废弃电子电器产品(电子垃圾)中含有大量重金属等有毒有害污染物,已成为全球范围内重金属的重要污染源之一[1]。电子垃圾处置不当造成的重金属污染及对生态系统和人类健康的不利影响已引起了高度关注[2-5]。由于严重的环境污染,我国在2010年前后取缔了粗犷的电子垃圾回收活动,许多电子垃圾拆解地被废弃[6]。最近的研究显示,这些废弃电子垃圾拆解地重金属污染依旧十分严重[6-8]。然而,废弃电子垃圾拆解地野生生物重金属暴露水平及其潜在的生态毒理学效应尚不清楚。
鸟类体温较高,新陈代谢旺盛,从环境中获取物质的数量和速率较大,重金属等环境污染物易于在鸟体内蓄积,从而对鸟类产生毒害效应[9]。此外,鸟类位于食物链中上层,生态位分化显著,对重金属污染敏感,是评价野生生物重金属暴露及毒性效应的良好指示类群[10]。
广东省汕头市贵屿镇是全球最大的电子垃圾拆解和回收中心之一,当地环境曾受到重金属等电子垃圾相关化合物的严重污染[11]。本文测定了该废弃电子垃圾拆解地5种野生鸟类肝脏中Cr、Cu、Cd、Pb和Zn等重金属的含量,探寻了鸟类食性、性别及居留类型对重金属蓄积的影响,检查了鸟类肝组织病理学形态,以期为废弃电子垃圾拆解地重金属污染防控和环境风险管理提供基础数据。
1 材料与方法(Materials and methods)
1.1 样品采集
经广东省林业局批准,2012年9—12月在广东省汕头市贵屿镇废弃电子垃圾拆解作坊附近用网捕法采集白头鹎(Pycnonotus sinensis)、棕背伯劳(Lanius schach)、北红尾鸲(Phoenicurus auroreus)、鹊鸲(Copsychus saularis)和普通翠鸟(Alcedo atthis)等5种野生鸟类。同时,在非电子垃圾拆解地(广东石门台自然保护区)采集了白头鹎(P. sinensis)和鹊鸲(C. saularis)作为对照样品。采集的鸟类均为成鸟,其样品数、食源、食性及居留类型见表1。样品低温运输至实验室,称量体质量后,放血并立即解剖,辨明性别后,取出肝脏。肝左叶置10%中性甲醛缓冲液中,用于组织病理学分析;肝右叶置-20 ℃冷冻保存,用于重金属含量分析。
表1 鸟类样品数、食源、食性及居留类型
Table 1 The sampling number and classification of the bird species collected according to their feeding habits and residence type

鸟类BirdsN食源Food source食性Feeding habits居留类型Residence type废弃电子垃圾拆解地Abandoned e-waste site白头鹎P. sinensis12陆源Terrestrial sources杂食性Omnivorous birds留鸟Resident bird棕背伯劳L. schach4陆源Terrestrial sources食虫鸟Insectivorous birds留鸟Resident bird北红尾鸲P. auroreus3陆源Terrestrial sources食虫鸟Insectivorous birds候鸟Migratory bird鹊鸲C. saularis2陆源Terrestrial sources食虫鸟Insectivorous birds留鸟Resident bird普通翠鸟A. atthis6水源Aquatic sources食鱼鸟Piscivorous birds留鸟Resident bird对照区Reference site白头鹎P. sinensis5陆源Terrestrial sources杂食性Omnivorous birds留鸟Resident bird鹊鸲C. saularis5陆源Terrestrial sources食虫鸟Insectivorous birds留鸟Resident bird
1.2 样品前处理及分析
1.2.1 重金属含量分析
肝脏样品经冷冻干燥后,用玻璃研钵研磨至粉状。取1 g左右样品置于聚四氟乙烯消解罐中,加入浓硝酸和浓盐酸混合液(1∶3, V∶V),在室温下消解12 h,放入微波消解仪消解。待样品冷却至室温后用去离子水稀释至10 mL待进一步分析。用电感耦合等离子体质谱仪(ICP-MS; Agilent 7900)对重金属进行定性定量测定,仪器工作参数设定参照文献[7]。
1.2.2 肝脏组织病理学分析
甲醛固定的肝脏组织经蒸馏水洗涤后,一部分切片后石蜡包埋,进行苏木精-伊红(HE)染色;一部分冰冻切片(5 μm),进行油红O染色。切片在光学显微镜(Nikon Eclipse E600 Microscope)下观察肝细胞形态。肝脏切片及HE和油红O染色操作参见文献[12]。
肝脏病理学改变程度分为4级[13]:0级,无病理学变化;1级,轻微损伤,病理变化面积<10%;2级,中等损伤,病理变化面积为10%~50%;3级,重度损伤,病理变化面积为50%~90%;4级,严重损伤,病理变化面积>90%。
1.3 质量保证与质量控制(QA/QC)
重金属含量分析过程中通过设置样品重复样和分析标准物质(鸡肉成分分析标准物质,GBW10018(GSB-9))来保证实验结果的精确性和准确性。标准物质中Cr、Cu、Cd、Pb和Zn的回收率为86.7%~106.7%。样品重复样(n=3)中目标金属含量的相对标准偏差(RSD)均<10%。Cr、Cu、Cd、Pb和Zn的检测限分别为4.55、0.72、0.12、15.98和0.01 ng·g-1。
1.4 统计分析
采用Shapiro-Wilk检验来确定数据是否符合正态分布。非正态分布数据经对数转换,以达到正态分布。采用独立样本T检验确定不同食源(陆源与水源)、不同食性(杂食性鸟类、食虫鸟和食鱼鸟)、不同性别及不同居留类型(留鸟与候鸟)鸟类重金属含量是否具有显著性差异。采用Pearson相关性分析来检验重金属含量的相关性。所有统计分析显著性水平设定为0.05。
2 结果与讨论(Results and discussion)
2.1 重金属残留水平
废弃电子垃圾拆解地及对照区鸟类肝脏中Cr、Cu、Cd、Pb和Zn等重金属的含量见表2。电子垃圾拆解地白头鹎和鹊鸲肝脏中Cr的含量分别是对照区鸟类的3.3倍和3.5倍,Pb的含量也分别是对照区的1.5倍和2.4倍。白头鹎肝脏中Cr的含量(均值14.07 mg·kg-1)比采集于广州市白云山的白头鹎(均值0.2 mg·kg-1) [14]高约2个数量级,但Pb、Cu和Zn的含量低于该鸟种肝脏中这些重金属含量的报道值[14-15]。本研究棕背伯劳肝脏中Pb(均值1.82 mg·kg-1)和Cd(均值0.31 mg·kg-1)的含量也高于采集于牡丹江市的红尾伯劳(Lanius cristatus)肝脏中这2种重金属的报道值(Pb: 1.0 mg·kg-1; Cd: 0.1 mg·kg-1) [16]。翠鸟肝脏中Pb的含量(均值1.59 mg·kg-1)高于采集于美国田纳西州橡树岭保护区的束带翠鸟(Ceryle alcyon)肝脏中Pb的含量(均值<0.4 mg·kg-1)2倍[17]以上。这些结果表明,废弃电子垃圾拆解地野生鸟类受到Cr、Pb等重金属污染。本研究电子垃圾拆解地主要进行废旧电子电器的拆解,而这些电子垃圾中电路板及电子元件中Cr和Pb的含量很高[18],这些重金属释放到环境后在鸟体内蓄积。重金属对电子垃圾拆解地及其周边地区鸟类及其他野生动物的健康影响应引起关注。
表2 废弃电子垃圾拆解地和对照区鸟类肝脏中重金属含量
Table 2 Concentrations of heavy metals in liver of birds from an abandoned e-waste recycling site and a reference site in South China (mg·kg-1)(以干质量计Based on dry mass)

CrCuCdPbZn废弃电子垃圾拆解地Abandoned e-waste site白头鹎P. sinensis14.1 (8.2~19.3)13.1(6.35~22.4)1.20(0.26~4.99)2.84(1.46~6.25)72.1(45.8~105)棕背伯劳L. schach10.6(8.70~14.2)8.71(7.73~11.4)0.31(0.22~0.46)1.82(1.42~2.21)51.9(41.6~68.6)北红尾鸲P. auroreus10.8(7.74~15.1)7.02(5.18~8.85)0.22(0.15~0.26)1.27(1.14~1.37)53.4(37.9~68.4)鹊鸲C. saularis14.0(7.99~20.0)7.79(4.04~11.6)0.30(0.29~0.32)2.07(1.65~2.48)60.1(41.1~79.2)普通翠鸟A. atthis12.4(7.78~21.1)12.1(6.05~22.1)0.36(0.09~0.73)1.59(0.83~2.30)57.5(37.8~88.2)对照区Reference site白头鹎P. sinensis4.30(3.96~4.56)18.4(10.8~25.6)3.60(1.24~5.40)1.93(1.03~2.94)71.4(57.0~87.0)鹊鸲C. saularis4.00(3.79~4.20)14.1(11.7~16.6)1.23(0.77~1.68)0.76(0.19~1.48)56.3(52.5~60.1)
鸟类肝脏中Cr-Cu、Cr-Zn、Cu-Zn、Cd-Pb、Cd-Zn和Cr-Cd等重金属含量之间具有相关性(图1),表明鸟体内这些重金属的来源可能相同(即电子垃圾)。此外,这种相关性还可能与2种金属在鸟体内具有相似的蓄积、转运或排除过程有关。前人的研究也发现,鸟类肝脏中Cu-Zn和Cd-Zn具有显著的正相关关系,可能与这些金属在鸟体内均与金属硫蛋白结合的方式进行代谢有关[19-21]。
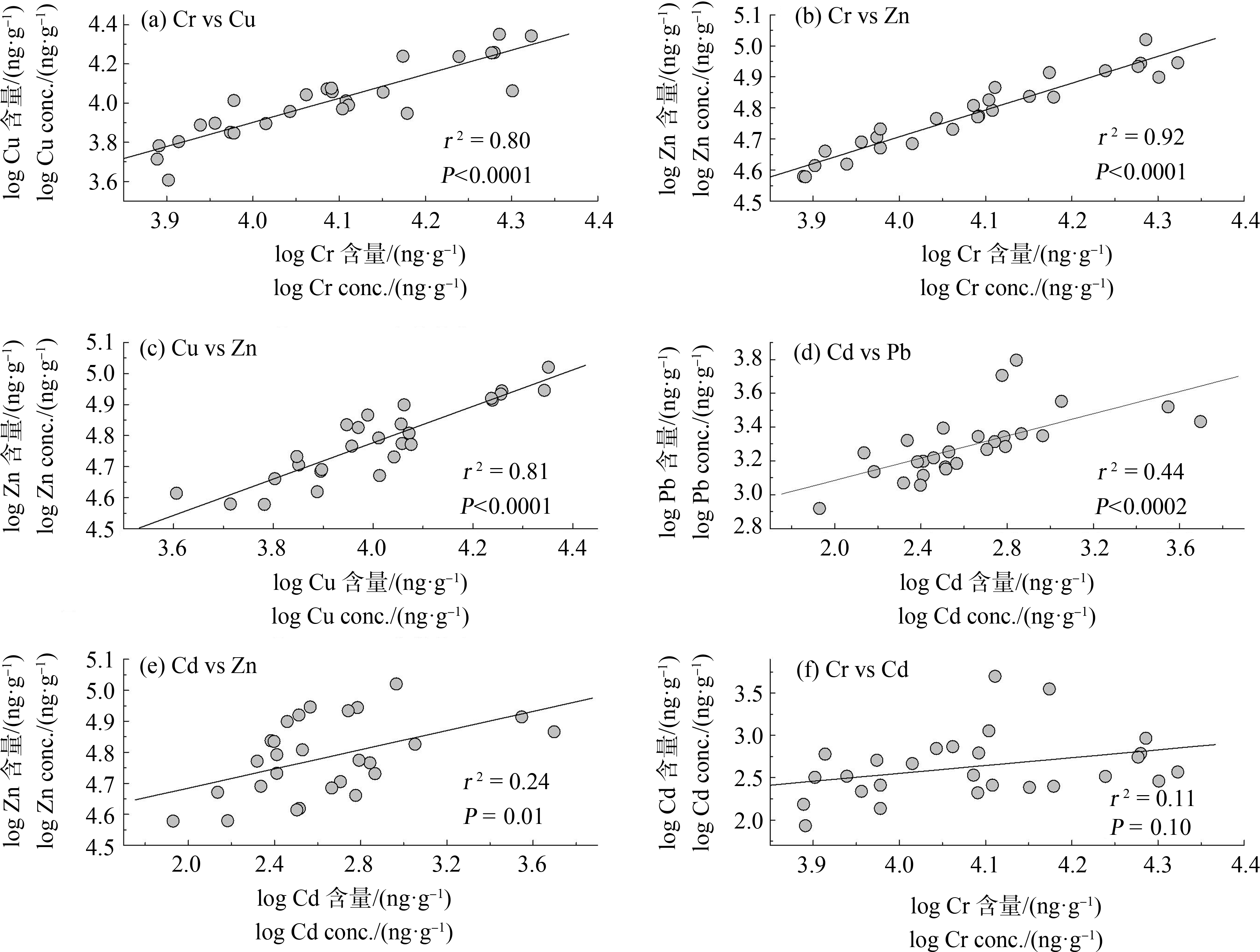
图1 电子垃圾拆解地鸟类肝脏中重金属含量的相关性
Fig. 1 The correlations between the metal concentrations in liver of birds inhabiting an abandoned e-waste recycling site in South China
2.2 鸟类食性、性别以及居留类型对重金属蓄积的影响
通过食物摄入是野生鸟类重金属暴露的主要途径,鸟类食源和食性差异可能造成其体内重金属含量不同[22-23]。本研究4种雀形目鸟类(白头鹎、北红尾鸲、鹊鸲和棕背伯劳)均摄食陆源性食物,而翠鸟以水源性食物(鱼类等)为主。统计分析结果显示,陆源性鸟类与水源性鸟类(翠鸟)肝脏中Zn (P=0.41)、Cr (P=0.78)、Cu (P=0.52)、Cd (P=0.16)和Pb (P=0.10)等重金属含量并无显著性差异。然而,杂食性鸟类(白头鹎)肝脏中Zn (P=0.02)、Cu (P=0.01)、Cd (P=0.02)和Pb (P=0.02)的含量均显著高于食虫鸟(北红尾鸲、鹊鸲和棕背伯劳),Cr的含量也高于食虫鸟,尽管无统计学上的显著性(P=0.11) (图2)。与食鱼鸟相比,杂食性鸟类肝脏中Zn、Cd和Pb的含量也显著增高(图2)。然而,食虫鸟与食鱼鸟肝脏中大多数重金属含量无显著性差异(图2)。本研究杂食性鸟类(白头鹎)主要以昆虫、浆果、嫩叶和蔬菜为食,其体内较高的重金属含量可能与电子垃圾拆解地植物中重金属污染有关[24]。本研究电子垃圾拆解地靠近山林和农田,电子垃圾释放的重金属会渗入这些场地的土壤中并被植物吸收,从而通过食物链在杂食性鸟类体内蓄积。

图2 不同食性鸟类肝脏中重金属含量比较
注:不同字母表示组间显著性差异(P<0.05),误差棒为标准误差(SE)。
Fig. 2 Comparisons of the hepatic concentrations of heavy metals in birds in relation to feeding habits
Note: Groups that do not share the same letter are significantly (P<0.05) different from each other; error bars represent one standard error (SE).
性别也是影响重金属在野生动物体内蓄积的重要因素之一[25]。电子垃圾拆解地雄性(n=7)白头鹎肝脏中Zn、Cr和Cu的含量显著高于雌性(n=5) (P<0.05)。但雄性与雌性肝脏中Cd和Pb的含量无显著性差异(图3)。雌性白头鹎体内较低的重金属(Zn、Cr和Cu)含量可能由于鸟类在产卵过程中把这些污染物传递到卵中,而Cd和Pb在雌鸟体内相对较难传递到卵中[26]。此外,雌鸟与雄鸟体内金属结合蛋白以及对于某些金属的结合途径等方面差异也会影响其在鸟体内的性别差异性蓄积[27]。关于麻雀和大山雀体内的重金属蓄积特征研究发现,雌鸟体内Zn、Cr或Cu的含量也较低[28-29],这与我们的研究结果相符。然而,较多研究发现,大多数鸟类对重金属的蓄积不具有性别差异性[25, 30-31]。
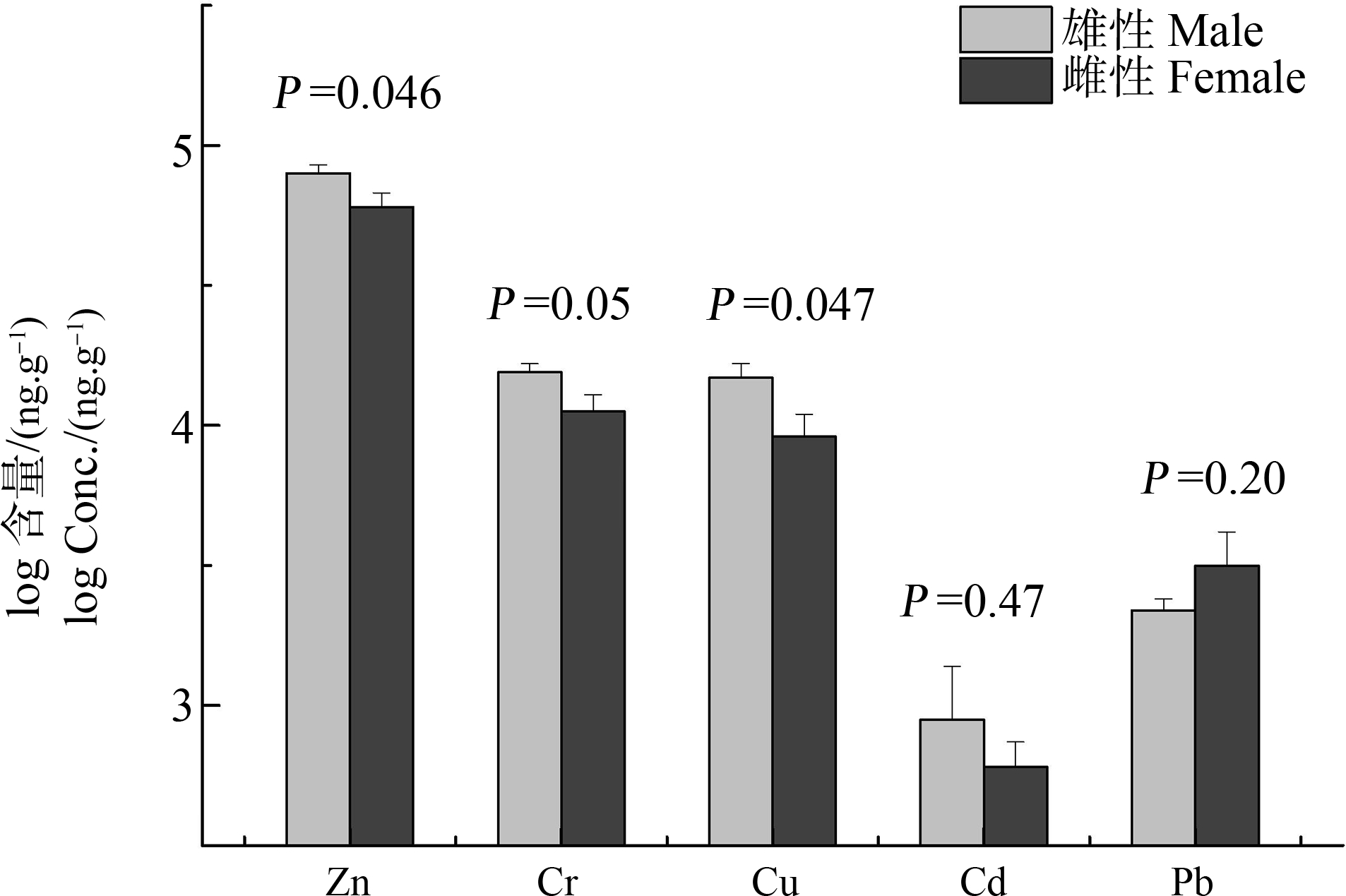
图3 重金属在白头鹎体内的性别差异性蓄积
注:误差棒为标准误差(SE)。
Fig. 3 Sex-dependent accumulation of heavy metals in light-vented bulbul from an abandoned e-waste recycling site in South China
Note: Error bars represent one standard error.
鸟类的居留类型也可能会影响重金属的蓄积[10, 32-33]。本研究棕背伯劳和鹊鸲为食虫性留鸟,而北红尾鸲为食虫性候鸟。统计分析发现(图4),留鸟体内的重金属含量均高于候鸟,尽管只有Pb具有统计学上的显著性(P<0.05),这可能与样本数较少有关。这一结果表明电子垃圾拆解地留鸟因长时间居住在污染地区,其体内重金属残留量较高。此外,留鸟的基础代谢率一般高于候鸟[34],较高的基础代谢率有利于重金属在鸟体内的蓄积,从而导致留鸟体内重金属浓度较高[35]。
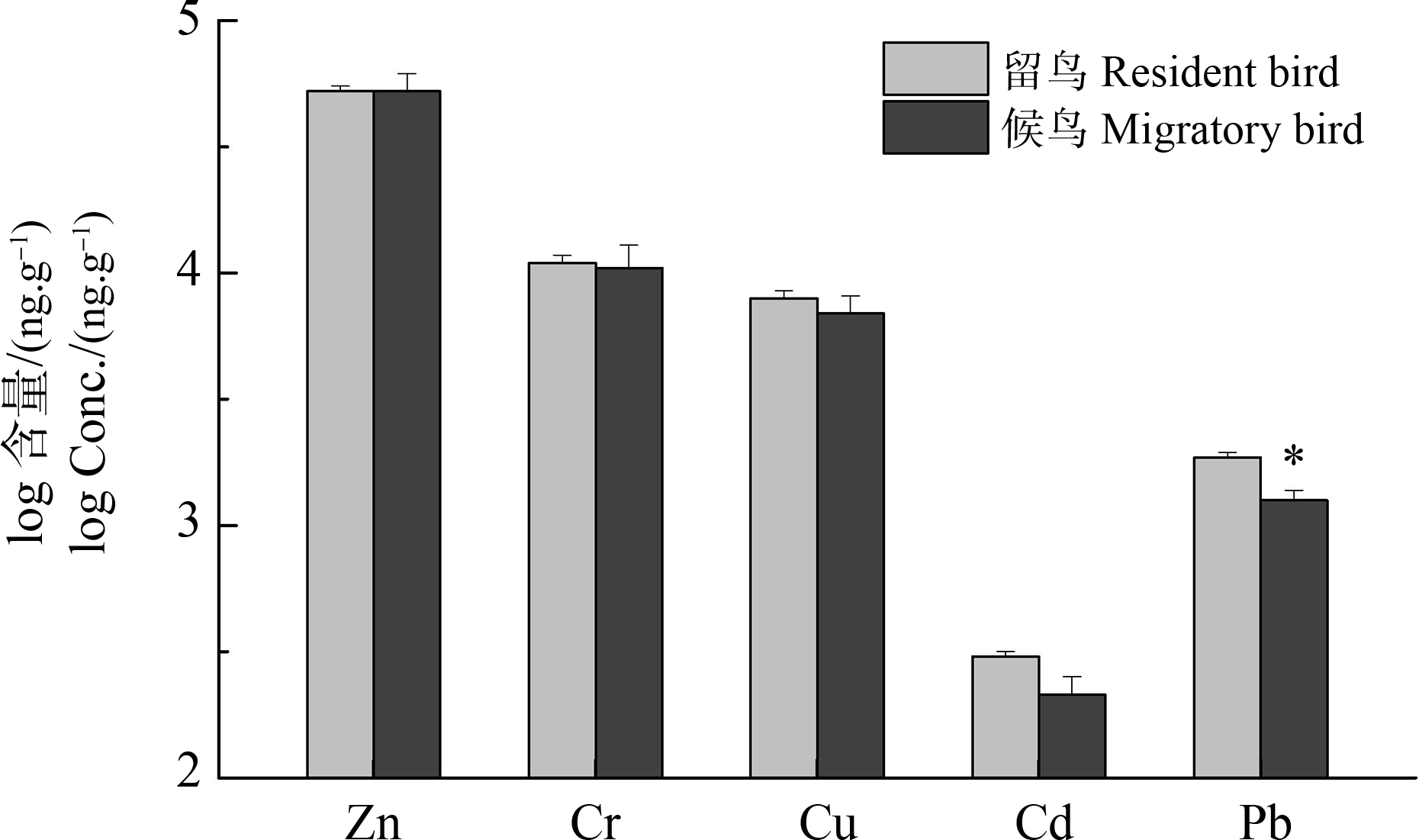
图4 不同居留类型鸟类肝脏中重金属含量比较
注:*表示组间有显著性差异(P<0.05)。
Fig. 4 Comparisons of the hepatic concentrations of heavy metals in birds in relation to residence type
Note: *above bars denotes significant difference between groups (P<0.05).
2.3 肝脏病理学改变
HE染色结果(图5(a))显示,66%的翠鸟肝细胞体积增大,胞浆中呈现大量空泡。油红O染色结果(图5(b))显示,肝组织中的肝细胞中呈现大面积红染,大小不一湿润浓深的橘色脂滴分布于肝细胞浆内。这些病理形态表明,废弃电子垃圾拆解地翠鸟的肝细胞发生了脂肪变性。肝细胞脂肪变性可进一步导致肝组织结构的改变、肝细胞坏死及肝功能紊乱等不良后果[36],从而影响鸟类的生长、发育和繁殖等过程[37]。

图5 废弃电子垃圾拆解地翠鸟肝脏组织HE(a)和油红O(b)染色结果
注:标尺为100 μm。
Fig. 5 Micrograph demonstrating steatosis in the liver of common kingfisher from an abandoned e-waste recycling site in South China by HE (a) and oil red O staining (b)
Note: Scale bar = 100 μm.
实验室暴露试验和野外研究均显示,Pb、Cd等重金属暴露可以导致鸟类肝细胞脂肪变性[36-38],其机制可能是由于重金属暴露增强了鸟类肝脏中活性氧(ROS)的形成并引起氧化抑制和脂质过氧化有关[37, 39]。研究表明,Pb、Cd、Cu、Zn和Cr等重金属均能增强鸟类肝脏中ROS的产生[40-42],从而可能导致鸟类肝脏组织发生病理性改变。本研究中翠鸟肝细胞发生脂肪变性的个体体内Zn、Cr、Cu和Cd等重金属残留均高于未发生脂肪变性个体(表3),且鸟类重金属含量越高,其肝细胞脂肪变性越严重(表3)。这些结果表明,翠鸟肝脏组织发生病理学改变可能与这些重金属暴露有关。废弃电子垃圾拆解地野生生物重金属暴露水平及潜在的生态毒理学效应应引起关注。
表3 翠鸟肝脏脂肪变性分级及重金属含量
Table 3 Classification of the degree of hepatic steatosis and the residue levels of heavy metals in common kingfisher from an abandoned e-waste site in South China

翠鸟编号Samples No.脂肪变性分级Classification of steatosis重金属含量/(mg·kg-1) (以干质量计)Heavy metal concentrations/(mg·kg-1) (Based on dry mass)ZnCrCuCdPb1#037.87.786.050.090.832#046.89.510.30.141.773#153.811.511.00.732.304#159.412.411.40.621.935#159.012.311.90.211.176#288.221.122.10.371.53
由于重金属在环境中的持久性,废弃电子垃圾拆解地野生动物重金属暴露水平可能较高,从而产生毒害效应。本文测定了某典型废弃电子垃圾拆解地野生鸟类肝脏中重金属的含量,探寻了鸟类的食性、性别及居留类型对重金属蓄积的影响,检查了鸟类肝组织病理学形态,得出如下主要结论:
(1) 尽管粗犷的电子垃圾回收活动已禁止多年,废弃电子垃圾拆解地野生鸟类仍受到Cr、Pb等重金属污染;
(2) 鸟类的食性、性别及居留类型影响其体内重金属残留水平,杂食性鸟类重金属残留高于食虫鸟和食鱼鸟,雄性白头鹎肝脏中重金属含量高于雌性,留鸟体内重金属含量高于候鸟;
(3) 翠鸟肝脏组织脂肪变性发生率为66%,且发生脂肪变性的个体肝脏中重金属的含量均高于未发生病理学改变的个体,鸟类肝脏组织病变可能与重金属暴露有关。
[1] Han Y, Tang Z W, Sun J Z, et al. Heavy metals in soil contaminated through e-waste processing activities in a recycling area: Implications for risk management [J]. Process Safety and Environmental Protection, 2019, 125: 189-196
[2] 彭平安, 盛国英, 傅家谟. 电子垃圾的污染问题[J]. 化学进展, 2009, 21(S1): 550-557
Peng P A, Sheng G Y, Fu J M. The pollution by electronic and electric wastes [J]. Progress in Chemistry, 2009, 21(S1): 550-557 (in Chinese)
[3] Yang S Y, He M J, Zhi Y Y, et al. An integrated analysis on source-exposure risk of heavy metals in agricultural soils near intense electronic waste recycling activities [J]. Environment International, 2019, 133(Pt B): 105239
[4] Xu R B, Zheng X B, Lin Y C, et al. Assessment of dust trace elements in an e-waste recycling area and related children’s health risks [J]. Science of the Total Environment, 2021, 791: 148154
[5] Zeng X, Zeng Z J, Wang Q H, et al. Alterations of the gut microbiota and metabolomics in children with e-waste lead exposure [J]. Journal of Hazardous Materials, 2022, 434: 128842
[6] Wu Q H, Leung J Y S, Du Y M, et al. Trace metals in e-waste lead to serious health risk through consumption of rice growing near an abandoned e-waste recycling site: Comparisons with PBDEs and AHFRs [J]. Environmental Pollution, 2019, 247: 46-54
[7] 吴江平, 陈小云, 韩义君, 等. 电子垃圾拆解地稻田土壤和稻米中重金属污染评估[J]. 环境科学学报, 2018, 38(4): 1628-1634
Wu J P, Chen X Y, Han Y J, et al. Assessment of heavy metal pollution in paddy soils and rice grains from an e-waste recycling area in South China [J]. Acta Scientiae Circumstantiae, 2018, 38(4): 1628-1634 (in Chinese)
[8] Du Y M, Wu Q H, Kong D G, et al. Accumulation and translocation of heavy metals in water hyacinth: Maximising the use of green resources to remediate sites impacted by e-waste recycling activities [J]. Ecological Indicators, 2020, 115: 106384
[9] Richard F J, Southern I, Gigauri M, et al. Warning on nine pollutants and their effects on avian communities [J]. Global Ecology and Conservation, 2021, 32: e01898
[10] Samaraweera M, Chandrajith R, Jayasena N. Birds of different feeding habits as biomonitors for trace elements in a wetland of the Central Asian Flyway, Sri Lanka [J]. Chemosphere, 2022, 306: 135602
[11] Li W L, Achal V. Environmental and health impacts due to e-waste disposal in China - A review [J]. Science of the Total Environment, 2020, 737: 139745
[12] Wu J P, Peng Y, Zhi H, et al. Contamination of organohalogen chemicals and hepatic steatosis in common kingfisher (Alcedo atthis) breeding at a nature reserve near e-waste recycling sites in South China [J]. The Science of the Total Environment, 2019, 659: 561-567
[13] Teh S, Zhang G, Kimball T, et al. Lethal and sublethal effects of esfenvalerate and diazinon on splittail larvae [J]. American Fisheries Society Symposium, 2004, 39: 243-253
[14] 邹发生, 杨琼芳, 谢美琪. 广州市白云山4种雀形目鸟类重金属残留分析[J]. 农村生态环境, 2005, 21(1): 51-54
Zou F S, Yang Q F, Xie M Q. Heavy metal residues in tissues of four species of Passeriformes at Baiyunshan, Guangzhou City, China [J]. Rural Eco-Environment, 2005, 21(1): 51-54 (in Chinese)
[15] 张丹, 张军, 欧阳盼, 等. 南昌市常见鸟类对环境中Cu、Pb、Cd重金属污染物的指示作用研究[J]. 江西师范大学学报(自然科学版), 2013, 37(3): 319-323
Zhang D, Zhang J, Ouyang P, et al. The environment indicative function of Cu, Pb, Cd heavy metal pollutants in common birds at Nanchang [J]. Journal of Jiangxi Normal University (Natural Science Edition), 2013, 37(3): 319-323 (in Chinese)
[16] 宫茜茜, 李自亲, 吕尤, 等. 牡丹江市夏季四种雀形目鸟体内铅和镉的残留测定[J]. 野生动物, 2008, 29(2): 79-83
Gong Q Q, Li Z Q, Lv Y, et al. Quantitative determination of Pb and Cd residue in 4 passerine species in Mudanjiang in summer [J]. Chinese Journal of Wildlife, 2008, 29(2): 79-83 (in Chinese)
[17] Baron L A, Ashwood T L, Sample B E, et al. Monitoring bioaccumulation of contaminants in the belted kingfisher (Ceryle alcyon) [J]. Environmental Monitoring and Assessment, 1997, 47(2): 153-165
[18] Huang W L, Shi X L, Wu K S. Human body burden of heavy metals and health consequences of Pb exposure in Guiyu, an e-waste recycling town in China [J]. International Journal of Environmental Research and Public Health, 2021, 18(23): 12428
[19] Hogstad O. Accumulation of cadmium, copper and zinc in the liver of some passerine species wintering in central Norway [J]. The Science of the Total Environment, 1996, 183(3): 187-194
[20] Wenzel C, Adelung D, Theede H. Distribution and age-related changes of trace elements in kittiwake Rissa tridactyla nestlings from an isolated colony in the German Bight, North Sea [J]. Science of the Total Environment, 1996, 193(1): 13-26
[21] Hernández-Moreno D, Ramos A, Romay C D, et al. Heavy metals content in great shearwater (Ardenna gravis): Accumulation, distribution and biomarkers of effect in different tissues [J]. Archives of Environmental Contamination and Toxicology, 2021, 80(3): 615-623
[22] Abbasi N A, Jaspers V L B, Chaudhry M J I, et al. Influence of taxa, trophic level, and location on bioaccumulation of toxic metals in bird’s feathers: A preliminary biomonitoring study using multiple bird species from Pakistan [J]. Chemosphere, 2015, 120: 527-537
[23] Liu W, Xu W X, Wei-kang Y, et al. Food habits of the great gerbil (Rhombomys opimus) in the southern Gurbantunggut Desert, Xinjiang, China [J]. Pakistan Journal of Zoology, 2012, 44: 931-936
[24] Luo C L, Liu C P, Wang Y, et al. Heavy metal contamination in soils and vegetables near an e-waste processing site, South China [J]. Journal of Hazardous Materials, 2011, 186(1): 481-490
[25] Burger J. A framework and methods for incorporating gender-related issues in wildlife risk assessment: Gender-related differences in metal levels and other contaminants as a case study [J]. Environmental Research, 2007, 104(1): 153-162
[26] Donaldson G M, Braune B M. Sex-related levels of selenium, heavy metals, and organochlorine compounds in American white pelicans (Pelecanus erythrorhyncos) [J]. Archives of Environmental Contamination and Toxicology, 1999, 37(1): 110-114
[27] Millán J, Mateo R, Taggart M A, et al. Levels of heavy metals and metalloids in critically endangered Iberian lynx and other wild carnivores from Southern Spain [J]. Science of the Total Environment, 2008, 399(1-3): 193-201
[28] Swaileh K M, Sansur R. Monitoring urban heavy metal pollution using the House Sparrow (Passer domesticus) [J]. Journal of Environmental Monitoring, 2006, 8(1): 209-213
[29] Dauwe T, Lieven B, Ellen J, et al. Great and blue tit feathers as biomonitors for heavy metal pollution [J]. Ecological Indicators, 2002, 1(4): 227-234
[30] Zaccaroni A, Amorena M, Naso B, et al. Cadmium, chromium and lead contamination of Athene noctua, the little owl, of Bologna and Parma, Italy [J]. Chemosphere, 2003, 52(7): 1251-1258
[31] Zarrintab M, Mirzaei R. Tissue distribution and oral exposure risk assessment of heavy metals in an urban bird: Magpie from Central Iran [J]. Environmental Science and Pollution Research International, 2018, 25(17): 17118-17127
[32] Durkalec M, Martinez-Haro M, Nawrocka A, et al. Factors influencing lead, mercury and other trace element exposure in birds from metal mining areas [J]. Environmental Research, 2022, 212: 113575
[33] Cooper Z, Bringolf R, Cooper R, et al. Heavy metal bioaccumulation in two passerines with differing migration strategies [J]. The Science of the Total Environment, 2017, 592: 25-32
[34] Jetz W, Freckleton R P, McKechnie A E. Environment, migratory tendency, phylogeny and basal metabolic rate in birds [J]. PLoS One, 2008, 3(9): e3261
[35] Cai F, Calisi R M. Seasons and neighborhoods of high lead toxicity in New York City: The feral pigeon as a bioindicator [J]. Chemosphere, 2016, 161: 274-279
[36] Kou H H, Ya J, Gao X B, et al. The effects of chronic lead exposure on the liver of female Japanese quail (Coturnix japonica): Histopathological damages, oxidative stress and AMP-activated protein kinase based lipid metabolism disorder [J]. Ecotoxicology and Environmental Safety, 2020, 190: 110055
[37] Amri N, Rahmouni F, Chokri M A, et al. Histological and biochemical biomarkers analysis reveal strong toxicological impacts of pollution in hybrid sparrow (Passer domesticus × Passer hispaniolensis) in southern Tunisia [J]. Environmental Science and Pollution Research International, 2017, 24(21): 17845-17852
[38] W ostowski T, Dmowski K, Bonda-Ostaszewska E. Cadmium accumulation, metallothionein and glutathione levels, and histopathological changes in the kidneys and liver of magpie (Pica pica) from a zinc smelter area [J]. Ecotoxicology, 2010, 19(6): 1066-1073
ostowski T, Dmowski K, Bonda-Ostaszewska E. Cadmium accumulation, metallothionein and glutathione levels, and histopathological changes in the kidneys and liver of magpie (Pica pica) from a zinc smelter area [J]. Ecotoxicology, 2010, 19(6): 1066-1073
[39] Koivula M J, Kanerva M, Salminen J P, et al. Metal pollution indirectly increases oxidative stress in great tit (Parus major) nestlings [J]. Environmental Research, 2011, 111(3): 362-370
[40] Espín S, Martínez-López E, Jiménez P, et al. Effects of heavy metals on biomarkers for oxidative stress in Griffon vulture (Gyps fulvus) [J]. Environmental Research, 2014, 129: 59-68
[41] Kanwal S, Abbasi N A, Chaudhry M J I, et al. Oxidative stress risk assessment through heavy metal and arsenic exposure in terrestrial and aquatic bird species of Pakistan [J]. Environmental Science and Pollution Research International, 2020, 27(11): 12293-12307
[42] Koim-Puchowska B, Drozdz-Afelt J M, Lamparski R, et al. Antioxidant defence barrier of great tit Parus major nestlings in response to trace elements [J]. Environmental Science and Pollution Research International, 2020, 27(16): 20321-20334