapolating from honeybees to bumblebees in pesticide risk assessment [J]. Ecotoxicology, 1999, 8(3): 147-166
[24] Organisation for Economic Co-operation and Development (OECD). Bumblebee, acute oral toxicity test [R]. Paris: OECD, 2017
[25] Auteri D, Arce A, Ingels B, et al. Analysis of the evidence to support the definition of Specific Protection Goals for bumble bees and solitary bees [J]. EFSA Supporting Publications, 2022, 19(1): 1-43
[26] 常菊花, 何月平. 应用Polo软件进行农药毒力数据的比较分析[J]. 浙江农业学报, 2014, 26(6): 1552-1557
Chang J H, He Y P. The analysis for comparing the pesticide toxicity data using the Polo software [J]. Acta Agriculturae Zhejiangensis, 2014, 26(6): 1552-1557 (in Chinese)
[27] European and Mediterranean Plant Protection Organization (EPPO). EPPO Bulletin: Chapter 10: Honeybees [M]. John Wiley &Sons, Ltd, 2010: 323-331
[28] European Food Safety Authority (EFSA). Guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees) [J]. EFSA Journal, 2013, 11(7): 3295
[29] 宋怀磊, 周婷, 王强, 等. 杀虫剂对蜜蜂的亚致死效应[J]. 中国蜂业, 2010, 61(6): 8-10
Song H L, Zhou T, Wang Q, et al. Research advance in sublethal effects of pesticides on honeybee [J]. Apiculture of China, 2010, 61(6): 8-10 (in Chinese)
[30] European Food Safety Authority (EFSA). Scientific opinion on the science behind the development of a risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees) [J]. EFSA Journal, 2012, 10(5): 2668
[31] Demirozer O, Uzun A, Gosterit A. Lethal and sublethal effects of different biopesticides on Bombus terrestris (Hymenoptera: Apidae) [J]. Apidologie, 2022, 53(2): 1-13
[32] Balfour N J, Al Toufailia H, Scandian L, et al. Landscape scale study of the net effect of proximity to a neonicotinoid-treated crop on bee colony health [J]. Environmental Science &Technology, 2017, 51(18): 10825-10833
[33] Barbosa W F, de Meyer L, Guedes R N, et al. Lethal and sublethal effects of azadirachtin on the bumblebee Bombus terrestris (Hymenoptera: Apidae) [J]. Ecotoxicology, 2015, 24(1): 130-142
[34] 尹令虹. 亚致死浓度噻虫啉对西方蜜蜂工蜂学习记忆的影响[D]. 北京: 中国农业科学院, 2021: 7-8
Yin L H. Effects of sublethal concentration of thiacloprid on learning and memory of Apis mellifera worker bees [D]. Beijing: Chinese Academy of Agricultural Sciences, 2021: 7-8 (in Chinese)
[35] Gradish A E, Scott-Dupree C D, Shipp L, et al. Effect of reduced risk pesticides for use in greenhouse vegetable production on Bombus impatiens (Hymenoptera: Apidae) [J]. Pest Management Science, 2010, 66(2): 142-146
[36] Morandin L A, Winston M L, Franklin M T, et al. Lethal and sub-lethal effects of spinosad on bumble bees (Bombus impatiens Cresson) [J]. Pest Management Science, 2005, 61(7): 619-626
[37] Klinger E G, Camp A A, Strange J P, et al. Bombus (Hymenoptera: Apidae) microcolonies as a tool for biological understanding and pesticide risk assessment [J]. Environmental Entomology, 2019, 48(6): 1249-1259
[38] Siviter H, Brown M J F, Leadbeater E. Sulfoxaflor exposure reduces bumblebee reproductive success [J]. Nature, 2018, 561(7721): 109-112
[39] Minnameyer A, Strobl V, Bruckner S, et al. Eusocial insect declines: Insecticide impairs sperm and feeding glands in bumblebees [J]. The Science of the Total Environment, 2021, 785: 146955
[40] Rothman J A, Russell K A, Leger L, et al. The direct and indirect effects of environmental toxicants on the health of bumblebees and their microbiomes [J]. Proceedings Biological Sciences, 2020, 287(1937): 20200980
[41] Motta E V S, Moran N A. The effects of glyphosate, pure or in herbicide formulation, on bumble bees and their gut microbial communities [J]. The Science of the Total Environment, 2023, 872: 162102
[42] Tang Q H, Li W L, Wang J P, et al. Effects of spinetoram and glyphosate on physiological biomarkers and gut microbes in Bombus terrestris [J]. Frontiers in Physiology, 2022, 13: 1054742
[43] Syromyatnikov M Y, Gureev A P, Starkova N N, et al. Method for detection of mtDNA damages for evaluating of pesticides toxicity for bumblebees (Bombus terrestris L.) [J]. Pesticide Biochemistry and Physiology, 2020, 169: 104675
[44] Weidenmüller A, Meltzer A, Neupert S, et al. Glyphosate impairs collective thermoregulation in bumblebees [J]. Science, 2022, 376(6597): 1122-1126
[45] Gill R J, Ramos-Rodriguez O, Raine N E. Combined pesticide exposure severely affects individual- and colony-level traits in bees [J]. Nature, 2012, 491(7422): 105-108
[46] Helander M, Lehtonen T K, Saikkonen K, et al. Field-realistic acute exposure to glyphosate-based herbicide impairs fine-color discrimination in bumblebees [J]. The Science of the Total Environment, 2023, 857(Pt 1): 159298
[47] 李正阳, 王玉波, 刘佩杭, 等. 5种设施常用农药对欧洲熊蜂的毒力及残毒测定[J]. 河北林果研究, 2017, 32(1): 61-64
Li Z Y, Wang Y B, Liu P H, et al. Toxicity determination and residual of five pesticides on Bombus terrestris in greenhouse [J]. Hebei Journal of Forestry and Orchard Research, 2017, 32(1): 61-64 (in Chinese)
[48] 王烁, 谢丽霞, 陈浩, 等. 八种新烟碱类杀虫剂对地熊蜂工蜂的毒性及风险评估[J]. 昆虫学报, 2020, 63(1): 29-35
Wang S, Xie L X, Chen H, et al. Toxicity and risk assessment of eight neonicotinoid insecticides to workers of Bombus terrestris (Hymenoptera: Apoidea) [J]. Acta Entomologica Sinica, 2020, 63(1): 29-35 (in Chinese)
[49] 王宏栋, 韩冰, 王玉赛, 等. 11种常用农药对地熊蜂工蜂的毒性和风险评估[J]. 昆虫学报, 2021, 64(11): 1350-1358
Wang H D, Han B, Wang Y S, et al. Toxicity and risk assessment of eleven pesticides to workers of Bombus terrestris (Hymenoptera: Apidae) [J]. Acta Entomologica Sinica, 2021, 64(11): 1350-1358 (in Chinese)
[50] 廖秀丽, 刘佳霖, 罗术东, 等. 5种设施农业常用农药对2种熊蜂的毒效评价[J]. 西北农业学报, 2013, 22(4): 191-195
Liao X L, Liu J L, Luo S D, et al. Evaluation the toxicity of five pesticides to two species of bumblebees [J]. Acta Agriculturae Boreali-Occidentalis Sinica, 2013, 22(4): 191-195 (in Chinese)
[51] Reid R J, Troczka B J, Kor L, et al. Assessing the acute toxicity of insecticides to the buff-tailed bumblebee (Bombus terrestris audax) [J]. Pesticide Biochemistry and Physiology, 2020, 166: 104562
[52] Rosenberger D W, Conforti M L. Native and agricultural grassland use by stable and declining bumble bees in Midwestern North America [J]. Insect Conservation and Diversity, 2020, 13(6): 585-594
[53] Hladik M L, Main A R, Goulson D. Environmental risks and challenges associated with neonicotinoid insecticides [J]. Environmental Science &Technology, 2018, 52(6): 3329-3335
[54] Wood T J, Goulson D. The environmental risks of neonicotinoid pesticides: A review of the evidence post 2013 [J]. Environmental Science and Pollution Research, 2017, 24(21): 17285-17325
[55] Gradish A E, van der Steen J, Scott-Dupree C D, et al. Comparison of pesticide exposure in honey bees (Hymenoptera: Apidae) and bumble bees (Hymenoptera: Apidae): Implications for risk assessments [J]. Environmental Entomology, 2019, 48(1): 12-21
[56] Kessler S C, Tiedeken E J, Simcock K L, et al. Bees prefer foods containing neonicotinoid pesticides [J]. Nature, 2015, 521(7550): 74-76
[57] Thompson L J, Smith S, Stout J C, et al. Bumblebees can be exposed to the herbicide glyphosate when foraging [J]. Environmental Toxicology and Chemistry, 2022, 41(10): 2603-2612
[58] Syromyatnikov M Y, Kokina A V, Lopatin A V, et al. Evaluation of the toxicity of fungicides to flight muscle mitochondria of bumblebee (Bombus terrestris L.) [J]. Pesticide Biochemistry and Physiology, 2017, 135: 41-46
[59] Conceição de Assis J, Eduardo da Costa Domingues C, Tadei R, et al. Sublethal doses of imidacloprid and pyraclostrobin impair fat body of solitary beeTetrapedia diversipes (Klug, 1810) [J]. Environmental Pollution, 2022, 304: 119140
[60] Jiang X G, Hansen H C B, Strobel B W, et al. What is the aquatic toxicity of saponin-rich plant extracts used as biopesticides? [J]. Environmental Pollution, 2018, 236: 416-424
[61] Pino-Otín M R, Ballestero D, Navarro E, et al. Ecotoxicity of a novel biopesticide from Artemisia absinthium on non-target aquatic organisms [J]. Chemosphere, 2019, 216: 131-146
[62] Switzer C M, Combes S A. The neonicotinoid pesticide, imidacloprid, affects Bombus impatiens (bumblebee) sonication behavior when consumed at doses below the LD50 [J]. Ecotoxicology, 2016, 25(6): 1150-1159
[63] Stanley D A, Raine N E. Chronic exposure to a neonicotinoid pesticide alters the interactions between bumblebees and wild plants [J]. Functional Ecology, 2016, 30(7): 1132-1139
[64] Straub F, Orih I J, Kimmich J, et al. Negative effects of the neonicotinoid clothianidin on foraging behavior and antennal sensitivity in two common pollinator species, Osmia bicornis and Bombus terrestris [J]. Frontiers in Ecology and Evolution, 2021, 9: 697355
[65] Hotchkiss M Z, Poulain A J, Forrest J R K. Pesticide-induced disturbances of bee gut microbiotas [J]. FEMS Microbiology Reviews, 2022, 46(2): 1-22
[66] Kwong W K, Moran N A. Gut microbial communities of social bees [J]. Nature Reviews Microbiology, 2016, 14(6): 374-384
[67] Su Q Z, Wang Q L, Mu X H, et al. Strain-level analysis reveals the vertical microbial transmission during the life cycle of bumblebee [J]. Microbiome, 2021, 9(1): 216
[68] Zhang Z J, Huang M F, Qiu L F, et al. Diversity and functional analysis of Chinese bumblebee gut microbiota reveal the metabolic niche and antibiotic resistance variation of Gilliamella [J]. Insect Science, 2021, 28(2): 302-314
[69] Cornet L, Cleenwerck I, Praet J, et al. Phylogenomic analyses of Snodgrassella isolates from honeybees and bumblebees reveal taxonomic and functional diversity [J]. mSystems, 2022, 7(3): e0150021
[70] 陈奕霏, 董志祥, 李还原, 等. 肠道菌群在蜜蜂健康与疾病中的作用研究进展[J]. 微生物学杂志, 2021, 41(2): 92-100
Chen Y F, Dong Z X, Li H Y, et al. Advances in the role of enteric duct microbial population (EDMP) in honeybees’ health and diseases [J]. Journal of Microbiology, 2021, 41(2): 92-100 (in Chinese)
[71] Engel P, Kwong W K, McFrederick Q, et al. The bee microbiome: Impact on bee health and model for evolution and ecology of host-microbe interactions [J]. mBio, 2016, 7(2): e02164-e02115
[72] Zheng H, Nishida A, Kwong W K, et al. Metabolism of toxic sugars by strains of the bee gut symbiont Gilliamella apicola [J]. mBio, 2016, 7(6): e01326-e01316
[73] Praet J, Parmentier A, Schmid-Hempel R, et al. Large-scale cultivation of the bumblebee gut microbiota reveals an underestimated bacterial species diversity capable of pathogen inhibition [J]. Environmental Microbiology, 2018, 20(1): 214-227
[74] Cariveau D P, Elijah Powell J, Koch H, et al. Variation in gut microbial communities and its association with pathogen infection in wild bumble bees (Bombus) [J]. The ISME Journal, 2014, 8(12): 2369-2379
[75] Koch H, Schmid-Hempel P. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite [J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(48): 19288-19292
[76] Zhang Q C, Wang Q L, Zhai Y F, et al. Impacts of imidacloprid and flupyradifurone insecticides on the gut microbiota of Bombus terrestris [J]. Agriculture, 2022, 12(3): 389
[77] Rothman J A, Russell K A, Leger L, et al. The direct and indirect effects of environmental toxicants on the health of bumblebees and their microbiomes [J]. Proceedings Biological Sciences, 2020, 287(1937): 20200980
[78] Powell E, Ratnayeke N, Moran N A. Strain diversity and host specificity in a specialized gut symbiont of honeybees and bumblebees [J]. Molecular Ecology, 2016, 25(18): 4461-4471
[79] Johnson R M. Honey bee toxicology [J]. Annual Review of Entomology, 2015, 60: 415-434
[80] Xu J H, Strange J P, Welker D L, et al. Detoxification and stress response genes expressed in a western North American bumble bee, Bombus huntii (Hymenoptera: Apidae) [J]. BMC Genomics, 2013, 14: 874
[81] 李佳欢, 齐素贞, 吴黎明, 等. 氟虫腈对意大利蜜蜂工蜂幼虫及幼龄工蜂的亚致死效应[J]. 生态毒理学报, 2021, 16(5): 314-325
Li J H, Qi S Z, Wu L M, et al. Sublethal effects of fipronil on larvae and young worker honey bees (Apis mellifera ligustica) [J]. Asian Journal of Ecotoxicology, 2021, 16(5): 314-325 (in Chinese)
[82] Haas J, Glaubitz J, Koenig U, et al. A mechanism-based approach unveils metabolic routes potentially mediating chlorantraniliprole synergism in honey bees, Apis mellifera L., by azole fungicides [J]. Pest Management Science, 2022, 78(3): 965-973
[83] Coleman T, Kirk A M, Chao R R, et al. Understanding the mechanistic requirements for efficient and stereoselective alkene epoxidation by a cytochrome P450 enzyme [J]. ACS Catalysis, 2021, 11(4): 1995-2010
[84] Feyereisen R. Insect Molecular Biology and Biochemistry [M]. San Diego: Academic Press, 2012: 236-316
[85] Shumyantseva V V, Kuzikov A V, Masamrekh R A, et al. From electrochemistry to enzyme kinetics of cytochrome P450 [J]. Biosensors &Bioelectronics, 2018, 121: 192-204
[86] Hu B D, Zhao X R, Wang E D, et al. Efficient heterologous expression of cytochrome P450 enzymes in microorganisms for the biosynthesis of natural products [J]. Critical Reviews in Biotechnology, 2023, 43(2): 227-241
[87] 朱江, 邱星辉. 昆虫抗药性相关细胞色素P450基因的表达调控机制[J]. 昆虫学报, 2021, 64(1): 109-120
Zhu J, Qiu X H. Molecular mechanisms of expression regulation of insect cytochrome P450 genes involved in insecticide resistance [J]. Acta Entomologica Sinica, 2021, 64(1): 109-120 (in Chinese)
[88] Manjon C, Troczka B J, Zaworra M, et al. Unravelling the molecular determinants of bee sensitivity to neonicotinoid insecticides [J]. Current Biology, 2018, 28(7): 1137-1143.e5
[89] Troczka B J, Homem R A, Reid R, et al. Identification and functional characterisation of a novel N-cyanoamidine neonicotinoid metabolising cytochrome P450, CYP9Q6, from the buff-tailed bumblebee Bombus terrestris [J]. Insect Biochemistry and Molecular Biology, 2019, 111: 103171
[90] Feyereisen R. Toxicology: Bee P450s take the sting out of cyanoamidine neonicotinoids [J]. Current Biology, 2018, 28(9): R560-R562
[91] Bebane P S A, Hunt B J, Pegoraro M, et al. The effects of the neonicotinoid imidacloprid on gene expression and DNA methylation in the buff-tailed bumblebee Bombus terrestris [J]. Proceedings Biological Sciences, 2019, 286(1905): 20190718
[92] Cresswell J E, Page C J, Uygun M B, et al. Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid) [J]. Zoology, 2012, 115(6): 365-371
[93] Zhang Q, Fu L L, Cang T, et al. Toxicological effect and molecular mechanism of the chiral neonicotinoid dinotefuran in honeybees [J]. Environmental Science &Technology, 2022, 56(2): 1104-1112
[94] Stoner K A. Current pesticide risk assessment protocols do not adequately address differences between honey bees (Apis mellifera) and bumble bees (Bombus spp.) [J]. Frontiers in Environmental Science, 2016, 4: 79
[95] Gong Y H, Diao Q Y. Current knowledge of detoxification mechanisms of xenobiotic in honey bees [J]. Ecotoxicology, 2017, 26(1): 1-12
[96] Johnson R M, Mao W F, Pollock H S, et al. Ecologically appropriate xenobiotics induce cytochrome P450s in Apis mellifera [J]. PLoS One, 2012, 7(2): e31051