2,4-二氯酚(2,4-DCP)是典型的酚类污染物,被广泛运用于农药、印染、石油化工、炼油、医药、机械制造和有机合成等诸多行业[1-2]。作为农业用除草剂或由于工业生产过程中储存、处置不善,2,4-DCP会通过废水、泄漏、大气沉降等途径进入环境中[3]。2,4-DCP具有很强的毒性,进入环境后会对动、植物造成不同强烈的毒害作用,由于其在水和土壤中半衰期长,难以被降解,易在机体中累积,直接或间接地影响人类健康[4-5]。
蚯蚓作为一种生物量大的无脊椎动物,在自然净化中起关键因素[6],其取食、排泄等生理活动对土壤中有机质的分解和矿化循环起重要作用,有“土壤生态工程师”的美誉[7]。蚯蚓是一种对外来污染物敏感的生物[8-9],体内的抗氧化酶活性和蛋白质等指标可及时反映各种化学品(重金属、有机物)的毒性效应,故具有评价土壤污染生态毒理的作用[10-11]。
有关重金属及有机物对蚯蚓的毒性效应已有很多研究[12-13],但因蚯蚓大小、蚯蚓种类及测试方法不同,结果也存在差异。自然界中,由于生物处于不同年龄,其对污染物的敏感程度也不同。王勤等[14]调查了白纹伊蚊对常用杀虫剂的抗性,结果表明各地白纹伊蚊幼虫对杀虫剂很敏感,而白纹伊蚊成蚊则具有不同程度的抗药性;黄轶等[15]在研究生态毒性数据质量评估时提出,以鱼和虫为试验对象时,幼鱼和幼虫通常对环境更敏感,而这在对蚯蚓的相关研究中鲜有报道。本研究采用滤纸接触法和两室趋避试验,揭示2,4-DCP对赤子爱胜蚓(若蚓期、成蚓期)2个生长阶段的毒性效应,可为深入研究2,4-DCP对蚯蚓及其生物生态系统的影响提供理论依据。
1 材料与方法(Materials and methods)
1.1 供试蚯蚓和试剂
试验蚯蚓为赤子爱胜蚓(Eisenia fetida),购自河南石家庄某蚯蚓养殖场,于温度(23±2) ℃、空气湿度70%、光暗比12 h∶12 h的实验室条件预驯养14 d后,挑选色泽红润、活力强的蚯蚓,以无明显生殖环的蚯蚓为若蚓组,体质量为150~250 mg;有明显生殖环的蚯蚓为成蚓组,体质量350~500 mg,分别代表蚯蚓生长过程的2个重要阶段(图1)。2,4-DCP(CAS号120-83-2,纯度99.5%)由润友化学有限公司提供,为白色结晶性粉末,分子式C6H4Cl2O。试验所用丙酮等试剂均为分析纯。

图1 赤子爱胜蚓若蚓组150~250 mg(a)和成蚓组350~500 mg(b)
Fig. 1 Eisenia fetida 150~250 mg in the growth stage earthworms group (a) and 350~500 mg in the adult stage earthworms group (b)
1.2 供试土壤
土壤采集于常州市滨江化工园区无污染的林地,去除石子树枝等杂物后自然风干,过10目筛备用。经测定,该土壤pH为7.52±0.17,有机质含量(17.69±1.08) g·kg-1,其中未检出2,4-DCP。
1.3 试验方法
1.3.1 蚯蚓清肠
将蚯蚓放于培养皿中,清水洗去表皮泥土及排泄物,纱布擦干多余水分。随后将蚯蚓置于经蒸馏水打湿的滤纸上,黑暗环境下清肠1 d。
1.3.2 滤纸法染毒及2,4-DCP浓度设计
滤纸染毒参考OECD No.207方法[16],称0.5 g的2,4-DCP溶于40 mL丙酮中制成12.5 g·L-1的2,4-DCP丙酮溶液,取若干直径9 cm培养皿,用移液枪吸取4 mL丙酮于各培养皿中,再分别吸取160、165、170、175、180、185、190、195、200、205 μL的2,4-DCP丙酮溶液于各培养皿中,即得70.74、72.95、75.16、77.37、79.58、81.79、84.00、86.21、88.42、90.63 μg·cm-2这10个浓度梯度(空白对照组不添加),放入裁剪好的滤纸使其刚好浸泡在培养皿底部。考虑2,4-DCP具有挥发性,待丙酮挥发完全后即在每个培养皿中滴加4 mL蒸馏水以保持滤纸完全湿润,随后于培养皿中投加5条若蚓期或成蚓期的蚯蚓,用带小孔的塑料薄膜封住,橡皮筋固定,放于温度(23±2) ℃、空气湿度70%、黑暗的培养箱中,每组试验3个平行,24、48和72 h观察蚯蚓状态及死亡情况,若针头刺激蚯蚓头部和尾部均无反应则认定死亡。
1.3.3 土壤染毒和两室趋避试验设计
土壤染毒方法参考OECD No.222方法[17]:取600 g风干后的土壤于若干带盖塑料盒中,分别吸取96、240、480、720、960、1 200 μL浓度为12.5 g·L-1的2,4-DCP丙酮溶液于各塑料盒中土壤表面,待丙酮挥发后通过搅拌和盖上盖子摇晃的方式使土壤与2,4-DCP混合均匀,即得浓度为2、5、10、15、20、25 mg·kg-1的2,4-DCP污染土壤。
两室趋避试验参考ISO 17512-1方法[18]:取长16.5 cm、宽11.9 cm、高6.2 cm的带盖塑料盒,于塑料盒中间插入一厚度0.5 cm隔板,两侧分别放入清洁土壤和不同浓度2,4-DCP染毒土壤600 g,控制含水率为35%后抽出隔板并投12条蚯蚓于原隔板处缝隙中,盖上盖子防止蚯蚓逃逸,在盖子上扎若干2 mm左右孔洞以保持通风,48 h后计算两侧的蚯蚓个数,处于原先隔板处的蚯蚓按半条计算,每组试验设3个平行。
1.3.4 蚯蚓组织切片制备
根据试验过程中所观察的蚯蚓形态学变化,采用苏木精-伊红染色制备蚯蚓组织切片以观察70.74 μg·cm-2和88.42 μg·cm-2浓度下滤纸法暴露24 h时蚯蚓表皮和肠道细胞变化。具体操作为:蚯蚓用0.86%(m∶V)NaCl溶液冲洗后,放于培养皿中,加入少量蒸馏水,滴入无水乙醇麻痹蚯蚓后,将蚯蚓拉直放于滤纸上,吸干表面水分用刀片于生殖环后1 cm处切取0.5 cm放于10%(V∶V)福尔马林溶液中固定24 h。在自动脱水机中脱水后,石蜡包埋,切片机切成5 μm左右切片,在200倍数光学显微镜(AxioVert.A1)下观察组织变化情况。
1.3.5 蚯蚓体内蛋白质含量、SOD活性、CAT活性测定
通过计算得出半致死浓度(LC50)后,于亚致死浓度13.26、26.52、39.79、53.05、66.32 μg·cm-2条件下,分别于24、48和72 h对蚯蚓进行蛋白质含量、SOD活性和CAT活性测定,测定均采用南京建成提供的试剂盒。在生化分析之前,先用蒸馏水清洗蚯蚓表面。加入蚯蚓质量9倍体积的0.86%(m∶V)NaCl溶液,于4 ℃下搅碎。随后将匀浆液以4 000 r·min-1离心10 min。保留上清液以测定蛋白质含量、SOD活性和CAT活性,酶活性单位为U·mg-1,以单位蛋白质量计。
1.3.6 数据处理
使用SPSS 27.0软件对实验数据进行统计分析。采用Probit概率单位回归法算出各浓度梯度及时间下2,4-DCP对蚯蚓的LC50值和置信区间。数值以平均数±标准差呈现,对同一时间不同浓度梯度暴露下蚯蚓各项生物指标进行单因素方差分析(ANOVA),若有显著差异则进行LSD多重比较来检验组间差异显著性,P<0.05、P<0.01表示差异显著。统计图均由Origin 2022软件绘制。
2 结果(Results)
2.1 2,4-DCP对蚯蚓的急性毒性
各浓度梯度下若蚓、成蚓死亡情况数据分别见表1、表2。
表1 2,4-DCP滤纸接触法若蚓死亡情况
Table 1 Death of earthworms of growth stage by 2,4-DCP filter paper contact method
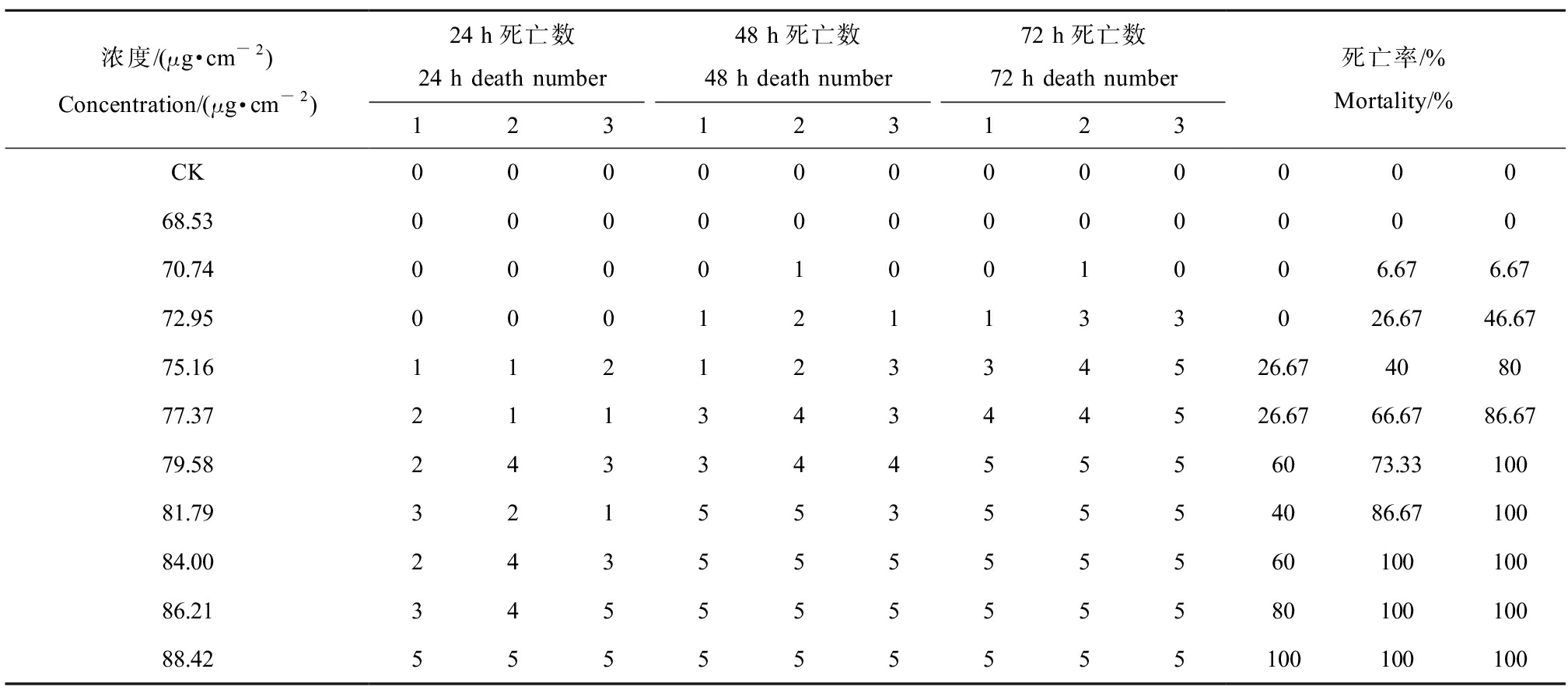
浓度/(μg·cm-2)Concentration/(μg·cm-2)24 h死亡数24 h death number48 h死亡数48 h death number72 h死亡数72 h death number123123123死亡率/%Mortality/%CK00000000000068.5300000000000070.7400001001006.676.6772.95000121133026.6746.6775.1611212334526.67408077.3721134344526.6766.6786.6779.582433445556073.3310081.793215535554086.6710084.002435555556010010086.213455555558010010088.42555555555100100100
注:CK为空白对照组,2,4-DCP表示2,4-二氯酚。
Note: CK is the blank control group, and 2,4-DCP stands for 2,4-dichlorophenol.
表2 2,4-DCP滤纸接触法成蚓死亡情况
Table 2 Death of earthworms of adult stage by 2,4-DCP filter paper contact method
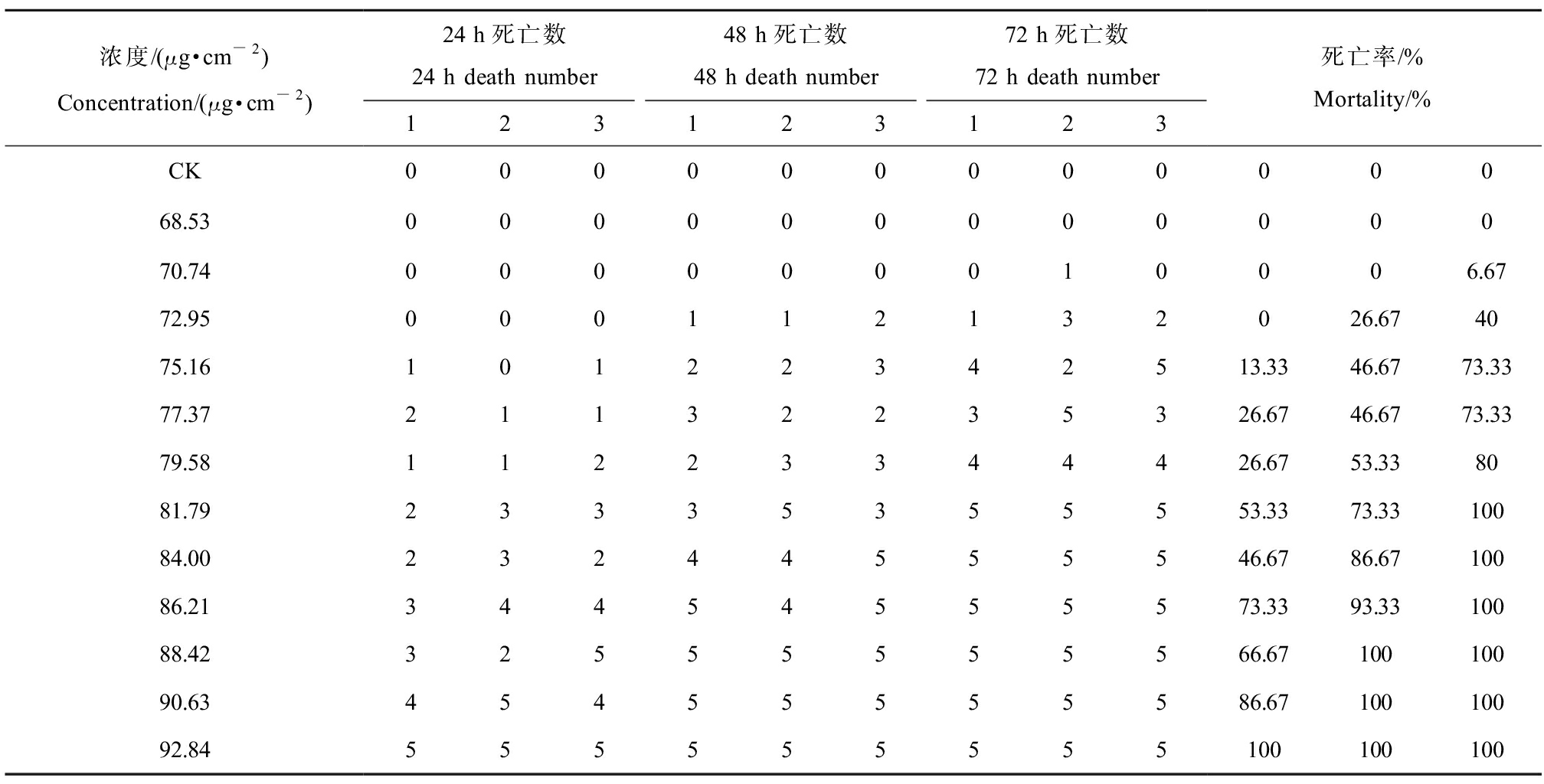
浓度/(μg·cm-2)Concentration/(μg·cm-2)24 h死亡数24 h death number48 h死亡数48 h death number72 h死亡数72 h death number123123123死亡率/%Mortality/%CK00000000000068.5300000000000070.74000000010006.6772.95000112132026.674075.1610122342513.3346.6773.3377.3721132235326.6746.6773.3379.5811223344426.6753.338081.7923335355553.3373.3310084.0023244555546.6786.6710086.2134454555573.3393.3310088.4232555555566.6710010090.6345455555586.6710010092.84555555555100100100
注:CK为空白对照组。
Note: CK is the blank control group.
将表1、表2数据使用SPSS 27.0中Probit程序进行回归性分析,结果如表3所示。当2,4-DCP浓度为70.74 μg·cm-2时,24 h内蚯蚓均无死亡现象,48 h时发现若蚓期蚯蚓出现个别死亡现象。随着2,4-DCP浓度的升高,蚯蚓的死亡率逐渐上升,当浓度高于88.42 μg·cm-2时,24 h后几乎无存活蚯蚓。由SPSS软件算出,若蚓期蚯蚓24 h、48 h、72 h的LC50分别为80.71、76.20、73.64 μg·cm-2,成蚓期蚯蚓24 h、48 h、72 h的LC50分别为82.88、77.63、74.65 μg·cm-2,略高于若蚓期,表明蚯蚓在不同生长阶段对2,4-DCP的耐受度略有差异。
表3 滤纸接触法测得2,4-DCP对若蚓和成蚓的LC50
Table 3 LC50 of 2,4-DCP on growth and adult stage earthworm measured by filter paper contact method
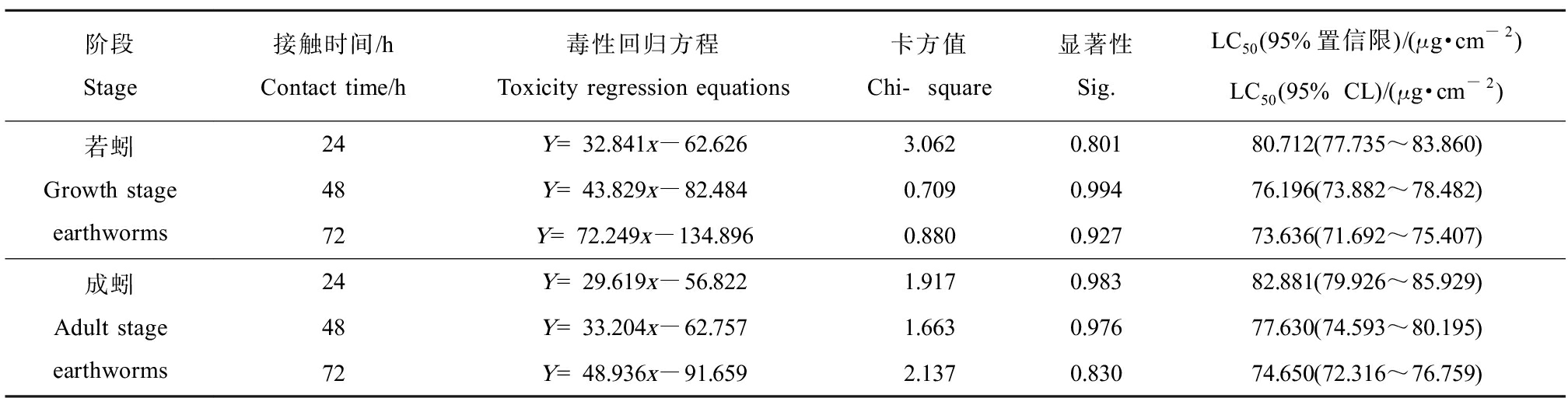
阶段Stage接触时间/hContact time/h毒性回归方程Toxicity regression equations卡方值Chi-square显著性Sig.LC50(95%置信限)/(μg·cm-2)LC50(95% CL)/(μg·cm-2)若蚓Growth stage earthworms24Y=32.841x-62.6263.0620.80180.712(77.735~83.860)48Y=43.829x-82.4840.7090.99476.196(73.882~78.482)72Y=72.249x-134.8960.8800.92773.636(71.692~75.407)成蚓Adult stage earthworms24Y=29.619x-56.8221.9170.98382.881(79.926~85.929)48Y=33.204x-62.7571.6630.97677.630(74.593~80.195)72Y=48.936x-91.6592.1370.83074.650(72.316~76.759)
如图2所示,随着2,4-DCP浓度升高,蚯蚓接触滤纸的反应产生显著变化。空白对照组(图2(a))中,蚯蚓未发生中毒和死亡现象;2,4-DCP暴露浓度为70.74~75.16 μg·cm-2时,虽蚯蚓无明显死亡现象,但滤纸周围出现可见的黄色液体,个别蚯蚓出现浮肿现象(图2(b)),且蚯蚓多出现在滤纸边缘和底部,具有明显逃避行为(图2(c));当2,4-DCP浓度为77.37~79.58 μg·cm-2时,蚯蚓出现蜷缩现象(图2(d)),并且死亡数目明显增多;当浓度为81.79~86.21 μg·cm-2时蚯蚓出现部分环节肿大、躯体颜色变透明、躯体萎缩、断节等现象并大幅度出现死亡(图2(e)、图2(f));当浓度达到88.42~92.48 μg·cm-2时基本均出现生殖环等环节肿大并渗血现象且死亡时间显著缩短(图2(g));试验还发现当2,4-DCP暴露浓度高于92.84 μg·cm-2时,蚯蚓的反应为不停扭动并排出大量黄色液体后死亡(图2(h))。
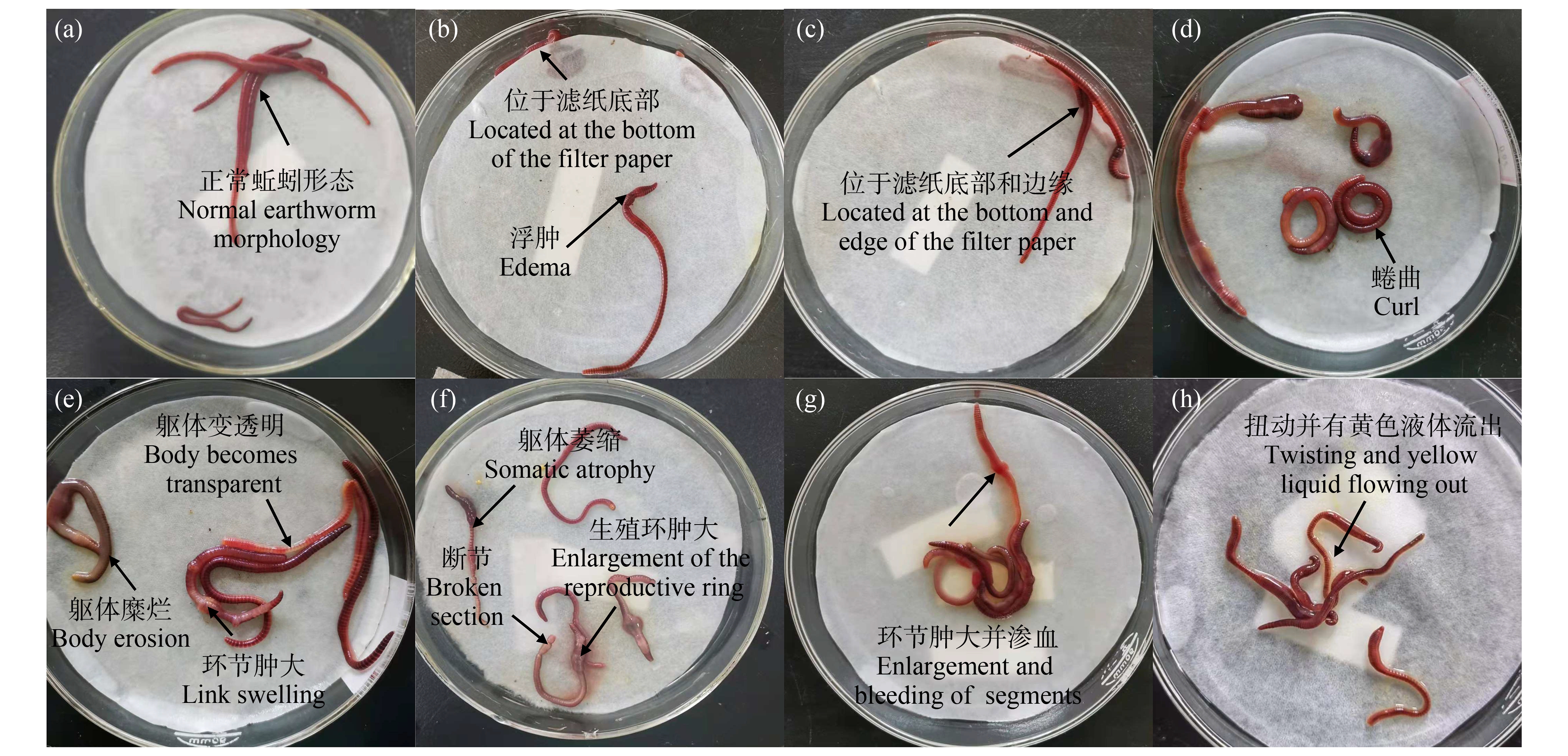
图2 2,4-DCP滤纸接触法蚯蚓中毒现象照片
注:(a) 空白对照组(即正常的蚯蚓);(b), (c) 70.74~75.16 μg·cm-2浓度组;(d) 77.37~79.58 μg·cm-2浓度组;(e), (f) 81.79~86.21 μg·cm-2浓度组;(g) 88.42~92.84 μg·cm-2浓度组;(h) 92.84 μg·cm-2以上浓度组。
Fig. 2 Photos of earthworm poisoning by 2,4-DCP filter paper contact method
Note: (a) CK; (b), (c) 70.74~75.16 μg·cm-2 concentration group; (d) 77.37~79.58 μg·cm-2 concentration group; (e), (f) 81.79~86.21 μg·cm-2 concentration group; (g) 88.42~92.84 μg·cm-2 concentration group; (h) 92.84 μg·cm-2 above concentration group.
2.2 蚯蚓对2,4-DCP污染土壤的趋避效应
两室趋避性试验结果如图3所示,由图3可知,若蚓和成蚓对试验中各浓度2,4-DCP污染土壤的趋避性结果无明显差异。空白对照组中,两室均为清洁土壤,设定此时蚯蚓于两室土壤中分布均匀。随着污染土壤中2,4-DCP浓度升高,清洁土壤中蚯蚓的占比逐渐增大,蚯蚓的趋避性越明显。污染土壤中2,4-DCP浓度达到10 mg·kg-1时,处于清洁土壤中的蚯蚓数目达到投放总数的80%。于15 mg·kg-1及以上浓度的2,4-DCP污染土壤中,也可观察到少数蚯蚓,其大多出现在原先隔板的缝隙处和污染土壤中靠近原先隔板处的土壤表面,并具有和滤纸法染毒相似的中毒症状。
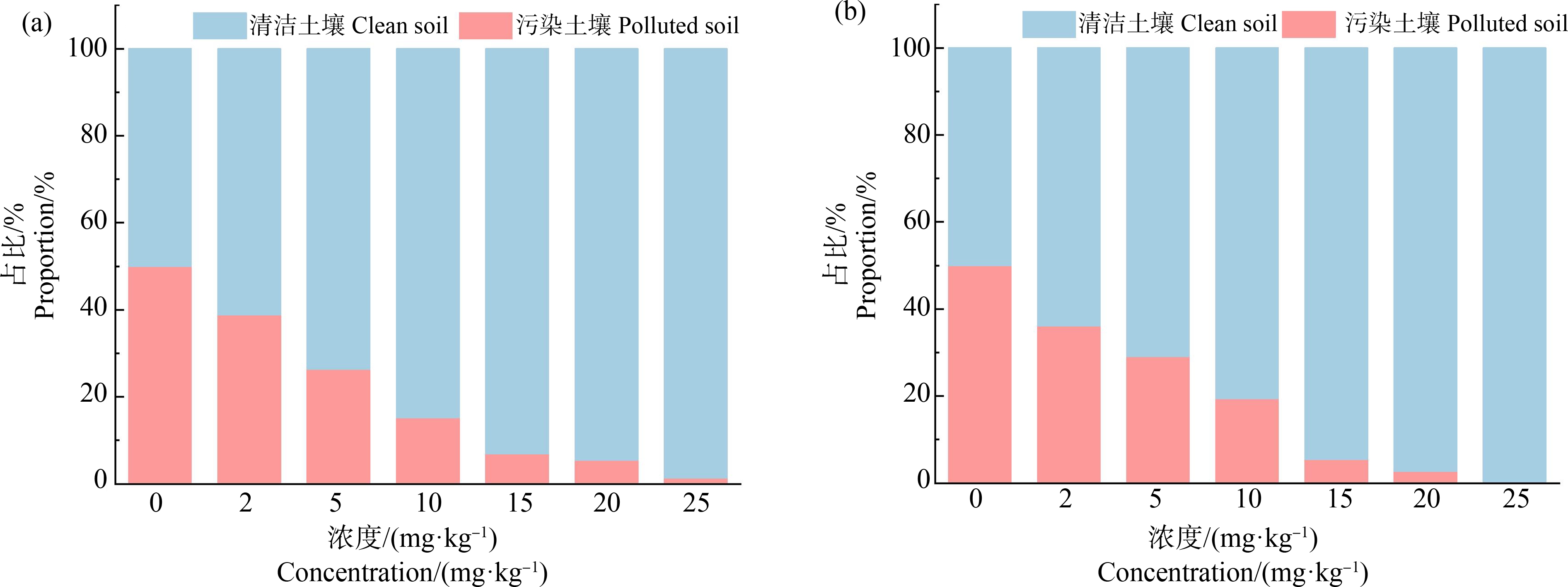
图3 若蚓(a)和成蚓(b)对不同浓度2,4-DCP污染土壤的趋避性
Fig. 3 The repellency of growth stage earthworms (a) and adult stage earthworms (b) from soils contaminated by 2,4-DCP at different concentrations
2.3 2,4-DCP对蚯蚓细胞组织的影响
暴露于2,4-DCP中的蚯蚓体壁和肠道组织细胞结构变化见图4。如图4(a)和图4(d)所示,空白对照组中蚯蚓体壁和肠道组织细胞具有规则和完整的细胞结构。如图4(b)和图4(c)所示,暴露于70.74 μg·cm-2和88.42 μg·cm-2浓度下24 h蚯蚓体壁组织细胞结构,与空白对照组相比,蚯蚓表皮受损,结构完整性消失,部分环肌出现细胞形态和肌纤维组织的消失和坏死,细胞间隙扩大,在纵肌也观察到相同变化,但环肌和纵肌变化均不明显(图4(b))。如图4(c)所示,蚯蚓表皮出现严重的溃烂和坏死,部分部位的上皮细胞近乎完全分解,与空白对照组比较,其表皮、纵肌的组织和染色特征均有显著变化,大面积坏死,横肌萎缩,纵肌出现大量空隙,结构完整性发生彻底变化。同时,如图4(e)和图4(f)所示,蚯蚓的黄色细胞和肠上皮组织也发生了组织病理学变化。在暴露于70.74 μg·cm-2和88.42 μg·cm-2浓度下24 h蚯蚓肠道组织细胞结构变化图中,可以观察到黄色细胞发生明显破损,细胞开始破裂并失去形状,但肠上皮细胞未受明显影响(图4(e))。随着浓度的升高,黄色细胞和肠上皮细胞均出现坏死迹象,且与空白对照组相比,黄色细胞和肠上皮细胞彻底变性,肠上皮细胞几乎完全坏死,绒毛变疏松并与肠壁分离(图4(f))。
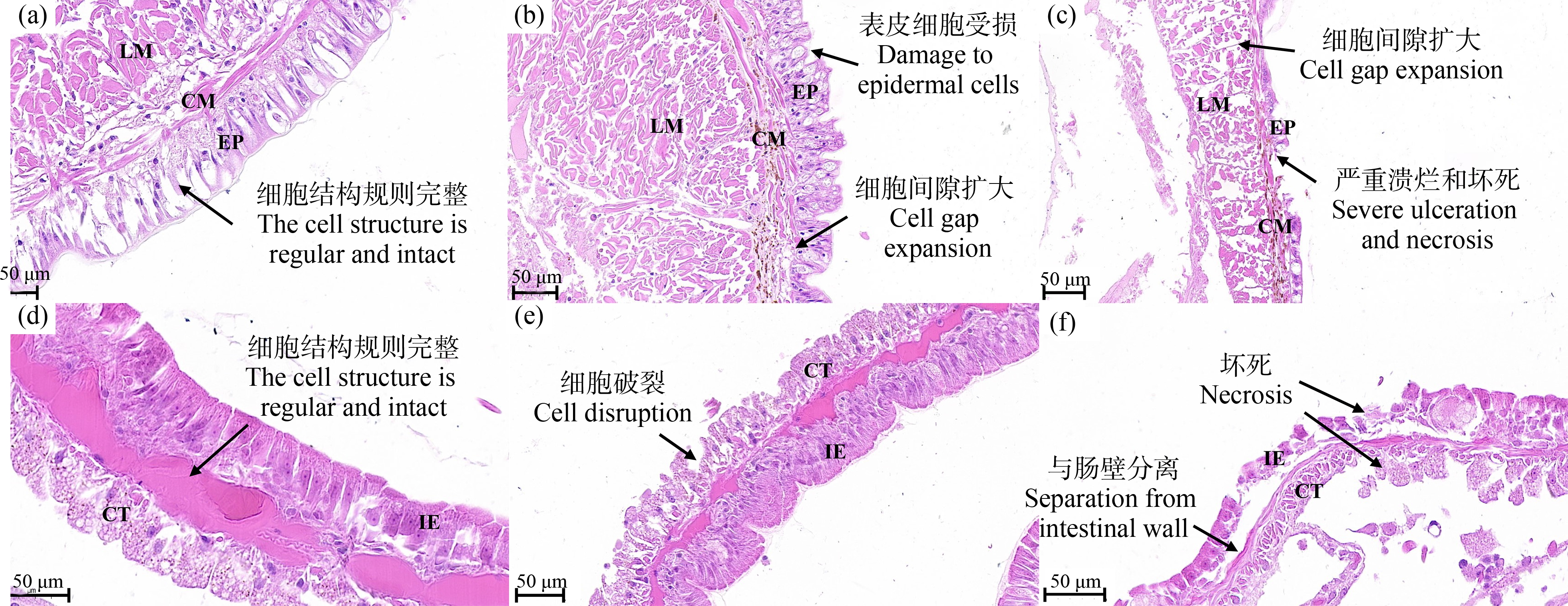
图4 蚯蚓表皮和肠道细胞组织变化图
注:(a), (d) 空白对照组(即正常的蚯蚓体壁和肠道结构);(b), (e) 70.74 μg·cm-2浓度暴露24 h蚯蚓表皮和肠道结构;(c), (f) 88.42 μg·cm-2浓度暴露24 h蚯蚓表皮和肠道结构;EP表示表皮,CM表示环肌,LM表示纵肌,IE表示肠上皮细胞,CT表示黄色细胞。
Fig. 4 Changes in the epidermis and intestinal cells of earthworms
Note: (a), (d) The body wall and intestinal structure of earthworm in CK; (b), (e) Epidermis and intestinal structure of earworms exposed to 70.74 μg·cm-2 for 24 h; (c), (f) Epidermis and intestinal structure of earworms exposed to 88.42 μg·cm-2 for 24 h; EP means epidermis; CM means circular muscle; LM means longitudinal muscle; IE means intestinal epithelium; CT means chloragogenous tissue.
2.4 2,4-DCP对蚯蚓体内蛋白质含量的影响
如图5所示,各浓度2,4-DCP暴露下,若蚓期和成蚓期蚯蚓接触滤纸24 h时,其体内的蛋白质含量均高于空白对照组,随浓度增加呈现先上升后降低的趋势,且均于39.79 μg·cm-2浓度达到峰值,与空白对照组具有显著差异(P<0.01);低浓度(13.26 μg·cm-2、26.52 μg·cm-2)暴露下,若蚓体内蛋白质含量高于成蚓,可见在试验前24 h内若蚓比成蚓对低浓度2,4-DCP表现更敏感。48 h和72 h蚯蚓体内的蛋白质含量随浓度增加无明显规律性,同一浓度下24、48和72 h之间差异随着浓度升高而增大,中、高浓度(39.79 μg·cm-2、53.05 μg·cm-2)暴露下其变化幅度明显高于低浓度。
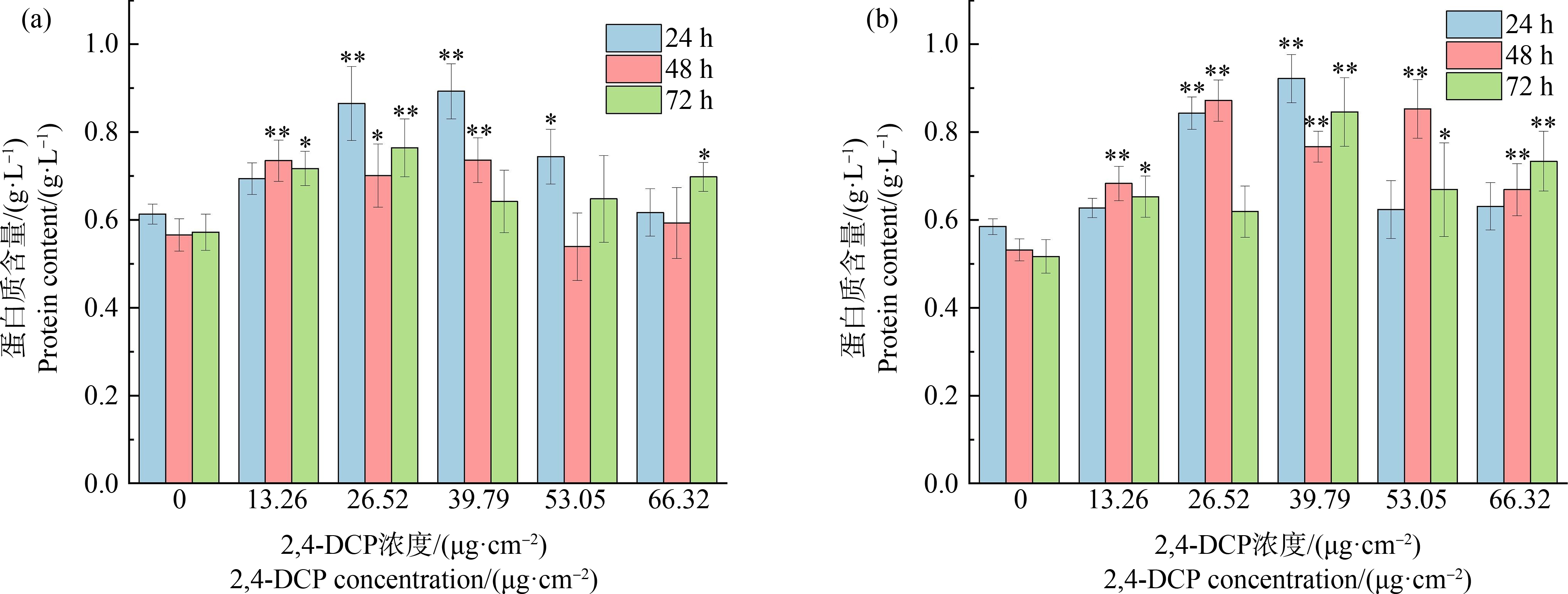
图5 若蚓(a)和成蚓(b)体内蛋白质含量的变化
注:与对照组相比差异显著,*表示P<0.05,**表示P<0.01。
Fig. 5 Changes of protein content in growth stage earthworms (a) and adult stage earthworms (b)
Note: Compare with the control, there is significant difference; *represents P<0.05, and **represents P<0.01.
2.5 2,4-DCP对蚯蚓体内SOD活性的影响
试验期间蚯蚓体内SOD活性变化见图6。如图6(a)所示,3个时间段若蚓体内SOD活性随浓度升高均呈现出先上升后下降的趋势。24 h时,低浓度(13.26 μg·cm-2、26.52 μg·cm-2)暴露下,若蚓体内SOD活性显著高于空白对照组(P<0.05),而高浓度(53.05 μg·cm-2、66.32 μg·cm-2)则显著低于空白对照组(P<0.01),表现出明显的低剂量刺激和高剂量抑制效应。随着暴露时间的延长,各浓度下蚯蚓体内SOD活性总体呈现下降趋势。成蚓体内SOD活性变化见图6(b),其总体变化规律与若蚓相似,暴露于13.26 μg·cm-2和39.79 μg·cm-2浓度下24 h时,若蚓体内SOD活性高于成蚓,而高浓度(53.05 μg·cm-2、66.32 μg·cm-2)暴露下各时间段却均低于成蚓。
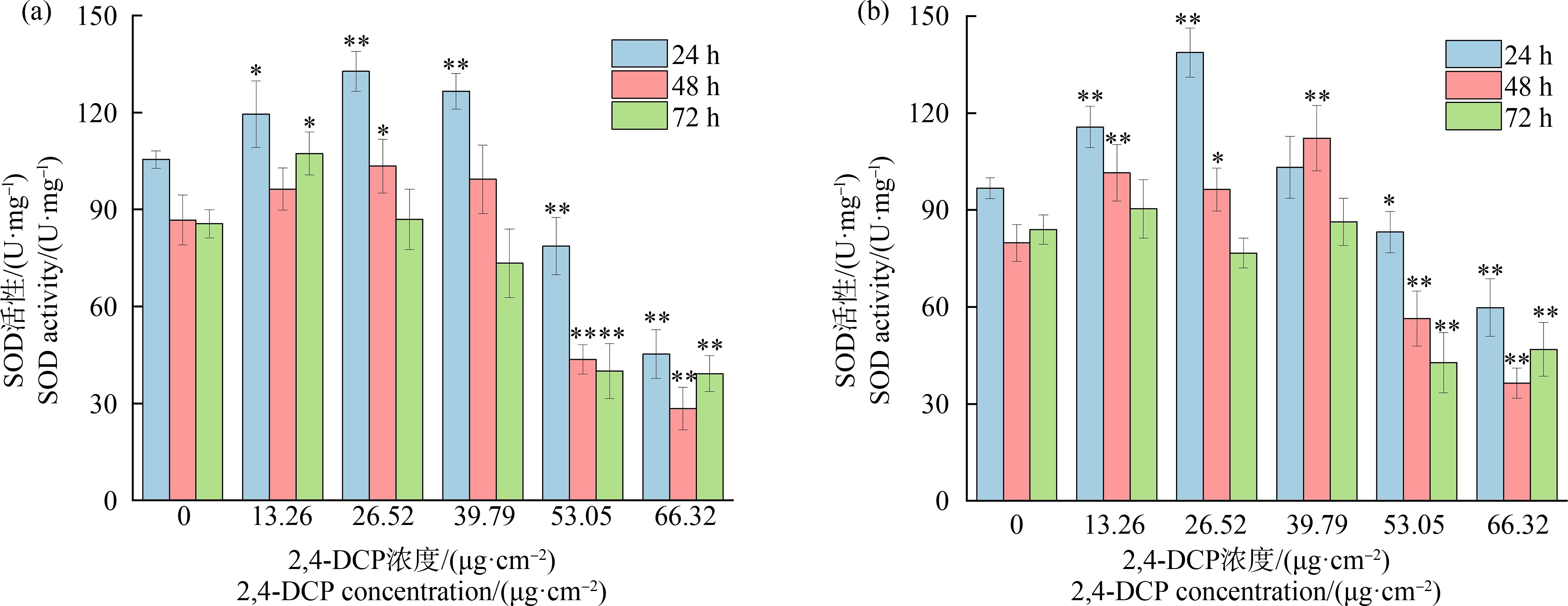
图6 若蚓(a)和成蚓(b)体内SOD活性变化
注:与对照组相比差异显著,*表示P<0.05,**表示P<0.01。
Fig. 6 Changes of SOD content in growth stage earthworms (a) and adult stage earthworms (b)
Note: Compare with the control, there is significant difference; *represents P<0.05, and **represents P<0.01.
2.6 2,4-DCP对蚯蚓体内CAT活性的影响
由图7可知,各浓度2,4-DCP暴露下,蚯蚓体内CAT活性基本均高于空白对照组,表明2,4-DCP激发了蚯蚓体内的CAT活性。同浓度梯度下,48 h时CAT活性均高于24 h,而48 h后大多有所下降。若蚓体内CAT活性变化如图7(a)所示,试验的3个时间段若蚓体内CAT活性随浓度升高均呈现先上升后降低趋势,24 h于53.05 μg·cm-2达到峰值,而48 h和72 h于39.79 μg·cm-2处达到峰值,均与空白对照组具有显著差异(P<0.01)。成蚓体内CAT活性变化如图7(b)所示,其各时间段变化趋势与若蚓相似,24 h成蚓体内CAT活性达到峰值处的2,4-DCP浓度与若蚓相同,但48 h和72 h达到峰值处浓度高于若蚓,为53.05 μg·cm-2。
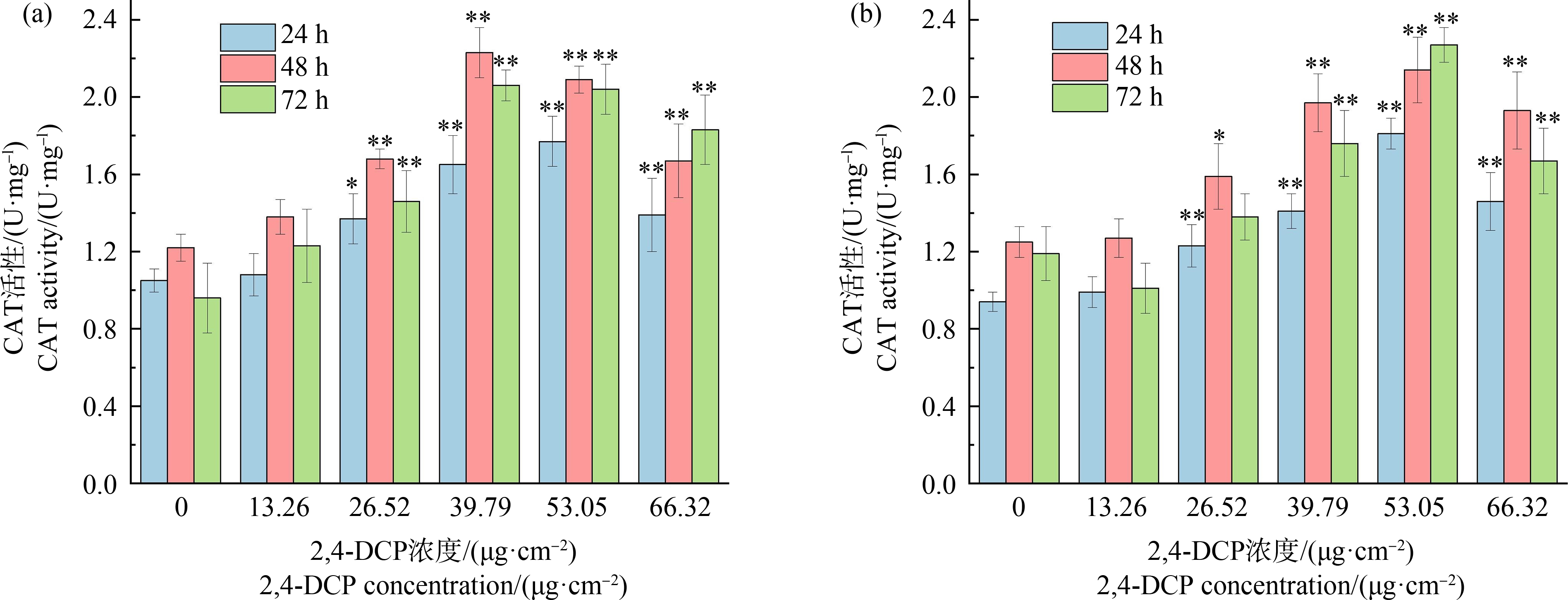
图7 若蚓(a)和成蚓(b)体内CAT活性变化
注:与对照组相比差异显著,*表示P<0.05,**表示P<0.01。
Fig. 7 Changes of CAT content in growth stage earthworms (a) and adult stage earthworms (b)
Note: Compare with the control, there is significant difference; *represents P<0.05, and **represents P<0.01.
3 讨论(Discussion)
2,4-DCP作为农药、除草剂和杀虫剂的主要合成原料之一,在我国被广泛使用,已普遍存在于农业土壤和自然水体中。Fan等[19]和Gao等[20]对近几年来我国地表水2,4-DCP的污染状况进行了调查,其中2,4-DCP在黄河、淮河和海河等流域中被频繁发现,具有不容忽视的潜在健康风险。目前,已有较多文献报道了2,4-DCP对水生动物的毒性效应,Tsukazawa等[21]的研究表明,斑马鱼幼鱼暴露于2,4-DCP浓度为2.5 mg·L-1的水体中5 d时,可观察到脂质积累和ROS诱导;Hu等[22]发现,2,4-DCP可通过干扰性激素合成来诱导鱼类雌性化。但目前关于2,4-DCP对土壤环境中生物的影响却鲜有报道,蚯蚓作为评价土壤环境的指示生物,其表现出的毒性效应对2,4-DCP污染的早期预警和生态危险评估具有重要作用。
本研究采用滤纸接触法和两室趋避试验观察了2,4-DCP对若蚓期和成蚓期2个生长阶段赤子爱胜蚓的毒性效应。滤纸接触法可使蚯蚓与滤纸上的污染物直接接触,从而快速、准确地评估出毒物对细胞的毒性效应[23]。两室趋避试验则可以灵敏地反映出蚯蚓对污染物的趋避响应,已广泛用于各种土壤污染评估中。
蚯蚓的很多感官细胞对化学刺激非常敏感,在滤纸接触试验中,污染物附着在滤纸的表面,并经由蚯蚓的皮肤进入体腔,被输送至躯体的各个部位,从而引起中毒[24]。试验过程中,随着2,4-DCP浓度的增加,蚯蚓出现了浮肿、蜷缩、生殖环等部分环节肿大并渗血、躯体萎缩、断节、黄色液体渗出和躯体颜色变透明等现象。主要原因为2,4-DCP在与蚯蚓皮肤接触后,与体腔内的细胞发生交互作用,从而产生上述现象。蚯蚓流出的黄色液体和血水,很有可能是体腔液中的一种成分,受到污染物刺激后,从角质膜的小孔中排出,浮肿、蜷曲等反应都是蚯蚓对抗外来污染物的自身防御机制[25]。
蚯蚓与潜在的致命和非致命源接触时,会产生巴普洛夫条件反射,从而避免污染,这就是所谓的“趋避”[26]。土壤生态风险评价中,蚯蚓对污染物的趋避效应较于急性毒性更为敏感,可快速诊断环境风险[27]。相关研究表明,趋避实验中,若清洁土壤中的蚯蚓数高于投加总数的80%,即趋避行为明显,该污染浓度下不宜蚯蚓生存[28]。本次两室趋避实验中,当土壤中2,4-DCP浓度达到10 mg·kg-1时,清洁土壤中若蚓和成蚓的比例均高于80%,即表现出明显的趋避行为;蚯蚓接触到各浓度2,4-DCP污染土壤的48 h内,做出了不同程度的趋避响应,土壤中2,4-DCP浓度越高,蚯蚓的趋避性越强;若蚓和成蚓对各浓度2,4-DCP的趋避性结果无明显差异,但试验过程中发现,48 h后于高浓度梯度下(20 mg·kg-1、25 mg·kg-1),隔板缝隙处仍然存在一些蚯蚓,其中若蚓较多,导致高浓度梯度下若蚓趋避性小于成蚓。此现象表明,若蚓趋避恶劣环境及生存能力可能不及成蚓。
近年来,蚯蚓的组织病理学改变在毒理学中的应用日益广泛。组织细胞的改变依赖于机体对损伤的修复能力、污染物的性质以及接触的持续时间[29]。表皮是抵抗外界污染的首要防线,一旦上皮细胞受到损伤,污染物很可能会堆积在表皮并侵入到生物的躯体里[30-31]。本试验中发现,同一时间下,随2,4-DCP浓度增加,蚯蚓表皮先于环肌和纵肌发生损伤,暴露浓度越高,蚯蚓表皮呈现出的损伤越大。目前已有研究证明,金属对暴露在污染土壤中的蚯蚓造成损伤,主要是透过表皮并在蚯蚓的环肌和纵肌中积累[32-33]。本试验中,70.74 μg·cm-2浓度2,4-DCP暴露下,蚯蚓表皮观察到明显损伤,环肌和纵肌变化均不显著,而随浓度升高(88.42 μg·cm-2)则出现蚯蚓表皮丧失完整性结构、环肌萎缩和纵肌处空隙增多等现象,具有与之相似效应。众所周知,肠道在吸收消化营养物质和维持蚯蚓足够的生物量方面起着重要作用,肠组织中的黄色细胞具有排泄功能,并参与氮代谢,除了可以积累外源物质外,还可储存糖原和脂肪[34]。相关研究表明,蚯蚓的黄色细胞不仅是聚集重金属的主要部位,也可以累积有机污染物[35-36],此外,黄色细胞在受到污染物损害后,也会降低其贮存污染物的能力[37]。本研究中,随着2,4-DCP暴露浓度和时间的增加,蚯蚓的黄色细胞层和肠上皮细胞均发生明显退化,且高浓度暴露下,黄色细胞和肠上皮细胞出现松散并脱落的现象,与Zhang等[38]研究多氯联苯(PCB)暴露下产生的结果相似。
蚯蚓遭受不良因素的迫害后,其躯体的各机能都会受到损伤,这种情况下,体内会产生一些应激蛋白,导致体内蛋白质含量增加[39-40],这也是本试验中低浓度(13.26 μg·cm-2、26.52 μg·cm-2)、短时间(24 h)内蚯蚓体内蛋白质增加的原因。本试验中,若蚓和成蚓体内蛋白质含量于24 h时随浓度升高均呈现出先上升后降低趋势,且低浓度长时间(48 h、72 h)与高浓度(53.05 μg·cm-2、66.32 μg·cm-2)短时间处数值相似,表明蚯蚓体内蛋白质受到2,4-DCP影响具有浓度与时间的综合作用。随着暴露时间的延长,蚯蚓的许多器官功能受到损害,破坏了体内蛋白质的合成途径[40],故各浓度下蚯蚓体内的蛋白质含量大多随时间呈现出不同程度的下降趋势。而48 h和72 h时各浓度下蚯蚓体内蛋白质含量相较于空白对照组无明显规律性,且个别浓度下两者差异性较大,这缘于长时间暴露下蚯蚓机体功能受损,体内环境紊乱,代谢活动不正常导致[41-42]。
活性氧(ROS)一般由正常的新陈代谢过程生成,在正常环境中,ROS的生成和消失维持着一个动态的平衡[43-44]。但是,一旦有机体接触到环境污染,就会破坏这种动态平衡[45]。SOD是一种在超氧阴离子向H2O2转化过程中起着关键作用的金属酶,是蚯蚓抗御ROS的首要防御机制[46]。图6中,低浓度2,4-DCP处理24 h导致蚯蚓SOD活性增加,而高浓度暴露下却抑制了SOD活性,表明在低浓度2,4-DCP处理24 h内,蚯蚓体内的SOD能够保护细胞免受ROS的损伤,此时蚯蚓体内具有清除ROS的能力。随着暴露浓度和时间的增加,蚯蚓体内SOD活性显著低于对照组,表现出明显的抑制作用,表明达到一定浓度或时间,超过了蚯蚓的自我修复能力,最后被ROS破坏,反映出蚯蚓自我修复体系的缺陷[47]。经过SOD催化超氧阴离子向H2O2的转化过程后,H2O2等自由基在其他重要酶如CAT等的作用下被清除[48]。CAT存在于线粒体、过氧化物酶体和细胞质中,可将H2O2分解为H2O和O2[49]。在本研究中,各浓度下所有时间段CAT活性几乎均高于空白对照组,说明在2,4-DCP的诱导下,蚯蚓体内CAT活性呈激活状态。24 h各浓度下CAT活性均低于48 h,表明24 h内蚯蚓所受2,4-DCP应激产生的H2O2少于48 h。66.32 μg·cm-2浓度下各时间段CAT活性显著低于53.05 μg·cm-2,表明高浓度暴露下CAT活性相对受到抑制,与SOD变化相似,这缘于此时细胞内存在大量的H2O2,导致CAT的大量消耗和清除能力下降所致[50]。
本试验中各生物标志物关系如图8所示,蚯蚓与2,4-DCP接触后,首先表现出趋避行为,当无法回避污染时,2,4-DCP通过蚯蚓的表皮进入到蚯蚓体内,2,4-DCP浓度越高,蚯蚓短时间内受到的刺激越大,这时体内产生大量的ROS,同时分泌出大量的应激蛋白以缓解环境压力。为消除体内多余的ROS,蚯蚓体内的抗氧化酶系统(SOD和CAT等)被激活,使躯体免受ROS的损伤。低水平暴露下2,4-DCP引起的氧化应激可被蚯蚓的抗氧化系统平衡和缓解,但当暴露浓度过高或时间较长时,蚯蚓体内的抗氧化系统便不足以完全消除大量的ROS,并受到抑制,从而造成细胞损伤,使机体的适应性和健康程度下降,进而引起中毒现象。
图8 各生物标志物关系图
Fig. 8 Relationship diagram of biomarker
史志明[51]研究发现,蚯蚓处于不同生长阶段时,其体内的抗氧化酶系统也存在差异。本研究中,在低浓度2,4-DCP胁迫下,若蚓体内蛋白质含量、SOD活性和CAT活性均高于成蚓,表明较于成蚓,若蚓体内的抗氧化系统更容易被激活。低浓度长时间暴露下,若蚓和成蚓的SOD活性、CAT活性与对照组相比基本无显著差异,表明若蚓和成蚓的抗氧化系统均可以缓解低浓度2,4-DCP胁迫造成的压力。而在高浓度长时间的2,4-DCP胁迫下,若蚓体内SOD活性、CAT活性却低于成蚓,表明若蚓的抗氧化系统更易受到抑制。同时,由图7可知,成蚓于48 h和72 h体内CAT活性达到峰值处2,4-DCP浓度均高于若蚓,峰值处的浓度即为蚯蚓体内CAT清除能力最强时的浓度,而超过该浓度则存在不同程度的抑制情况,同样可以证实上述观点。两室趋避试验结果中,虽若蚓和成蚓表现出的趋避性差异不明显,但面对高浓度2,4-DCP污染时,若蚓趋避恶劣环境及生存能力却不及成蚓。目前,关于若蚓和成蚓及蚯蚓其他生长阶段的研究尚少。当若蚓和成蚓处于同一污染环境下,两者受到相同的环境污染胁迫时,其体内要产生与污染程度相抗衡的解毒机制以避免躯体受到损伤,由于若蚓体积相对成蚓小以及抗氧化系统的差异,其体内产生的解毒效应需高于成蚓,随着污染物浓度和暴露时间的增加,若蚓体内的解毒机制便先于成蚓达到极限,出现崩盘局面,从而使若蚓先于成蚓受到污染物的损伤。本试验数据表明,若蚓期和成蚓期这2个生长阶段的蚯蚓对2,4-DCP具有不同的应激效应,其耐受能力也存在差异。
[1] Dubey M, Kumar R, Srivastava S K, et al. ZnO/α-MnO2 hybrid 1D nanostructure-based sensor for point-of-care monitoring of chlorinated phenol in drinking water [J]. Materials Today Chemistry, 2022, 26: 101098
[2] 杨柳, 王名威, 张耀斌. 磁铁矿负载生物炭强化厌氧微生物处理2,4-二氯苯酚废水[J]. 化工进展, 2022, 41(9): 5065-5073
Yang L, Wang M W, Zhang Y B. Magnetite-loaded biochar for enhanced anaerobic microbial treatment of 2,4-dichlorophenol wastewater [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 5065-5073 (in Chinese)
[3] Fernandez M E, del Rosario Morel M, Clebot A C, et al. Effectiveness of a simple biomixture for the adsorption and elimination of 2,4-dichlorophenoxyacetic acid (2,4-D) herbicide and its metabolite, 2,4-dichlorophenol (2,4-DCP), for a biobed system [J]. Journal of Environmental Chemical Engineering, 2022, 10(1): 106877
[4] Dubey M, Kumar R, Srivastava S K, et al. Visible light induced photodegradation of chlorinated organic pollutants using highly efficient magnetic Fe3O4/TiO2 nanocomposite [J]. Optik, 2021, 243: 167309
[5] Prabhu K, Malode S J, Kulkarni R M, et al. Electro-sensing base for hazardous pesticide 2,4-DCP and its quantification in real samples at ZnO@Cu core-shell nanoparticles in the presence of cationic surfactant [J]. Materials Chemistry and Physics, 2022, 278: 125705
[6] Zeb A, Li S, Wu J N, et al. Insights into the mechanisms underlying the remediation potential of earthworms in contaminated soil: A critical review of research progress and prospects [J]. The Science of the Total Environment, 2020, 740: 140145
[7] 邵将, 张宗鹏, 谢宇震, 等. 蚯蚓在生态毒理试验中的应用研究[J]. 农业与技术, 2020, 40(16): 24-26
Shao J, Zhang Z P, Xie Y Z, et al. Study on the application of earthworm in ecotoxicological experiment [J]. Agriculture and Technology, 2020, 40(16): 24-26 (in Chinese)
[8] He F L, Wan J Q, Chu S S, et al. Toxic mechanism on phenanthrene-triggered cell apoptosis, genotoxicity, immunotoxicity and activity changes of immunity protein in Eisenia fetida: Combined analysis at cellular and molecular levels [J]. The Science of the Total Environment, 2022, 819: 153167
[9] 宋国欣. 现代生物技术在环境监测中的应用探究[J]. 科技风, 2021(18): 125-126
[10] García-Gómez C, Babín M, García S, et al. Joint effects of zinc oxide nanoparticles and chlorpyrifos on the reproduction and cellular stress responses of the earthworm Eisenia andrei [J]. Science of the Total Environment, 2019, 688: 199-207
[11] 郭佳葳, 周世萍, 刘守庆, 等. 蚯蚓生物标志物在土壤生态系统监测中的应用研究进展[J]. 生态毒理学报, 2020, 15(5): 69-81
Guo J W, Zhou S P, Liu S Q, et al. Advances in application of earthworm biomarkers in monitoring soil ecosystem [J]. Asian Journal of Ecotoxicology, 2020, 15(5): 69-81 (in Chinese)
[12] Tang W T, Wang G Y, Zhang S R, et al. Physiochemical responses of earthworms (Eisenia fetida) under exposure to lanthanum and cerium alone or in combination in artificial and contaminated soils [J]. Environmental Pollution, 2022, 296: 118766
[13] Wu R L, Zhou T T, Wang J, et al. Oxidative stress and DNA damage induced by trifloxystrobin on earthworms (Eisenia fetida) in two soils [J]. Science of the Total Environment, 2021, 797: 149004
[14] 王勤, 侯春霞, 程亚媛, 等. 茂名市白纹伊蚊对常用杀虫剂的抗性调查[J]. 中华卫生杀虫药械, 2021, 27(4): 318-320
Wang Q, Hou C X, Cheng Y Y, et al. Resistance of Aedes albopictus to the commonly used insecticides in Maoming City [J]. Chinese Journal of Hygienic Insecticides &Equipments, 2021, 27(4): 318-320 (in Chinese)
[15] 黄轶, 闫振广, 张天旭, 等. 我国水质基准制定中生态毒性数据质量评估方法研究[J]. 环境工程技术学报, 2021, 11(1): 122-128
Huang Y, Yan Z G, Zhang T X, et al. Evaluation method of ecotoxicity data quality for deriving water quality criteria in China [J]. Journal of Environmental Engineering Technology, 2021, 11(1): 122-128 (in Chinese)
[16] Organization for Economic Co-operation and Development. Guideline for Testing of Chemicals No.207, Earthworm Acute Toxicity Tests [S]. Paris: Organization for Economic Co-operation and Development, 1984
[17] Organization for Economic Co-operation and Development. Guideline for Testing of Chemicals No 222, Earthworm Reproduction Test (Eisenia fetida/andrei) [S]. Paris: Organization for Economic Co-operation and Development, 2016
[18] International Organization for Standardization. Draft: Soil Quality-Avoidance Test for Determining the Quality of Soils and Effects of Chemicals on Behaviour-Part 1: Test with Earthworms (Eisenia fetida/andrei) [S]. Geneva: International Organization for Standardization, 2008
[19] Fan B, Wang X N, Xie Z Y, et al. Aquatic life criteria &human health ambient water quality criteria derivations and probabilistic risk assessments of 7 benzenes in China [J]. Chemosphere, 2021, 274: 129784
[20] Gao J J, Liu L H, Liu X R, et al. Levels and spatial distribution of chlorophenols - 2,4-dichlorophenol, 2,4,6-trichlorophenol, and pentachlorophenol in surface water of China [J]. Chemosphere, 2008, 71(6): 1181-1187
[21] Tsukazawa K S, Li L, Tse W K F. 2,4-dichlorophenol exposure induces lipid accumulation and reactive oxygen species formation in zebrafish embryos [J]. Ecotoxicology and Environmental Safety, 2021, 230: 113133
[22] Hu Y, Li D, Ma X, et al. Effects of 2,4-dichlorophenol exposure on zebrafish: Implications for the sex hormone synthesis [J]. Aquatic Toxicology, 2021, 236: 105868
[23] Zhang L J, Hu C W, Wang W L, et al. Acute toxicity of multi-walled carbon nanotubes, sodium pentachlorophenate, and their complex on earthworm Eisenia fetida [J]. Ecotoxicology and Environmental Safety, 2014, 103: 29-35
[24] Jeyaprakasam A, Muniyandi B, James A J P, et al. Assessment of earthworm diversity and pesticide toxicity in Eudrilus eugeniae [J]. Environmental Chemistry and Ecotoxicology, 2021, 3: 23-30
[25] Duo L, Wang Y L, Zhao S L. Individual and histopathological responses of the earthworm (Eisenia fetida) to graphene oxide exposure [J]. Ecotoxicology and Environmental Safety, 2022, 229: 113076
[26] Wilson W J, Ferrara N C, Blaker A L, et al. Escape and avoidance learning in the earthworm Eisenia hortensis [J]. PeerJ, 2014, 2: e250
[27] 刘嫦娥, 孟祥怀, 秦媛儒, 等. 乙草胺胁迫下蚯蚓活动对土壤-作物系统影响研究[J]. 环境生态学, 2020, 2(12): 8-14
Liu C E, Meng X H, Qin Y R, et al. Effects of acetochlor stress on soil-crop system in the presence of Eisenia foetida [J]. Environmental Ecology, 2020, 2(12): 8-14 (in Chinese)
[28] 李芬, 林雪儿, 黄慧雯, 等. 探究蚯蚓对食用油污染土壤的回避行为[J]. 中学生物教学, 2020(23): 67-69
Li F, Lin X E, Huang H W, et al. Explore the avoidance behavior of earthworms to edible oil contaminated soil [J]. Teaching of Middle School Biology, 2020(23): 67-69 (in Chinese)
[29] Haschek W M, Rousseaux C G, Wallig M A, et al. Chapter 1-Toxicologic Pathology: An Introduction [M]//Haschek W M, Rousseaux C G, Wallig M A, et al. Haschek and Rousseaux’s Handbook of Toxicologic Pathology (Fourth Edition). Academic Press. 2022: 1-12
[30] Adeel M, Shakoor N, Hussain T, et al. Bio-interaction of nano and bulk lanthanum and ytterbium oxides in soil system: Biochemical, genetic, and histopathological effects on Eisenia fetida [J]. Journal of Hazardous Materials, 2021, 415: 125574
[31] Shi Y J, Shi Y J, Zheng L S. Individual and cellular responses of earthworms (Eisenia fetida) to endosulfan at environmentally related concentrations [J]. Environmental Toxicology and Pharmacology, 2020, 74: 103299
[32] Wang G H, Xia X Q, Yang J, et al. Exploring the bioavailability of nickel in a soil system: Physiological and histopathological toxicity study to the earthworms (Eisenia fetida) [J]. Journal of Hazardous Materials, 2020, 383: 121169
[33] Adeel M, Ma C X, Ullah S, et al. Exposure to nickel oxide nanoparticles insinuates physiological, ultrastructural and oxidative damage: A life cycle study on Eisenia fetida [J]. Environmental Pollution, 2019, 254: 113032
[34] 杨斌, 徐阳, 段睿洁, 等. 氯氰菊酯对蚯蚓处理蔬菜废弃物过程中的毒性效应研究[J]. 云南农业大学学报(自然科学), 2021, 36(4): 640-647
Yang B, Xu Y, Duan R J, et al. Study on the toxic effect of cypermethrin on using earthworm to treat vegetable waste [J]. Journal of Yunnan Agricultural University (Natural Science), 2021, 36(4): 640-647 (in Chinese)
[35] Giovanetti A, Fesenko S, Cozzella M L, et al. Bioaccumulation and biological effects in the earthworm Eisenia fetida exposed to natural and depleted uranium [J]. Journal of Environmental Radioactivity, 2010, 101(6): 509-516
[36] Gautam K, Seth M, Dwivedi S, et al. Soil degradation kinetics of oxybenzone (benzophenone-3) and toxicopathological assessment in the earthworm, Eisenia fetida [J]. Environmental Research, 2022, 213: 113689
[37] 段晓尘. 重金属和有机污染物对赤子爱胜蚓(Eisenia fetida)的生态毒理效应及机制差异[D]. 南京: 南京农业大学, 2015: 76-78
Duan X C. Ecotoxicological effects and different mechanisms of heavy metal and organic pollutants on earthworm (Eisenia fetida) [D]. Nanjing: Nanjing Agricultural University, 2015: 76-78 (in Chinese)
[38] Zhang J Y, He M Y, Liu Y X, et al. Chlorine substitution-dependent toxicities of polychlorinated biphenyls to the earthworm Eisenia fetida in soil [J]. Journal of Environmental Sciences (China), 2023, 128: 171-180
[39] 高晨昕, 朱艳, 郭梦炜, 等. 杀螟丹与Pb对赤子爱胜蚓的联合毒性效应[J]. 生态毒理学报, 2020, 15(6): 212-222
Gao C X, Zhu Y, Guo M W, et al. Joint toxicity of cartap and Pb on earthworms (Eisenia foetida) [J]. Asian Journal of Ecotoxicology, 2020, 15(6): 212-222 (in Chinese)
[40] 王雪佳. 蚯蚓-高羊茅联合修复镉污染土壤研究[D]. 杨凌: 西北农林科技大学, 2022: 27-29
Wang X J. Study on the effect of combined remediation by earthworm-Festuca arundinacea on soil contaminated cadmium [D]. Yangling: Northwest A &F University, 2022: 27-29 (in Chinese)
[41] 孙仕仙, 陶瑞, 张庆蛟, 等. 乐果和杀虫双污染对蚯蚓体内蛋白质含量的影响[J]. 江西农业大学学报, 2012, 34(2): 298-303
Sun S X, Tao R, Zhang Q J, et al. Effects of dimethoate and dimehypo pollution on protein content of earthworm [J]. Acta Agriculturae Universitatis Jiangxiensis, 2012, 34(2): 298-303 (in Chinese)
[42] 王银翠, 周国娜, 张斌, 等. 油松毛虫取食和剪叶刺激胁迫下油松的蛋白质表达差异分析[J]. 林业科学, 2016, 52(8): 68-75
Wang Y C, Zhou G N, Zhang B, et al. Difference in protein expression of Pinus tabulaeformis induced by Dendrolimus tabulaeformis feeding and leaf-cutting stimulation [J]. Scientia Silvae Sinicae, 2016, 52(8): 68-75 (in Chinese)
[43] Wen S F, Liu C, Wang Y W, et al. Oxidative stress and DNA damage in earthworm (Eisenia fetida) induced by triflumezopyrim exposure [J]. Chemosphere, 2021, 264(Pt 2): 128499
[44] Cheng Y L, Zhu L S, Song W H, et al. Combined effects of mulch film-derived microplastics and atrazine on oxidative stress and gene expression in earthworm (Eisenia fetida) [J]. The Science of the Total Environment, 2020, 746: 141280
[45] He F L, Liu Q, Jing M Y, et al. Toxic mechanism on phenanthrene-induced cytotoxicity, oxidative stress and activity changes of superoxide dismutase and catalase in earthworm (Eisenia foetida): A combined molecular and cellular study [J]. Journal of Hazardous Materials, 2021, 418: 126302
[46] Liu J B, Qin J J, Zhu L, et al. The protective layer formed by soil particles on plastics decreases the toxicity of polystyrene microplastics to earthworms (Eisenia fetida) [J]. Environment International, 2022, 162: 107158
[47] Li B, Song W H, Cheng Y L, et al. Ecotoxicological effects of different size ranges of industrial-grade polyethylene and polypropylene microplastics on earthworms Eisenia fetida [J]. The Science of the Total Environment, 2021, 783: 147007
[48] Li M Y, Ma X X, Wang Y R, et al. Ecotoxicity of herbicide carfentrazone-ethyl towards earthworm Eisenia fetida in soil [J]. Comparative Biochemistry and Physiology Toxicology &Pharmacology, 2022, 253: 109250
[49] Soares C, de Sousa A, Pinto A, et al. Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake in Solanum nigrum L. under Ni stress [J]. Environmental and Experimental Botany, 2016, 122: 115-125
[50] He F L, Li X X, Huo C Q, et al. Evaluation of fluorene-caused ecotoxicological responses and the mechanism underlying its toxicity in Eisenia fetida: Multi-level analysis of biological organization [J]. Journal of Hazardous Materials, 2022, 437: 129342
[51] 史志明. 菲在蚯蚓体内的分布及其对蚯蚓抗氧化防御体系的影响[D]. 南京: 南京农业大学, 2013: 59-71
Shi Z M. Distribution of phenanthrene in earthworms and its effects on the anti-oxidant defence system of earthworm [D]. Nanjing: Nanjing Agricultural University, 2013: 59-71 (in Chinese)