草甘膦(glyphosate)是1971年由Monsanto公司开发的一种内吸传导的非选择性芽后除草剂,其原理是通过抑制植物莽草酸(shikimic acid)途径中关键酶的合成,从而影响植物芳香族氨基酸(aromatic amino acid)的生物合成,使蛋白质合成受到抑制,导致植物死亡。由于这条生化途径在高等生物中并不存在,通常认为草甘膦对包括人类在内的哺乳动物是安全的,美国环境保护局(United States Environmental Protection Agency, US EPA)将草甘膦列为第三类有毒物质(低毒性)[1]。草甘膦因具有高效、低毒、广谱的特点,成为世界上应用最广、使用量最大的农药品种,年销售值一直居农药之首[2]。
除草剂多为有一定残效期(1~5年)的化合物,可在生物机体内富集,对水体中的鱼类构成严重威胁[3]。在中国,每年有145万t的各种类型农药被使用[4],仅有1%农药有效作用于标靶生物[5],其余大部分通过沉降、雨水冲刷等途径进入土壤和农田水环境。水环境中草甘膦分布较广,在河流、饮用水源水、海水、地下水和雨水中都曾检测到草甘膦的存在。2011年范瑾煜等[6]测定太湖中草甘膦含量为0.113 μg·L-1。在美国的河流中检测到草甘膦质量浓度达到了2.2 μg·L-1[7];在加拿大南部的安大略湖的最高浓度达40.8 μg·L-1[8],在德国的江河中检出最高浓度3.2 μg·L-1[9];在法国雨水管道中检出最高浓度75~90 μg·L-1[10]。草甘膦及制剂对生物及人体的危害不可忽视[11],越来越多的研究表明,草甘膦及其制剂对多种水生生物和哺乳动物都具有毒性作用[12-14]。草甘膦及其制剂低浓度暴露显示出对鲫鱼(Carassius auratus)的生态毒性[15];不同浓度的草甘膦对鲫鱼染毒后,血红蛋白和血红细胞形成受抑制[16];泥鳅(Misgurnus anguillicaudatus)暴露于草甘膦后,外周血淋巴细胞和粒细胞均升高[17];草甘膦制剂对银鲶鱼(Rhamdia quelen)的新陈代谢和酶活性产生显著影响[18];草甘膦可导致银鲶鱼机体自由基增加、天冬氨酸氨基转移酶(aspartate aminotransferase, AST)活力升高和线粒体损伤[19];将金鱼暴露于低剂量(2.5~20 mg·L-1)草甘膦96 h后,金鱼组织中超氧化物歧化酶(superoxidase dismutase, SOD)、谷胱甘肽过氧化物酶(glutathione peroxidase, GST)、谷胱甘肽还原酶(glutathione reductase, GR)和葡萄糖-6-磷酸脱氢酶(glucose-6-phosphate dehydrogenase, G-6-PD)活力受到抑制[20-21]。草甘膦对青鳉(Oryzias latipes)暴露显出肝脏毒性[22];罗非鱼(Oreochromis mossambicus)暴露于浓度为15 mg·L-1的草甘膦后可导致其肾脏病变[23];草甘膦暴露可导致虹鳟(Oncorhynchus mykiss)幼鱼出现损害,且表现时间-效应关系[24];成年雌性斑马鱼(Danio rerio)暴露于65 μg·L-1草甘膦15 d后可使类固醇生成因子-1(steroidogenic factor, SF-1)表达升高[25]。
随着草甘膦及其代谢物在水环境中的残留量逐年增加,生物富集作用使其影响更加长期,对水环境造成严重威胁,其潜在致毒作用有必要长期关注。研究显示,草甘膦对草鱼(Ctenopharyngodon idella)、鲢鱼(Hypophthalmichthys molitrix)、鲫鱼(Carassius auratus auratus)和斑马鱼(Danio rerio)的96 h-LC50分别为0.2518、0.2588、0.2599和0.879 mg·L-1[26-27],依照毒性分类标准属于高毒农药。
斑马鱼与人类基因同源性高达85%[28],且具有发育周期短、体外受精、胚胎透明易观察、单次产卵数高等优势,已成为研究环境污染物对水生生物影响的良好模型[29-31]。研究表明,斑马鱼的胚胎发育受多种基因调控,心脏及骨胳发育在胚胎至仔鱼阶段可初步完成,而胚胎时期心脏及骨骼发育与相关基因表达量直接相关,Tbx5是心肌细胞分化与心脏系统传导功能重要因子[32];Nkx2.5是触发初始心肌细胞分化的关键因素,Tnnt2编码心肌肌钙蛋白[33];Bmp2b是骨骼发育中背板早期形成的重要因子;Shh、Ihh与早期软骨组织形成关系密切[34]。本课题根据环境中检测出的草甘膦浓度范围[6-10]设置浓度梯度,研究其对斑马鱼胚胎的毒性效应,并以Tbx5、Nkx2.5、Tnnt2、Bmp2b、Shh、Ihh等为目标基因,采用聚合酶链式反应(polymerase chain reaction, PCR)初步分析其分子机制,对草甘膦的生态风险评估以及水体污染的生物监测提供指导,期望为进一步制定草甘膦的毒性判断标准提供参考数据。
1 材料与方法(Materials and methods)
1.1 主要试剂
草甘膦(纯度 99%,山东西亚化学股份有限公司),先以纯水配制浓度为1 mg·L-1储备液,4 ℃避光保存。蛋白含量测定、ATP酶活性测定、RNA提取、逆转录、荧光定量PCR等试剂盒均购于南京建成生物工程研究所,引物由福州擎科生物技术有限公司合成;其他试剂均为分析纯试剂。
99%,山东西亚化学股份有限公司),先以纯水配制浓度为1 mg·L-1储备液,4 ℃避光保存。蛋白含量测定、ATP酶活性测定、RNA提取、逆转录、荧光定量PCR等试剂盒均购于南京建成生物工程研究所,引物由福州擎科生物技术有限公司合成;其他试剂均为分析纯试剂。
1.2 主要仪器
体视显微镜(尼康,SMZ745T),光照培养箱(上海一恒,THZ-100),酶标仪(上海闪谱生物科技有限公司,ReadMax-1200),实时荧光定量PCR仪(Archimed,TMX4),全自动数码凝胶成像分析仪(培清科技,JS-2012),琼脂糖凝聚电泳仪(北京六一生物科技有限公司,DYY-8C),低温高速离心机(SCILOGEX,CF1524R)。
1.3 实验用鱼
斑马鱼(AB品系,购于福州百维斯生物科技有限公司),根据Westerfield[35]的方法饲养于武夷学院圣农实验中心的斑马鱼养殖循环系统(上海海圣)。循环养殖水(曝气除氯并经活性炭过滤的自来水),水温(26±1) ℃,光照周期为14 h光照/10 h黑暗,喂食新鲜孵化的丰年虫(Artemia nauplii),日常每天早晚喂2次,计划配鱼前一周,每天早中晚喂3次。
1.4 胚胎收集
实验前1 天晚上,将雌雄鱼按照1∶1比例放置在孵化槽中,并用隔板将雌雄分开,第2天早上约9点打开隔板,开灯给予光照刺激使其交配产卵。0.5~1 h内收集鱼卵,用斑马鱼养殖液清洗并在显微镜下观察,选取受精胚胎用于暴露实验。
1.5 暴露试验
设置终浓度为1、10、100 μg·L-1 3个试验组,1个空白对照组,每组设3个平行(n=3)。实验前将草甘膦用培养液(3.5 g·L-1 NaCl,0.05 g·L-1 NaHCO3,0.05 g·L-1 KCl,0.05 g·L-1 CaCl2)配制染毒液,分别置于直径90 mm培养皿(暴露培养液30 mL,50枚斑马鱼胚胎)。暴露期间(28±0.5) ℃恒温培养,每隔12 h更换等量等浓度的培养液并清理死亡胚胎,72 hpf (hours post-fertilization)收样。
1.6 相关形态学指标统计及计算
于斑马鱼胚胎12、24、36、48、72 hpf时,观察胚胎的存活情况(肉眼观察胚胎变白或者显微镜下观察胚胎絮状凝结、心脏不跳动即可判定死亡),统计存活率。48 hpf时,统计孵化率(胚胎出膜并能游动视为已孵化),72 hpf时统计畸形率(主要指心包水肿、卵黄囊水肿和脊柱弯曲),并测量心率(heart rate, HR)。
存活率=存活胚胎数/总胚胎数×100%;孵化率=已孵化胚胎数/总胚胎数×100%;畸形率=畸形胚胎数/存活胚胎数×100%。
心率的统计方法:每个处理组设3个平行,每个平行随机测5条仔鱼。在40倍焦距光学显微镜下,计算不同实验组的心跳速率,显微镜下对心房和心室的搏动次数进行记录并摄影,记录胚胎20 s内心跳次数,计算1 min心率[36]。
1.7 ATP酶活性测定
采用暴露72 hpf的仔鱼以超纯水荡洗后用液氮速冻,保存于-80 ℃冰箱。实验前将样品于冰盒上解冻,按1∶9的比例加入生理盐水,在匀浆器内仔细研磨。准备好的匀浆液在冷冻离心机上,在4 ℃下,以4 000 r·min-1离心10 min,取上清液测定蛋白含量和酶活性。
蛋白含量测定、Na+/K+-ATP酶活性、Ca2+-ATP酶活性的测定均按试剂盒说明进行操作。计算酶活力时,定义每小时每毫克组织蛋白的组织中ATP酶分解ATP产生1 μmol无机磷的量为一个ATP酶活力单位(μmol·mg-1·h-1)。
1.8 荧光定量PCR测定
RNA提取、逆转录、荧光定量PCR测定均使用试剂盒。内参基因选择ef1α,各基因引物采用primer5软件自行设计(表1),经Basic Local Alignment Search Tool (BLAST)比对后,送由福州擎科生物技术有限公司合成。荧光定量PCR的结果采用相对定量法计算,基因的表达量F=2-△△CT。
表1 荧光定量PCR序列
Table 1 Sequences of forward and reverse primers used for RT-PCR
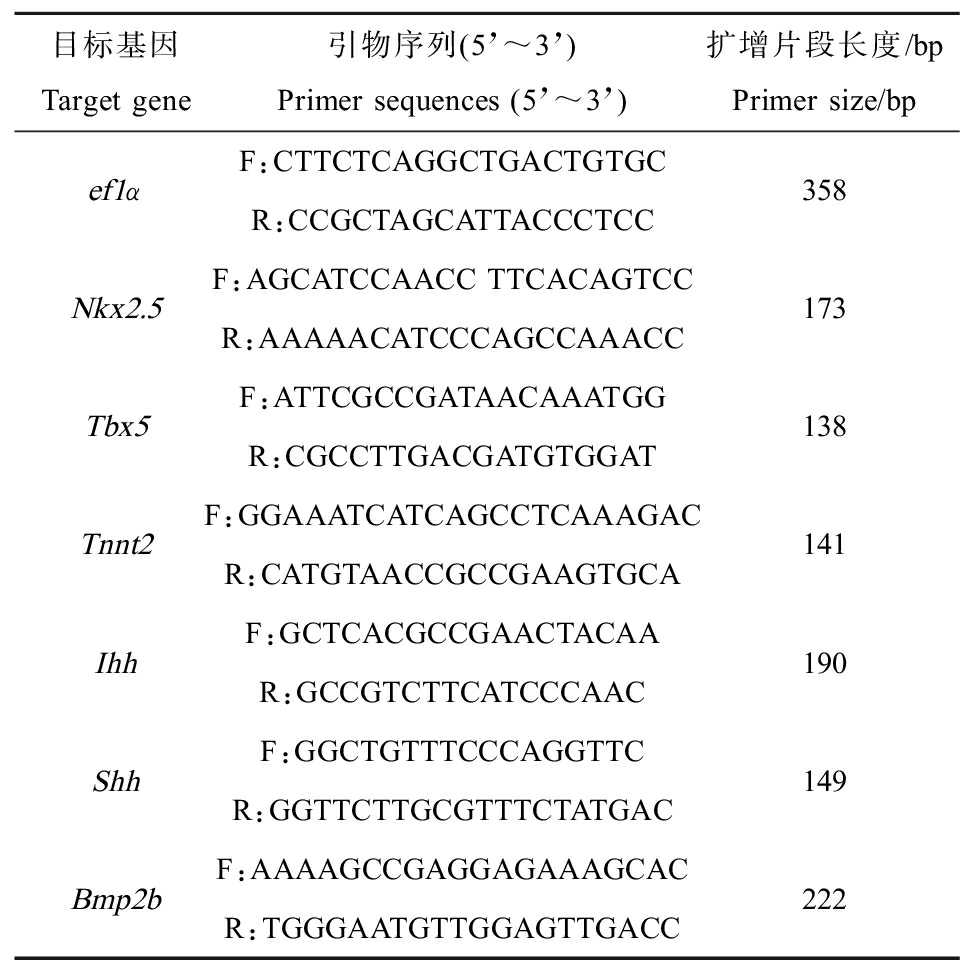
目标基因Target gene引物序列(5’~3’)Primer sequences (5’~3’)扩增片段长度/bpPrimer size/bpef1αF:CTTCTCAGGCTGACTGTGCR:CCGCTAGCATTACCCTCC358Nkx2.5F:AGCATCCAACC TTCACAGTCCR:AAAAACATCCCAGCCAAACC173Tbx5F:ATTCGCCGATAACAAATGGR:CGCCTTGACGATGTGGAT138Tnnt2F:GGAAATCATCAGCCTCAAAGACR:CATGTAACCGCCGAAGTGCA141IhhF:GCTCACGCCGAACTACAAR:GCCGTCTTCATCCCAAC190ShhF:GGCTGTTTCCCAGGTTCR:GGTTCTTGCGTTTCTATGAC149Bmp2bF:AAAAGCCGAGGAGAAAGCAC R:TGGGAATGTTGGAGTTGACC222
1.9 数据分析
结果采用平均值±标准误差(Mean±SE)表示,应用SPSS软件对数据进行单因素方差分析(one-way ANOVA),组间数据用Duncan法进行显著性水平分析,设定P<0.05表示差异显著。
2 结果(Results)
2.1 草甘膦暴露对斑马鱼胚胎存活率及孵化率的影响
斑马鱼胚胎经草甘膦暴露48 h,胚胎存活率与空白对照组(94%)比较,100 μg·L-1暴露组显著降低,降低至82%(图1)。胚胎孵化率空白对照组为82%,经草甘膦暴露后1 μg·L-1组无显著差异,10 μg·L-1、100 μg·L-1组孵化率显著降低,分别降至42%和37% (P<0.05) (图2)。
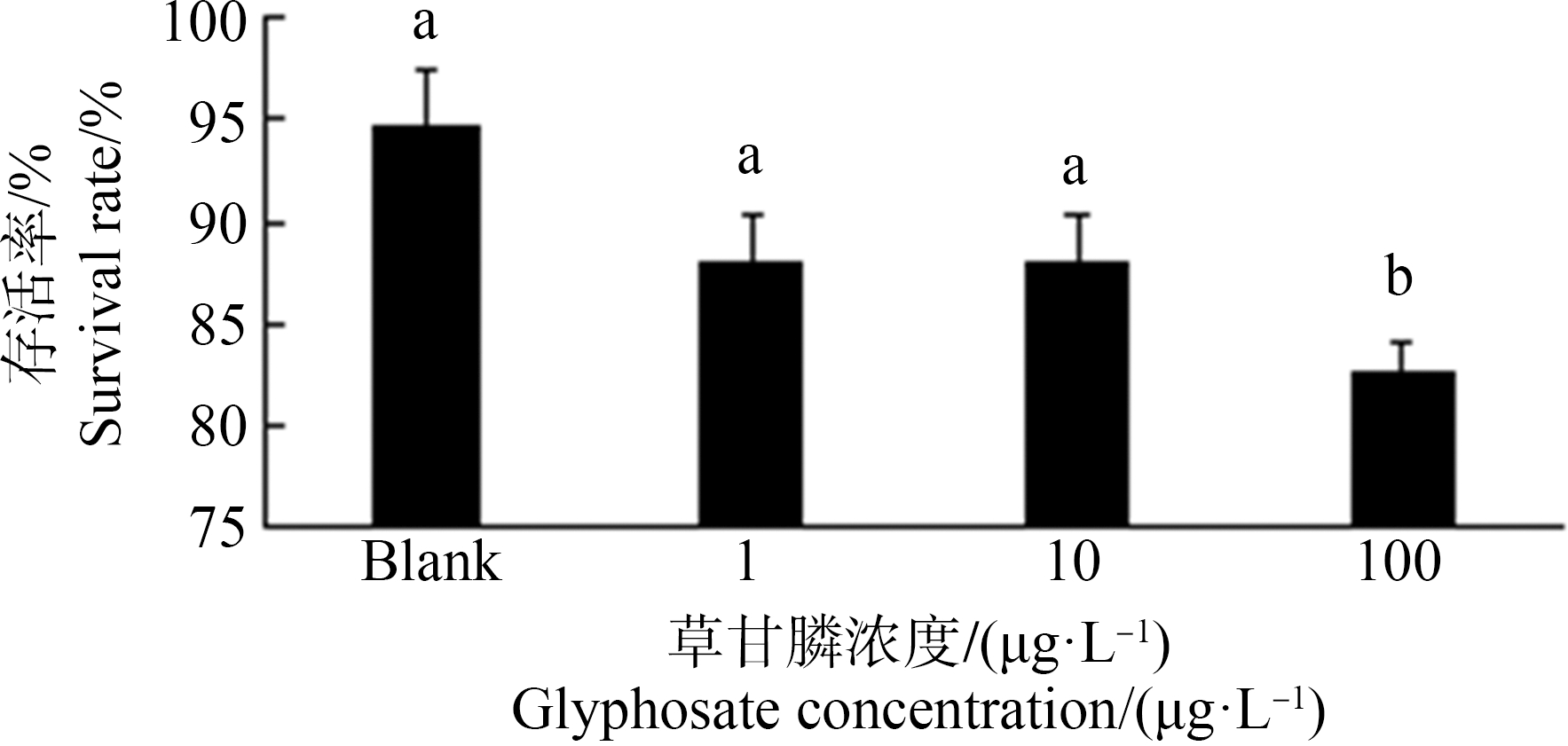
图1 暴露48 h对斑马鱼胚胎存活率的影响
注:数据表示为平均值±标准误差,n=3;上标字母不同的组间具有显著性差异(P<0.05)。
Fig. 1 Mortality rate in zebrafish embryos exposed for 48 h
Note: Data are presented as mean±S.E., n=3; means of the treatment not sharing a common letter are significantly different (P<0.05).
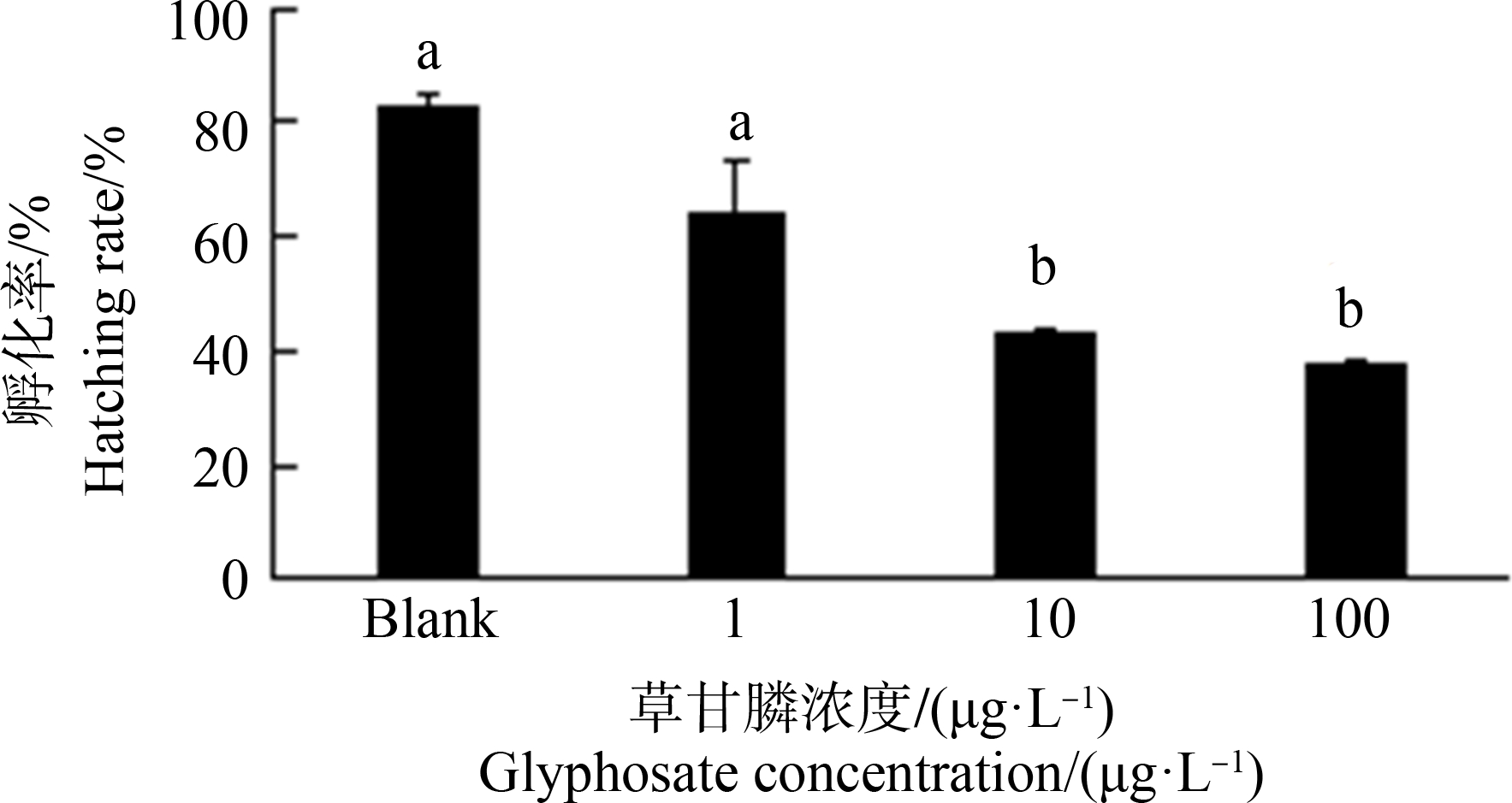
图2 暴露48 h对斑马鱼胚胎孵化率的影响
注:数据表示为平均值±标准误差,n=3;上标字母不同的组间具有显著性差异(P<0.05)。
Fig. 2 Hatching rate in zebrafish embryos exposed for 48 h
Note: Data are presented as mean±S.E., n=3; means of the treatment not sharing a common letter are significantly different (P<0.05).
2.2 草甘膦暴露对斑马鱼仔鱼脊柱弯曲率及卵黄囊水肿率的影响
斑马鱼胚胎在空白组及1 μg·L-1暴露组末出现脊柱弯曲。10 μg·L-1、100 μg·L-1组出现脊柱弯曲,且随着草甘膦暴露浓度增大,脊柱弯曲率显著上升,10 μg·L-1、100 μg·L-1暴露组分别为6.6%、7.3%。卵黄囊水肿发生率随着草甘膦暴露浓度增大而显著增大,10 μg·L-1、100 μg·L-1暴露组分别增大26.5%、33.3% (P<0.05)(图3~图6)。

图3 暴露72 h斑马鱼仔鱼脊柱弯曲
Fig. 3 Spinal curvature in zebrafish larvae exposed for 72 h
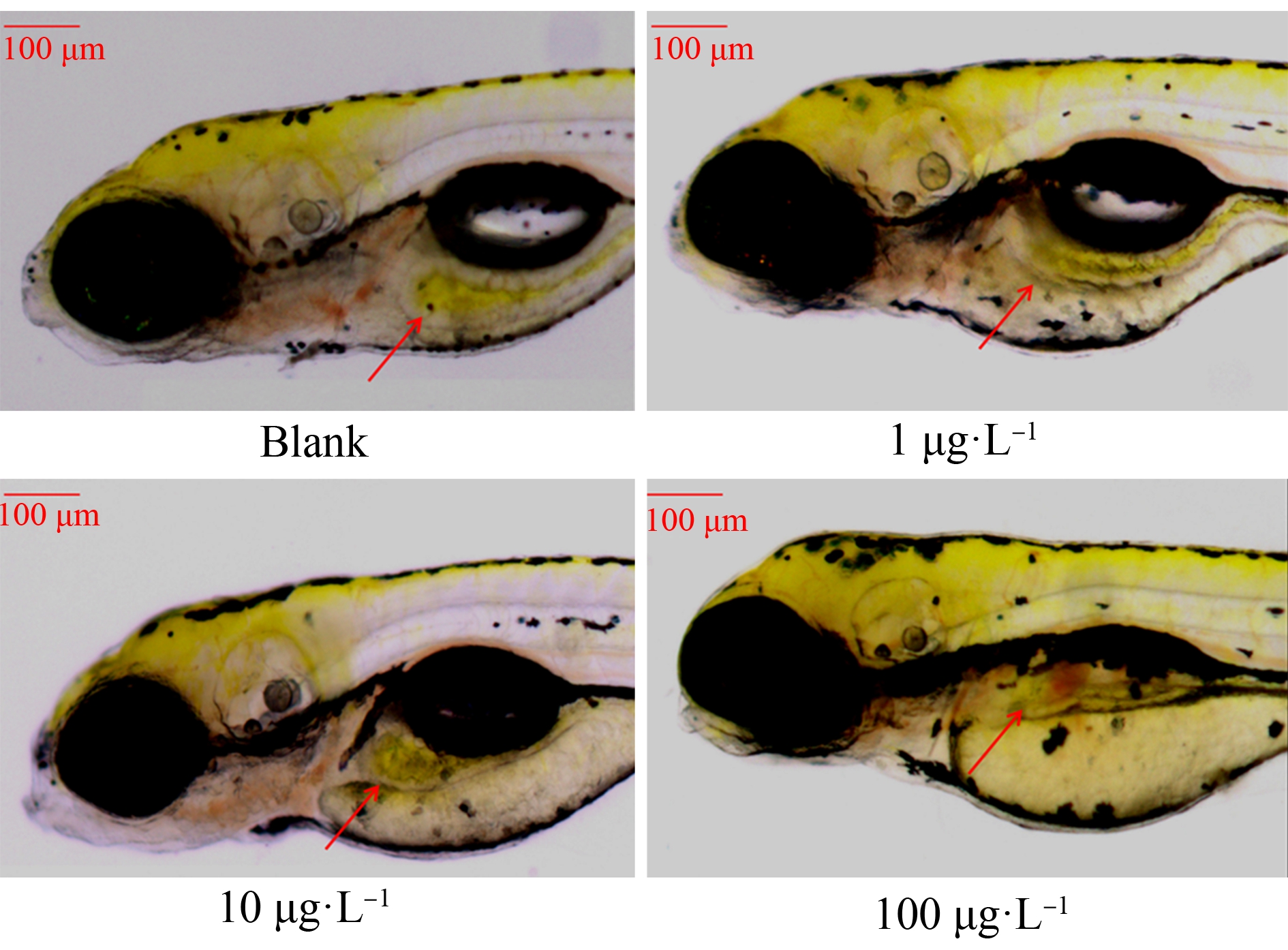
图4 暴露72 h斑马鱼仔鱼卵黄囊水肿
Fig. 4 Yolk cyst in zebrafish larvae exposed for 72 h
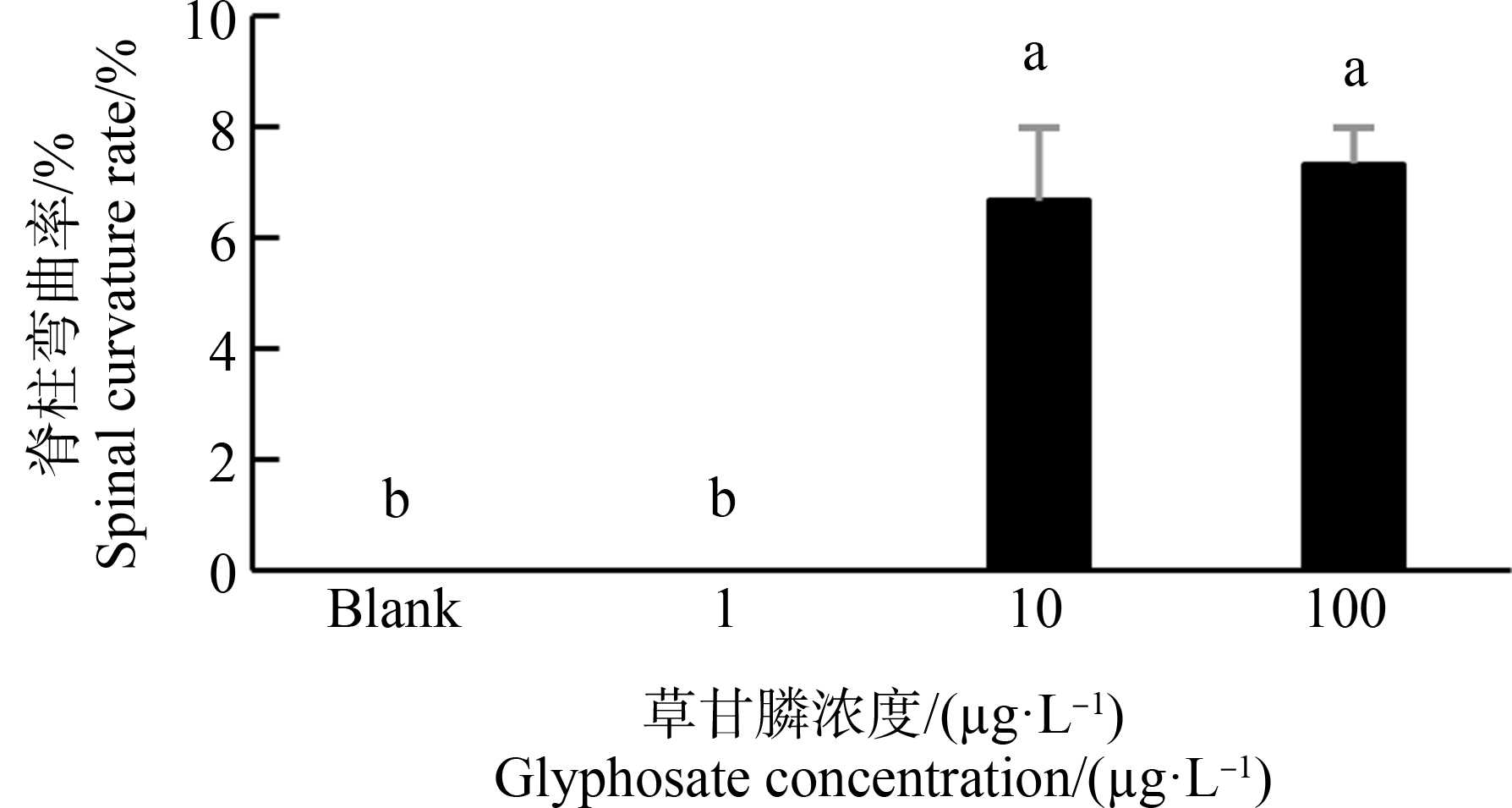
图5 暴露72 h对斑马鱼仔鱼脊柱弯曲率的影响
注:数据表示为平均值±标准误差,n=3;上标字母不同的组间具有显著性差异(P<0.05)。
Fig. 5 Spinal curvature rate in zebrafish larvae exposed for 72 h
Note: Data are presented as mean±S.E., n=3; means of the treatment not sharing a common letter are significantly different (P<0.05).
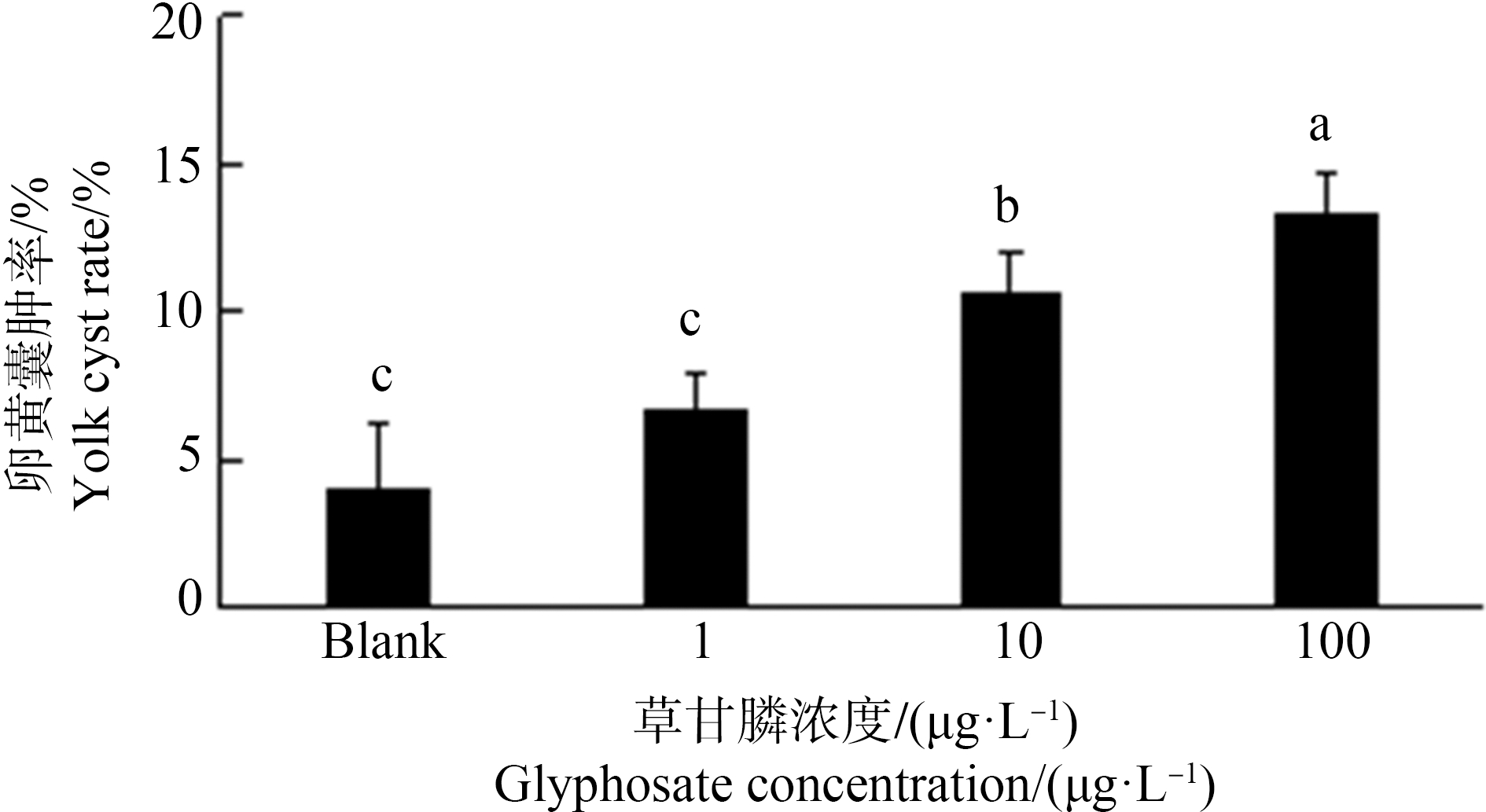
图6 暴露72 h对斑马鱼仔鱼卵黄囊水肿率的影响
注:数据表示为平均值±标准误差,n=3;上标字母不同的组间具有显著性差异(P<0.05)。
Fig. 6 Yolk cyst rate in zebrafish larvae exposed for 72 h
Note: Data are presented as mean±S.E., n=3; means of the treatment not sharing a common letter are significantly different (P<0.05).
2.3 草甘膦暴露对斑马鱼仔鱼心率及心包水肿率的影响
由图7~9可知斑马鱼仔鱼心率及心包水肿情况。草甘膦暴露72 h斑马鱼仔鱼心率与空白对照组相比,仅100 μg·L-1组显著上升12.2% (P<0.05)(图8)。由图9可知,空白对照组及1 μg·L-1暴露组中未出现心包水肿,在10 μg·L-1及100 μg·L-1暴露组出现心包水肿,但发生率均不高,分别仅为0.6%和2.6%,且由图7可知,10 μg·L-1组心包水肿程度较100 μg·L-1组轻微。
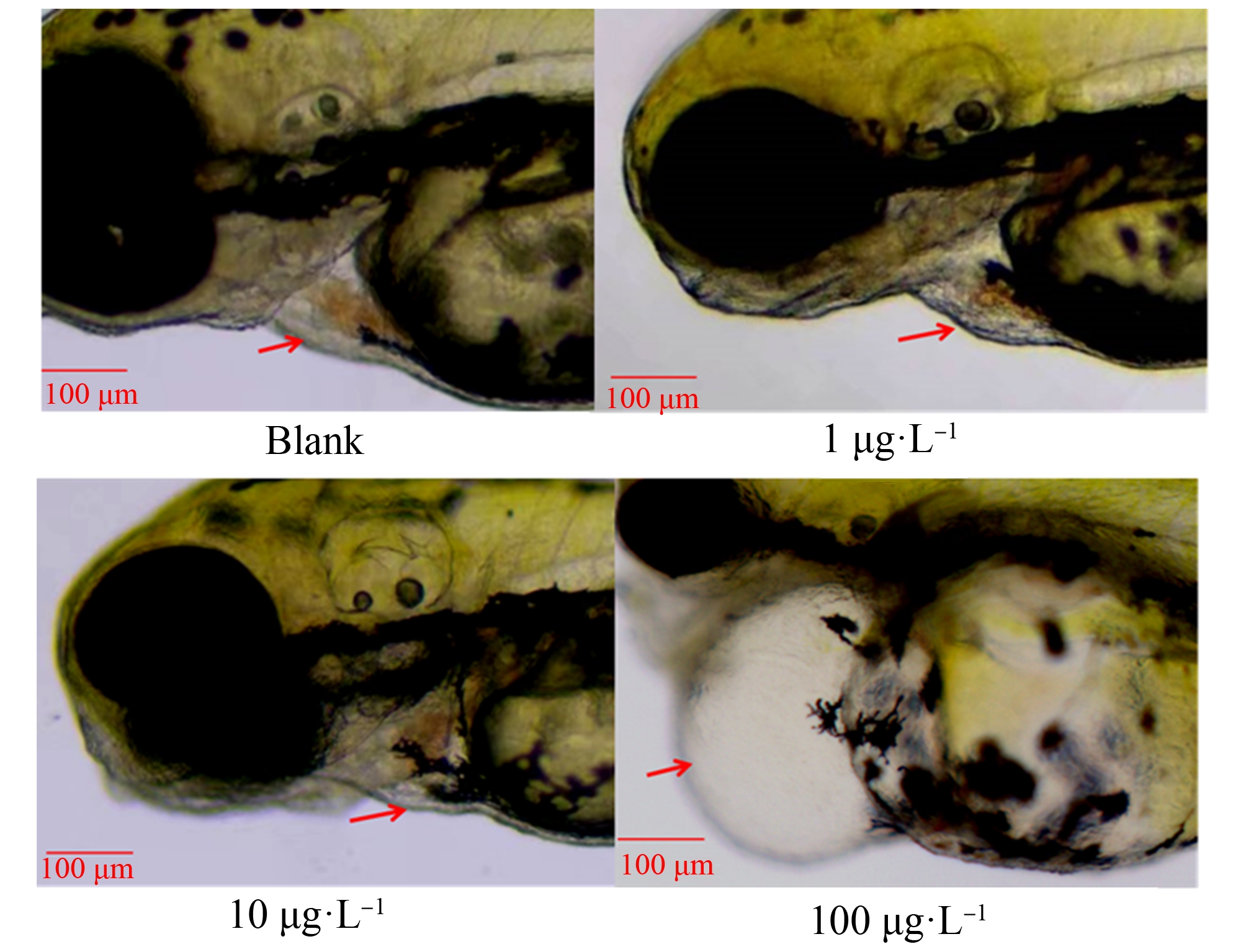
图7 暴露72 h斑马鱼仔鱼心包囊水肿
Fig. 7 Pericardial cyst in zebrafish larvae exposed for 72 h
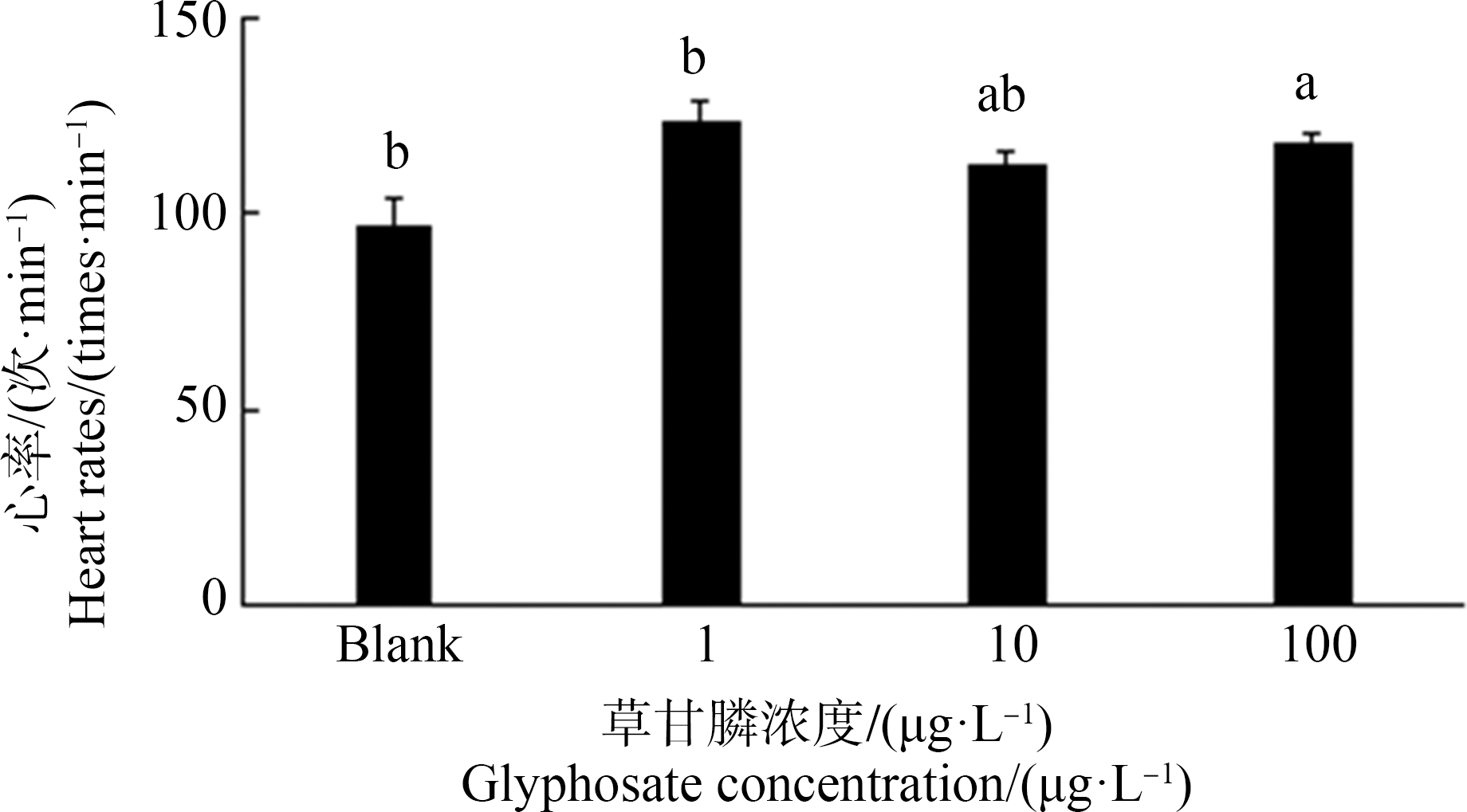
图8 暴露72 h对斑马鱼仔鱼心率的影响
注:数据表示为平均值±标准误差,n=3;上标字母不同的组间具有显著性差异(P<0.05)。
Fig. 8 Heart rates in zebrafish larvae exposed for 72 h
Note: Data are presented as mean±S.E., n=3; means of the treatment not sharing a common letter are significantly different (P<0.05).
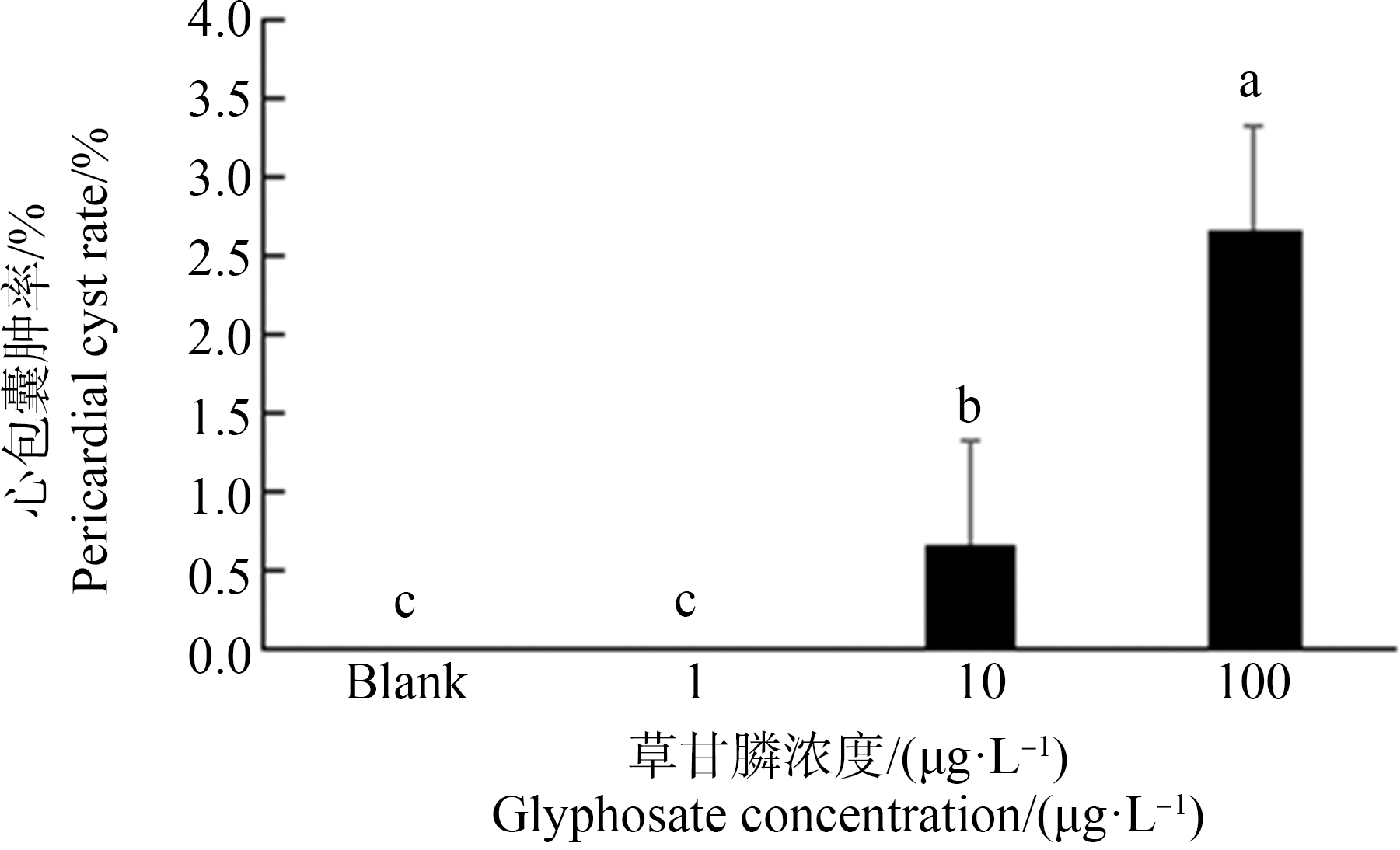
图9 暴露72 h对斑马鱼仔鱼心包囊肿率的影响
注:数据表示为平均值±标准误差,n=3;上标字母不同的组间具有显著性差异(P<0.05)。
Fig. 9 Pericardial cyst rate in zebrafish larvae exposed for 72 h
Note: Data are presented as mean±S.E., n=3; means of the treatment not sharing a common letter are significantly different (P<0.05).
2.4 草甘膦暴露对斑马鱼仔鱼ATP酶活性的影响
斑马鱼胚胎经草甘膦暴露72 h,在1 μg·L-1暴露组仔鱼组织Na+/K+-ATP酶活性及Ca2+-ATP酶活性都与空白对照组无差异;而在10 μg·L-1及100 μg·L-1暴露组均显著降低(P<0.05)(图10)。

图10 暴露72 h对斑马鱼仔鱼Na+/K+-ATP酶和Ca2+-ATP酶活性的影响
注:数据表示为平均值±标准误差,n=3;上标字母不同的组间具有显著性差异(P<0.05)。
Fig. 10 Activity of Na+/K+-ATPase and Ca2+-ATPase in zebrafish larvae exposed for 72 h
Note: Data are presented as mean±S.E., n=3; means of the treatment not sharing a common letter are significantly different (P<0.05).
2.5 草甘膦对斑马鱼仔鱼相关基因表达的影响
斑马鱼胚胎暴露72 hpf后相关基因表达量如图11所示,草甘膦暴露对仔鱼的多个基因产生了不同程度的影响。其中,在低浓度(1 μg·L-1)暴露组中,Bmp2b、Tbx5、Tnnt2和Shh等4个基因表达量上升(上升量分别为28%、45%、87%和86%,P<0.05),而Ihh表达水平降低(下降量为25%,P<0.05),Nkx2.5表达水平与空白组无差异。但当草甘膦浓度增大时,在10 μg·L-1及100 μg·L-1暴露组中各相关基因表达水平均出现下调,且存在剂量-效应关系,Nkx2.5表达量下调比例最大,达82%(P<0.05)。
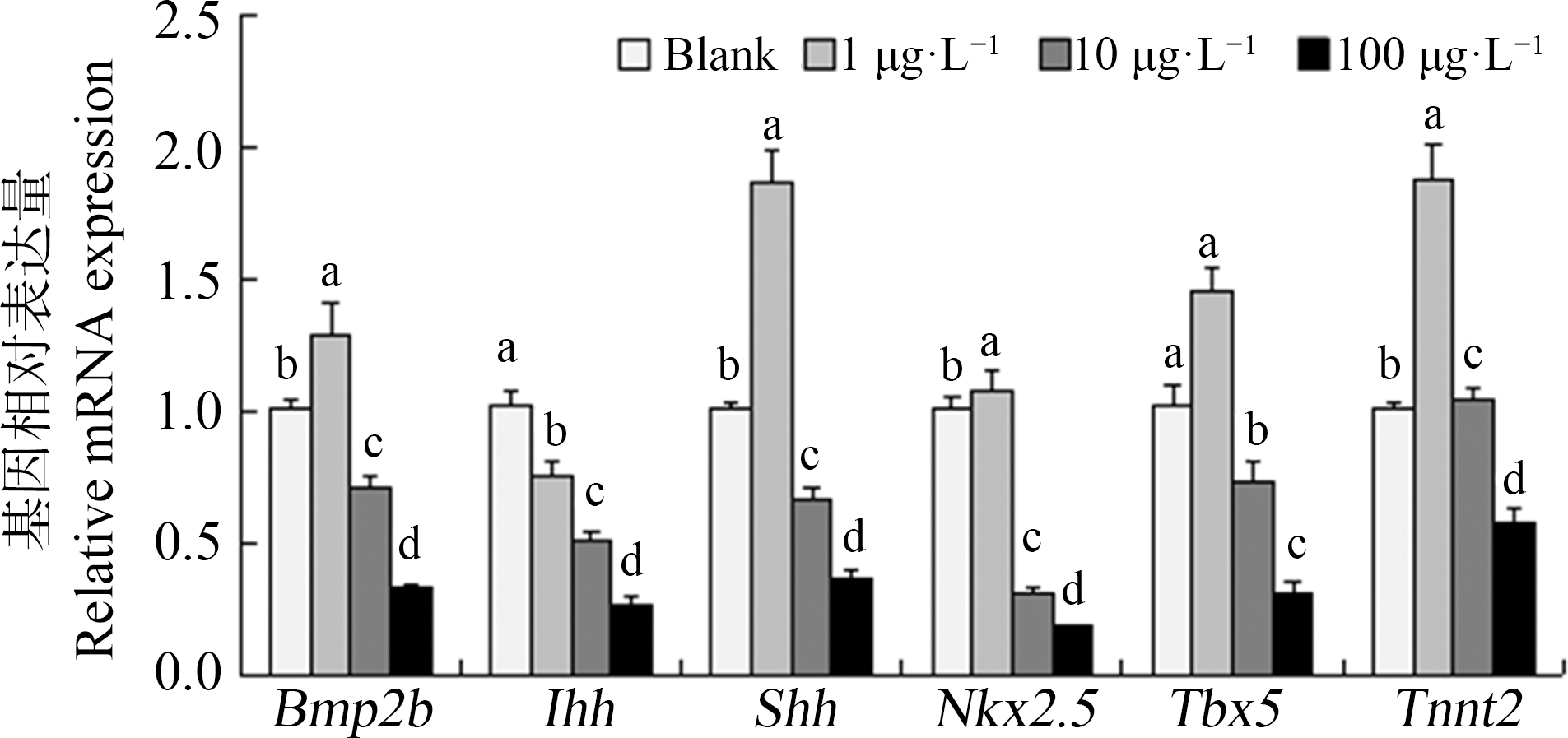
图11 暴露72 h对斑马鱼仔鱼基因表达的影响
注:数据表示为平均值±标准误差,n=3;上标字母不同的组间具有显著性差异(P<0.05)。
Fig. 11 The transcription of genes in zebrafish larvae exposed for 72 h
Note: Data are presented as mean±S.E., n=3; means of the treatment not sharing a common letter are significantly different (P<0.05).
3 讨论(Discussion)
鱼类毒性试验是评价污染物环境风险的主要方法之一,斑马鱼胚胎毒性检测实验中常出现的毒性反应是心包水肿、脊柱弯曲、卵黄囊水肿等发育畸形[37]。有研究表明,水肿会引起生物体代谢障碍,损害器官功能,是生物体抵御外界有毒物质入侵的一种反应[38]。实际上,脊柱弯曲、心包水肿、心脏形态和功能缺陷在暴露于杀菌剂和杀虫剂等化学物质的鱼类胚胎中相当常见[39-42]。本试验中,斑马鱼胚胎经草甘膦暴露后仔鱼有出现心包水肿、卵黄囊水肿及脊柱弯曲等反应,显示草甘膦对斑马鱼胚胎具有显著的发育毒性。
心脏作为胚胎发育过程中最早发挥功能的器官,也是鱼类胚胎试验的重要毒理学终点[43-44],完善的循环功能对于胚胎的正常发育起着关键作用[45]。对于斑马鱼胚胎发育而言,正常生长条件下,48 hpf时主要的器官系统完成形态发生并开始破膜孵化,这是发育进程的一个重要转折点。当生物体内能量供给不足时,心脏的正常工作会受到影响[46]。有研究表明污染物的暴露会使斑马鱼胚胎心率上升或下降以适应心脏对能量的供给[47]。本试验中,斑马鱼胚胎经草甘膦暴露后仔鱼心率升高,可推测此表现是为了满足心脏发育进程中能量需要的适应性反应。
ATP酶在细胞内维持离子平衡和渗透压稳态方面具有重要作用[48]。Na+/K+-ATP酶是组成Na+/K+泵的主要部分,Ca2+-ATP酶是质膜上钙离子转运系统中最重要的部分。有研究分析,受污染物低浓度暴露时,鱼体为了使机体免受氧化损伤,抗氧化酶会被激活,清除自由基;但当污染物浓度超过机体耐受阈值,产生的自由基过多会使细胞衰老加速,导致酶活性降低[49],这也能反映能量代谢的变化[50]。本试验结果显示低浓度(1 μg·L-1)草甘膦暴露中仔鱼的Na+/K+-ATP酶和Ca2+-ATP酶的活性无显著变化,但在高浓度(10 μg·L-1、100 μg·L-1)组中2种ATP酶活性均下降。有研究认为ATP酶的作用是水解高能化合物ATP而释放供生命活动所需的能量[51],造成胚胎发育异常的原因可能与ATP酶的活性抑制有关。本试验结果提示草甘膦暴露引起Na+/K+-ATP酶和Ca2+-ATP酶活性的降低也是影响斑马鱼胚胎心脏发育畸形的原因。
斑马鱼胚胎发育由多种基因控制,Tbx5是心脏发育的重要调节剂[32,52],在心肌细胞的分化[53]以及心脏传导系统功能[54]中起着至关重要的作用。有研究表明,Tbx5表达异常导致小鼠严重畸形[55],人类Tbx5基因的突变导致心脏发育缺陷[56]。本试验结果显示较高浓度草甘膦暴露后仔鱼出现Tbx5基因表达量下降,这可能是心包发育异常的主要机制。
骨形态发生蛋白(bone morphogenetic protein, BMP)在脊椎动物中是骨骼形成的关键调节因子,Bmp2是转化生长因子-β(TGF-β)超家族中的一员。在斑马鱼生长发育过程Bmp2直接参与了背板的早期形成[34]。Nkx2.5是触发初始心肌细胞分化的关键因素[57],可定性和定量确定心室的特征[58-59]。心肌肌钙蛋白(由Tnnt2编码)参与心脏收缩功能,是斑马鱼心肌分化所必需的[33]。本课题组前期研究表明,环境污染物通过干扰斑马鱼胚胎的转录会导致斑马鱼胚胎心脏发育和功能缺陷[41-42]。本试验显示,高浓度组(100 μg·L-1)中Nkx2.5表达量下调且比例最大,而Bmp2b、Tnnt2表达量上调。Bmp2b是Nkx2.5的上游基因[51],Nkx2.5表达量降低表明草甘膦可能会阻止心肌细胞的发育,从而引起心脏发育畸形[34],由图7可知,高浓度组出现极大程度的心包水肿,推测此畸形出现与Nkx2.5表达量受抑制关系最大。
Hh(Hedgehog)基因家族在胚胎发育中对软骨形成起着重要作用,Shh(Sonic hedgehog)、Ihh (Indian hedgehog)是Hh基因家族2个重要成员,早期胚胎发育中这2个基因在内胚层的正确表达与软骨组织相关[33]。课题组前期的研究表明污染物暴露干扰斑马鱼Shh、Ihh和Bmp2的转录水平从而引起骨发育缺陷[60]。本试验结果显示草甘膦暴露出现脊柱弯曲,尤其是高浓度组发生率最高(7.3%)且弯曲程度最大(图3)。高浓度组仔鱼Bmp2b、Nkx2.5的表达量受抑制,表明草甘膦暴露导致心脏发育紊乱,进而影响骨骼的发育。
环境浓度的草甘膦暴露导致斑马鱼胚胎明显的胚胎毒性,包括存活率下降、脊柱弯曲、心包水肿、卵黄囊肿;酶活性测定显示,心包水肿和骨骼畸形与Na+/K+-ATPase和Ca2+-ATPase活性的降低有关;荧光定量PCR分析显示,草甘膦暴露改变了与胚胎心脏发育(Tbx5、Nkx2.5、Tnnt2)、骨骼发育(Bmp2b、Shh、Ihh)相关基因的转录。值得注意的是,不同浓度的草甘膦对斑马鱼胚胎发育毒性强弱有差异,较低浓度(1 μg·L-1)对斑马鱼胚胎相对安全,但浓度达10 μg·L-1,甚至100 μg·L-1时出现显著毒性效应。可以认为,环境中的草甘膦残留量达到一定浓度(≥10 μg·L-1)时对环境生物构成潜在威胁。依据本试验结果,以斑马鱼胚胎为对象,草甘膦的最低观察效应浓度(lowest observed effect concentration, LOEC)可表示为10 μg·L-1,无观察效应浓度(no observed effect concentration, NOEC)可表示为1 μg·L-1。该指标可为草甘膦风险评估提供参考值,在草甘膦的实际应用中应全面考察环境可能残留浓度。
[1] 孙克. 全球十大除草剂的市场与展望[J]. 农药, 2013, 52(5): 317-322
Sun K. Market and outlook of the top ten herbicides in the world [J]. Agrochemicals, 2013, 52(5): 317-322 (in Chinese)
[2] Williams A L, Watson R E, DeSesso J M. Developmental and reproductive outcomes in humans and animals after glyphosate exposure: A critical analysis [J]. Journal of Toxicology and Environmental Health Part B, Critical Reviews, 2012, 15(1): 39-96
[3] Bisson M, Hontela A. Cytotoxic and endocrine-disrupting potential of atrazine, diazinon, endosulfan, and mancozeb in adrenocortical steroidogenic cells of rainbow trout exposed in vitro [J]. Toxicology and Applied Pharmacology, 2002, 180(2): 110-117
[4] Wang C, Lu G H, Cui J. Responses of AChE and GST activities to insecticide coexposure in Carassius auratus [J]. Environmental Toxicology, 2012, 27(1): 50-57
[5] 宋秀杰, 陈博. 北京市农药化肥非点源污染防治的技术措施[J]. 环境保护, 2001, 29(9): 30-32
Song X J, Chen B. Technical measures for prevention and control of non-point source pollution of pesticides and fertilizers in Beijing [J]. Environmental Protection, 2001, 29(9): 30-32 (in Chinese)
[6] 范瑾煜, 耿金菊, 王晓蓉. 离子色谱法测定太湖水体中草甘膦含量[C]// 中国化学会环境化学专业委员会.第六届全国环境化学大会暨环境科学仪器与分析仪器展览会摘要集. 上海: 中国化学会环境化学专业委员会, 2011: 222-223
[7] Contardo-Jara V, Klingelmann E, Wiegand C. Bioaccumulation of glyphosate and its formulation Roundup Ultra in Lumbriculus variegatus and its effects on biotransformation and antioxidant enzymes [J]. Environmental Pollution, 2009, 157(1): 57-63
[8] Stachowski-Haberkorn S, Becker B, Marie D, et al. Impact of Roundup on the marine microbial community, as shown by an in situ microcosm experiment [J]. Aquatic Toxicology, 2008, 89(4): 232-241
[9] Marchand J, Tanguy A, Charrier G, et al. Molecular identification and expression of differentially regulated genes of the European flounder, Platichthys flesus, submitted to pesticide exposure [J]. Marine Biotechnology, 2006, 8(3): 275-294
[10] Botta F, Lavison G, Couturier G, et al. Transfer of glyphosate and its degradate AMPA to surface waters through urban sewerage systems [J]. Chemosphere, 2009, 77(1): 133-139
[11] Vandenberg L N, Blumberg B, Antoniou M N, et al. Is it time to reassess current safety standards for glyphosate-based herbicides? [J]. Journal of Epidemiology and Community Health, 2017, 71(6): 613-618
[12] 赵伟, 曹曦予, 吴艳萍, 等. 草甘膦致小鼠机体氧化损伤作用的研究[J]. 毒理学杂志, 2011, 25(5): 364-366
[13] Astiz M, de Alaniz M J T, Marra C A. Antioxidant defense system in rats simultaneously intoxicated with agrochemicals [J]. Environmental Toxicology and Pharmacology, 2009, 28(3): 465-473
[14] 朱国念, 楼正云, 孙锦荷. 草甘膦对水生生物的毒性效应及环境安全性研[J]. 浙江大学学报(农业与生命科学版), 2000, 26(3): 309-312
Zhu G N, Lou Z Y, Sun J H. Study on toxicity and environmental safety of glyphosate to aquatic organisms [J]. Journal of Zhejiang Agricultural University (Agric &Life Sci), 2000, 26(3): 309-312 (in Chinese)
[15] 范瑾煜, 耿金菊, 王晓蓉. 草甘膦及Roundup诱导鲫鱼肝脏自由基的产生及其氧化应激[C]// 中国化学会. 中国化学会第28届学术年会第2分会场摘要集. 成都: 中国化学会, 2012: 78
[16] 南旭阳. 除草剂“草甘膦”对鲫鱼血细胞及血红蛋白影响的研究[J]. 甘肃科学学报, 2002, 14(4): 66-70
Nan X Y. A study of hemoglobin and blood cells in Carassius auratus induced by the herbicide of glyphosate [J]. Journal of Gansu Sciences, 2002, 14(4): 66-70 (in Chinese)
[17] 南旭阳, 张艳丹, 黄小莲. 除草剂“草甘膦”对泥鳅白细胞的影响[J]. 温州师范学院学报(自然科学版), 2003, 24(2): 72-74
Nan X Y, Zhang Y D, Huang X L. Effect of Misgurnus anguillicaudatus induced by glyphosate [J]. Journal of Wenzhou Teachers College, 2003, 24(2): 72-74 (in Chinese)
[18] Glusczak L, Miron D D S, Moraes B S, et al. Acute effects of glyphosate herbicide on metabolic and enzymatic parameters of silver catfish (Rhamdia quelen) [J]. Comparative Biochemistry and Physiology Toxicology &Pharmacology, 2007, 146(4): 519-524
[19] Murussi C R, Costa M D, Leitemperger J W, et al. Exposure to different glyphosate formulations on the oxidative and histological status of Rhamdia quelen [J]. Fish Physiology and Biochemistry, 2016, 42(2): 445-455
[20] Çava T, Könen S. Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus test and the comet assay [J]. Mutagenesis, 2007, 22(4): 263-268
T, Könen S. Detection of cytogenetic and DNA damage in peripheral erythrocytes of goldfish (Carassius auratus) exposed to a glyphosate formulation using the micronucleus test and the comet assay [J]. Mutagenesis, 2007, 22(4): 263-268
[21] Lushchak O V, Kubrak O I, Storey J M, et al. Low toxic herbicide Roundup induces mild oxidative stress in goldfish tissues [J]. Chemosphere, 2009, 76(7): 932-937
[22] Folmar L C, Sanders H O, Julin A M. Toxicity of the herbicide glyphosate and several of its formulations to fish and aquatic invertebrates [J]. Archives of Environmental Contamination and Toxicology, 1979, 8(3): 269-278
[23] Jiraungkoorskul W, Upatham E S, Kruatrachue M, et al. Biochemical and histopathological effects of glyphosate herbicide on Nile tilapia (Oreochromis niloticus) [J]. Environmental Toxicology, 2003, 18(4): 260-267
[24] Topal A, Atamanalp M, Uçar A, et al. Effects of glyphosate on juvenile rainbow trout (Oncorhynchus mykiss): Transcriptional and enzymatic analyses of antioxidant defence system, histopathological liver damage and swimming performance [J]. Ecotoxicology and Environmental Safety, 2015, 111: 206-214
[25] Armiliato N, Ammar D, Nezzi L, et al. Changes in ultrastructure and expression of steroidogenic factor-1 in ovaries of zebrafish Danio rerio exposed to glyphosate [J]. Journal of Toxicology and Environmental Health Part A, 2014, 77(7): 405-414
[26] 傅建炜, 史梦竹, 李建宇, 等. 草甘膦对草鱼、鲢鱼和鲫鱼的毒性[J]. 生物安全学报, 2013, 22(2): 119-122
Fu J W, Shi M Z, Li J Y, et al. Toxicity of glyphosate on grass carp (Ctenopharyngodon idellus), chub (Hypophthalmictuthys molitrix) and crucian (Carassius auratus) [J]. Journal of Biosafety, 2013, 22(2): 119-122 (in Chinese)
[27] 廖艺钰, 惠吕佳, 严吉祥, 等. 草甘膦农药对斑马鱼的急性毒性和慢性毒性研究[J]. 广州化工, 2020, 48(21): 66-68
Liao Y Y, Hui L J, Yan J X, et al. Study on acute and chronic toxicity of glyphosate to zebrafish [J]. Guangzhou Chemical Industry, 2020, 48(21): 66-68 (in Chinese)
[28] Clark M D, Hennig S, Herwig R, et al. An oligonucleotide fingerprint normalized and expressed sequence tag characterized zebrafish cDNA library [J]. Genome Research, 2001, 11(9): 1594-1602
[29] Schulte C, Nagel R. Testing acute toxicity in the embryo of zebrafish, Brachydanio rerio, as an alternative to the acute fish test: Preliminary results [J]. Alternatives to Laboratory Animal, 1994, 22: 12-19
[30] Oberemm A. The use of a refined zebrafish embryo bioassay for the assessment of aquatic toxicity [J]. Lab Animal, 2000, 29(7): 32-40
[31] Organisation for Economic Co-operation and Development (OECD). Draft proposal for a new guideline fish two generation test guideline [R]. Paris: OECD, 2002
[32] Hiroi Y, Kudoh S, Monzen K, et al. Tbx5 associates with Nkx2-5 and synergistically promotes cardiomyocyte differentiation [J]. Nature Genetics, 2001, 28(3): 276-280
[33] Mak K K, Kronenberg H M, Chuang P T, et al. Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy [J]. Development, 2008, 135(11): 1947-1956
[34] Kruithof B P, Xu J W, Fritz D T, et al. An in vivo map of bone morphogenetic protein 2 post-transcriptional repression in the heart [J]. Genesis, 2011, 49(11): 841-850
[35] Westerfield M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) [M]. Eugene: University of Oregon Press, 2000: 28-45
[36] 吴艳娣. 几种典型持久性有机污染物对斑马鱼胚胎发育的毒性效应研究[D]. 武汉: 华中农业大学, 2008: 17-18
Wu Y D. Study on the toxicity of several typical POPs exposed to zebrafish (Danio rerio) embryos [D]. Wuhan: Huazhong Agricultural University, 2008: 17-18 (in Chinese)
[37] Walker M K, Peterson R E. Aquatic Toxicity of Dioxins and Related Chemicals [M]//Schecter A. Dioxins and Health. Boston, MA: Springer, 1994: 347-387
[38] 陈粉丽, 张松林, 陈红梅, 等. 氯代苯类化合物对斑马鱼胚胎的单一急性毒性[J]. 环境与职业医学, 2010, 27(8): 472-475
Chen F L, Zhang S L, Chen H M, et al. Individual acute toxicity of chlorobenzenes on zebrafish embryo [J]. Journal of Environmental &Occupational Medicine, 2010, 27(8): 472-475 (in Chinese)
[39] Goetz A K, Ren H Z, Schmid J E, et al. Disruption of testosterone homeostasis as a mode of action for the reproductive toxicity of triazole fungicides in the male rat [J]. Toxicological Sciences: An Official Journal of the Society of Toxicology, 2007, 95(1): 227-239
[40] Mu X Y, Pang S, Sun X Z, et al. Evaluation of acute and developmental effects of difenoconazole via multiple stage zebrafish assays [J]. Environmental Pollution, 2013, 175: 147-157
[41] Wu Y Q, Zuo Z H, Chen M, et al. The developmental effects of low-level procymidone towards zebrafish embryos and involved mechanism [J]. Chemosphere, 2018, 193: 928-935
[42] Wu Y Q, Yang Q H, Chen M, et al. Fenbuconazole exposure impacts the development of zebrafish embryos [J]. Ecotoxicology and Environmental Safety, 2018, 158: 293-299
[43] Organization for Economic Cooperation and Development (OECD). Guidelines for the testing of chemicals. Section 2: Effects on biotic systems test No.236: Fish embryo acute toxicity (FEAT) test [S]. Paris: OECD, 2013
[44] Glickman N S, Yelon D. Cardiac development in zebrafish: Coordination of form and function [J]. Seminars in Cell &Developmental Biology, 2002, 13(6): 507-513
[45] Barrionuevo W R, Burggren W W. O2 consumption and heart rate in developing zebrafish (Danio rerio): Influence of temperature and ambient O2 [J]. The American Journal of Physiology, 1999, 276(2): R505-R513
[46] Kodde I F, van der Stok J, Smolenski R T, et al. Metabolic and genetic regulation of cardiac energy substrate preference [J]. Comparative Biochemistry and Physiology Part A, Molecular &Integrative Physiology, 2007, 146(1): 26-39
[47] Limas C J, Olivari M T, Goldenberg I F, et al. Calcium uptake by cardiac sarcoplasmic reticulum in human dilated cardiomyopathy [J]. Cardiovascular Research, 1987, 21(8): 601-605
[48] Yadwad V B, Kallapur V L, Basalingappa S. Inhibition of gill Na+ K+-ATPase activity in dragonfly larva, Pantala flavesens, by endosulfan [J]. Bulletin of Environmental Contamination and Toxicology, 1990, 44(4): 585-589
[49] 王重刚, 余群, 郁昂, 等. 苯并(a)芘和芘暴露对梭鱼肝脏超氧化物歧化酶活性的影响[J]. 海洋环境科学, 2002, 21(4): 10-13
Wang C G, Yu Q, Yu A, et al. Effect of benzo(a)pyrene and pyrene exposure on hepatic superoxide dismutase in Mugil so-iuy [J]. Marine Environmental Science, 2002, 21(4): 10-13 (in Chinese)
[50] 张玉, 张建明, 刘海涛, 等. 内蒙古不同水质湖泊瓦氏雅罗鱼Na+, K+-ATP酶活性的研究[J]. 内蒙古农业大学学报(自然科学版), 2013, 34(1): 1-4
Zhang Y, Zhang J M, Liu H T, et al. Inner Mongolian different lake Valsalva jarraud fish study of blood physiological parameters [J]. Journal of Inner Mongolia Agricultural University (Natural Science Edition), 2013, 34(1): 1-4 (in Chinese)
[51] 夏勇, 傅剑云, 郑云燕, 等. 丁草胺对斑马鱼及其胚胎发育毒性影响的研究[J]. 浙江预防医学, 2011, 23(1): 11-16
Xia Y, Fu J Y, Zheng Y Y, et al. Study on toxicity of butachlor on zebrafish and its embryo development [J]. Zhejiang Journal of Preventive Medicine, 2011, 23(1): 11-16 (in Chinese)
[52] Garg V, Kathiriya I S, Barnes R, et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5 [J]. Nature, 2003, 424(6947): 443-447
[53] Takeuchi J K, Bruneau B G. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors [J]. Nature, 2009, 459(7247): 708-711
[54] Moskowitz I P, Pizard A, Patel V V, et al. The T-Box transcription factor Tbx5 is required for the patterning and maturation of the murine cardiac conduction system [J]. Development, 2004, 131(16): 4107-4116
[55] Chapman D L, Garvey N, Hancock S, et al. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development [J]. Developmental Dynamics: An Official Publication of the American Association of Anatomists, 1996, 206(4): 379-390
[56] Bruneau B G, Nemer G, Schmitt J P, et al. A murine model of holt-oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease [J]. Cell, 2001, 106(6): 709-721
[57] Reiter J F, Verkade H, Stainier D Y R. Bmp2b and oep promote early myocardial differentiation through their regulation of gata5 [J]. Developmental Biology, 2001, 234(2): 330-338
[58] Balci M M, Akdemir R. NKX2.5 mutations and congenital heart disease: Is it a marker of cardiac anomalies? [J]. International Journal of Cardiology, 2011, 147(3): e44-e45
[59] Targoff K L, Colombo S, George V, et al. Nkx genes are essential for maintenance of ventricular identity [J]. Development, 2013, 140(20): 4203-4213
[60] Wu Y Q, Xu Z Y, Xu X B, et al. Exposure to metalaxyl disturbs the skeletal development of zebrafish embryos [J]. Bulletin of Environmental Contamination and Toxicology, 2020, 104(4): 432-437