抗生素是一类由微生物产生的次生代谢产物以及人工合成的类似化合物,能抑制其他微生物的生长[1-2],是治疗细菌感染的特效药物并可作为动物生长促进剂,广泛应用于医疗卫生、畜禽和水产养殖等方面[3-4]。由于生物机体对抗生素的吸收和代谢率较低,大量抗生素随尿液和粪便排出体外[5-6],经污水管网的收集进入城市污水处理厂[7-8]。虽然城市污水处理厂是抗生素进入自然水环境之前的最后一道屏障,但目前主流的城市污水处理工艺是以去除化学需氧量(COD)、氮、磷等常规污染物为主要目的,而并非针对抗生素。研究者普遍认为城市污水处理厂无法有效去除污水中的抗生素[9-12],但污水处理工艺和处理单元对抗生素的去除率还不明确,甚至还存在一些争议[13-16]。
城市污水处理厂每天都有大量的处理出水排放至受纳水体,由于抗生素并不能被完全去除,城市污水处理厂就成为自然水体中抗生素的重要来源[17-18]。尽管经过水体的稀释,抗生素的浓度极低,但长期暴露仍会对受纳水体的水生生物产生不良影响[19-22],且有可能通过食物链对人类健康产生潜在影响[23]。在2022年,我国发布了《新污染物治理行动方案》,特别指出要加强对环境介质中抗生素的监测和治理[24-25]。因此,城市污水处理厂受纳水体中的抗生素生态风险是十分值得关注的问题。然而由于抗生素种类繁多,且各地区使用情况不尽相同,加之城市污水处理工艺多样,目前这方面的研究还有待补充。
本研究选取西安市2座城市污水处理厂进行取样调查,对常见的3大类9种抗生素进行定量检测,利用风险商值法评价污水处理厂排放的抗生素对受纳水体造成的生态风险,以揭示城市污水处理工艺对抗生素的去除效果及其对受纳水体水生态的影响,以期为污水处理技术改进和城市水环境质量提升提供科学依据。
1 材料与方法(Materials and methods)
1.1 样品采集
本研究选取西安市的2座污水处理厂(WWTP-C和WWTP-E)进行水样采集,WWTP-C以奥贝尔氧化沟为主,处理量为20万m3·d-1,出水排放至年径流量为2.22亿m3的浐河;WWTP-E则是以AAO(厌氧-缺氧-好氧)作为核心处理工艺,处理量为50万m3·d-1,年径流量为5.86亿m3的灞河为其受纳水体,2座污水处理厂主要接纳和处理生活污水。在2021年4月—6月,采集各处理单元水样,采样点的布设位置如图1所示,标三角符号处即为采样点。使用预先润洗的采样瓶,在每个采样点采集1 L水样,现场测定pH和溶解氧。水样置于含有冰袋的保温箱中,在4 h内运回实验室并进行处理。在实验室测定氨氮、硝酸盐氮、总溶解性固体(TDS)、COD等常规水质指标[26-27]。
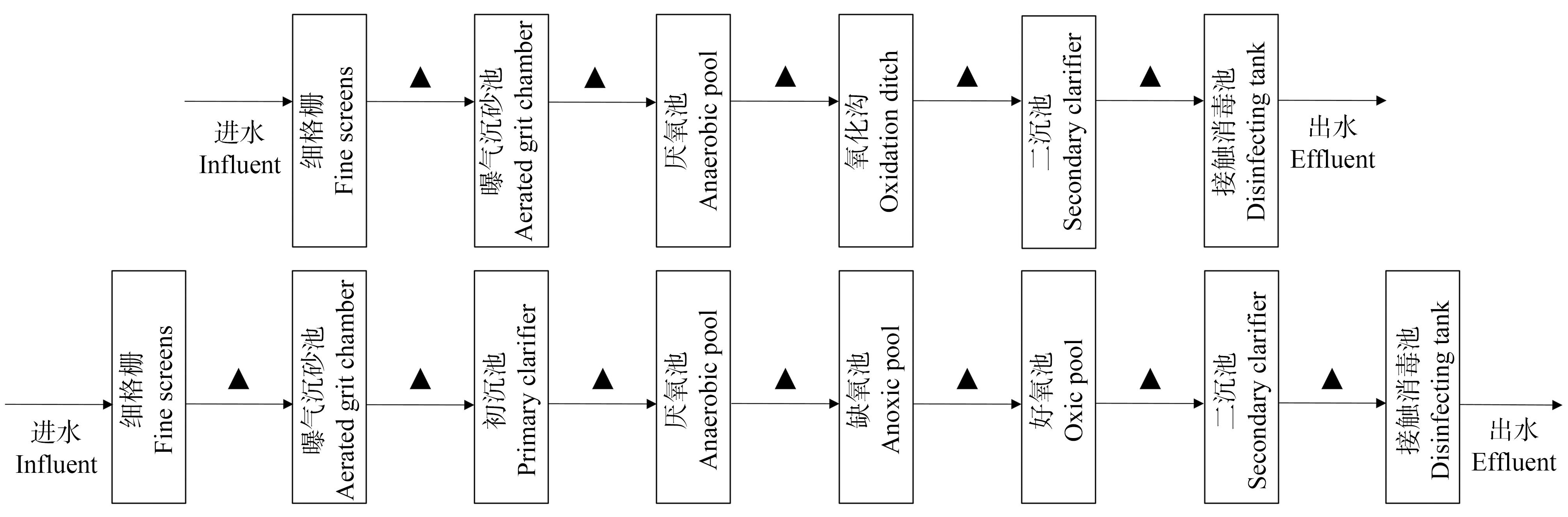
图1 污水厂流程图及采样点位置示意图
注:▲为采样点;(a) WWTP-C,(b) WWTP-E。
Fig. 1 Schematic diagram of the WWTPs and the sampling site locations
Note: ▲ is the sampling collection point; (a) WWTP-C, (b) WWTP-E.
1.2 实验试剂
选取9种目标抗生素,分别为:四环素(tetracyclines, TET)、金霉素(chlortetracycline, CTC)、土霉素(oxytetracycline, OTC)、环丙沙星(ciprofloxacin, CIP)、诺氟沙星(norfloxacin, NOR)、恩诺沙星(enrofloxacin, ENR)、磺胺嘧啶(sulfadiazine, SDZ)、磺胺甲基嘧啶(sulfamerazine, SMR)、磺胺甲恶唑(sulfamethoxazole, SMZ)。各抗生素标准品浓度均为1 000 μg·mL-1,纯度>99%(天津阿尔塔科技有限公司,中国)。乙腈、甲醇、甲酸为色谱纯(美国Fisher公司),无水硫酸钠、氯化钠、十二水合磷酸二氢钠、乙二酸四乙酸二钠等均为化学纯(国药集团化学试剂有限公司),检测用水为美国密理博公司Synergy型超纯水系统制备的超纯水。
1.3 抗生素的定量检测
水样预处理方法在以往研究[28-30]的基础上进行了调整:水样经0.45 μm滤膜过滤,向200 mL滤过液中加入1 mL Na2EDTA-Mcllvaine缓冲溶液,涡旋混匀(IKA MS3 Basic型涡旋仪,广州艾卡仪器设备有限公司,中国)。利用Smart prep全自动固相萃取装置(美国Horizon公司),以5 mL·min-1的流速通过HLB固相萃取柱(美国Waters公司),利用6 mL的超纯水进行洗脱,再用12 mL甲醇进行洗脱。经氮气将洗脱液吹至干后,用0.1%甲酸水溶液-乙腈(体积比9∶1)定容至1 mL,再用0.22 μm针头滤器过滤后装入棕色小瓶待测。
取预处理后的样品进行UPLC-MS/MS(QTRAPTM 5500 LC/MS/MS系统,美国SCIEX公司)测定,使用沃特世Cortecs T3色谱柱(2.1 mm×100 mm, 2.7 μm),液相色谱进样量2 μL,流速0.3 mL·min-1,柱温40 ℃,使用流动相为0.1%甲酸水溶液-乙腈梯度洗脱。质谱离子源为电喷雾电离(electrospray ionization, ESI)(+),离子源接口电压5 500 V,气帘气206.84 kPa,源温度550 ℃;雾化气310.26 kPa,辅助气310.26 kPa。采用多反应监测模式(multiple reactions monitoring, MRM)进行扫描。
1.4 抗生素去除率计算和数据统计分析
分别采用式(1)和式(2)计算抗生素的去除率、某单元对抗生素去除的贡献率:
Rn=[(C0-Cn)/C0]×100%
(1)
Pn=Rn/RT×100%
(2)
式中:Rn为不同处理阶段对抗生素的去除率,C0为进水抗生素浓度,Cn为不同处理阶段的出水中抗生素浓度,Pn为不同处理阶段对抗生素去除的贡献率,RT为抗生素的总去除率。
采用Microsoft Excel 2016和SPSS 23.0对数据进行处理和分析,采用Origin 8.5对数据图像进行处理优化。选择独立样本t检验对不同样品之间的差异性进行分析,利用Spearman相关性分析对水质指标和抗生素浓度之间的关联性进行分析。
1.5 生态风险评价
利用风险商值法评价污水处理厂排放抗生素对受纳水体造成的生态风险[31-32]。计算公式如下:
RQS=MEC/PNEC
(3)
PNEC=EC50/AF
(4)
式中:RQs为风险商值,MEC为受纳水体中抗生素的估算浓度(ng·L-1),PNEC为预测无效应浓度(ng·L-1),EC50为半数效应浓度(ng·L-1),AF为评估因子。根据Hernando等[33]提出的RQ分类方法,表征生态风险的不同程度可将RQ分为4类:RQ<0.01为无风险,0.01≤RQ<0.1为低风险,0.1≤RQ<1为中风险,RQ≥1为高风险.不同抗生素的EC50和PNEC通过在ECOTOX数据库(US EPA)中查找获得[34-35],每种抗生素对应的水生生物毒性敏感数据如表1所示。基于最坏情况考虑,PNEC根据最敏感物种计算。
表1 目标抗生素对应最敏感物种的毒理数据
Table 1 Aquatic toxicity data of antibiotics to the most sensitive aquatic species
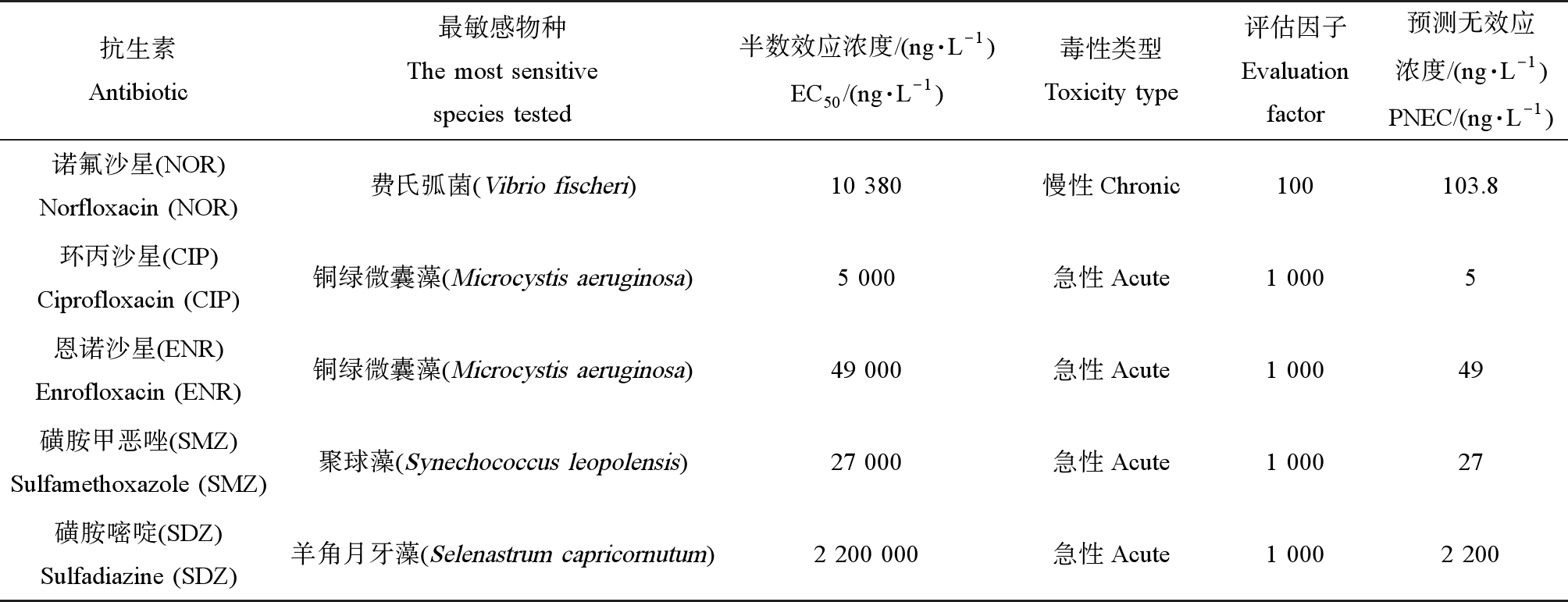
抗生素Antibiotic最敏感物种The most sensitivespecies tested半数效应浓度/(ng·L-1)EC50/(ng·L-1)毒性类型Toxicity type评估因子Evaluationfactor预测无效应浓度/(ng·L-1)PNEC/(ng·L-1)诺氟沙星(NOR)Norfloxacin (NOR)费氏弧菌(Vibrio fischeri)10 380慢性Chronic100103.8环丙沙星(CIP)Ciprofloxacin (CIP)铜绿微囊藻(Microcystis aeruginosa)5 000急性Acute1 0005恩诺沙星(ENR)Enrofloxacin (ENR)铜绿微囊藻(Microcystis aeruginosa)49 000急性Acute1 00049磺胺甲恶唑(SMZ)Sulfamethoxazole (SMZ)聚球藻(Synechococcus leopolensis)27 000急性Acute1 00027磺胺嘧啶(SDZ)Sulfadiazine (SDZ)羊角月牙藻(Selenastrum capricornutum)2 200 000急性Acute1 0002 200
2 结果与讨论(Results and discussion)
2.1 抗生素在污水处理厂中的含量水平
2座具有不同主体工艺的污水处理厂,各单元出水中抗生素的检出浓度如图2所示。在WWTP-C污水处理厂共检出4种抗生素,分别是诺氟沙星(NOR)42.5~129.1 ng·L-1、环丙沙星(CIP)37.0~113.0 ng·L-1、恩诺沙星(ENR)28.0~45.8 ng·L-1和磺胺甲恶唑(SMZ)30.5~212.5 ng·L-1;在WWTP-E污水处理厂共检出5种抗生素,分别是NOR 61.4~349.7 ng·L-1、CIP 45.3~259.7 ng·L-1、ENR 27.3~51.7 ng·L-1、SMZ 47.3~163.7 ng·L-1和磺胺嘧啶(SDZ)26.0~110.0 ng·L-1;3种四环素类抗生素在2座污水处理厂中都没有检出。细格栅仅拦截水中粗大悬浮物和漂浮杂质,故可以使用细格栅出水来代表污水处理厂进水水质。尽管2座污水处理厂进水中浓度最高的抗生素种类有所差别,但总体来看NOR、CIP和SMZ是城市污水厂进水中最主要的3种抗生素,其浓度都在100 ng·L-1以上。在污水厂进水中所检出的多种抗生素,在消毒后的排放出水中仍然被检出,这表明城市污水处理工艺并不能完全去除抗生素[36-37]。

图2 不同抗生素在各污水厂的浓度
注:(a) WWTP-C中的抗生素浓度,(b) WWTP-E中的抗生素浓度;图中C1~C6分别代表进水、曝气沉砂池、厌氧池、氧化沟、二沉池和最终出水;E1~E8分别代表进水、曝气沉砂池、初沉池、厌氧池、缺氧池、好氧池、二沉池和最终出水。
Fig. 2 The abundance of different antibiotics in two WWTPs
Note: (a) WWTP-C, (b) WWTP-E; C1~C6 represent influent water, aerated grit chamber, anaerobic chamber, oxic chamber, secondary sedimentation tank and effluent water, respectively; E1~E8 represents influent water, aerated grit chamber, primary sedimentation tank, anaerobic chamber, anoxic chamber, oxic chamber, secondary sedimentation tank and effluent water, respectively.
2座污水处理厂各单元对抗生素的去除率如图3所示。整体来看,WWTP-E对NOR和CIP的去除率约80%,优于WWTP-C;而WWTP-C则对SMZ有较高的去除率(85.64%);2座污水处理厂对ENR的去除效果不佳,去除率都不足50%。在WWTP-C中,二级生物处理单元对NOR和CIP的去除率分别为(0.43±0.07)%和(0.50±0.04)%;在WWTP-E中,二级生物处理单元对NOR和CIP的去除率分别为(0.38±0.03)%和(0.70±0.02)%。因此,城市污水处理厂对NOR和CIP的去除主要来自二级生物处理单元,贡献率分别高达46.21%~64.09%和76.71%~89.21%。SMZ的去除情况却与此大相径庭,WWTP-C和WWTP-E对SMZ的去除率分别为76.36%和85.64%,但SMZ的浓度在二级生物处理单元前后没有明显变化。例如在WWTP-E中,初沉池出水中SMZ浓度为116.0 ng·L-1,二沉池出水SMZ浓度为72.3 ng·L-1。一级处理和氯消毒是SMZ去除的主要原因,其去除贡献率分别为30.49%~40.97%和32.69%~57.31%。

图3 不同抗生素在各污水厂的去除率
注:(a) WWTP-C;(b) WWTP-E。
Fig. 3 The removal rate of different antibiotics in two WWTPs
Note: (a) WWTP-C; (b) WWTP-E.
由此可见,城市污水处理工艺以及处理单元对不同抗生素的去除效果差别很大。这主要与抗生素的物理化学性质和污水处理设施的特性有关[38]。有研究表明,在污水处理过程中喹诺酮类抗生素从水相去除的主要途径是污泥吸附[39],通过调整工艺参数和运行条件,有望获得良好的去除效果。本研究的结果显示出二级生物处理单元对喹诺酮类抗生素中的典型代表——NOR和CIP有良好的去除效果,且以AAO为主体的生物处理工艺效果更优,一般来说AAO工艺能把硝化功能发挥得比较好,故该工艺对NOR和CIP的较高去除率可能与硝化过程有一定联系。目前,有关使用传统工艺的污水处理厂对磺胺类抗生素的去除作用研究并不充分,但很多研究者都认为在不使用特定菌种的情况下生物处理难以对污水中的磺胺类抗生素起到有效的降解[40-41]。在本研究的2座污水处理厂中,均显示出氯消毒对SMZ有较高的去除率。丁朋飞等[42]的研究表明次氯酸钠对磺胺二甲氧嗪的降解效率在95%,且反应速率常数随着余氯初始浓度增大而增大,这可能与氯消毒的氯代机制[43]和磺胺类抗生素的分子结构有关[44-45]。
值得注意的是,厌氧处理对于SMZ不仅没有去除作用,反而会使水中的SMZ浓度有所升高。对于SDZ,也是同样的结果。类似的现象在以往的研究中也有报道,例如崔迪等[15]的综述指出部分污水处理厂中的磺胺类抗生素经处理后浓度有所增加,这与邵天华等[46]得到的研究结果相类似,而Göbel等[47]也发现,污水厂出水中磺胺类抗生素浓度反而比进水浓度升高1倍,出现“负去除”现象。这可能是由于磺胺类抗生素在好氧段被转化为其他物质,而这些物质到了厌氧段又重新变为磺胺类,导致出水中浓度升高。污水处理过程中确实存在与之类似的现象,例如Zonja等[48]研究发现拉莫三嗪与其代谢产物LMG-N2-G在生物处理过程中会发生相互转化,导致出现“负去除”现象,Wang和Gardinali[49]发现污水厂中卡马西平去除率存在波动的主要原因是其代谢物在污水处理过程中转化为了母体。此外,包裹在粪便、污泥颗粒中的抗生素在污水处理过程中会逐渐释放到水里[50],导致抗生素去除效果不佳。
2.2 常规水质指标与抗生素浓度的相关性
通过对各水样中的常规水质指标和抗生素浓度的相关性分析发现,氨氮与2座污水处理厂检出的所有抗生素浓度均呈显著正相关(P<0.05),这是一个值得关注的结果。以往一些研究[51-53]也报道过类似的现象。Fan等[54]曾指出,低浓度的抗生素可以促进细菌的脱氮能力,但浓度较高时会呈现抑制效果。Dorival-García等[55]也发现硝化过程对氟喹诺酮类抗生素降解率能高达60%。这些都表明抗生素的去除可能与生物脱氮具有某种联系。2座污水处理厂检出的各种抗生素也与COD存在显著正相关(P<0.05)。从一级处理到二级生物处理,污水中的COD随着污水处理流程持续下降,抗生素浓度变化也呈现出相同的趋势(图4)。故从污水处理的整体流程上看,它们有显著的相关性。有一些指标,例如硝氮、pH、TDS,在某座污水处理厂或对某些抗生素也呈现出相关性。但由于其变化范围较小,确认它们与抗生素存在某种联系还有待更深入的研究。
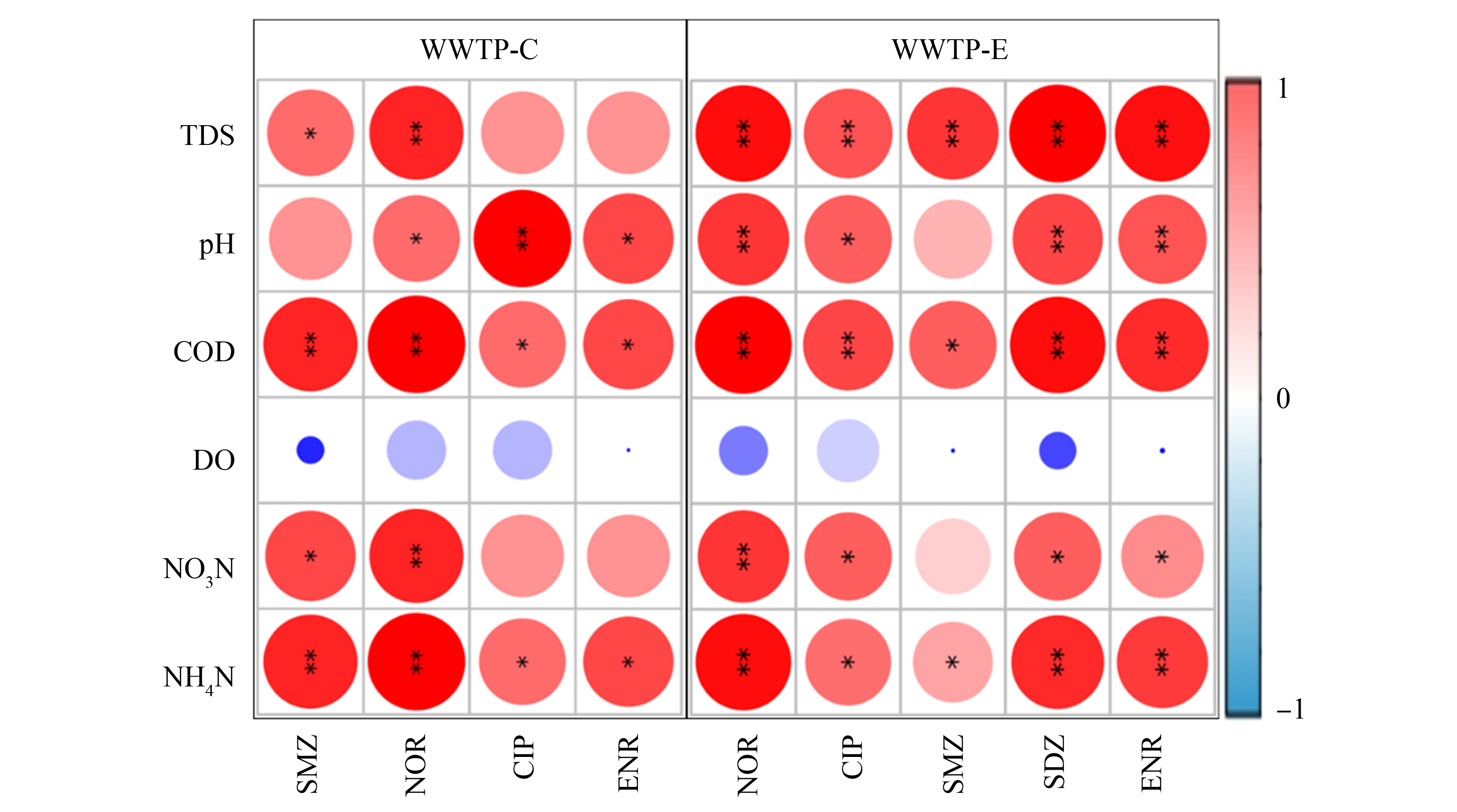
图4 不同污水厂抗生素浓度与常规水质指标的Spearman相关性
注:TDS表示总溶解性固体,COD表示化学需氧量,DO表示溶解氧;*和**分别表示P<0.05、P<0.01显著相关。
Fig. 4 Spearman correlations between antibiotic abundance and water quality parameters in two WWTPs
Note: TDS represents total dissolved solids, COD represents chemical oxygen demand and DO represents dissolved oxygen;*and **denote significant correlation at P<0.05 and P<0.01.
2.3 受纳水体抗生素的生态风险评价
2.3.1 污水处理厂抗生素排放量
对于本研究检出的抗生素,WWTP-C和WWTP-E每日排放总量分别为28.80 g和109.70 g。其中,喹诺酮类抗生素日排放量分别为22.70 g和73.03 g,磺胺类抗生素日排放量分别为6.10 g和36.67 g(图5)。显然,随污水厂处理出水排放至受纳水体的喹诺酮类抗生素最值得关注。
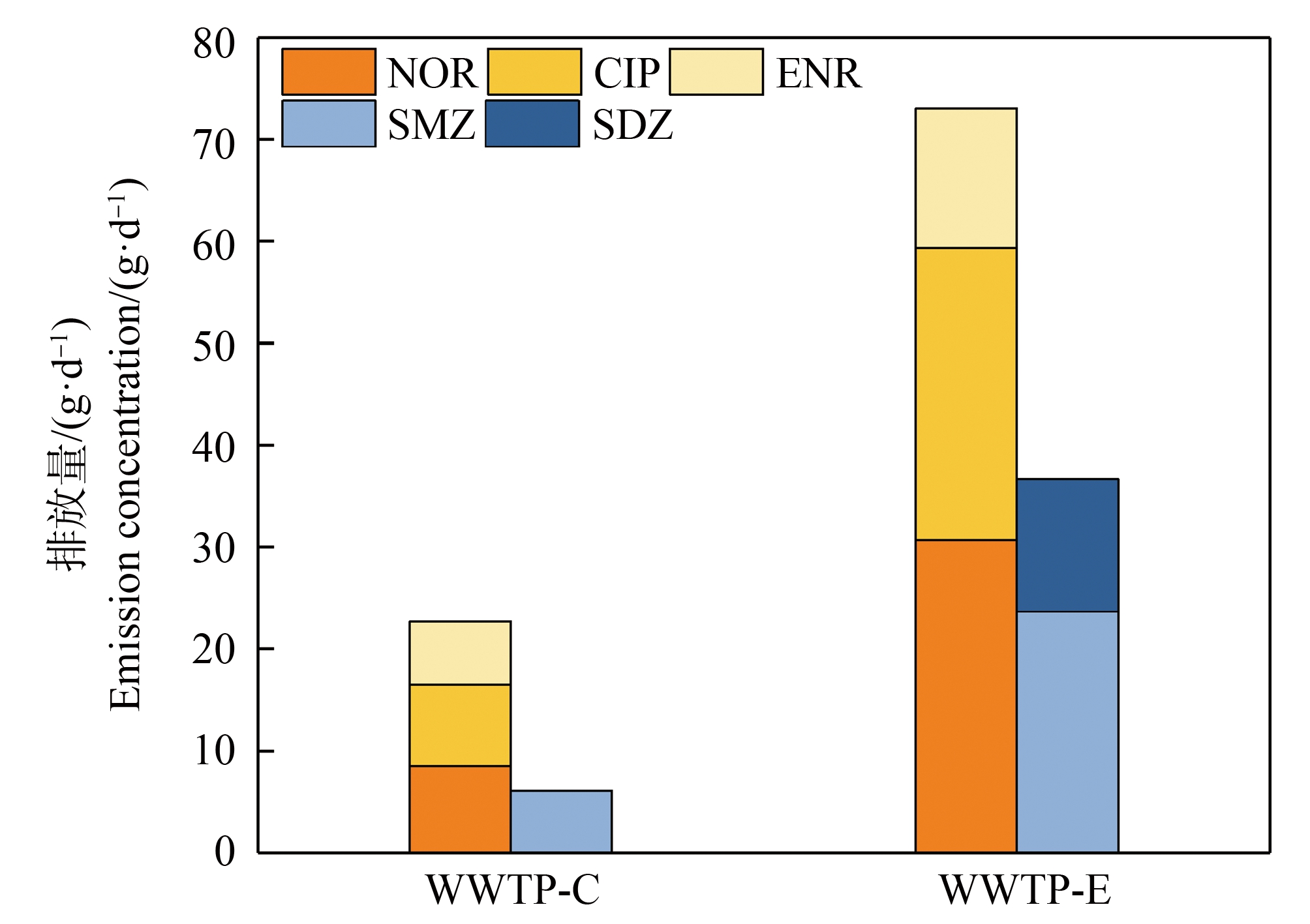
图5 污水处理厂喹诺酮类和磺胺类抗生素的排放量
Fig. 5 Quantity of sulfonamide and fluroauinolone antibiotics in WWTP effluent
2.3.2 受纳水体中抗生素污染物的生态风险评价
对于这2座污水处理厂的受纳水体,抗生素造成的生态风险商值如表2所示。NOR、ENR和SMZ的RQ均在0.1~1范围内,为中风险;而CIP在浐河和灞河的RQ则分别高达2.631和3.571,属于高风险。SDZ在灞河的RQ值仅为0.004,为低风险。
表2 受纳水体抗生素风险商值
Table 2 Antibiotic risks quotient in receiving waters

注:ND为由于实测未检出,故无法计算。
Note: ND can not be calculated because it is not detected in waters.
抗生素Antibiotics浐河the Chanhe River灞河the Bahe River抗生素估算浓度(MEC)/(ng·L-1)Measured environmental concentration (MEC)/(ng·L-1)风险商值(RQ)Risk quotient (RQ)抗生素估算浓度(MEC)/(ng·L-1)Measured environmental concentration (MEC)/(ng·L-1)风险商值(RQ)Risk quotient (RQ)平均风险商值(RQ)Mean risk quotient (RQ)风险等级Risk levelNOR13.9750.13519.1220.1840.160中风险Medium riskCIP13.1532.63117.8543.5712.101高风险High riskENR10.1940.2088.5110.1740.191中风险Medium riskSMZ10.0290.37114.7400.5460.459中风险Medium riskSDZNDND8.0970.0040.004低风险Low risk
总体来看,相较磺胺类抗生素,2处受纳水体中喹诺酮类抗生素的生态风险更高,对水生态影响更大。在我国其他地区地表水和地下水的调研中也发现了喹诺酮类抗生素具有较高的生态风险,例如张晓娇等[56]对辽河流域中的抗生素进行风险分析,发现河水中CIP、NOR、ENR等喹诺酮类抗生素均处于高风险,魏红等[57]发现渭河表层水NOR、CIP也均处于高风险,而杨大杰等[58]的调研结果表明华北和西南地区地表水和地下水中的喹诺酮类抗生素存在高生态风险。
从本研究结果来看,污水处理厂排放出水是受纳水体中喹诺酮类抗生素的重要来源,其造成的生态风险应得到重视。在喹诺酮类抗生素中,尤其需要关注CIP。由表2可知,虽然CIP在受纳水体中的MEC低于NOR,但其生态风险却较高。这也意味着CIP对水生生物具有较高的毒性。
综上所述,本研究结果表明:
(1)有5种抗生素在城市污水处理厂中被检出,分别为诺氟沙星、环丙沙星、恩诺沙星、磺胺甲恶唑和磺胺嘧啶,检出浓度范围在25.0~349.7 ng·L-1之间。诺氟沙星、环丙沙星和磺胺甲恶唑是城市污水厂进水中最主要的3种抗生素,其浓度都在100 ng·L-1以上,且在出水中依然存在。
(2)二级生物处理单元对诺氟沙星和环丙沙星有良好的去除效果,氯消毒对磺胺甲恶唑有较高的去除率。在城市污水处理工艺的厌氧处理段,水中磺胺类抗生素浓度会出现升高。污水中的氨氮浓度和COD,与检出的抗生素存在显著相关性。
(3)污水处理厂受纳水体中,诺氟沙星、恩诺沙星、磺胺甲恶唑造成的生态风险等级均在中风险水平(0.1≤RQ<1),而环丙沙星为高风险(RQ=3.10>1),可能对受纳水体水生态系统产生较大影响。
通信作者简介:张崇淼(1978—),男,博士,教授,博士生导师,研究方向为污染物毒理与风险控制。
[1] Demain A L, Sanchez S. Microbial drug discovery: 80 years of progress [J]. The Journal of Antibiotics, 2009, 62(1): 5-16
[2] Ben Y J, Fu C X, Hu M, et al. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review [J]. Environmental Research, 2019, 169: 483-493
[3] Zhu Y G, Johnson T A, Su J Q, et al. Diverse and abundant antibiotic resistance genes in Chinese swine farms [J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(9): 3435-3440
[4] Rodriguez-Mozaz S, Chamorro S, Marti E, et al. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river [J]. Water Research, 2015, 69: 234-242
[5] Qin Y H, Ma S T. Recent advances in the development of macrolide antibiotics as antimicrobial agents [J]. Mini Reviews in Medicinal Chemistry, 2020, 20(7): 601-625
[6] Zhou X Q, Cuasquer G J P, Li Z F, et al. Occurrence of typical antibiotics, representative antibiotic-resistant bacteria, and genes in fresh and stored source-separated human urine [J]. Environment International, 2021, 146: 106280
[7] Monahan C, Harris S, Morris D, et al. A comparative risk ranking of antibiotic pollution from human and veterinary antibiotic usage - An Irish case study [J]. The Science of the Total Environment, 2022, 826: 154008
[8] Tran N H, Hoang L, Nghiem L D, et al. Occurrence and risk assessment of multiple classes of antibiotics in urban canals and lakes in Hanoi, Vietnam [J]. The Science of the Total Environment, 2019, 692: 157-174
[9] Osińska A, Korzeniewska E, Harnisz M, et al. Small-scale wastewater treatment plants as a source of the dissemination of antibiotic resistance genes in the aquatic environment [J]. Journal of Hazardous Materials, 2020, 381: 121221
[10] Renau-Pru onosa A, García-Menéndez O, Ibá
onosa A, García-Menéndez O, Ibá![]() ez M, et al. Identification of aquifer recharge sources as the origin of emerging contaminants in intensive agricultural areas. La Plana de castellón, Spain [J]. Water, 2020, 12(3): 731
ez M, et al. Identification of aquifer recharge sources as the origin of emerging contaminants in intensive agricultural areas. La Plana de castellón, Spain [J]. Water, 2020, 12(3): 731
[11] de Santiago-Martín A, Meffe R, Teijón G, et al. Pharmaceuticals and trace metals in the surface water used for crop irrigation: Risk to health or natural attenuation? [J]. Science of the Total Environment, 2020, 705: 135825
[12] Fan J J, Wang S, Tang J P, et al. Bioaccumulation of endocrine disrupting compounds in fish with different feeding habits along the largest subtropical river, China [J]. Environmental Pollution, 2019, 247: 999-1008
[13] Grandclément C, Seyssiecq I, Piram A, et al. From the conventional biological wastewater treatment to hybrid processes, the evaluation of organic micropollutant removal: A review [J]. Water Research, 2017, 111: 297-317
[14] Tran N H, Chen H J, Reinhard M, et al. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes [J]. Water Research, 2016, 104: 461-472
[15] 崔迪, 邓红娜, 庞长泷, 等. 生物法去除水环境中磺胺甲恶唑的研究进展[J]. 中国给水排水, 2019, 35(24): 32-38
Cui D, Deng H N, Pang C L, et al. Progress in the study of the removal of sulfamethoxazole by biological methods in water environment [J]. China Water &Wastewater, 2019, 35(24): 32-38 (in Chinese)
[16] Ramírez-Morales D, Masís-Mora M, Montiel-Mora J R, et al. Occurrence of pharmaceuticals, hazard assessment and ecotoxicological evaluation of wastewater treatment plants in Costa Rica [J]. The Science of the Total Environment, 2020, 746: 141200
[17] Russell J N, Yost C K. Alternative, environmentally conscious approaches for removing antibiotics from wastewater treatment systems [J]. Chemosphere, 2021, 263: 128177
[18] Fu J M, Zhao Y Q, Yao Q, et al. A review on antibiotics removal: Leveraging the combination of grey and green techniques [J]. The Science of the Total Environment, 2022, 838(Pt 3): 156427
[19] Segura P A, François M, Gagnon C, et al. Review of the occurrence of anti-infectives in contaminated wastewaters and natural and drinking waters [J]. Environmental Health Perspectives, 2009, 117(5): 675-684
[20] Zhou J, Yun X, Wang J T, et al. A review on the ecotoxicological effect of sulphonamides on aquatic organisms [J]. Toxicology Reports, 2022, 9: 534-540
[21] Kovalakova P, Cizmas L, McDonald T J, et al. Occurrence and toxicity of antibiotics in the aquatic environment: A review [J]. Chemosphere, 2020, 251: 126351
[22] Duan W Y, Cui H W, Jia X Y, et al. Occurrence and ecotoxicity of sulfonamides in the aquatic environment: A review [J]. The Science of the Total Environment, 2022, 820: 153178
[23] 王慧, 王晨, 田业超, 等. 城市污水处理厂及其受纳水体中典型PPCPs的分布特征及其生态风险评价[J]. 环境科学学报, 2023, 43(4): 339-349
Wang H, Wang C, Tian Y C, et al. Distribution characters and ecological risk assessment of typical PPCPs in sewage treatment plant and its receiving water [J]. Acta Scientiae Circumstantiae, 2023, 43(4): 339-349 (in Chinese)
[24] 姚丽君, 莫凌, 庄僖, 等. 海南省典型饮用水源地水体与沉积物中抗生素的残留及风险评价[J]. 生态毒理学报, 2022, 17(5): 349-361
Yao L J, Mo L, Zhuang X, et al. Residual and risk assessment of antibiotics in water and sediments of typical drinking water sources in Hainan Province [J]. Asian Journal of Ecotoxicology, 2022, 17(5): 349-361 (in Chinese)
[25] 中华人民共和国国务院办公厅. 新污染物治理行动方案[R]. 北京: 国务院办公厅, 2022
[26] Zhang C M, Liang J, Liu W Y. Comparative study on the bacterial diversity and antibiotic resistance genes of urban landscape waters replenished by reclaimed water and surface water in Xi’an, China [J]. Environmental Science and Pollution Research International, 2021, 28(30): 41396-41406
[27] Li Y Q, Zhang C M, Mou X, et al. Distribution characteristics of antibiotic resistance bacteria and related genes in urban recreational lakes replenished by different supplementary water source [J]. Water Science and Technology: A Journal of the International Association on Water Pollution Research, 2022, 85(4): 1176-1190
[28] 李彦文, 莫测辉, 赵娜, 等. 菜地土壤中磺胺类和四环素类抗生素污染特征研究[J]. 环境科学, 2009, 30(6): 1762-1766
Li Y W, Mo C H, Zhao N, et al. Investigation of sulfonamides and tetracyclines antibiotics in soils from various vegetable fields [J]. Environmental Science, 2009, 30(6): 1762-1766 (in Chinese)
[29] Azanu D, Styrishave B, Darko G, et al. Occurrence and risk assessment of antibiotics in water and lettuce in Ghana [J]. Science of the Total Environment, 2018, 622-623: 293-305
[30] Zhang R J, Tang J H, Li J, et al. Occurrence and risks of antibiotics in the coastal aquatic environment of the Yellow Sea, North China [J]. The Science of the Total Environment, 2013, 450-451: 197-204
[31] Zou M Y, Tian W J, Zhao J, et al. Quinolone antibiotics in sewage treatment plants with activated sludge treatment processes: A review on source, concentration and removal [J]. Process Safety and Environmental Protection, 2022, 160: 116-129
[32] Chen H Y, Jing L J, Teng Y G, et al. Characterization of antibiotics in a large-scale river system of China: Occurrence pattern, spatiotemporal distribution and environmental risks [J]. Science of the Total Environment, 2018, 618: 409-418
[33] Hernando M D, Mezcua M, Fernández-Alba A R, et al. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments [J]. Talanta, 2006, 69(2): 334-342
[34] 李佳乐, 王萌, 胡发旺, 等. 江西锦江流域抗生素污染特征与生态风险评价[J]. 环境科学, 2022, 43(8): 4064-4073
Li J L, Wang M, Hu F W, et al. Antibiotic pollution characteristics and ecological risk assessment in Jinjiang River Basin, Jiangxi Province [J]. Environmental Science, 2022, 43(8): 4064-4073 (in Chinese)
[35] 吴天宇, 李江, 杨爱江, 等. 赤水河流域水体抗生素污染特征及风险评价[J]. 环境科学, 2022, 43(1): 210-219
Wu T Y, Li J, Yang A J, et al. Characteristics and risk assessment of antibiotic contamination in Chishui River Basin, Guizhou Province, China [J]. Environmental Science, 2022, 43(1): 210-219 (in Chinese)
[36] 王依琳, 张蕊, 张强英, 等. 污水中抗生素的分布、来源及去除研究进展[J]. 再生资源与循环经济, 2022, 15(3): 36-41
Wang Y L, Zhang R, Zhang Q Y, et al. Research progress on the occurrence and removal of antibiotics in sewage [J]. Recyclable Resources and Circular Economy, 2022, 15(3): 36-41 (in Chinese)
[37] 耿冲冲, 王亚军. 污水中抗生素生化去除研究进展[J]. 环境监测管理与技术, 2019, 31(3): 12-16, 56
Geng C C, Wang Y J. Research progress of biochemical treatment on antibiotics removal from wastewater [J]. The Administration and Technique of Environmental Monitoring, 2019, 31(3): 12-16, 56 (in Chinese)
[38] Michael I, Rizzo L, McArdell C S, et al. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review [J]. Water Research, 2013, 47(3): 957-995
[39] Oberoi A S, Jia Y Y, Zhang H Q, et al. Insights into the fate and removal of antibiotics in engineered biological treatment systems: A critical review [J]. Environmental Science &Technology, 2019, 53(13): 7234-7264
[40] Joss A, Zabczynski S, Göbel A, et al. Biological degradation of pharmaceuticals in municipal wastewater treatment: Proposing a classification scheme [J]. Water Research, 2006, 40(8): 1686-1696
[41] Rosal R, Rodríguez A, Perdigón-Melón J A, et al. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation [J]. Water Research, 2010, 44(2): 578-588
[42] 丁朋飞, 陆金鑫, 汤慧俐, 等. 次氯酸钠消毒中磺胺二甲氧嗪的降解与风险评价[J]. 土木与环境工程学报(中英文), 2023, 45: 1-10
Ding P F, Lu J X, Tang H L, et al. Degradation and risk assessment of sulfadimethoxine during sodium hypochlorite disinfection process [J]. Journal of Civil and Environmental Engineering, 2023, 45: 1-10 (in Chinese)
[43] 杨帅, 余晓敏, 郭学博, 等. 二氧化氯对典型磺胺类抗生素的降解机制[J]. 环境化学, 2019, 38(1): 34-41
Yang S, Yu X M, Guo X B, et al. Degradation mechanisms of sulfonamides by chlorine dioxide [J]. Environmental Chemistry, 2019, 38(1): 34-41 (in Chinese)
[44] Yang Y J, Shi J C, Yang Y, et al. Transformation of sulfamethazine during the chlorination disinfection process: Transformation, kinetics, and toxicology assessment [J]. Journal of Environmental Sciences, 2019, 76: 48-56
[45] Wang M, Helbling D E. A non-target approach to identify disinfection byproducts of structurally similar sulfonamide antibiotics [J]. Water Research, 2016, 102: 241-251
[46] 邵天华, 贲伟伟, 苏都, 等. 城市污水处理厂污水及污泥中典型药物及其代谢产物的定量检测[J]. 环境科学学报, 2020, 40(6): 2136-2141
Shao T H, Ben W W, Su D, et al. Quantitative determination of typical pharmaceuticals and their metabolites in municipal wastewater treatment plants [J]. Acta Scientiae Circumstantiae, 2020, 40(6): 2136-2141 (in Chinese)
[47] Göbel A, McArdell C S, Joss A, et al. Fate of sulfonamides, macrolides, and trimethoprim in different wastewater treatment technologies [J]. The Science of the Total Environment, 2007, 372(2-3): 361-371
[48] Zonja B, Pérez S, Barceló D. Human metabolite lamotrigine-N(2)-glucuronide is the principal source of lamotrigine-derived compounds in wastewater treatment plants and surface water [J]. Environmental Science &Technology, 2016, 50(1): 154-164
[49] Wang J, Gardinali P R. Identification of phase Ⅱ pharmaceutical metabolites in reclaimed water using high resolution benchtop Orbitrap mass spectrometry [J]. Chemosphere, 2014, 107: 65-73
[50] 温明铎, 陈文兵, 高自豪, 等. 污水处理厂新兴污染物赋存及末端控制技术进展[J]. 净水技术, 2022, 41(5): 14-22
Wen M D, Chen W B, Gao Z H, et al. Technological progress in occurrence and end control of emerging contaminants in WWTP [J]. Water Purification Technology, 2022, 41(5): 14-22 (in Chinese)
[51] 杨尚乐, 王旭明, 王伟华, 等. 松花江哈尔滨段及阿什河抗生素的分布规律与生态风险评估[J]. 环境科学, 2021, 42(1): 136-146
Yang S L, Wang X M, Wang W H, et al. Distribution and ecological risk assessment of antibiotics in the Songhua River Basin of the Harbin section and Ashe River [J]. Environmental Science, 2021, 42(1): 136-146 (in Chinese)
[52] 王嘉玮, 魏红, 杨小雨, 等. 渭河西安段磺胺类抗生素的分布特征及生态风险评价[J]. 环境化学, 2017, 36(12): 2574-2583
Wang J W, Wei H, Yang X Y, et al. Occurrence and ecological risk of sulfonamide antibiotics in the surface water of the Weihe Xi’an section [J]. Environmental Chemistry, 2017, 36(12): 2574-2583 (in Chinese)
[53] Bao Y Y, Li F F, Chen L J, et al. Fate of antibiotics in engineered wastewater systems and receiving water environment: A case study on the coast of Hangzhou Bay, China [J]. The Science of the Total Environment, 2021, 769: 144642
[54] Fan N S, Fu J J, Huang D Q, et al. Resistance genes and extracellular proteins relieve antibiotic stress on the anammox process [J]. Water Research, 2021, 202: 117453
[55] Dorival-García N, Zafra-Gómez A, Navalón A, et al. Removal and degradation characteristics of quinolone antibiotics in laboratory-scale activated sludge reactors under aerobic, nitrifying and anoxic conditions [J]. Journal of Environmental Management, 2013, 120: 75-83
[56] 张晓娇, 柏杨巍, 张远, 等. 辽河流域地表水中典型抗生素污染特征及生态风险评估[J]. 环境科学, 2017, 38(11): 4553-4561
Zhang X J, Bai Y W, Zhang Y, et al. Occurrence, distribution, and ecological risk of antibiotics in surface water in the Liaohe River Basin, China [J]. Environmental Science, 2017, 38(11): 4553-4561 (in Chinese)
[57] 魏红, 王嘉玮, 杨小雨, 等. 渭河关中段表层水中抗生素污染特征与风险[J]. 中国环境科学, 2017, 37(6): 2255-2262
Wei H, Wang J W, Yang X Y, et al. Contamination characteristic and ecological risk of antibiotics in surface water of the Weihe Guanzhong section [J]. China Environmental Science, 2017, 37(6): 2255-2262 (in Chinese)
[58] 杨大杰, 欧阳友, 李炳华, 等. 我国水环境中喹诺酮类抗生素赋存特征及生态风险评估[J]. 人民黄河, 2022, 44(8): 97-102, 108
Yang D J, Ouyang Y, Li B H, et al. Occurrence characteristics and ecological risk assessment of quinolone antibiotics in Chinese aquatic environment [J]. Yellow River, 2022, 44(8): 97-102, 108 (in Chinese)