全/多氟烷基类物质(PFASs)已成为国际社会广泛关注的一类新污染物。根据经济合作与发展组织(OECD)的最新定义,PFASs是至少含有一个—CF3—或—CF2—(不含任何H/Cl/Br/I原子)的含氟化合物[1]。PFASs分子热稳定好,且疏水疏油,因此常作为添加剂应用于防火材料、防水面料和不粘锅涂层等产品[2]。其中,全氟烷基羧酸(PFCAs)是一类典型的PFASs。PFCAs是典型的环境持久性、生物蓄积性、有毒(PBT)化学物质:一些PFCAs的环境降解半减期高达半年以上[3],在环境及生物介质中广泛检出[4],如在我国20个沿海城市饮用水中检出PFCAs的总浓度为39.8~127mg·L-1[5];PFCAs在水生食物链/网中产生生物累积和放大效应[6];活体动物实验和流行病学研究表明,PFCAs可能破坏人体免疫功能,产生甲状腺毒性、肝毒性,还会影响生殖发育,甚至引发癌症[7]。因此人和生物体暴露PFCAs的途径及风险值得关注。
PFCAs暴露来源大致分为2类:饮食摄入、皮肤接触等直接来源,以及经PFCAs前体代谢转化的间接来源[8]。研究表明,前体代谢转化可贡献PFCAs整体暴露的60%[9]。氟调聚醇(FTOH)、多氟烷基磷酸酯(PAP)和氟调聚磺酸(FTSA)是常见的PFCAs前体。FTOH是生产表面活性剂和防污聚合物的中间体[10],被大量用于防雾剂产品中[11];PAPs多用于食品包装纸中的防油脂剂,而FTSA用于油漆、涂料和工业清洁产品[12]。细胞色素P450酶是生物体内转化PFCAs前体的主要酶系[13]。P450酶是以血红素为活性中心的蛋白酶,能够催化75%的药物代谢以及95%的生物氧化还原反应[14]。PFCAs前体的P450酶代谢转化可能产生毒性产物,如FTOH经P450酶催化转化的中间产物(如醛类物质)对大鼠细胞的毒性显著增强[15]。因此研究P450酶催化的PFCAs前体代谢转化路径及动力学对评价其生物暴露途径及毒性风险具有重要意义。
研究表明,PAP, FTOH及FTS等前体在生物体内可被P450酶转化为PFCAs,然而相关的反应路径及产物尚不清楚。例如,Zhao等[16]在粗P450酶提取液中加入6:2氟调聚磺酸(6:2 FTSA),检测到代谢产物全氟戊酸(PFPeA)和全氟己酸(PFHxA)等PFCAs。Zhao等[17]使南瓜暴露于6:2 FTSA并添加P450酶抑制剂氨基苯并三唑,发现PFCAs浓度降低,表明P450酶参与6:2 FTSA的生物转化过程,可将其转化为PFCAs。Li等[18]研究了8∶2 FTOH在人体重组P450酶中的离体代谢过程,观测到8∶2 FTAL产物,而在大鼠肝微粒体中6∶2 FTOH的主要代谢产物为6∶2 FTCA[19]。Rand和Mabury[20]使大鼠口服暴露6∶2 diPAP,在肝脏中发现代谢产物PFHxA。总体上,PFCAs前体的P450酶促转化产物各有差异,不同前体分子官能团结构(如磷酸基、磺酸基和羟基等)对反应活性的影响尚不清楚,不同碳链长度PFCAs前体的反应特征差异也有待研究。
基于活体、离体实验难以检测高反应活性的中间产物,且逐一测试种类众多的PFCAs前体花费大、周期长。相比之下,量子化学计算方法可以阐明电子结构特征,揭示反应路径,在环境有机污染物代谢研究方面发挥着重要作用。前人基于量子力学密度泛函理论(DFT)计算,成功阐明了P450酶催化典型外源化合物的代谢机制[21],如农药(狄氏剂)[22]、抗生素(替达霉素)[23]以及酚类抗氧化剂(丁羟基茴香醚和二丁基羟基甲苯)[24]等。
P450酶活性中心卟啉环π轨道上存在单电子,形成最低非占据分子轨道(LUMO)。根据前线分子轨道理论,当与底物发生代谢反应时,LUMO轨道会捕获底物中最高占据分子轨道(HOMO)的电子。DFT计算时通常建立三维团簇模型来模拟酶活性中心及底物分子,通过模型定义的原子类型和坐标,近似求解薛定谔方程(Schrödinger equation)并进行电子布居分析(population analysis),获得体系的电子轨道分布与势能极小值。由此构筑体系势能随反应坐标变化的势能剖面,结合过渡态理论,实现电子转移模式及产物结构预测的目的。在此基础上,通过产物分子的理化性质(如辛醇水分配系数),借助定量构效关系模型软件(如ECOSAR)预测其水生急性毒性,考察代谢转化对底物毒性的影响。
因此,本研究以6∶2 FTOH, 6∶2 PAP和6∶2 FTS为前体模型化合物,采用DFT计算,揭示P450酶活性中心催化不同PFCAs前体的转化反应。上述3种前体在环境介质及生物体内检出率较高,且具有部分代谢实验研究,相关结果可作为计算结果的佐证。研究阐明了PFCAs前体转化生成PFCAs的内暴露途径,发现不同结构前体的P450酶促转化存在动力学差异,分子氢键及氟代碳链长度可能对反应能垒及产物产生影响,结果为阐明PFCAs的生物归趋、暴露及健康风险奠定科学依据。
1 材料与方法(Materials and methods)
1.1 计算模型
使用简化的团簇模型Compound I (Cpd Ⅰ)模拟P450酶活性中心,结构如图1所示。Cpd Ⅰ由铁-卟啉环(血红素去除烷基侧链的简易结构),轴向的Fe![]() O键及巯基配体(模拟半胱氨酸配体)构成。
O键及巯基配体(模拟半胱氨酸配体)构成。
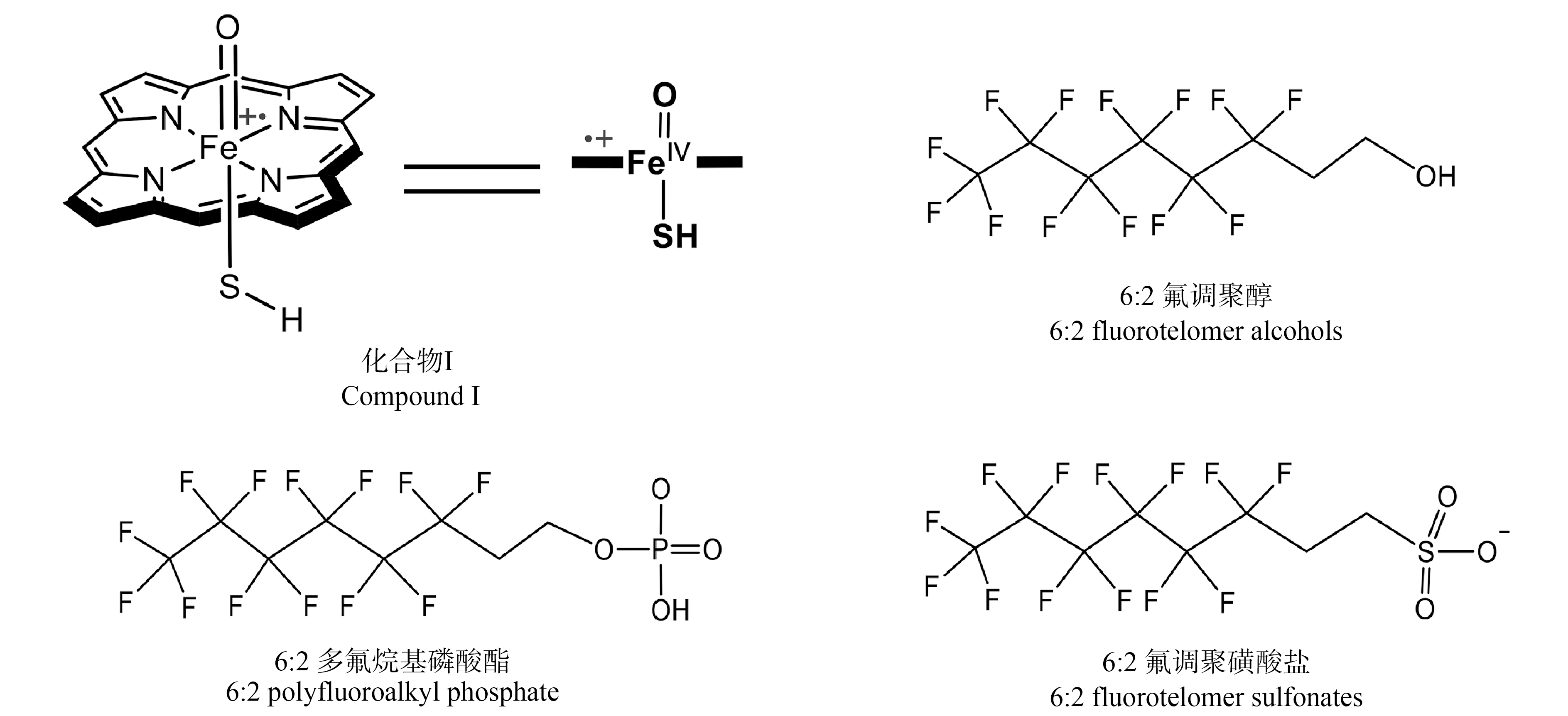
图1 本研究采用的P450酶活性中心模型(化合物Ⅰ)及底物(6∶2 PAP, 6∶2 FTOH和6∶2 FTS)结构图
Fig. 1 Structures of the active center model of P450 enzymes (Cpd Ⅰ) and substrates (6∶2 PAP, 6∶2 FTOH and 6∶2 FTS) used in this study
虽然不同物种、不同亚型的P450酶蛋白结构存在差异,但本质上均共有类似的血红素活性中心。因此在相对精确的DFT计算水平下处理P450酶活性中心,不仅能有效回答酶反应中局部构型和电子结构等问题,也可提供反应途径的细节。理论研究已经证实采用Cpd Ⅰ模型阐明P450酶催化反应机制,具有较高的可靠性[25-27]。基态的Cpd Ⅰ包含3个单电子,其中2个自旋向上的单电子位于Fe![]() O的π反键轨道上,另一个单电子位于卟啉环的a2u轨道上,自旋向上或向下都有可能,分别对应高自旋(HS)四重态及低自旋(LS)二重态,形成了2个能量简并的自旋态[28]。
O的π反键轨道上,另一个单电子位于卟啉环的a2u轨道上,自旋向上或向下都有可能,分别对应高自旋(HS)四重态及低自旋(LS)二重态,形成了2个能量简并的自旋态[28]。
本研究中,考虑了Cpd Ⅰ模型2种自旋状态下催化3种PFCAs前体转化的分子机制。FTOH, PAP和FTS的分子结构如图1所示,由全氟代烷烃连接亚甲基及相应的官能团构成。由于磷酸及磺酸根属于强酸基团,采用半经验软件MOPAC预测得到6∶2 FTS和6∶2 PAP的解离常数(pKa)分别为-2.83和-0.16[29-30],表明这2种前体在生理环境下完全电离,因此采用阴离子态底物进行模拟计算。
1.2 反应途径猜测
根据已有的烷烃羟基化反应的机制研究[21],推测3种PFCAs前体与Cpd Ⅰ的反应可能遵循以下路径(图2):(a) Cpd Ⅰ催化前体分子的Cα和Cβ位氢原子转移(HAT)形成中间体;(b) 中间体经羟基反弹生成醇产物,醇产物在非酶环境下可发生氢转移分子内重排过程;(c1)羟基化6∶2 PAP脱磷酸基生成6∶2 FTAL和磷酸二氢根![]() 羟基化6∶2 FTS通过脱磺酸基生成6∶2 FTAL和亚硫酸氢根离子
羟基化6∶2 FTS通过脱磺酸基生成6∶2 FTAL和亚硫酸氢根离子![]() 羟基化6∶2 FTOH通过分子内重排脱水生成6∶2 FTAL。
羟基化6∶2 FTOH通过分子内重排脱水生成6∶2 FTAL。

图2 6∶2 PAP, 6∶2 FTS和6∶2 FTOH被P450酶活性中心(化合物Ⅰ)催化转化反应的可能路径
注:HAT代表氢原子转移,R代表—C6F13基团,紫色区域表示酶环境下的反应过程,绿色区域表示非酶环境下的反应过程。
Fig. 2 Possible pathways for the transformation of 6∶2 PAP, 6∶2 FTOH and 6∶2 FTS catalyzed by the active species of cytochrome P450 enzymes (Cpd Ⅰ)
Note: HAT represents hydrogen atom transfer, and R represents —C6F13 group; the purple region represents the reaction process in the enzymatic environment, while the green region represents the reaction process in the non-enzymatic environment.
1.3 密度泛函理论(DFT)计算方法
所有计算在Gaussian 09软件包中进行[31]。因为反应是开壳层体系,使用非限制的B3LYP泛函结合2种基组进行计算。优化几何构型时采用基组LACVP(Fe)/6-31G*(C, H, O, N, S, F, P),记作B1。单点能(SPE)的计算采用了更高水平的基组LACV3P+*(Fe)/6-311+G**(C, H, O, N, S, F, P),记作B2。经典的B3LYP泛函没有考虑色散弱作用对反应的影响,因此使用DFT-D3来校正色散能量的影响[32]。在B1水平上进行频率计算以表征过渡态并获得零点能量(ZPE)。以上泛函和基组在P450酶催化转化外源化合物计算研究方面被证实是有效的[24,33-34]。
计算得到的过渡态构型有且仅有一个虚频,且沿反应坐标方向振动,并通过内禀反应坐标(IRC)计算得到了验证,其它能量极小值点优化构型无虚频。使用隐式溶剂化模型SMD模拟体系的溶剂效应,采用氯苯溶剂(介电常数为5.6)模拟酶环境,水溶剂模拟非酶环境(介电常数为78.4)。势能面图采用UB3LYP/B2//B1水平的相对SPE能量(包含ZPE和色散能量校正)构建。
2 结果(Results)
2.1 不同PFCAs前体的反应路径
计算结果表明,Cpd Ⅰ与6∶2 PAP首先形成反应复合物(RC),而后Cpd Ⅰ的氧原子摘取烷基C—H键的氢原子,经反应过渡态(TS),并伴随羟基反弹过程,生成羟基化产物PCHα。由图3可知,摘氢反应是该反应的速率控制步骤,而羟基反弹是无垒的。
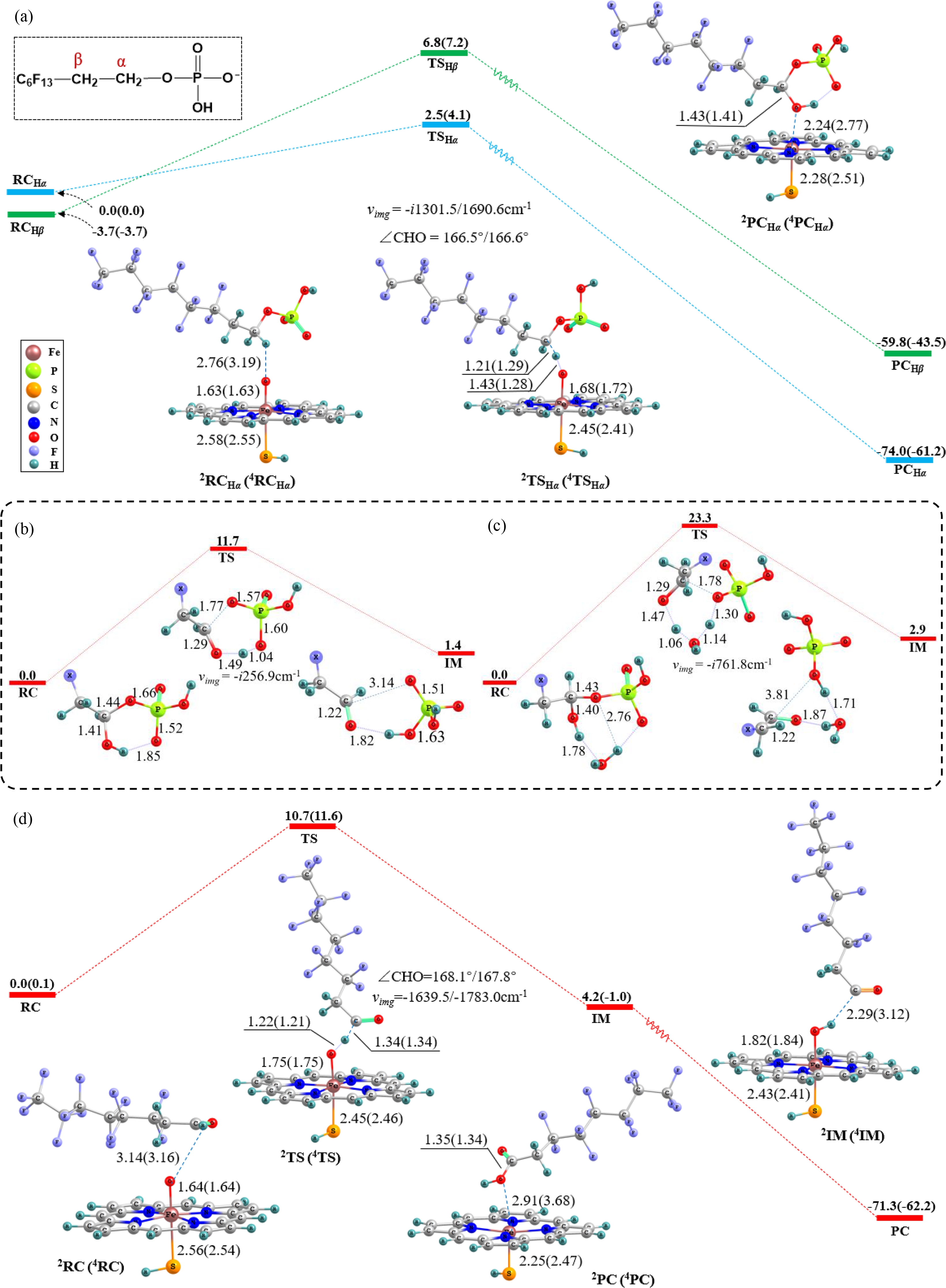
图3 Cpd Ⅰ催化6∶2 PAP转化生成6∶2 FTCA的反应能量及中间物种构型图
注:(a)显示Cpd Ⅰ催化6∶2 PAP羟基化反应过程;(b)显示6∶2 PAP羟基化产物P![]() O辅助的氢转移重排过程;(c)显示6∶2 PAP羟基化产物P—O辅助的氢转移重排过程;(d)显示Cpd Ⅰ催化6∶2 FTAL的羟基化反应过程;X基团表示—C6F13,括号内为四重态值,键长单位为Å (0.1 nm),键角单位为(°),振动频率为i cm-1,能量单位为kcal·mol-1(1 kcal = 4.1859 kJ)是酶反应能垒常用的量纲。
O辅助的氢转移重排过程;(c)显示6∶2 PAP羟基化产物P—O辅助的氢转移重排过程;(d)显示Cpd Ⅰ催化6∶2 FTAL的羟基化反应过程;X基团表示—C6F13,括号内为四重态值,键长单位为Å (0.1 nm),键角单位为(°),振动频率为i cm-1,能量单位为kcal·mol-1(1 kcal = 4.1859 kJ)是酶反应能垒常用的量纲。
Fig. 3 Optimized geometries of the key species and reaction barriers for the transformation of 6∶2 PAP to 6∶2 FTCA catalyzed by Cpd Ⅰ
Note: (a) Displays the hydroxylation process of 6∶2 PAP catalyzed by Cpd Ⅰ; (b) Displays the P![]() O assisted H-transfer rearrangement of the hydroxylated 6∶2 PAP; (c) Displays the P—O assisted H-transfer rearrangement of the hydroxylated 6∶2 PAP; and (d) Displays the hydroxylation process of 6∶2 FTAL catalyzed by Cpd Ⅰ; the X group represents —C6F13, quartet state values are in parentheses, bond distances are in angstroms (Å, i.e. 0.1 nm), angles in degrees (°), vibrational frequencies (ν) in i cm-1 and energy in kcal·mol-1(1 kcal = 4.1859 kJ) that is the common scale of enzyme reaction energy barriers.
O assisted H-transfer rearrangement of the hydroxylated 6∶2 PAP; (c) Displays the P—O assisted H-transfer rearrangement of the hydroxylated 6∶2 PAP; and (d) Displays the hydroxylation process of 6∶2 FTAL catalyzed by Cpd Ⅰ; the X group represents —C6F13, quartet state values are in parentheses, bond distances are in angstroms (Å, i.e. 0.1 nm), angles in degrees (°), vibrational frequencies (ν) in i cm-1 and energy in kcal·mol-1(1 kcal = 4.1859 kJ) that is the common scale of enzyme reaction energy barriers.
P450酶催化的C—H键羟基化有2种可能的反应机制:自旋选择反应机制(SSM)和双态反应机制(TSR)[35],计算发现6∶2 PAP的Cβ—H羟基化反应在二、四重态上的整体反应能垒接近,遵循TSR机制[36],而Cα—H羟基化反应遵循SSM机制,与氨基相连的Cα—H羟基化过程类似[37],可能是由于Cα—H靠近富电子的磷酸基团[38-39]。从反应构型上看,过渡态时∠C—H—O分别为166.5°和166.6°,几乎呈直线。随着反应进行,Fe—O距离伸长(由1.63到1.68 Å,0.163~0.168 nm),Fe—S距离缩短(由2.58到2.45 Å,0.258~0245 nm),上述反应结构特征与前人报道的Cpd Ⅰ催化烷烃摘氢反应路径相符[40]。
比较不同反应位点发现,Cβ—H摘氢反应能垒为10.7 kcal·mol-1(二、四重态平均值,1 kcal = 4.1859 kJ)显著高于Cα—H位点(3.3 kcal·mol-1,1 kcal = 4.1859 kJ),这可能是因为Cβ—H相比Cα—H更靠近碳氟长链,而—C6F13的强吸电子作用导致Cβ—H位点的电子密度降低,电荷量增大,使其反应活性降低。电荷分析结果表明,Cβ—H位点电荷QHβ≈ 0.42,高于Cα—H位置电荷QHα≈ 0.34(均为二、四重态的均值)。由表1可知,Cpd Ⅰ催化的6∶2 PAP羟基化反应是一个双电子转移过程,底物上的电子首先转移到Fe![]() O的π轨道上(ρFe+O由2.11到1.57,ρsub由0到0.56),使得FeⅣ变为FeⅢ,此时卟啉环上仍存在单电子(ρpor+SH ≈ ±1),会接受底物上转移的第2个电子(ρsub由0.56到0,ρpor+SH由±1到0),完成催化氧化过程。
O的π轨道上(ρFe+O由2.11到1.57,ρsub由0到0.56),使得FeⅣ变为FeⅢ,此时卟啉环上仍存在单电子(ρpor+SH ≈ ±1),会接受底物上转移的第2个电子(ρsub由0.56到0,ρpor+SH由±1到0),完成催化氧化过程。
表1 Cpd Ⅰ催化6∶2 PAP羟基化反应过程中关键物种的密立根自旋密度和原子电荷
Table 1 Mulliken spin densities and charges for the key species in the hydroxylation of 6∶2 PAP catalyzed by Cpd Ⅰ
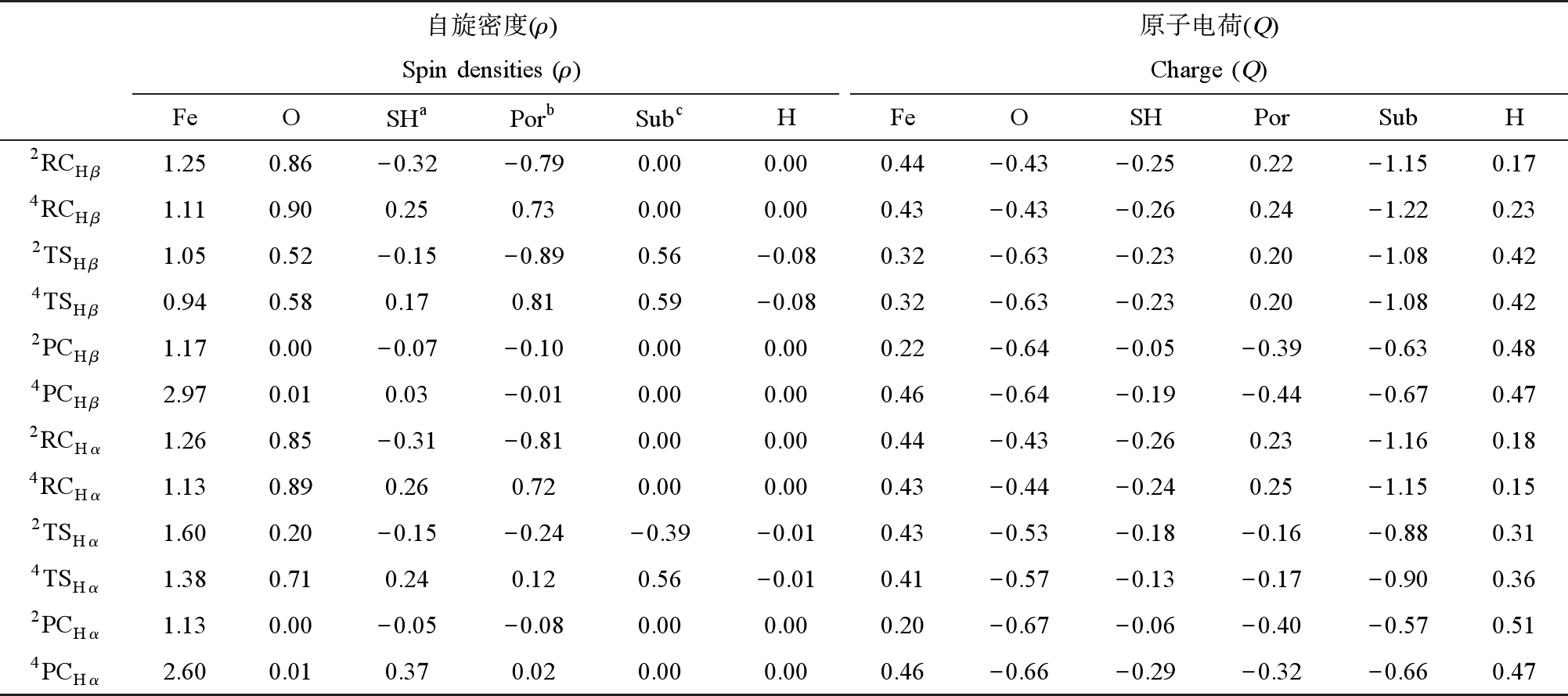
注:a代表巯基配体;b代表除Fe原子外卟啉环;c代表底物6∶2 PAP。
Note∶ a represents the thiol ligand; b represents the porphyrin ring except for the Fe atom; c represents the substrate 6∶2 PAP.
自旋密度(ρ)Spin densities (ρ)原子电荷(Q)Charge (Q)FeOSHaPorbSubcHFeOSHPorSubH2RCHβ1.250.86-0.32-0.790.000.000.44-0.43-0.250.22-1.150.174RCHβ1.110.900.250.730.000.000.43-0.43-0.260.24-1.220.232TSHβ1.050.52-0.15-0.890.56-0.080.32-0.63-0.230.20-1.080.424TSHβ0.940.580.170.810.59-0.080.32-0.63-0.230.20-1.080.422PCHβ1.170.00-0.07-0.100.000.000.22-0.64-0.05-0.39-0.630.484PCHβ2.970.010.03-0.010.000.000.46-0.64-0.19-0.44-0.670.472RCHα1.260.85-0.31-0.810.000.000.44-0.43-0.260.23-1.160.184RCHα1.130.890.260.720.000.000.43-0.44-0.240.25-1.150.152TSHα1.600.20-0.15-0.24-0.39-0.010.43-0.53-0.18-0.16-0.880.314TSHα1.380.710.240.120.56-0.010.41-0.57-0.13-0.17-0.900.362PCHα1.130.00-0.05-0.080.000.000.20-0.67-0.06-0.40-0.570.514PCHα2.600.010.370.020.000.000.46-0.66-0.29-0.32-0.660.47
研究表明,羟基化产物由于极性增强,较容易脱离活性空腔,进入非酶环境[41]。此时,由于氢键作用,醇羟基氢原子会转移到P![]() O键的氧(图3(b))或P—O的氧原子(图3(c))上,引发C—O键断裂,生成6∶2氟调聚醛(6∶2 FTAL)和磷酸二氢根
O键的氧(图3(b))或P—O的氧原子(图3(c))上,引发C—O键断裂,生成6∶2氟调聚醛(6∶2 FTAL)和磷酸二氢根![]() 离子。计算结果显示,氢原子更容易转移到P
离子。计算结果显示,氢原子更容易转移到P![]() O的氧原子上,该反应能垒低于P—O介导的氢原子迁移过程。生成的6∶2 FTAL会进一步与Cpd Ⅰ发生醛基C—H键摘氢反应(图3(d)),经羟基反弹后生成6∶2氟调聚羧酸(6∶2 FTCA)。计算发现,反应能垒四重态(11.5 kcal·mol-1,1 kcal = 4.1859 kJ)显著高于二重态(6.3 kcal·mol-1,1 kcal = 4.1859 kJ),表明该反应具有自旋选择性,更容易在二重态上发生。Chai等[33]通过DFT计算得到Cpd Ⅰ氧化醛生成羧酸的反应能垒为8.5 kcal·mol-1(1 kcal = 4.1859 kJ),高于本研究的计算能垒,可能由于—C6F13基团的吸电子作用,使得C—H键变弱,能垒降低。Chen等[42]使鲤鱼暴露于diPAP,在肝脏中检出了FTCA产物,侧面证实了本研究的计算结果。
O的氧原子上,该反应能垒低于P—O介导的氢原子迁移过程。生成的6∶2 FTAL会进一步与Cpd Ⅰ发生醛基C—H键摘氢反应(图3(d)),经羟基反弹后生成6∶2氟调聚羧酸(6∶2 FTCA)。计算发现,反应能垒四重态(11.5 kcal·mol-1,1 kcal = 4.1859 kJ)显著高于二重态(6.3 kcal·mol-1,1 kcal = 4.1859 kJ),表明该反应具有自旋选择性,更容易在二重态上发生。Chai等[33]通过DFT计算得到Cpd Ⅰ氧化醛生成羧酸的反应能垒为8.5 kcal·mol-1(1 kcal = 4.1859 kJ),高于本研究的计算能垒,可能由于—C6F13基团的吸电子作用,使得C—H键变弱,能垒降低。Chen等[42]使鲤鱼暴露于diPAP,在肝脏中检出了FTCA产物,侧面证实了本研究的计算结果。
类似地,Cpd Ⅰ与6∶2 FTS也可能在Cα—H和Cβ—H位点发生摘H反应,生成碳中心自由基和Fe—OH中间体(IM),而后反应路径在不同位点有所差别(图4(a)):在Cβ—H位点,IM经过无垒的羟基反弹生成醇产物;而在Cα—H位点,除了羟基反弹之外,IM还可能发生二次摘氢反应,摘取邻碳上氢原子,生成6∶2氟调聚不饱和磺酸(6∶2 FTUSA)。Yang等[43]在培养菌株(RHA1)时加入6∶2 FTSA作为硫源,发现RHA1生长过程中P450酶高度表达,并检测到6∶2 FTUSA代谢产物,与本研究预测的产物吻合。计算发现,在非酶环境下,生成的醇产物也可能发生分子内氢转移重排,醇羟基氢原子通过氢键作用,转移到磺酸基团的氧上,引发C—S键断裂,生成6∶2 FTAL和亚硫酸氢根离子![]() 同样地,水分子可以催化该过程,使反应能垒降低1.6 kcal·mol-1(1 kcal = 4.1859kJ)(图4(b))。
同样地,水分子可以催化该过程,使反应能垒降低1.6 kcal·mol-1(1 kcal = 4.1859kJ)(图4(b))。
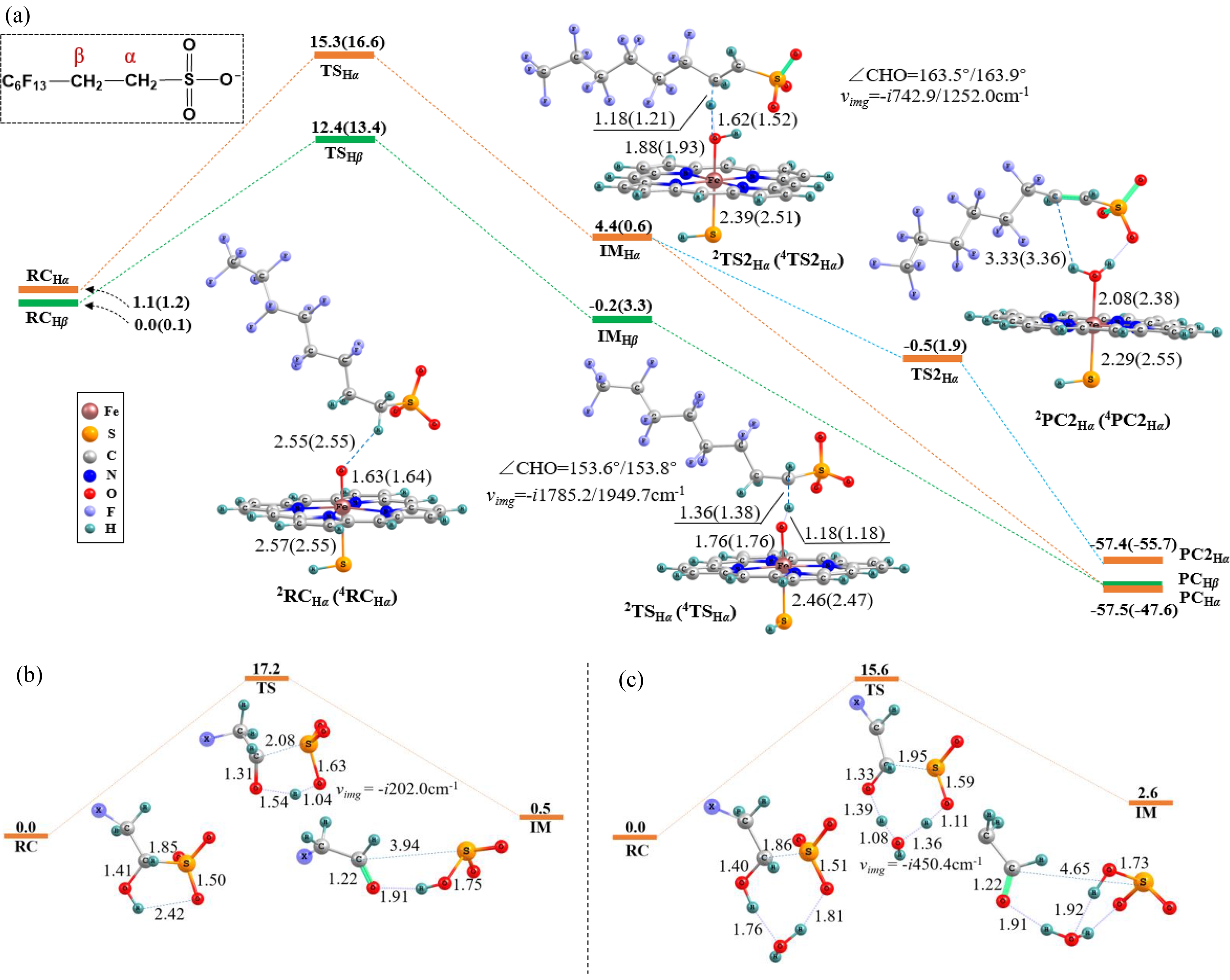
图4 Cpd Ⅰ催化6∶2 FTS转化生成6∶2 FTAL的反应能量及中间物种构型图
注:(a)显示Cpd Ⅰ催化6∶2 FTS转化生成6∶2 FTUSA反应过程;(b)显示6∶2 FTS羟基化产物S—O辅助的氢转移重排过程;(c)显示水分子催化的6∶2 FTS羟基化产物S—O辅助的氢转移重排过程;X基团表示—C6F13,括号内为四重态值,键长单位为Å (0.1 nm),键角单位为(°),振动频率为i cm-1,能量单位为kcal·mol-1 (1 kcal = 4.1859 kJ)是酶反应能垒常用的量纲。
Fig. 4 Optimized geometries of the key species and reaction barriers for the transformation of 6∶2 FTS to 6∶2 FTAL catalyzed by Cpd Ⅰ
Note: (a) Displays the transformation of 6∶2 FTS to 6∶2 FTUSA catalyzed by Cpd Ⅰ; (b) Displays the S—O assisted H-transfer rearrangement of the hydroxylated 6∶2 FTS; (c) Displays the S—O assisted H-transfer rearrangement of the hydroxylated 6∶2 FTS with the assistance of water; the X group represents —C6F13, quartet state values are in parentheses, bond distances are in angstroms (Å, i.e. 0.1 nm), angles in degrees (°), vibrational frequencies (ν) in i cm-1 and energy in kcal·mol-1 (1 kcal = 4.1859 kJ) that is the common scale of enzyme reaction energy barriers.
由图5可知,Cpd Ⅰ与6∶2 FTOH可能有3个反应位点,即Cα—H, Cβ—H和醇羟基H。结果发现,相比于其他2个位点,Cpd Ⅰ催化的醇羟基摘氢是反应的优势路径,能垒为14.8 kcal·mol-1(1 kcal = 4.1859 kJ),其次为Cα—H位点。反应生成氧中心自由基和Fe—OH中间体(IM),而后Fe—OH进一步摘取Cα—H形成6∶2 FTAL和H2O。Cα—H和Cβ—H位点发生摘氢反应后,伴随着无能垒的OH反弹过程,生成二醇产物。值得注意的是,Cα—H位点摘氢反应产物为偕二醇,可经过脱水反应生成醛。计算表明,直接脱水的反应能垒较高(图5(b)),相比之下,1个水分子催化的脱水反应容易发生(图5(c)),而脱水反应的能垒均高于酶催化的羟基化过程。由此可见,氟调聚醛是FTOH的P450酶代谢过程中的稳定中间产物。Li等[18]采用人重组P450酶研究8∶2 FTOH的转化过程,也检测到了醛类产物,与本研究的计算结果一致。

图5 Cpd Ⅰ催化6∶2 FTOH转化生成6∶2 FTAL的反应能量及中间物种构型图
注:(a)显示Cpd Ⅰ催化6∶2 FTOH转化生成6∶2 FTAL反应过程;(b)显示偕二醇产物脱水重排过程;(c)显示水分子辅助偕二醇产物脱水重排过程;X基团表示—C6F13,括号内为四重态值,键长单位为Å (0.1 nm),键角单位为(°),振动频率为i cm-1,能量单位为kcal·mol-1(1 kcal = 4.1859 kJ)是酶反应能垒常用的量纲。
Fig. 5 Optimized geometries of the key species and reaction barriers for the transformation of 6∶2 FTOH to 6∶2 FTAL catalyzed by Cpd Ⅰ
Note: (a) Displays the transformation of 6∶2 FTOH to 6∶2 FTAL catalyzed by Cpd Ⅰ; (b) Displays the dehydration of the gem-diol; (c) Displays the dehydration of the gem-diol with the assistance of water; the X group represents —C6F13, quartet state values are in parentheses, bond distances are in angstroms (Å, i.e. 0.1 nm), angles in degrees (°), vibrational frequencies (ν) in i cm-1 and energy in kcal·mol-1(1 kcal = 4.1859 kJ) that is the common scale of enzyme reaction energy barriers.
2.2 氢键作用对反应的影响
值得注意的是,6∶2 PAP的Cα—H羟基化过程中,Cpd Ⅰ氧原子可能受到6∶2 PAP羟基的氢键影响(图6)。此时,由于氢键的稳定作用,RC的能量较低,过渡态反应能垒为6.8 kcal·mol-1 (1 kcal = 4.1859 kJ),高于没有氢键作用时的能垒(4.7 kcal·mol-1)(1 kcal = 4.1859 kJ)。可见,氢键的形成导致反应活性降低。此外,氢键的存在也改变了反应路径,Cpd Ⅰ摘取Cα—H后,不再发生羟基回弹,而是进一步摘取磷酸根羟基的氢原子,实现了2个氢原子的共同转移,伴随着O—P键断裂,生成6∶2 FTAL,水和偏磷酸根离子![]() 可见,氢键的作用使得醛产物FTAL通过酶内反应直接生成。Zhang等[44]研究发现,胆固醇上羟基和Cpd Ⅰ的氧形成氢键会显著影响摘氢反应能垒(增加了约2 kcal·mol-1,1 kcal = 4.1859 kJ),与本研究计算结果一致[44]。可见,在P450酶促反应研究中,有必要考察分子间氢键对反应机制的影响。
可见,氢键的作用使得醛产物FTAL通过酶内反应直接生成。Zhang等[44]研究发现,胆固醇上羟基和Cpd Ⅰ的氧形成氢键会显著影响摘氢反应能垒(增加了约2 kcal·mol-1,1 kcal = 4.1859 kJ),与本研究计算结果一致[44]。可见,在P450酶促反应研究中,有必要考察分子间氢键对反应机制的影响。
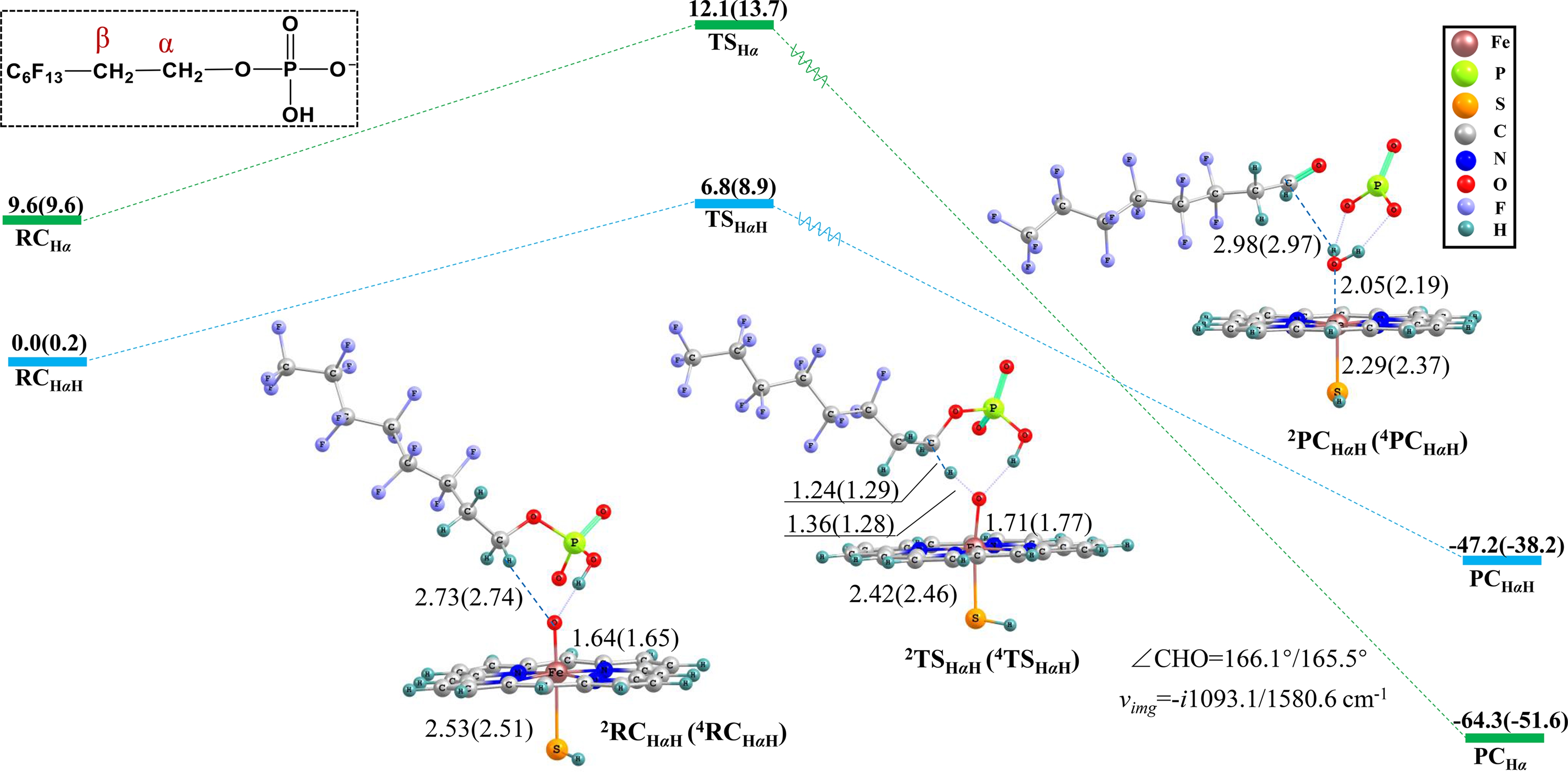
图6 有无氢键作用时Cpd Ⅰ催化6∶2 PAP Cα—H摘氢反应的能量过程图及关键中间物种的优化构型
注:X基团表示—C6F13,括号内为四重态值,键长单位为Å (0.1 nm),键角单位为(°),振动频率为i cm-1,能量单位为kcal·mol-1(1 kcal = 4.1859 kJ)是酶反应能垒常用的量纲。
Fig. 6 Optimized geometries of key species and reaction barriers for Cα—H abstraction of 6∶2 PAP catalyzed by Cpd Ⅰ with or without hydrogen bonding
Note: The X group represents —C6F13, quartet state values are in parentheses, bond distances are in angstroms (Å, i.e. 0.1 nm), angles in degrees (°), vibrational frequencies (ν) in i cm-1 and energy in kcal·mol-1(1 kcal = 4.1859 kJ) that is the common scale of enzyme reaction energy barriers.
2.3 不同PFCAs前体反应动力学差异
通过计算4∶2 PAP, 6∶2 PAP, 8∶2 PAP和4∶2 FTS, 6∶2 FTS, 8∶2 FTS以及4∶2 FTOH, 6∶2 FTOH, 8∶2 FTOH与Cpd Ⅰ反应速率控制步骤(氢原子转移反应)的能垒,考察了不同碳链长度对反应活性的影响。结果发现,随碳链长度增加,PAP与Cpd Ⅰ反应的能垒升高(图7),反应变得愈发困难。Lee等[45]使虹鳟鱼(Oncorhynchus mykiss)分别暴露于碳原子数为6、8和10的全氟烷基膦酸(PFPAs),发现随碳链长度增加,PFPAs在鱼体内的代谢半减期增大,与本研究的动力学计算结果吻合。FTS和FTOH与Cpd Ⅰ反应的能垒随碳链长度增加有所差别,但未呈现线性规律。总体上看,计算得到的不同链长PAP,FTS,FTOH反应的平均能垒(分别为4.57、14.1和10.3 kcal·mol-1,1 kcal = 4.1859 kJ)从大到小依次为:氟调聚磺酸>氟调聚醇>多氟烷基磷酸酯,表明PAP最易被转化成PFCAs,其次为FTOH和FTS。Just等[46]研究了PFCAs前体在玉米根部的降解速率,发现PAP降解速率高于FTOH,与计算的反应动力学结果一致。
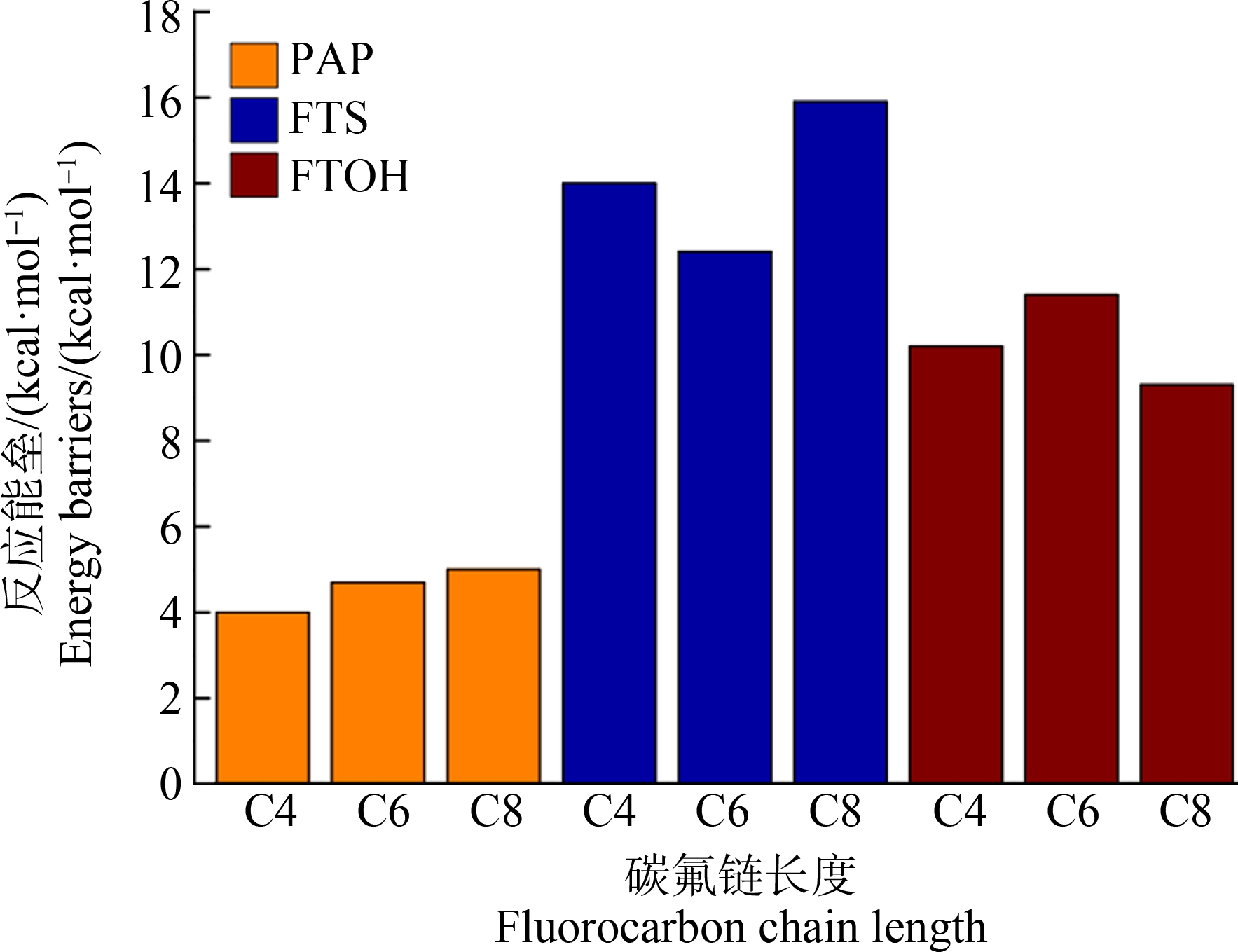
图7 不同碳链长度全氟烷基羧酸(PFCAs)前体与化合物Ⅰ反应能垒(1 kcal = 4.1859 kJ)
Fig. 7 Calculated reaction barriers of perfluorinated carboxylic acids (PFCAs) precursors with different fluorocarbon chain lengths catalyzed by Cpd Ⅰ(1 kcal = 4.1859 kJ)
2.4 反应产物的生态毒性预测
通过ECOSAR软件[47]预测了PFCAs前体及P450转化中间体/产物对鱼的急性毒性(半数致死浓度(LC50)),结果如图8所示。整体上随碳链增长,中间产物的毒性增强。Mitchell等[48]检测了FTCA对水藻的毒性,发现FTCA毒性随着碳链长度的增加而增加,与ECOSAR预测结果一致。此外,对碳链长度相同的PFCAs来说,中间体(如FTAL)毒性显著强于前体化合物及产物。Rand等[49]将PFCAs中间体代谢物FTALs、氟调聚物不饱和醛(FTUALs)、FTCAs和氟调聚物不饱和羧酸(FTUCAs)与人肝上皮细胞(THLE-2)一起孵育,发现中间体代谢物(FTALs, FTUALs, FTCAs和FTUCAs)毒性与官能团有关,强弱顺序为:FTUALs ≥ FTALs >FTUCAs ≥ FTCAs >PFCAs,与预测结果一致。因此,在PFCAs的风险评估中,代谢中间体及产物的毒性值得关注。但当前体的疏水性较大时(如8∶2 FTOH和8∶2 FTAL的辛醇水分配系数logKow预测值均>5),中间体的毒性反而小于前体,这可能是由于化合物分子量过大,难以跨膜进入生物体,导致其生物积累性及毒性减小。
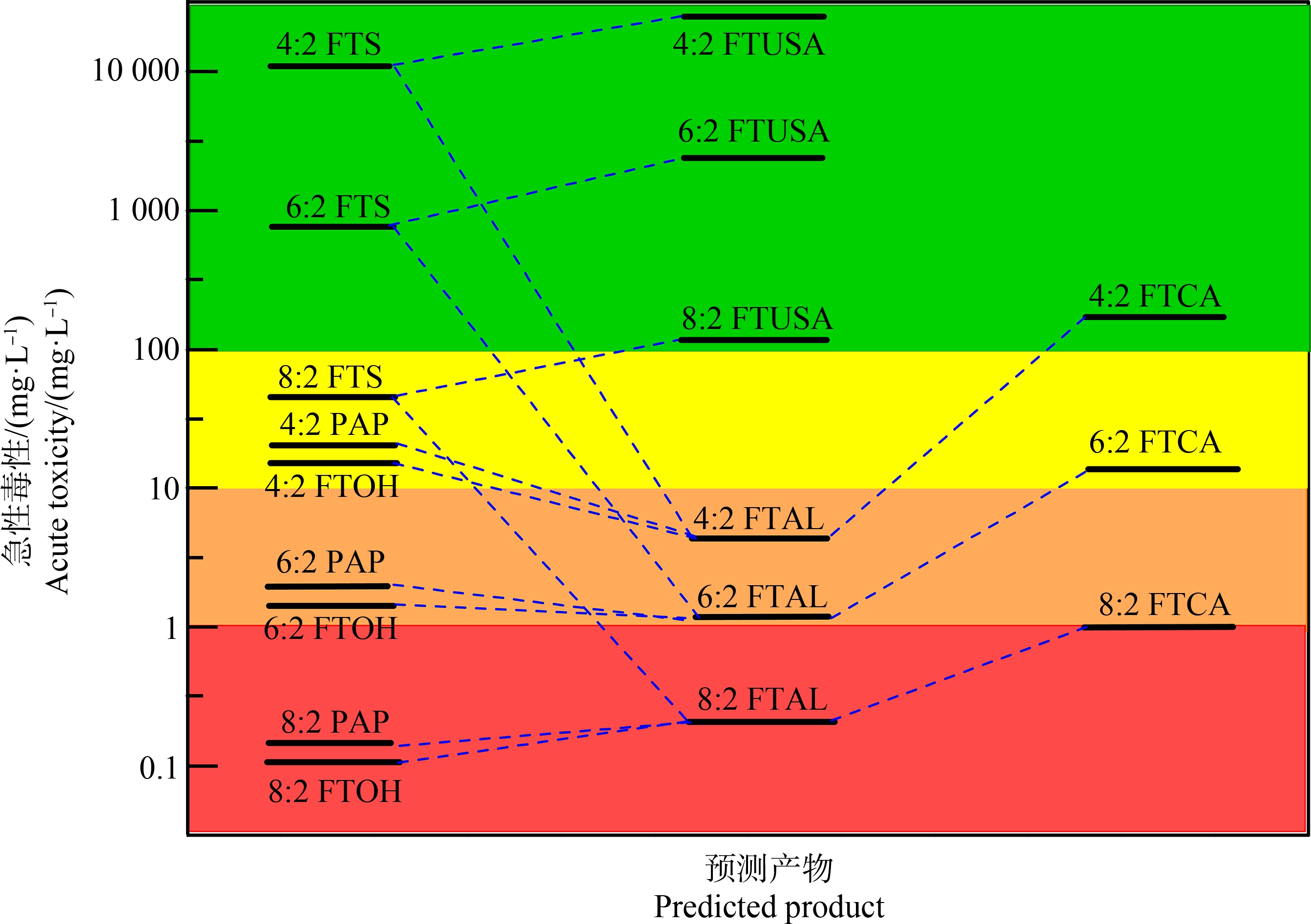
图8 PFCAs前体P450酶促转化中间体及产物的鱼水生急性毒性预测结果
注:红色区域表示剧毒,橙色区域表示有毒,黄色区域表示有害,绿色区域表示无害。
Fig. 8 Predicted acute toxicity of fish for transformation intermediates and products of PFCAs precursors by P450s
Note: The red region indicates very toxic, the orange region indicates toxic, the yellow region indicates harmful and the green region indicates not harmful.
3 讨论(Discussion)
基于密度泛函理论计算,阐明了P450酶活性中心催化转化典型PFCAs前体的反应机制。结果发现FTAL是PFCAs前体P450酶代谢转化过程的共同中间产物。Cpd Ⅰ先通过C—H键羟基化将前体转化为醇中间体,而后经非酶环境分子内重排,脱除磺酸基/磷酸基/羟基生成FTAL,FTAL进一步与Cpd Ⅰ发生二次转化生成FTCAs,相关产物得到体外实验的证实。发现前体化合物分子的磷酸基、磺酸基以及羟基可能通过给电子作用、氢键作用影响反应复合物或过渡态的稳定性,进而改变反应路径和产物。整体上,3种PFCAs前体被Cpd Ⅰ转化反应存在动力学差异,反应的难易程度依次为FTS
PFCAs前体的生物转化途径复杂,除P450酶催化氧化外,还涉及水解、氧化/还原脱氟等过程。例如,D’Eon和Mabury[50]发现PAP在小鼠肠道中可水解生成FTOH。Martin等[51]将分离的大鼠肝细胞与8∶2 FTOH一起孵育,检测到了脱氟反应产物8∶2 FTUCA。FTCA脱氟矿化被认为是其代谢脱毒的重要途径[52]。因此,PFCAs前体的其他生物转化途径也值得关注。此外,PFCAs前体的生物转化是多种生物酶参与的过程。Li等[53]的研究表明,氟乙酸脱卤素酶(FAcD)可催化2-氟丙酸发生Cα—F键断裂。Peskett和Rand[54]研究发现,人肝脏S9以及小肠细胞具有水解酶和氧化酶,使8∶2 monoPAP发生转化生成PFCAs产物。因此需要研究其他生物酶催化转化PFCAs的反应,以全面揭示PFCAs的生物转化过程。
基于量子化学计算研究P450酶催化的PFCAs前体代谢反应,虽可准确预测转化产物及反应机制,但在实际环境中,不同物种及亚型的P450酶活性中心处蛋白环境也存在差异,均可能对反应的动力学产生影响。这方面,量子力学/分子力学(QM/MM)多尺度模拟可以探究真实蛋白环境的影响,已成为研究酶化学反应的重要工具。Lonsdale等[55]通过QM/MM计算发现双氯芬酸在过渡态时能与CYP2C9 Arg108侧链形成氢键,导致代谢实验产物与反应优势产物不同。Yadav等[56]基于QM/MM计算发现,不同亚型P450酶因活性中心附近残基差异导致脂肪酸转化产物不同,P450BSβ通过常规的反弹机制生成羟基化产物,P450OleT通过去羧基化生成烯烃产物。未来考虑使用QM/MM方法,以探究PFCAs前体在真实酶环境下的转化反应过程及影响因素。
获取种类众多PFASs物质的毒代动力学参数(如肝脏固有清除率CLint)是评价其生物积累及毒性的前提。由于计算理论及效率的限制,基于量子力学的方法难以直接计算上述参数。考虑到P450酶是生物体内污染物代谢清除的主要酶系,可以在计算不同结构PFASs的P450酶代谢活化能垒基础上,探索建立毒代动力学参数与反应活性的定量构效关系(QSAR)模型,进而为PFAS毒理及健康风险提供高效准确的预测方法。
通信作者简介:傅志强(1989—),男,博士,主要研究方向为新污染物的生物代谢转化行为及毒理效应的模拟预测。
[1] Organisation for Economic Co-operation and Development. Reconciling terminology of the universe of per- and polyfluoroalkyl substances: Recommendations and practical guidance [R/OL]. [2023-02-02]. https://www.foodpackagingforum.org/news/oecd-report-reconciles-pfas-terminology
[2] Glüge J, Scheringer M, Cousins I T, et al. An overview of the uses of per- and polyfluoroalkyl substances (PFAS) [J]. Environmental Science Processes &Impacts, 2020, 22(12): 2345-2373
[3] Evich M G, Davis M J B, McCord J P, et al. Per- and polyfluoroalkyl substances in the environment [J]. Science, 2022, 375(6580): eabg9065
[4] Zheng G M, Schreder E, Dempsey J C, et al. Per- and polyfluoroalkyl substances (PFAS) in breast milk: Concerning trends for current-use PFAS [J]. Environmental Science &Technology, 2021, 55(11): 7510-7520
[5] Cao X Z, Xin S H, Liu X X, et al. Occurrence and behavior of per- and polyfluoroalkyl substances and conversion of oxidizable precursors in the waters of coastal tourist resorts in China [J]. Environmental Pollution, 2023, 316(Pt 1): 120460
[6] Lewis A J, Yun X Y, Spooner D E, et al. Exposure pathways and bioaccumulation of per- and polyfluoroalkyl substances in freshwater aquatic ecosystems: Key considerations [J]. The Science of the Total Environment, 2022, 822: 153561
[7] Fenton S E, Ducatman A, Boobis A, et al. Per- and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research [J]. Environmental Toxicology and Chemistry, 2021, 40(3): 606-630
[8] 杨琳, 李敬光. 全氟化合物前体物质生物转化与毒性研究进展[J]. 环境化学, 2015, 34(4): 649-655
Yang L, Li J G. Perfluorinated compound precursors: Biotransformation and toxicity [J]. Environmental Chemistry, 2015, 34(4): 649-655 (in Chinese)
[9] Gebbink W A, Berger U, Cousins I T. Estimating human exposure to PFOS isomers and PFCA homologues: The relative importance of direct and indirect (precursor) exposure [J]. Environment International, 2015, 74: 160-169
[10] Liu X Y, Guo Z S, Folk E E, et al. Determination of fluorotelomer alcohols in selected consumer products and preliminary investigation of their fate in the indoor environment [J]. Chemosphere, 2015, 129: 81-86
[11] Herkert N J, Kassotis C D, Zhang S, et al. Characterization of per- and polyfluorinated alkyl substances present in commercial anti-fog products and their in vitro adipogenic activity [J]. Environmental Science &Technology, 2022, 56(2): 1162-1173
[12] Jiao X C, Shi Q Y, Gan J. Uptake, accumulation and metabolism of PFASs in plants and health perspectives: A critical review [J]. Critical Reviews in Environmental Science and Technology, 2021, 51(23): 2745-2776
[13] Daramola O, Rand A A. Emerging investigator series: Human CYP2A6 catalyzes the oxidation of 6: 2 fluorotelomer alcohol [J]. Environmental Science Processes &Impacts, 2021, 23(11): 1688-1695
[14] Rendic S, Guengerich F P. Survey of human oxidoreductases and cytochrome P450 enzymes involved in the metabolism of xenobiotic and natural chemicals [J]. Chemical Research in Toxicology, 2015, 28(1): 38-42
[15] Martin J W, Chan K T, Mabury S A, et al. Bioactivation of fluorotelomer alcohols in isolated rat hepatocytes [J]. Chemico-Biological Interactions, 2009, 177(3): 196-203
[16] Zhao S Y, Liu T Q, Zhu L Y, et al. Formation of perfluorocarboxylic acids (PFCAs) during the exposure of earthworms to 6: 2 fluorotelomer sulfonic acid (6: 2 FTSA) [J]. The Science of the Total Environment, 2021, 760: 143356
[17] Zhao S Y, Liang T K, Zhu L Y, et al. Fate of 6: 2 fluorotelomer sulfonic acid in pumpkin (Cucurbita maxima L.) based on hydroponic culture: Uptake, translocation and biotransformation [J]. Environmental Pollution, 2019, 252: 804-812
[18] Li Z M, Guo L H, Ren X M. Biotransformation of 8: 2 fluorotelomer alcohol by recombinant human cytochrome P450s, human liver microsomes and human liver cytosol [J]. Environmental Science Processes &Impacts, 2016, 18(5): 538-546
[19] Ruan T, Sulecki L M, Wolstenholme B W, et al. 6: 2 fluorotelomer iodide in vitro metabolism by rat liver microsomes: Comparison with[1,2-14C]6: 2 fluorotelomer alcohol [J]. Chemosphere, 2014, 112: 34-41
[20] Rand A A, Mabury S A. Protein binding associated with exposure to fluorotelomer alcohols (FTOHs) and polyfluoroalkyl phosphate esters (PAPs) in rats [J]. Environmental Science &Technology, 2014, 48(4): 2421-2429
[21] Shaik S, Kumar D, de Visser S P, et al. Theoretical perspective on the structure and mechanism of cytochrome P450 enzymes [J]. Chemical Reviews, 2005, 105(6): 2279-2328
[22] Chai L H, Ji S J, Zhang S B, et al. Biotransformation mechanism of pesticides by cytochrome P450: A DFT study on dieldrin [J]. Chemical Research in Toxicology, 2020, 33(6): 1442-1448
[23] Espinoza R V, Maskeri M A, Turlik A, et al. Epoxidation and late-stage C-H functionalization by P450 TamI are mediated by variant heme-iron oxidizing species [J]. ACS Catalysis, 2022, 12(6): 3731-3742
[24] Zhang H N, Song R Q, Guo F J, et al. Using physical organic chemistry knowledge to predict unusual metabolites of synthetic phenolic antioxidants by cytochrome P450 [J]. Chemical Research in Toxicology, 2022, 35(5): 840-848
[25] Ma G C, Yu H Y, Xu T, et al. Computational insight into the activation mechanism of carcinogenic N’-nitrosonornicotine (NNN) catalyzed by cytochrome P450 [J]. Environmental Science &Technology, 2018, 52(20): 11838-11847
[26] Guo F J, Chai L H, Zhang S B, et al. Computational biotransformation profile of emerging phenolic pollutants by cytochromes P450: Phenol-coupling mechanism [J]. Environmental Science &Technology, 2020, 54(5): 2902-2912
[27] Yang R Y, Ye Y X, Chen Y, et al. First insight into the formation of in vivo transformation products of 2-ethylhexyl diphenyl phosphate in zebrafish and prediction of their potential toxicities [J]. Environmental Science &Technology, 2023, 57(1): 451-462
[28] 倪君秀, 徐婷, 温家乐, 等. 细胞色素P450酶催化烟草特异性亚硝胺N’-亚硝基新烟草碱和N’-亚硝基假木贼碱代谢活化的分子机制研究[J]. 生态毒理学报, 2019, 14(4): 73-82
Ni J X, Xu T, Wen J L, et al. Mechanistic insight into cytochrome P450-catalyzed activation of tobacco-specific N’-nitrosoanatabine and N’-nitrosoanabasine [J]. Asian Journal of Ecotoxicology, 2019, 14(4): 73-82 (in Chinese)
[29] Rayne S, Forest K. Estimated pKa values for the environmentally relevant C1 through C8 perfluorinated sulfonic acid isomers [J]. Journal of Environmental Science and Health Part A, Toxic/Hazardous Substances &Environmental Engineering, 2016, 51(12): 1018-1023
[30] Xiao F. Emerging poly- and perfluoroalkyl substances in the aquatic environment: A review of current literature [J]. Water Research, 2017, 124: 482-495
[31] Frisch M, Trucks G, Schlegel H, et al. Gaussian 09 Revision D.01 [CP]. Wallingford CT: Gaussian, Incorporation, 2013
[32] Lonsdale R, Harvey J, Mulholland A. Inclusion of dispersion effects significantly improves accuracy of calculated reaction barriers for cytochrome P450 catalyzed reactions [J]. Journal of Physical Chemistry Letters, 2010, 1: 3232-3237
[33] Chai L H, Zhang H N, Song R Q, et al. Precision biotransformation of emerging pollutants by human cytochrome P450 using computational-experimental synergy: A case study of tris(1,3-dichloro-2-propyl) phosphate [J]. Environmental Science &Technology, 2021, 55(20): 14037-14050
[34] Zhang Q, Ji S J, Chai L H, et al. Metabolic mechanism of aryl phosphorus flame retardants by cytochromes P450: A combined experimental and computational study on triphenyl phosphate [J]. Environmental Science &Technology, 2018, 52(24): 14411-14421
[35] Wang X B, Chen J W, Wang Y, et al. Transformation pathways of MeO-PBDEs catalyzed by active center of P450 enzymes: A DFT investigation employing 6-MeO-BDE-47 as a case [J]. Chemosphere, 2015, 120: 631-636
[36] Shaik S, de Visser S P, Ogliaro F, et al. Two-state reactivity mechanisms of hydroxylation and epoxidation by cytochrome P-450 revealed by theory [J]. Current Opinion in Chemical Biology, 2002, 6(5): 556-567
[37] Wang Y, Kumar D, Yang C L, et al. Theoretical study of N-demethylation of substituted N, N-dimethylanilines by cytochrome P450: The mechanistic significance of kinetic isotope effect profiles [J]. The Journal of Physical Chemistry B, 2007, 111(26): 7700-7710
[38] Schyman P, Usharani D, Wang Y, et al. Brain chemistry: How does P450 catalyze the O-demethylation reaction of 5-methoxytryptamine to yield serotonin? [J]. The Journal of Physical Chemistry B, 2010, 114(20): 7078-7089
[39] Wang Z Y, Fu Z Q, Yu Q, et al. Oxidation reactivity of 1,2-bis(2,4,6-tribromophenoxy)ethane (BTBPE) by compound Ⅰ model of cytochrome P450s [J]. Journal of Environmental Sciences (China), 2017, 62: 11-21
[40] Fu Z Q, Wang Y, Wang Z Y, et al. Transformation pathways of isomeric perfluorooctanesulfonate precursors catalyzed by the active species of P450 enzymes: In silico investigation [J]. Chemical Research in Toxicology, 2015, 28(3): 482-489
[41] Fu Z Q, Wang Y, Chen J W, et al. How PBDEs are transformed into dihydroxylated and dioxin metabolites catalyzed by the active center of cytochrome P450s: A DFT study [J]. Environmental Science &Technology, 2016, 50(15): 8155-8163
[42] Chen M, Guo T T, He K Y, et al. Biotransformation and bioconcentration of 6∶2 and 8∶2 polyfluoroalkyl phosphate diesters in common carp (Cyprinus carpio): Underestimated ecological risks [J]. The Science of the Total Environment, 2019, 656: 201-208
[43] Yang S H, Shi Y, Strynar M, et al. Desulfonation and defluorination of 6: 2 fluorotelomer sulfonic acid (6: 2 FTSA) by Rhodococcus jostii RHA1: Carbon and sulfur sources, enzymes, and pathways [J]. Journal of Hazardous Materials, 2022, 423: 127052
[44] Zhang X Q, Liu Y F, Wang Y. The influence of the adjacent hydrogen bond on the hydroxylation processes mediated by cytochrome P450 side-chain cleavage enzyme [J]. Theoretical Chemistry Accounts, 2014, 133(6): 1485
[45] Lee H, De Silva A O, Mabury S A. Dietary bioaccumulation of perfluorophosphonates and perfluorophosphinates in juvenile rainbow trout: Evidence of metabolism of perfluorophosphinates [J]. Environmental Science &Technology, 2012, 46(6): 3489-3497
[46] Just H, Göckener B, Lämmer R, et al. Degradation and plant transfer rates of seven fluorotelomer precursors to perfluoroalkyl acids and F-53B in a soil-plant system with maize (Zea mays L.) [J]. Journal of Agricultural and Food Chemistry, 2022, 70(29): 8920-8930
[47] ECOSAR v2.0. [CP]. Wallingford, CT: U.S. Environmental Protection Agency (US EPA). https://www.epa.gov/tsca-screening-tools/ecological-structure-activity-relationships-ecosar-predictive-model (2023-03-06)
[48] Mitchell R J, Myers A L, Mabury S A, et al. Toxicity of fluorotelomer carboxylic acids to the algae Pseudokirchneriella subcapitata and Chlorella vulgaris, and the amphipod Hyalella azteca [J]. Ecotoxicology and Environmental Safety, 2011, 74(8): 2260-2267
[49] Rand A A, Rooney J P, Butt C M, et al. Cellular toxicity associated with exposure to perfluorinated carboxylates (PFCAs) and their metabolic precursors [J]. Chemical Research in Toxicology, 2014, 27(1): 42-50
[50] D’Eon J C, Mabury S A. Exploring indirect sources of human exposure to perfluoroalkyl carboxylates (PFCAs): Evaluating uptake, elimination, and biotransformation of polyfluoroalkyl phosphate esters (PAPs) in the rat [J]. Environmental Health Perspectives, 2011, 119(3): 344-350
[51] Martin J W, Mabury S A, O’Brien P J. Metabolic products and pathways of fluorotelomer alcohols in isolated rat hepatocytes [J]. Chemico-Biological Interactions, 2005, 155(3): 165-180
[52] Lim X. Can microbes save us from PFAS? [J]. ACS Central Science, 2021, 7: 3-6
[53] Li Y W, Yue Y, Zhang H X, et al. Harnessing fluoroacetate dehalogenase for defluorination of fluorocarboxylic acids: In silico and in vitro approach [J]. Environment International, 2019, 131: 104999
[54] Peskett S T, Rand A A. The human fecal microbiome contributes to the biotransformation of the PFAS surfactant 8: 2 monosubstituted polyfluoroalkyl phosphate ester [J]. Environmental Science Processes &Impacts, 2022, 24(10): 1758-1768
[55] Lonsdale R, Houghton K T, ![]() J, et al. Quantum mechanics/molecular mechanics modeling of regioselectivity of drug metabolism in cytochrome P450 2C9 [J]. Journal of the American Chemical Society, 2013, 135(21): 8001-8015
J, et al. Quantum mechanics/molecular mechanics modeling of regioselectivity of drug metabolism in cytochrome P450 2C9 [J]. Journal of the American Chemical Society, 2013, 135(21): 8001-8015
[56] Yadav S, Shaik S, Siddiqui S A, et al. Local electric fields dictate function: The different product selectivities observed for fatty acid oxidation by two deceptively very similar P450-peroxygenases OleT and BSβ [J]. Journal of Chemical Information and Modeling, 2022, 62(4): 1025-1035